1. Introduction
For photocatalytic applications, titania (TiO2) is the primary selection due to its physical and chemical stability in reactive environments, such as in air cleaning and water purification system [1]. Especially, the capability of volatile organic compounds (VOCs) destruction in the presence of the ultraviolet (UV) ray irradiation is a crucial function of the TiO2 materials for the environmental application. Since Dibble and Raupp [2] did their works on the destruction of gas phase trichloroethylene (TCE) in air by using TiO2 powder and UV ray irradiation, many workers developed various types of TiO2/UV catalytic system for air pollution control [3]. For this kind of application, TiO2 must have high specific surface area and special crystalline structure for the photocatalytic reaction to be done efficiently. Most of TiO2 powder products available on the market satisfy these requirements. But the currently developed applications of TiO2 powders for destruction of air pollutants still have many limitations mainly caused by the low activity of TiO2 with respect to the light sources. Synthesis of TiO2 based materials by adding the dopants to utilize the solar energy for enhancing the photocatalytic activation is one of major concerns to improve the conversion efficiency of the air cleaning system used for the destruction of VOCs. In addition to this crucial concern, the alteration of particle morphology of the base powder, which occurs while the base powder is being immobilized for the applications, is also a barrier for the maximized utilization of the base TiO2 powder. For using the commercial TiO2 powder products in the TiO2/UV catalytic system to be used for the air cleaning, the proper immobilization of the TiO2 powder on the substrate is necessarily required. In this fixation process, the original physical property of the base powder may be easily altered, and the resultant system performance also may be degraded. Once the UV ray is selected as the source of the activation energy in the TiO2/UV catalytic system for the air cleaning, the physical and chemical characteristics of the TiO2 materials would have a dominant role to determine the final conversion efficiency of the system though there still left a design problem concerning about the diffusion gas flow in the system. The specific area and crystalline structure of the materials coated on the substrate will be the key parameters in determining the performance of the TiO2/UV catalytic system.
The current study is about how to prepare the TiO2 powder and to immobilize it on the substrate by using the premixed flame synthesis to get the desired properties, such as the particle morphology and crystalline structure. The premixed flame aerosol synthesis has many advantages in preparation of TiO2 powers for the TiO2/UV catalytic system for the air cleaning system [4]. As the TiO2 powder formed in the flame synthesis can be directly coated on the substrate without losing the base property of the powder, the final characteristics of the powder coated on the substrate will be conserved without losing those original properties of particles as determined in the flame synthetic process. The flame-synthesized powder can be produced in form of nano-sized particles which have higher specific surface area. So if the flame process is selected as the synthetic process for the TiO2 preparation, the major concern left will be how to control the synthetic process to get the desired powder properties. In the flame synthetic process, the particle morphology and the crystalline structure are controlled simultaneously by controlling the reaction process variables. In the current study, the TiO2 powder was manufactured by the oxidation of the TiCl4 vapor precursor in the premixed flame aerosol reactor. The morphology and the crystalline phase of TiO2 particles were characterized by controlling the reaction variables in the flame aerosol reactor, and these morphological controls resulted in the determination of the specific surface area.
