AbstractThis study reinforced polyvinyl alcohol/sodium alginate (PVA/SA) gel beads immobilizing aerobic sludge with graphene oxide (GO) as a nanofiller. The different types of beads were made by various concentrations of GO (0.02, 0.2, 2, 20, and 200 mg/L). The effect of GO on beads structure was observed by comparing the mechanical properties of chemical oxygen demand (COD) and NH4+-N removal efficiencies. The results showed that the PVA/SA/GO gel carriers have excellent reinforcement properties compared to non-GO gel carriers, while sphericity factor and methylene blue absorption are the same. The strongest carrier contains 200 mg/L GO, referred to as GO6. In addition, the highest COD and NH4+-N removal efficiencies of GO6 gel carriers were 97 and 96.67%, respectively, which is higher than a non-GO PVA/SA (GO1) gel carrier. The addition of GO (200 mg/L) was thus an effective way to improve the mechanical strength of PVA/SA gel beads due to the strong interaction between GO and the PVA/SA matrix. It can also promote COD and NH4+-N removal efficiency of immobilized bacteria inside the beads
Graphical Abstract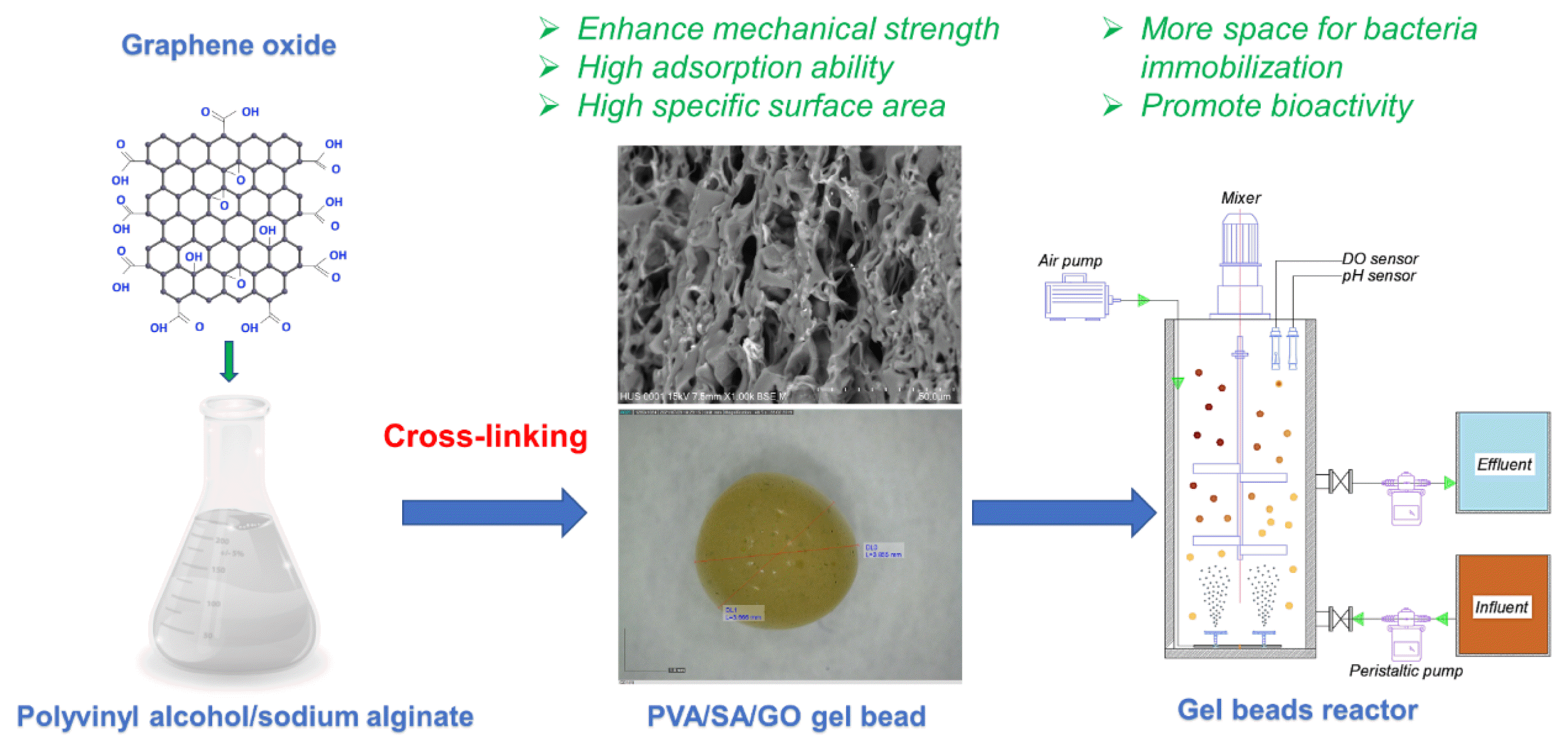
1. IntroductionWastewater discharge (e.g., industrial wastewater or brine) can degrade water quality, preventing it from being used directly for potable water (via desalination) and industrial applications. Therefore, wastewater treatment is crucial to protect the environment and water recovery and reuse [1–3]. Biological treatment is one of the most popular and effective in conventional wastewater treatment systems. Besides, the immobilization of activated sludge in spherical polymeric matrices has recently been widely studied in biological wastewater treatment because it can improve conventional biological wastewater treatment processes. Compared with conventional free-cell processes, immobilization has several advantages: it can maintain a high concentration of biomass and protect microorganisms from the negative impact of the environment [4], and it has reusability and relative ease of liquid-solid phase separation [5, 6].
Polyvinyl alcohol (PVA) is one of the most suitable materials for activated sludge immobilization because it is non-toxic and has biocompatibility, substrate absorption, and low manufacturing cost. Besides, PVA has usually been combined with sodium alginate (SA) to reduce agglomeration, so PVA gel beads can be used in a suspended biological reactor for treating wastewater [5]. On the other hand, PVA gels have poor mechanical properties, limiting their long-term operation. This limitation has been resolved by methods, as shown in Table 1. However, these methods can have adverse effects on immobilized bacteria. Testing new methods to improve PVA’s durability is necessary to put this technology into practice.
