AbstractMarble industry is one of the pillars of modern buildings and structures but marble industry’s effluents, organic pollutants and heavy metals are affecting natural ecosystem. In present work, fourteen bacteria were isolated from the rhizo-surface soil, receiving marble industry effluent from Hattar industrial area. The soil was heavily polluted with manganese (Mn). Among isolated strains Rothia sp, Methylobacterium sp, Pantoea eucrina and Bacillus safensis were found strong phosphate solublizer, indole acetic and organic acids producers. B. safensis and P. eucrina exhibited strong tolerance at 1750 ppm and 2000 ppm of MnSO4; and were applied alone and in consortium to naturally polluted soil collected from Hattar in a green house on maize (Zea mays L.). Consortium + recommended dose of fertilizers (RDF) and different concentrations of MnSO4 improved 12–25% of plant biomass. Plants grown in naturally polluted soil of marble industry exhibited 50–60% increases in growth indices in single inoculation, and these increases were 65% in consortium application. Plants grown in naturally polluted soil exhibited 30–45% increases in physiological indices. It is inferred that Mn stressed soil may be reclaimed by the application of B. safensis and P. eucrina.
Graphical Abstract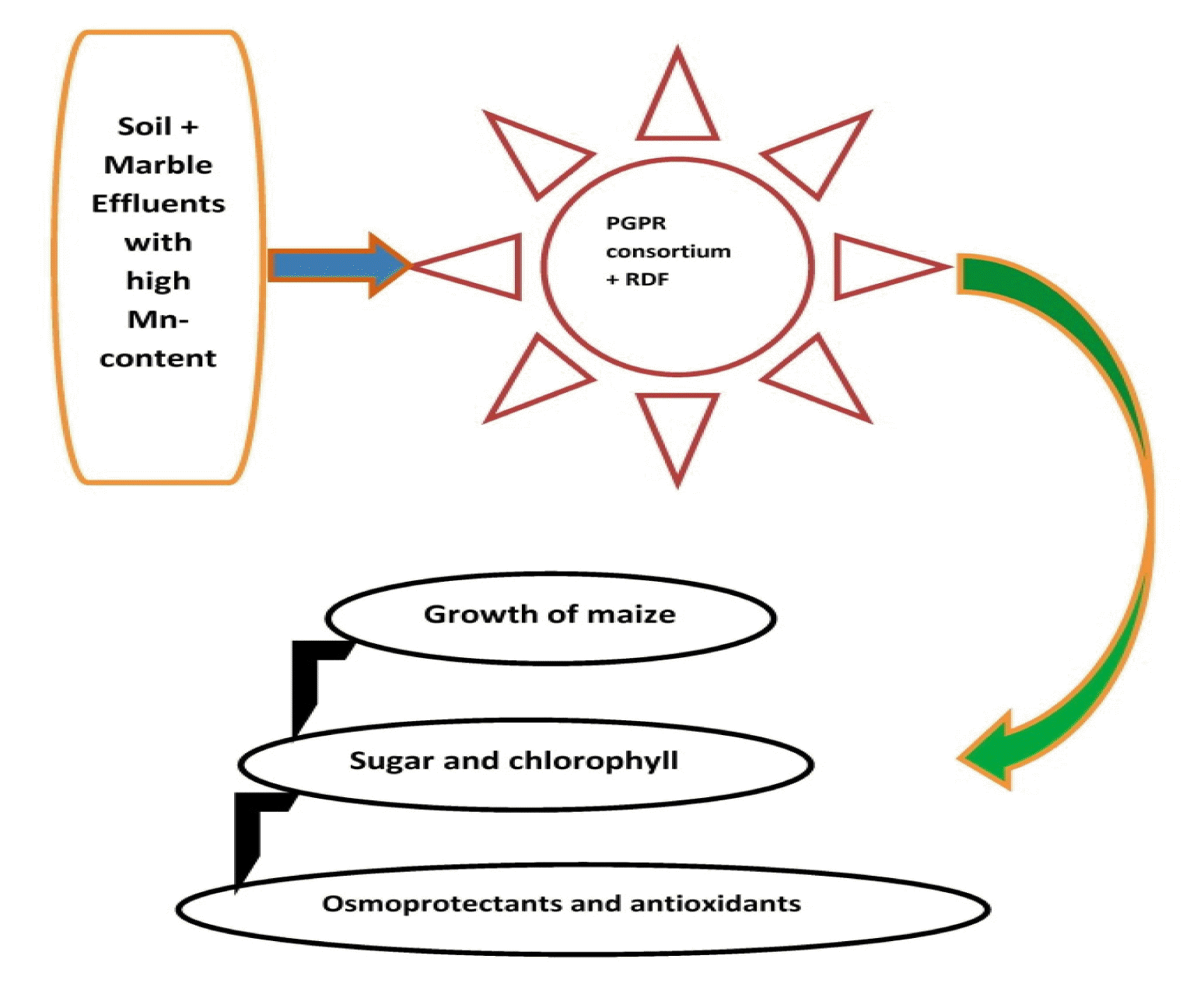
1. IntroductionMan has revolutionized architectural science for its comfort. Use of marble in structures has long been used by emperors, but with the advent of modernization and excessive use of marble; processing of marble industry has now become a major contributor of toxic heavy metals. It is estimated that 40% of marble industry wastes are drained in farmland, rivers and road sides [1]. Ground water and effluent of marble industries contain high level of lead (Pb), nickel (Ni), arsenic (As), cadmium (Cd), copper (Cu), mercury (Hg) and manganese (Mn), as documented by United States Environmental Protection Agency (USEPA) [2].
Bioremediation is emerging and effective method to remove excessive metals from soil. It is non-hazardous, environmental friendly, cost effective, and non-laborious practice to remove toxic metals [3]. Plant growth promoting rhizobacteria (PGPR) having heavy metal tolerances potential are vital partner of bioremediation [4]. Screening of heavy metal tolerant bacteria has proved beneficial, because they not only alleviate heavy metals toxicity, but also improve plant growth and yield [5]. Recent studies reveal that PGPR having ability to improve plant growth by P-solubilization, nitrogen fixation and phytohormones production are also able to cope with heavy metal stresses [6–7].
Metals found in trace amount like Mn are indispensable micronutrients in metabolic pathways of plants, but the excessive accumulation of these trace elements has deleterious consequences on plants, animals and humans. Heavy metal toxicity may result in several human disorders including damages in blood components and vital organs. It also leads to reduced energy levels and impaired or reduced mental and central nervous function [8]. Beyond certain limit, deposition of these metals becomes toxic to the plants [9].
Mn is micronutrient which is required for plant health and its presence become more important when plants are growing in acidic soil [10]. Excessive accumulation of Mn in low pH soil leads to phytotoxicity of Mn, as it prevent translocation of important elements by blocking root xylem and damaging chlorophyll machinery; hence leading to chlorosis. In these conditions higher oxidized Mn and oxidized phenolics accumulates in leaves and apoplast which result in spotted and necrotic brown leaves [11]. Excessive uses of Mn rich food leads to brain diseases like Manganism and Parkinson disease in human [12].
P-solubilizing soil microorganisms (PSM) release P from organic and inorganic pools of P by mineralization and solubilization and improve crops yields [13]. The affectivity of Bacillus safensis in P-solubilization in sandy fluvo-aquic soils and its impact on growth of wheat has been previously revealed [14]. P-solubilizing Pantoea agglomerans as part of consortium of other PSM was found to increase growth and nutrient uptake in ryegrass [15]. These microorganisms are capable to release various organic acids in soil which are responsible for completing this task [16].
The roles of phytohormones in decreasing heavy metal stresses have been previously documented [17]. The Indole-3-acetic acid (IAA) producing PGPR positively impacted on growth and yields of different plants and cope deleterious effects of heavy metals [18]. IAA has been reported as stress hormone as it enabled plant to cope with metal stresses effectively [19].
Pakistan has agriculture based economy but still 32500 hectares of land is irrigated with industrial toxic waste water, which is fetal for soil health [20]. The Hattar industrial area, Haripur district is situated in northwest of Islamabad at a distance of 60 kilometer. Many industries like pharmaceuticals, heavy electrical engineering, textiles, steel, chemicals, marble tiles and edible oils are working in Hattar. Effluents of these industries accumulate various heavy metals in soil.
