AbstractMesopores of copper hexacyanoferrate (II) (M-CuHCF-II) nanomaterials used for the cesium (Cs+) removal were synthesized by the chemical coprecipitation method. M-CuHCF-II has a cubic structure (space group F-43m), a surface area of 12.80 m2/g, and a pore width of about 34.49 nm. At pH=6, the adsorption capacity of Cs+ ions by CuHCF-II reached the maximum within 15 minutes. Langmuir, Redlich–Peterson, and Sips models are suitable to describe the adsorption of Cs+ ions by M-CuHF-II materials. The maximum absorption capacity of M-CuHCF-II was determined at 318 K corresponding to the value of 197.72 mg g−1. The Gibbs free energy change (ΔG°) of Cs+ adsorption process with ranging values from −37.47 to −40.74, and ΔH0 = −8.28 (kJ mol−1) at temperatures from 298–348 K. Na+, NH4+, Ca2+, and K+ ions in solution affect the removal of Cs+ ions when they occur at high concentrations. M-CuHCF-II materials are easy to prepare, low-cost, have high adsorption capacity for Cs+ ions, and are easy to apply. In addition, the adsorption mechanism of Cs+ ions by M-CuHCF-II is also proposed and discussed.
Graphical Abstract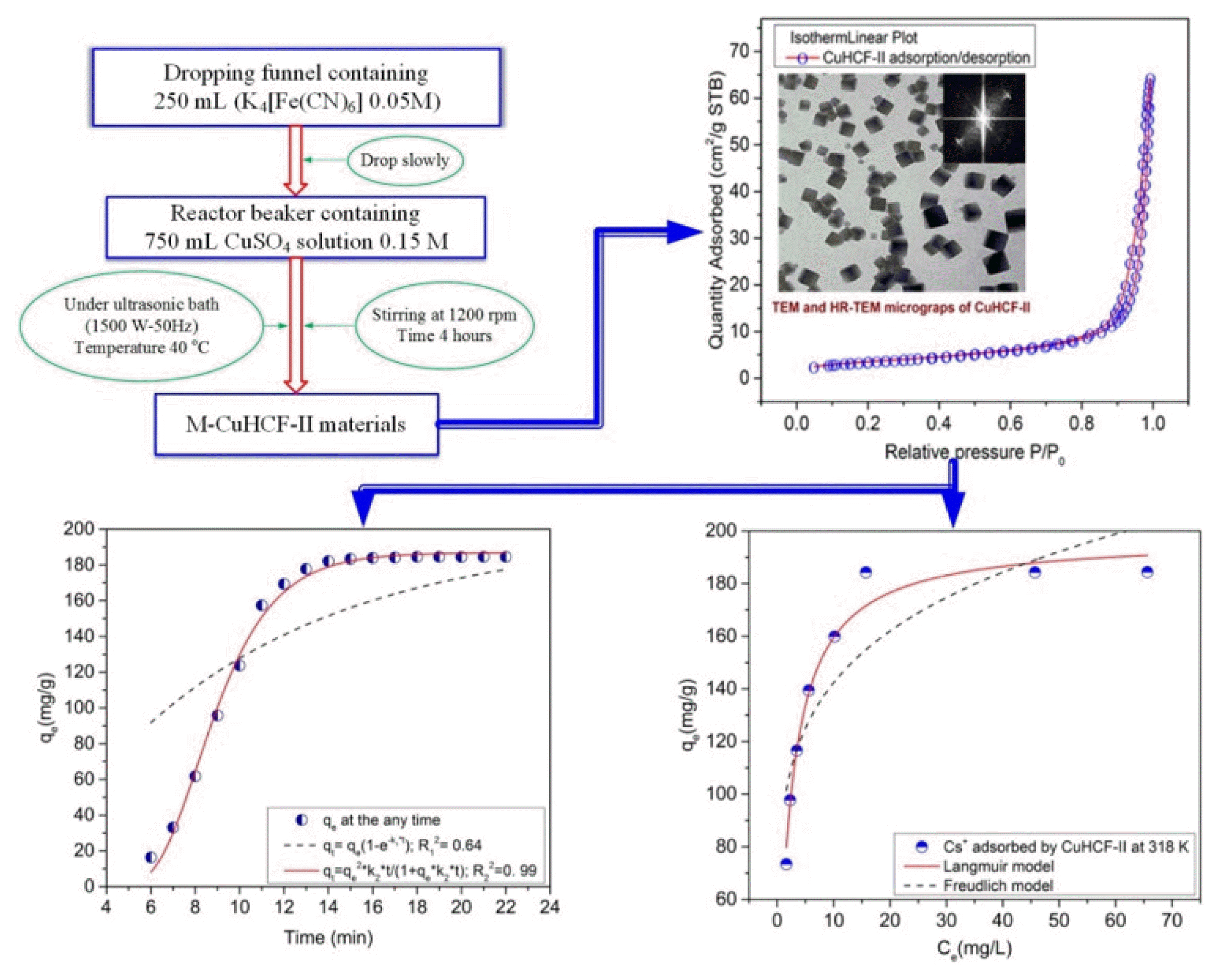
1. IntroductionIn recent years, with the rapid development of the global economy, energy demand is also increasing. The problem of supplying energy sources for the world's manufacturing industry is posing a great challenge to the energy industry. In addition to other energy sources, the energy nuclear industry has played an important role in responding to the increasing energy requirements of rapid economic development. However, radioactive elements that exist in nature or are generated from the operation of nuclear power plants, random incidents, or nuclear disasters have caused great harm to human health and adverse effects on the environment [1, 2]. The radioactive wastewater from the Dalat nuclear reactor in Vietnam, as well as some nuclear reactors in some other countries, is generated by the purification process, the cleaning of the storage pool, and water leaks. In the wastewater of nuclear power plants, there are soluble and insoluble compounds, including radioactive substances dissolved in solution. The radioactive substances in the solution of most interest are isotopes of Cs+ ions, they have long half-lives such as 137Cs: T1/2 30.17 years, 135Cs: T1/2 2.3 106 years, 134Cs: T1/2 2.06 years have the potential to cause great harm to humans and the environment. The 137Cs enter the body through the food chain, they can spread to soft tissues in the body. This is the major cause of blood cell damage and depressed blood factors, leading to increased incidences of benign and malignant neoplasms in various tissues and organs. In addition, it can cause thyroid cancer [2, 3]. For terrestrial ecosystems, including invertebrates such as insects: butterflies [4], grasshoppers [5], aquatic insects [6], earthworms [7], and spiders [8] they are located in the food chain, the accumulation of radioactive substances in them also increases the accumulation of radioactive substances in insectivorous animals such as birds. In addition, radioactive substances also contaminate the soil [9], trees [10], and animals [11]. Due to serious harm to health and the ecosystem, discharge management must strictly comply with radiation safety. Radioactive wastewater contains enormous amounts of soluble and stable radioactive compounds and other substances. Therefore, it is difficult to recover low radionuclide concentrations of liquid metals and solutions with a high salt content [12, 13]. The main processes for radioactive recovery include the decontamination and reduction of radioactive wastewater, followed by the conditioning of concentrated wastewater for storage and disposal. In radioactive wastewater solutions, there are many dissolved anions and cations, so the separation of Cs+ ions in this solution is difficult and challenging work [14, 15]. Many techniques and methods to remove radioactive Cs+ ions from nuclear wastewater have been developed by experts and scientists in recent decades, including co-precipitation, solid-phase extraction, solvent extraction involving crown ethers methods, and the ion exchange and/or adsorption processes [16–18].
The radioactive wastewater treatment methods listed below have the following advantages and disadvantages. The precipitation method is suitable for large volumes and high salt content waste, easy, and non-expensive operations, however, this method has a limitation as the decontamination factor and efficiency depend on the solid-liquid separation step [19, 20]. The evaporation method is performed with well-established technology, and a high-volume reduction factor, but this method has limitations such as process limitations (scaling, foaming, corrosion, volatility of certain radionuclides) and high operation and capital costs. The reverse osmosis method is a modern method that has the advantages of removing dissolved salts, is economical, and is established easily for large-scale operation. However, when operating, it also faces some difficulties such as a high-pressure system, limited by osmotic pressure, and non-back washable, subject to fouling. Therefore, the ion exchange/adsorption method for removing Cs+ ions from radioactive wastewater is one of the most widely used methods [20]. In the ion exchange/adsorption methods, the selection of adsorbent material is very important.