Adding nanofiller can overcome the limitations of PVA [7], such as metals, silicon dioxide, zinc oxide, copper oxide, and carbon nanotubes [8]. Graphene oxide (GO) is also attractive as a reinforcement nanofiller because of its low density and high mechanical strength. GO has a carbon skeleton structure with many hydrophilic oxygen-containing functional groups. Therefore, a high-performance composite material can be prepared with high strength, modulus, low thickness, and lightweight [9]. Seira Morimune (2012) [10] found that adding GO made the PVA/GO nanocomposite have significantly high mechanical strength and thermal properties. Besides, other studies revealed that GO is a super biocompatible material that can create a suitable or nontoxic environment for bacteria. Its large specific surface area can also provide higher hydrophilicity and reactive activity for carriers. Sha (2020) [11] reported that the NH4+-N removal efficiency increased with 5–25 mg/L of GO in a sequencing batch reactor (SBR). Guizhong Zhou [12] successfully tested the application of GO-modified PVA gel beads to immobilize microbial biomass treating high-salinity wastewater. To the best of my knowledge, there are currently very few studies that have conducted experiments to select the appropriate GO concentration for making PVA/SA gel beads and have not evaluated at the same time the mechanical strength as well as the bioremediation efficiency of the beads.
This study was thus to evaluate the utilization of GO with different concentrations as a nanofiller to enhance the mechanical strength and other physical properties of PVA/SA gel beads for immobilizing aerobic bacteria. From the results, the most suitable GO concentration for gel fabrication was selected to obtain gel beads with high mechanical strength. Finally, the COD and NH4+-N removal efficiency of gel beads was measured in batch experiments to know the biocompatibility of GO with immobilized bacteria.
2. Materials and Methods2.1. MaterialsPolyvinyl alcohol was bought from Kuraray Co., ltd India (Grade is 98%–100%, polymerization degree is 2000). Sodium alginate (Shanghai Zhanyun Chemical Co., Ltd), boric acid, sodium sulfate, and calcium chloride were bought from Sigma-Aldrich USA. All compounds were utilized as supplied with no additional purification. The GO nanosheet synthesized by Hummer methods, as described in the report of Dinh [13] was provided by Phenikaa University Nano Institute, Ha Noi, Viet Nam.
2.2. Gel Beads PreparationThe activated sludge was collected from an anaerobic/anoxic/ anaerobic wastewater treatment system in Ha Noi, Viet Nam. Then the sludge was mixed with distilled water and collected after the settling step. This procedure was repeated until the concentrations of COD, NH4+, NO2−, and NO3− in the sludge were nearly zero, so it could not affect the results of the experiments. The final mixed liquor suspended solids (MLSS) and mixed liquor volatile suspended solid (MLVSS) of the sludge were 18,410±350 mg/L and 13,650±264 mg/L.
The immobilization process was described in our previous study [14]. A mixture of 15% PVA (w/v) and 2% SA (w/v) was dissolved at 121°C for 20 minutes. Different volumes of 1.5 g/L GO solution were added to get a PVA/SA/GO blend to get GO concentrations of 0 (GO1), 0.02 (GO2), 0.2 (GO3), 2 (GO4), 20 (GO5), 200 (GO6) mg/L. Then, these blends were mixed with the same volume of the sludge and crosslinked in boric acid 7% and CaCl2 2% (w/v) solution for bead formation. These beads were dipped for 1 hour with magnetic stirring, and immersed in 0.5 Na2SO4 for an hour. Lastly, a large amount of water was used to clean gel beads before being preserved at 4°C in water [14]. The selection of the right gel bead formulation was made based on the comparative results of the physicochemical characteristics of the gel beads, ensuring a balance between mechanical strength and absorption capacity.
2.3. Characteristics of Gel Beads2.3.1. Sphericity factor of the gel beadsA digital camera (Dino-2Mp-1000X) was used to capture images of gel beads (Fig. S1). The diameter of the beads was determined by software (MicroCapture) to measure the sphericity factor (SF) as Eq. (1).
where dmax and dmin are the maximum and minimum diameters of gel beads.
2.3.2. Swelling ratioPVA/SA/GO gel beads were immersed and gently mixed in a beaker with distilled water at ambient temperature. The diameter of gel beads was measured to calculate the volume until they reached the equilibrium state. The swelling ratio (SR) was then calculated by Eq. (2). [5].
where V0 and Vt are volumes of the beads on the first and final days, respectively.
2.3.3. Mechanical strengthOne hundred gel beads were mixed by a high-speed agitation unit (1000 rpm) with four blades for 10 minutes in 100 ml of distilled water. Counting the number of intact gel beads is very hard, while the total chemical oxygen demand (TCOD) concentration is caused by debris after mixing [15]. Therefore, the TCOD of the mixed solution was used to represent the mechanical strength of the beads as a dependent variable. The supernatant was sampled to measure TCOD by standard methods (method 5220 D) [16].
2.3.4. Compressive strength of gel beadsThe compressive strength of gel beads was determined by an Instron 6900 Universal Testing system. The system includes a stainless steel plate to place the bead and a cylindrical steel plate to compress the bead. The latter moved continuously during the compressive test at a 1 mm/min speed. Each type of bead was tested three times under the same conditions. After the beads were broken, the plate was controlled to return to the first position. The displacement of the bead was measured base on the distance between the first and final position of the test.
2.3.5. Methylene blue absorption100 gel beads were immersed in a methylene blue (MB) solution (20 mg/L). A magnetic stir was used to mix the mixture for 6 hours at room temperature to reach equilibrium. Then, the supernatant was collected and measured for absorbance at 665 nm by a UV–Vis spectrophotometer (HACH DR600). The changes in absorbance were recorded to calculate the absorption capacity coefficient (qMB) and the MB removal efficiency (RMB) using Eq. (3) and (4), respectively [17]:
where C0 and Ce are the first and final concentrations (mg/L); V is the volume of samples (L), and m is the weight of beads (g).
