AbstractIn this study, La-Ce/γ-Al2O3 materials for the catalytic ozonation of phenol were prepared by impregnation. In order to analyze the physicochemical properties, BET, SEM, XRD, XPS, and FTIR were used to characterize the catalysts. The removal performance of phenol under different reactive conditions (catalyst dosage, initial pH value, initial phenol concentration, and ozone concentration) was investigated, and the possible catalytic oxidation mechanism and conversion pathway of phenol were proposed through the analysis of ROS and intermediates in solution. The results indicated that the conversion rate of 2.5% La-Ce/γ-Al2O3 increased by 20.53% within 20 min compared with ozonation alone. Moreover, the CO2 mineralization rate was more than twice that of ozonation alone within 250 min, reaching 92.53%, and superior CO2 mineralization was maintained after repeated use. The CO2 mineralization rate of phenol after adding 3 g/L 2.5% La-Ce/γ-Al2O3 catalyst reached almost 100%, and could be maintained in a solution with a wide pH range of 3~9.5. From the characterization results, it could be seen that the 2.5% La-Ce/γ-Al2O3 catalyst obtained higher adsorbed oxygen and hydroxyl species content than γ-Al2O3 and Ce/γ-Al2O3. In the process of phenol catalyzed ozonation, the •O2− species in the solution were deduced to be the key ROS.
Graphical Abstract
1. IntroductionImportant intermediates and raw ingredients in many industrial manufacturing processes are phenolic compounds, mainly used in coal carbonization, oil refining, plastics production, dyes synthesis, pharmaceutical production, etc. [1, 2]. As a result, phenolic compounds are common in industrial wastewater. Due to its properties of high toxicity and being difficult for biodegradation, it has been listed as one of the key hazardous wastewater for water pollution prevention in the world [3]. The U.S. Environmental Protection Administration also lists it as one of 129 priority pollutants for control [4]. In China, the maximum discharge concentration of phenolic pollutants in wastewater must be controlled below 1 mg/L [5]. Therefore, the removal of phenolic compounds from wastewater is overwhelming imperative.
For the past few years, advanced oxidation processes have been reported to be widely used in wastewater treatment [6, 7], such as Fenton oxidation [8], Photocatalytic oxidation [9], Electrochemical oxidation [10], Wet air oxidation [11], and Ozone oxidation [12–15]. Among them, ozone oxidation has attracted many attentions because ozone has strong oxidizing properties, and it can decompose various organic compounds through direct or indirect reactions [16]. Although ozone oxidation is considered as an effective phenolic pollutants removal method, it hardly reacts with some intermediates (such as aldehydes and carboxylic acids) produced during phenolic oxidation, resulting in a low mineralization rate [17–19]. Mu et al. [20] evaluated the effect of ozonation alone on bisphenol A (BPA) removal. The BPA conversion efficiency reached 99.9% within 20 minutes, but the mineralization rate of BPA was only 27.2%. Fang et al. [21] found that after ozonation alone for 60 min, the removal rate of phenol reached 70%, but only 10.0% of TOC. Therefore, in order to improve the mineralization rate of phenolic pollutants, various catalysts were employed to absorb ozone and promote its decomposition to generate more active free radicals for reactions, which has been proven to be an effective method [22–26].
Due to the advantages of low cost, good thermal stability and environmental friendliness of rare earth metals and transition metals, they have become hot spots in the research of catalyst development in recent years [27–30]. Among them, CeO2 has a special oxygen defect structure and two valence states of Ce3+/Ce4+, which make it have a high oxygen storage capacity [31]. Qu et al. [32] prepared Ce/SEP catalysts, and found that high oxygen vacancy accelerated the Ce4+/Ce3+ cycle, which significantly improved the TOC removal of p-nitrophenol (PNP) compared with SEP alone. Similarly, a nano-CeO2-doped ZSM-5 catalyst was prepared for catalytic ozonation to sulfamethoxazole (SMX), and its optimal TOC removal rate was twice that of O3 alone [33]. Besides, it was found that the compounding of other metal oxides with CeO2 could increase the active sites and generate active radicals, further improving the catalytic performance [34–37]. Ma et al. [38] concluded that COD removal rate of MnxCe1−xO2/γ-Al2O3/O3 reached 70.2%, while that of CeO2/γ-Al2O3/O3 was only 65.8%. Additionally, Mohebali et al. [39] prepared Fe3O4@Ce-UiO-66 composites and evaluated their degradation and mineralization ability for acetaminophen removal. They found that 54.9% of TOC could be removed within 10 min, 17.8% higher than that of Ce-UiO-66. It also has been shown that La-doped Ce can increase the generation of surface oxygen defect structure of Ce-based catalyst, which further promote Ce4+/Ce3+ cycling and improve oxygen mobility [40–43]. Li et al. [44] found that La-doped Ce microspheres had a better catalytic performance for toluene oxidation, and T90 (the reaction temperature when conversion reaches 90%), T50 and T10 were 52 °C, 32 °C and 14 °C lower than that of CeO2, respectively. Similarly, a high specific surface area La-doped nano-CeO2 catalyst had high oxygen vacancy content and indicated that the conversion rate of CO reaches 50% at 170 °C, thus proving that the catalyst had excellent catalytic activity [45]. According to previous studies, the combination of La and Ce is mainly used in the catalysis oxidation of gaseous organic pollutants, but there is almost no report on liquid organic pollutants. Considering the obvious difference between the catalytic interface reaction in the gas phase and liquid phase, it is necessary to study the catalytic effect of La and Ce in wastewater containing organic pollutants.
In this study, different amounts of La-modified Ce/γ-Al2O3 catalysts were prepared by equal volume impregnation. The catalysts were characterized by using N2 adsorption-desorption, scanning electron microscopy (SEM), X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), and Fourier transform infrared spectroscopy (FTIR) to analyze the physicochemical properties. Taking phenol as the target pollutant, the catalytic performance of different catalysts via catalytic ozonation was evaluated by the conversion and the mineralization. The effects of catalyst dosage, initial pH value, initial phenol concentration, and ozone concentration on phenol removal performance were investigated, and possible mechanisms of phenol degradation were proposed.