Bacteria surviving in marble effluents with high deposition of Mn may act as Mn-tolerant bacteria and prove as an effective tool for reclamation of Mn stressed soil. Such bacteria having plant growth promoting features may be handful as bio-inoculants for crops growing in Mn stressed soil. Some recent researches revealed the capability of Bacillus safensis and Pantoea sp in improving growth and physiology of different plants, either by phosphate solubilization or phytohormone production ([14–15]. However, the role of P. Eucrina and B. safensis as PGPR under heavy metal stress has not been explored so far. The aim of this study was to isolate, characterized and explore Mn tolerant bacteria from soil, affected by effluents of marble industry. The remediation and plant growth promoting ability of P. eucrina and B. safensis, under induced Mn-toxicity and naturally contaminated were determined. The potential of these bacteria in improving growth, and physiology of maize was checked by the bio-inoculation of these bacteria.
2. Materials and Methods2.1. Isolation and Screening of BacteriaTwelve soil samples were collected from Hattar industrial area (33.92° N, 72.88° E) in the month of March and stored at 4°C. Collections were made from the land receiving marble effluent and industrial deposits. The temperature, humidity and rain fall of the area were 32 °C, 51% and 68.6 mm, respectively at the time of collection. Samples were collected from three different zones i.e from surface, at the depth of 10–15 cm and from rhizosphere of Conyza canadensis, Euphorbia sp, Parthenium hysterophorus and Amaranthus viridis growing in polluted soil.
Several bacteria were isolated from these samples. Among these, four different isolates were screened out on the basis of Mn tolerance, P-solublization and (Table S1). Pure colonies of these isolates were sent for sequencing to Macrogen, Inc., Seoul, Korea. On the basis of 16S rRNA sequencing results, isolates were identified as Rothia sp, Methylobacterium sp, Pantoea eucrina and B. safensis. Nucleotide sequences were submitted to NCBI and accession numbers MH605007 for Rothia sp, MH603683 for Methylobacterium sp, MH 603867 for P. eucrina and MH603952 for B. safensis were obtained. Two isolates viz. P. eucrina and B. safensis were selected for bio-inoculation on maize under greenhouse condition.
2.1.1. Mn tolerance of bacteriaMn tolerance of bacterial isolates was determined by the method of [21]. Stock solution of MnSO4 (1000 ppm) was prepared, and from this stock solution working solutions of 1500 ppm, 1750 ppm and 2000 ppm were made. Luria Bertani (LB) agar media was prepared in these working solutions and different sets of petri plates containing different ppm concentration of MnSO4 were made. Decimal concentration 109, 107 and 105 of bacterial cultures (30 ml) were spread over the plates. Plates were incubated at 28°C for 24–48 h and colony forming unit (cfu) were calculated according to the formula given by [22]. The experiment was reiterated in triplicate and agar plates without heavy metal salts were taken as negative controls.
2.1.2. Phosphate solubilization abilityAll bacterial isolates were checked for phosphate solubilizing character on Pikovskaya’s agar medium and National Botanical Research Institute Phosphate media (NBRIP) media as expressed by [23]. NBRIP supplemented with different ingredients containing glucose, 10 g; Ca3 (PO4)2, 5 g; MgCl2·6H2O, 5 g; MgSO4.7H2O, 0.25 g; KCl, 0.2 g and (NH4)2SO4, 0.1 g; Fe3 PO4 (2.0 g L−1), Aluminium phosphate AlPO4; 5g L−1 and soy lecithin (15.0 g L−1). Fe3PO4 and AlPO4 were supplemented because Mn rich soil are acidic and accumulate these forms of phosphate. Autoclaved culture media (25 ml) was poured in each petri plate and 10 μL bacterial suspensions were placed at the center. For assessing solublization zone and colony diameters, plates were incubated at 28 °C for 15 days and results were interpreted on the bases of different sizes of both.
2.1.3. Organic acid production of PGPROrganic acid production and identification were determined by the method explain by [24]. 1 ml aliquots from 24 h old bacterial culture were centrifuged at 3500 × g. Media was filtered through 0.45 millipore filter (Greiner, NC, USA) and centrifuged again. Supernatant was collected in a 96-well plate (Greiner, NC, USA). Organic acids were analyzed by HPLC (Shimadzu, C-R4A Chromatopac; SCL-6B system controller) equipped with a binary pump, a UV and RI detector, and a HPX-87H column (Bio-Rad, CA, USA). H2SO4 (10m mM) was used as mobile phase and flow rate was maintained at 0.5 ml min−1, column compartment was 85 °C, while RI detector was operated at 55 °C. External calibration was used to identify and quantify by different organic acid standards (formic, isobutyric, acetic, lactic, butyric, malic, propionic, gluconic). Identification of different organic acids was made by comparing retention times and the peak areas of chromatograms of samples with their subsequent standards.
2.1.4. Quantification of indole acetic acid (IAA) on Salkowski reagent and high pressure liquid chromatography (HPLC)The bacterial culture was grown in a 250 ml Erlenmeyer flask containing 50 ml of nutrient broth for 48–72 hours at 28°C. The development of pink color indicated IAA production. The flasks were inoculated with different bacterial strains, respectively. The fully cultured culture was centrifuged at 3000 × g for 30 minutes. Two drops of orthophosphoric acid and 4 ml of Salkowski reagent (50 ml, 35% perchloric acid, 1 ml, 0.5 M FeCl3 solution) were mixed with the supernatant (2 ml). The development of pink indicates the production of IAA. The optical density (OD) of each sample was determined by spectrophotometer at 530 nm. The quantification of IAA was made by standard IAA curve.
For the confirmation of IAA production bacterial cultures were also subjected to high pressure liquid chromatography (HPLC). Yeast Manitol Agar (YMA) broth media (10 mg/100 ml) was inoculated with 24 h old bacterial cultures and placed on a shaker (ECELLA E24, USA) at 100 × g for 5 d. Bacterial cells were harvested by centrifugation at 10,000 × g for 15 min at 4°C and supernatant was collected as described by [25]. The sample was analyzed on HPLC (Shimadzu, C-R4A Chromatopac; SCL-6B system controller) equipped with UV detector and C-18 column (39 × 300 mm) for identification of hormones. Prior to injection in HPLC column, the sample (100 μl) was filtered through 0.45 millipore filter. Methanol, acetic acid and water (29: 1: 70) were used at flow rate (0.5 ml/min) for an average run of 15 min/sample. Pure IAA (sigma, USA) was used as standard and IAA was identified on the basis of retention time and peak area. The detection of IAA was made at 280 nm.
2.2. Green House ExperimentFor bio-inoculation experiments, maize (Zea mays L.) cv. Azam was obtained from Baffa Research Station, Mansehra. Garden soil and soil naturally polluted with marble effluents were collected from Hazara University and Hattar industrial area, respectively. Soils were autoclaved and poured into pots with 3:1 ratio of soil and sand. Seventeen treatments were made and each treatment had five replicates. Different concentrations of MnSO4 were provided with irrigation water before sowing. Recommended dose of chemical fertilizers (0.082 g/10kg DAP and 0.035g /10kg of urea) was added to all the treatments except control and pots containing naturally contaminated soil at the time of sowing. In each pot 7–10 seeds were sown and thinned to three plants per pot.
P. eucrina and B. safensis were inoculated alone as well as in consortium with three different concentrations (1500 ppm, 1750 ppm and 2000 ppm) of MnSO4. Controlled plants had no inoculation but were stressed with three concentrations of MnSO4. P. eucrina and B. safensis were also inoculated alone and in consortium in naturally polluted soil collected from Hattar. Control plants were also grown in same soil but they did not receive any bio-inoculant.
Seeds were sterilized according to the method of [26]. Sterilized seeds of maize were soaked for 3 h in microbial culture of P. eucrina and B. safensis having 107cell/ ml. Seeds were shade dried for 45 min and sown in pots. For second set pot experiment seeds were soaked in microbial culture along with different concentrations (1500 ppm, 1750 ppm and 2000 ppm) of MnSO4 for 3 h and then sown after shade drying. Control seeds were soaked in autoclaved distilled water before sowing. Plants were harvested at two-leaf stage, after 60 days of sowing for growth and physiological parameters.