2.3.6. Morphology, chemical structure, and specific surface area of gel beadsAs described by Zhang, a drying procedure using phosphate buffer, glutaraldehyde, and ethanol solutions was applied to pretreat gel beads [18]. Then, the surface morphology and cross-sectional images of GO6 and GO1 gel beads were observed using TM-4000 (Hitachi, Japan) scanning electron microscopy (SEM) at 15kV. The functional groups of pure PVA, pure SA, and PVA/SA/GO composite were confirmed by a Fourier-transform infrared spectrometer (Jasco FT-IR model 4600, Japan). The specific surface area and pore distribution of gel beads were analyzed using the Brunauer–Emmett–Teller (BET) equation using the NOVAtouch LX4 analyzer (Quantachrome Instruments, USA).
2.4. Biological Activity of Immobilized Activated Sludge in Gel Beads2.4.1. Oxygen uptake rate – OUR200 ml of gel beads were immersed in an air-tight reactor containing 800 ml of wastewater (2000 mg/L COD and 100 mg/L NH4+-N). The reactors were aerated to get saturated dissolved Oxygen (DO) through an air diffuser. Then the aeration was stopped, and DO was measured by a DO meter (YSI5000-230V). A DO-t curve was drawn after the experiment was finished. The OUR can be calculated by Eq. (5). [18]:
where OUR is the oxygen uptake rate (mgO2/L.min); DO1, and DO2 are saturated, and the final DO value (mgO2/L); t is time (min).
2.4.2. Batch experiment procedureTwo 3L reactors with 30% of the beads were used for a batch experiment in 15 days, as described in Fig. 1. Each batch of the experiment included 4 steps: fill, aeration, settle and withdraw. The exchange ratio of the reactors was 50%. Peristaltic pumps (Masterflex) were used to fill wastewater into the reactor (through the bottom valve) and withdraw treated water through the middle valve, pumping wastewater into the reactors and collecting the effluent of the PN reactor. Disslove oxygen for the aeration step was supplied by an air compressor and air diffuser (at the bottom of the reactors). An air flowmeter controlled the air flow rate of the reactors and DO was maintained at the value of higher than 2 mg/L. DO, PH, and temperature sensors (HACH) were equipped to monitor these parameters of the reactors. All the equipment of the system was controlled through an electric control box.
A cycle of 24 hours with 30 minutes of filling, 23 hours of aeration, 10 minutes of sedimentation, and 20 minutes of the drain was applied. Thus the hydraulic retention time (HRT) was 48 hours. The organic loading rate and nitrogen loading rate of the reactors were 0.5 kgCOD/m3/day and 0.04 kgN/m3/day, respectively. The synthetic wastewater contained: COD 2000 mg/L; NH4+-N 120 mg/L; KHCO3 600 mg/L; KH2PO4 23 mg/L; CaCl2·2H2O 30 mg/L, MgSO4·7H2O 20 mg/L; FeSO4 6 mg/L; EDTA 3 mg/L and 1 mL/L of trace element solution as described in previous studies [14].
The concentrations of NH4+-N, NO3−-N, NO2−-N, and COD were measured by spectrophotometer (methods 4500 F) according to standard methods [16] ((methods 4500-NH3 F, 4500-NO3 B, 4500-NO2 B, and 5220 D, respectively) to evaluate the performance of bacteria inside the beads.
2.5. Statistical AnalysesIn this study, SPSS IBM 26 was applied for statistical analysis. The normality of data was examined by The Kolmogorov Smirnov. T-test and one-way ANOVA were applied to compare the differences between data in experiments. Tukey’s post-hoc test was applied to determine the significant differences between samples. Spearman’s rank correlation coefficients measured the relationship between two variables in SR and mechanical strength experiments. The 0.05 significance level was chosen for all tests.
3. Results and Discussion3.1. Effect of Graphene Oxide Concentration on Characteristics of Gel Beads3.1.1. Sphericity factorAll types of gel beads showed a pear-shape with uniform size and had SF values higher than 0.15, as shown in Fig. 2. Gel beads are nearly spherical if the sphericity coefficient is less than 0.05, while the value of an elongated bead is 0.1 [19]. The shape of the gel beads has a significant impact on their mechanical strength. Compared to spherical beads, nonspherical beads have lower mechanical strength [20]. Qi Gao [21] observed the deformation and breakage of the tear-shape gel beads with tails and irregular shape gel beads, causing the immobilized biomass to be released.
On the other hand, the results showed a significant difference between 0.23±0.01 SF of GO3 and 0.17 ±0.01 SF of GO6 (p<0.05), while there was no significant difference between SF of the others type of beads. However, this result does not clearly show the effect of GO on the shape of the gel beads. The major parameters of gel beads preparation that have a major impact on gel beads shape were surface tension, and viscosity of polymer solution, dripping tip size, dropping gap, and properties of gelation bath [22].
3.1.2. Swelling ratioFor a long-term operation in wastewater, the gel beads must be highly stable in water. The absorption of too much water can reduce the mechanical strength of the gel beads. Examining the SR of gel beads is thus very important. Gel bead swelling suppression may prevent the gel from deforming and maintain its mechanical strength [23]. The SR value of different hydrogel beads is shown in Fig. 2. The SR of GO3 beads was the largest, with a value of 324.67±3.3%. However, the SR of the PVA/SA/GO beads decreased when the GO concentration grew from 0.2 to 200mg/L. The SR of GO6 gel beads was 231±2.16%, 1.35 times lower than GO1, which was 313±2.94. The lower was probably due to the hydrogen bonds between functional groups of GO and PVA macromolecules [24]. This interfacial interaction could limit the water absorption of the polymer matrix in water [25], leading to a lower SR of the GO6 gel beads. A significant correlation between SR and GO concentration proved the interaction, as is shown in Table S1.
On the other hand, there are two possible reasons for the high SR of GO2 to GO4 gel beads: (1) under low concentration of GO, their hydrophilic groups may combine with more water molecules [7], and (2) PVA has a low degree of physical crosslinking [22]. The GO6 had the lowest SR, so that it may have better mechanical strength than the others. Thus the GO6 was more stable and could not be readily destroyed.