2. Materials and Methods
2.1. Preparation of Particulate Films by Using the Premixed Flame Aerosol Reactor
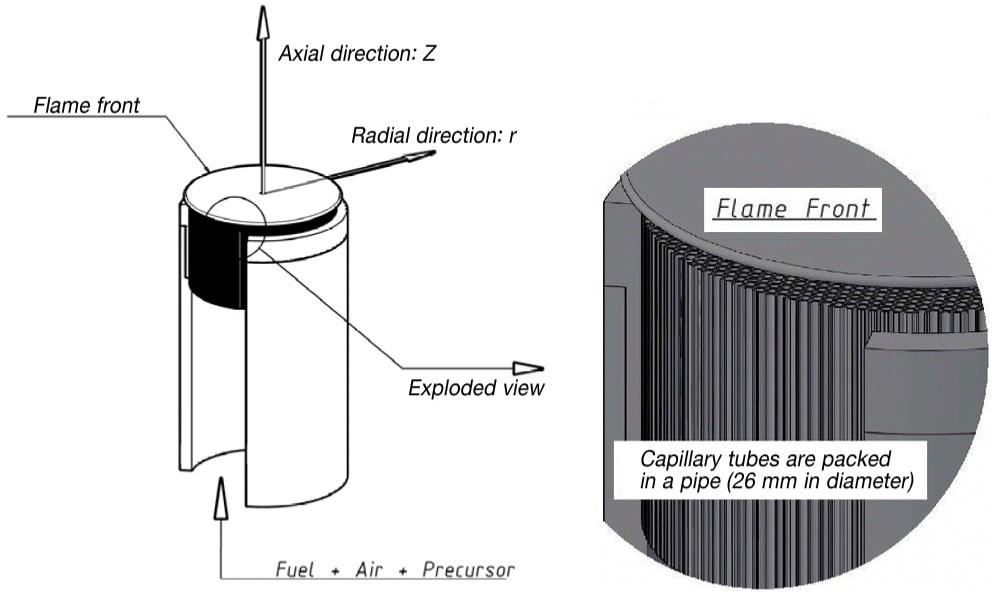
Fig. 1 shows the schematic of the flame aerosol reactor used in the experiment, which is the same arrangement with that of our previous work [5]. The morphology of aggregated particles in the flame zone were estimated by using the thermophoretic sampling method [6], and the results of estimation were used to control the morphology of particles immobilized on the substrate as the flame generated particles are directly deposited on the target substrate. Using the transmission electron microscope (TEM, Model H-600; Hitachi, Tokyo, Japan) system and the scanning electron microscope (SEM, Model S-4100; Hitachi) system, the real shape of aggregates were obtained and the photometric algorithms described in references were used to get the technical data [7, 8]. We counted the primary particle sizes by checking the gray level in the TEM images. In the image analysis, we determined the boundary of each primary particle in each aggregates and found the equivalent spherical shapes of primary particle in each aggregate to determine its diameter. To distinguish between primary particle and aggregates, we exploited the built-in image processing algorithm in a commercial software package (SigmaScan Pro ver. 5.0; SPSS Inc., Chicago, IL, USA). Using the Brunauer-Emmett-Teller (BET) surface analyzer (Model Gemini 2371; Micromeritics, Norcross, GA, USA), we checked the variation of the specific surface area of the particulate film which was caused by the particulate parameters, such as the size of primary particles and the size distribution of the aggregates. The X-ray diffractometer (XRD, Model D/Max-2500H; Rigaku, Tokyo, Japan) analysis was used for the estimation of crystalline structure.