2. Experimental Methods2.1. Catalyst PreparationLa-Ce/γ-Al2O3 catalysts were prepared by equal volume impregnation. First, γ-Al2O3 powder (0.2 μm) was washed with C2H5OH and deionized water. Subsequently, the washed γ-Al2O3 powder was dried at 110 °C for 6 h. Finally, it was calcined at 500 °C for 3 h and cooled for standby. A certain amount of La(NO3)3·6H2O and Ce(NO3)3·6H2O were dissolved simultaneously in a suitable amount of deionized water. Then evenly loaded on the corresponding quality of the γ-Al2O3 powder prepared previously by equal volume impregnation method. The impregnation time was 12 h. Subsequently, the drying and calcination conditions were consistent with the above mentioned γ-Al2O3. Finally, the La-Ce/γ-Al2O3 catalyst was cooled to obtain. In this study, La-modified Ce/γ-Al2O3 catalysts with different loading capacities were prepared, namely x% La-Ce/γ-Al2O3 (1% La-Ce/γ-Al2O3, 2% La-Ce/γ-Al2O3, 2.5% La-Ce/γ-Al2O3, and 3% La-Ce/γ-Al2O3). Ce content remained unchanged at 2% based on the previous works (Fig. S2).
2.2. Experimental Setup and Methods
Fig. S1 is used to investigate the catalytic ozonation of phenol in this experiment. The experimental setup is mainly divided into ozone generation system, catalytic reaction system and analysis system. Ozone (O3) is supplied by O2 DBD (Dielectric barrier discharge) using a pulsed power supply. The catalytic ozonation reactor is a 100 mL bubbling bottle with phenol solution and catalyst, which are stirred with a magnetic stirrer to facilitate effective mixture.
Except of influencing parameter experiment, the reaction conditions are: 2 g/L of catalyst, 100 mL of phenol solution, 200 mg/L of initial concentration, 7 of initial pH and 24 mg/L of O3 concentration. Before experiment, add the catalyst to the phenol solution and stir to achieve an adsorption-desorption equilibrium for 30 min. Every 5 minutes, the gas sample and liquid sample were analyzed, respectively. During the experiment, each sample was tested three times, and its average value was taken.
The ozone analyzer measures the mass concentration analysis of ozone from the DBD reactor. The tail gas of catalytic ozonation reactor was injected into gas chromatography (GC-2014, Shimadzu, Japan; 2 m Porpark N column). Every 5 minutes, we took samples and recorded the peak area of CO2. During the experiment, 0.2 mL solution was removed from the catalytic ozonation reactor and then the change of phenol concentration in the reaction solution was analyzed by HPLC (Shimadzu, Japan; Eclipse Plus C18 column). The liquid sample was filtered with a filter (0.22 μm) before analyzed. The intermediates of phenol removal solution were qualitatively analyzed by LC-MS (Agilent, USA; Waters BEH C18 column).
The phenol conversion rate is calculated by:
C0 refers to the initial phenol concentration; C1 refers to the residual phenol concentration at a certain time t (mg/L).
The CO2 mineralization rate is calculated by:
m refers to the cumulative amount of phenol conversion into CO2 (g); Cti refers to the amount of CO2 (ppm) produced at a specific time ti; υ refers to O3 traffic (mL/min); M refers to the relative molecular mass of phenol; 22.4 refers to the molar volume (L) of the gas in the standard state; 6 refers to the number of C atoms of phenol.
3. Results and Discussion3.1. Evaluation of Phenol Removal Activity of Different Catalysts
Fig. 1 shows the effect of La-Ce/γ-Al2O3 catalysts on the catalytic ozonation performance for phenol removal. Fig. 1(a) shows that the phenol conversion under different catalysts was very fast, and the conversion rate reached more than 99% after 60 min. However, the addition of catalysts improved the phenol conversion, and 2.5% La-Ce/γ-Al2O3 obtained the highest conversion rate of phenol at the same time. Specifically, for the x% La-Ce/γ-Al2O3 (x = 1, 2, 2.5, and 3) catalysts, the conversion rate of phenol reached 77.50%, 82.91%, 83.92% and 80.60% within 20 min, respectively. By contrast, the conversion rate of phenol was 78.89% over the Ce/γ-Al2O3 catalyst, and only 66.04% for ozone alone. However, the conversion rate of phenol during the catalytic ozonation only reflects the preliminary oxidation capacity of catalytic ozonation, and the deep oxidation requires further study of the CO2 mineralization rate.
As shown in Fig. 1(b), when ozone oxidation alone was performed, the CO2 mineralization rate was only 35.76% after 250 min of reaction. By contrast, the CO2 mineralization rate of the Ce/γ-Al2O3 catalyst increased to 70.54%. As the x% La-Ce/γ-Al2O3 (x = 1, 2, 2.5, and 3) catalyst was dosed, the CO2 mineralization rate furtherly increased to 78.77%, 87.13%, 92.53% and 82.02%, respectively. The CO2 mineralization over 2.5% La-Ce/γ-Al2O3 was more than twice that of ozonation alone, and the deep oxidation effect of phenol was greatly improved. The results proved that the addition of La in the catalytic ozonation of phenol played a leading role, and 2.5% La-Ce/γ-Al2O3 catalyst in this experiment was the best.
3.2. Catalyst Characterization3.2.1. BET and SEM analysis of the samplesN2 adsorption-desorption isotherm was used to measure the pore structure and data of the samples. It could be seen from Fig. 2 that these three catalysts exhibited a typical type IV isotherm with H2-hysteresis loop in the range of 0.6–1.0 relative pressure (P/P0), indicating that the catalysts still maintained the mesoporous structure of the γ-Al2O3 vector after loading Ce and La. The specific pore structure data for different catalysts are compiled in Table S1. When La was introduced, the pore data of the catalyst decreased, probably because some metal oxides were embedded in the pores of the γ-Al2O3 support, leading to pore blockage. However, the catalytic activity was greatly improved as demonstrated (Fig. 1), indicating that the pore structure may not be the key factor.
In order to detect the surface morphology of the catalysts, SEM is used to characterize them. Fig. 3 shows that the three catalysts exhibited similar morphology. In combination with the results of N2 adsorption-desorption isotherms, suggesting that although the metal loading caused some specific surface area loss, they did not greatly change the basic structure of the γ-Al2O3 support. Besides, 2.5% La-Ce/γ-Al2O3 catalyst had less surface aggregation and more evenly grain distribution than Ce/γ-Al2O3 catalyst.