3.1.3. FTIRThe FT-IR spectrum of gel beads was analyzed to observe the interaction between functional groups of PVA, SA, and GO in gel beads. All samples exhibited a board peak at 3427 and 2944 cm−1 corresponding to −OH stretch and −CH stretch, respectively, as shown in Fig. 3. In the figure of SA, the peaks near 1606 and 1420 cm−1 were caused by symmetric and asymmetric stretching vibrations of −COO, and peaks at 1035 cm−1 related to −CO groups. With PVA, the peaks at 1717 cm−1 and 1635 cm−1 corresponded to the stretching of C=O and C-O from the acetate group [26]. The peak at 1099 indicated the presence of secondary alcohols −CO stretch vibration and −OH bending vibration in the amorphous regions [27], which are sensitive to hydrogen bonding. These peaks shifted to a lower wavenumber, and their intensity slightly decreased in the spectrum of PVA/SA (GO1). These changes depicted a possible hydrogen bond formation between PVA and SA [28]. FTIR spectrums of GO2 to GO6 were similar to GO1, except for a slight decrease of −OH stretching peak of GO1 from 3421 cm−1 to 3406-3396 cm−1. Based on these results, hydrogen bonds must existed between the PVA/SA matrix and the GO [29].
3.1.4. SEM and specific surface area
Fig. 5a and 5c show that GO1 gel beads had a nearly spherical shape and a uniform grain distribution with small holes (under 1 μm) evenly distributed on a smooth surface. Besides, they had a reticulate structure. These characteristics favored the diffusion of substrates and metabolites for the growth of bacteria immobilized inside beads. In contrast, GO6 beads showed a rough surface and had no apparent spherical shape, while they also had a uniform grain distribution. In particular, they had a lamellar structure instead of a reticulate structure, which can support the attachment and growth of bacteria. These differences in the morphology and structure of gel beads demonstrated the presence of graphene oxide and the formation of an improved degree of crosslinking. The uniform dispersion and high interaction of GO nanosheet with the PVA/SA polymer chain via hydrogen bonds could be the main reason for the improved cross-linking degree [30].
As shown in Table 2, the average pore size of non-GO gel beads was 12 nm, which was three times higher than GO6 gel beads (3 nm). However, the specific surface area and pore volume of GO6 beads were significantly higher than those of the GO1 beads, shifting from 12.2655 m2/g and 0.0487343 cc/g to 19.6874 m2/g and 0.0785862 cc/g, respectively. These results indicated that the GO nanosheet dispersion also affects the specific surface area and pore size of gel beads since GO nanosheet is well known for its large specific surface area [31]. On the other hand, GO6 beads had smaller pores, which might reduce the SR of beads [30], as observed in Fig. 2. GO6 beads also could provide an ideal condition for preventing the washout of immobilized bacteria and promoting their growth because of their mesoporous structure and high surface area. Moreover, few studies reported that PVA/SA/GO structure could help immobilized bacteria maintain high bioactivity under adverse environment conditions, such as high salinity wastewater [12], and low temperature [5].
3.1.5. Methylene blue absorptionThe water absorption and nutrient diffusion are directly related to the absorption capacity of the PVA/SA gel beads due to the high number of −OH groups. The results showed that adding GO to the PVA/SA gel beads did not cause significant differences in qMB and RMB. There was a slight increase in qMB and RMB when the GO concentration rose from GO2 to GO6. The highest absorption coefficient was observed in GO5 (0.8795±0.05 mg/g). The highest removal rate was observed in GO5 and GO6 (Fig. 3). When GO concentration rose, more crosslinking points in PVA/SA hydrogels were created by hydrogen bonding interactions between the GO sheets and PVA/SA chains, resulting in reduced pore size and increased absorption sites [32]. These changes were also observed in this study, as dicussed in section 3.1.4, and they could be the reason for the slight increase in the absorption ability of the gel beads. Cuiyun Liu [32] also reported that many functional groups were included in GO nanosheets. These groups can also interact with MB molecules via electrostatic or hydrogen bonding interactions, improving MB absorption. However, statistical analysis revealed that these differences were unrelated to GO concentration (p>0.05) or the presence of GO did not affect significantly the absorption capacity of the PVA/SA gel beads in this study. This could be due to the lower concentration of GO compared with other studies, so the MB adsorption capacity of GO is not clearly shown. Beside, BanachWiśniewska (2021) [25] also observed that temperature could significantly affect the RMB, but not GO concentration. A pH lower than 4 could affect the surface charge of the PVA/SA/GO matrix and the MB ionization degree, leading to a reduction of MB absorption capacity of the beads [33].
3.2. Effect of Graphene Oxide Concentration on Mechanical Properties of Gel Beads3.2.1. The mechanical strengthThe mechanical strength (Fig. S2) was improved with the increase of GO concentration from 0 to 200 mg/L. The highest mechanical strength (GO6 with 200 mg/L of GO) was equivalent to the lowest COD concentration of a stirred solution (34.33±1.7). In contrast, the highest COD concentration was of GO1 hydrogel beads with a value of 160.67±2.49 mg/L. This significant correlation between GO concentration and mechanical strength was also observed in Table S2. Morimune (2012) [10] reported no apparent impact of GO on the crystallinity degree of gel beads. The compatibility between the PVA and the GO was the main reason for the high mechanical strength of PVA/SA/GO hydrogel beads compared with non-GO PVA/SA beads. Strong interactions provided higher load transfer between the PVA and the GO and leaded to the mechanical enhancement of gel beads. However, the agglomeration of GO nanosheet can occur, and GO can not fully disperse into the PVA/SA matrix when the GO concentration is too high. As a result, there are not enough hydrogen bonds to create the reinforcement effects on the shear stress of the PVA/SA/GO beads [7]. This phenomenon was observed when the GO concentration was higher than 0.8% [34]. This value is much higher than the GO concentration in this study. Therefore, the increase in the gel beads’ mechanical strength and GO concentration are consistent in this study.
