AbstractThis study aims to develop three-dimension reduced graphene oxide aerogel supported nano zero-valent iron (rGOA-nZVI) by hydrothermal and liquid phase reduction approach and applies it to the removal of two typical dyes, methylene blue (MB) and methyl orange (MO) from aqueous solutions. The obtained magnetic rGOA-nZVI composites were characterized by scanning electron microscopy (SEM), atomic force microscopy, Brunauer-Emmett-Teller, X-ray diffraction and Fourier transform infrared spectroscopy, and its removal efficiencies for MB and MO from aqueous solutions were evaluated. The removal processes of MB and MO could be completed within 30 min, which were well described by the pseudo-second order kinetic model. The rGOA-nZVI nanocomposites exhibited remarkable performance for the removal of MB (the maximum adsorption amount, qmax = 3918 mg/g) and MO (qmax = 667 mg/g) in a wide pH range (pH 1.0–10.0). Furthermore, rGOA-nZVI is stable and recyclable with high dye removal efficiencies (> 90%) after five cycles. The removal mechanisms were demonstrated with SEM and XRD analyses before and after MB and MO removal. This study showed that rGOA acts as an excellent carrier for nZVI, and the rGOA-nZVI nanocomposites are efficient materials for the advanced treatment of dye wastewater over a wide pH range.
Graphical Abstract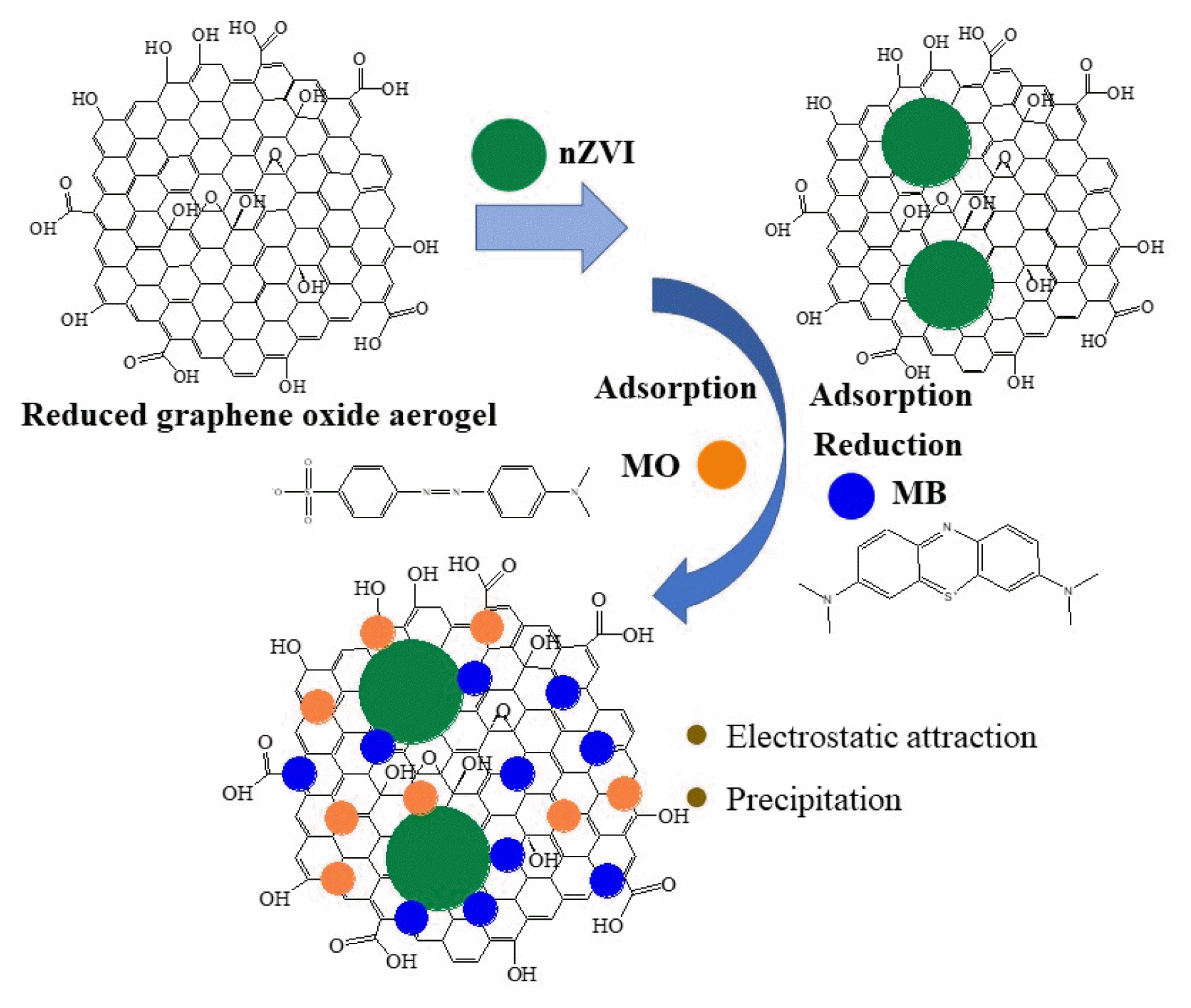
1. IntroductionThe safety and accessibility of water resource is a basic need for human society. On the surface of the Earth, less than 3% of total water is freshwater and only one third of this freshwater is available for drinking, industry and agriculture activities and ecosystem functions [1]. With the rapid development of industry, the accidental and purposive dumping of industrial wastes (e.g., heavy metal ions and organic dyes) has led to serious water pollution [2, 3]. Due to the obvious colorization of water as well as adverse effects like inhibiting photosynthesis of hydrophytes and the activities of adenosine triphosphate and monoamine oxidase of mammal organisms, etc., the pollution control technology of organic dyes has received much attention. Many organic dyes (e.g., methylene blue (MB) and methyl orange (MO)) are stable to heat and sunlight, generally not degradable under natural conditions due to their complex (aromatic) structure [4–7]. Several advanced technologies have been developed for the removal of organic dyes from wastewater, such as catalyst oxidation, ultrasound oxidation process, membrane separation, microbiological decomposition and adsorption [8–10]. Among those above methods, adsorption has been most frequently adopted due to the low cost, simplicity and high efficiency of the adsorbent [11].
In recent years, graphene, composed of monolayer sp2-hybridized hexagonal carbon atoms, has drawn significant interest due to its thermal stability, large surface area and remarkable thermal, electrical and mechanical properties [12, 13]. However, due to the relatively low density of surface functional groups and poor water dispersibility caused by aggregation via van der Waals interactions, the performance of graphene for the pollutant removal is rather limited. To overcome this shortage, graphene oxide (GO) and reduced graphene oxide (rGO) with large numbers of O-containing functional groups were raised to remove pollutants from aqueous solutions [14–16]. Those O-containing functional groups (e.g., -OH, -COOH, -C=O) not only improve the hydrophilicity of GO and rGO, but also act as high-energy adsorption sites, enhancing their adsorption capacity for pollutants. Additionally, enhanced dispersibility was achieved for GO and rGO, which ensures the preservation of an ultra-large surface area of these modified graphene. However, the difficulty in the recovery of two-dimension (2D) GO and rGO may hinder their massive practical application in wastewater treatment [17, 18]. To resolve this problem, three-dimension (3D) graphene was more recently proposed and has been reported to be easily retrieved from water body and has excellent adsorption capacities for various contaminants [19–21]. The 3D graphene presents large accessible surface area, continuously interconnected networks and channels, low mass density, abundant adsorption sites and superior mechanical flexibility [22, 23].
Easy separation of the adsorbent from the environment or water treatment media is an important issue for adsorption technology, which often requires suitable magnetic adsorbents. Due to its magnetic properties, high reactivity and relatively large specific surface area, nano zero-valent iron (nZVI) particles have drawn much research interest [24–26]. However, there are still some technical challenges in nZVI application for water treatment, for example, the agglomeration of nZVI particles limit its dispersivity, durability, mobility, mechanical strength and reactivity. The 3D graphene has large specific area and can therefore serves as an ideal carrier for supporting nZVI. Recently, 3D graphene foam was employed to support the nZVI particles to enhance their reactivity and reduce the particle agglomeration [25]. The nZVI/rGO material was synthesized to remove U(VI), which showed an efficient removal capability and can be a candidate for the in-situ remediation of U(VI) contaminated water [24]. More recently, Pu et al. prepared nZVI@rGO composite and employed it for nitrate-nitrogen removal in aqueous solutions. The results showed that 90% of nitrate-nitrogen can be removed within 1 h [27]. Nevertheless, to our best knowledge, few studies have been currently reported on stabilizing nZVI particles onto 3D rGO aerogel (rGOA-nZVI).