In recent years, research into transitional metal-hexacyanoferrates (TM-HCF) has attracted attention. These complex substances are capable of cleaning or adsorbing Cs+ ions in nuclear wastewater [19–21]. TM-HCF has special structures and electron arrangements, which allow them to adsorb Cs+ ions efficiently and selectively. The precipitation processes on the surface of TM-HCF or chemical interactions between the transitional metal and hexacyanoferrates group with larger hydrated radii can enhance the adsorption performance to remove Cs+ ions from the wastewater solution [19, 22]. TM-HCF as analogs or nanoparticles has promising applications, such as the degradation of toxic PAHs [23], catalysis [24], and Cs+ adsorption [25, 26]. The porous framework of CuHCF-II has a small free volume and is very stable [27]. However, there are few studies on the application of these materials to separate Cs+ ions from radioactive solutions. Nanoparticles have different physical and chemical properties than traditional materials. Nanomaterials are reported in many notable chemical applications, including analytical chemistry, environmental chemistry, materials chemistry, chemical technology, nuclear fuel cells, and solar cell materials. Most atoms on the surface of nanoparticles are not saturated and may easily bind to other atoms. The specific surface and no internal diffusion resistance endow nanoscale adsorbents with superior efficacy for Cs+ ions removal [28–30]. Many experts have focused on studying the application of TM-HCF for Cs+ ions removal with the highest possible efficiency [19, 31–34]. However, the material structure (surface area, porosity), adsorption kinetics, adsorption thermodynamics, and adsorption mechanism, are still issues that need further research. Depending on the intended use and synthesis method, the TM-HCF adsorbent is obtained at the macro or nanoscale, it has a monoclinic, hexagonal, or cubic structure [27, 28].
Based on the above considerations, in this study, we investigated and reported a cube adsorbent (F-43m) mesopores of copper hexacyanoferrate (II) (M-CuHCF-II) nanoscale prepared by a simple and relatively inexpensive chemical coprecipitation method. The behavior of M-CuHCF-II adsorbent for Cs+ adsorption was investigated. The effects of adsorption time and pH on the adsorption of Cs+ ions in the presence of competing ions, thermodynamic studies, and adsorption isotherms were also investigated and evaluated. Remarkably, the principal adsorption mechanism of M-CuHCF-II for Cs+ ions removal was investigated using total reflection X-ray fluorescence (TXRF) analytical methods.
2. Materials and Methods2.1. MaterialsAll chemicals in this experiment were used in pure chemical form (MerK Germany): Stocsolution cesium chloride, CAS no. 7647-17-8; K4[Fe(CN)6].3H2O, CAS no. 14459-95-1. CuSO4.5H2O, CAS no. 7758-98-7. NH4Cl, CAS no. 12125-02-9; KCl, CAS no. 7447-40-7; NaCl CAS no.7647-14-5; CaCl2, CAS no.10043-52-4. Double distilled water was used throughout the experiment to synthesize materials. NaOH (0.01–0.1 N) and HNO3 (0.01–0.1 N) were used to correct for changes in the pH value of the solutions.
2.2. MethodsTo study the Cs+ adsorption processes by M-CuHCF-II. The first step is to synthesize adsorbent materials and analyze their properties. The second is to investigate the factors affecting the adsorption processes.
2.2.1. Preparation of M-CuHCF-II nanoparticleThe process of synthesizing adsorbent materials was carried out according to the process diagram as shown in Fig. 1[34, 35].
The M-CuHCF-II was prepared according to the following procedure. The reactor beaker containing 750 mL CuSO4 solution 0.15M, was placed in an ultrasonic bath (Elma S300H 1500W–50Hz). The reactor beaker was combined with a dropping funnel and a stirring machine. 250 mL K4[Fe(CN)6] solution 0.05 M from the dropping funnel, it was slowly dropped into the reaction beaker, under sonication at 50Hz, 1500W, and stirred at 1200 rpm. The temperature was maintained at 40°C, the reaction time was 4 hours. After the end of the reaction, the products were separated by centrifuging (Universal 320-Germany) at 10000 rpm, washed five times with double-distilled water, and then dried at 60°C. After that, the dried materials were finely ground to conduct further experiments.
2.2.2. Analytical characterization of M-CuHCF-II and the concentration of Cs+ ions in solutionTo analyze the properties and morphology of M-CuHCF-II materials, we performed the following: FTIR spectra were analyzed using Thermo Scientific, Nicolet iS10 (USA), and FTIR spectra of the samples were recorded in the range of 400–4000 cm−1 by using KBr pellets. XRD patterns were analyzed by using Shimadzu XD-3A device (Japan), Scintag XDS-2000 Bruker D8 software, under condition advanced diffractometer using CuKα radiation (λ = 1.5405 Å) in the focused beam within the range of 10° to 80°. TEM and HR-TEM images were taken with JEM 2100, HSX: JEOL (Japan). EDS-mapping images were taken with JEOL JSM-6510LV (Japan) with field emission scanning electron microscopy. The surface area and pore volume of M-CuHCF-II were measured by using a Micromeritics-TriStar II 3020 3.02 (USA) under gas (Nitrogen) adsorption/desorption isotherms at 77K conditions.
The concentration of Cs+ ions in the solutions before and after adsorption was analyzed by AA-7000, Shimadzu (Japan). The analysis conditions were in the flame (Type Air-C2H2) atomic absorption method, the addition of potassium nitrate (0.1% potassium) as an ionization inhibitor improved the sensitivity (Shimadzu No. A446), and the calibration curve method (LOD = 0.017mg L−1) was used to analyze the Cs+ content. In addition, to study the adsorption mechanism, the chemical composition of the solutions before and after adsorption was analyzed by TXRF S2 Picofox Bruker (Germany), and gallium was used as the internal standard.
2.3. Adsorption of Cs+ by M-CuHCF-IITo study the adsorption process of Cs+ by CuHCF-II. The 250 mL Erlenmeyer flask contained 0.1g M-CuHCF-II and 100 mL of Cs+ solution, Cs+ ions in the solution have a concentration in the range of 75–250 mg L−1. The reaction vessel was shaken at 270 rpm for 24 h. The adsorption process was carried out in the temperature range from 298 to 348 Kelvin (K). HNO3 0.01N or NaOH 0.01N was used to adjust pH. At the end of the adsorption process, the samples were centrifuged at 10000 rpm for 5 min. The supernatant solution (after being filtered through a 0.22 μm membrane) was used for Cs+ ions analysis. This procedure was adopted for all adsorption experiments, including evaluations of the isotherms, pH effect, effects of competing ions, and mechanism. The Cs+ adsorption capacity of the adsorbent can be calculated according to Eq. (1) [36]:
In there, V is the volume of solution (L) used; Ci is the Cs+ concentrations (mg L−1) before the Cs+ adsorption process; Ce is the Cs+ concentrations (mg L−1) after the Cs+ adsorption process; qe is the Cs+ adsorption capacity by M-CuHCF-II (in mg g−1 of M-CuHCF-II); M is the mass (g) of M-CuHCF-II used for Cs+ adsorption process.
2.3.1. Effect of solution pHTo evaluate the effects of pH in the solution on the Cs+ adsorption process of M-CuHCF-II materials. We conducted the experiments by varying the pH value from 1 to 9 while keeping the original Cs+ concentration of 140 mg L−1. All experiments were carried out according to section 2.2 above, with a reaction time of 24 hours and a temperature of 318 K. After the Cs+ adsorption reaction was complete, the solution pH value was measured again.
2.3.2. Effect of the initial Cs+ concentrationAll experiments were performed under the procedure in section 2.3, the initial Cs+ concentration was selected in the concentration range of 75–250 mg L−1. The pH of the solutions was the optimum pH based on section 2.3.1 above.
2.3.3. Adsorption isotherm studyBased on the study results in section 2.3.2, the absorbance values of qe corresponding to each initial concentration of Cs+ solutions, are the basis for the study of the following isothermal adsorption processes at 318 K. The Langmuir adsorption and Freundlich adsorption are calculated based on Eqs. (2) and (3) [36]:
In there, qe is the amount of Cs+ ions adsorbed by CuHCF-II (in mg g−1); qm is the maximum of Cs+ adsorption capacity (in mg g−1); Ce is the Cs+ concentration at the point of Cs+ adsorption (in mg L−1), KL is the Langmuir constant (L mg−1), and
and n is the Freundlich constant.
The Redlich–Peterson isotherm is an empirical isotherm incorporating three parameters. Sips proposed a formula similar to the Freundlich formula, but this formula has a finite limit with the concentration being sufficiently high. The Redlich–Peterson and the Sips isotherm can be calculated based on Eq. (4) [36]:
In there, A is the Redlich–Peterson isotherm constant (L g−1), B is the constant with units of (L mg−1)n, and n is an exponent (n = 0 ÷ 1).
In there, qmS is the Sips maximum sorption capacity (mg g−1), KS is the Sips equilibrium constant (L mg−1)m, and m is the exponent of the Sips model.
2.3.4. Thermodynamic studiesThe adsorption of Cs+ ions by M-CuHCF-II has been performed as section 2.3, temperature range 298–348 K. Based on the Langmuir constant (KL), thermodynamic parameters as ΔG0, ΔH0, and ΔS0 can be computed by using the Van’t Hoff equation.