In the flame aerosol reactor, the precursor in the combustion zone undergoes different oxidation history due to the different temperature profile, and the crystalline phases of the TiO2 particulates are determined in specific manner with respect to the temperature profiles. In the flame aerosol reactor shown in Fig. 1, the temperature profile along the flow direction is controlled by changing the combustion equivalence ratio. The liquid phase of TiCl4 was supplied into the evaporator that was immersed in the hot water bath as shown in Fig. 2. In the evaporator, TiCl4 was completely evaporated and it is supplied to the flame aerosol reactor in vapor phase. The heating cable was wrapped along the tubing from the evaporator to the flame aerosol reactor to inhibit the condensation of the precursor. The liquid feed rate of precursor was exactly controlled with the automatic syringe pump (Model KDS100; KD Scientific, Japan). In the flame region, TiCl4 is oxidized to TiO2 by the reaction [9],
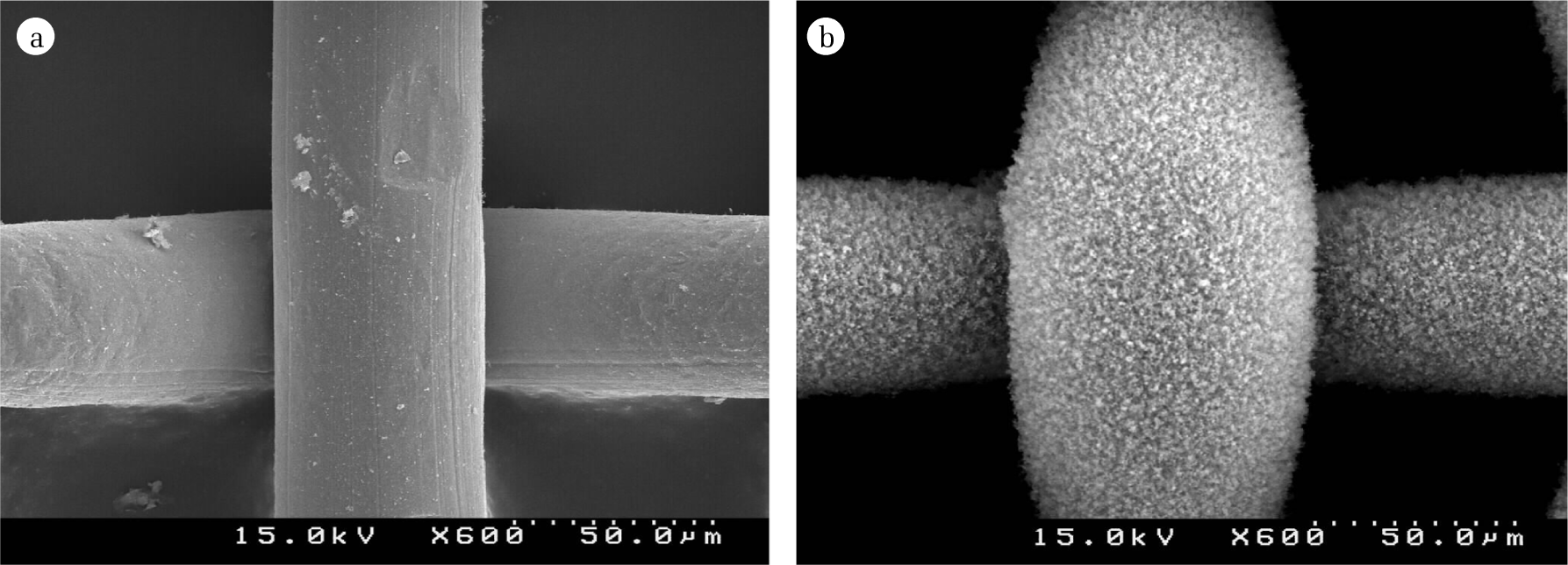
In the oxidation process across the flame front, the newly formed TiO2 monomers will form the aggregates. The final shape and crystalline phase of TiO2 aggregates were determined by controlling the reaction variables in the flame aerosol reactor. Such as the combustion equivalence ratio and precursor concentrations were the main control variables for determining the characteristic of TiO2 particles. The aggregates formed in flame region were directly coated on the substrate. In the experiment, the stainless steel mesh was used as the substrate. By controlling the speed of the linear carrier, the coating thickness was controlled. The coated surface shown in Fig. 3(b) was obtained after 300 linear sweepings of the stainless steel mesh substrate (138 mm×505 mm, mesh number 200) across the flame flow in speed of 2 mm/sec under the condition of 0.7 in the combustion equivalence ratio, φ. Using the SEM photos, thickness of the TiO2 film layer on the substrate was estimated. In Table 1, the experimental conditions are summarized. Fig. 3 shows the TiO2 film layer on the substrate prepared by the coating process. In our experiment, the TiO2 film layer on the substrate was 11.2 μm which was prepared under condition at φ = 0.7. The local exhaust gas ventilation and gas control facilities were installed as the precaution to the exposure of toxic materials, such as the particulate matter and chlorine in the exhaust.