In this study, the feasibility of preparation of rGOA-nZVI was investigated for highly efficient removal of two typical dyes (MB and MO) from aqueous solutions. Hydrothermal and liquid phase reduction approaches were applied successively to synthesize magnetic rGOA-nZVI nanocomposites, which were characterized systematically. To investigate the interaction mechanisms, the kinetics, isothermals and thermodynamics were studied for the removal of MB and MO by rGOA-nZVI. The recycling and stability of rGOA-nZVI were also evaluated to explore the potential of practical applications for dyes removal.
2. Materials and Methods2.1. Materials and ChemicalsGraphite powder (< 30 μm), analytical grade ferrous sulfate heptahydrate (FeSO4·7H2O, 99.5%), hydrochloric acid (HCl, 35–37%), sulfuric acid (H2SO4, 98%), permanganate (KMnO4, 99.5%), MB (99.8%) and MO (99.8%) were all purchased from Shanghai Lingfeng Chemical Reagent Company (Shanghai, China). Sodium borohydride (NaBH4, 98%) was obtained from Sinopharm Chemical Reagent Company, China. Ethanol (CH3CH2OH, 99.7%) was obtained from Shanghai Titan Scientific Company, China. The stock solution of 5000mg/L MB or 2500mg/L MO were prepared by dissolving solid dyes in deionized water and applied in the batch experiments.
2.2. Synthesis of rGOA-nZVI NanocompositesThe synthesis methods for rGOA-nZVI nanocomposites were the same as described in our recently published papers, which used rGOA-nZVI nanocomposites as catalyst for persulfate oxidative removal of organophosphorus pesticides [28]. The preparation approach can be found as follow.
2.2.1. Preparation of GOThe synthesis of GO was achieved by chemical exfoliation of graphite according to a previously reported method [29]. Briefly, 1.0 g of graphite powder and 0.5 g of NaNO3 were added into 23 mL concentrated H2SO4 solution under vigorous stirring in ice-water bath, then 3.0 g of KMnO4 was fractionally added over about 2 h. The suspension was continually mechanically stirred for 30 min at 35°C, and then the suspension temperature was raised to 98°C after 46 mL deionized water was added into the suspension and this was kept for 15 min with mechanical stirring. The above suspension was diluted with 140mL deionized water, and then about 30 mL of H2O2 (30wt%) was added to remove the residual MnO4− until the brown color turned yellow. The yellow suspension was separated by filter and the collected solid was washed with 500mL HCl (5%) and freeze-dried for 24 h.
2.2.2. Synthesis of rGOAThe synthesis of rGOA was carried out according to Chen et al. (2017) [12]. One gram of GO was ultrasonicated in 300 mL ultra-pure water for 2 h, and then 10.0 g of NaBH4 was added into the GO suspension. The reaction mixture was ultrasonicated for 1 h and then heated for 1 h at 95°C in a water bath. The prepared material was washed with deionized water 3 times and freeze-dried for 24 h to obtain rGOA [12].
2.2.3. Synthesis of rGOA-nZVI nanocompositesThe synthesis of rGOA-nZVI nanocomposites was implemented according to Wang et al. (2015) [25]. One gram of as-synthesized rGOA was mixed with 5.0, 10.0, 15.0, 20.0 and 25.0 g of FeSO4 in 300 mL deionized water for 12 h to achieve nZVI theoretical loading capacities of 50.0%, 66.6%, 75.0%, 80.0% and 83.3% (wt%), which were recorded as rGOA-nZVI-1, rGOA-nZVI-2, rGOA-nZVI-3, rGOA-nZVI-4, and rGOA-nZVI-5, respectively. After sufficient adsorption of Fe(II) on rGOA, desired amounts of NaBH4 (1.82, 3.64, 5.46, 7.28 and 9.10 g) dissolved in 50 mL deionized water was added into the suspension at room temperature. The mixture was then stirred by a magnetic stirrer for 30 min. After the reaction, the black suspension was separated by filter, and then the collected black solid was washed with deionized water and ethanol (1:1, v/v) three times, respectively. The final product was freeze-dried for 24 h. The synthesis of nZVI nanoparticles was similar to that of rGOA-nZVI but without the addition of rGOA.
2.3. Characterization of NanocompositesThe structures of the nanocomposites were systematically analyzed by scanning electron microscopy (SEM), atomic force microscopy (AFM), Brunauer-Emmett-Teller (BET), X-ray diffraction (XRD) and Fourier transform infrared spectroscopy (FTIR). The surface characteristics and morphology of the composites were studied using the NoVa™ Nano SEM 250 (FEI, Hillsboro, OR, USA). The AFM images were recorded with a scanning probe microscope (Dimension Icon, Bruker, USA) in tapping mode. The BET method was applied to measure the specific surface area and pore size distribution of rGOA-nZVI nanocomposites with a surface area analyzer (Micromeritics Instrument Corporation, Norcross, USA). A crystallinity study of the samples was performed by XRD analysis (Ulitma IV, Rigaku, Tokyo, Japan) with a scanning scope ranging from 5° to 90° and a scanning rate of 10° min−1 via Cu-Kα (λ =0.154 nm) radiation. The Fe contents in rGOA-nZVI-1 and rGOA-nZVI-3 were measured by the acid pickling method [30]. Briefly, the nanocomposites were soaked in HCl:HNO3 (3:1, v/v) for 48 h, and then the suspension was separated by water filter membrane. The collected filtrate was analyzed by inductively coupled plasma-mass spectrometry (ICP-MS, Agilent 7700ce, Agilent, USA).
2.4. Batch Adsorption ExperimentsBatch adsorption experiments were carried out in 50-mL centrifuge tubes containing 50 mL of MB or MO solution (with designed initial concentrations of 200–2500mg/L) and 30mg nanocomposites, and the suspensions were tumbled on a reciprocating shaker at 150 rpm with designed temperature (15–55°C) and contact time (0–90 min). The pH of the suspension was adjusted to a range of 1–10 with 0.1M HCl and 0.1M NaOH solutions to check the effects of pH. After equilibrium or designed time, the nanocomposites were separated from the aqueous solutions by a magnet (expect for GO and rGOA, which were separated by centrifugation) and the filtrate MB or MO concentrations in aqueous solutions were analyzed by an ultraviolet-visible spectrophotometer (UVWin 5.0, Puxi, China). The maximum absorbance wavelength of MB and MO were 664 nm and 464 nm, respectively. The removal capacity (qe) of MB or MO by the nanocomposites was calculated using Eq. (1) and Eq. (2) at equilibrium time and time t. The removal efficiency (R) of MB or MO from aqueous solutions at different times was calculated using Eq. (3) (Tan et al., 2015).
where qe (mg/g) is the amount of MB or MO removed by the nanocomposites at equilibrium time, qt (mg/g) is the amount of MB or MO removed by the nanocomposites at time t, m (mg) is the mass of nanocomposites used, V (L) is the volume of the solution, and C0, Ct and Ce (mg/L) are concentrations of MB or MO in the solution at the beginning, time t and equilibrium, respectively.
2.5. Recycling and Stability of rGOA-nZVIThe rGOA-nZVI nanocomposites were separated by a magnet after MB and MO adsorption at 50 mL of dye solution with initial concentration of 2500 mg/L and 200 mg/L, respectively, and then the collected nanocomposites were washed with deionized water and ethanol 2–3 times. Fresh MB or MO solution was added into 50-mL centrifuge tubes containing the recycled nanocomposites to check the dye removal capacities of the recycled nanocomposites. The recycling experiment was repeated for 5 cycles. Besides, the stability of nZVI particles in rGOA-nZVI was examined by measuring the loss of total dissolved Fe species in resultant solutions at the end of MB and MO removal experiments. The supernatant was collected by filtration with 0.45 μm filter membrane and then acidified immediately for Fe analysis with ICP-MS.
2.6. Data AnalysisThe removal kinetics of the MB by rGOA-nZVI-3 and MO by rGOA-nZVI-1 were fitted by four commonly used models, i.e., pseudo-first-order, pseudo-second-order, intra-particle diffusion model and the Elovich model, which are described in detail in the Text S1 of supplementary materials [31].
