1. Introduction
Quintinite is a carbonate mineral with a hexagonal crystal system. It has the chemical formula of Mg4Al2(OH)12CO3 · 3H2O, which is included in the family of hydrotalcite-like (HTL) particles [1]. Hydrotalcite is a carbonate mineral that is rare in nature, with the general formula of Mg6Al2(CO3)(OH)16·4(H2O). Hydrotalcite mineral is a layered double hydroxide (LDH), with carbonate anions lying between the structural layers [2]. The HTL particles are similar to hydrotalcite mineral, which can be easily synthesized in the laboratory. They have the general formula of [M(II)1-xM(III)x (OH)2]x+[An−x/n]x− · mH2O, where M(II) is the divalent cation (Mg, Ni, Zn, Co, Mn, etc.), M(III) is the trivalent cation (Al, Fe, Cr, V, etc.), An− is the interlayer anion (Cl−, NO3−, CO32−, SO42−, etc.) of valence n, and x is the molar ratio of M(III)/[M(II)+M(III)]. They consist of positively charged brucite [Mg(OH)2]-like layers [M(II)1−xM(III)x(OH)2]x+ and negatively-charged interlayers [An−x/n]x− mH2O. They are a class of anionic clays with high surface area and large anion exchange capacity [3, 4]. The HTL particles have been used as adsorbents for the removal of oxyanions, including chromate, phosphate, nitrate, borate, arsenate/arsenite, and selenite/selenite [5].
Fluoride is an essential micronutrient for human health that prevents dental caries, and helps dental enamel calcification. However, at concentrations greater than 1.5 mg/L, fluoride can cause dental/skeletal fluorosis and neurological damage [6]. The contamination of fluoride in drinking water resources is a serious environmental problem worldwide. Fluoride contamination in surface and ground water can come from natural geological sources (fluorite, biotites, granite, basalt, etc.), and from industrial waste-water (semiconductor manufacturing, electroplating, glass and ceramic production, etc.) [7]. In many countries, fluoride occurs naturally in groundwater at concentrations exceeding the guidelines of the World Health Organization (1.5 mg/L), causing serious health problems [8]. Adsorption is widely used for fluoride removal from aqueous solutions, due to its cost effectiveness and simplicity of operation. Various adsorbents have been applied for fluoride removal, including activated alumina, activated carbon, granular ferric hydroxide, limestone, fly ash, and clay [9, 10].
Recently, some researchers used Mg/Al, Zn/Al, and Mg/Al/Fe type HTL particles for fluoride removal [6, 11]. Batistella et al. [12] evaluated the effect of acid activation on the removal of fluoride by Mg/Al type particles. Mandal and Mayadevi [13] performed kinetic, equilibrium, and thermodynamic studies for fluoride removal by Zn/Al type particles. Others used mixed metal oxides (MMO) derived from the various HTL particles, via calcination for fluoride removal [14–19]. Cai et al. [7] examined the competitive adsorption of fluoride and phosphate by Mg/Al MMO. Zhou et al. [20] analyzed the characteristics of Li/Al MMO in fluoride adsorption in water. In addition, Mandal and Mayadevi [21] used Zn/Al MMO for fluoride adsorption.
The aim of this study was to characterize quintinite in fluoride removal from aqueous solutions. The characteristics of synthetic quintinite particles were elucidated using transmission electron microscopy (TEM), X-ray diffractometry (XRD), nitrogen gas (N2) adsorption-desorption experiment, and X-ray photoelectron spectroscopy (XPS). Batch experiments were performed to examine the effect of adsorbent dosage, reaction time, initial fluoride concentration, and initial solution pH on the adsorption of fluoride to quintinite. Kinetic and equilibrium isotherm models were used to analyze the batch experimental data.
2. Materials and Methods
2.1. Synthesis and Characterization of Quintinite Particles
All chemicals used for the experiments were purchased from Sigma-Aldrich. Quintinite particles were prepared by the following procedures. The particles were synthesized by co-precipitating mixtures of magnesium nitrate [Mg(NO3)2 · 6H2O] and aluminum nitrate [Al(NO3)3 · 9H2O]. A 700 mL solution (Mg/Al molar ratio=2) of Mg(NO3)2 · 6H2O (1 mol) and Al(NO3)3 · 9H2O (0.5 mol) was added drop-wise, using a peristaltic pump (QG400; Fasco, Springfield, MO, USA), at 3 mL/min, into 1,000 mL of alkali solution (pH=13) consisting of sodium hydroxide (NaOH) and sodium carbonate (Na2CO3), with intensive stirring at room temperature. The resulting precipitates were aged at 65°C for 18 hr in mother liquor. The precipitates were thoroughly washed with deionized water to remove excess sodium, and then final suspensions were centrifuged at 8,500 rpm for 20 min. The washed precipitates were oven-dried again at 65°C for 24 hr, and then pulverized in a ball mill. The pulverized precipitates were passed through US Standard Sieve No. 100 (grain size=0.149 mm). The particles used for the experiments were finally obtained through oven drying at 105°C.
