AbstractNanoparticles are defined as structures of size ranges from 1–100 nm in diameter in at least one direction. Metallic nanoparticles are considered the most potent agents in the modern world because they are used in every field of life, including the biotechnology, medical, textile, and food industries. Owing to their increased application, humans are constantly exposed to these nanoparticles. Their physiochemical characteristics, as their large surface to volume ratio might enhance their toxicity, increased surface area of NPs exhibit increased biological activity i.e reactive oxygen species generation when compared to large particles of the same mass. Consequently, their biosafety is a matter of major concern. The liver is the primary organ for detoxification and the major organ exposed to nanoparticles. After penetrating the body, nanoparticles enter the bloodstream and are internalized by hepatocytes. After penetration, they cause genotoxicity, oxidative stress and histopathology in hepatic tissues.
Graphical Abstract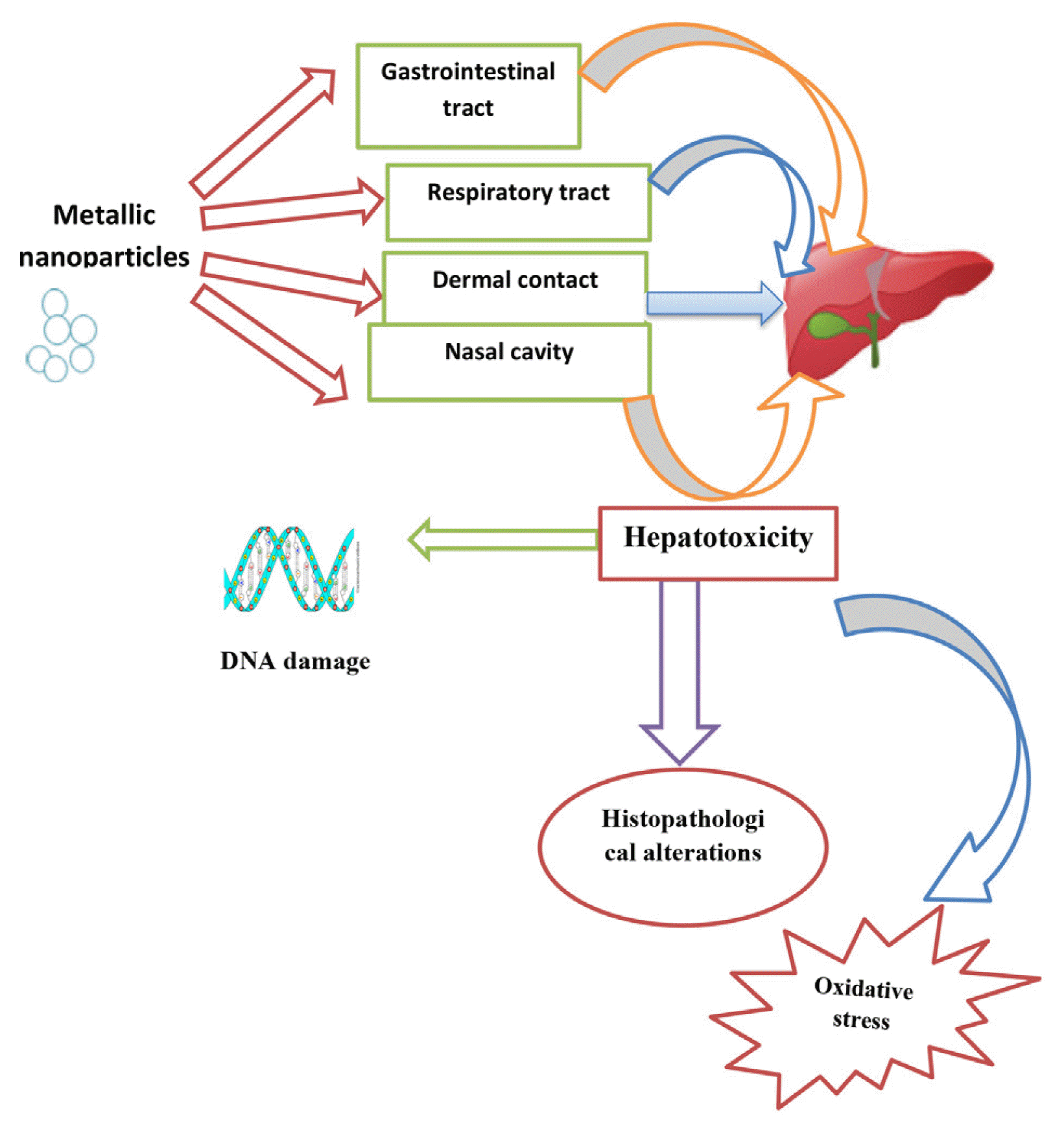
1. IntroductionNano-objects or nanoparticles (NPs) are defined as structures of different shapes that are ≤100 nm in size in at least one direction. Nano-objects exhibit distinctive physical, chemical, electronic, optical, and mechanical properties owing to their small size and high surface-to-volume ratio [1]. Based on their external dimensions, nano-objects can be divided into three categories: a) nanoparticles, with three dimensions <100 nm in size; b) nanotubes or nano-filaments, with only two dimensions at the nano-scale and have a much higher third dimension; and c) nanosheets, with only one dimension at the nanoscale [2]. NPs are used in many fields of science and technology. Based on their physicochemical properties, NPs can be divided into three classes: i) organic NPs, ii) inorganic NPs, and iii) carbon-based NPs [3].