2.2.1. Density of bacteria in rhizospheric soil
P. eucrina and B. safensis were re isolated at 60 DAS and 155 DAS from treated soil by serial dilution preparation [27].
2.2.2. Heavy metal analysis of soilSoil samples affected with marble effluents were analyzed for different heavy metals by Ammonium Bicarbonate-DTPA method described by [28]. 1.97 g of 0.005 M diethylenetriamine penta acetate (DTPA) was mixed with 79 g of ammonium bicarbonate (NH4HCO3) and 800 ml of distilled water. Volume of the solution was raised up to 1 L using distilled water, and the pH was adjusted to 7.6 by using ammonium hydroxide.
Air-dried soil (10 g) was added in 10 ml of above solution (extraction solution) and shaken for 15 minutes in a shaker at 180 cycles/minute. The extract was filtered through Whatman No. 42 filter paper. The filtrate was analyzed for the presence of Mn through atomic absorption spectrophotometer (Shimadzu AA-700).
2.2.4. Biological concentration factor (BCF), biological accumulation coefficient (BAC), and translocation factor (TF)2.2.5. Chlorophyll, soluble sugar and proline contents of leaves2.2.6. Superoxide dismutase assayThe SOD activity was determined by the method of [35], which determines the inhibition of photochemical reduction of nitrotetrazolium (NBT) as measure of SOD activity. A single unit of SOD defined as the amount of enzyme, which reduced the absorbance reading by 50% as compared to the control (lacking enzyme). For the quantification of peroxidase, method devised by [36] was applied. Peroxidase activity was recorded by the change in absorbance at 485 nm at 1 min and after 3 minutes with spectrophotometer and articulated as ΔOD485nm/min mg proteins.
2.3. Statistical AnalysisTreatments were statistically analyzed by Statistix 8.1 software for two ways of ANOVA. All the statistical analyses were carried out on the raw data except antibiotic results, which was transformed before analysis. Completely randomized design (CRD) was applied. Duncan’s multiple range test was performed to determine Analysis of variance (ANOVA) and least significant difference (LSD) among treatment at P≤ 0.05.
3. Results and Discussion3.1. Mn tolerance of PGPRAll bacterial strains were tolerant against Mn at 1500 ppm, 1750 ppm and 2000 ppm. P. eucrina and B. safensis were more tolerant than Methylobacterium sp and Rothia sp (Fig. 1). At 2000 ppm, P. eucrina exhibited highest (2.45×106 CFU), while B. safensis 2.2 ×106 CFU at same concentration of Mn. Results indicates that P. eucrina and B. safensis had 96% greater resistance to Mn than Methylobacterium sp and Rothia sp. Similar trend was observed at 108 decimal dilutions (Fig. 2).
3.1.1. Phosphate solublization potentialPhosphate solublization potential measured by inhibition zone of P. eucrina and B. safensis was greater than Methylobacterium sp and Rothia sp (Fig. S1). P. eucrina had 1.6 mm inhibition zone, while Methylobacterium sp had smallest (1.05 mm) zone of inhibition. P. eucrina and B. safensis had 43% and 52% greater zone than Methylobacterium sp.
3.1.2. Organic acids production
P. eucrina, B. safensis, Methylobacterium sp and Rothia sp produced different concentrations of gluconic acid, maliac acid, acetic acid, formic acid, oxalic acid, lectic acid, propinic acid and isobutyric acid (Fig. 4). Among four PGPR organic acids production was higher in P. eucrina and lowest in Rothia sp. Formic acid and gluconic acid production was 62% and 70% higher in P. eucrina as compare to Rothia sp. P. eucrina produced 50%, 37%, 41%, 68%, 71% and 80% higher maliac acid, acetic acid, oxalic acid, lectic acid, propinic acid and isobutyric acid than Rothia sp, respectively. Similarly, B. safensis produced 51%, 27%, 58%, 28%, 35%, 51%, 60% and 40% higher gluconic acid, maliac acid, formic acid, acetic acid, oxalic acid, lectic acid, propinic acid and isobutyric acid than Rothia sp, respectively.
3.1.3. Indole-3-acetic acid potentialIndole-3-acetic acid production was observed in all strains (Fig. 3). Among bacterial strains, B. safensis produced the highest amount (30.5 and 52 μg ml−1) on Salkowski media and HPLC, respectively. Rothia sp produced lowest (21 and 42 μg ml−1). P. eucrina and B. safensis showed 43% and 56% greater IAA than Methylobacterium sp. B. safensis produced 37% greater IAA than P. eucrina.
3.2. Bacterial Density of Inoculated Bacteria in SoilA gradual decline in survival of P. eucrina and B. safensis was observed at 60 DAS as compared to CFU at 0 DAS (8.3 × 109) and with increase in Mn concentrations (Fig. S2). However, CFU in single inoculation of P. eucrina and B. safensis was increased by 4–6% as compared to CFU recorded at 0 DAS. Highest increases (8–12%) in CFU were observed in single inoculation of P. eucrina and B. safensis inoculated in naturally polluted soil. Both isolates had lowest CFU in soil received 2000 ppm of Mn.
3.2.1. Effects of PGPR inoculation on bioremediation of MnAffectivity of P. eucrina to remediate Mn from soil was greater than B. safensis (Table 1 & 2). P. eucrina and B. safensis decreased 28% and 24% Mn in the soil which received 1500 ppm–2000 ppm of Mn stress with RDF as compared to un-inoculated soil with 1500 ppm–2000 ppm of Mn stress and RDF. In similar cases, decrease in Mn was 30% when consortium was applied with different concentrations of Mn and RDF.
In naturally polluted soil, inoculation of P. eucrina decreased 29% Mn over naturally polluted control soil B. safensis decreased 27% of Mn over control while consortium of P. eucrina and B. safensis decreased 31% Mn in soil.
3.2.2. Mn accumulation in plant partsMaize roots and leaves accumulated 23–28% and 25% higher Mn when P. eucrina was applied with different concentration of Mn and RDF as compare to un-inoculated control with different concentrations of Mn and RDF (Table 1). B. safensis inoculation with different concentrations of MN and RDF exhibited 17% higher Mn in roots and 20–24% higher Mn in leaves. Accumulation of Mn in roots and leaves were 28–36% higher in consortium treatment over un-inoculated control.
In plants growing in soil contaminated with marble industry, application of P. eucrina increased 28% Mn in roots and 37% in leaves while B. safensis showed 22% and 33% greater Mn in plant roots and leaves over control. Consortium of B. safensis and P. eucrina accumulated 33% and 40% greater Mn in roots and leaves of maize plant.
BAC and BCF of Mn was highest (0.8) and (0.45) when P. eucrina and B. safensis were inoculated with 2000 ppm of Mn and RDF (Table 2). TF of Mn was highest (2.3) in single inoculation of P. eucrina and B. safensis as well as in consortium. In naturally polluted soil increase in BAC and TF was significant (13–15%) in inoculation treatments over control.
3.2.3. Effects of PGPR inoculation on maize growthIncrease in plant height and fresh weight measured at 60 DAS was 20% and 18% over control following the single inoculation of B. safensis and P. eucrina (Table 3). Co-inoculation of PGPR comprising B. safensis and P. eucrina increased plant height by 26% over control. Plant height and fresh weight were 25–30% higher in inoculation treatments as compared to the applications of B. safensis and P. eucrina with different concentration of Mn + RDF. Plants growing in soil contaminated with marble industry had 11–12% higher plant height and 20% higher fresh weight in single inoculation of P. eucrina and B. safensis over control. Consortium of B. safensis and P. eucrina exhibited 64% and 32% greater plant height and fresh weight over control, respectively.
Single inoculation of P. eucrina and B. safensis increased shoot dry weight by 11–13% over control. Consortium comprising P. eucrina and B. safensis had 21% higher shoot dry weight. Under induced Mn stress, consortium application enhanced 14% dry weight. In plants growing in naturally contaminated soil of marble industry, single inoculation of P. eucrina and B. safensis as well as consortium increased dry weight by 55–60% over control.