TEM (JEM-1010; JEOL Ltd., Tokyo, Japan) was used to take images of the particles. The particle size was determined by analysis of the TEM image (number of particle=71) using ImageJ 1.43u software (National Institutes of Health, Bethesda, MD, USA). The mineralogical and crystalline structural properties were examined using XRD (D8 ADVANCE; Bruker, Germany) with a CuKα radiation of 1.5406 Ǻ, at a scanning speed of 0.6°/sec. N2 adsorption-desorption experiments were performed using a surface area analyzer (BELSORP-max; BEL Japan Inc., Osaka, Japan), after the sample was pretreated at 120oC. From the N2 adsorption-desorption isotherms, the specific surface area, total pore volume, and mesopore volume were determined by Brunauer-Emmett-Teller (BET) and Barrett-Joyner-Halenda (BJH) analyses. XPS (XPS Sigma Probe; Thermo VG, West Sussex, UK) measurement was performed with monochromatic Al Kα radiation.
2.2. Fluoride Adsorption Experiments
The desired fluoride solution was prepared by diluting the stocking fluoride solution (1,000 mg/L), which was made from sodium fluoride (NaF). The first batch experiments were performed at different dosages of adsorbent (quintinite), ranging from 0.1 to 2.0 g in 30 mL solution. Adsorbent was added to 30 mL of fluoride solution (initial concentration=10 mg/L), in 50 mL polypropylene conical tubes. The tubes were shaken at 25°C and 100 rpm, using a culture tube rotator (MG-150D; Mega Science, Seoul, Korea). The samples were collected 6 hr after the reaction, and were filtered through a 0.45-μm membrane filter. The fluoride concentration was measured using a fluoride ion selective electrode (9609BNWP; Thermo Scientific, Waltham, MA, USA). For the fluoride measurement, total ionic strength adjustment buffer solution (58 g of NaCl, 57 mL of CH3COOH, and 150 mL of 6 M NaOH in 1,000 mL of deionized water) was used to prevent the interference of other ions.
Based on the results from the above tests, further batch experiments were conducted, at the adsorbent dose of 1.5 g in 30 mL solution. The second batch experiments were performed at the initial fluoride concentrations of 10 mg/L, to examine the effect of contact time on fluoride removal. In the experiments, the samples were collected 1, 2, 3, 6, 9, 12, and 24 hr after the reaction. The third batch experiments were conducted at fluoride concentrations of 10–1,000 mg/L, to examine the effect of initial fluoride concentrations on fluoride removal. The samples were collected for fluoride measurement 6 hr after the reaction. The fourth batch experiments were performed to examine the effect of the initial solution pH, which was adjusted to the desired value with 0.1 M NaOH and/or 0.1 M HCl. All experiments were performed in triplicate.
2.3. Data Analysis
All of the parameters of the models were estimated using MS Excel 2010, with the solver add-in function incorporated into the program. The model parameter values were determined by nonlinear regression. The determination coefficient (R2), chi-square coefficient (χ2), and sum of square error (SSE) were used to analyze the data, and confirm the fit to the model. The expressions of R2, χ2, and SSE are given below:
where, yc is the calculated adsorption capacity from the model,
is the measured adsorption capacity from the experiment, and is the average of the measured adsorption capacity.