3.2.2. XRD, FTIR and XPS analysis of the samples
Fig. 4(a) shows the XRD patterns of the different catalysts. All these three samples indicated diffraction peaks of 2θ at 66.8°, 60.5°, 45.8°, 39.5°, 37.6°, 31.9°, and, 19.6°, corresponding to γ-Al2O3 (PDF#29-0063) [46], indicating that the γ-Al2O3 structure remained stable. No diffraction peaks of CeO2 were observed of Ce/γ-Al2O3, demonstrating that CeO2 species were highly dispersed on γ-Al2O3 [47]. However, after the further introduction of La on Ce/γ-Al2O3, obvious diffraction peaks appeared near 29.1° and 55.9°, corresponding to La2O3 (PDF#74-2430). Therefore, we thought that these two peaks could be diffraction peaks of La2O3.
The chemical groups on the catalyst were identified by infrared spectroscopy (Fig. 4(b)). The bands at 1630 cm−1 and 3435 cm−1 which attributed to the bending vibration (δ) and stretching vibration (ν) of the H2O adsorption and surface hydroxyl groups, respectively, were clearly observed [48]. The peak intensities followed the sequence of 2.5% La-Ce/γ-Al2O3 > Ce/γ-Al2O3 > γ-Al2O3, indicating that the presence of cerium oxides and lanthanum oxides brought about more hydroxyl groups to be produced on the surface of the catalyst. The highest surface hydroxyl content of the 2.5% La-Ce/γ-Al2O3 should be related to its superior catalytic activity [47].
Fig. 4(c) shows the results of XPS O 1s. The O 1s was deconvoluted into adsorbed oxygen (Oads), lattice oxygen (Olatt) and hydroxyl (OH), corresponding binding energies of 530.2 eV, 531.5 eV and 532.9 eV, respectively [49]. Hydroxyl groups were important surface reactive oxygen species, which acted as an active site to improve the activity of catalytic [50–52]. The relative ratios of various oxygen species were calculated (Table S2). 2.5% La-Ce/γ-Al2O3 catalyst compared with Ce/γ-Al2O3 catalyst, the results of XPS O 1s showed that the content of Olatt decreased from 50.3% to 37.7%, the content of Oads increased from 32.5% to 43.8%, and the content of OH also increased from 17.2% to 18.5%. A higher ratio of hydroxyl groups represented more surface active sites of the catalyst, and helped to improve the phenol catalyzed ozonation [53].
3.3. Influence of Experimental ParametersFurthermore, the effects of 2.5% La-Ce/γ-Al2O3 catalyst on the conversion and mineralization of phenol under different catalyst dosage, initial pH value, initial phenol concentration, and ozone concentration were studied by catalytic ozonation, so as to optimize the reaction process for phenol removal.
3.3.1. Catalyst dosageThe effects of different 2.5% La-Ce/γ-Al2O3 catalyst dosages (1, 2, 3, and 4 g/L) on the removal of phenol were studied. Fig. S3(a) shows that the conversion rate improves from 92% to 95.52% at 30 min with the increase of catalyst dosage from 1g/L to 3g/L. But the catalyst dosage further increased to 4 g/L, and the phenol conversion rate was hardly unchanged at 30 min. The catalyst dosage had almost no impact on the preliminary oxidation of phenol. In Fig. S3(b), after catalytic ozonation for 250 min, the CO2 mineralization was 83.16% (1 g/L), 92.53% (2 g/L), 99.69% (3 g/L) and 91.8% (4 g/L), respectively. The catalyst dosage had a vital effect on the CO2 mineralization rate, and the best phenol mineralization was reached at a 3 g/L dosage of 2.5% La-Ce/γ-Al2O3 catalyst. All in all, with the catalyst dosage increased, the conversion rate and CO2 mineralization rate of phenol raised. Probably because the increase of catalyst dosage could provide more active sites, which promotes O3 decomposition, being conducive to produce more active free radicals. Nevertheless, the CO2 mineralization rate decreased when the dosage was increased to 4 g/L. This was probably because the excessive catalyst leading to the active sites close to saturation, and even aggregating with each other catalyst, thereby reducing the efficient contact between the catalyst surface with O3 and the pollutants [28, 54, 55]. As a result, ozone conversion and mineralization were inhibited, and the catalytic activity was greatly suppressed. The results indicated that the catalyst dosage distinctly affected the phenol removal, and the catalyst dosage in subsequent experiments was recognized to be 3 g/L.
3.3.2. Initial pHDuring the process of water treatment, the initial pH is an important condition. Herein, in order to adjust the initial pH of the solution, HCl (0.1 mol/L) and NaOH (0.1 mol/L) were added to the solution. The catalytic ozonation effect of phenol under different pH conditions was studied. As shown in Fig. S3(c) and (d), the change in pH had little effect on the phenol conversion when the initial pH was between 3 and 9.5. The conversion rates of phenol could all reach more than 99.5% under different initial pH after 60 min of reaction. After 250 min of reaction, the CO2 mineralization rate also reached more than 99%. Therefore, the 2.5% La-Ce/γ-Al2O3 catalyst is capable of catalytic ozonation of phenol in a wide range of pH, which reduces the pH adjustment process of water treatment.
3.3.3. Initial phenol concentrationAfter 30 min of reaction, Fig. S3(e) shows that the conversion rates of phenol were 99.09%, 97.58%, 95.52%, 88.56%, and 82.13%, when the initial phenol concentration was 100, 150, 200, 250 and 300 mg/L. With the increase of the initial phenol concentration, the results indicated that the conversion rate gradually decreased. In addition, the CO2 mineralization rates after 85 min of reaction were 99.76%, 76.59%, 54.7%, 46.47%, and 38.65% (Fig. S3(f)), respectively, showing that the different initial phenol concentrations significantly influenced the CO2 mineralization rate. Both the conversion rate and CO2 mineralization rate of phenol decreased significantly with the increase of the initial phenol concentration. The higher the phenol concentration implied the more phenol molecules in the solution, while the production number of active free radicals was almost the same as the O3 addition unchanged. This suggests that more active free radicals were involved in the preliminary oxidized phenol molecules with the increasing phenol concentration, and the deep oxidation of phenol conversion was restrained [53, 54].