B. safensiss and P.eucrina inoculation increased root length and dry weight by 11% over control (Table 3). Root length and fresh weight of maize plants were 15% higher over control when PGPR consortium was applied. Under induced Mn stress increased in root length and root fresh weight was 14–18% higher as compare to un-inoculated plant receiving same concentration of heavy metal and RDF. Maize plants grown in naturally contaminated soil had 52% and 34% greater root length and fresh weight over control when inoculated with P. eucrina or B. safensis. Similarly, 52% and 43% higher root length and root fresh weight were observed in plants inoculated with Consortium.
3.2.4. Effects of PGPR inoculation on physiology of maizeThe percentage of increase in soluble sugar content was 33% and 16% over control in single inoculation of P. eucrina and B. safensis, respectively (Table 4). Consortium increased 40% sugar content in maize leaves over control. P. eucrina increased soluble sugar content by 60–65% in the presence of different concentration of Mn + RDF as compared to control. B. safeness in the presence of Mn + RDF showed 20–35% greater sugar content over control. The consortium application with Mn + RDF increased 65–75% soluble sugar as compare to control. Maize plants grown in naturally contaminated soil had 20% and 22% greater soluble sugar over control when inoculated with P. eucrina and B. safensis, respectively while consortium exhibited 31% higher sugar contents.
Proline content was 12% higher in single inoculation of P. eucrina and B. safensis. This increase was 26% over control in consortium treatment. P. eucrina with different concentration of Mn + RDF increased 30–48% proline over control. B. safensis showed 36–40% increase proline content in leaves in the presence of Mn + RDF. Consortium with 1500 ppm, 1750 ppm and 2000 ppm of Mn + RDF induced 52% increase in proline content. Maize plants grown in naturally contaminated soil had 24% greater proline over control when inoculated with P. eucrina or B. safensis while consortium exhibited 28% higher sugar contents
The SOD and POD activities were 21% and 24% higher than control following the inoculation of P.eucrina. B.safensis increased SOD and POD by 21% and 16%, respectively while accumulation of SOD and POD were 26% and 32% in consortium treatment. Application of P. eucrina with different concentration of Mn and RDF increased 39–73% and 44–68% SOD and POD over control while B. safensis with different concentration of Mn and RDF increased SOD and POD by 40–56%. Consortium application with different concentrations of Mn and RDF increased 69–100% SOD and 52–80% POD over control. Maize plants grown in naturally contaminated soil and inoculated with P. eucrina had 29% and 19% greater SOD and POD over control while B. safensis exhibited 27% and 16% higher SOD and POD. Consortium of B. safensis and P. eucrina accumulated 32% higher SOD and 22% higher POD in leaves.
4. Discussion4.1. Isolation of Heavy Metal Tolerant Bacteria
Rothia sp, P. eucrina, Methylobacterium sp and B. safensis were isolated from the effluent of marble industry, having high deposition of Mn. Marble industry effluents have drastic effects on microbial biodiversity and only few bacterial species can survive in high Mn Stress [37]. Soil health and growth indices of plants are also affected by marble effluents as studied in tomato (Lycopersicon esculentum) [38]. Pantoea sp commonly colonized soil as well as endophytes in different plant species including onion, orchid, eucalyptus and brassica [39–40]. Soil contaminated with heavy metals are reported to harbor different Pantoea sp [41–42].
4.1.1. Solublization of phosphorus by bacteriaAll isolates were competent to solublize phosphorus (P) and produce IAA. Since IAA production and P-solublization is peculiarity of PGPR and it has been revealed in many researches [43]. It has been demonstrated that under heavy metals stress, B. Safensis compensate deleterious effects by high solublization of phosphorus and IAA production. B. Safensis was found more effective than other isolates under Mn stress, because of its spore producing ability [44].
4.1.2. Mn toxicity in soilMn being trace element exists in different oxidative states and it becomes available to plants at low pH [45]. Bacterial community is capable of producing different organic acids, which help to solublise soil P, decrease soil pH and enable plants to survive at high concentration of Mn. Our results also confirm that all the strains were P-solubliser and capable of producing different organic acids. Decrease in Mn contents is correlated with production of higher oxalic acid which is considered as good Mn solublizer [46].
All the bacterial isolates were tolerant to different concentrations of Mn (1500 ppm, 1750 ppm and 2000 ppm) as determined by CFU. Mn tolerant bacteria Serratia marcescent and Lysinibacillus sp were isolated from Mn stressed soil [47–48]. It was reported previously that survival of Bacillus thuringiensis at 4000 mg Kg−1 in manganese ore having PGPR properties [49]. Different studies prove that methylotrophic bacteria are resistant to high concentrations of heavy metals [50].
4.2. Occurrence of Mn in MaizeTranslocation of Mn from soil to roots and from roots to leaves was increased following the inoculation of PGPR. This was also evident by BAC, BCF and TF. Decrease in soil Mn content is attributed with better solublization of phosphorus and increased production of organic acids ([13, 51, 52]; thereby increasing solublization of Mn [53]. Most of bacterial species are good Mn solublizers at higher or neutral pH, but data about Mn solublizers and Mn oxidizing bacteria in acidic soil is limited [54]. It was found that Mesorhizobium australicum had potential to oxidize Mn at low pH [53]. Application of Pantoea sp and Bacillus increased translocation and accumulation of Mn in plant parts of strawberry [55].
4.2.1. Recovery of inoculated bacteria from soilResult indicates that CFU of inoculated P. eucrina and B. safensis decreased with increasing Mn concentrations when Mn was induced at 60 DAS. However, increases in CFU of both inoculated bacteria were observed when they were applied in single inoculation to control soil as well as to naturally polluted soil. Various factors including inoculum density, inoculation methods, soil humidity, pH, temperature and host plant physiology determines survival of inoculated bacteria [56]. According to [57] root exudate, plant age and genotype also effect survival capabilities of bacteria in soil and these may be causes of variation in CFU in different treatments. Greater decreases in CFU of P. eucrina and B. safensis in the soil treated with different concentrations of Mn may be attributed to increase in proliferation of hazardous ions in the soil.
4.2.2. Improvement in growth indices of maize due to inoculation under Mn stressMarked increases were observed in term of plant height, shoot and root fresh, and dry weight of maize. Increase in growth responses following the inoculation of PGPR is mediated by better acquisition of P and IAA production [58]. Same mechanism was described under drought stress where Pantoea alhagi was found effective to increase growth of wheat [59]. Bio-inoculation of Pantoea sp on Brassica napus has been documented to improve shoot and root biomass [40]. Affectivity of B. safensis was also experimented for growth responses in maize and soybean [60, 61].
4.2.3. Improvement in physiology of maize due to inoculation under Mn stressIncreases in sugar, proline and antioxidative enzyme activities were observed when plants were treated with PGPR and these increases were further augmented in consortium treatment. Sugar, proline and antioxidants are the major osmoprotectants and indicators of stresses [58]. Onset of abiotic stress triggers accumulation of different types of sugars (mannitol, trehalose and galactinol) in plants [62]. Similarly, imino acid proline accumulation is primary indicator to abiotic stresses including heavy metal stresses [63].
Under heavy metal stress, antioxidant enzymes are activated for quenching reactive oxygen species (ROS) which are produced in abundant and damage macromolecules [64]. Application of Bacillus species under Cr stress increased sugar and proline contents of leaves of wheat [65]. Similarly, under heavy metal stress, bio-inoculation of Rhizobia leguminosarum, Pseudomonas fluorescens, Luteibacter sp, Sinorhizobium meliloti and Variovorax sp exhibited high sugar and proline in Lathyrus sativus.
5. ConclusionsSoil receiving marble effluent had higher Mn content which is harmful for plants. Contaminated soil harbors Mn tolerant bacteria and among these most important were P. eucrina and B. Safensis. Theses bacteria were tolerant to high concentration of Mn found in naturally contaminated soil having marble effluents as well as induced form i.e MnSO4. P. eucrina and B. safensis have a variety of plant growth promoting qualities including phosphate solubilization and IAA and organic acid production. Bio-inoculation of PGPR alone and in consortium effectively reclaimed Mn polluted soil by accumulating high concentrations of Mn in plant parts. Application of these PGPR and their inoculation with other PGPR may be helpful for reclamation of Mn polluted soil and recovery of soil health for agriculture practices. Since the experiment was conducted in a greenhouse, it is recommended that research should be extended to field condition also for attaining better insight of these bacteria.
