1. Introduction
Boron is an indispensable component for human and animal taking its major roles in human are for brain function and perception temporary memory [1]. Also boron has protective characteristics against prostate cancer [2]. In the desalination process for seawater, boron is not easily removed because pH of about 6.5 should be maintained by adding acid such as sulfuric acid in order to remove scales such as calcium and silica. Therefore, the normal practice is to convert seawater into fresh water by using the RO (Reverse Osmosis) membrane of two stages, but such practice shows to be inefficient due to high costs of maintenance [3].
Boron can become toxic when its amount is slightly greater than the required amount. The common boron toxication symptoms are nausea, vomiting, diarrhea, dermatitis and lethargy [4]. The WHO guideline of standard boron concentration in drinking water is 0.3 mg/L in 1990. It is difficult to comply to this standard due to the available treatment technology reality. Hence, the guideline of WHO was raised to 0.5 mg/L in 1998. The average concentration of boron of freshwater of less than 0.1 mg/L and below 2 mg/L in ground water become acceptable [5]. That of seawater of boron at around 4.5 mg/L is acceptable [6]. The criteria of the amount of boron in drinking water, established by WHO, is 0.5 mg/L. Every country has distinct criteria based on WHO. For instance, the criteria for United States, although it varies by each states, is approximately 0.6 mg/L, and for Europe, Japan and Korea are 1 mg/L; Australia and Canada have relatively high standards which is about 4~5 mg/L. If adequate amount of boron existing in seawater could be removed during the pre-treatment process, the experiments would only need to carry out the first stage of RO membrane to make it drinkable. The pre-treatment process tested the efficiency of boron removal by injecting the Mineral cluster, which acts as an adsorbent that is extracted from natural mineral. Seawater desalination system was reexamined based on the Lab scale experiment [7] using aqueous solution and Pilot plant experiment using Sea water.
2. Materials and Methods
2.1. Experimental Apparatus
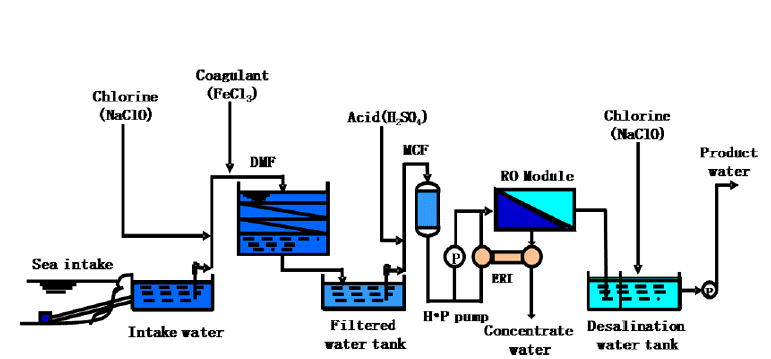
The general process of the seawater desalination is shown in Fig. 1. MF membrane was used in to remove the suspended solids and the colloidal material the pre-treatment process; The FI (Fouling index) was approximately 3.0 in this experiment. The removal efficiency of boron was examined using RO membrane after using the MF membrane in the pre-treatment process. The RO membrane of Spiral type was used, and for the experimental condition for RO membrane, the pressure of inlet water was 55~60 kgf/cm2, the Flux was 0.2~2 m3/m2·day, and the recovery rate was 40%. The boron concentration in the experiment was measured by using the Curcumin method [8] from ‘Standard Method (SMWW)’.
2.2. Condition of Original Seawater
The lab scale experiment used an aqueous solution; all bulk water containing 4.5 mg/L of boron was adjusted to pH 7, 8, 9, 10 solutions with sodium hydroxide (NaOH) and sulfuric acid (H2SO4) respectively. The bulk solution was kept stirred rapidly for 2 minutes to encourage flocculation. The solution was stopped stirring to settle down for 60 minutes at room temperature then filtered with 0.45 μm membrane.
The pilot experiment of spiral wound RO system was used to estimate the boron removal efficiency of Mineral cluster in desalination process. Combining the efficiency of both Mineral cluster and RO system enabled to calculate the final boron removal efficiency.
The seawater samples used in this Pilot plant experiment were attained from the Somuido coast in the western sea of Incheon in South Korea. The electrical conductivity contained in the sea-water was 41000 (μs/cm), and the components of the seawater are as listed in the following Table 1.
2.3. Materials (Mineral cluster)
The Mineral cluster in this experiment, a solution extracted from the Black Mica, Illite using the following device of Fig.2 was used as the coagulant of the pre-treatment process for removing boron. In order to suppress the occurrence of the scale component occurring on the surface of RO membrane, the sulfuric acid was injected into seawater while maintaining the pH of sea water 6.8±0.5. The pilot plan experiment used Mineral cluster instead of Sulfuric acid. The manufacturing process of the Mineral cluster is as the following, and the components are as Table 2.