3.3.4. Ozone concentrationThe addition of ozone provided a continuous supply of reactive radicals for the entire catalytic system and promoted the oxidation of phenol, so the effects of ozone concentration were studied on the conversion and mineralization of phenol. The ozone concentration was set to 8, 16, 24 and 32 mg/L, respectively. As shown in Fig. S3(g), indicating that with the ozone concentration increased from 8 to 24 mg/L, the conversion rate of phenol raised from 60.94% to 97.01% within 35 min. However, the ozone concentration was further increased to 32 mg/L, and the conversion rate only raised by 0.49%. At this time, ozone was more involved in the deep oxidation of phenol, which further improved the CO2 mineralization. As shown in Fig. S3(h), as the ozone concentration increased from 24 to 32 mg/L, the CO2 mineralization rates of phenol within 100 min were raised from 61.64% to 81.4%. Obviously, the increase in ozone concentration could significantly improve the CO2 mineralization of phenol. This was mainly because the active radicals decomposed from ozone increase when the ozone concentration increased, which promoted phenol oxidation.
3.4. Catalyst StabilityThe stability of 2.5% La-Ce/γ-Al2O3 catalysts was verified by repeated experiments. The used catalyst was washed with C2H5OH and deionized water for several times, and then dried at 60 °C for 6 h for reuse. Fig. 5 shows that the catalytic activity of the 2.5% La-Ce/γ-Al2O3 catalyst hardly reduced after four cycles. After 40 min, the conversion rates of phenol were 98.51%, 97.91%, 97.41%, and 96.86%, respectively. After 250 min, the CO2 mineralization rate still remained at 94.61% after four cycles, indicating that the 2.5% La-Ce/γ-Al2O3 catalyst was quite stable during the phenol removal process.
3.5. Possible Removal Mechanisms of Phenol3.5.1. Identification of ROS (Radical Oxygen Species) in solutionSince the phenol in the solution was almost not adsorbed on the catalyst, its oxidation reaction mainly occurred in the solution. In order to illustrate the oxidation mechanism of phenol in the catalytic ozonation process of 2.5% La-Ce/γ-Al2O3, p-BQ and TBA were added as probes for the detection of superoxide radicals (•O2−) and hydroxyl radicals (•OH), respectively [56, 57]. As shown in Fig. 6, the phenol conversion rate was 91.37% after 30 min of reaction in the presence of TBA, which decreased by 4.15% compared with the absence of TBA. After 60 min of reaction, there was no obvious difference in conversion rate. This indicates that •OH species in solution are not the main ROS produced in the catalytic ozonation process. The conversion rate of phenol greatly decreased from 99.5% to 50.5% after 60 min after the addition of p-BQ, which showed that the •O2− in the solution may be the active oxygen group that played a dominant role in catalyzing ozone oxidation.
3.5.2. Possible mechanisms of •O2− formationBased on the above experimental results and previous studies, the possible catalytic mechanism of ROS generation in the La-Ce/γ-Al2O3/O3 system was proposed and shown in Fig. 7. As O3 is introduced, the hydroxyl groups on the catalyst surface (M-OH) react with O3 to generate •HO2 and •O2− (Eq. (4)) [48], and the consumed surface hydroxyl group would be recovered through the decomposition of an adsorbed water molecule on the surface of the catalyst [38]. The •HO2 species are unstable and would easily decompose into •O2− and H+ (Eq. (5)) [58]. In addition, •HO2 also reacts with dissolved O3 in solution to form •OH radical (Eq. (6)) [47], which is less important in the catalytic ozonation of phenol based on the results of section 3.5.1. Meanwhile, the Ce3+/Ce4+ cycle plays an important role. Ce3+ species are oxidized by O3 to form Ce4+ and •O2− species (Eq. (7)), and the oxygen vacancy on the surface of La-Ce/γ-Al2O3 promotes the reduction of Ce4+ to Ce3+ (Eq. (8)) [33]. During the Ce3+ and Ce4+ cycle, the released electrons would combine with O2 to form the reactive •O2− species (Eq. (9)) [59].
3.5.3. Possible conversion pathwaysThe main intermediate products of phenol conversion were detected and characterized by LC-MS (30 min), as shown in Fig. S4. Based on the previous studies and experimental results, the main intermediate products and possible pathways of phenol conversion are speculated and shown in Fig. 8.
Pathway I: Phenol is attacked by •OH to generate hydroquinone/resorcinol/catechol (m/z = 110). Pathway II: Phenol is attacked by •O2− to form p-benzoquinone/o-benzoquinone (m/z = 108). Meanwhile, p-benzoquinone/o-benzoquinone (m/z = 108) and hydroquinone/resorcinol/catechol (m/z = 110) are interconvertible. The aromatic ring of the above intermediates is further attacked by •O2−, resulting in the opening of the ring and maleic acid (m/z = 116) is produced. Maleic acid is a macromolecular organic acid, which further degrades into short-chain acids, such as oxalic acid (m/z = 90) and malonic acid (m/z = 104). Short-chain acids further degrade into formic acid (m/z = 46). Finally, formic acid is oxidized into CO2 and H2O [60].
4. ConclusionsThe conversion rate of phenol could be significantly increased in the catalyst presence compared with ozonation alone, and the conversion rate of 2.5% La-Ce/γ-Al2O3 increased by 20.53% within 20 min. Moreover, the CO2 mineralization rate of phenol over 2.5% La-Ce/γ-Al2O3 catalyst was more than twice that of ozonation alone within 250 min, reaching 92.53%, and superior CO2 mineralization was maintained after repeated use. The CO2 mineralization rate of phenol after adding 3 g/L 2.5% La-Ce/γ-Al2O3 catalyst reached almost 100%, and could be remained in a solution with a wide pH range (pH = 3~9.5). The increase of the initial phenol concentration was not conducive to its oxidation, while the increase of ozone concentration significantly improved the conversion and mineralization of phenol.
The best 2.5% La-Ce/γ-Al2O3 catalyst obtained a higher amount of adsorbed oxygen and hydroxyl species compared with Ce/γ-Al2O3 and γ-Al2O3, which were essential for the CO2 mineralization of phenol. The •O2− species in the solution were deduced to be the key ROS during the catalytic ozonation of phenol, and they were continuously generated between the reactions of M-OH and O3, and the Ce3+/Ce4+ cycle. The ring-opening and continuous oxidation of phenolic substances were mainly achieved by •O2− species. According to the high removal efficiency, catalytic activity and stability of La-Ce/γ-Al2O3 catalyst, the catalytic system provides a theoretical basis for the advanced treatment of phenol containing wastewater by ozone oxidation and has a good practical application prospect and value.