NotesAuthor contributions T.H. and M.H. conceived ideas, and along with I.N. designed the methodology; A.A. managed and coordinated greenhouse experiment. T.H., M.H., K.R. did laboratory work and collected data. A.A. analyzed the data; K.R. wrote initial draft; all authors contributed critically and gave final approval for publication. References1. Waseem A., Arshad J., Iqbal F., Sajjad A., Mehmood Z., Murtaza G.. Pollution status of Pakistan: a retrospective review on heavy metal contamination of water, soil, and vegetables. BioMed Res. Int. 2014;2015:1–29.813206. 10.1155/2014/813206
2. Afzal M.S., Ashraf A., Nabeel M.. Characterization of industrial effluents and groundwater of Hattar industrial estate, Haripur. Adv. Agric. Environ. Sci. 2018;1(2)70–77. 10.30881/aaeoa.00013
3. Azubuike C.C., Chikere C.B., Okpokwasili G.C.. Bioremediation techniques-classification based on site of application: principles, advantages, limitations and prospects. World J. Microbiol Biotechnol. 2016;32(11)1–18.
doi.org/10.1007/s11274-016-2137-x
4. Robas M., Jiménez P.A., González D., Probanza A.. Bio-Mercury Remediation Suitability Index: A Novel Proposal That Compiles the PGPR Features of Bacterial Strains and Its Potential Use in Phytoremediation. Int. J. Environ. Res. Public. Health. 2021;18(8)1–14.
doi.org/10.3390/ijerph18084213
5. Manoj S.R., Karthik C., Kadirvelu K.. Understanding the molecular mechanisms for the enhanced phytoremediation of heavy metals through plant growth promoting rhizobacteria. J. Environ Manage. 2020;254:1–14. 10.1016/j.jenvman.2019.109779
6. Yan A., Wang Y., Tan S.N., Mohd Yusof M.L., Ghosh S., Chen Z.. Phytoremediation: A Promising Approach for Revegetation of Heavy Metal-Polluted Land. Front. Plant Sci. 2020;11:1–15. 10.3389/fpls.2020.00359
7. Kang S.M., Asaf S., Khan A.L., Lubna K.A., Mun B.G., Khan M.A., Gul H., Lee I.J.. Complete Genome Sequence of Pseudomonas p
sychrotolerans CS51, a Plant Growth-Promoting Bacterium, Under Heavy Metal Stress Conditions. Microorganisms. 2020;8:360–382. 10.3390/microorganisms8030382
8. Obasi P.N., Akudinobi B.B.. Potential health risk and levels of heavy metals in water resources of lead–zinc mining communities of Abakaliki, southeast Nigeria. Appl. Water Sci. 2020;10(184)1–23.
doi.org/10.1007/s13201-020-01233-z
9. Balkhair K.S., Ashraf M.A.. Field accumulation risks of heavy metals in soil and vegetable crop irrigated with sewage water in western region of Saudi Arabia. Saudi J. Biol. Sci. 2016;23(1)32–44.
doi.org/10.1016/j.sjbs.2015.09.023
10. Alejandro S., Höller S., Meier B., Peiter E.. Manganese in Plants: From Acquisition to Subcellular Allocation. Front. Plant Sci. 2020;11:1–23.
doi.org/10.3389/fpls.2020.00300
11. Andresen E., Peiter E., Küpper H.. Trace metal metabolism in plants. J. Exp. Bot. 2018;69(5)909–954.
doi.org/10.1093/jxb/erx465
12. Harischandra D.S., Ghaisas S., Zenitsky G., Jin H., Kanthasamy A., Anantharam V., Kanthasamy A.G.. Manganese-Induced Neurotoxicity: New Insights into the Triad of Protein Misfolding, Mitochondrial Impairment, and Neuroinflammation. Front. Neurosci. 2019;13:1–19.
doi.org/10.3389/fnins.2019.00654
13. Billah M., Khan M., Bano A., Hassan T.U., Munir A., Gurmani A.R.. Phosphorus and phosphate solubilizing bacteria: Keys for sustainable agriculture. Geomicrobiol. J. 2019;36:904–916. 10.1080/01490451.2019.1654043
14. Wang Z., Zhang H., Liu L., Li S., Xie J., Xue X., Jiang Y.. Screening of phosphate-solubilizing bacteria and their abilities of phosphorus solubilization and wheat growth promotion. BMC Microbiol. 2022;22:1–15.
doi.org/10.1186/s12866-022-02715-7
15. Amri M., Mateus D., Gatrouni M., Rjeibi M.R., Asses N., Abbes C.. Co-Inoculation with Phosphate-Solubilizing Microorganisms of Rock Phosphate and Phospho gypsum and Their Effect on Growth Promotion and Nutrient Uptake by Ryegrass. Appl Biosci. 2022;1(2)179–197.
doi.org/10.3390/applbiosci1020012
16. Tian J., Ge F., Zhang D., Deng S., Liu X.. Roles of Phosphate Solubilizing Microorganisms from Managing Soil Phosphorus Deficiency to Mediating Biogeochemical P Cycle. Biology (Basel). 2021;10(2)1–19.
doi.org/10.3390/biology10020158
17. Saini S., Kaur N., Pati P.K.. Phytohormones: Key players in the modulation of heavy metal stress tolerance in plants. Ecotoxicol Environ. Saf. 2021;223:1–14.
doi.org/10.1016/j.ecoenv.2021.112578
18. Nazli F., Wang X., Ahmad M., Hussain A., Bushra Dar A., Nasim M., Jamil M., Panpluem N., Mustafa A.. Efficacy of Indole Acetic Acid and Exopolysaccharides-Producing Bacillus safensis Strain FN13 for Inducing Cd-Stress Tolerance and Plant Growth Promotion in Brassica juncea (L.). Appl. Sci. 2021;11(9)1–19.
doi.org/10.3390/app11094160
19. Rehman B., Hassan T.U., Bano A.. Potential of indole-3-acetic acid-producing rhizobacteria to resist Pb toxicity in polluted soil. Soil Sediment contam. 2019;28(1)101–121. 10.1080/15320383.2018.1539947
20. Mahfooz Y., Yasar A., Guijian L., Islam Q.U., Akhter A.B.T., Rashid R., Irshad S., Naeem U.. Critical risk analysis of metals toxicity in wastewater irrigated soil and crops: a study of a semi-arid developing region. Sci. Rep. 2020;1–10.
doi.org/10.1038/s41598-020-69815-0
21. Washington J.A.H., Sutter V.L.. Dilution sus-ceptibility test: Agar and macro-broth dilution procedures. Lennette E.H., Balows A., Hausler W.J., Truant J.P., editorsManual of Clinical Microbiology. Washington DC: American Society for Microbiology; 1980. 453–458.
https://www.scirp.org/(S(351jmbntvnsjt1aadkposzje))/reference/referencespapers.aspx?referenceid=247477
22. James G.C.. Natalic Sherman Rockland Community College, State University of New York: The Benjamin/Coming Publishing Company. Inc; 1978. 75–80. 1978.
http://prr.hec.gov.pk/jspui/bitstream/123456789/2419/1/3026S.pdf
23. Nautiyal C.S.. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol. Lett. 1999;170:265–270.
doi.org/10.1111/j.1574-6968.1999.tb13383.x
24. Sharon J.A., Hathwaik L.T., Glenn G.M., Imam S.H., Lee C.C.. Isolation of efficient phosphate solubilizing bacteria capable of enhancing tomato plant growth. J. Soil Sci. Plant Nutr. 2016;16(2)525–536.
doi.org/10.4067/S0718-95162016005000043
25. Tien T.M., Gaskins M.H., Hubbell D.H.. Plant growth substances produced by Azospirillum brasilense and their effect on the growth of pearl millet (Pennisetum americanum L). Appl Environ. Microbiol. 1979;37:1016–1024. 10.1128/aem.37.5.1016-1024.1979
26. Miché L., Balandreau J.. Effects of rice seed surface sterilization with hypochlorite on inoculated Burkholderia vietnamiensis. Appl. Environ. Microbiol. 2001;67(7)3046–3052. 10.1128/AEM.67.7.3046-3052.2001
27. Johnson L.F., Curl E.A.. Methods for research on ecology of soil-borne plant pathogens. Minneapolis: Burgess publishing company; 1972. 206–207.