3.2.2. The compressive strength of gel beadsIn the compressive strength experiment, a gel bead was compressed until it broken, and then the compressional plate returned to zero states. Fig. S3 shows relative compressive force-displacement curves of different types of gel beads. Regardless of the rise in GO concentration, the curves had the same overall form. However, the maximum compressive force and displacement differed between types of beads. The former increased with the rise of GO concentration. GO6 gel beads had the highest maximal force (8.01 N) compared to 2.1 N of GO1 gel beads. In contrast, the displacement decreased with the reduction of GO concentration. The displacement of GO6 (80%) was marginally lower than GO5 and GO4 (84% and 86%) and considerably lower than GO1 (90%). In other words, the beads were broken at lower displacement and higher GO concentration. These results suggested that the PVA/SA/GO beads are stiffer and less elastic than PVA/SA gel beads. This property can be confirmed with Young’s Modulus value, as shown in Fig. S3. Young’s Modulus rised with the increase of GO concentration from 2.14 Mpa of GO1 to 7.34, 11.58, 13.91 of GO4, GO5, and GO6, respectively. This correlation has also been observed in various reports studying PVA and GO composites [35–37], and can be attributed to the dispersion and interaction of GO nanosheets in the hydrogel network of the PVA/SA matrix. As a result, the movement of the polymer chains had been limited, and higher stress was required to change the form of gel beads. Besides, the beads became stiffer because of the higher occupation of GO nanosheets in the hydrogel network than water molecules.
3.3. Effects of Graphene Oxide on the Wastewater Treatment Efficiency of Activated Sludge Immobilized in the Gel BeadsThe biological wastewater treatment process using activated sludge is based on the activity of microorganisms, many of which are active in aerobic conditions. There are two possible ways for activated sludge to consume oxygen: (1) heterotrophic bacteria use oxygen to oxide organic compounds, and (2) autotrophic bacteria use oxygen to oxide ammonia and nitrite. Therefore, assessing the access to free oxygen of microorganisms in gel beads is crucial to ensure their activities. As mentioned in the description of the experiment (2.4), the reactors were air-tight to prevent the loss of oxygen to the environment. At this time, the decline in oxygen levels was mainly related to the activities of immobilized bacteria. The difference in DO consumption between beads could represent the difference in the activity of bacteria inside the gel beads, as shown in Fig. S4. OUR was thus calculated based on DO consumption to express the activity of bacteria. The OUR of GO1 and GO6 were 12 mgO2/L/min and 18.6 mgO2/L.min, respectively (Table S3). A higher OUR could be due to increased immobilized activated sludge concentration [38]. However, the activated sludge concentration of GO1 and GO6 were the same in this study. It is possible that the bacteria in the matrix of GO6 could oxide more COD and ammonia than the bacteria in the GO1 matrix. Finally, these beads were applied in a batch experiment to prove this prediction.
GO6 and GO1 gel beads were filled in separated biological reactors to evaluate the effect of GO on the COD and NH4+-N removal efficiency of immobilized activated sludge. The COD and NH4+-N removal efficiency of the two bead types during a cycle is illustrated in Fig. 6. In the GO6 reactor, the COD concentration fell sharply from 2000 to 818±2 mg/L after 9 hours. At that point this figure started to fall gradually to 117±6 mg/L after 24 hours. At the same time, the COD concentration in the GO1 reactor was always lower than in GO6, which reveals that GO6 can remove COD faster than GO1 biomass. In contrast, the NH4+-N concentration of NH4+-N of GO1 and GO6 reactors decreased slightly from 119.8±0.65 mgN/L and 120.5±0.7 mgN/L to 87.15±0.95 and 90.5±0.5 mgN/L, respectively after 16 hours, followed by a significant drop to 5.5 and 12.75 mgN/L at the end of the cycle. The batch experiment was kept running for 15 days to evaluate the gel beads’ performance stability. The COD removal efficiency (95.83–97%) and NH4+-N removal efficiency (94.44–96.89%) of GO6 were higher than that of GO1 (94–96.43% and 90.16–94.6%, respectively). In an aerobic reactor with gel beads, a decrease in COD could be due to bio-degradation, absorption, and air stripping. An reported that organic compounds were mainly removed by immobilized activated sludge, while less than 10% of the organic compounds were treated by absorption and air stripping [4]. Thus, it can be revealed that much of the COD and ammonium removal efficiency was achieved due to immobilized microorganisms’ activity.
On the other hand, the results suggested that GO6 beads achieved a higher and faster substrate removal efficiency. The first is that the high specific surface areas of GO6 beads can provide more space for bacteria can survive and grow. Zhou [12] reported that the lamellar structure of PVA/SA/GO beads could be more useful for supporting the entrapment and growth of bacteria than the structure of pure PVA hydrogels. This type of structure was also observed in this study, as shown in Fig. 4F. The second reason is that a small amount of GO can positively affect bacteria growth due to the relationship of GO and extracellular polymeric substances (EPS) [11, 39, 40]. Dong Wang reported that the ammonium removal efficiency also increased when the presence of GO in water with concentrations between 0.05 and 0.1 mg/L increased the EPS content of the activated sludge [40]. EPS are the important components of activated sludge. EPS can significant affects the aggregation, settling, and dewatering properties of sludge; EPS also affects microbial metabolism and pollution clearance by transferring mass between cells and surroundings.
With significantly improved mechanical strength and microbial activity, GO/PVA/SA gel beads have the potential for wide application in the field of wastewater treatment. The gel beads can be used in aerobic, anoxic, and anaerobic conditions without secondary settling tanks. As a result, investment costs can be significantly reduced, and the system becomes more compact. Besides, immobilization by high-strength GO/PVA/SA gel also has the potential to replace the moving bed biofilm reactor (MBBR), which is a technology that often uses carriers made from non-biodegradable plastic such as high-density polyethylene (HDPE). When these carriers are no longer used, they become plastic waste and pollute the environment. Meanwhile, PVA and SA are both biodegradable materials; thus their use will contribute to sustainable development in the environmental field. However, gel beads were initially tested on synthetic wastewater and the operation time was short. In further studies, it is necessary to test the application of PVA/SA/GO gel beads treating some real wastewater and long-term operation to find the appropriate technical parameters for optimal treatment performance of the gel beads.