2.2. Testing the Performance of Particulate Film Layers
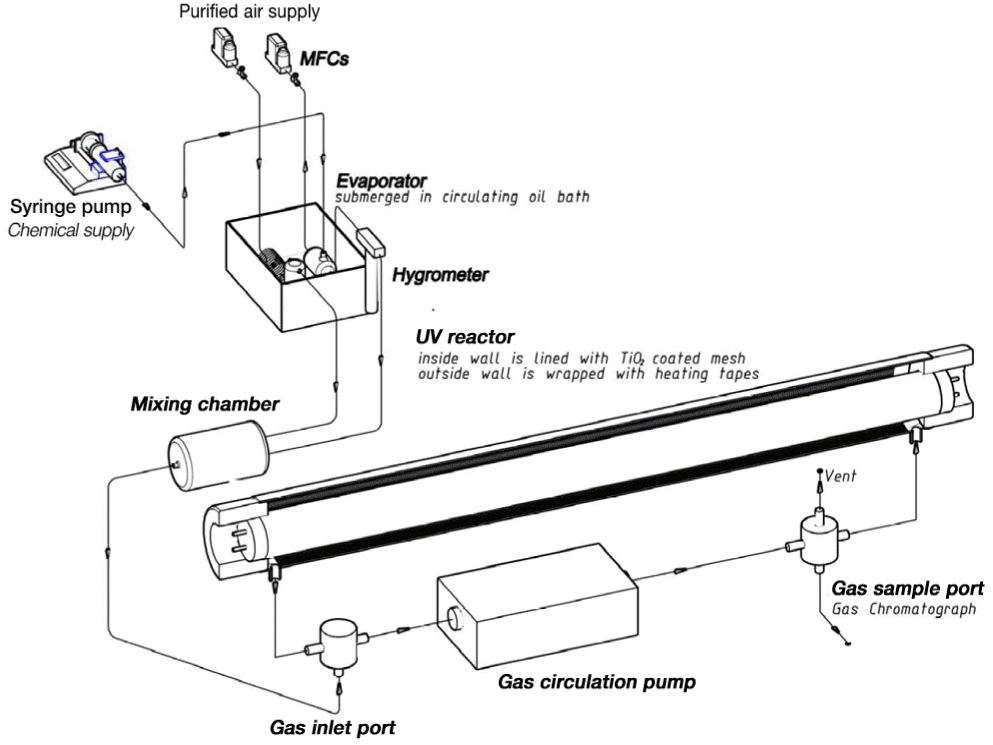
As shown in Fig. 4, the TiO2 layered steel mesh was installed inside wall of the glass tube of 44 mm in inside diameter and 520 mm in length. Based on the study [6], the UV-C lamp (20W, Model GL20; Sanyo Denki, Japan) which has 250–260 nm in wavelengths was selected for the UV-TiO2 photocatalytic system. In our application, the selected UV lamp was installed in the center of tubular reactor. The outside diameter of the UV lamps was 32 mm. For checking the light spectra of the UV lamp, a mono chromator (Model SpectraPro-150; Acton Research Corp., Acton, MA, USA) was used which has the specialized silicon photodetector (Model OSD35-7Q; Centronic LLC, Houston, TX, USA). In the TiO2/UV reactor, the gas flows through the annular slut between the lamp and tubular glass reactor where the UV ray is directly irradiated on the TiO2 coated substrate. The inside temperature of the tubular reactor is being heated up to 40°C after UV lamp is turned on. So there may be the role of thermal decomposition in TiO2/UV reactor. The heating band was wrapped around the tubular reactor and the temperature is controlled to 60°C to exclude the thermal decomposition effect which is caused by the heat of UV lamp itself. The formaldehyde and benzene contaminated air was sent through the tubular reactor respectively for testing the performance of the flame synthesized TiO2 particles. Formaldehyde and benzene vapor was injected separately by the syringe pump, which is entrained into dry air which goes through the bubbler that was immersed in temperature-controlled hot water. The other air was supplied to the mixing chamber after it entrains the water vapor for enhancing the conversion rate of VOCs in the UV-TiO2 reactor. Once the flow condition is stabilized across the inlet and vent of the TiO2/UV reactor, the VOCs contaminated air is circulated around the TiO2/UV reactor with respect to the reaction time. While the contaminated air circulates around the TiO2/UV reactor, gas samplings were done with respect to time intervals. In the Fig. 4, the volume of in the tubing around the tubular TiO2/UV reactor is 5% of the main volume. A gas sample of 40 μL was used for each analysis in the gas chromatography flame ionization detector (Model CP 3800; Varian Inc., Palo Alto, CA, USA) system.