doi.org/10.2136/sssaj.1972.03615995003600040002x
28. Soltanpour P.N., Schwab A.P.. A new soil test for simultaneous extraction of macro- and micronutrients in alkaline soils. Commun. Soil Sci. Plant Anal. 1977;8:195–207.
doi.org/10.1080/00103627709366714
29. Piper C.S.. Soil and plant analysis. Adelaide Australia; University of Adelaide press S.A: 1947. 368.
http://www.sciepub.com/reference/294879
30. Yoon J., Cao X., Zhou Q., Ma L.Q.. Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Sci. Total Environ. 2006;368:456–464. 10.1016/j.scitotenv.2006.01.016
31. Cui S., Zhou Q., Chao L.. Potential hyper-accumulation of Pb, Zn, Cu and Cd in endurant plants distributed in an old smeltery, northeast China. Environ. Geol. 2007;51:1043–1048. 10.1007/S00254-006-0373-3
32. Li M.S., Luo Y.P., Su Z.Y.. Heavy metal concentrations in soils and plant accumulation in a restored manganese mineland in Guangxi, South China. Environ. Pollut. 2007;147:168–175. 10.1016/j.envpol.2006.10.016
33. Dubois M., Giles K.A., Hamilton J.K., Rebers P.A., Smith F.. Calorimetric method for determination of sugars and related substances. Anal. Chem. 1983;28:350–356.
doi.org/10.1021/ac60111a017
34. Bates L.S., Waldern R.P., Teare I.D.. Rapid determination of free proline for water status studies. Plant Soil. 1973;39:205–207.
doi.org/10.1007/BF00018060
35. Beauchamp C., Fridovich I.. Superoxide dismutase: improved assay and an assay applicable to acrylamide gels. Anal. Biochem. 1971;44:276–287.
doi.org/10.1016/0003-2697(71)90370-8
36. Vetter J.L., Steinberg M.P., Nelson A.I.. 1958;Enzyme assay, quantitative determination of peroxidase in sweet corn. J. Agric. Food Chem. 2007;6:39–41.
doi.org/10.1021/jf60083a006
37. Iqbal M., Akbar F., Ullah S., Anwar I., Khan M.T., Nawab A., Bacha M.S., Rashid W.. The effects of marble industries effluents on water quality in Swat, Northern Pakistan. J. Bio. Environ Sci. 2018;13(1)35–43.
doi.org/10.3390/su14073831
38. Neelab N., Ullah S., Faiz P., Rashid S., Rahim K., Akbar F., Yousufzai M.T., Lashari A.H., Rashid W., Hussain Z.. Effects of Marble Industry Effluents on Soil Quality, Growth and Productivity of Tomato (Lycopersicon esculentum) in District Swat, Khyber Pakhtunkhwa, Pakistan. Pol. J. Environ. Stud. 2022;31(4)3263–3270.
doi.org/10.15244/pjoes/144659
39. Brady C., Cleenwerck I., van der Westhuizen L..
Pantoea rodasii sp. nov., Pantoea rwandensis sp. nov.and Pantoea wallisii sp. nov., three novel Pantoea species isolated from Eucalyptus. Int. J. Syst. Evol. Microbiol. 2012;62:1457–1464. 10.1099/ijs.0.032615-0
40. Trifi H., Ben S., Kolsi B., Amine F., Clara C., Wafa A., tham S., Mouldi S.. Effectiveness of plant growth-promoting rhizobacterium Pantoea sp. BRM17 in enhancing canola growth on phosphogypsum amended soil. Pedosphere. 2020;30(4)570–576. 10.1016/S1002-0160(17)60454-5
41. Khan M.U., Sessitsch A., Harris M., Fatima K., Imran A., Arslan M., Shabir G., Khan Q.M., Afzal M.. Cr-resistant rhizo- and endophytic bacteria associated with Prosopis juliflora and their potential as phytoremediation enhancing agents in metal-degraded soils. Front. Plant. Sci. 2015;1–10.
doi.org/10.3389/fpls.2014.00755
42. Audu K.E., Adeniji S.E., Obidah J.S.. Bioremediation of toxic metals in mining site of Zamfara metropolis using resident bacteria (Pantoea agglomerans): A optimization approach. Heliyon. 2020;14(8)1–7. 10.1016/j.heliyon.2020.e04704
43. Fahsi N., Mahdi I., Mesfioui A., Biskri L., Allaoui A.. Plant Growth-Promoting Rhizobacteria Isolated from the Jujube (Ziziphus lotus) Plant Enhance Wheat Growth, Zn Uptake, and Heavy Metal Tolerance. Agriculture. 2021;11(4)1–18.
doi.org/10.3390/agriculture11040316
44. Ramírez V., Baez A., López P., Bustillos R., Villalobos MÁ., Carreño R., Contreras J.L., Muñoz-Rojas J., Fuentes L.E., Martínez J., Munive J.A.. Chromium Hyper-Tolerant Bacillus sp. MH778713 Assists Phytoremediation of Heavy Metals by Mesquite Trees (Prosopis l
aevigata). Front. Microbiol. 2019;1–12. 10.3389/fmicb.2019.01833
45. Jeyakumar P., Debnath C., Vijayaraghavan R., Muthuraj M.. Trends in Bioremediation of Heavy Metal Contaminations. Environ. Eng. Res. 2023;28(4)1–19.220631.
doi.org/10.4491/eer.2021.631
46. Panhwar Q.A., Shamshuddin J., Naher U.A., Radziah O., Mohd Razi I.. Biochemical and molecular characterization of potential phosphate-solubilizing bacteria in acid sulfate soils and their beneficial effects on rice production. PLoS One. 2014;9(10)1–14.
doi.org/10.1371/journal.pone.0097241
47. Tang W., Gong J., Wu L., Li Y., Zhang M., Zeng X.. DGGE diversity of manganese mine samples and isolation of a Lysinibacillus sp. efficient in removal of high Mn (II) concentrations. Chemosphere. 2016;165:277–283. 10.1016/j.chemosphere.2016.08.134
48. Barboza N.R., Morais M.M.C.A., Queiroz P.S., Amorim S.S., Guerra-Sá R., Leão V.A.. High Manganese Tolerance and Biooxidation Ability of Serratia marcescens Isolated from Manganese Mine Water in Minas Gerais, Brazil. Front. Microbiol. 2017;9(8)1–11.
doi.org/10.3389/fmicb.2017.01946
49. Huang H., Zhao Y., Xu Z., Ding Y., Zhou X., Dong M.. A high Mn(II)-tolerance strain, Bacillus thuringiensis HM7, isolated from manganese ore and its biosorption characteristics. PeerJ. 2020;19:1–24.
doi.org/10.7717/peerj.8589
50. Dourado M.N., Ferreira A., Araújo W.L., Azevedo J.L., Lacava P.T.. The diversity of endophyticmethylotrophic bacteria in an oil-contaminated and an oil-free mangrove ecosystem and their tolerance to heavy metals. Biotechnol. Res. Int. 2012;2012:1–8. 10.1155/2012/759865
51. Elhaissoufi W., Ghoulam C., Barakat A., Zeroual Y., Bargaz A.. Phosphate bacterial solubilization: A key rhizosphere driving force enabling higher P use efficiency and crop productivity. J. Adv. Res. 2022;38:13–28. doi.10.1016/j.jare.2021.08.014
52. Afzal A., Bahader S., Hassan T.U., Naz I., Aziz-ud-Din . Rock Phosphate Solubilization by Plant Growth-Promoting Bacillus v
elezensis and Its Impact on Wheat Growth and Yield. Geomicrobiol J. 2022;40(2)131–142. 10.1080/01490451.2022.2128113
53. Bohu T., Santelli C.M., Akob D.M., Neu T.R., Ciobota V., Rösch P., Popp J., Nietzsche S., Küsel K.. Characterization of pH dependent Mn(II) oxidation strategies and formation of a bixbyite-like phase by Mesorhizobium australicum T-G1. Front. Microbiol. 2015;6:1–15. 10.3389/fmicb.2015.00734
54. Jofré I., Matus F., Mendoza D., Nájera F., Merino C.. Manganese-Oxidizing Antarctic Bacteria (Mn-Oxb) Release Reactive Oxygen Species (ROS) as Secondary Mn(II) Oxidation Mechanisms to Avoid Toxicity. Biology (Basel). 2021. 610p. 1–16.