The equilibrium adsorption data were fitted using four commonly used isotherm models, including Langmuir, Freundlich, Temkin, and Dubinin-Radushkevich (D-R) isotherms. Statistical analyses were carried out to determine the validity of the isotherm models on the basis of four error functions, including R2, chi square test (x2), average percentage errors (APE), the sum of absolute errors (SAE) [32]. The detail can be referred in the Text S2 of supplementary materials.
Each experiment was conducted in triplicate and the data shown are the average values and error bars. The relative errors of the experiment data were all < 5%.
3. Results and Discussion3.1. Characterization of NanocompositesThe morphological and microstructure of rGOA, rGOA-nZVI-1, rGOA-nZVI-2, rGOA-nZVI-3, rGOA-nZVI-4 and rGOA-nZVI-5 were analyzed by SEM (Fig. 1). The nanosheets of GO as building units were assembled into layers and further overlapped together, and the finally rendered rGOA possessed porous structure without any collapse, which had well defined and interconnected 3D porous networks (Fig. 1a). This was also demonstrated by the AFM image (Fig. S1) that after the ultrasonic treatment, GO was exfoliated into thin GO nanosheets with a thickness of about ~1.8 nm. The GO nanosheets were expected to be thick because of the I ntroduction of covalently bound oxygen and the displacement of the sp3-hybridized carbon atoms on the original graphene plane [33]. Moreover, few nZVI particles dispersed uniformly on the surface of rGOA at nZVI/rGOA= 1:1 (named as rGOA-nZVI-1) with a nZVI loading capacity of 37.4 wt% (Fig. 1b), while nZVI kept distributed uniformly on the surface of rGOA when the ratio of nZVI further increased, i.e., at nZVI/rGOA=3:1 (named as rGOA-nZVI-3) with a nZVI loading capacity of 52.7 wt% (Fig. 1d). The Fe content of rGOA-nZVI-3 were higher than those of other reported materials [34–36]. Rough nZVI existed in the form of prominent chain-like aggregates and assembled severely on the surface of rGOA for rGOA-nZVI-5 (Fig. 1f). With increasing nZVI loading in the rGOA-nZVI composites from mass ratio of 1:1 to mass ratio of 3:1, nZVI particles were more evenly dispersed, and thus providing more reactive sites. However, excessive nZVI (4:1 and 5:1) loading could possibly block the active sites on rGOA surfaces, resulting in the decrease of the reactivity of the iron particles. The BET results indicated that the incorporation of nZVI (SA: 5.49 m2/g) onto rGOA decreased the SA of rGOA (SA: 83.2 m2/g) by different extents with rGOA-nZVI-2 having the lowest SA of 16.9 and rGOA-nZVI-3 having the highest SA of 31.5 m2/g (Table S1). The pore size distributions of nZVI, GO, rGOA, rGOA-nZVI-1, and rGOA-nZVI-3 were all about 3.83 nm, which clearly demonstrated the porous structures of the as-synthesized materials.
The XRD patterns of nZVI, GO, rGOA, rGOA-nZVI-1, rGOA-nZVI-2, rGOA-nZVI-3, rGOA-nZVI-4, and rGOA-nZVI-5 are illustrated in Fig. 2. The strong characteristic peak for GO appeared at 11°, and based on this value, the interlayer spacing (001) of GO was calculated to be 0.81 nm (d value) according to Bagg’s equation [37]. This confirms the oxidation of graphite to the GO [38]. However, the strong peak (11°) was not observed for rGOA but a new broad diffraction peak appeared at 25° (d=0.36 nm). The d value of rGOA was much lower than that of GO but slightly higher than that of natural graphite (d = 0.335 nm). The difference between rGOA and GO and natural graphite in d value can be attributed to the p-p stacking interaction between the graphene sheets in rGOA, which resulted in the formation of the agglomerates [39]. Three diffraction peaks at 44.7°, 65.0°, and 82.5° corresponding to the formation of nZVI can be observed in the XRD patterns of nZVI, rGOA-nZVI-1 and rGOA-nZVI-3, which were indexed to the (110), (200) and (211) planes of cubic Fe (Gu et al., 2018). There was no obvious difference in the XRD patterns of nZVI, rGOA-nZVI-1, rGOA-nZVI-2, rGOA-nZVI-3, rGOA-nZVI-4, and rGOA-nZVI-5.
The FTIR spectra of the nZVI, GO, rGOA, rGOA-nZVI-1, rGOA-nZVI-2, rGOA-nZVI-3, rGOA-nZVI-4, and rGOA-nZVI-5 samples are shown in Fig. 3. The broad adsorption band exhibited in the region of 3665–3265 cm−1 corresponds to the O-H stretching vibration, which was attributed to the interlayer water molecules and the hydroxyl group present on the GO surface. GO shows 1053, 1224, 1385, 1625 and 1730 cm−1, which were attributed to C=O, C=C, O-H, C-OH and C-O stretching vibration, respectively [40]. Compared with GO, the peak of C=O at 1730 cm−1 disappeared for rGOA, indicating the fully reduction of GO by NaBH4. There was no obvious difference in the FTIR spectra of nZVI, rGOA-nZVI-1, rGOA-nZVI-2, rGOA-nZVI-3, rGOA-nZVI-4, and rGOA-nZVI-5.
3.2. Adsorption Kinetics of DyesThe removal of the two dyes, i.e., MB and MO, by rGOA-nZVI was faster initially and proceeded gradually afterwards, and no obviously additional removal occurred after 60 min (Fig. S2a and 2b). The final removal rates of the two dyes were all higher than 94.8% with slight differences between the different nanocomposites with varied nZVI ratios. As for MB, the decolorization rates were in the order of rGOA-nZVI-3 (98.4%) > rGOA-nZVI-2 (97.8%) > rGOA-nZVI-4 (97.2%) > rGOA-nZVI-1(95.0%) > rGOA-nZVI-5 (94.8%), while the final removal rates of MO were in the order of rGOA-nZVI-1 (99.6%) > rGOA-nZVI-4 (96.1%) > rGOA-nZVI-5 (96.0%) > rGOA-nZVI-2 (95.7%) = rGOA-nZVI-3 (95.7%). Considering the removal efficiency, rGOA-nZVI-3 and rGOA-nZVI-1 were chosen for the further study on the removal of MB and MO, respectively.
The removal kinetics of the MB by rGOA-nZVI-3 and MO by rGOA-nZVI-1 were fitted by four commonly used models, i.e., pseudo-first-order, pseudo-second-order, intra-particle diffusion model, and the Elovich model. A comparison of the kinetic models for the MB and MO removal by rGOA-nZVI is illustrated in Fig. 4 and the calculated kinetics parameters are presented in Table S2. The R2 values of the pseudo-second-order kinetic model are 0.9999 and 0.9991 for MB and MO removal, respectively, which are much higher than those of the pseudo-first-order model and the Elovich model (Fig. 4b and c). The theoretical MB and MO removal capacities calculated from the pseudo-second-order kinetic equation were 826 and 341mg/g, being close to the experimental removal capacities (Table. S2), demonstrating that the pseudo-second-order model was applicable to the MB and MO removal by rGOA-nZVI. This result showed that the removal of the dyes was primarily determined by a chemical removal process, and it was deduced that the transfer, exchange or sharing electrons between the dye molecules and the rGOA-nZVI nanocomposites occurred and then a chemical bond was formed (Ho, 2006). In order to further investigate the diffusion of MB and MO onto rGOA-nZVI, the intraparticle diffusion model was employed to discuss the diffusion mechanism during the removal process (Fig. 4d). The result indicated that there are two stages during the removal process of MB and MO onto rGOA-nZVI nanocomposites. The slopes of the first stage (134 mg·g−1·min−1/2 for MB and 55.0 mg·g−1·min−1/2 for MO) were substantially higher than those of the second stage (3.09 for MB and 4.60 for MO) due to the external diffusion and the instantaneous availability of large active adsorption sites on the surface of rGOA-nZVI [41]. The second stage was a slow adsorption stage, which corresponded to the intraparticle diffusion of the MB and MO from the external surface into the pores of the rGOA-nZVI [42]. These results illustrated that the external diffusion played a main role in the initial period of removal kinetics of the dyes by rGOA-nZVI nanocomposites.