3. Results and Discussion
3.1. Evaluation of Particle Morphology
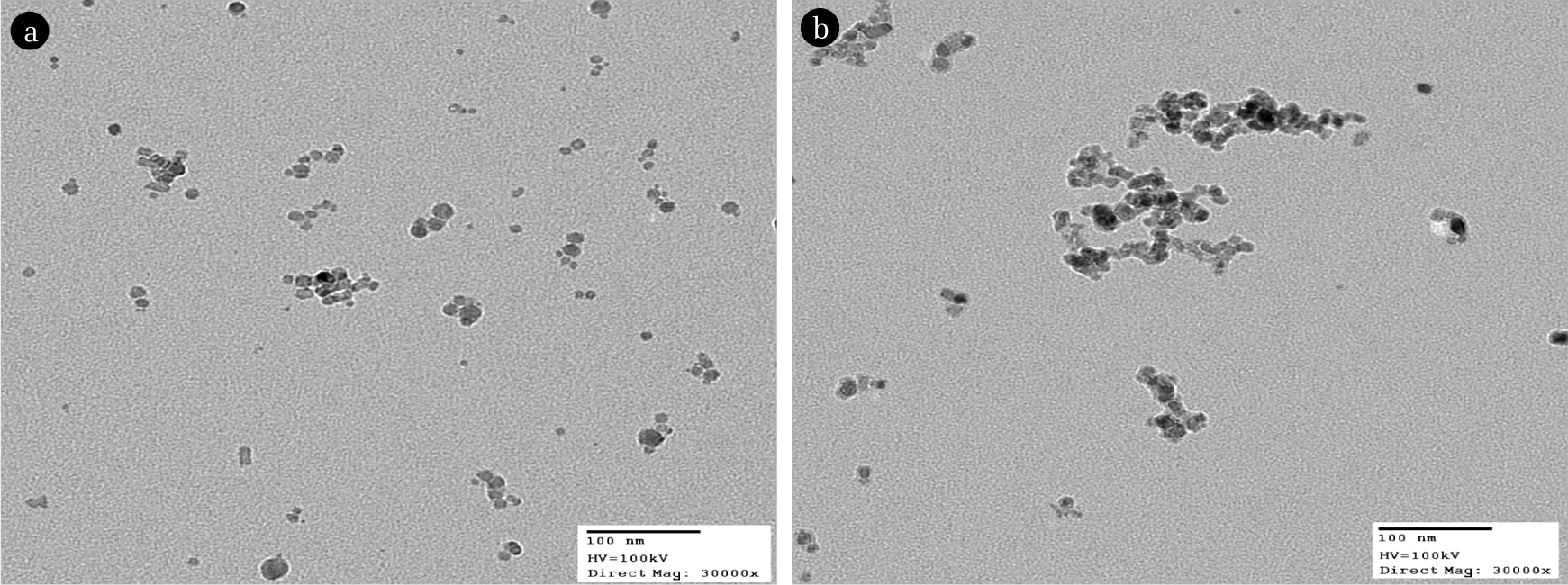
Under the various conditions of combustion, the particles were sampled by using the thermophoretic sampler [6] at the location of z = 50 mm of the flame flow to check the particle morphological characteristics with respect to the equivalence ratios of combustion and flame temperature. By the preliminary experimental works, it was known that the particle characteristics above of location z = 50 mm was almost same. In the TiO2 synthetic process, the key parameter for controlling the final particle characteristics was the equivalence ratios. Though the flame temperature itself can be a key parameter in oxidation process of the precursor, it is not a control variable in our flame aerosol reactor for TiCl4 oxidation as the flame temperature is a dependent variable on the equivalence ratio. Based on the TEM pictures, the image analysis methods [7, 8] were used to estimate the morphology of the sampled particles. Fig. 5 shows the morphological shapes under different preparation conditions of φ = 0.98 and φ = 0.7 at z = 50 mm, and Table 2 is the summary of the morphological measurements. Data in Table 2 show that the size of primary particle in an aggregate becomes larger with increasing the equivalence ratio, which results in lower specific surface area of the particulate aggregates as summarized in Table 3. Lowered oxygen concentration in higher equivalence ratio causes the slow down of the oxidation rate of TiCl4, and the primary particles become bigger as the newly formed solid monomer are continuously attached on the previously formed particle by the so-called the reaction limited aggregation [5]. Though there could be coagulation between the particles, the flux rate of monomer transfer on to the previous generated particles is much higher to determine the final morphology of each aggregate. There could be the additional growth by the coalescence of particle as the aggregates are exposed to higher flame temperature in higher equivalence ratio. In case of lower value of equivalence ratio, the oxygen concentration in the reaction is higher hence the conversion of precursor to monomer is much easier and faster. The primary particles formed from monomers do not grow easily due to the lower flame temperature. Instead the particle coagulation mechanism does a stronger role and the aggregates become more chain like shape in lowered fractal number. For application of the catalytic materials, the specific surface area is a very important characteristic. The particle morphology has direct relation with the specific