doi.org/10.3390/biology10101004
55. Ipek M., Pirlak L., Esitken A., Dönmez M.F., Turan M., Sahin F.. Plant Growth-Promoting Rhizobacteria (PGPR) Increase Yield, Growth And Nutrition Of Strawberry Under High-Calcareous Soil Conditions. J. Plant Nutr. 2014;37(7)990–1001. 10.1080/01904167.2014.881857
56. Msimbira L.A., Smith D.L.. The roles of plant growth promoting microbes in enhancing plant tolerance to acidity and alkalinity stresses. Front. Sustain. Food Syst. 2020;4:1–14.
doi.org/10.3389/fsufs.2020.00106
57. Lopes M.J.S., Dias-Filho M.B., Gurgel E.S.C.. Successful Plant Growth-Promoting Microbes: Inoculation Methods and Abiotic Factors. Front. Sustain. Food Syst. 2021;5:1–13.
doi.org/10.3389/fsufs.2021.606454
58. Sharma A., Shahzad B., Kumar V.. Phytohormones Regulate Accumulation of Osmolytes Under Abiotic Stress. Biomolecules. 2019;9(7)1–36. 10.3390/biom9070285
59. Chen C., Xin K., Liu H..
Pantoea alhagi, a novel endophytic bacterium with ability to improve growth and drought tolerance in wheat. Sci. Rep. 2017;7:1–14. 10.1038/srep41564
60. Breedt G., Labuschagne N., Coutinho T.A.. Seed treatment with selected plant growth-promoting rhizobacteria increases maize yield in the field. Ann. Appl. Biol. 2017;171(2)229–236.
doi.org/10.3389/fsufs.2020.00136
61. Rufus J., Akinrinlola , Yuen G.Y., Drijber R.A., Adesemo A.O.. Evaluation of Bacillus Strains for Plant Growth Promotion and Predictability of Efficacy by In Vitro Physiological Traits. Int J. Microbiol. 2018;2018:1–11.
doi.org/10.1155/2018/5686874
62. Darko E., Végh B., Khalil R., Marček T., Szalai G., Pál M.. Metabolic responses of wheat seedlings to osmotic stress induced by various osmolytes under iso-osmotic conditions. PLoS ONE. 2019;14(12)1–19.
doi.org/10.1371/journal.pone.0226151
63. Anjum S.A., Ashraf U., Tanveer M., Khan I., Hussain S., Shahzad B., Zohaib A., Abbas F., Saleem M.F., Ali I.. Drought Induced Changes in Growth, Osmolyte Accumulation and Antioxidant Metabolism of Three Maize Hybrids. Front. Plant Sci. 2017;8:1–12. 10.3389/fpls.2017.00069
Fig. 1Manganese solublization potential of PGPR (Log CFU at 106) on agar nutrient media. Results are mean of two replicates. Different alphabets heading the bars represent significant difference among the treatment with ± standard error. 