Metal NPs are among the most extensively used and are a major component of nanotechnology and nanoscience. NPs are employed in every field, including medical, biomedical, and pharmaceutical applications of nanotechnology. They are effectively used in drug delivery procedures, enzymology, surface coatings, biosensors, and diagnostics [4]. Further, metal NPs are used in the clinic to treat metabolic disorders, in industries, and in everyday products, such as sunscreen, deodorant, and cosmetics. They are extensively used in the textile industry, food production, antibiotic manufacturing, and electronic components [5]. Owing to their increased application, they are released into the environment, and humans are directly exposed to them [6]. Despite its advantages, the broad application of nanotechnology poses serious health risks to humans. They enter the body in various ways. After penetrating the human body, NPs upset the normal biological system and cause kidney, liver, lung, and nerve damage [7].
The liver is positioned in the upper right portion of the abdomen and is the vital organ of the body for detoxification and blood cleaning as it removes excretory metabolites. As the primary detoxifying organ, the liver is subjected to extreme damage, even in the absence of external factors [8]. NPs affect many organs, including the spleen, kidneys, heart, brain, and lungs. However, the liver is the most affected by NPs because it is involved in detoxifying excretory metabolites [2].
Among the NPs entering the body, 80% are retained in the blood by the liver, which acts as a biological filtration system. Therefore, the effects of NPs on the liver need to be considered seriously. The results of recent studies summarized in this review indicate that metallic NPs have substantial toxic effects on the liver and hepatic functions. This review presents findings that strongly support the widespread notion that using metallic NPs (MNPs) in daily life can cause hepatotoxicity and affect liver function. Overall, this review summarizes and discusses how MNPs influence the liver at the cellular level and highlight their different mechanisms of action in inducing hepatotoxicity.
1.1. The LiverThe liver is located in the upper right abdomen, has a high capacity for regeneration, and is crucial for survival. It constitutes 1/50th of the human body weight [9]. The liver comprises four functional units or lobules, and each lobule is composed of interconnected plates of hepatocytes separated by endothelium-lined sinusoids. Hepatocytes are large cells with a central nucleus, prominent nucleolus, and abundant cytoplasm and are the primary cell type in the liver.[10].
The other three cell types include (i) endothelial cells of liver sinusoids that assist hepatocytes in accessing plasma nutrients; (ii) Kupffer cells, also known as hepatic macrophages; and (iii) stellate cells, which are storage sites of vitamin A, because they are loaded with lipid droplets [11]. The liver is considered one of the most perfused organs because it receives 1.5 L of blood per minute from the hepatic artery and the portal vein. Oxygen-rich blood enters the liver through the hepatic artery and is distributed to perihepatic organs via the portal vein [12].
The liver plays various roles within the body that are vital for a healthy life. It is a vital organ in digestion because it produces bile. Bile is a mixture of water, bile salts, bile pigments, amino acids, lipids, and mucopolysaccharides that digest the dietary lipids in food upon contact. Bile is produced in the hepatocytes, deposited in the gall bladder, and eliminated in the duodenum. The liver also serves as an organ for glucose, fructose, and galactose storage. The release of these sugars from the liver is controlled by insulin, which regulates blood sugar levels. Further, the liver is the main organ in which lipids, such as cholesterol, are synthesized and stored. Moreover, vitamins A, D, E, K, and B12 and several minerals are stored in the liver [13].
The phagocytic ability of Kupffer cells in the liver makes them vital for immunity because they can capture and eliminate different parasites, bacteria, and other cellular debris. This is a crucial function of the liver because it receives a large quantity of blood, thus providing an efficient filtration system [14]. The liver has a specific enzymatic system that enables detoxification. Organisms are constantly subjected to different xenobiotics from the environment or diet; therefore, the liver is pivotal to their purification and filtration. Enzymes, most likely the cytochrome family of enzymes present in the liver, directly eliminate or transform xenobiotics into less toxic forms for excretion in bile or urine [15].
1.2. Metallic NanoparticlesMNPs are composed of two components, a metallic core composed of an inorganic metal or metal oxide and an outer shell composed of organic or inorganic materials or metal oxides. MNPs have gained much attention because they are an essential part of everyday life. They are used in shampoos, creams, toothpaste, the textile industry, footwear, and plastic utensils [16]. They can enter the human body through inhalation, dermal contact, and the gastrointestinal tract and then circulate in the body through the blood or lymphatic system, thus accumulating in different organs [17]. MNPs are used everywhere despite their toxicity to humans and the environment. They can also affect biological systems at the cellular level [18].
MNPs cause toxicity through different mechanisms. Some MNPs may cause acute toxicity by damaging the cell membrane because of their abrasive nature. Cell contents leak upon cell membrane damage, which eventually results in cell death. However, this type of cell membrane injury is more noticeable in unicellular organisms [19]. Owing to their high surface area-to-volume ratio, small size, and high chemical reactivity, MNPs have a strong capability to generate reactive oxygen species such as free radicals, oxygen ions, and peroxides. ROS production may cause oxidative stress in cells through different mechanisms, as different body components, such as fats, proteins, and nucleic acids, lose their functional capabilities [20]. Changes in liver coefficients may occur because of the inflammation caused by MNPs. For example, gold NPs (AuNPs) activate hepatic macrophages and consequently stimulate the occurrence of immune hepatitis and liver dysfunction [21].
Based on their composition, nanoparticles are divided into two main categories i) Organic nanoparticles ii) Inorganic nanoparticles. Organic nanoparticles are made of proteins, carbohydrates, lipids, polymers, or any other organic compounds. This class comprises NPs that not made of carbon or organic materials. The typical examples of this class are metal, ceramic, and semiconductor NPs. Metal NPs are purely made of metal precursors. All metallic nanoparticles along with their applications are discussed one by one.