2.3.5. Effects of competing ions on the Cs+ adsorption processThe interference of K+, NH4+, Na+, and Ca2+ ions on the Cs+ adsorption was studied, the initial Cs+ ions concentration was 200 mg L−1. The M-CuHCF-II was used in 1 g L−1, and the pH = 6, at 318 K. The NH4+, Ca2+, K+, and Na+ competing cations were used in the experiments ranging from 1 to 120 mg L−1.
3. Results and Discussion3.1. Characterisation of M-CuHCF-II Adsorbent3.1.1. EDS-mapping of M-CuHCF-IIThe EDS-mapping image of M-CuHCF-II is shown in Fig. 2. From the results of the EDS-mapping analysis, the main ingredient of M-CuHCF-II was the Cu, Fe, K, C, N, and O elements. It was also consistent with FTIR analysis results next section, that in the composition of the complex, there contain water molecules, and thus, the materials that have just been synthesized were M-CuHCF-II.xH2O [21]. Depending on the method and conditions of synthesizing, we can see whether the structure of copper hexacyanoferrate compounds contains water molecules or not [21, 37, 38]. K1.97CuII1.0Fe(CN)6 [33] or K2/3Cu[Fe(CN)6]2/3.zH2O [36]. The M-CuHCF-II was synthesized by the following chemical reactions (6) and (7):
The compositions of M-CuHCF-II materials were analyzed by EDS and shown in Fig. 2. When high-energy electron beams were projected onto the M-CuHCF-II materials, the beams penetrated deeply into the atoms of M-CuHCF-II, and it interacted with the inner electron layers of the atoms, they were components of M-CuHCF-II materials. This interaction leads to the generation of X-rays with a characteristic wavelength proportional to the atomic number (Z) of the atom according to Mosley's law. The results showed that there were emitted two elements copper and iron in the L series. Meanwhile, elements such as carbon, oxygen, nitrogen, and potassium were emitted at the K series.
From the results of Fig. 2, we can see that Cu L, Fe L, K K, C K, N K, and O K series appeared in the EDS-mapping, corresponding to Figs. 2 (a), (b), (c), (d), (e), and (f). They could overlap with each other, but the emission image (see Fig. 2 (c)) of the element potassium was different from the ones mentioned above, they have interspersed points. The K2Cu[Fe(CN)6] obtained from reaction (7) was dissolved in solution [38]. K2Cu[Fe(CN)6] was removed after the as-prepared M-CuHCF-II was washed with distilled water. Therefore, the K+ ions in the adsorbent can be adsorbed by M-CuHCF-II. The above results also correspond to the publications of Jiang P, et al. 2017 [39] and Moloney MP, et al. 2020 [40].
3.1.2 FTIR analysisThe frequency values and the FTIR spectra of M-CuHCF-II were described in Table S1 and Fig. 3(d), respectively. Results of Fig. 3(d) and Table S1 showed the ν(OH), [Fe(CN)6]4−: ν(CN), δ(Fe-CN), and ν(Fe-C) vibrations coexist in an octahedral unit [41]. At the same time, the existence of one type of crystal binding to water molecules demonstrated the successful preparation of M-CuHCF-II. xH2O. The FTIR spectra obtained in this study are also similar to the research results by the Avila M group [27].
3.1.3. TEM and HR-TEM micrographs of M-CuHCF-IIThe morphology of M-CuHCF-II materials is shown in Fig. 3 (a). From this result, the structure of "cubic particles" was very clear, and the sizes were relatively similar. Depending on the intended use, the material synthesis conditions, the composition of the reactants, and the surfactants added to the reaction solution, the obtained products had different morphology [22, 27, 40]. In our material synthesis method, no surfactant was required, and no other additives were used in the reaction solution. We only affected the reaction with stirring and ultrasonic waves, that's why the resulting product had a cubic shape, and the material was nano-sized. HR-TEM micrograph of the unit cell showed that our synthesized product was a face-centered cubic.
3.1.4. XRD of M-CuHCF-IIBy using FullProf Suite software with standard 1010359.cif, XRD patterns were analyzed and shown in Fig. 3(c) [42]. When comparing the XRD patterns (red line) of M-CuHCF-II with the peaks of the standard (blue line), the main peaks of M-CuHCF-II coincided with the peaks of the standard. The XRD analysis results showed that the main cubic structure comprised eight sub-cubic structures with the Miller indices d[h, k, l] as follows: = 5.01; and . [43]. The M-CuHCF-II had a cubic structure (space group F-43m) with a = b = c and α = β = γ = 90°. The crystalline size (D) of
M-CuHCF-II nanoparticles used in the adsorption experiments can be calculated by the Debye–Scherrer equation [44]:
3.1.5. Surface area and pore area of M-CuHCF-IITo examine the surface area and the pore characteristics of M-CuHCF-II, N2 adsorption/desorption measurements were performed under the following conditions: Analysis, adaptive by N2 (Nitrogen); Analysis bath temperature at 77.350 K; Sample mass of 0.3587 g; Warm free space about of 9.4024 cm3; Cold free space about of 28.2383 cm3, calibration interval of 10 s; Sample density of 1.000 g/cm3. The obtained gas N2 adsorption/desorption curves were shown in Fig. 4 (a). The isothermal plots of N2 adsorption/desorption for M-CuHCF-II showed type IV isotherms [47, 48]. Regarding the relative pressure (P/Po) range from 0.05 to 0.8 (see Fig. 4(a)). The adsorption branch contained a low slope region associated with multilayer adsorption on pore walls and material surfaces. When the related pressure (P/Po) increased from 0.8 to 0.99, the adsorption branch contains a high slope region, that region is the absorption of nitrogen gas by the mesopores of the materials [49].
The pore size distributions of M-CuHCF-II were calculated from adsorption and desorption data using the Barrett–Joyner–Halenda (BJH) model. Figs. 4 (b) and (c) showed that most of the peaks were in the range of 2–50 nm and the pore size of M-CuHCF-II was mostly mesopores (pore width 2–50 nm). The results of the physical properties analysis of M-CuHCF-II materials by the BET method were described in detail in Table S2.
3.2. Effects of pH on Adsorption
Fig. 4 (d) showed the Cs+ adsorption onto M-CuHCF-II as a function of pH when an initial concentration of 140 mg L−1 at 318K. The Cs+ absorption process was affected and depended on the pH. From the results of Fig. 4(d), it is shown that the maximum adsorption capacity of M-CuHCF-II occurred at a pH of 6 [50]. To explain this phenomenon, Fig. 5(a) showed the point of zero charges (PZC) of the adsorbent.
The PZC defined the conditions of the solution for which the M-CuHCF-II surface density of positive charges equals that of negative charges, which means, ΔpH = pHf – pHi = 0. In there, pHi was the initial pH value, (pHi from 1.0 to 9.0); pHf was the pH value after Cs+ ions were absorbed by M-CuHCF-II. When ΔpH > 0, the surface of the adsorbent had a positively charged due to the surface of M-CuHCF-II becoming protonated. At pHi = 1, ΔpH > 0, the adsorption process by M-CuHCF-II of Cs+ ions did not happen. When ΔpH < 0, the surface of the adsorbent had a negatively charged due to the surface of M-CuHCF-II becoming deprotonated. At pHi = 6, ΔpH had the most negative value of −0.28. In solution, the Cs+ ions existed as positive ions, therefore, if the surface of the adsorbent has a negative charge, the Cs+ adsorption process took place more favorably. Interesting in this study was that we determined a pH value of 6 was the best Cs+ absorption condition.
3.3. Effect of Cs+ Concentration on Adsorption at Different Temperatures from 298 to 348KThe adsorption capacities of Cs+ ions by M-CuHCF-II at different temperatures from 298 to 348K were described in detail in Table S3. From the analysis results presented in Table S3, we can see that when the initial concentration of Cs+ ions was increased, the adsorption capacity of Cs+ ions by M-CuHCF-II was also increased significantly. With an initial concentration of Cs+ ions of 230 mg L−1, the adsorption capacity of M-CuHCF-II at 298K reached 184 mg g−1. When increasing the temperatures from 298 to 318 K, the adsorption capacity of M-CuHCF-II was insignificantly changed. Continuing to increase the temperatures from 333 to 348K, the adsorption capacity of M-CuHCF-II was slightly decreased. Figs. 5 (b) and (c) showed the Cs+ adsorption isotherms were explored using the Langmuir, Freundlich, Redlich–Peterson, and Sips isotherm models at 318K. And the Langmuir, Freundlich, Redlich–Peterso, and Sips model parameters of the isotherm were listed in Table 1.