3.3. Adsorption Isotherms of Dyes
Fig. S3a and S3b demonstrate the adsorption isotherms of the two dyes on rGOA-nZVI-1 and rGOA-nZVI-3, respectively, obtained with different initial MB (500 to 2500 mg/L) and MO (200 to 500 mg/L) concentrations. It can be seen that the removal capacity increased with increasing equilibrium concentrations of the dyes in the aqueous solutions and reached a platform at a high concentration.
The equilibrium adsorption data were fitted using four commonly used isotherm models, including Langmuir, Freundlich, Temkin, and Dubinin-Radushkevich (D-R) isotherms. The isotherm parameters and fitting results are shown in Table S3 and Fig. S3c. The R2 values of Langmuir isotherms for MB and MO are higher than those of the Freundlich, Temkin and D-R isotherms, which indicated that single molecular layer chemisorption occurred on the homogeneous surface of the composites [14]. Based on the Langmuir isotherm, the maximum removal capacities of rGOA-nZVI-3 toward MB and rGOA-nZVI-1 toward MO are 3918 mg/g and 667 mg/g, respectively, which are significantly higher than those of reported materials (Table 1). Besides, the values of RL are usually adopted to determine the conformance of adsorption to a specific adsorbate-adsorbent system, which could be obtained from parameters in Langmuir model (Eq 6 in the SI). The relationships between initial MB and MO concentrations and RL values are shown in Fig. S4 and the RL values were all less than 1.0 demonstrating that the removal processes of MB and MO were highly favorable and the interactions between the dye molecules and rGOA-nZVI were rather strong [40].
3.4. Thermodynamics AnalysisThe removal capacities of MB by rGOA-nZVI-3 and MO by rGOA-nZVI-1 increased with increasing temperature (288K to 328K) (Fig. S5a and S5b), which implied the endothermic nature of the removal processes. However, the removal capacity of MB by rGOA-nZVI-3 decreases over 308K (Fig. S5a), which may be due to the oxidation of nZVI.
The changes in distribution coefficient (Kd) with temperature can be used to determine the thermodynamic parameters of dye removal process [49]. The thermodynamics parameters are determined as:
where Kd is the distribution coefficient (mL/g) of MB and MO, which can be calculated by qe/Ce; ΔH (kJ mol−1), ΔS (kJ mol−1 K−1) and ΔG (kJ mol−1) are the change in enthalpy, entropy and Gibbs free energy during adsorption process, respectively; T (K) is the solution temperature and R (J mol−1 K−1) is the universal gas constant.
The ΔH and ΔS values are calculated from the intercept and slope of the linear plot of ln(Kd) against 1/T (Fig. S5c and d), which are listed in Table S4. The positive values of ΔH implied that the removal processes were endothermic in nature. In addition, the positive values of ΔS demonstrated increased randomness at the solid-solution interface and good affinity of MB and MO with rGOA-nZVI. The negative values of ΔG indicated the feasibility and spontaneity of the MB and MO removal processes [50].
3.5. pH EffectpH can affect the speciation of the target contaminants and the charge distribution on material surface, and hence show great influence on the charge transfer on the solid/liquid interface and the removal of ionized compounds from aqueous solutions [49]. The effect of solution pH on the MB and MO removal by rGOA-nZVI was investigated at a pH range from 1 to 10 (Fig. 5). To investigate the removal mechanism for MB and MO by rGOA-nZVI, zeta potential was measured for rGOA-nZVI-1 and rGOA-nZVI-3 (Fig. S6). The surface charges of rGOA-nZVI were negative in pH range of 1–10. At low pH range, the surface charges of the nanocomposites decreased with increasing pH, to the lowest value of −36.2 mV for rGOA-nZVI-1 and −34.0 mV for rGOA-nZVI-3 at pH=6. This can be attributed to the ionization of the multiple oxygen-containing functional groups like carboxyl, phenolic hydroxyl [51]. After pH of 6, the surface charges of rGOA-nZVI increased with increasing pH but were still below zero. This can be ascribed to the accumulation of Fe precipitation. The pKa of MB and MO are 4.40 and 3.76, respectively, indicating that MB and MO molecules are predominantly in cationic form when the pH was < 4.40 and 3.76 and changes to neutral molecules as the pH becomes > 4.40 and 3.76 [52, 53]. No obvious change of MB and MO removal efficiencies by rGOA-nZVI was observed under different pH values (Fig. 5), indicating that the MB and MO removal by rGOA-nZVI were pH independent. This is an advantage of the nanocomposites, which can perform efficiently in wastewater with a wide pH range.
3.6. Recycling and Stability of rGOA-nZVIThe regeneration and stability of nanocomposites are vital factors for evaluating their potential for practical applications [7]. Deionized water and ethanol were employed to desorb the sorbed MB (with initial concentration of 2500 mg/L) and MO (with initial concentration of 200 mg/L) from rGOA-nZVI composites. The recycled rGOA-nZVI nanocomposites were measured five times for their capacities for dye removal. The results showed that the removal efficiencies of dyes using regenerated rGOA-nZVI were still rather high after five cycles, and more than 90% of the removal efficiencies were retained for MB and MO (Fig. 6). Table 2 shows the leaching percentages of Fe from rGOA-nZVI nanocomposites after MB and MO removal. The percentages of Fe leaching from rGOA-nZVI were less than 1% after five cycles reuse, which demonstrated that rGOA-nZVI nanocomposites are very stable. Therefore, in all aspects, rGOA-nZVI can be an ideal candidate for the practical applications in dye removal due to the distinguished removal efficiency, good recyclability and excellent stability.
3.7. Removal MechanismsSeveral processes, such as the electrostatic attraction, redox reactions and precipitation could be involved in the MB and MO removal by rGOA-nZVI [54]. At low pH, MB (cation) adsorption is mainly electrostatic attraction, as the surface of rGOA-nZVI-3 is negatively charged based on zeta potential measurements, which demonstrated that the adsorption capacity of MB was higher than that of MO by rGOA-nZVI. Furthermore, the large SSA and high pore size distribution of rGOA-nZVI also contribute to physical adsorption of MB and MO. Iron hydroxides or oxides could be generated on the surface of rGOA-nZVI, which could also enhance their adsorption capacities in the rGOA-nZVI system.
Both SEM and XRD were employed to analyze the reaction products to infer the removal mechanisms. The SEM images of rGOA-nZVI-3 and rGOA-nZVI-1 after adsorption of MB and MO are presented in Fig. S7a-S7b. Before the reaction, the iron particles are in a shape of spherical and in a scale of nanometer uniformly (Fig. 1b and 1d). This indicates that most of the iron particles were distributed on the surface of rGOA-nZVI evenly and randomly due to the large surface area of the rGOA-nZVI, even though a very small amount of nZVI particles existed in the form of small agglomerates. After reducing MB by rGOA-nZVI-3, the products were agglomerated together tightly, indicating the generation of the Fe(II)/Fe(III)(oxy)hydroxides, which also enhancing the removal efficiency of rGOA-nZVI-3. On the contrary, this phenomenon was not observed in the removal of MO by rGOA-nZVI-1, which indicated that the removal mechanism of MO is only adsorption.
To further investigate the removal mechanism, XRD spectra of rGOA-nZVI-3 and rGOA-nZVI-1 were observed. As demonstrated in Fig. 2 and Fig. S7c, obvious changes can be found after MB adsorption by rGOA-nZVI-3. The Fe0 peak disappeared in the XRD spectrum of rGOA-nZVI-3 after MB adsorption, while the new peak present at the 2θ angles of 35.6° (Fe2O3 and Fe3O4) [55]. The appearance of Fe2O3 and Fe3O4 enhanced the adsorption of rGOA-nZVI-3 and the disappearance of Fe0 demonstrated the redox reactions between nZVI and MB. However, the Fe0 peak obviously existed in the XRD spectrum of rGOA-nZVI-1 after MO removal, which also confirmed that the removal mechanism for MO is only adsorption.
4. ConclusionsThe rGOA-nZVI nanocomposites were successfully prepared by precipitating nZVI on 3D rGOA, which were obtained by reduction of GO under ultrasonication. The obtained rGOA-nZVI nanocomposites showed excellent performance on azo dyes removal, the adsorption could be finished in 30 min with the maximum adsorption capacities being 3918mg/kg for MB by rGOA-nZVI-3 and 667 mg/kg for MO by rGOA-nZVI-1. The adsorptions of the MB and MO onto rGOA-nZVI were a monolayer chemisorption process, which fit the pseudo-second-order and Langmuir isotherm models. The adsorptions of MB and MO onto rGOA-nZVI nanocomposites at varied temperatures indicated that the adsorptions are spontaneous and endothermic, and the randomness increases with the adsorptions of MB and MO. Moreover, rGOA-nZVI showed satisfactory regeneration performance and reusability, which can significantly reduce the wastewater treatment expenses. Based on these results, rGOA-nZVI is an excellent nanomaterial for efficient removal of MB and MO from aqueous solutions. Furthermore, it is worthwhile to point out that rGOA-nZVI is not limited to dye removal but can also be explored to the removal of other pollutants by adsorption and redox reactions.