2. Effects of MNPs on Different Cellular OrganellesA cell is composed of small organ-like structures called organelles, which are structurally different, perform distinct functions within the cell, and are the basic units of all living organisms. The fate of cells depends on the integrity of organelles.
2.1. Liver Cell MembraneThe liver cell membrane is highly delicate and sensitive to lipid peroxidation and free radicals because they may cause increased permeability and decreased fluidity. For instance, ZnO NPs accumulate in the microvilli of cell membranes in the hepatocytes of catfish [22]. As cell membranes contain different receptors for enzyme activation, altered cell membrane fluidity can inactivate different liver enzymes, thus inhibiting hepatocyte function. MNPs can alter the internal structure of the cell membrane by altering its permeability. For instance, silver nanoparticles (AgNPs) damage the hepatocyte membrane, thus causing diminished albumin synthesis owing to disrupted alanine transaminase (ALT) activity [23]
2.2. NucleusThe nucleus is the most crucial part of the cell because it contains genetic material and controls cell growth, proliferation, differentiation, and other metabolic activities. MNPs can access the cell nucleus and destroy DNA, consequently damaging cellular morphology and gene expression [24].
ZnO NPs cause shrinkage of the nucleus in hepatocytes along with chromatin material condensation into a structureless mass with an irregular nuclear membrane. Moreover, they also cause hepatocyte apoptosis [25]. In mice orally administered TiO2 NPs, metabolic genes such as Cyp2b10 and Cyp2c37 in hepatocytes were upregulated and genes involved in DNA damage and repair, such as p21 and mdm2, were downregulated. DNA strands were broken down in the hepatocytes of exposed animals. [26] Further, MNPs can induce different activities, resulting in hepatocyte apoptosis.
2.3. MitochondriaMNPs also influence the mitochondria in cells. They induce structural changes in mitochondria and produce reactive oxygen species (ROS), leading to oxidative stress, diminished enzyme activities, decreased calcium content, inhibition of the electron transport chain and respiration, and decreased ATP synthesis, eventually leading to hepatocyte necrosis and apoptosis [27]. In one study, mice were exposed to ZnO NPs, which caused structural deviations, such as inflammation and elongation of hepatocyte mitochondria, as well as absence and rupture of cristae [28]. In addition to structural changes, MNPs can diminish mitochondrial enzyme activity, reduce mitochondrial membrane potential, suppress mitochondrial gene expression for the dynamin and fusion proteins, and induce oxidative stress in hepatocyte mitochondria [29].
2.4. Endoplasmic Reticulum (ER)The hepatocyte endoplasmic reticulum (ER) is involved in protein synthesis, steroid hormone synthesis, calcium accumulation, and lipid metabolism. Alterations induced by MNPs in the ER of hepatocytes include strain, distension, changes in protein synthesis, and misfolded proteins. These changes lead to a decline in the detoxification function of the liver [30]. ZnO NPs can shorten stacks, inhibit parallel arrays, and enhance the expression of protein kinase genes. ER damage caused by MNPs is an early sign of MNP-induced toxicity [31].
3. Hepatotoxicity Induced by MNPs3.1. Oxidative StressCellular metabolism produces some active molecules, such as superoxide anions, hydrogen peroxide, and hydroxyl radicals, which are called reactive oxygen species (ROS). When produced within limits, these helps maintain cell physiology. Excessive ROS production can disturb redox balance and induce oxidative stress, thus damaging normal cell functioning and eventually leading to cell death. Oxidative stress, caused by excessive ROS production, is the leading cause of many liver diseases, including hepatitis, liver failure, alcoholic liver disease, and other conditions [32]. NP exposure can cause oxidative stress in hepatic tissues via different mechanisms. Exposure to MNPs can cause their accumulation in the liver, consequently disturbing the activities of antioxidant enzymes and causing oxidative stress in the liver. Mice exposed to silver NPs (Ag NPs) exhibited elevated hydrogen peroxide and altered liver enzyme levels, suggesting cell membrane disruption in liver cells after exposure to AgNPs [33]. Similar results were observed in another study in which quails were exposed to AgNPs; this resulted in elevated plasma fibrinogen levels and decreased serum aspartate aminotransferase (AST) activity. Further, the hepatocytes of AgNP-exposed quails exhibited increased lipid peroxidation and decreased catalase activity and lipid vacuolization [34].
A previous study has revealed the impact of metal oxide NPs on antioxidant systems involving liver enzymes such as glutathione peroxidase (GPx), glutathione reductase (GR), glutathione S-transferase (GST), superoxide dismutase (SOD), and catalase (CAT). When Nile fish (Oreochromis niloticus) were exposed to Al2O3, CuO, and TiO2 NPs for 14 days, GR and GPx levels remained unchanged, whereas the levels of CAT and SOD significantly declined. However, GST activity was enhanced, indicating that the NPs level increased in the liver over time and caused oxidative stress in fish [35].
Oral administration of TiO2 NPs and AgNPs to rats induced oxidative stress in the liver by disturbing the antioxidant system. These NPs decreased the GSH/GSSG ratio and enhanced the production of reactive species [36].
Metal oxides, such as TiO2 NPs, are currently used in the cosmetics and paint industries. Therefore, using these products may cause oxidative stress in the liver. These findings were confirmed in a study wherein rats were exposed to TiO2, which enhanced the levels of AST and ASP, causing lipid peroxidation and, ultimately, cell death in the liver [37]. Further, ROS generation and oxidative stress were observed in the livers and kidneys of animals exposed to Al2O3 and ZnO NPs; both these NPs acted synergistically, causing more pronounced hepatorenal toxicity. These NPs act by damaging the defense system of the body against antioxidants and enhancing cell death by necrosis and apoptosis [38].