The determination coefficients (R2) of the Langmuir, Freundlich, Redlich–Peterson, and Sips isotherms were 0.97, 0.87, 0.98, and 0.98, respectively. The Langmuir, Redlich–Peterson, and Sips models could describe the adsorption process of Cs+ ions by M-CuHCF-II, demonstrated by the high determination coefficient. The data were better described by the Langmuir, Redlich–Peterson, and Sips models. The maximum adsorption capacities of various adsorbents for Cs+ ions were compared in Table S4. Based on this comparison, the M-CuHCF-II used in this study had a high uptake capacity for Cs+ ions.
3.4. Thermodynamic StudiesFrom the experimental results in Table S3, the parameters of Langmuir and Freundlich isotherm adsorption processes according to the temperature ranges from 298 to 348K were calculated and described in detail in Table S5. The Gibbs free energy change (ΔG0) during adsorption is an important factor to be investigated. The adsorption process at a given temperature value, this process acts automatically when the ΔG0 value is negative. The ΔG0 value of the adsorption process can be determined according to the following formula:
In there, R = 8.314 (J Mol–1 K–1) is the gas constant; T is the temperature (K); KC = 132. 905*1000*55.5*KL is the equilibrium constant [51, 52], and KL is the Langmuir constant (KL) (see Table S5). From the Van’t Hoff equation, the ΔG0, ΔH0, and ΔS0 thermodynamic parameters were calculated by using Eq. (12) [53]:
In there, ΔH0 is the enthalpy change and ΔS0 is the entropy change. KC value can also be determined based on the Clausius–Clapeyron equation Eq. (13):
The values of ΔH0, ΔS0, and ΔG0 were calculated and described in detail in Table S6 and Fig. 5 (d). When the temperature changed from 298 to 348 K, ΔG0 had a negative value, varying from −37.47 to −42.27, respectively. Therefore, the adsorption of Cs+ ions by M-CuHCF-II was considered to be a spontaneous process [54]. From the results of Table S6, ΔS0 = 96.21 (J Mol−1) was a positive value, this result is evidence of the random increase of Cs+ ions on the surface of M-CuHCF-II. ΔH0 = −8.28 (KJ Mol−1) was a negative value, therefore, the adsorption process was exothermic [55, 56]. The Cs+ adsorption process by M-CuHCF-II was exothermic, so when the heat was supplied more than necessary, the opposite results.
3.5. Effect of Adsorption TimeThe adsorption kinetics is an important factor in determining the efficiency of the adsorption process. Therefore, in this study, we conducted kinetic analysis for the absorption of Cs+ ions, and the results of this analysis are described in Fig. 6 (a). The results of Fig. 6(a) showed that the adsorption of Cs+ ions was time-depended, the adsorption of Cs+ ions took place rapidly in the first 10 min, and the adsorption approached equilibrium after 15 min. Meanwhile, Parajuli D et al. [57] reported and suggested the optimal pH was 4–8 and that equilibrium was reached within 2h [57]. These adsorption kinetics were determined by integrating the Eq. (14) [58, 59]:
In there, qt and qe (mg g−1) are the amounts of Cs+ adsorbed at time t (min) and equilibrium, respectively; Kn (min−1) is the pseudo-in-the-order rate constant. Pseudo first-order (PFO) adsorption and Pseudo second-order (PSO) adsorption are commonly used to evaluate and test the adsorption kinetics data.
PFO and PSO adsorptions were used to test the adsorption kinetics data. Lagergren described the formula for PFO, corresponding to n = 1 as Eq. (15) [58, 59]:
Integrate equation (15) with the boundary conditions (t = 0, qt = 0 and t = t, qe = qt) we get the linear Eq. (16):
In there, qe and qt are the amounts of Cs+ uptake per mass of the CuHCF-II at equilibrium and at any time t (min), respectively, and k1(min−1) is the rate constant of the PFO equation, which can be rearranged in a PFO nonlinear form:
Eq. (14) is rearranged in linear form as follows:
Integrate Eq. (14) with the boundary conditions (t = 0, qt = 0 and t = t, qe = qt) we get the PSO non-linear form:
In there, qe (mg g−1) and qt (mg g−1) are the amounts of Cs+ adsorbed at equilibrium and any t (min), respectively, and k2 (g/mg min) is the PSO equation constant rate.
Table S7 described compositions of PFO and PSO adsorption rate constants in non-linear form. The R2 value of the PFO adsorption model was 0.64. While that, values of R2 for the PSO adsorption reached 0.99, the R2 value of PSO was extremely high. For the adsorption of Cs+ ions, the experimental data fit the PSO model better than the PFO model. The PSO model was well-chosen to describe the adsorption kinetics of Cs+ ions by M-CuHCF-II. The adsorption rate of Cs+ ions depends on the concentration gradient and driving force. For the PFO, the adsorption rate of Cs+ ions was proportional to the concentration gradient (ΔC), and for the PSO the adsorption rate of Cs+ ions was proportional to the square of the concentration gradient (ΔC2). So, adsorption equilibrium was reached in about 15 min, this was not at all contradictory with the adsorption isotherms.
3.6. Cs+ Adsorption in the Presence of Competing IonsIn radioactive wastewater, there are many other soluble elements present in the solution, which can competitively adsorb with Cs+ ions present in the solution. In this study, the K+, NH4+, Ca2+, and Na+ ions were selected to test the influence of components coexisting in the solution. The results of this study showed that when occurring at high concentrations NH4+, Ca2+, Na+, and K+ ions had little influence on Cs+ absorption by M-CuHCF-II. Results of Fig. 6 (b) showed that NH4+, Ca2+, Na+, and K+ ions were present in the solution with the concentration increasing from 0 to 120 mg L−1, and the adsorption capacity of Cs+ ions by M-CuHCF-II materials decreased by 1.01, 0.72, 0.01, and 1.06 %, respectively.
To explain the above finding, hydrated radii (MZ+ (H2O)n, Z = 1, 2, 3) of ions were also mentioned by some authors. Hydrated radii of various ions (nm) includes: Cs+ (0.329), NH4+ (0.331), Ca2+ (0.412), Na+ (0.358), and K+ (0.331) had researched and published by Volkov AG et al. 1997 [60]. The close similarity of the ions' hydration radii was also mentioned by Zong Y et al. 2017 [30]. While Ca2+ ions had a larger hydration radius than Cs+ ions they did not compete for adsorption with Cs+ ions by M-CuHCF-II, but they could be adsorbed on the surface of M-CuHCF-II materials. This statement was also similar to the statements of the Zong Y group [30] and our recent studies [34, 35].
Meanwhile, H. Gad's research group studied and reported that Na+ and Ca2+ affect the absorption of Cs+ ions by M-CuHCF-II [58]. The difference and interesting point in our research results compared to the study of the Gad H group [58] are that the NH4+, Ca2+, Na+, and K+ ions only affect Cs+ adsorption when occurring at high concentrations.
3.7. Mechanism of Cs+ Adsorption ProcessMechanisms of Cs+ adsorption by M-CuHCF-II are complex and dependent on both the compositions and physical properties of the adsorbent. There were differences between the two spectra of Figs. 7(a) and (b) when analyzing the solution before and after Cs+ adsorption by M-CuHCF-II. The TXRF analysis of the solution before the Cs+ adsorption process was shown in Fig. 7(a). The TXRF spectrum featured exhibited a strong peak for Cs+ ions corresponding at La1 = 4, 28 KeV. Fig. 7(b) shows the TXRF analysis of the solution after the Cs+ adsorption process. Peaks of Cu2+ and K+ ions corresponding to the TXRF spectrum at ~8.04 KeV and ~3.31 KeV were observed. The peak of Cs+ ions corresponding to the TXRF spectrum at La1 = 4, 28 KeV was absent. This proves that both Cu2+ and K+ ions were released into the solution. The amounts of K+ and Cu2+ ions released into the solution of 0.55 mEq/L and 0.59 mEq/L, respectively. The total concentrations of both K+ and Cu2+ ions in the solution were 1.14 mEq/L. At 318K, with an initial Cs+ concentration of 200 mg L−1, the capacity of Cs+ adsorption achieved 1.39 mEq/g. Total equivalent concentrations of K+ and Cu2+ were smaller than the equivalent concentration of Cs+ adsorbed by M-CuHCF-II about 0.25 mEq/L.
Interesting in this study, that was the non-stoichiometric amount of Cs+ ions was adsorbed when compared with the total amounts of K+ and Cu2+ ions exchanged, meanwhile, previous studies did not mention this aspect. Such as Ayrault S et al. [31] reported the adsorption mechanism was complex; Zong Y et al. [30] only mentioned the ion exchange between Cs+ and K+ ions; Ishizaki M et al. [32] reported a Cs+ adsorption mechanism in which Cs+ was adsorbed by lattice defect sites of the metal hexacyanoferrates filled with coordinated and crystallized water molecules [32]. The adsorption of Cs+ ions by M-CuHCF-II follows two mechanisms: (i) The main mechanism was the ion exchange process between Cs+ ions with two K+ and Cu2+ ions; (ii) The Cs+ ions were adsorbed onto the surface or into the pores of the crystal lattice M-CuHCF-II.