AcknowledgmentsThis work was supported by National Key R&D Program of China (2018YFC1802001), and the Major Research plan of the Shandong Science Foundation (ZR2020ZD19), Science and Technology Major Project of Tianjin (22YFZCSN00050) and Ministry of Education of China (T2017002).
References1. Alotaibi KM, Shiels L, Lacaze L, et al. Iron supported on bioinspired green silica for water remediation. Chem. Sci. 2017;8(1)567–576.
https://doi.org/10.1016/j.jiec.2013.05.024
2. Fan MY, Hu J, Cao R, Ruan W, Wei X. A review on experimental design for pollutants removal in water treatment with the aid of artificial intelligence. Chemosphere. 2018;200:330–343.
https://doi.org/10.1016/j.chemosphere.2018.02.111
3. Huang LJ, He M, Chen BB, Hu B. Magnetic Zr-MOFs nanocomposites for rapid removal of heavy metal ions and dyes from water. Chemosphere. 2018;7(1)435–444.
https://doi.org/10.1016/j.chemosphere.2018.02.019
4. Cui KX, Yan B, Xie YJ, et al. Regenerable urchin-like Fe3O4@ PDA-Ag hollow microspheres as catalyst and adsorbent for enhanced removal of organic dyes. J. Hazard. Mater. 2018;350:66–75.
https://doi.org/10.1016/j.jhazmat.2018.02.011
5. Geng ZG, Lin Y, Yu XX, et al. Highly effiient dye adsorption and removal: a functional hybrid of reduced grapphene oixed-Fe3O4 nanoparticles as easily regenerative adsorbent. J. Mater. Chem. 2012;22:3527–3535.
https://doi.org/10.1039/C2JM15544C
6. Rahimi K, Mirzaei R, Akmad A, Mirghaffari N. Preparation of nanoparticle-modified polymeric adsorbent using wastage fuzzes of mechanized carpet and its application in dye removal from aqueous solution. J. Clean. Prod. 2018;178:373–383.
https://doi.org/10.1016/j.jclepro.2017.12.213
7. Xu R, Mao J, Peng N, Luo XG, Chang CY. Chitin/clay microspheres with hierarchical architecture for highly efficient removal of organic dyes. Carbohyd. Polym. 2018;188:143–150.
https://doi.org/10.1016/j.carbpol.2018.01.073
8. Forgacs E, Cserhati T, Oros G. Removal of synthetic dyes from wastewaters: a review. Environ. Int. 2004;30:953–971.
https://doi.org/10.1016/j.envint.2004.02.001
9. Deng SJ, Xu HJ, Jiang XS, Yin J. Poly(vinyl alcohol) (PVA)-enhanced hybrid hydrogels of hyperbranched poly(ether amine)s (hPeAs) for slective adsorption and seperation of dyes. Macromolecules. 2014;46:2399–2406.
https://doi.org/10.1021/ma302330w
10. Paz A, Carballo J, Perez MJ, Dominguez JM. Biological treatment of model dyes and textile wastewaters. Chemosphere. 2017;181:168–177.
https://doi.org/10.1016/j.chemosphere.2017.04.046
11. Wu Y, Chen L, Long XW, Zhang XL, Pan BC, Qian JS. Multi-functional magnetic water purifier for disinfection and removal of dyes and metal ions with superior reusability. J. Hazard. Mater. 2018;347:160–167.
https://doi.org/10.1016/j.jhazmat.2017.12.037
12. Chen FY, An WJ, Liu L, Liang YH, Cui WQ. Highly efficient removal of bisphenol A by a three-dimensional graphene hydrogel-AgBr@rGO exhibiting adsorption/photocatalysis synergy. Appl. Catal. B-Environ. 2017;217:65–80.
https://doi.org/10.1016/j.apcatb.2017.05.078
13. Fan MY, Hu JW, Cao RS, Xiong KN, Wei XH. Modeling and prediction of copper removal from aqueous solutions by nZVI/rGO magnetic nanocomposites using ANN-GA and ANN-PSO. Sci. Rep. 2017;7(1)18040.
https://doi.org/10.1038/s41598-017-18223-y
14. Wang L, Zhang JP, Wang AQ. Fast removal of methylene blue from aqueous solution by adsorption onto chitosan-g-poly (acrylic acid)/attapulgite composite. Desalination. 2011;266(1)33–39.
https://doi.org/10.1016/j.desal.2010.07.065
15. Fan MY, Li TJ, Hu JW, et al. Synthesis and characterization of reduced graphene oxide-supported nanoscale zero-valent iron (nZVI/rGO) composites used for Pb(II) removal. Materials. 2016;9(8)687.
https://doi.org/10.3390/ma9080687
16. Zou YD, Wang XX, Ai YJ, et al. Coagulation behavior of graphene oxide on nanocrystallined Mg/Al layered double hydroxides: batch experimental and theoretical calculation study. Environ. Sci. Technol. 2016;50:3658–3667.
https://doi.org/10.1021/acs.est.6b00255
17. Zhang X, Guo H, Jin L, Xu M, Miller JF, Zhou ZH. Atomic structure of bordetella bacteriophage reveals a jellyroll fold in cementprotein and a topologically distinct HK97-like fold in major capsid protein. Microsc. Microanal. 2012;18(S2)72–73.
https://doi.org/10.1017/S1431927612002218
18. Fan MY, Li TJ, Hu JW, et al. Artificial neural network modeling and genetic algorithm optimization for cadmium removal from aqueous solutions by reduced graphene oxide-supported nanoscale zero-valent iron (nZVI/rGO) composites. Materials. 2017;10(5)544.
https://doi.org/10.3390/ma10050544
19. Bi HC, Xie X, Yin KB, et al. Graphene: Spongy graphene as a highly efficient and recyclable sorbent for oils and organic solvents. Adv. Funct. Mater. 2012;22(21)4421–4425.
https://doi.org/10.1002/adfm.201290127
20. Liu TH, Li YH, Du QJ, et al. Adsorption of methylene blue from aqueous solution by graphene. Colloid. Surfaces B. 2012;90(1)197–203.
https://doi.org/10.1016/j.colsurfb.2011.10.019
21. Liu Y, Luo C, Sun J, Li HZ, Sun ZB, Yan SQ. Enhanced adsorption removal of methyl orange from aqueous solution by nanostructured proton-containing delta-MnO2
. J. Mater. Chem. A. 2015;3(10)5674–5682.
https://doi.org/10.1039/c4ta07112c
22. Wu ZS, Sun Y, Tan YZ, Yang S, Feng X, Müllen K. Three-dimensional graphene-based macro- and mesoporous frameworks for high-performance electrochemical capacitive energy storage. J. Am. Chem. Soc. 2012;134(48)19532–19535.
https://doi.org/10.1021/ja308676h
23. Shi QR, Cha Y, Song Y, et al. 3D graphene-based hybrid materials: synthesis and applications in energy storage and conversion. Nanoscale. 2016;8(34)15414–15447.
https://doi.org/10.1039/c6nr04770j
24. Sun YB, Ding CC, Cheng WC, Wang XK. Simultaneous adsorption and reduction of U(VI) on reduced graphene oxide-supported nanoscale zerovalent iron. J. Hazard. Mater. 2014;280:399–408.
https://doi.org/10.1016/j.jhazmat.2014.08.023
25. Wang W, Cheng YL, Kong T, Cheng GS. Iron nanoparticles decoration onto three-dimensional graphene for rapid and efficient degradation of azo dye. J. Hazard. Mater. 2015;299:50–58.
https://doi.org/10.1016/j.jhazmat.2015.06.010
26. Asfaram A, Ghaedi M, Hajati S, Goudarzi A. Synthesis of magnetic γ-Fe2O3-based nanomaterial for ultrasonic assisted dyes adsorption: Modeling and optimization. Ultrason. Sonochem. 2016;32:418–431.