NPs affect different organs differently. Haseeb et al. measured the levels of oxidative stress markers, such as reduced glutathione (GSH) and malondialdehyde (MDA), in the liver, heart, and lungs of rats exposed to gold NPs (AuNPs) for 3 and 7 days. They demonstrated that AuNPs caused an increase in MDA levels in the liver, but there was no impact on the MDA levels in the heart and lungs of exposed animals. Therefore, they concluded that AuNPs did not cause oxidative stress in the lungs and heart, and that liver was the only affected organ [39].
Sharma et al. studied the effect of orally administered ZnO NPs on liver enzymes in mice. They reported that ZnO NPs induced elevation in the serum levels of ALP and ALT, subsequently causing cellular injuries in the liver. They also concluded that NP exposure causes lipid peroxidation, leading to oxidative stress and DNA deformities in hepatocytes [40]. These alterations in the antioxidant system were further confirmed in a study wherein rats were exposed to copper NPs for 14 days. These NPs disturbed the antioxidant activities of glutathione, CAT, SOD, and MDA. These results confirm that NP exposure causes liver toxicity, mainly due to oxidative stress (Anreddy, 2018).
Overall, the available data on oxidative stress in the liver caused by metallic NPs suggest that, in most cases, it might be caused by alterations in the antioxidant system of the liver. Further investigation is required to understand the other possible mechanisms of oxidative stress induction in the liver.
3.2. Histopathological Alterations Caused by MNPs in the LiverLiver injury induced by metallic NPs at the histological level has been reported in various in vivo studies. Mice exposed to ZnO NPs exhibited a significant increase in ALT and AST activity, indicating NPs as a possible cause of hepatic injury. Furthermore, cellular necrosis, congestion, and glycogen clumping have been observed in hepatic tissues, suggesting severe damage [42]. These results were further confirmed by another study that investigated the toxic effects of AgNPs on juvenile common carp. After the experiment, euthanized animals revealed a massive buildup of AgNPs in the liver and other organs. Tissue lesions, cellular swelling, and granuloma formation were observed in the organs of exposed animals, which increased with an increase in the AgNP dose [43].
Desouqy et al. reported histopathological alterations in the liver of crayfish after administering 25–250 mg/L of TiO2 NPs for 28 days. They observed NP accumulation in the liver and other organs. These NPs enhanced the levels of CAT, glutathione, and glutathione peroxidase, and induced histopathological changes through tubular disruption and inflammatory infiltration [44].
Biomarkers of liver injury were also detected in Oreochromis niloticus exposed to aluminum oxide NPs for seven days. ASP, ALT, and alkaline phosphatase levels were elevated in the plasma of treated animals, along with melanomacrophage aggregation and hepatic tissue necrosis [45]. In another study, Tohamy et al. measured the downregulation of antioxidant genes and upregulation of inflammatory mediators in rats exposed to copper NPs (CuO-NPs); further, they demonstrated swollen hepatocytes with nuclei replacing the cytoplasm and hepatocellular necrosis [46].
In another study, iron oxide NPs caused histopathological effects in a dose-dependent manner in rats administered iron oxide NPs at doses of 25, 50,75,150, and 300 μg/g body weight. Although significant histopathological changes were observed in the 25 and 50 μg/g groups, an increase in the dose to 75 and 150 μg/g resulted in hepatocyte necrosis with lymphocyte and neutrophil infiltration. The hepatocytes of these high-dose groups exhibited hypertrophy, and central venous congestion was observed in their livers [47]. These findings were confirmed by another study indicating that iron oxide nanoparticles caused histopathological alterations in hepatocytes, wherein hepatocytes from treated animals exhibited excessive lipid accumulation [48].
The above findings suggest that data on the effects of NPs on biochemical markers diverge significantly from each other, regardless of the type of NPs considered. Moreover, mechanism may of histopathology may differ due to route of exposure, time duration and physiochemical properties of diiferent nanoparticles.
3.3. GenotoxicityAlteration in genetic material is called genotoxicity, including DNA strand breakage at the chromosomal or genomic level, and can exert severe health defects. MNPs exhibit high genotoxic potential. Genotoxicity might be reparable or can induce permanent alterations in genetic material. When these permanent alterations occur in germ cells, they are inherited by the next generation and can cause genetic disorders. In contrast, somatic mutations lead to other pathologies, such as cancer; thus, every mutagen is considered carcinogenic [54].
MNPs may induce genotoxicity through different mechanisms, such as primary or secondary genotoxicity. In primary genotoxicity, MNPs come into direct contact with the genome and cause DNA deformities, strand breakage, DNA abrasions, and chromosomal damage [55]. In secondary genotoxicity, MNPs induce toxicity by producing reactive oxygen species by inflammatory cells. MNPs activate phagocytes upon their entry into the body, resulting in an oxidative burst, which eventually leads to inflammation, the first defense mechanism of the body against foreign particles. This mechanism helps the body eliminate foreign material. Failure of this clearance mechanism leads to a chronic immune response by cells [56].
Genotoxicity can be assessed using different methods, including the comet assay and micronucleus test. Different MNPs can cause DNA damage through different mechanisms. DNA disruption caused by SiO2, TiO2, and ZnO NPs has also been investigated [57]. A study investigating genotoxicity induced by titanium dioxide NPs demonstrated that exposure to these NPs enhanced ROS production and decreased glutathione levels. Further, increased levels of p53, BAX, caspase-9, and caspase-3 with reduced levels of Bcl-2 were observed, resulting in liver cell apoptosis [58].