4. ConclusionsThe M-CuHCF-II adsorbent used for Cs+ ion removal was successfully synthesized by the co-precipitation method. The M-CuHCF-II had a cubic structure with space group F-43m, and nanometers in size. The M-CuHCF-II had a surface area of 12.80 m2/g and average pore width of about 34.49 nm. At pH = 6, the adsorption capacity of Cs+ ions by M-CuHCF-II reached the maximum within 15 minutes. The Langmuir, Redlich–Peterson, and Sips models could describe the adsorption process of Cs+ ions by M-CuHCF-II. The maximum absorption capacity of M-CuHCF-II reached 197.72 (mg g−1) at 318 K. The Cs+ adsorption process by M-CuHCF-II was spontaneous and follows two mechanisms: (i) The ion exchange process between Cs+ with K+ and Cu2+ ions; (ii) Cs+ was adsorbed onto the surface or into the pores of the crystal lattice M-CuHCF-II. The M-CuHCF-II materials are easy to prepare, low cost, have high adsorption capacity for Cs+ ions, and are easy to apply. Therefore, M-CuHCF-II is a promising potential material in the treatment of radioactive wastewater.
AcknowledgmentsThis research is funded by the Vietnam Ministry of Education and Training (MOE) under grant number B2020-DLA 01, and the authors are thankful to the DaLat University and Van Lang University.
References1. Koo YH, Yang YS, Song KW. Radioactivity release from the Fukushima accident and its consequences: A review. Prog. Nucl. Energy. 2014;74:61–70. http://dx.doi.org/10.1016/j.pnucene.2014.02.013
2. Sangvanich T, Sukwarotwat V, Wiacek RJ, et al. Selective capture of cesium and thallium from natural waters and simulated wastes with copper ferrocyanide functioned mesoporous silica. J. Hazard. Mater. 2010;182:225–231. https://doi.org/10.1016/j.jhazmat.2010.06.019
3. Nguyen DT, Le VTA, Truong DP, et al. Copper hexacyanoferrate (II): synthesis, characterization, and cesium, strontium adsorbent application. Dalat Univ. J. Sci. 2021;11:76–97. https://doi.org/10.37569/DalatUniversity.11.4.901(2021)
4. Hiyama A, Nohara C, Kinjo S, et al. The biological impacts of the Fukushima nuclear accident on the pale grass blue butterfly. Sci. Rep. 2012;2:1–11. https://doi.org/10.1038/srep00570
5. Mitsuhashi R, Mizuno H, Saeki S, et al. Radioactive caesium contamination in Inago, and sustainability of Inago cuisine in Fukushima. Shokuhin Eiseigaku Zasshi. 2013;54:410–414. https://doi.org/10.3358/shokueishi.54.410
6. Yoshimura M, Akama A. Radioactive contamination of aquatic insects in a stream impacted by the Fukushima nuclear power plant accident. Hydrobiologia. 2014;722:19–30. https://doi.org/10.1007/s10750-013-1672-9
7. Hasegawa M, Ito MT, Kaneko S, Kiyono Y, Ikeda S, Makino SI. Radioactivity concentrations in epigeic earthworms at various distances from the Fukushima Nuclear Power Plant 6 months after the 2011 accident. J Environ Radioact. 2013;126:8–13. https://doi.org/10.1016/j.jenvrad.2013.06.006
8. Ayabe Y, Kanasashi T, Hijii N, Takenaka C. Radiocesium contamination of the web spider Nephila clavata (Nephilidae: Arachnida) 1.5 years after the Fukushima Dai-Ichi nuclear power plant accident. J Environ Radioact. 2014;127:105–110. https://doi.org/10.1016/j.jenvrad.2013.10.010
9. Ogura SI, Suzuki T, Saito M. Distribution of radioactive cesium in soil and its uptake by herbaceous plants in temperate pastures with different management after the Fukushima Dai-Ichi Nuclear Power Station accident. Soil Sci. Plant Nutr. 2014;60:790–800. https://doi.org/10.1080/00380768.2014.954269
10. Tanaka K, Iwatani H, Sakaguchi A, Takahashi Y, Onda Y. Local distribution of radioactivity in tree leaves contaminated by the fallout of the radionuclides emitted from the Fukushima Daiichi Nuclear Power Plant. J. Radioanal. Nucl. Chem. 2013;295:2007–2014. https://doi.org/10.1007/s10967-012-2192-1
11. Tanoi K, Uchida K, Doi C, et al. Investigation of radiocesium distribution in organs of wild boar grown in Iitate, Fukushima after the Fukushima Daiichi nuclear power plant accident. J. Radioanal. Nucl. Chem. 2016;307:741–746. https://doi.org/10.1007/s10967-015-4233-z
12. Yasunari TJ, Stohl A, Hayano RS, Burkhart JF, Eckhardt S, Yasunari T. Cesium-137 deposition and contamination of Japanese soils due to the Fukushima nuclear accident. Proc. Natl. Acad. Sci. U.S.A. 2011;108:19530–19534. https://doi.10.1073/pnas.1112058108
13. Panagopoulos A. Techno-economic assessment and feasibility study of a zero liquid discharge (ZLD) desalination hybrid system in the Eastern Mediterranean. Chem. Eng. Process. Process Intensif. 2022;178:109029. https://doi.org/10.1016/j.cep.2022.109029
14. Panagopoulos A. Brine management (saline water & wastewater effluents): Sustainable utilization and resource recovery strategy through Minimal and Zero Liquid Discharge (MLD & ZLD) desalination systems. Chem. Eng. Process. Process Intensif. 2022;176:108944. https://doi.org/10.1016/j.cep.2022.108944
15. Panagopoulos A, Giannika V. Comparative techno-economic and environmental analysis of minimal liquid discharge (MLD) and zero liquid discharge (ZLD) desalination systems for seawater brine treatment and valorization. Sustain. Energy Technol. Assess. 2022;53:102477. https://doi.org/10.1016/j.seta.2022.102477
16. Awual MR, Yaita T, Taguchi T, Shiwaku H, Suzuki S, Okamoto Y. Selective cesium removal from radioactive liquid waste by crown ether immobilized new class conjugate adsorbent. J. Hazard. Mater. 2014;278:227–235. https://doi.10.1016/j.jhazmat.2014.06.011
17. Avramenko V, Bratskaya S, Zheleznov V, Sheveleva I, Voitenko O, Sergienko V. Colloid stable sorbents for cesium removal: Preparation and application of latex particles functionalized with transition metals ferrocyanides. J. Hazard. Mater. 2011;186:1343–1350. https://doi.10.1016/j.jhazmat.2010.12.009
18. Nilchi A, Saberi R, Moradi M, Azizpour H, Zarghami R. Adsorption of cesium on copper hexacyanoferrate–PAN composite ion exchanger from aqueous solution. Chem. Eng. J. 2011;172:572–580. https://doi.10.1016/j.cej.2011.06.011
19. Haas PA. A Review of information on ferrocyanide solids for removal of Cesium from solutions. Sep Sci Technol. 1993;28:2479–2506. https://doi.10.1080/01496399308017493
20. International Atomic Energy Agency. Handling and processing of radioactive waste from nuclear applications. Technical Reports Series No. 402. October 2001. IAEA; Vienna, Austria:
21. Kiener J, Limousy L, Jeguirim M, et al. Activated Carbon/Transition metal (Ni, In, Cu) hexacyanoferrate nanocomposites for Cesium adsorption. Materials. 2019;12:1253. https://doi.10.3390/ma12081253
22. Ding D, Zhang Z, Chen R, Cai T. Selective removal of cesium by ammonium molybdophosphate-polyacrylonitrile bead and membrane. J. Hazard. Mater. 2017;324:753–761. https://doi.org/10.1016/j.jhazmat.2016.11.054
23. Shanker U, Jassal V, Rani M. Degradation of toxic PAHs in water and soil using potassium zinc hexacyanoferrate nanocubes. J. Environ. Manage. 2017;204:337–348. https://doi.10.1016/j.jenvman.2017.09.015
24. Ali SR, Bansal VK, Khan AA, Jain SK, Ansari MA. Growth of zinc hexacyanoferrate nanotubes and their potential as heterogeneous catalyst for solvent-free oxidation of benzyl alcohol. J Mol Catal A Chem. 2009;303:60–64. https://doi.10.1016/j.molcata.2008.12.020
25. Yang H, Sun L, Zhai J, Li H, Zhao Y, Yu H. In situ controllable synthesis of magnetic Prussian blue/graphene oxide nanocomposites for removal of radioactive cesium in water. J. Mater. Chem. A. 2014;2:326–332. https://doi.10.1039/c3ta13548a
26. Ke Y, Li Y, Zhu LJ, Zhou YZ, Liu DB. Rapid enrichment of cesium ions in aqueous solution by copper ferrocyanide powder. SN Appl. Sci. 2020;2:522. https://doi.10.1007/s42452-020-2337-8
27. Avila M, Reguera L, Rodríguez-Hernández J, Balmaseda J, Reguera E. Porous framework of T2Fe(CN)6].xH2O with T = Co, Ni, Cu, Zn, and H2 storage. J Solid State Chem. 2008;181:2899–2907. https://doi.org/10.1016/j.jssc.2008.07.030