AcknowledgementsThis work was financially supported by the National Natural Science Foundation of China (No.22075032).
NotesAuthor Contributions Q.J.L. (Master student) took charge of investigation, scientific experiments, data curation, formal analysis and writing; E.H.G. (Lecturer) revised the manuscript; J.L. (Associate Professor) and J.L.Z. (Lecturer) did a formal analysis; S.F. (Professor) and S.L.Y. (Professor) took charge of investigation; Z.L.W. (Professor) provided supervision, theoretical foundation, and experimental guidance. References1. Huang J, Chang Q, Ding YB, Han XY, Tang HQ. Catalytic oxidative removal of 2,4-dichlorophenol by simultaneous use of horseradish peroxidase and graphene oxide/Fe3O4 as catalyst. Chem. Eng. J. 2014;254:434–442. https://doi.org/10.1016/j.cej.2014.05.136
2. Tang Y, Jiang SL, Li WY, et al. Confined construction of COF@Cu-nanozyme with high activity and stability as laccase biomimetic catalyst for the efficient degradation of phenolic pollutants. Chem. Eng. J. 2022;448:137701. https://doi.org/10.1016/j.cej.2022.137701
3. Li GQ, Chai SQ, Zhang GJ. Deactivation characteristics of Ce-modified Cu-based carbon materials for catalytic wet air oxidation of phenol wastewater. J. Environ. Chem. Eng. 2022;10:108228. https://doi.org/10.1016/j.jece.2022.108228
4. Davi ML, Gnudi F. Phenolic compounds in surface water. Water Res. 1999;33:3213–3219. https://doi.org/10.1016/S0043-1354(99)00027-5
5. Wang JL, Zhuan R. Degradation of antibiotics by advanced oxidation processes: An overview. Sci. Total. Environ. 2020;701:135023. https://doi.org/10.1016/j.scitotenv.2019.135023
6. Wang JL, Xu LJ. Advanced Oxidation Processes for Wastewater Treatment: Formation of Hydroxyl Radical and Application. Crit. Rev. Env. Sci. Tec. 2012;42:251–325. https://doi.org/10.1080/10643389.2010.507698
7. Xin L, Hu JW, Wu XL, Huang KW, Huang XF. Fenton-like degradation of carmine dyes based on artificial intelligence modeling and optimization of reduced graphene oxide loaded iron-cobalt-nickel trimetallic nanocomposites. Mater. Today Commun. 2022;31:103463. https://doi.org/10.1016/j.mtcomm.2022.103463
8. Dong WY, Lee CW, Lu XC. Synchronous role of coupled adsorption and photocatalytic oxidation on ordered mesoporous anatase TiO2-SiO2 nanocomposites generating excellent degradation activity of RhB dye. Appl. Catal. B Environ. 2010;95:197–207. https://doi.org/10.1016/j.apcatb.2009.12.025
9. Tang Y, He D, Guo Y, et al. Electrochemical oxidative degradation of X-6G dye by boron-doped diamond anodes: Effect of operating parameters. Chemosphere. 2020;258:127368. https://doi.org/10.1016/j.chemosphere.2020.127368
10. Wang PH, Liang YN, Zhong ZY, Hu X. Nano-hybrid bimetallic Au-Pd catalysts for ambient condition-catalytic wet air oxidation (AC-CWAO) of organic dyes. Sep. Purif. Technol. 2020;233:115960. https://doi.org/10.1016/j.seppur.2019.115960
11. Yan PW, Shen JI, Zhou YC, et al. Interface mechanism of catalytic ozonation in an α-Fe0.9Mn0.1OOH aqueous suspension for the removal of iohexol. Appl. Catal. B Environ. 2020;277:119055. https://doi.org/10.1016/j.apcatb.2020.119055
12. Guo L, Zhang M, Xie SQ. Catalytic ozonation of high-salinity wastewater using salt-resistant catalyst Fe-Bi@γ-Al2O3. J. Water Process Eng. 2022;49:103160. https://doi.org/10.1016/j.jwpe.2022.103160
13. Xu ML, Zhang YB, Yin HQ, Wang JN, Li A, Corvini PF. Efficient catalytic ozonation over Co-ZFO@Mn-CN for oxalic acid degradation: Synergistic effect of oxygen vacancies and HOO-Mn-NX bonds. Appl. Catal. B Environ. 2023;322:122085. https://doi.org/10.1016/j.apcatb.2022.122085