Further, significant DNA damage has been reported in a study wherein mice were intravenously administered titanium dioxide NPs at 50 mg/kg. DNA strand breakage was observed in the hepatocytes of the treated animals as determined using a comet assay. Further, the treated animals exhibited altered gene expression in the hepatocytes, consequently disrupting metabolic homeostasis [59]. Similar results were obtained in another study suggesting that titanium dioxide NPs induced genotoxicity in mouse liver cells ROS production and induction of inflammation in hepatocytes [60].
MNPs used in the cosmetic industry are studied extensively because of their potential to cause genotoxicity. ZnO NPs are major constituents in the cosmetic industry. These NPs have been reported to induce DNA damage in hepatocytes. ZnO NPs are stored in the liver after exposure, which causes pathological abrasions in the liver by increasing the serum levels of ALT and ALP. These NPs also induce oxidative stress through ROS production. These findings were evaluated using an Fpg-modified comet assay. Overall, these results indicate that DNA lesions in liver cells might be induced by oxidative stress [40]. ZnO NPs also cause genotoxicity in human liver cells. For instance, ZnO NPs induce DNA damage, possibly mediated via reactive oxygen species in HepG2 liver cells. These NPs also induce apoptosis in human liver cells [61]
Intravenous exposure of mice to silica NPs is also reported to cause DNA damage. Pathological alterations in DNA damage are assessed using the comet and micronucleus assays. These results suggested that DNA damage might be due to inflammation or immune responses [62]. Mice were exposed to a 0.25 mg/kg dose of SiNPs. These NPs induced DNA damage in hepatocytes, as evaluated using the comet assay [63].
Elje et al. compared the effects of titanium dioxide, zinc oxide, and silver NPs on human hepatocytes and observed DNA lesions and breakage after exposure to all NPs as evaluated using the comet assay [64].
Further, to assess the cytotoxic and genotoxic effects of AgNPs in mouse hepatocytes, a 0.5–20 mg/kg dose of AgNPs was administered intravenously to mice, followed by hepatocyte genotoxicity and cytotoxicity of hepatocytes evaluation using micronucleus and comet assays. The results revealed significant DNA strand breaks and lesions in hepatocytes [65].
In conclusion, data on liver inflammatory effects and the liver genotoxicity process are less well described for metallic nanoparticles. Further investigation is required to find more mechanisms of actions of DNA damage induced by metallic nanoparticles.
3.4. InflammationNecrosis of parenchymal cells in organs is mediated by inflammation, which enhances the buildup of extracellular matrix in tissues. Fibrosis occurs when the damage is not severe, whereas severe damage causes morphological alterations in tissues and organs. Induction of inflammation is one of the most pronounced effects of MNPs on the body. Livers of nanoparticle-exposed animals mostly indicate inflammatory cell infiltration. This was supported by a comparative study in which Wistar rats were exposed to ZnO, TiO2, and AgNPs. The results revealed inflammatory cell infiltration in the livers of exposed animals. Necrosis of the central part of the hepatic lobule and steatosis of liver cells were also detected. Further, the serum levels of IL-1β, a cytokine responsible for host cell defense mechanisms, were significantly increased. In contrast, the IFN-γ and TNF-α levels were significantly decreased in MNP-exposed animals [69].
The liver contains a type of cell called Kupffer cells, which are natural macrophages. MNPs entering the liver are mostly ingested by Kupffer cells along with some hepatocytes, consequently inducing inflammation and increasing IL-1β release [26].
Exposure of human hepatocytes to C3a significantly elevated the levels of IL-8, IL-1R1, inflammatory protein 2, and tumor necrosis factor α [70]. The formation of the NLR family pyrin domain containing 3 (NLRP3) inflammasome, an inflammatory corpuscle, is the primary mechanism of inducing inflammation in the liver. NLRP3 activation and production are mediated by MAPK, NF-κB, and ROS signaling.
Exposure to CuNPs increased NF-κb, consequently decreasing the liver index, resulting in oxidative stress and inflammation in the liver [71]. These findings were supported by another study in which AgNPs enhanced the levels of MAPK and PKB pathway components, leading to their activation, which mediated the ROS pathways for inducing inflammation in HepG2 liver cells [72].
4. ConclusionAdvances in nanotechnology have resulted in increased exposure to metallic NPs. Therefore, there is an urgent need to examine the possibility of organ damage, the mechanism of action, and other detrimental effects. Investigating the toxic effects of MNPs on the liver is the basis for their safety assessment. Currently, research on MNP-induced hepatotoxicity is preliminary. The liver is the primary organ affected by MNPs because it is the site of NP accumulation. Despite ample data on liver injury caused by oxidative stress, inflammation, and apoptosis, we lack comprehensive elucidation of the factors involved in energy, protein, and lipid metabolism. To evaluate the hepatotoxicity induced by MNPs, a detailed understanding of the entry, distribution, and metabolism of NPs in the liver and the possible alterations in liver function, degree of liver injury, and recovery of liver function in vivo are required.
Current research on the toxicity of MNPs has been limited to animal experiments in vivo and in vitro; thus, the relationship between subcellular damage and related mechanisms remains unclear. Therefore, the toxicology of MNPs must be studied in depth to improve their quality and safety. To reduce the nanoparticle toxic effects on human liver, the toxic species can be replaced with less toxic elements that have similar properties, the nanoparticle can be capped with a shell material, the morphology of the nanoparticle can be chosen to minimize surface area and thus minimize dissolution. These alterations can reduce the impact of MNPs on human liver.