4. ConclusionsThe different concentrations of GO were introduced to improve the physical properties of PVA/SA beads, which were used as biomass carriers for immobilizing activated sludges. The addition of GO did not affect SF, but it could limit the swell of the bead in the water. The lowest SR value was 231±2.16% of GO6 gel beads, while FTIR images confirmed the strong interactions between GO and PVA/SA matrix functional groups. These results demonstrated the ability to improve the mechanical strength of gel beads by using GO as a nanofiller. The mechanical strength of gel beads increased when the GO concentration increased from 0 to 200 mg/L. GO6 gel beads have the highest maximal compressive force (8.01 N) compared to 2.1 N of GO1 gel beads. In addition, the SEM figure also showed that GO also transforms the structure of the PVA/SA gel beads. GO6 beads had smaller pores (3 nm) and a higher specific surface area (19.6874 m2/g) than GO1. As a result, the adverse effects of GO on bacteria were reduced, while the bioactivity of immobilized bacteria was improve. The reactor using GO6 gel beads obtained higher removal efficiency than the GO1 reactor. The highest COD and NH4+-N removal efficiencies of the GO6 reactor were 97% and 96.89%, respectively. These results indicated that PVA/SA/GO can be a promising technology to improve conventional biological wastewater treatment processes. Significantly, the addition of GO with 200 mg/L of concentration could thus improve both the mechanical strength of gel beads and the bioactivity of bacteria, unlike the other methods (Table 1). Future studies may need to test the use of PVA/SA/GO gel beads in immobilizing specific groups of bacteria such as Anammox and apply the gel beads on real wastewater for a long time of operation to evaluate the effectiveness and stability of PVA/SA/GO gel beads.
AcknowledgementsNguyen Van Tuyen was funded by Vingroup Joint Stock Company and supported by the Ph.D. Scholarship Programme of Vingroup Innovation Foundation (VINIF), Vingroup Big Data Institute (VINBIGDATA), code 2021.TS.112.
NotesAuthor contributions D.H.A (Professor) and T.DQ (Professor) provided research ideas and supervised the experiments. T.N.V (Ph.D. student) conducted the experiments, wrote and edit the manuscript. T.T.H (Ph.D), Q.C.X (Ph.D) and T.H.V ((Ph.D) assisted the data analysis and manuscript writing. H.G.K (Ph.D) reviewed the manuscript and suggested modifications. References1. Panagopoulos A. Brine management (saline water & wastewater effluents): Sustainable utilization and resource recovery strategy through Minimal and Zero Liquid Discharge (MLD & ZLD) desalination systems. Chem. Eng. Process.: Process Intensif. 2022;176:108944.
https://doi.org/10.1016/j.cep.2022.108944
2. Panagopoulos A, Giannika V. Decarbonized and circular brine management/valorization for water & valuable resource recovery via minimal/zero liquid discharge (MLD/ZLD) strategies. J. Environ. Manage. 2022;324:116239.
https://doi.org/10.1016/j.jenvman.2022.116239
3. Panagopoulos A. Process simulation and analysis of high-pressure reverse osmosis (HPRO) in the treatment and utilization of desalination brine (saline wastewater). Int. J. Energy Res. 2022;46(15)8607.
https://doi.org/10.1002/er.8607
4. An M, Lo KV. Activated sludge immobilization using the PVA-alginate-borate method. J. Environ. Sci. Health A. 2001;36(1)101–115.
https://doi.org/10.1081/ESE-100000475
5. Takei T, Ikeda K, Ijima H, Kawakami K. Fabrication of poly (vinyl alcohol) hydrogel beads crosslinked using sodium sulfate for microorganism immobilization. Process Biochem. 2011;46(2)566–571.
https://doi.org/10.1016/j.procbio.2010.10.011
6. Pattaraporn K, Chaiwat R, Panida N, Tawan L. Application of cell immobilization technology to promote nitritation: A review. Environ. Eng. Res. 2020;25(6)807–818.
https://doi.org/10.4491/eer.2019.151
7. Tao CA, Hao Z, Fang W, Hui Z, Xiaorong Z, Jianfang W. Mechanical properties of graphene oxide/polyvinyl alcohol composite film. Polym. Polym. Compos. 2017;25(1)11–16.
https://doi.org/10.1177/096739111702500102
8. Aslam M, Kalyar MA, Raza ZA. Polyvinyl alcohol: A review of research status and use of polyvinyl alcohol based nanocomposites. Polym. Eng. Sci. 2018;58(12)2119–2132.
https://doi.org/10.1002/pen.24855
9. Wu X, Xie Y, Xue C, et al. Preparation of PVA-GO composite hydrogel and effect of ionic coordination on its properties. Mater. Res. Express. 2019;6(7)075306.
https://doi.org/10.1088/2053-1591/ab11ee
10. Morimune S, Nishino T, Goto T. Poly (vinyl alcohol)/graphene oxide nanocomposites prepared by a simple eco-process. Polym. J. 2012;44(10)1056–1063.
https://doi.org/10.1038/pj.2012.58
11. Sha Y, Liu J, Yu J, et al. Effect of graphene oxide on the ammonia removal and bacterial community in a simulated wastewater treatment process. Int. J. Environ. Eng. 2020;146(9)04020097.
https://doi.org/10.1061/(ASCE)EE.1943-7870.0001781
12. Zhou G, Wang Z, Li W, Yao Q, Zhang D. Graphene-oxide modified polyvinyl-alcohol as microbial carrier to improve high salt wastewater treatment. Mater. Lett. 2015;156:205–208.
https://doi.org/10.1016/j.matlet.2015.05.110
13. Dinh NX, Pham TN, Tran QH, et al. Ultrasensitive determination of chloramphenicol in pork and chicken meat samples using a portable electrochemical sensor: effects of 2D nanomaterials on the sensing performance and stability. New. J. Chem. 2021;45(17)7622–7636.
https://doi.org/10.1039/D1NJ00582K
14. Nguyen VT, Ryu JH, Yae JB, Kim HG, Hong SW, Ahn DH. Nitrogen removal performance of anammox process with PVA–SA gel bead crosslinked with sodium sulfate as a biomass carrier. J. Ind. Eng. Chem. 2018;67:326–332.
https://doi.org/10.1016/j.jiec.2018.07.004
15. Bae HK, Yang HJ, Choi MK, Chung YC, Lee SH, Yoo YJ. Optimization of the mechanical strength of PVA/alginate gel beads and their effects on the ammonia-oxidizing activity. Desalin. Water. Treat. 2015;53(9)2412–2420.
https://doi.org/10.1080/19443994.2014.927118
16. Baird R, Bridgewater L. Standard methods for the examination of water and wastewater. 23rd edWashington, DC: American Public Health Association; 2005.