3. Results and Discussion
3.1. Characteristics of Quintinite Particles
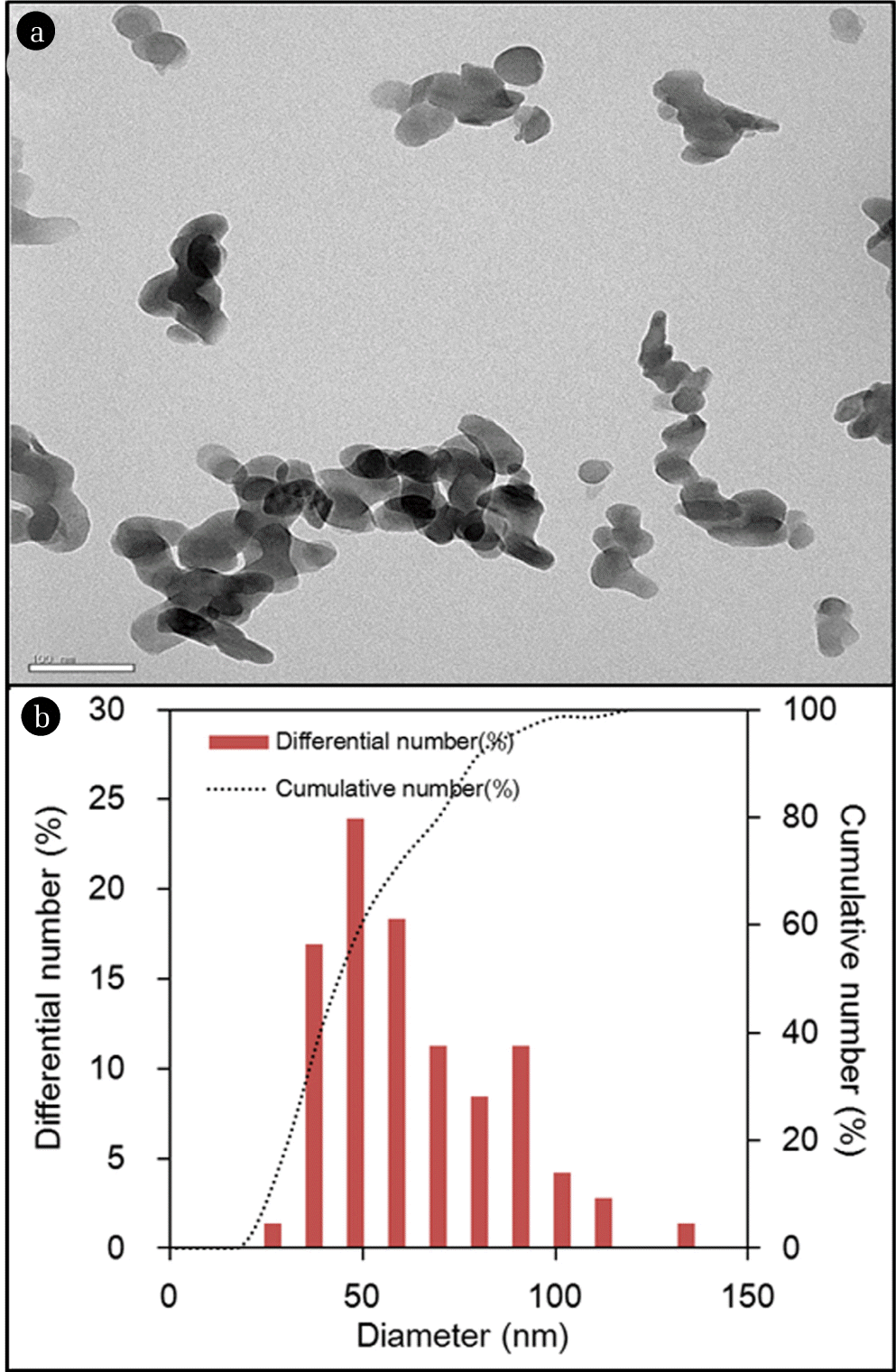
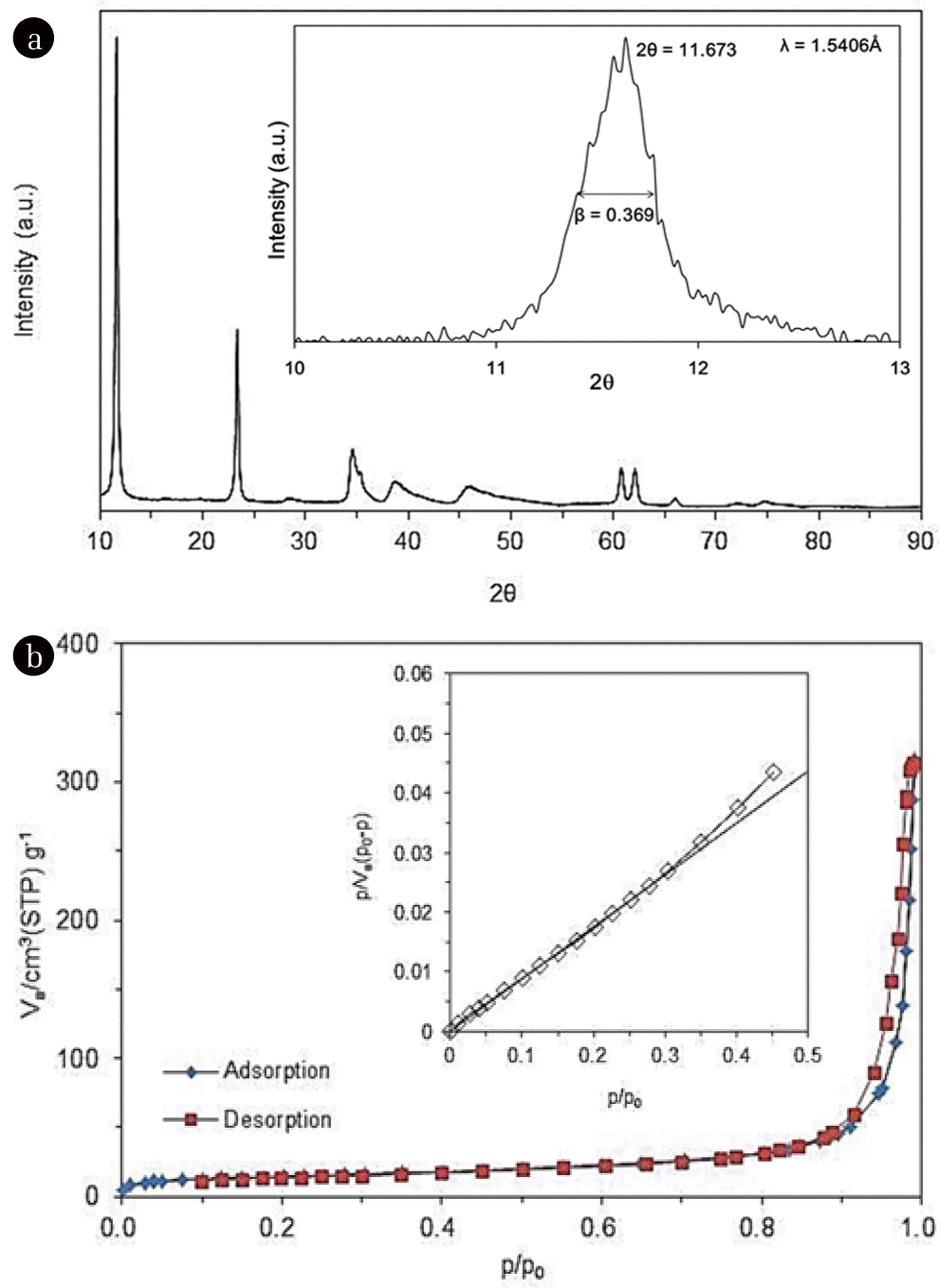
The characteristics of quintinite particles are presented in Figs. 1 and 2. The TEM image (Fig. 1(a)) demonstrates that the particles were nano-sized. The particle size distribution (Fig. 1(b)) determined from the TEM image shows that the particle size was in the range from 20 to 120 nm, with a mean particle size of 59 nm. According to the XRD pattern (Fig. 2(a)), the particle had a layered structure, with sharp and intense lines at low 2θ, and less intense lines at high 2θ. The peaks observed at 2q=11.673, 23.469, 34.437, 35.978, 38.428, 41.652, 45.520, 60.675, and 62.025 (JCPDS 87-1138) correspond well with those of quintinite particles found in the literature [1]. The particle had a chemical formula of Mg4Al2(OH)12 CO3 · 3H2O with hexagonal crystal system (a=5.283 Å; c=15.159 Å). Based on the XRD pattern, the average crystal size (d) of the particle was estimated by the Debye-Scherrer formula [22]:
where, λ is the wavelength of the X-ray (=1.5406 Å), β is the full width at half maximum width of the diffraction peak (=0.369°), and θ is the diffraction angle (=11.673°/2). From the Debye-Scherrer analysis (inset in Fig. 2(a)), the value of d was calculated to be 21.6 nm. Fig. 2(b) presents N2 adsorption and desorption isotherms. According to the BET analysis (inset in Fig. 2(b)), the particles had a specific surface area of 49.8 m2/g, and total pore volume of 0.4582 cm3/g. From the BJH analysis, the mesopore volume was determined to be 0.4522 cm3/g.