surface area. If the size of primary particle is smaller in the aggregates, the specific surface area becomes larger. In the experiment, the resultant specific areas of the TiO2 aggregates were between 39–56 m2/g. Those results were comparable with that of the P-25 (Degussa, 55 m2/g) which is the standard referenced TiO2 particle for the commercial application. More detailed experimental conditions and results on the particle preparation are reported in our previous work [5].
Based on the information of the particle morphology obtained under the various combustion conditions, the substrate coating device shown in Fig. 2 was moved back and forward across the flame flow at the axial position of z = 100 mm to get the same particle morphology determined in the preliminary morphological study. Though the coated surface was uniform, the stickiness of the particle on the substrate was not good compare with that of the chemical vapor deposition or the aerosol-assisted chemical vapor deposition processes [10].
3.2. Evaluation of Crystalline Structure of Particles
The XRD analysis to estimate the crystalline structure of the flame generated TiO2 is shown in Fig. 6 with that of the Degussa P-25. Compared with the P-25, the XRD graphs of the flame generated TiO2 particles shows more noise. The anatase composition of flame generated TiO2 can be obtained about 71.4% at the condition of φ = 0.98, and much higher anatase composition ratio can be obtained at the lower values of φ. The XRD and BET analysis summarized in Table 3 shows the specific surface areas and crystalline structures with respect to the flame synthetic conditions [10]. The mass fraction of anatase in the particle aggregates becomes higher with decreasing the flame temperature and the equivalence ratios of the combustion. As the mass fraction of anatase is important for the higher photocatalytic activity, the higher yield rate of anatase composition in the preparation process is very important. In the current particulate preparation process, the peculiar crystalline structure was easily determined under the designated chemical reaction condition and temperature history with respect to the location of gas flow. These results were mainly caused by the rapid cooling process after the flame front of the premixed flame aerosol reactor. For the up-scaled preparation of the material, the current technical information can be applied without major modification. These results agree with the previous works done under different conditions [9–11]. In the equivalence ratio of higher temperature region, the rutile mass fraction becomes higher due to the thermal instability.