https://doi.org/10.1016/j.ultsonch.2016.04.011
27. Pu SY, Deng DL, Wang KX, et al. Optimizing the removal of nitrate from aqueous solutions via reduced graphite oxide–supported nZVI: synthesis, characterization, kinetics, and reduction mechanism. Environ. Sci. Pollut. R. 2019;26:3932–3945.
https://doi.org/10.1007/s11356-018-3813-1
28. Fan MY, Zhang P, Wang CP, Tang JC, Sun HW. Tailored design of three-dimensional rGOA-nZVI catalyst as an activator of persulfate for degradation of organophosphorus pesticides. J. Hazard. Mater. 2022;428:128254.
https://doi.org/10.1016/j.jhazmat.2022.128254
29. Hummers W, Offeman R. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958;80(6)1339.
https://doi.org/10.1021/ja01539a017
30. Santoro A, Held A, Linsinger TPJ, Perez A, Ricci M. Comparison of total and aqua regia extractability of heavy metals in sewage sludge: The case study of a certified reference material. Trac-Trend. Anal. Chem. 2017;89:34–40.
https://doi.org/10.1016/j.trac.2017.01.010
31. Dil EA, Ghaedi M, Asfaram A, Hajati S, Mehrabi F, Goudarzi A. Preparation of nanomaterials for the ultrasound-enhanced removal of Pb2+ ions and malachite green dye: Chemometric optimization and modeling. Ultrason. Sonochem. 2017;34:677–691.
https://doi.org/10.1016/j.ultsonch.2016.07.001
32. Rahman N, Haseen U. Equilibrium modeling, kinetic, and thermodynamic studies on adsorption of Pb(II) by a hybrid inorganic-organic material: Polyacrylamide zirconium(IV) iodate. Ind. Eng. Chem. Res. 2014;53(19)8198–8207.
https://doi.org/10.1021/ie500139k
33. Stankovich S, Piner RD, Nguyen SBT, Ruoff RS. Synthesis and exfoliation of isocyanate-treated graphene oxide nanoplatelets. Carbon. 2006;44(15)3342–3347.
https://doi.org/10.1016/j.carbon.2006.06.004
34. Liu FL, Yang JH, Zuo JN, et al. Graphene-supported nanoscale zero-valent iron: Removal ofphosphorus from aqueous solution and mechanistic study. J. Environ. Sci. 2014;26:1751–1762.
https://doi.org/10.1016/j.jes.2014.06.016
35. Chen WJ, Wang XJ, Yuan ZQ. Preparation of nanoscale zero-valent iron particles with different coatings for removal of Cr(VI) from water. Int. J. Nanotechnol. 2016;13:923.
https://doi.org/10.1504/IJNT.2016.080365
36. Jia ZZ, Shu YH, Huang RL, Liu JG, Liu LL. Enhanced reactivity of nZVI embedded into Supermacroporouscryogels for highly efficient Cr(VI) and total Cr removal from aqueous solution. Chemosphere. 2018;199:232–242.
https://doi.org/10.1016/j.chemosphere.2018.02.021
37. Deerattrakul V, Dittanet P, Sawangphruk M, Kongkachuichay P. CO2 hydrogenation to methanol using Cu-Zn catalyst supported on reduced graphene oxide nanosheets. J. CO2 Util. 2016;16:104–113.
https://doi.org/10.1016/j.jcou.2016.07.002
38. Liu ZH, Wang ZM, Yang X, Ooi K. Intercalation of organic ammonium ions into layered graphite oxide. Langmuir. 2002;18(12)4926–4932.
https://doi.org/10.1021/la011677i
39. Jiang Y, Chowdhury S, Balasubramanian R. Nitrogen-doped graphene hydrogels as potential adsorbents and photocatalysts for environmental remediation. Chem. Eng. J. 2017;327:751–763.
https://doi.org/10.1016/j.cej.2017.06.156
40. Liu CY, Liu HY, Xu AR, Tang KY, Huang Y, Lu C. In situ reduced and assembled three-dimensional graphene aerogel for efficient dye removal. J. Alloy. Compd. 2017;714:522–529.
https://doi.org/10.1016/j.jallcom.2017.04.245
41. Liu F, Chung S, Oh G, Seo TS. Three-dimensional graphene oxide nanostructure for fast and efficient water-soluble dye removal. Acs Appl. Mater. Inter. 2012;4(2)922–927.
https://doi.org/10.1021/am201590z
42. Zhu HY, Jiang R, Xiao L, Li W. A novel magnetically separable Fe2O3/crosslinked chitosan adsorbent: preparation, characterization and adsorption application for removal of hazardous azo dye. J. Hazard. Mater. 2010;179:251–257.
https://doi.org/10.1016/j.jhazmat.2010.02.087
43. Haque E, Jun JW, Jhung SH. Adsorptive removal of methyl orange and methylene blue from aqueous solution with a metal-organic framework material, iron terephthalate (MOF-235). J. Hazard. Mater. 2011;185(1)507–511.
https://doi.org/10.1016/j.jhazmat.2010.09.035
44. Zhao Q, Zhu XY, Chen BL. Stable graphene oxide/poly(ethyleneimine) 3D aerogel with tunable surface charge for high performance selective removal of ionic dyes from water. Chem. Eng. J. 2017;334:1119–1127.
https://doi.org/10.1016/j.cej.2017.11.053
45. Bagheri AR, Ghaedi M, Asfaram A, et al. Modeling and optimization of simultaneous removal of ternary dyes onto copper sulfide nanoparticles loaded on activated carbon using second-derivative spectrophotometry. J. Taiwan Inst. Chem. E. 2016;65:212–224.
https://doi.org/10.1016/j.jtice.2016.05.004
46. Asfaram A, Ghaedi M, Goudarzi A, Hajati S. Ternary dye adsorption onto MnO2 nanoparticle-loaded activated carbon: Derivative spectrophotometry and modeling. RSC Adv. 2015;5(88)72300–72320.
https://doi.org/10.1039/C5RA10815B
47. Goscianska J, Marciniak M, Pietrzak R. Mesoporous carbons modified with lanthanum(III) chloride for methyl orange adsorption. Chem. Eng. J. 2014;247(6)258–264.
https://doi.org/10.1016/j.cej.2014.03.012
48. Ruan X, Chen Y, Chen H, Qian G, Frost RL. Sorption behavior of methyl orange from aqueous solution on organic matter and reduced graphene oxides modified Ni-Cr layered double hydroxides. Chem. Eng. J. 2016;297:295–303.
https://doi.org/10.1016/j.cej.2016.01.041
49. Shao L, Wang XF, Ren YM, et al. Facile fabrication of magnetic cucurbit [6] uril/graphene oxide composite and application for uranium removal. Chem. Eng. J. 2016;286:311–319.
https://doi.org/10.1016/j.cej.2015.10.062
50. Varadwaj G, Oyetade O, Rana S, Martincigh B, Jonnalagadda S, Nyamori V. Facile synthesis of three-dimensional Mg-Al layered double hydroxide/partially reduced graphene oxide nanocomposites for the effective removal of Pb2+ from aqueous solution. ACS Appl. Mater. Inter. 2017;9:17290–17305.
https://doi.org/10.1021/acsami.6b16528
51. Li D, Müller MB, Gilje S, Kander RB, Wallace GG. Processable aqueous dispersions of graphene nanosheets. Nat. Nanotechnol. 2008;3:101–105.
https://doi.org/10.1038/nnano.2007.451
52. Pfaffen V, Ortiz PI, Torresi SICD, Torresi RM. On the pH dependence of electroactivity of poly(methylene blue) films. Electrochim. Acta. 2010;55:1766–1771.
https://doi.org/10.1016/j.electacta.2009.10.062
53. Mu’azu ND, Jarrah N, Zubair M, et al. Mechanistic aspects of magnetic MgAlNibarium-ferrite nanocomposites enhanced adsorptiveremoval of an anionic dye from aqueous phase. J. Saudi Chem. Soc. 2020;24:715–732.
https://doi.org/10.1016/j.jscs.2020.08.001
54. Dong HR, Deng JM, Xie YK, et al. Stabilization of nanoscale zero-valent iron (nZVI) with modified biochar for Cr(VI) removal from aqueous solution. J. Hazard. Mater. 2017;332:79–86.
https://doi.org/10.1016/j.jhazmat.2017.03.002
55. Zhang X, Shen L, Chen ZL, Megharaj M, Naidu R. Kaolinite-supported nanoscale zero-valent iron for removalof Pb2+ from aqueous solution: Reactivity, characterizationand mechanism. Water Res. 2011;45(11)3481–3488.
https://doi.org/10.1016/j.watres.2011.04.010
Fig. 1SEM images of (a) rGOA, (b) rGOA-nZVI-1, (c) rGOA-nZVI-2, (d) rGOA-nZVI-3, (e) rGOA-nZVI-4 and (f) rGOA-nZVI-5. 