3.2. Characteristics of Fluoride Sorption to Quintinite Particles
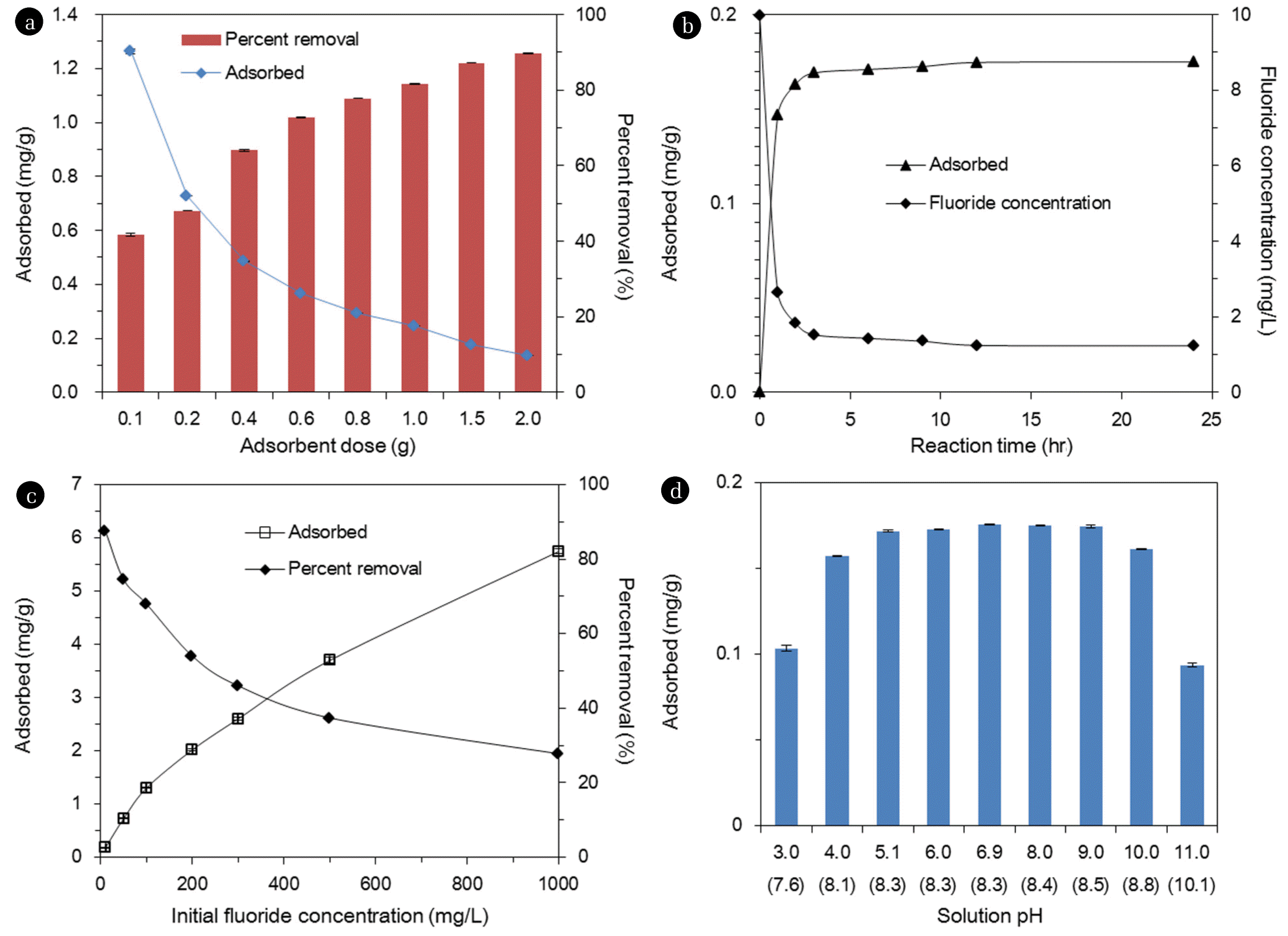
Fig. 3(a) shows the effect of adsorbent dose on fluoride removal. The percent removal increased from 41.7%±0.3% to 89.8%±0.2% with increasing adsorbent doses from 0.1 to 2.0 g in 30 mL of solution. Meanwhile, the sorption capacity decreased from 1.26±0.01 to 0.14±0.01 mg/g with increasing adsorbent doses. Results indicate that the initial fluoride concentration of 10 mg/L could be reduced to <1.5 mg/L at the adsorbent dose ≥1.5 g. Fig. 3(b) provides the effect of reaction time on the removal of fluoride. The fluoride concentrations decreased rapidly with increasing reaction time, until the equilibrium was reached at 6 hr. The fluoride concentration dropped sharply to 2.7 mg/L (initial fluoride concentration=10 mg/L) at 1 hr of reaction time, and further decreased to 1.4 mg/L at 6 hr. Then, the fluoride concentration reached 1.2 mg/L at 24 hr of reaction time. Meanwhile, the sorption capacity increased from 0.15 to 0.18 mg/g, with increasing reaction time from 1 to 24 hr.