NotesAuthors Contribution A.W. (PhD student) wrote the original manuscript. S.S. (Professor) revised and edited the manuscript. S.N. (Associate Professor) reviewed and revised the original manuscript. F.M. (Professor) supervised the manuscript. F.J. (Assistant Professor) visualized and reviewed data. M.H. (Professor) reviewed and edited the manuscript. Y.J.C. (Associate Professor) reviewed and revised the original manuscript. Y-K.P. (Professor) supervised the manuscript. References1. Conde JA, Dias JT, Graz? V, Moros M, Baptista PV. Revisiting 30 years of biofunctionalization and surface chemistry of inorganic nanoparticles for nanomedicine. Front. Chem. 2014;2:3389.
https://doi.org/10.3389/fchem.2014.00048
2. Cornu R, Béduneau A, Martin H. Influence of nanoparticles on liver tissue and hepatic functions: A review. Toxicology. 2020;430:152344.
https://doi.org/10.1016/j.tox.2019.152344
3. Darr JA, Zhang J, Makwana NM, Weng X. Continuous hydrothermal synthesis of inorganic nanoparticles: applications and future directions. Chem. Rev. 2017;117:11125–11238.
https://doi.org/10.1021/acs.chemrev.6b00417
4. Jaleh B, Nasrollahzadeh M, Nasri A, Eslamipanah M, Moradi A, Nezafat Z. Biopolymer-derived (nano) catalysts for hydrogen evolution via hydrolysis of hydrides and electrochemical and photocatalytic techniques: A review. Int. J. Biol. Macromol. 2021;182:1056–1090.
https://doi.org/10.1016/j.ijbiomac.2021.04.087
5. Ajdary M, Keyhanfar F, Moosavi MA, Shabani R, Mehdizadeh M, Varma RS. Potential toxicity of nanoparticles on the reproductive system animal models: A review. J. Reprod. Immunol. 2021;148:103384.
https://doi.org/10.1016/j.jri.2021.103384
6. Ishii S, Bell J, Marshall F. Phytotoxic risk assessment of ambient air pollution on agricultural crops in Selangor State, Malaysia. Environ. Pollut. 2007;150:267–279.
https://doi.org/10.1016/j.envpol.2007.01.012
7. Brohi RD, Wang L, Talpur HS, et al. Toxicity of Nanoparticles on the Reproductive System in Animal Models:A Review. Front. Pharmacol. 2017;8:
https://doi.org/10.3389/fphar.2017.00606
8. Addolorato G, Abenavoli L, Dallio M, et al. Alcohol associated liver disease. A clinical practice guideline by the Italian Association for the Study of the Liver (AISF). Dig. Liver. Dis. 2020;52:374–391.
https://doi.org/10.1016/j.dld.2019.12.008
9. Abdel-Misih SR, Bloomston M. Liver anatomy. Surg. Clin. 2010;90:643–653.
https://doi.org/10.1016%2Fj.suc.2010.04.017
10. Chandrasoma P, Taylor CR. Chapter 42. The Liver: I. Structure & Function; Infections. Concise Pathology. 3rd edThe McGraw-Hill Companies; 1998.
11. Bogdanos DP, Gao B, Gershwin ME. Liver immunology. Compr. Physiol. 2013;3:567.
https://doi.org/10.1002%2Fcphy.c120011
12. Kan Z, Madoff DC. Liver anatomy: microcirculation of the liver. Seminars in interventional radiology. Thieme Medical Publishers; 2008.
13. Boyer F, Le Rousseau J. Carleman estimates for semi-discrete parabolic operators and application to the controllability of semi-linear semi-discrete parabolic equations. Annales de l'Institut Henri Poincare (C) Non Linear Analysis. 2014. Elsevier;
14. Kubes P, Jenne C. Immune responses in the liver. Annu. Rev. Immunol. 2018;36:247–277.
https://doi.org/10.1146/annurev-immunol-051116-052415
15. Guengerich FP. Mechanisms of cytochrome P450 substrate oxidation. MiniReview. Biochem. Mol. Toxicol. 2007;21:163–168.
http://dx.doi.org/10.1002/jbt.20174
16. Diegoli S, Manciulea AL, Begum S, Jones IP, Lead JR, Preece JA. Interaction between manufactured gold nanoparticles and naturally occurring organic macromolecules. Sci. Total. Environ. 2008;402:51–61.
https://doi.org/10.1016/j.scitotenv.2008.04.023
17. Yah CS, Iyuke SE, Simate GS. A review of nanoparticles toxicity and their routes of exposures. Iran. J. Pharm. Sci. 2012;8:299–314. retrieved from http://www.ijps.ir/article_2193.html
18. Brunner TJ, Wick P, Manser P, et al. In vitro cytotoxicity of oxide nanoparticles: comparison to asbestos, silica, and the effect of particle solubility. Environ. Sci. Technol. 2006;40:4374–4381.
https://doi.org/10.1021/es052069i
19. Morones JR, Elechiguerra JL, Camacho A, et al. The bactericidal effect of silver nanoparticles. Nanotechnology. 2005;16:2346.
http://dx.doi.org/10.1088/0957-4484/16/10/059
20. Fahmy B, Cormier SA. Copper oxide nanoparticles induce oxidative stress and cytotoxicity in airway epithelial cells. Toxicol. In Vitro. 2009;23:1365–1371.
https://doi.org/10.1016%2Fj.tiv.2009.08.005
21. Bartneck M, Ritz T, Keul HA, et al. Peptide-functionalized gold nanorods increase liver injury in hepatitis. ACS. Nano. 2012;6:8767–8777.