14. Wang JL, Chen H. Catalytic ozonation for water and wastewater treatment: Recent advances and perspective. Sci. Total Environ. 2020;704:135249. https://doi.org/10.1016/j.scitotenv.2019.135249
15. Orge CA, Pereira MFR, Faria JL. Photocatalytic ozonation of model aqueous solutions of oxalic and oxamic acids. Appl. Catal. B Environ. 2015;174:113–119. https://doi.org/10.1016/j.apcatb.2015.02.038
16. Nawrocki J, Kasprzyk B. The efficiency and mechanisms of catalytic ozonation. Appl. Catal. B Environ. 2010;99:27–42. https://doi.org/10.1016/j.apcatb.2010.06.033
17. Ke LJ, Liu J, Sun L, Pan F, Yuan XJ, Xia DS. A non-specific surface area dominated catalytic ozonation with CuO modified β-MnO2 in efficient oxalic acid degradation. J. Water Process Eng. 2022;46:102535. https://doi.org/10.1016/j.jwpe.2021.102535
18. Ghuge SP, Saroha AK. Ozonation of Reactive Orange 4 dye aqueous solution using mesoporous Cu/SBA-15 catalytic material. J. Water Process Eng. 2018;23:217–229. https://doi.org/10.1016/j.jwpe.2018.04.009
19. Mu J, Li S, Wang J, et al. Efficient catalytic ozonation of bisphenol A by three-dimensional mesoporous CeOX-loaded SBA-16. Chemosphere. 2021;278:130412. https://doi.org/10.1016/j.chemosphere.2021.130412
20. Fang C, Gao X, Zhang X. Facile synthesis of alkaline-earth metal manganites for the efficient degradation of phenolic compounds via catalytic ozonation and evaluation of the reaction mechanism. J. Colloid Interf. Sci. 2019;551:164–176. https://doi.org/10.1016/j.jcis.2019.05.010
21. Ikhlaq A, Brown DR, Kasprzyk B. Mechanisms of catalytic ozonation: An investigation into superoxide ion radical and hydrogen peroxide formation during catalytic ozonation on alumina and zeolites in water. Appl. Catal. B Environ. 2013;129:437–449. https://doi.org/10.1016/j.apcatb.2012.09.038
22. Nie YL, Hu C, Li NN, Yang L, Qu JH. Inhibition of bromate formation by surface reduction in catalytic ozonation of organic pollutants over β-FeOOH/Al2O3. Appl. Catal. B Environ. 2014;147:287–292. https://doi.org/10.1016/j.apcatb.2013.09.005
23. Nie YL, Li NN, Hu C. Enhanced inhibition of bromate formation in catalytic ozonation of organic pollutants over Fe-Al LDH/Al2O3. Sep. Purif. Technol. 2015;151:256–261. https://doi.org/10.1016/j.seppur.2015.07.057
24. Nemati O, Navaei AA, Yazdani M, Taghavi M. Catalytic ozonation of ciprofloxacin using γ-Al2O3 nanoparticles in synthetic and real wastewaters. J. Water Process Eng. 2019;32:100894. https://doi.org/10.1016/j.jwpe.2019.100894
25. Wang JL, Bai ZY. Fe-based catalysts for heterogeneous catalytic ozonation of emerging contaminants in water and wastewater. Chem. Eng. J. 2017;312:79–98. https://doi.org/10.1016/j.cej.2016.11.118
26. He Y, Wang LJ, Chen Z. Novel catalytic ceramic membranes anchored with MnMe oxide and their catalytic ozonation performance towards atrazine degradation. J. Membrane Sci. 2022;648:120362. https://doi.org/10.1016/j.memsci.2022.120362
27. Li Y, Zhu JY, Hu JY. Catalytic ozonation for effective degradation of aniline by sulfur-doped copper-nickel bimetallic oxide in aqueous solution. J. Environ. Chem. Eng. 2021;9:104953. https://doi.org/10.1016/j.jece.2020.104953
28. Shao SJ, Li ZX, Gao KC, Zhang JW, Liu YZ, Jiao WZ. Preparation of Cu-MnOX/γ-Al2O3 by high gravity-assisted impregnation method for heterogeneous catalytic ozonation of nitrobenzene. Sep. Purif. Technol. 2022;280:119896. https://doi.org/10.1016/j.seppur.2021.119896
29. Zhang Z, Ai H, Fu ML, et al. Oxygen vacancies enhancing performance of Mg-Co-Ce oxide composite for the selective catalytic ozonation of ammonia in water. J. Hazard. Mater. 2022;436:129000. https://doi.org/10.1016/j.jhazmat.2022.129000