28. Baig N, Kammakakam I, Falath W. Nanomaterials: A review of synthesis methods, properties, recent progress, and challenges. Mater. Adv. 2021;2:1821–1871. https://doi.org/10.1039/d0ma00807a
29. Kolahalam LA, Viswanath IVK, Diwakar BS, Govindh B, Reddy V, Murthy YLN. Review on nanomaterials: Synthesis and applications. Materials Today: Proceedings. 2019;18:2182–2190. https://doi.org/10.1016/j.matpr.2019.07.371
30. Zong Y, Zhang Y, Lin X, Ye D, Qiao D, Zeng S. Correction: Facile synthesis of potassium copper ferrocyanide composite particles for selective cesium removal from wastewater in the batch and continuous processes. RSC Adv. 2017;7:33974–33974. https://doi.10.1039/c7ra90079a
31. Ayrault S, Jimenez B, Garnier E, Fedoroff M, Jones D, Loos-Neskovic C. Sorption mechanisms of Cesium on CuII2FeII (CN)6 and CuII3FeIII (CN)6]2 Hexacyanoferrates and their relation to the crystalline structure. J Solid State Chem. 1998;141:475–485. https://doi.10.1006/jssc.1998.7997
32. Ishizaki M, Akiba S, Ohtani A, et al. Proton-exchange mechanism of specific Cs+ adsorption via lattice defect sites of Prussian blue filled with coordination and crystallization water molecules. Dalton Trans. 2013;42:16049. https://doi.10.1039/c3dt51637g
33. Loos-Neskovic C, Ayrault S, Badillo V, et al. Structure of Copper-Potassium hexacyanoferrate (II) and sorption mechanisms of Cesium. J Solid State Chem. 2004;177:1817–1828. https://doi.10.1016/j.jssc.2004.01.018
34. Trung ND, Ping N, Lan LTH, Dan HK. Synthesis, characterization, and caesium adsorbent application of trigonal zinc hexacyanoferrate (II) nanoparticles. J. Environ. Chem. Eng. 2021;9:106772. https://doi.org/10.1016/j.jece.2021.106772
35. Trung ND, Ping N, Dan HK. Synthesis, characterization, and the effectiveness of cobalthexacyanoferrate nanoparticles in Cs+ adsorbent application. Nanotechnol. Environ. Eng. 2022;7:893–905. https://doi.org/10.1007/s41204-022-00265-x
36. Ayawei N, Ebelegi AN, Wankasi D. Modelling and interpretation of adsorption isotherms. J Chem. 2017;2017:1–11. https://doi.org/10.1155/2017/3039817
37. Wang L, Feng M, Liu C, et al. Supporting of Potassium Copper hexacyanoferrate on porous activated carbon substrate for cesium separation. Sep Sci Technol. 2009;44:4023–4035. https://doi.10.1080/01496390903183253
38. Vincent T, Vincent C, Guibal E. Immobilization of Metal hexacyanoferrate ion-exchangers for the synthesis of metal ion sorbents-A mini-review. Molecules. 2015;20:20582–20613. https://doi.10.3390/molecules201119718
39. Jiang P, Shao H, Chen L, et al. Ion-selective copper hexacyanoferrate with an open-framework structure enables high-voltage aqueous mixed-ion batteries. Mater. Chem. A. 2017;5:16740–16747. https://doi.10.1039/c7ta04172a
40. Moloney MP, Massoni N, Grandjean A. Tuning the thermal stability of copper(II) hexacyanoferrate (II) nanoparticles. J. Therm. Anal. Calorim. 2020;145:2353–2362. https://doi:10.1007/s10973-020-09823-4
41. Nakamoto K. Infrared and Raman spectra of Inorganic and coordination compounds. Handbook of Vibrational Spectroscopy. New York: John Wiley & Sons; 2006. 1872–1892. https://doi.org/10.1002/0470027320.s4104
42. Murray-Rust P. Open-access collection of crystal structures of organic, inorganic, metal-organics compounds and minerals, excluding biopolymers. cited 10 september 2022]. Available from: http://www.crystallography.net/cod/
43. Sun SD, Zhang XC, Cui J, Liang SH. Identification of the Miller indices of crystallographic plane: A tutorial and comprehensive review on fundamental theory, universal methods based on different case studies and matters needing attention. Nanoscale. 2020;12:16657–16677. https://doi.org/10.1039/D0NR03637D
44. Dan HK, Zhou DC, Wang RF, et al. Energy transfer and upconversion emission of Er3+/Tb3+/Yb3+ co-doped transparent glass-ceramics containing Ba2LaF7 nanocrystals under heat treatment. Opt. Mater. 2014;36:639–644. http://dx.doi.org/10.1016/j.optmat.2013.10.045
45. Dan HK, Zhou DC, Wang RF, et al. Effect of Mn2+ ions on the enhancement upconversion emission and energy transfer of Mn2+/Tb3+/Yb3+ tri-doped transparent glass-ceramics. Mater. Lett. 2015;150:76–80. http://dx.doi.org/10.1016/j.matlet.2015.03.005
46. Bragg WH, Bragg WL. The reflexion of X-rays by crystals. Proc. Math. Phys. Eng. Sci. 1913;88:428–438. https://doi.org/10.1098/rspa.1913.0040
47. Peng Y, Shen X, Wang L, et al. Preparation of porous TiO2 photocatalyts with different crystal phases and high catalytic activity by simple calculation of titanate nanofibers. RSC Adv. 2017;7:45742–45745. https://doi.org/10.1039/c7ra08381e
48. Kadam AN, Salunkhe TT, Kim H, Lee SW. Biogenic synthesis of mesoporous N–S–C tri-doped TiO2 photocatalyst via ultrasonic-assisted derivatization of biotemplate from expired egg white protein. Appl. Surf. Sci. 2020;518:146194. https://doi.org/10.1016/j.apsusc.2020.146194
49. Sotomayor FJ, Cychosz KA, Thommes M. Characterization of micro/mesoporous Materials by physisorption: Concepts and case studies. Acc. Mater. Surf. Res. 2018;3:34–50. https://www.hyomen.org
50. Clarke TD, Wai CM. Selective Removal of Cesium from Acid solutions with immobilized copper ferrocyanide. Anal. Chem. 1998;70:3708–3711. https://doi.10.1021/ac971138b
51. Takahashi A, Kitajima A, Parajuli D, et al. Radioactive cesium removal from ash-washing solution with high pH and high K+-concentration using potassium zinc hexacyanoferrate. Chem Eng Res Des. 2016;109:513–518. https://doi.10.1016/j.cherd.2016.02.027
52. Zhou XY, Zhou X. The unit problem in the thermodynamic calculation of adsorption using the Langmuir equation. Chem Eng Commun. 2014;2011:459–1467. https://doi.org/10.1080/00986445.2013.818541
53. Mondal NK, Samanta A, Dutta S, Chattoraj S. Optimization of Cr(VI) biosorption onto Aspergillus niger using 3-level Box-Behnken design: equilibrium, kinetic, thermodynamic and regeneration studies. J Genet Eng Biotechnol. 2017;15:151–160. https://doi.org/10.1016/j.jgeb.2017.01.006
54. Abd El-Rahman KM, El-Kamash AM, El-Sourougy MR, Abdel-Moniem NM. Thermodynamic modeling for the removal of Cs+, Sr2+, Ca2+ and Mg2+ ions from aqueous waste solutions using zeolite A</p> </p>. J. Radioanal. Nucl. Chem. 2006;268:221–230. https://doi.org/10.1007/s10967-006-0157-y
55. Emeniru DC, Neminebor J, Ikirigo J, Sogbara F. Perspective view on sorption thermodynamics: basic dye uptake on Southern Nigerian Clay. European Scientific J. 2017;13:355–372. http://dx.doi.org/10.19044/esj.2017.v13n18p355
56. Hwang KS, Park CW, Lee KW, Park SJ, Yang HM. Highly efficient removal of radioactive cesium by sodium-copper hexacyanoferrate-modified magnetic nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2017;516:375–382. https://doi.10.1016/j.colsurfa.2016.12.052
57. Parajuli D, Takahashi A, Noguchi H, et al. Comparative study of the factors associated with the application of metal hexacyanoferrates for environmental Cs decontamination. Chem. Eng. J. 2016;283:1322–1328. https://doi.10.1016/j.cej.2015.08.076
58. Gad H, Elsanafeny H, Ali M, Lasheen Y, Abdelwahed M. Factors affecting the sorption behavior of Cs+ and Sr2+ using biosorbent material. Russ. J. Appl. Chem. 2016;89:988–999. https://doi.org/10.1134/S1070427216060240
59. Ke Y, Li Y, Zhu L, Zhou Y, Liu D. Rapid enrichment of cesium ions in aqueous solution by copper ferrocyanide powder. SN Appl. Sci. 2020;2:1–12. https://doi.10.1007/s42452-020-2337-8
60. Volkov AG, Paula S, Deamer DW. Two mechanisms of permeation of small neutral molecules and hydrated ions across phospholipid bilayers. Bioelectrochemistry Bioenergetics. 1997;42:153–160. https://doi.org/10.1016/S0302-4598(96)05097-0
Fig. 3(a) TEM image of M-CuHCF-II; (b) HR-TEM image of a unit cell of M-CuHCF-II; (c) Comparing the XRD patterns (red line) of M-CuHCF-II with the peaks of the standard (blue line); (d) The FTIR spectra of M-CuHCF-II. 