2.4. Experimental Method
2.4.1. Pre-treatment process using Mineral cluster
In order to investigate the optimal pH and the injection amount of the Mineral cluster, the Jar test was carried out. Sodium Hydroxide (NaOH) and Sulfuric acid (H2SO4) was used with the pH adjusted to all 7, 8, 9 and 10. The Mineral cluster was diluted according to the ratios of 1:100, 1:500, 1:1000 and 1:2500 into the aqueous solution and seawater of 250 mL, and the boron concentrations according to each pH condition were measured.
2.4.2. Boron removal using RO membrane
The experiment of the RO membrane was divided into two types. The first experiment is to verify how much boron would be removed when using solely RO membrane. The second experiment combines RO and Mineral cluster to treat RO membrane after using the Mineral cluster in the pre-treatment process. Cohesion, absorption, oxidation and filtration, etc. are the widely practiced pre-treatment process of RO membrane, but this experiment treated with MF after injecting Mineral cluster.
3. Results and Discussion
3.1. Boron Removal Rate according to pH
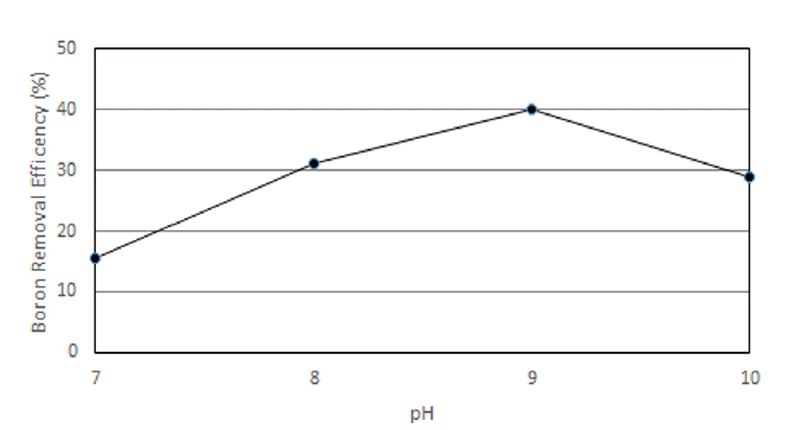
The experiment was carried out to obtain the optimum value of pH condition during the boron removal process. Boron is completely dissociated with B(OH)4− at pH higher than 11 and is formed as H3BO3 at pH lower than 7 [9]. The samples of the western sea of Incheon containing boron concentration of 4.5 mg/L were adjusted to pH of 7, 8, 9, and 10 with 1: 100 of Mineral cluster. Boron removal efficiency showed that it increased with pH value between 8 and 9. The highest efficiency of boron removal is 40.0% and 2.7 mg/L at pH 9 and boron removal is 31.3% and 3.1 mg/L at pH 8 in Fig 3. 28.9% (3.2 mg/L) and 15.6% (3.8 mg/L) of boron was removed at pH 10 and 7 respectively.
Boron removal mechanism can be explained with the coagulation and adsorption mechanism of Alum and Iron. Alum coagulant is successfully removed at the pH range of 5.5 and 8 and Iron does best at pH range of 5.0 and 11.0 [10]. Mineral cluster solution contains a variety of minerals especially Alum (530 mg/L) and Iron (373 mg/L) in high extent amount, which took major roles in the coagulation process. Therefore the optimum efficiency can be deduced to around pH 9 in Mineral cluster.
According to major interactions and forces of atoms and molecules, boron adsorption mechanism can be inferred from those types of molecular and atomic interactions and bonding, such as hydrophilic, hydrophobic and electrostatic interaction but also hydrogen bonding [11]. Hydrogen bonding and hydrophobic interaction can be major roles in the adsorption mechanism of boron which can be formed with boric acid and borates ions from the physicochemical natures of boron particle surfaces. All the particle surfaces of boron have the suitable functional groups enabling hydrogen bonding with boric acid or borate ions which can enhance the adsorption process in well-adjusted aqueous media of pH condition. The equal number of boric acid and borates ions can be formed at the well-adjusted aqueous pH 9 solution which promoted increased hydrogen bonding and electrostatic attraction but also hydrophobic interactions. The relations between molecules containing non polar group and water can be described with hydrophobic interaction in aqueous solution. Thus, the adsorption was reduced due to the lack of hydrophobic interaction at higher pH than 9, but also at the lower pH than 9 the lack of electrostatic force makes the adsorption decrease in substantial extent. Boron removal efficiency clearly improved with increasing Mineral cluster dosage and the optimum pH condition was clarified with pH 9 in the above experiment.
3.2. Optimal Injection Amount of Mineral Cluster
Fig. 4 shows the experimental results of boron removal efficiency according to the amount of Mineral cluster in pH 9 as presented in the above experimental condition. As a result, the boron concentrations were 4.5 mg/L in pH 9 were 4.5 mg/L, 4.3 mg/L, 4.0 mg/L, and 3.7 mg/L when the Mineral cluster was diluted to 100 times, 500 times, 1000 times and 2500 times respectively.