https://doi.org/10.1021/nn302502u
22. Esnouf A, Latrille É, Steyer JP, Helias A. Representativeness of environmental impact assessment methods regarding Life Cycle Inventories. Sci. Total. Environ. 2018;621:1264–1271.
https://doi.org/10.1016/j.scitotenv.2017.10.102
23. Jin Y, Zhang S, Tao R, et al. Oral exposure of mice to cadmium (II), chromium (VI) and their mixture induce oxidative-and endoplasmic reticulum-stress mediated apoptosis in the livers. Environ. Toxicol. 2016;31:693–705.
https://doi.org/10.1002/tox.22082
24. Chen R, Hu B, Liu Y, Xu J, Yang G, Xu D, Chen C. Beyond PM2. 5: The role of ultrafine particles on adverse health effects of air pollution. Biochim. Biophys. 2016;1860:2844–2855.
https://doi.org/10.1016/j.bbagen.2016.03.019
25. Almansour M, Sajti L, Melhim W, Jarrar B. Ultrastructural Hepatic Alterations Induced by 35 nm Zinc Oxide Nanoparticles. Nanosci. Nanotechnol. Lett. 2015;7:763–769.
http://dx.doi.org/10.1166/nnl.2015.2028
26. Mirshafiee V, Sun B, Chang CH, et al. Toxicological profiling of metal oxide nanoparticles in liver context reveals pyroptosis in Kupffer cells and macrophages versus apoptosis in hepatocytes. ACS. Nano. 2018;12:3836–3852.
http://dx.doi.org/10.1021/acsnano.8b01086
27. Nguyen KC, Rippstein P, Tayabali AF, Willmore WG. Mitochondrial toxicity of cadmium telluride quantum dot nanoparticles in mammalian hepatocytes. Toxicol. Sci. 2015;146:31–42.
http://dx.doi.org/10.1016/j.freeradbiomed.2013.10.773
28. Almansour M, Sajti L, Melhim W, Jarrar B. Ultrastructural hepatic alterations induced by 35 nm zinc oxide nanoparticles. Nanosci. Nanotechnol. Lett. 2015;7:763–769.
http://dx.doi.org/10.1166/nnl.2015.2028
29. Natarajan V, Wilson CL, Hayward SL, Kidambi S. Titanium dioxide nanoparticles trigger loss of function and perturbation of mitochondrial dynamics in primary hepatocytes. PloS. One. 2015;10:e0134541.
https://doi.org/10.1371/journal.pone.0134541
30. Yu KN, Sung JH, Lee S, et al. Inhalation of titanium dioxide induces endoplasmic reticulum stress-mediated autophagy and inflammation in mice. Food. Chem. Toxicol. 2015;85:106–113.
http://dx.doi.org/10.1016/j.fct.2015.08.001
31. Vickers NJ. Animal communication: when i’m calling you, will you answer too? Curr. Biol. 2017;27:R713–R715.
https://doi.org/10.1016/j.cub.2017.05.064
32. Liu H, Ma L, Liu J, Zhao J, Yan J, Hong F. Toxicity of nano-anatase TiO2 to mice: liver injury, oxidative stress. Environ. Toxicol. Chem. 2010;92:175–186.
https://doi.org/10.1080/02772240902732530
33. Yousof SM, Erfan H, Hosny MM, Shehata SA, El-Sayed K. Subacute toxic effects of silver nanoparticles oral administration and withdrawal on the structure and function of adult Albino Rats’ hepatic tissue. Saudi. J. Biol. Sci. 2022;29:3890–3898.
https://doi.org/10.1016/j.sjbs.2022.02.054
34. Rezaei A, Farzinpour A, Vaziry A, Jalili A. Effects of silver nanoparticles on hematological parameters and hepatorenal functions in laying japanese Quails. Biol. Trace. Elem. Res. 2018;185:475–485.
https://link.springer.com/article/10.1007/s12011-018-1267-4
35. Canli EG, Canli M. Effects of aluminum, copper and titanium nanoparticles on the liver antioxidant enzymes of the Nile fish (Oreochromis niloticus). Energy Rep. 2020;6:62–67.
https://doi.org/10.1016/j.egyr.2020.10.047
36. Pereira LC, Pazin M, Franco-Bernardes MF, et al. A perspective of mitochondrial dysfunction in rats treated with silver and titanium nanoparticles (AgNPs and TiNPs). Trace. Elem. Med. Biol. 2018;47:63–69.
http://dx.doi.org/10.1016/j.jtemb.2018.01.007
37. Hassanein KM, El-Amir YO. Ameliorative effects of thymoquinone on titanium dioxide nanoparticles induced acute toxicity in rats. Int. J. Vet. Sci. 2018;6:16–21.
https://doi.org/10.1016%2Fj.ijvsm.2018.02.002
38. Yousef MI, Mutar TF, Kamel MAE-N. Hepato-renal toxicity of oral sub-chronic exposure to aluminum oxide and/or zinc oxide nanoparticles in rats. Toxicol. Rep. 2019;6:336–346.
http://dx.doi.org/10.1016/j.toxrep.2019.04.003
39. Khan HA, Abdelhalim MAK, Al-Ayed MS, Alhomida AS. Effect of gold nanoparticles on glutathione and malondialdehyde levels in liver, lung and heart of rats. Saudi. J. Biol. Sci. 2012;19:461–464.
https://doi.org/10.1016/j.sjbs.2012.06.005
40. Sharma V, Singh P, Pandey AK, Dhawan A. Induction of oxidative stress, DNA damage and apoptosis in mouse liver after sub-acute oral exposure to zinc oxide nanoparticles. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2012;745:84–91.