Fig. 4(a) Isothermal plots of N2 adsorption/desorption for M-CuHCF-II; (b) BJH adsorption dV/dlog(W) pore area; (c) BJH desorption dV/dlog(W) pore area; (d) Cs+ adsorption onto CuHCF-II (1 g L−1) at various initial pH conditions. 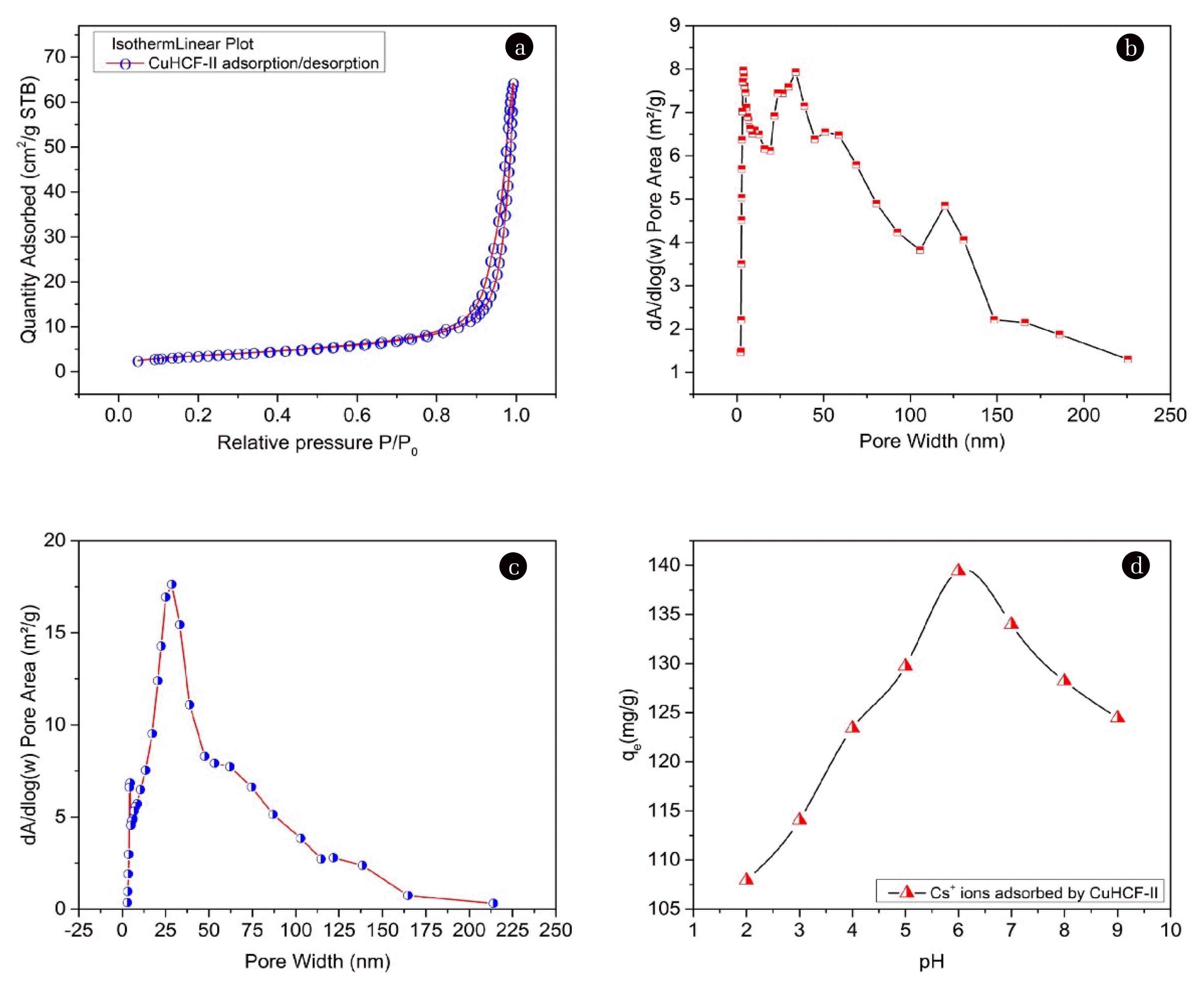
Fig. 5(a) Plot of the variation in ΔpH = pHf – pHi with pHi at a Cs+ concentration of 140mg L−1; (b) Langmuir and Freundlich isotherms; (c) Redlich Peterson and Sips isotherms; (d) The plot of Ln(KC) vs. 1/T. 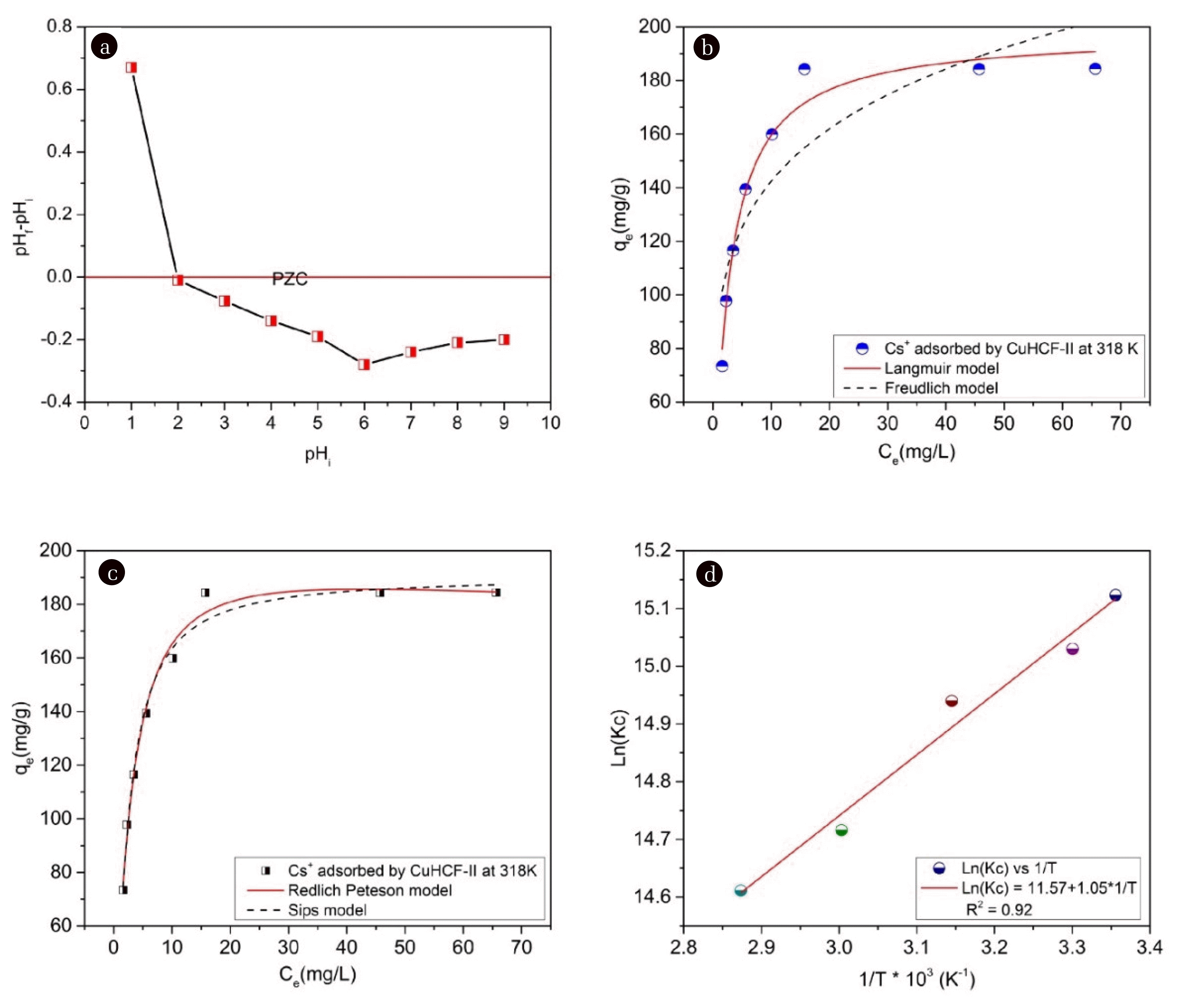