Fig. 3(c) presents the effect of initial fluoride concentration on fluoride removal. At the lowest concentration of 10 mg/L, the percent removal was 87.6% and at the fluoride concentration of 100 mg/L, then decreased to 68.0%. At the highest concentration of 1,000 mg/L, the percent removal further decreased to 27.8%. Meanwhile, with increasing fluoride concentrations from 10 to 1,000 mg/L, the sorption capacity increased from 0.18 to 5.75 mg/g. Fig. 3(d) demonstrates the effect of initial solution pH on fluoride removal. The sorption capacity was 0.10 mg/g at pH 3, and increased to 0.17 mg/g at pH 5. The sorption capacity remained relatively constant at 0.17–0.18 mg/g, between pH 5 and pH 9. The sorption capacity dropped to 0.16 mg/g at pH 10, and then further decreased sharply to 0.09 mg/g.
Results demonstrate that the fluoride sorption in quintinite particles do not vary much at initial pH 4–10. This result could be related to the fact that during the sorption experiments, the final (equilibrium) pH converged to 8.1–8.8 (initial pH 4–10). Similar findings were reported in the literature by Kim et al. [14], showing that the fluoride sorption capacity of calcined Mg/Al LDH did not vary greatly between pH 4 and pH 9; the sorption capacity changed from 15.6 to 14.1 mgP/g, with increasing pH from 4 to 9. Han et al. [23] also reported that the removal of phosphate in the alginate beads containing calcined Mg/Al LDH was not sensitive to solution pH. They demonstrate that the percent removal of phosphate decreased slightly from 98.6% to 95.5%, as the solution pH increased from 4.9 to 8.9.
In our experiments, the fluoride sorption capacity considerably decreased at highly acidic (pH < 3) and alkaline pH (pH > 11) conditions. The fluoride sorption could decrease at highly acidic pH, due to the formation of hydrofluoric acid (HF), which is not favorable for adsorption onto the surfaces of adsorbent. According to MINTEQ calculation (Visual MINTEQ 3.0), HF species is formed at 35% of total F species (HF and F-) at pH 3. In addition, metal cations in quintinite might be dissolved at highly acidic pH, which results in the decrease of fluoride sorption [11]. At highly alkaline pH, the fluoride sorption could decrease, due to the competition between F- and OH- on the sorption sites. The contribution of NaF formation to the decrease of fluoride sorption might be negligible at highly alkaline pH. According to MINTEQ calculation, NaF species is only 0.2% of total F species (F- and NaF) at pH 11. Note that alkaline pH was obtained by adding 0.1 M NaOH in the experiments. Lv et al. [11] reported that phosphate removal in Mg/Al HTL decreased sharply from 110 to 5 mg/g, with increasing pH from 5 to 10. Mandal and Mayadevi [21] showed that phosphate removal in Zn/Al HTL had an increasing tendency between pH 2.5 and pH 6.2, but a decreasing one between pH 6.2 and pH 9.8.
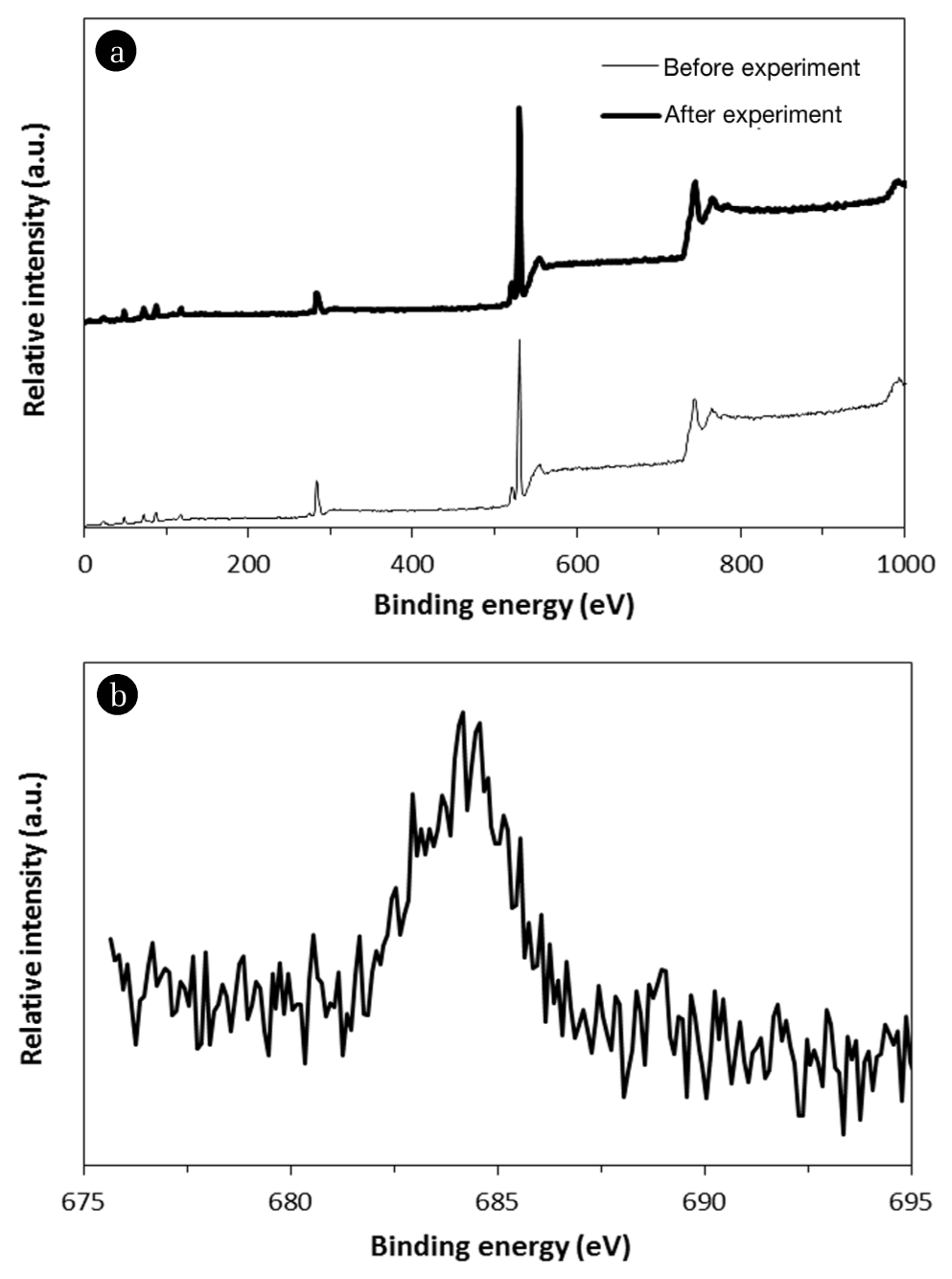