Fig. 2Manganese solublization potential of PGPR (Log CFU at 108) on agar nutrient media. Results are mean of two replicates. Different alphabets heading the bars represent significant difference among the treatment with ± standard error. 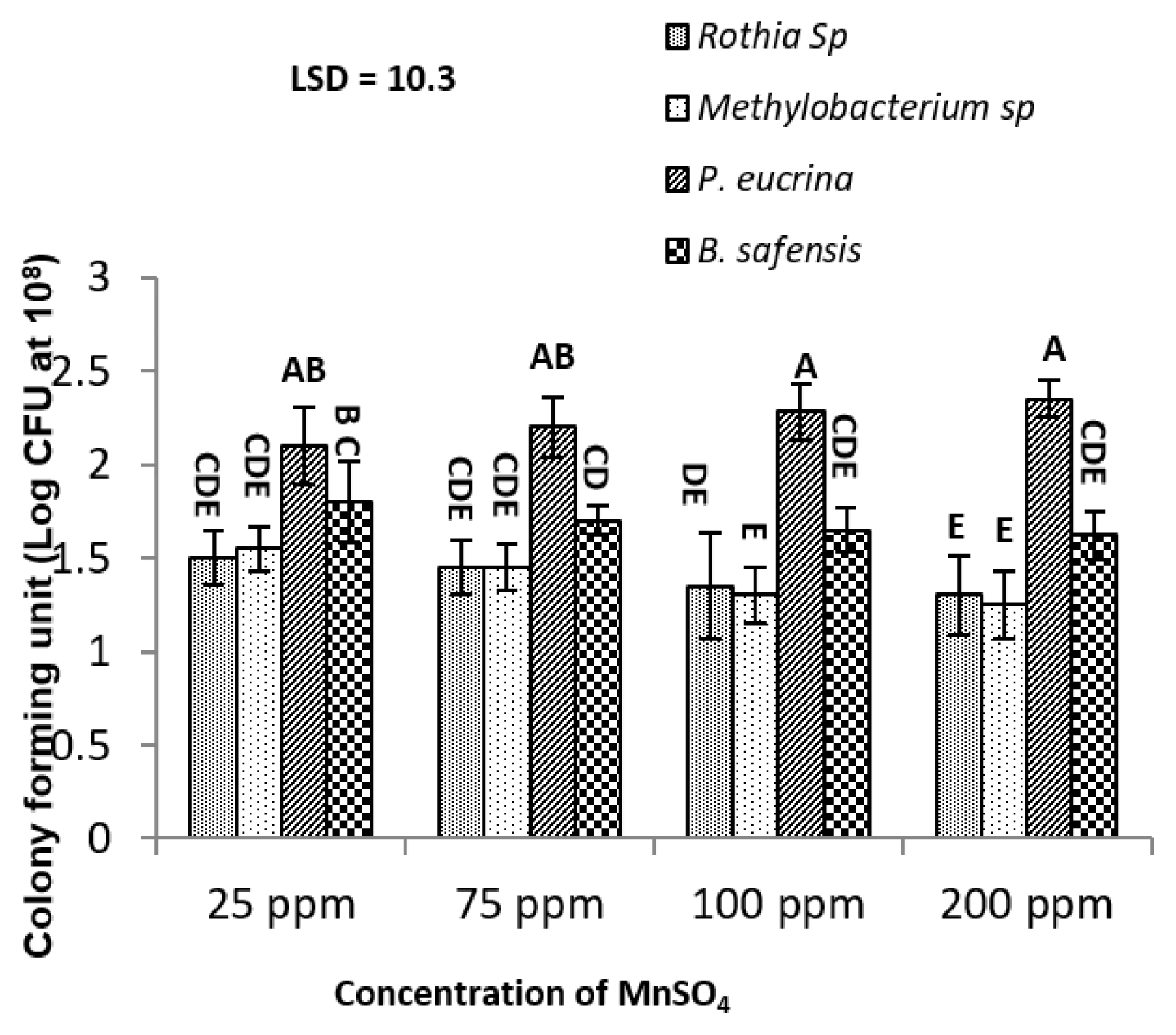
Fig. 3Indole acetic acid production (μg ml−1) of PGPR by clororimetric and High pressure liquid chromatography (HPLC). Values are mean of three replicates with ± standard bar. Values heading the bar with different alphabet reflect significant difference at (p=0.05). 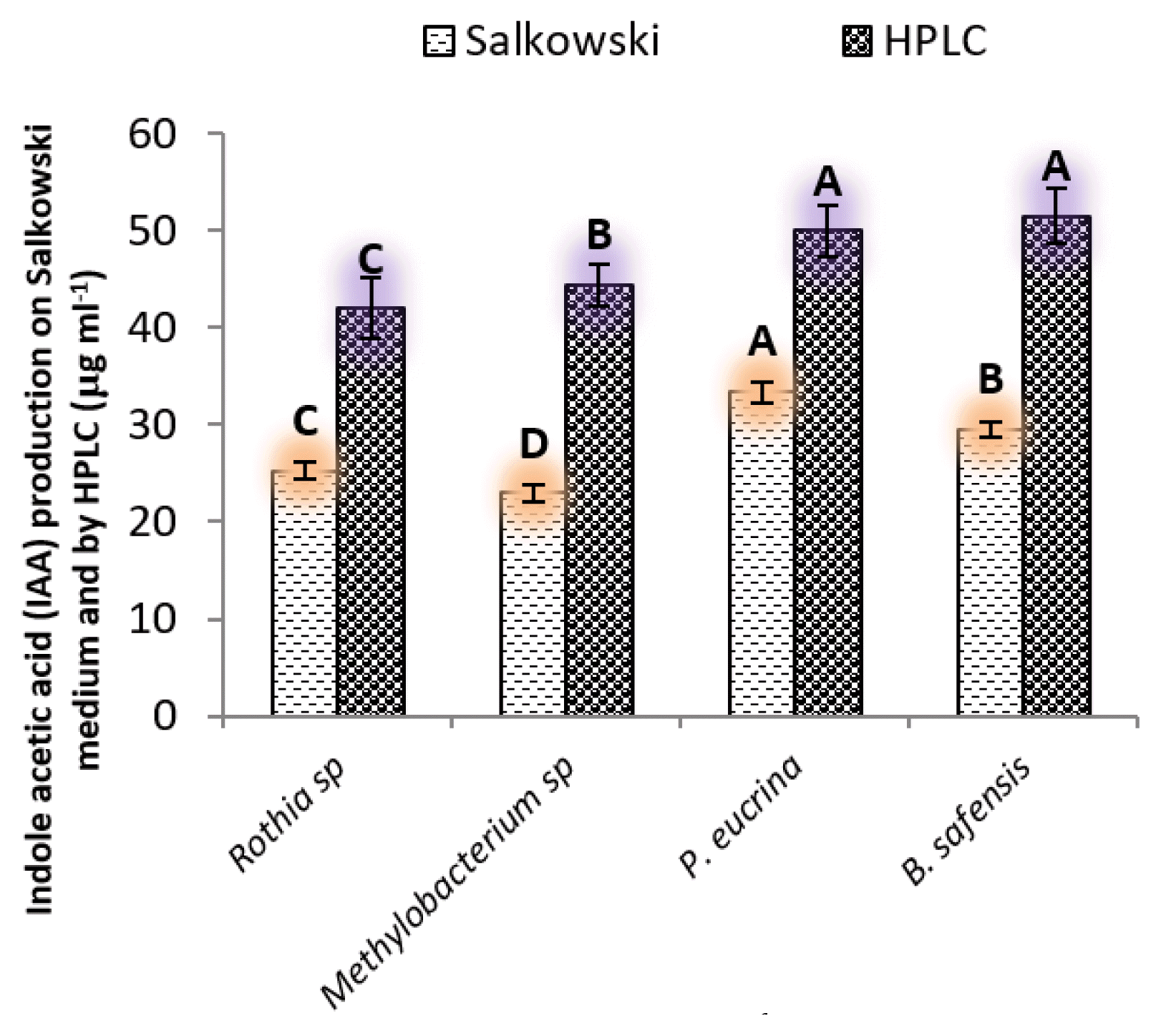
Fig. 4Organic acid production (μg ml−1) by different PGPR. Values are mean of four replicates with ± standard bar. Values heading the bar with different alphabet reflect significant difference at (p=0.05). 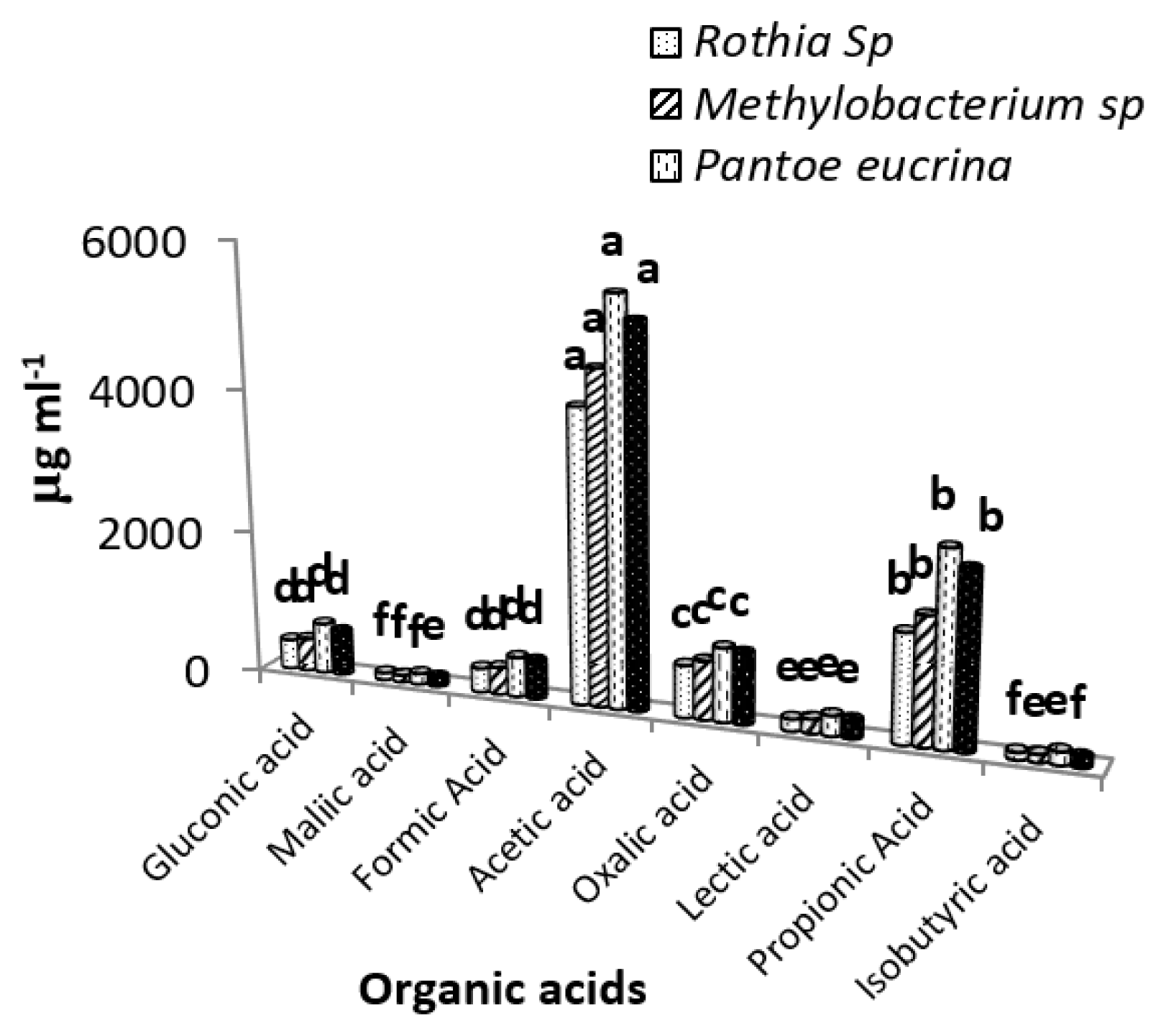
Table 1Effects of Different Treatments on Mn Contents of Soil, Roots and Leaves of Maize in a Greenhouse Experiment. Measurements were Made After 60 Days of Sowing. Values are Mean of Two Replicates with ± Standard Error. Different Alphabets Following the Mean Values Represent Significant Difference (p = 0.05) Table 2Effects of Different Treatments on Biological Accumulating Coefficient (BAC), Biological Concentration Factor (BCF), and Translocation Factor (TF) of Mn Contents Maize in a Greenhouse Experiment. Measurements were Made After 60 Days of Sowing. Values are Mean of Two Replicates with ± Standard Error. Different Alphabets Following the Mean Values Represent Significant Difference (p = 0.05) Table. 3Effects of Different Treatments on Growth Parameters of Maize Grown Under Mn Stress in Greenhouse Experiment. Measurements were Made After 60 Days of Sowing. Values are Mean of Two Replicates with ± Standard Error. Different Alphabets Following the Mean Values Represent Significant Difference (p = 0.05) Table. 4Effects of Different Treatments on Physiology of Maize Grown Under Mn Stress in Greenhouse Experiment. Measurements were Made After 60 Days of Sowing. Values are Mean of Two Replicates with ± Standard Error. Different Alphabets Following the Mean Values Represent Significant Difference (p = 0.05) |
|
||||||||||||||||||||||||||||||||||||