Fig. 6(a) Non-linear form of PFO and PSO; (b) Effect of other constituents on Cs+ adsorption by M-CuHCF-II 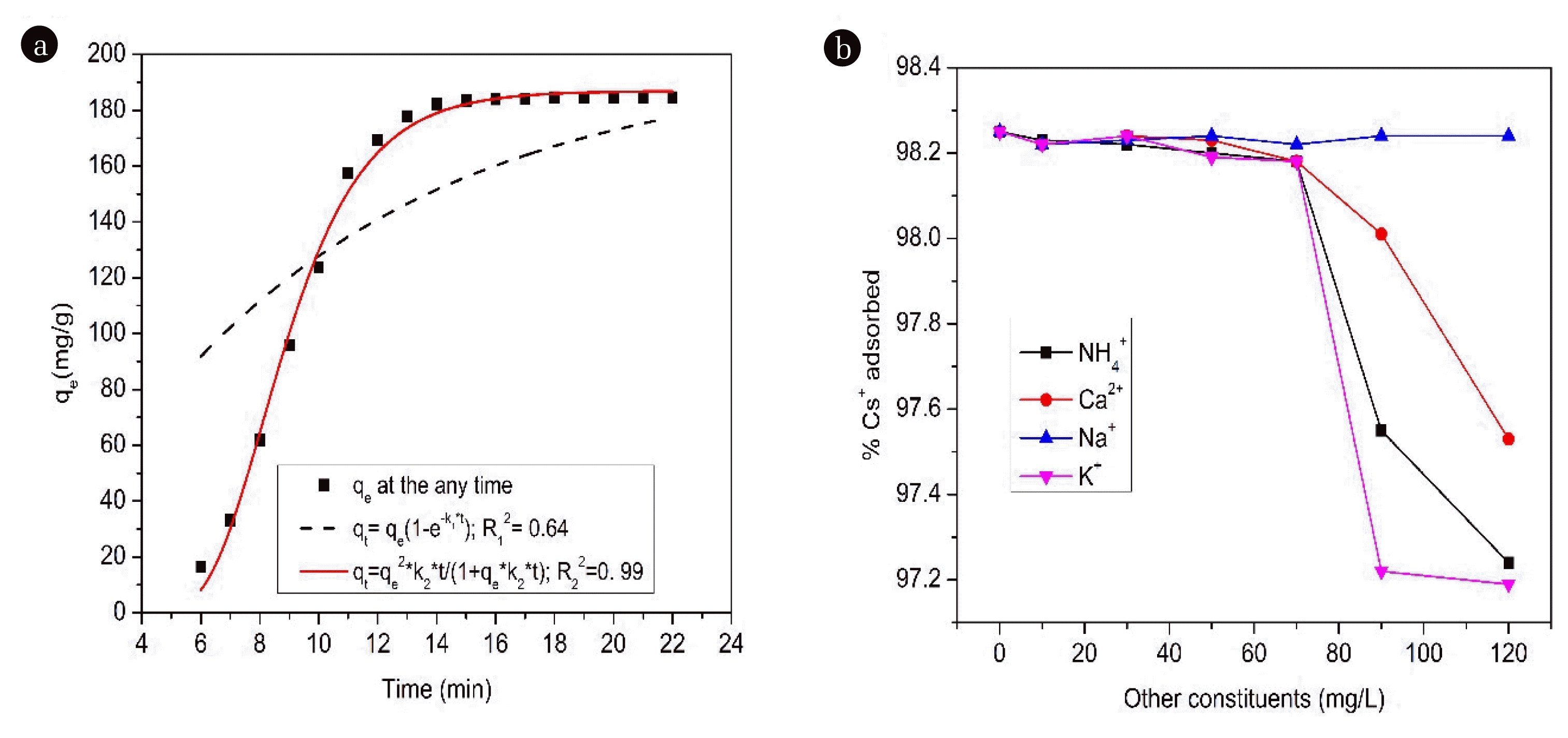
Fig. 7(a) TXRF analysis of the solution before the Cs+ adsorption process; (b) TXRF analysis of the solution after the Cs+ adsorption process. 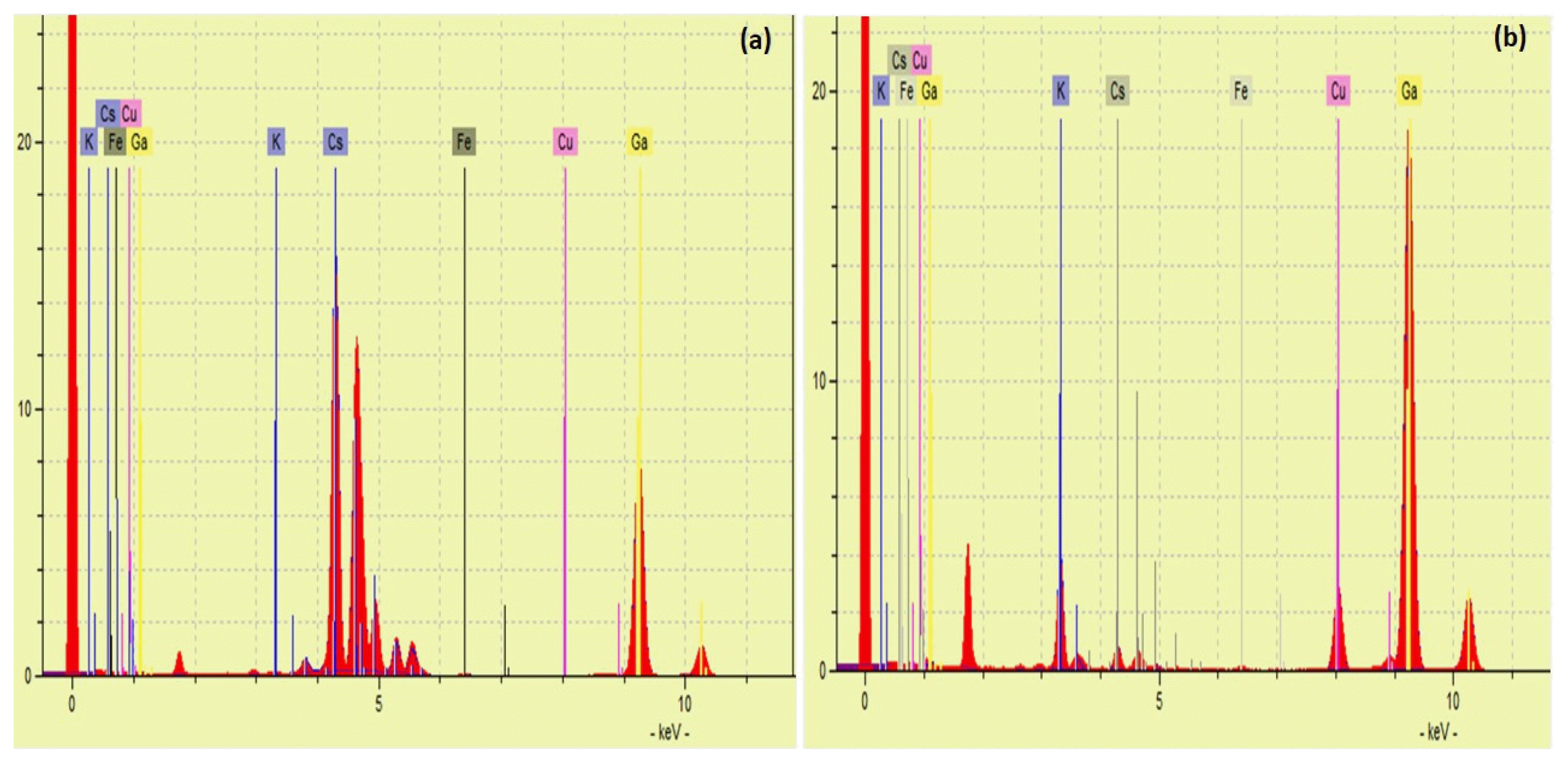
Table 1Langmuir, Freundlich, Redlich Peterson, and Sips Isotherm Parameters for Cs+ Adsorption on M-CuHCF-II at 318K |
|
||||||||||||||||||||||||||||||||||||