Fig. 4 presents XPS spectra. In the wide scan before and after fluoride sorption experiments (Fig. 4(a)), the peaks at binding energy of 49.15 and 73.20 eV are assigned to Mg 2p and Al 2p, respectively. In the high-resolution scan of the F 1s region after fluoride sorption experiment (Fig. 4(b)), the peak at 683.4 eV is ascribed to adsorbed fluoride ion on the surfaces of quintinite [20]. It is known that the two mechanisms of interlayer anion exchange and surface adsorption could contribute to the removal of fluoride by quintinite particles [5]. In the anion exchange process, the charge balancing anion (carbonate) in the interlayer region is replaced by fluoride ion. In surface adsorption, the negatively charged fluoride could adsorb to the positively charged brucite-like layer, via electrostatic interaction [5].
3.3. Kinetic and Equilibrium Model Analyses
The reaction time data (Fig. 3(b)) were analyzed, using the following nonlinear forms of pseudo-first-order (Eq. (5)), pseudo-second-order (Eq. (6)), and Elovich (Eq. (7)) kinetic models:
where, qt is the amount of fluoride removed at time t, qe is the amount of fluoride removed per unit mass of adsorbent at equilibrium, k1 is the pseudo-first-order rate constant, k2 is the pseu-do-second-order velocity constant, α is the initial adsorption rate constant, and β is the Elovich adsorption constant.
The kinetic data and model fits for fluoride sorption to quintinite are shown in Fig. 5. Model parameters for the pseudo-first-order, pseudo-second-order, and Elovich models are provided in Table 1. In the pseudo-first-order model, the value of qe was 0.17 mg/g, and the value of k1 was 1.85 (1/hr). The value of qe from the pseudo second-order model was similar to that from the pseudo-first-order model. The value of qe was 0.18 mg/g, and the value of k2 was 28.80 g/mg/hr. In the Elovich model, the values of α and β were 1.90E+06 mg/g/hr and 125.0 g/mg, respectively. The values of R2, χ2, and SSE indicate that the pseudo-second-order model was the most suitable for describing the data. This finding indicates that chemisorption is involved in the adsorption of fluoride to quintinite. In the literature, Cai et al. [7] reported that the kinetic data for fluoride sorption to calcined Mg/Al LDH were found to fit very well the pseudo-second-order model. Zhou et al. [20] also reported that the pseudo-second-order model was suitable for describing the adsorption kinetics of fluoride on calcined Li/Al LDH.
The fluoride concentration data (Fig. 3(c)) were analyzed using the following nonlinear forms of the Freundlich (Eq. (8)), Langmuir (Eq. (9)), and Redlich-Peterson (Eq. (10)) isotherm models:
where, Ce is the concentration of fluoride in the aqueous solution at equilibrium, KF is the distribution coefficient, 1/n is the Freundlich constant, Qm is the maximum mass of fluoride removed per unit mass of adsorbent (removal capacity), KL is the Langmuir constant related to the binding energy, KR is the Redlich-Peterson constant related to the adsorption capacity, aR is the Redlich-Peterson constant related to the affinity of the binding sites, and g is the Redlich-Peterson constant related to the adsorption intensity.