17. Wu Y, Qi H, Shi C, Ma R, Liu S, Huang Z. Preparation and adsorption behaviors of sodium alginate-based adsorbent-immobilized β-cyclodextrin and graphene oxide. RSC. Adv. 2017;7(50)31549–31557.
https://doi.org/10.1039/C7RA02313H
18. Zhang LS, Wu WZ, Wang JL. Immobilization of activated sludge using improved polyvinyl alcohol (PVA) gel. J. Environ. Sci. 2007;19(11)1293–1297.
https://doi.org/10.1016/S1001-0742(07)60211-3
19. Voo WP, Ooi CW, Islam A, Tey BT, Chan ES. Calcium alginate hydrogel beads with high stiffness and extended dissolution behaviour. Eur. Polym. J. 2016;75:343–353.
https://doi.org/10.1016/j.eurpolymj.2015.12.029
20. Al-Hajry HA, Al-Maskry SA, Al-Kharousi LM, El-Mardi O, Shayya WH, Goosen MF. Electrostatic encapsulation and growth of plant cell cultures in alginate. Biotechnol. Prog. 1999;15(4)768–774.
http://doi.org/10.1021/bp990069e
21. Gao Q, Li SW, Xie YJ, Zheng MX, et al. Rapid cultivation of anammox sludge based on Ca-alginate cell beads. Water Sci. Technol. 2022;85(10)2899–2911.
https://doi.org/10.2166/wst.2022.161
22. Lee BB, Ravindra P, Chan ES. Size and shape of calcium alginate beads produced by extrusion dripping. Chem. Eng. Technol. 2013;36(10)1627–1642.
http://doi.org/10.1002/ceat.201300230
23. Zhuang Y, Yu F, Chen H, Zheng J, Ma J, Chen J. Alginate/graphene double-network nanocomposite hydrogel beads with low-swelling, enhanced mechanical properties, and enhanced adsorption capacity. J. Mater. Chem. A. 2016;4(28)10885–10892.
https://doi.org/10.1039/C6TA02738E
24. Zhang Y, Ye L. Improvement of permeability of poly (vinyl alcohol) hydrogel by using poly (ethylene glycol) as porogen. Polym. Plast. Technol. Eng. 2011;50(8)776–782.
https://doi.org/10.1080/03602559.2010.551443
25. Anna BW, Mariusz T, Mohamed SH, Aleksandra ZB. Effect of biomass immobilization and reduced graphene oxide on the microbial community changes and nitrogen removal at low temperatures. Sci. Rep. 2021;11(1)1–12.
https://doi.org/10.1038/s41598-020-80747-7
26. Fan J, Shi Z, Lian M, Li H, Yin J. Mechanically strong graphene oxide/sodium alginate/polyacrylamide nanocomposite hydrogel with improved dye adsorption capacity. J. Mater. Chem. A. 2013;1(25)7433–7443.
https://doi.org/10.1039/C3TA10639J
27. Mansur HS, Sadahira CM, Souza AN, Mansur AA. FTIR spectroscopy characterization of poly (vinyl alcohol) hydrogel with different hydrolysis degree and chemically crosslinked with glutaraldehyde. Mater. Sci. Eng: C. 2008;28(4)539–548.
https://doi.org/10.1016/j.msec.2007.10.088
28. Mirzaie Z, Reisi-Vanani A, Barati M. Polyvinyl alcohol-sodium alginate blend, composited with 3D-graphene oxide as a controlled release system for curcumin. J. Drug. Deliv. Sci. Technol. 2019;50:380–387.
https://doi.org/10.1016/j.jddst.2019.02.005
29. Thayumanavan N, Tambe P, Joshi G, Shukla M. Effect of sodium alginate modification of graphene (by ‘anion-π’type of interaction) on the mechanical and thermal properties of polyvinyl alcohol (PVA) nanocomposites. Compos. Interfaces. 2014;21(6)487–506.
https://doi.org/10.1080/15685543.2014.879512
30. Zhuang Y, Kong Y, Han K, Hao H, Shi B. A physically cross-linked self-healable double-network polymer hydrogel as a framework for nanomaterial. New. J. Chem. 2017;41(24)15127–15135.
https://doi.org/10.1039/C7NJ03392C
31. Zhang S, Wang H, Liu J, Bao C. Measuring the specific surface area of monolayer graphene oxide in water. Mater. Lett. 2020;261:127098.
https://doi.org/10.1016/j.matlet.2019.127098
32. Liu C, Liu H, Xiong T, Xu A, Pan B, Tang K. Graphene oxide reinforced alginate/PVA double network hydrogels for efficient dye removal. Polymers. 2018;10(8)835.
https://doi.org/10.3390/polym10080835
33. Ma YX, Li X, Shao WJ, Kou KL, Yang HP, Zhang DJ. Fabrication of 3D porous polyvinyl alcohol/sodium alginate/graphene oxide spherical composites for the adsorption of methylene blue. J. Nanosci. Nanotechnol. 2020;20(4)2205–2213.
http://doi.org/10.1166/jnn.2020.17193
34. Zhang L, Wang Z, Xu C, Li Y, Gao J, Wang W, Liu Y. High strength graphene oxide/polyvinyl alcohol composite hydrogels. J. Mater. Chem. A. 2011;21(28)10399–10406.
https://doi.org/10.1039/C0JM04043F
35. Yi X, Sun F, Han Z, et al. Graphene oxide encapsulated polyvinyl alcohol/sodium alginate hydrogel microspheres for Cu(II) and U(VI) removal. Ecotoxicol. Environ. Saf. 2018;158:309–318.