Of the four dilution ratios 1:100, 1:500, 1:1000 and 1:2500, the ratio 1:2500 showed the greatest amount of removal. Because the Mineral cluster itself is a strong acid its pH is lowers when great amounts are injected into the sea water with pH of 7 and 8. In this experiment the boron removal efficiency by Mineral cluster was performed at pH 9. As the quantity of the Mineral cluster increased the removal efficiency decreased and vice versa-due to its acidic nature. Table 2 represents the major components of the Mineral Cluster which are Ca, Fe, Mg, Al, Na, K and Si. Of the components Al is the most with the percentage of 35.3%, and Fe is the second most with 23%. Al and Fe are normally used as coagulants and adsorbents [12]; Aluminum sulfate, ferrous sulfate and ferric sulfate are the commonly used materials containing Al and Fe. Clay minerals are normally used as adsorbents since their porous structure [13, 14]. Therefore, Mineral cluster can remove boron via adsorbing and aggregating.
3.3. Boron Removal Efficiency by RO Membrane System
3.3.1. Boron removal via RO membrane
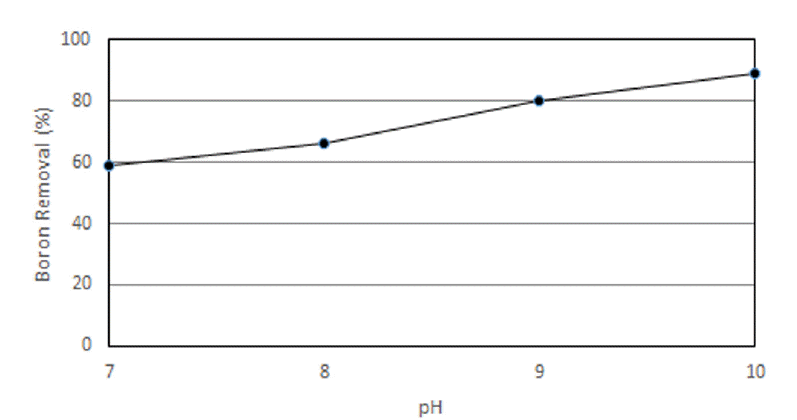
The purpose of this experiment is to investigate the removal efficiency of boron contained in seawater through RO membrane [15]. Fig. 5 shows the measurements of the boron concentration after processing the original seawater collected at Somuido in Incheon. The initial concentration of boron in sea water was 4.5 mg/L. By adjusting the pH of the sea water to pH of 7, 8, 9, 10 the boron concentration changed to 1.85 mg/L (removal rate 59%), 1.53 mg/L(66%), 0.9 mg/L(80%) and 0.5 mg/L(89%), respectively. When the pH of sea water is above 9, boron could be removed up to 1.0 mg/L which is near drinking water quality. In order to solely remove boron, an additional process to pH adjustment is required.
3.3.2. Efficiency of the combination process of mineral cluster and RO membrane system
This experiment examined the boron removal efficiency by removing certain amount of boron through Mineral cluster injection in the pre-treatment process and removing the rest through RO membrane in Fig. 6. The removal of boron in seawater at pH 7, 8, 9, and 10 were 0.96 mg/L (removal rate 74%), 0.65 mg/L (82%), 0.3 mg/L (92%), and 0.1 mg/L (97%) respectively. As shown the above the removal rate was above 90% when pH was adjusted to 9 or higher. When solely processed through RO membrane, the boron concentration was 1.85 mg/L at pH 7. However the concentration turned out to be 0.90 mg/L when the Mineral cluster was injected at the ratio 1:2500. Such value does not concord with the WHO criteria that is 0.5 mg/L but it comply to Korea’s standard, 1.0 mg/L. pH is maintained as a weak acid to prevent the scale in the seawater desalination process. Simple Mineral cluster injection diluted at 1: 2500 is required to lower the pH condition. Therefore, if the desalination process combines Mineral cluster injection in the pre-treatment process and the RO membrane, the whole process would proceed much efficiently and economically.
4. Conclusions
The following results were attained when the process combined Mineral cluster in the pre-treatment process and RO membrane process. Boron removal is depended largely on pH condition and it is difficult to remove by common water treatment method due to its different chemical formation. The solution of pH 9 is best condition for boron removal by Mineral cluster coagulant. Boron can be eliminated until 80% with 1:2500 Mineral clusters at this pH value. The original sea water could be desalinated to drinking water quality, 1 mg/L, without any pH adjustments if the desalination process combines the pre-treatment process with Mineral cluster diluted at the ratio of 1:2500 and the RO membrane process. The desalination process would be much safer, efficient and economical if the Mineral cluster was added without any other chemicals for adjusting pH. Thus the Mineral cluster injection process was well suited to the desalination system.