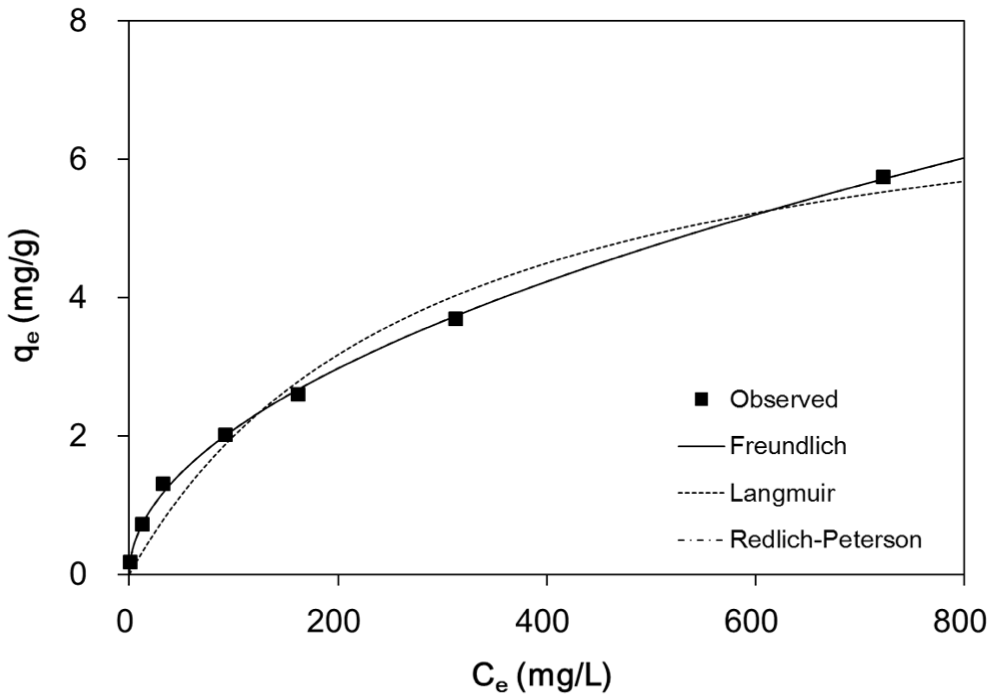
Fig. 6 shows the equilibrium data and isotherm model fits for fluoride sorption to quintinite. Table 2 summarizes the equilibrium isotherm parameters for the Langmuir, Freundlich, and Redlich-Peterson models. The values of R2, χ2, and SSE indicate that both the Freundlich and Redlich-Peterson models were suitable for describing the data. Note that the Redlich-Peterson model fit was superimposed on the Freundlich fit (Fig. 6). The Redlich-Peterson model can be reduced to the Freundlich model, if KR and aR are much greater than unity [24]. In the Freundlich model, the value of KF was 0.20 L/g, which corresponded well to the value of KR/aR in the Redlich-Peterson model. The value of 1/n was equivalent to the value (0.51) of (1–g). The adsorption capacity (qm) was calculated from KF and 1/n, using the following equation [25]:
The value of qm was calculated to be 6.78 mg/g.
Table 3 summarizes the fluoride sorption capacities of hydrotalcite-like particles or mixed metal oxides from the literature. The maximum adsorption capacity (qm) of quintinite in this study was determined to be 7.71 mg/g from the Langmuir model, which in Table 3 is in the low range of adsorption capacity. In order to improve the fluoride sorption capacity of quintinite, thermal treatment of quintinite at high temperatures (≥300°C) is recommended, which results in the increase of the BET surface area and fluoride sorption capacity [14]. Consequently, it is expected that the cost effectiveness of the adsorbent in fluoride removal can be improved.
4. Conclusions
In this study, the sorption of fluoride to quintinite was examined, using batch experiments. Results showed that the maximum adsorption capacity of fluoride to quintinite was 7.71 mg/g. The adsorption of fluoride to quintinite was not changed at pH 5–9, but decreased considerably in the highly acidic (pH < 3) and alkaline (pH > 11) solution conditions. Kinetic model analysis showed that the pseudo second-order model was the most suitable for describing the kinetic data. Equilibrium isotherm model analysis demonstrated that both the Freundlich and Redlich-Peterson models were suitable for describing the equilibrium data. This study demonstrated that quintinite could be used as an adsorbent for the removal of fluoride from aqueous solutions.