30. Li Y, Wu LC, Wang Y, Ke P, Xu J, Guan BH. γ-Al2O3 doped with cerium to enhance electron transfer in catalytic ozonation of phenol. J. Water Process Eng. 2020;36:101313. https://doi.org/10.1016/j.jwpe.2020.101313
31. Qu ZJ, Xu XS, Ren HF, Sun T, Huang LH, Gao ZH. Effective mineralization of p-nitrophenol in water by heterogeneous catalytic ozonation using Ce-loaded sepiolite catalyst. J. Environ. Chem. Eng. 2022;10:108184. https://doi.org/10.1016/j.jece.2022.108185
32. Zuo X, Ma S, Wu Q, et al. Nanometer CeO2 doped high silica ZSM-5 heterogeneous catalytic ozonation of sulfamethoxazole in water. J. Hazard. Mater. 2021;411:125072. https://doi.org/10.1016/j.jhazmat.2021.125072
33. Liu H, Gao Y, Wang J, Pan J, Gao B, Yue Q. Catalytic ozonation performance and mechanism of Mn-CeOX@γ-Al2O3/O3 in the treatment of sulfate-containing hypersaline antibiotic wastewater. Sci. Total Environ. 2022;807:150867. https://doi.org/10.1016/j.scitotenv.2021.150867
34. Zhang M, Zhang L, Wang H, Bian Z. Hybrid electrocatalytic ozonation treatment of high-salinity organic wastewater using Ni-Ce/OMC particle electrodes. Sci. Total. Environ. 2020;724:138170. https://doi.org/10.1016/j.scitotenv.2020.138170
35. Xu LJ, Wang JL. Magnetic nanoscaled Fe3O4/CeO2 composite as an efficient Fenton-like heterogeneous catalyst for degradation of 4-chlorophenol. Environ. Sci. Technol. 2012;46:10145–10153. https://doi.org/10.1021/es300303f
36. Xu LJ, Wang JL. Degradation of chlorophenols using a novel Fe0/CeO2 composite. Appl. Catal. B Environ. 2013;142:396–405. https://doi.org/10.1016/j.apcatb.2013.05.065
37. Ma N, Ru Y, Weng M, Chen L, Chen W, Dai Q. Synergistic mechanism of supported Mn-Ce oxide in catalytic ozonation of nitrofurazone wastewater. Chemosphere. 2022;308:136192. https://doi.org/10.1016/j.chemosphere.2022.136192
38. Mohebali H, Moussavi G, Karimi M, Giannakis S. Catalytic ozonation of Acetaminophen with a magnetic, Cerium-based Metal-Organic framework as a novel, easily-separable nanocomposite. Chem. Eng. J. 2022;434:134614. https://doi.org/10.1016/j.cej.2022.134614
39. He DD, Chen DK, Hao HS. Structural/surface characterization and catalytic evaluation of rare-earth (Y, Sm and La) doped ceria composite oxides for CH3SH catalytic decomposition. Appl. Surf. Sci. 2016;390:959–967. https://doi.org/10.1016/j.apsusc.2016.08.129
40. Lee J, Lee MW, Kim MJ. Effects of La incorporation in catalytic activity of Ag/La-CeO2 catalysts for soot oxidation. J. Hazard. Mater. 2021;414:125523. https://doi.org/10.1016/j.jhazmat.2021.125523
41. Lim WF, Cheong KY. Oxygen vacancy formation and annihilation in lanthanum cerium oxide as a metal reactive oxide on 4H-silicon carbide. Phys. Chem. Chem. Phys. 2014;16:7015–7022. https://doi.org/10.1039/c3cp55214d
42. Zhang B, Li D, Wang XY. Catalytic performance of La-Ce-O mixed oxide for combustion of methane. Catal. Today. 2010;158:348–353. https://doi.org/10.1016/j.cattod.2010.04.019
43. Li HF, Lu GZ, Wang YQ, Guo Y, Guo YL. Synthesis of flower-like La or Pr-doped mesoporous ceria microspheres and their catalytic activities for methane combustion. Catal. Commun. 2010;11:946–950. https://doi.org/10.1016/j.catcom.2010.04.006
44. Singhania A. High Surface Area M (M = La, Pr, Nd, and Pm)-Doped Ceria Nanoparticles: Synthesis, Characterization, and Activity Comparison for CO Oxidation. Ind. Eng. Chem. Res. 2017;56:13594–13601. https://doi.org/10.1021/acs.iecr.7b03143
45. Ren SD, Liang WJ, Li QL, Zhu YX. Effect of Pd/Ce loading on the performance of Pd-Ce/γ-Al2O3 catalysts for toluene abatement. Chemosphere. 2020;251:126382. https://doi.org/10.1016/j.chemosphere.2020.126382