https://doi.org/10.1016/j.ecoenv.2018.04.039
36. Jiwei L, Jianwei M, Shaojuan C, Yudong H, Jinmei H. Adsorption of lysozyme by alginate/graphene oxide composite beads with enhanced stability and mechanical property. Mater. Sci. Eng: C. 2018;89:25–32.
https://doi.org/10.1016/j.msec.2018.03.023
37. Nešović K, Janković A, Perić-Grujić A, et al. Kinetic models of swelling and thermal stability of silver/poly (vinyl alcohol)/ chitosan/graphene hydrogels. J. Ind. Eng. Chem. 2019;77:83–96.
https://doi.org/10.1016/j.jiec.2019.04.022
38. Nguyen TM, Choi MK, Park NB, Bae HK. Critical design factors for polyvinyl alcohol hydrogel entrapping ammonia-oxidizing bacteria: biomass loading, distribution of dissolved oxygen, and bacterial liability. Environ. Eng. Res. 2021;26(2)200010.
https://doi.org/10.4491/eer.2020.010
39. Ruiz ON, Fernando KS, Wang B, et al. Graphene oxide: a non-specific enhancer of cellular growth. ACS. nano. 2011;5(10)8100–8107.
https://doi.org/10.1021/nn202699t
40. Wang D, Wang G, Zhang G, Xu X, Yang F. Using graphene oxide to enhance the activity of anammox bacteria for nitrogen removal. Bioresour. Technol. 2013;131:527–530.
https://doi.org/10.1016/j.biortech.2013.01.099
41. Zhang Y, Hui B, Ye L. Reactive toughening of polyvinyl alcohol hydrogel and its wastewater treatment performance by immobilization of microorganisms. RSC. Adv. 2015;5(111)91414–91422.
https://doi.org/10.1039/C5RA20495J
Fig. 4SEM images of gel beads (A, B: morphologies of GO1 and GO6; C, D: the surface of GO1 and GO6; E, F: Cross-section of GO1 and GO6). 
Fig. 5Absorption capacity coefficient (qMB) and the MB removal efficiency (RMB) of gel beads under different concentrations of GO. 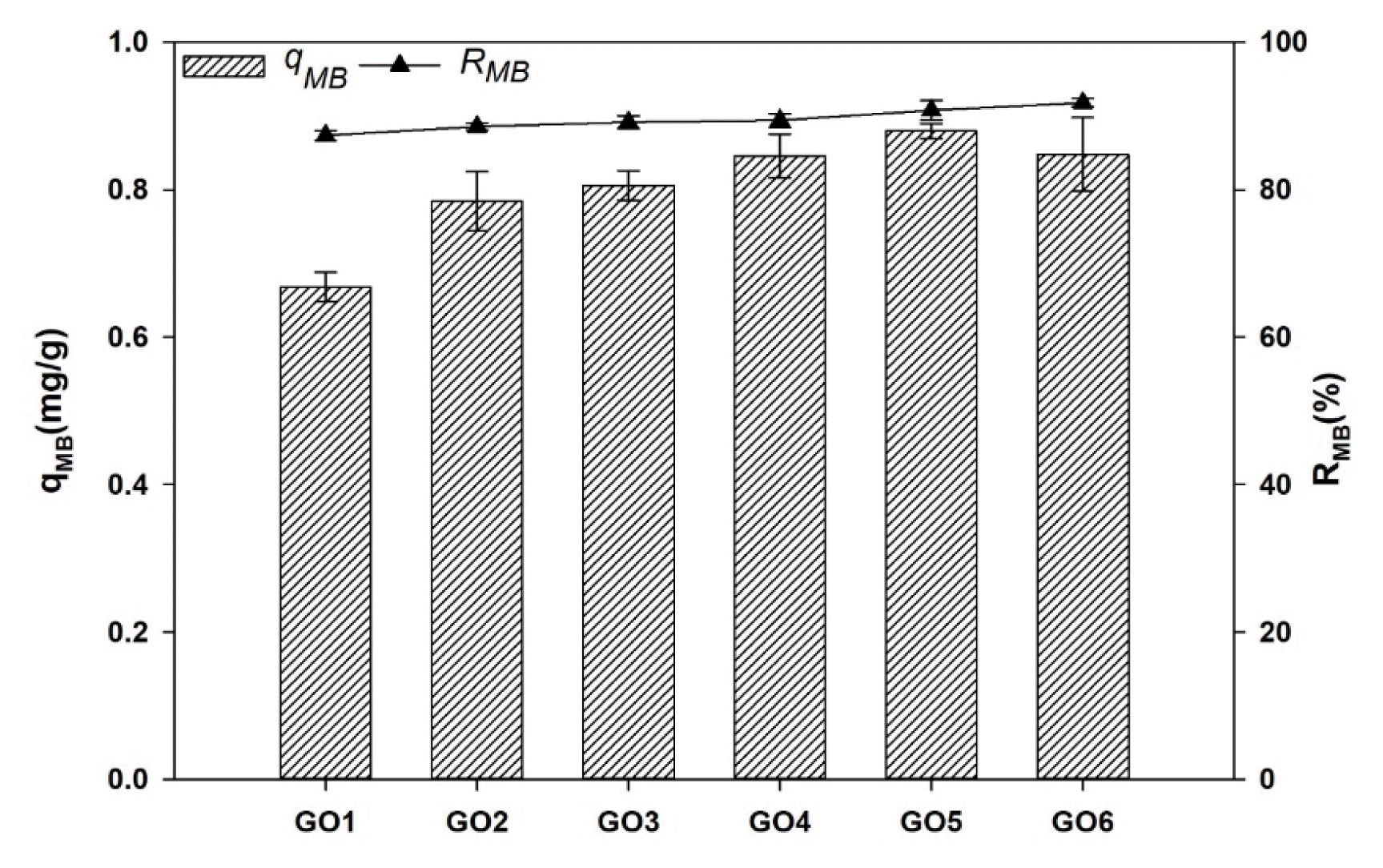
Table 1Recent Studies about Enhancement of PVA/SA Gel Beads
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||