3.3. The UV-TiO2 System for Destruction of Benzene Compound
The UV absorbance of synthesized TiO2 particles which were obtained under the various the equivalence ration of the combustion were estimated [5]. Though the pattern of UV absorbance of TiO2 particles obtained under the different particulate preparation conditions is similar, there is a minor blue shift with respect to the equivalence of the combustion. This may be the quantum size effect [12] caused by the size change of the primary particles in the TiO2 particle aggregates. In the case of fine primary particles that have below 10 nm in diameters, the quantum size effect becomes stronger. But in our flame process, the primary particles obtained have 20–30 nm in diameters. So the transition of the UV absorbance is not noticeable as the size change is not so wide. Anpo et al. [13] reported same results. Though the spectral transition was not noticeable, there was a tendency that the UV absorbance becomes stronger with increasing the size of primary particles within the wavelength of 300 nm below. This tendency of higher UV absorbance results in higher conversion efficiency of VOCs in TiO2/UV system. But the tendency of the UV absorbance is a contradictory result for application in VOCs destruction by using the TiO2/UV process. Though the larger primary particle is better in UV absorbance, it causes the decreased specific surface area of particle aggregates. So the decision on the optimal condition for the TiO2 preparation is required for the proper use of the TiO2/UV system. The intensity of photon energy from the irradiation ray is a key factor for the decomposition VOCs [3]. The band gap energy for TiO2 is 3.2 eV and the ray of 385 nm below in wavelength is required for the effective activation.
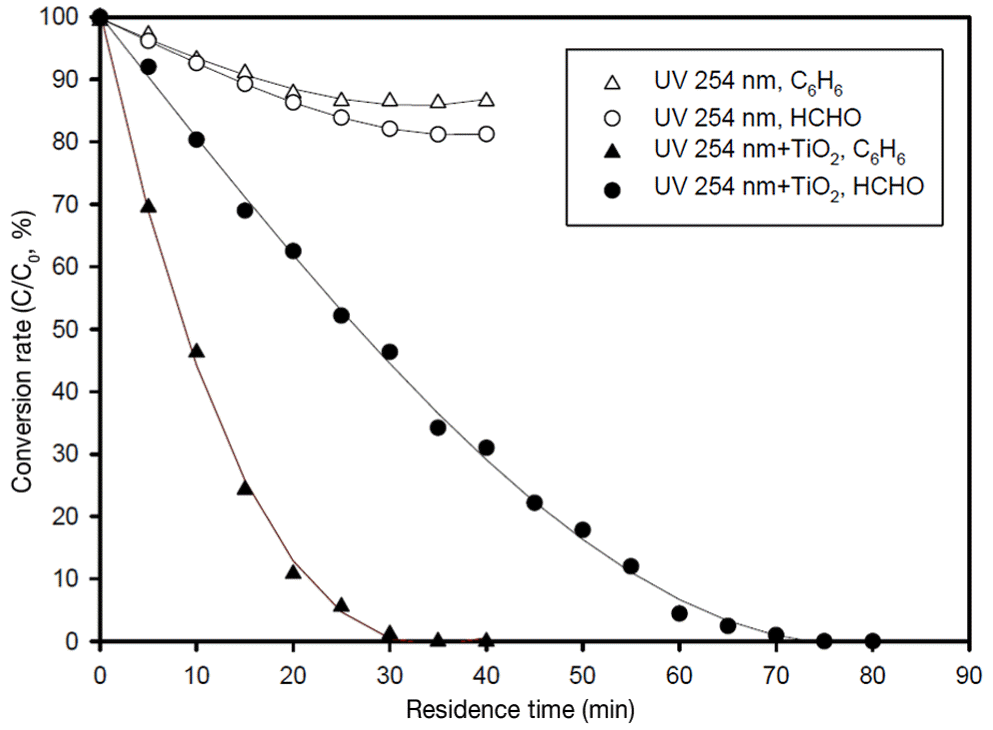
In the previous experimental works [6], the black light lamp (UV-A, peak intensity at 352 nm) and the germicidal lamp (UV-C, peak intensity at 254 nm) were used for testing the activation of TiO2 particles in TiO2/UV system. The UV-C lamp showed higher activity on the TiO2 layers and it was adopted for our experimental works. In the current experiment, TiO2 particles generated at φ = 0.7 were layered on the steel mesh to get the best system performance of photocatalytic material. Fig. 7 shows the application of TiO2 decomposition rate of formaldehyde and benzene in the UV-TiO2 layers reactor. On the same condition of the UV-TiO2 system, the decomposition rate of benzene is much higher than that of formaldehyde. To estimate the role of initial condition of VOCs on the decomposition rates, more experimental works are required under various conditions. Our work does not include the extended work, instead the previous workers already showed the kinetics and the chemistry [14, 15] under different conditions. Though the previous workers did good job in their analysis, their preparation process of TiO2 coated substrate was not well established as the process was done by the wet method. Our study credits the preparation of TiO2 based VOCs decomposition reactor, and the system works well. Our method is the dry method, and coating process is controllable by controlling the flame conditions. In our experiment, the initial concentrations of benzene and formaldehyde were 295 ppmv and 480 ppmv, respectively. To test the destruction rate of VOCs under UV irradiation only, the blank tests were done without the installation of the TiO2 coated substrate in the tubular glass reactor. As shown in the Fig. 7, without the participation of TiO2 for benzene destruction in the tubular glass reactor, the destruction rates of benzene and formaldehyde were about 14% and 19% after 40 