46. Shao SJ, Li ZX, Zhang JW, Gao KC, Liu YZ, Jiao WZ. Preparation of Ce-MnOX/γ-Al2O3 by high gravity-assisted impregnation method for efficient catalytic ozonation. Chem. Eng. Sci. 2022;248:117246. https://doi.org/10.1016/j.ces.2021.117246
47. Yu Y, An HZ, Zhao Y, et al. MnFe2O4 decorated graphene as a heterogeneous catalyst for efficient degradation of di-n-butyl phthalate using catalytic ozonation in water. Sep. Purif. Technol. 2021;259:118097. https://doi.org/10.1016/j.seppur.2020.118097
48. Comisso N, Rancan M, Armelao L. Investigation on the oxide-oxide galvanic displacement reactions employed in the preparation of electrocatalytic layers. Electrochim. Acta. 2020;341:136056. https://doi.org/10.1016/j.electacta.2020.136056
49. Wang BW, Chi CM, Xu M, Wang C, Meng DJ. Plasma-catalytic removal of toluene over CeO2-MnOX catalysts in an atmosphere dielectric barrier discharge. Chem. Eng. J. 2017;322:679–692. https://doi.org/10.1016/j.cej.2017.03.153
50. Zhao RQ, Wei XL, Chu BX, et al. Multi-phase coexisting metal oxide derived by MOFs for the CO-SCR reaction at low temperature and in situ DRIFTS study on reaction mechanism. Appl. Surf. Sci. 2022;580:152277. https://doi.org/10.1016/j.apsusc.2021.152277
51. Wen NN, Su YX, Deng WY, Zhou H, Hu MT, Zhao BT. Synergy of CuNiFe-LDH based catalysts for enhancing low-temperature SCR-C3H6 performance: Surface properties and reaction mechanism. Chem. Eng. J. 2022;438:135570. https://doi.org/10.1016/j.cej.2022.135570
52. Song JS, Ma NW, Chen WQ, Chen JM, Dai QZ. Insights into mechanism of catalytic ozonation of cinnamyl alcohol over core–shell Fe3O4@SiO2@La2O3 catalyst. Sep. Purif. Technol. 2022;282:119969. https://doi.org/10.1016/j.seppur.2021.119969
53. Liu XH, Li HP, Fang Y, Yang ZG. Heterogeneous catalytic ozonation of sulfamethazine in aqueous solution using maghemite-supported manganese oxides. Sep. Purif. Technol. 2021;274:118945. https://doi.org/10.1016/j.seppur.2021.118945
54. Liu ZY, Teng Y, Xu YH, et al. Ozone catalytic oxidation of biologically pretreated semi-coking wastewater (BPSCW) by spinel-type MnFe2O4 magnetic nanoparticles. Sep. Purif. Technol. 2021;278:118277. https://doi.org/10.1016/j.seppur.2020.118277
55. Zhang F, Wu K, Zhou H, et al. Ozonation of aqueous phenol catalyzed by biochar produced from sludge obtained in the treatment of coking wastewater. J. Environ. Manage. 2018;224:376–386. https://doi.org/10.1016/j.jenvman.2018.07.038
56. Wang JL, Wang SZ. Reactive species in advanced oxidation processes: Formation, identification and reaction mechanism. Chem. Eng. J. 2020;401:126158. https://doi.org/10.1016/j.cej.2020.126158
57. Shen TD, Bao HJ, Su WT, Jiang Y, Tong SP. Manganese containing oxides catalytic ozonation in aqueous solution: Catalytic mechanism on acid sites. Sep. Purif. Technol. 2022;282:120053. https://doi.org/10.1016/j.seppur.2021.120053
58. Zhang YT, Li QW, Long YJ, et al. Catalytic ozonation benefit from the enhancement of electron transfer by the coupling of g-C3N4 and LaCoO3: Discussion on catalyst fabrication and electron transfer pathway. Appl. Catal. B Environ. 2019;254:569–579. https://doi.org/10.1016/j.apcatb.2019.05.019
59. Andrade LS, Laurindo EA, Oliveira RV, Rocha RC, Cass QB. Development of a HPLC method to follow the degradation of phenol by electrochemical or photoelectrochemical treatment. J. Brazil. Chem. Soc. 2006;17:369–373. https://doi.org/10.1590/s0103-50532006000200022
Fig. 1Catalytic ozonation of phenol conversion (a) and CO2 mineralization (b) over a series of La-modified Ce/γ-Al2O3 catalyst. 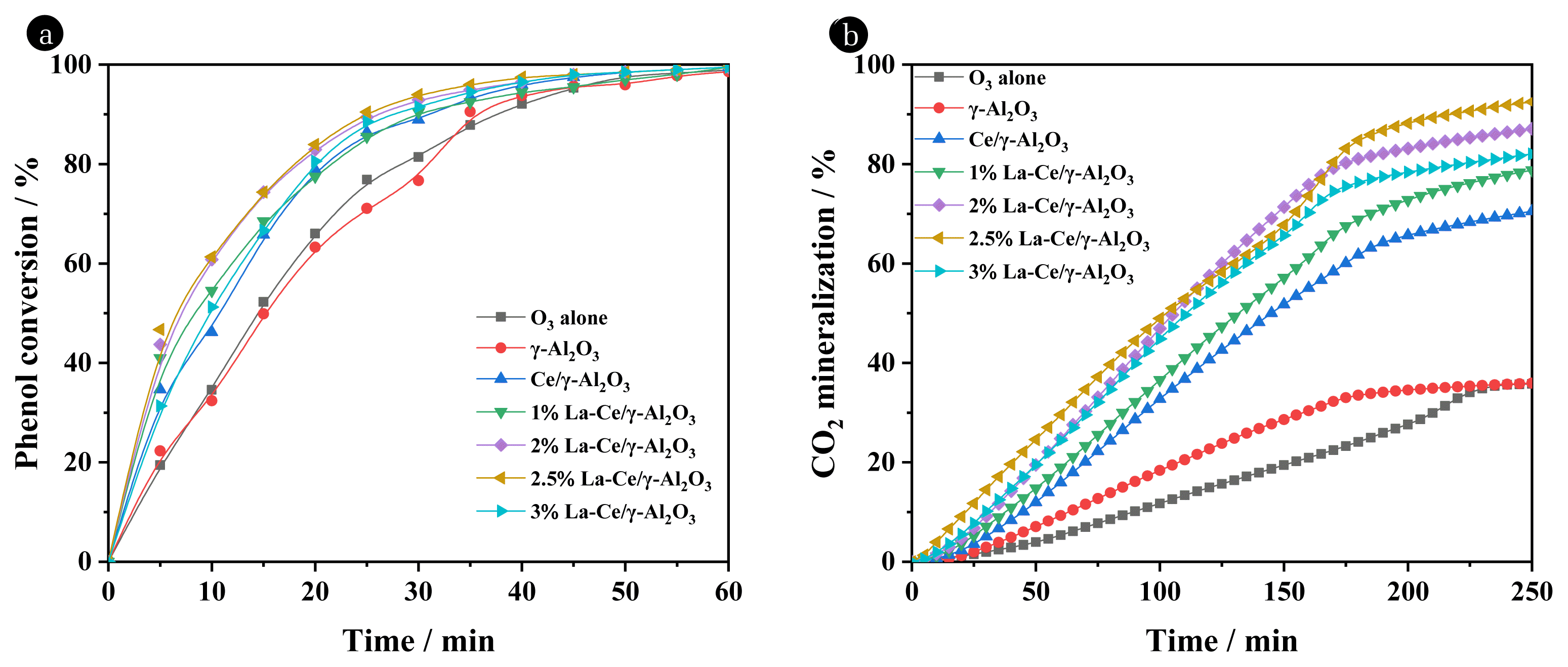
Fig. 5Stability evaluation of 2.5% La-Ce/γ-Al2O3 catalyst: phenol conversion (a) and CO2 mineralization (b). 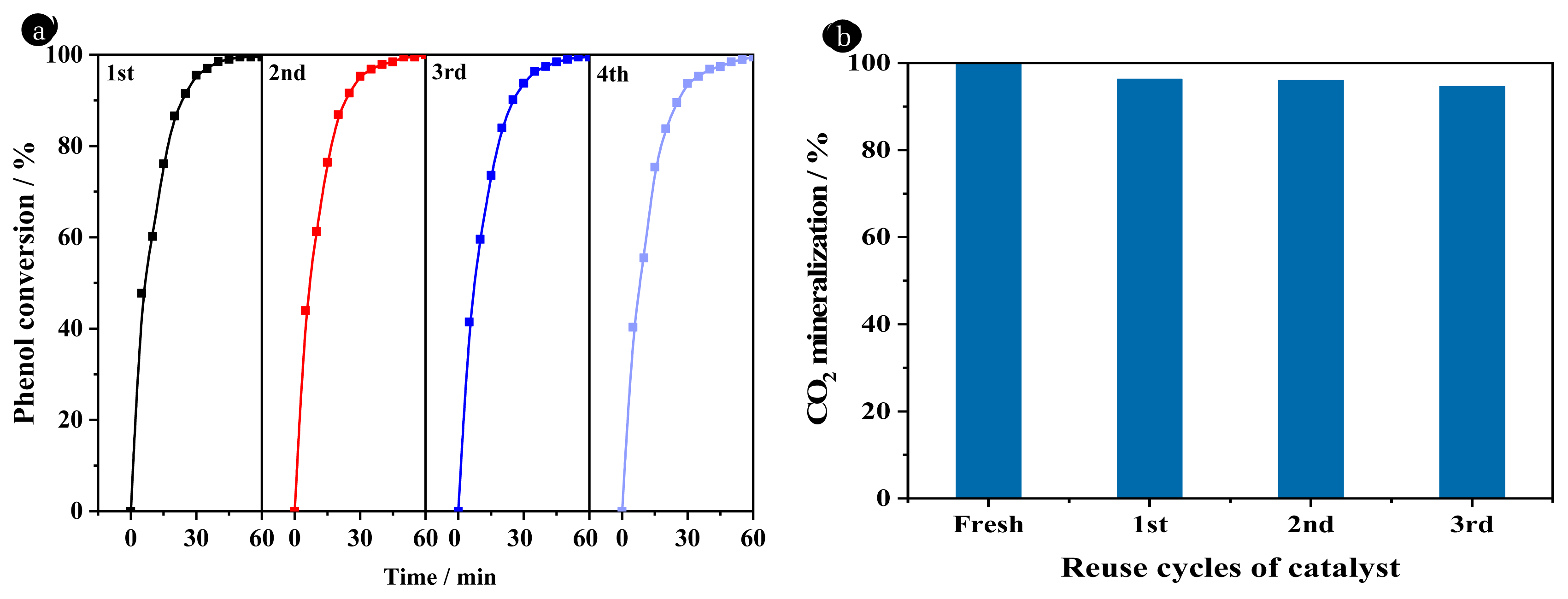
|
|
||||||||||||||||||||||||||||||||||||||||