Fig. 2XRD patterns of nZVI, GO, rGOA, rGOA-nZVI-1, rGOA-nZVI-2, rGOA-nZVI-3, rGOA-nZVI-4 and rGOA-nZVI-5. 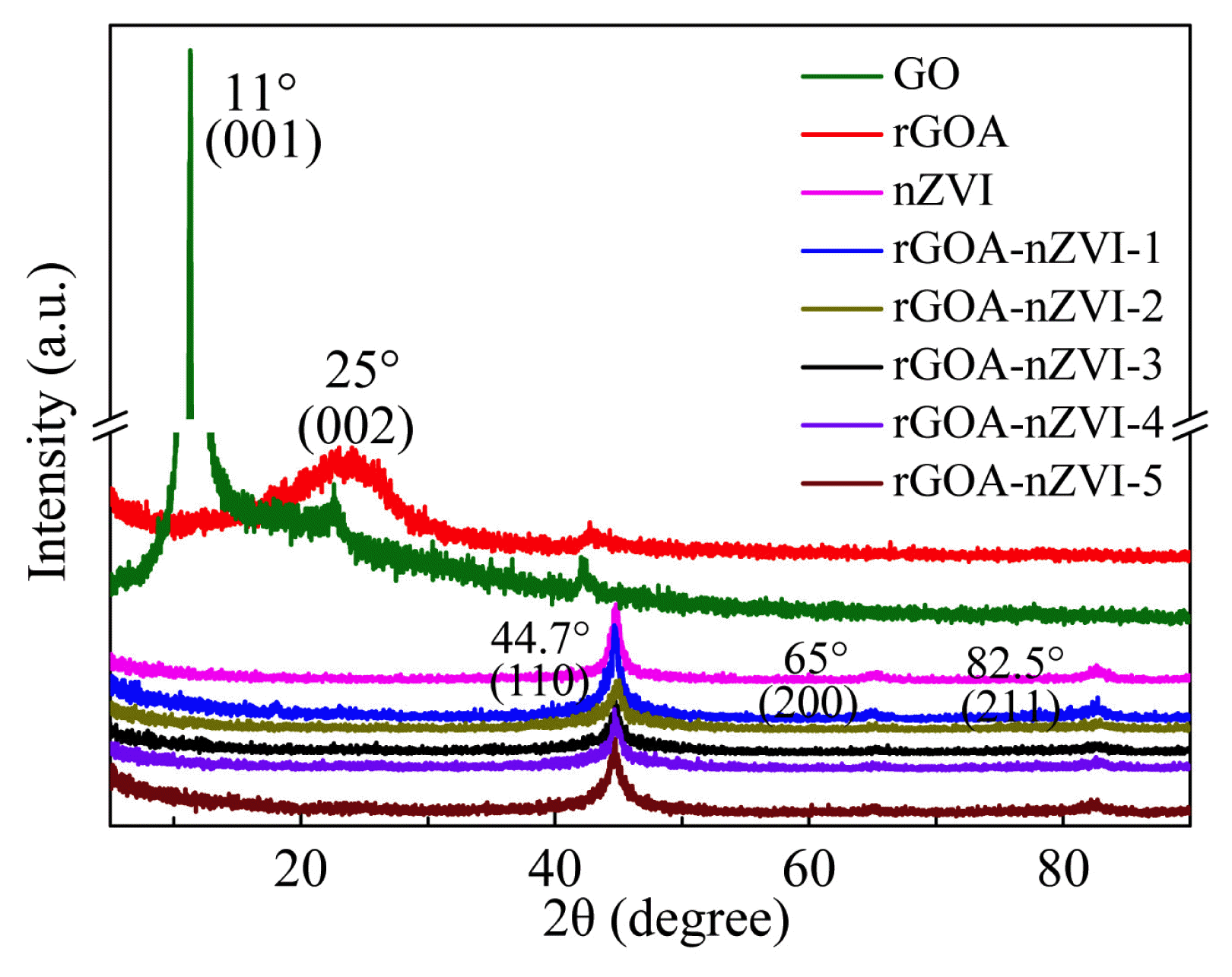
Fig. 3FTIR spectra of nZVI, GO, rGOA, rGOA-nZVI-1, rGOA-nZVI-2, rGOA-nZVI-3, rGOA-nZVI-4, and rGOA-nZVI-5. 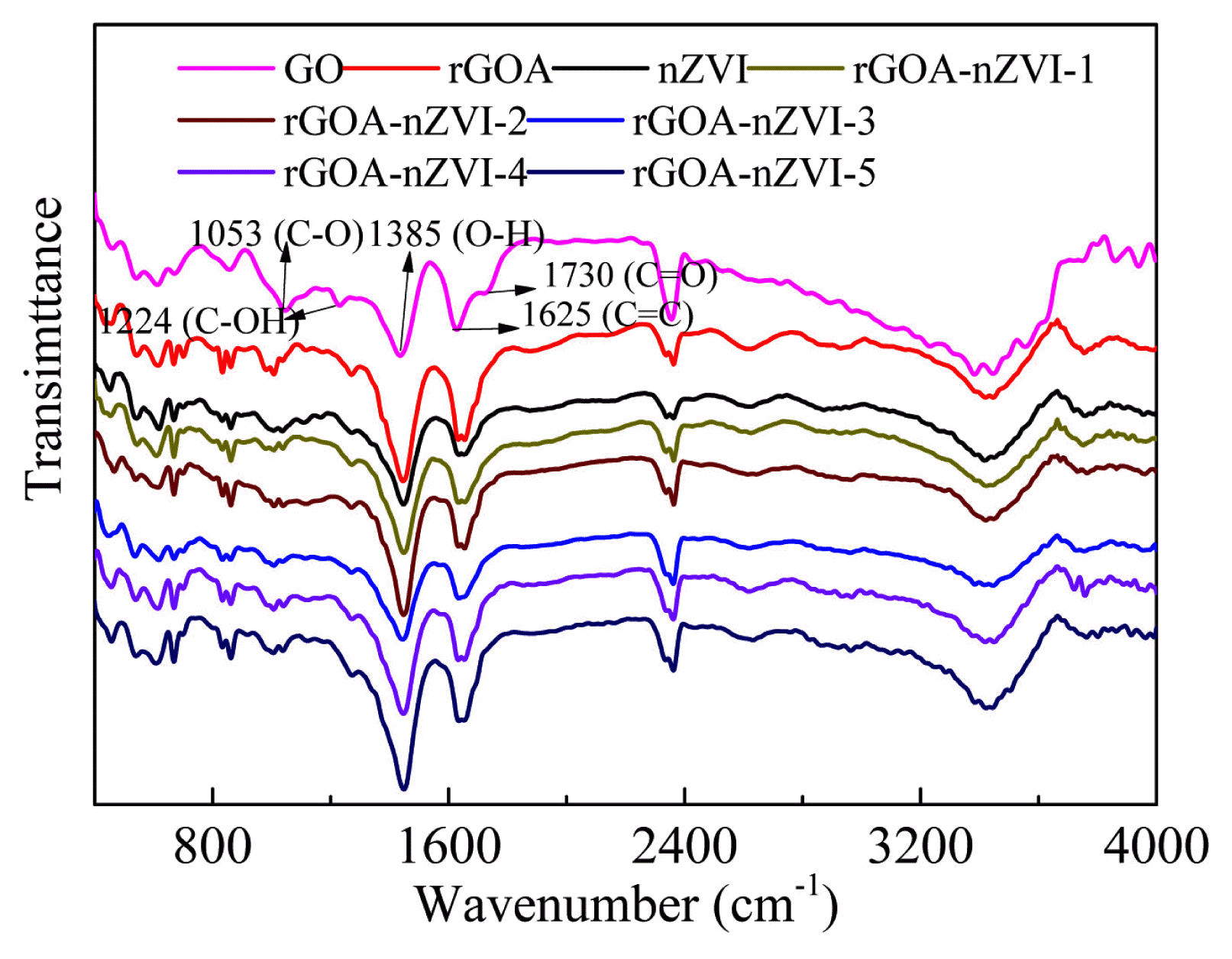
Fig. 4(a) The removal kinetics of MB by rGOA-nZVI-3 and MO by rGOA-nZVI-1 and fitting results by(b) pseudo-first-order, (c) pseudo-second-order, (d) intra-particle diffusion model, and(e) Elovich model. (MB concentration=500 