min running of the system. As the system was wrapped with the temperature controlled heating tapes to be 60°C, the destruction in the tubular reactor was solely caused by the direct photolysis of the UV irradiation. Though the thermal role in the decomposition of VOCs in the reactor is not clarified, the role of UV-C lamp was noticeable. In the repeated experiments in the UV-TiO2 system under same flow conditions, the destruction rate of VOCs are enhanced very much due to the role of photocatalytic function of TiO2 particulate layers. In the case of UV-C lamp and TiO2 combination, the benzene destruction was almost finished within 35 min, and the formaldehyde is decomposed within 80 min after the operation. In the particulate preparation process, the differences in mass loading on the substrate were within 6% for 300 sweeps of the TiO2 coating so the mass loading difference effect on the photocatalytic reaction can be estimated within the error value.
In the oxidation and reduction process, the water vapor does role for enhancing the reaction process [16, 17]. As shown in Fig. 4, the water vapor is supplied to the reactor by entraining in the nitrogen gas. In the experiment, the relative humidity was 40%–60%. The oxidation and reduction process in the UV-TiO2 reactor can be written in several stages and more detailed analysis is need for full description [18]. But the decomposition rate shown in Fig. 7 can be fitted with a simple exponential decay form which is the result of the first order reaction as
For the decompositions of benzene and formaldehyde, the reaction rate k were 9.35×10−2 min and 3.46×10−2 min, respectively, at 333 K for our UV-TiO2 reactor. The decomposition rate of VOCs in the UV-TiO2 reactor should be dependent on several variables, such as absorption rate and crystalline structures of TiO2 on the substrate including the operational variable. So the expression for the reaction rate of VOCs in Eq. (2) may not proper, but it can be used for estimation of the system performance. In our study, the decomposition rate of benzene was much higher than that of formaldehyde under the same thermal and characteristic conditions of TiO2 particles.
4. Conclusions
An experimental study on the photocatalytic destruction of VOCs by using the TiO2 particles which were synthesized in a flame aerosol reactor was done. The TiO2 particles that were synthesized in the flame aerosol reactor can be directly coated on the substrate without changing the original particle morphology. The photocatalytic performance can be improved by maintaining the particulate characteristics, such as the size of primary particles and the specific surface area of the aggregates. In the flame reactor, the particle morphology and crystalline structures were controlled by controlling the oxidation rate of the chemical precursor. At the lowered combustion equivalence ratio, the favorable anatase phase was generated easily with the enhancement of the particle morphology. In the lowered equivalence ratios, the abundant oxygen favors the conversion of precursor and the resultant particles have the smaller primary particles. The anatase type of crystalline structures were also easily obtained in the premixed flame aerosol reactor as the cool down process after the flame front is more sharp that that of conventional diffusion flame aerosol reactor. The experimental results showed that the TiO2 particle generation by the premixed flame aerosol reactor and the direct coating on the substrate is good combination to construct the UV-TiO2 decomposition system for the destruction of VOCs. In our system, the decomposition rate of gaseous benzene is much higher than that of formaldehyde.