mg/L, MO = 200 mg/L, amount of rGOA-nZVI-1 (for MO) and rGOA-nZVI-3 (for MB) = 0.03 g, solution pH=6.0, temperature =25°C. 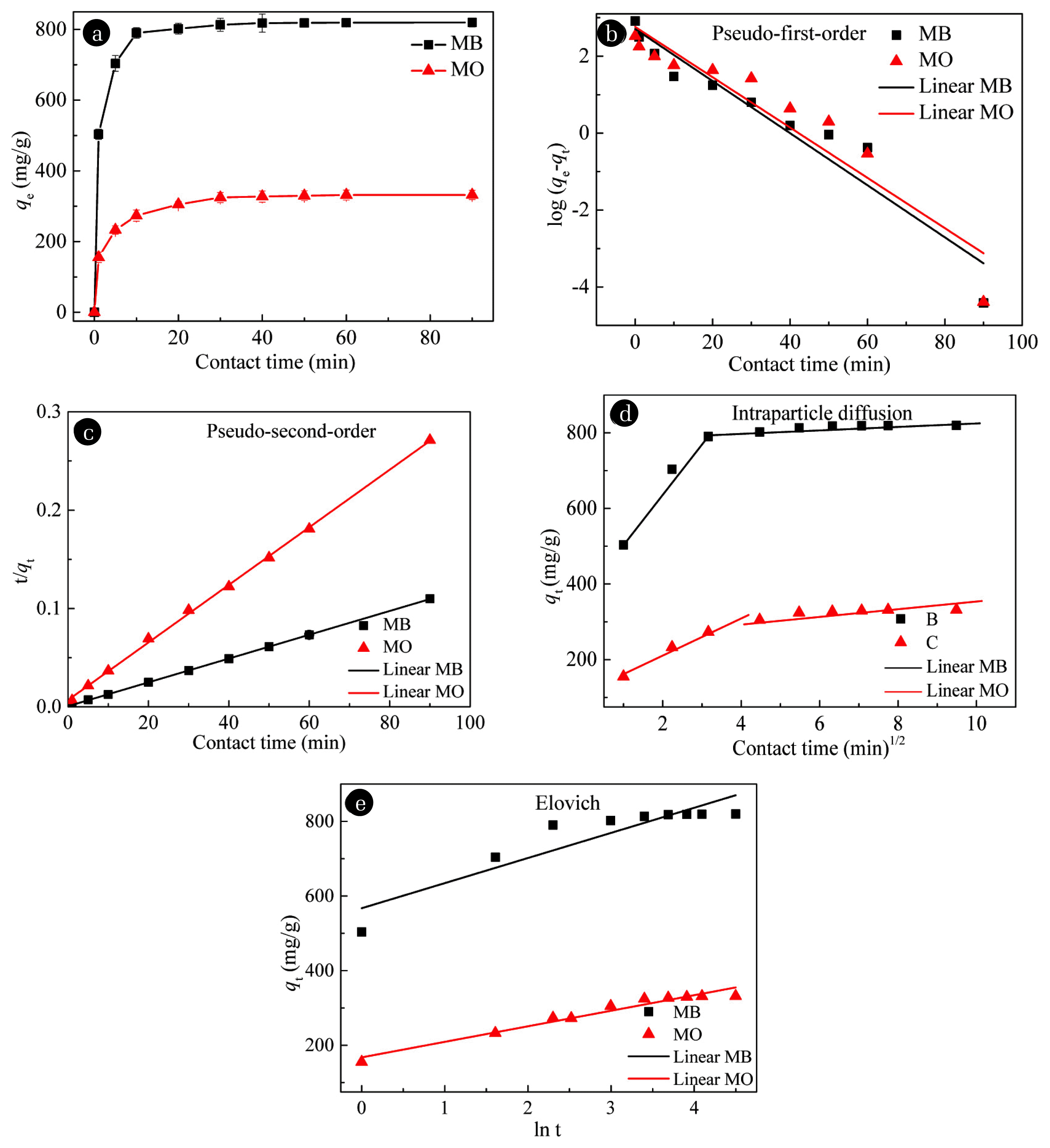
Fig. 6The removal efficiencies of MB and MO by rGOA-nZVI-3 and rGOA-nZVI-1 with five recycling cycles. 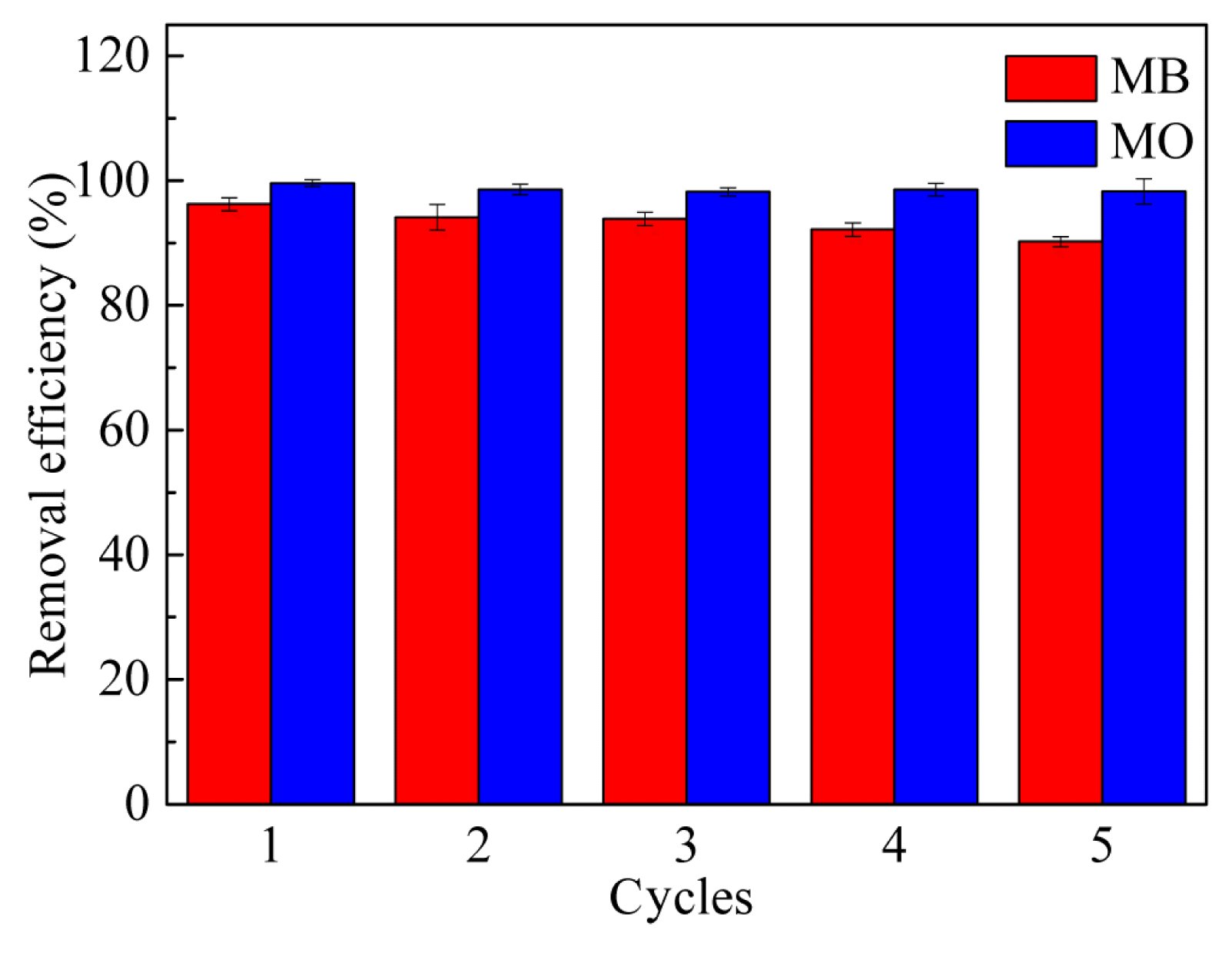
Table 1Comparison of removal capacities of MB and MO by different materials
Table 2The concentrations and percentages of Fe leaching from rGOA-nZVI |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||