https://doi.org/10.1016/j.mrgentox.2011.12.009
41. Anreddy RNR. Copper oxide nanoparticles induces oxidative stress and liver toxicity in rats following oral exposure. Toxicol. Rep. 2018;5:903–904.
http://dx.doi.org/10.1016/j.toxrep.2018.08.022
42. Esmaeillou M, Moharamnejad M, Hsankhani R, Tehrani AA, Maadi H. Toxicity of ZnO nanoparticles in healthy adult mice. Environ. Toxicol. Pharmacol. 2012;35:67–71.
https://doi.org/10.1016/j.etap.2012.11.003
43. Khosravi-Katuli K, Shabani A, Paknejad H, Imanpoor MR. Comparative toxicity of silver nanoparticle and ionic silver in juvenile common carp (Cyprinus carpio): accumulation, physiology and histopathology. J. Hazard. Mater. 2018;359:373–381.
http://dx.doi.org/10.1016/j.jhazmat.2018.07.064
44. Desouqy MMA. Effects of titanium dioxide nanoparticles on red swamp crayfish, Procambarus clarkii: Bioaccumulation, oxidative stress andhistopathological biomarker. Egypt. J. Aquat. Res. 2019;45:11–18.
https://doi.org/10.1016/j.ejar.2019.01.001
45. Massoud E, El-Kott A, Morsy K, Abdel-Khalek AA. Assessment of hepatotoxicity induced by aluminum oxide nanoparticles in oreochromis niloticus using integrated biomarkers: exposure and recovery. Bull. Environ. Contam. Toxicol. 2021;106:970–977.
https://link.springer.com/article/10.1007/s00128-021-03190-y
46. Tohamy HG, El Okle OS, Goma AA, Abdel-Daim MM, Shukry M. Hepatorenal protective effect of nano-curcumin against nanocopper oxide-mediated toxicity in rats: Behavioral performance, antioxidant, anti-inflammatory, apoptosis, and histopathology. Life. Sci. 2022;292:120296.
http://dx.doi.org/10.1016/j.lfs.2021.120296
47. Parivar K, Fard FM, Bayat M, Alavian SM, Motavaf M. Evaluation of iron oxide nanoparticles toxicity on liver cells of BALB/c rats. IRCMJ. 2016;18:
https://doi.org/10.5812/ircmj.28939
48. Saadi S, Hooshmandi Z. The effect of Short-Term intraperitoneal injection of Fe2O4Zn nanoparticle on liver enzymes and tissue in male wistar laboratory rats. IJMRHS. 2016;5:92–100. Retrieved from http://www.ijmrhs.com/
49. Parang Z, Moghadamnia D. Effects of silver nanoparticles on the functional tests of liver and its histological changes in adult male rats. J. Nanomed. Res. 2018;3:146–153.
https://doi.org/10.22034/nmrj.2018.03.005
50. Ibrahim KE, Al-Mutary MG, Bakhiet AO, Khan HA. Histopathology of the liver, kidney, and spleen of mice exposed to gold nanoparticles. Molecules. 2018;23:1848.
https://doi.org/10.3390%2Fmolecules23081848
51. Jalili P, Huet S, Lanceleur R, et al. Genotoxicity of aluminum and aluminum oxide nanomaterials in rats following oral exposure. Nanomaterials. 2020;10:305.
https://doi.org/10.3390/nano10020305
52. Ansari MA, Khan HM, Khan AA, Alzohairy MA. Biochemical and histopathological ultrastructural changes caused by ZnO nanoparticles in mice. Environ. Toxicol. Chem. 2015;97:1025–1040.
http://dx.doi.org/10.1080/02772248.2015.1077960
53. Yu Y, Li Y, Wang W, et al. Acute toxicity of amorphous silica nanoparticles in intravenously exposed ICR mice. PloS One. 2013;8:e61346.
http://dx.doi.org/10.1371/journal.pone.0061346
54. Kohl Y, Rundén-Pran E, Mariussen E, et al. Genotoxicity of nanomaterials: advanced in vitro models and high throughput methods for human hazard assessment—a review. Nanomaterials. 2020;10:1911.
https://doi.org/10.3390/nano10101911
55. Pfuhler S, Downs TR, Allemang AJ, Shan Y, Crosby ME. Weak silica nanomaterial-induced genotoxicity can be explained by indirect DNA damage as shown by the OGG1-modified comet assay and genomic analysis. Mutagenesis. 2017;32:5–12.
http://dx.doi.org/10.1093/mutage/gew064
56. Evans SJ, Clift MJ, Singh N, et al. Critical review of the current and future challenges associated with advanced in vitro systems towards the study of nanoparticle (secondary) genotoxicity. Mutagenesis. 2017;32:233–241.
https://doi.org/10.1093%2Fmutage%2Fgew054
57. Yazdimamaghani M, Moos PJ, Dobrovolskaia MA, Ghandehari H. Genotoxicity of amorphous silica nanoparticles: Status and prospects. Nanotechnol. Biol. Med. 2019;16:106–125.
https://doi.org/10.1016/j.nano.2018.11.013
58. Shukla RK, Kumar A, Gurbani D, Pandey AK, Singh S, Dhawan A. TiO2 nanoparticles induce oxidative DNA damage and apoptosis in human liver cells. Nanotoxicology. 2013;7:48–60.
https://doi.org/10.3109/17435390.2011.629747
59. Li Y, Yan J, Ding W, Chen Y, Pack LM, Chen T. Genotoxicity and gene expression analyses of liver and lung tissues of mice treated with titanium dioxide nanoparticles. Mutagenesis. 2017;32:33–46. 10.1093/mutage/gew065
60. Relier C, Dubreuil M, Lozano Garcìa O, et al. Study of TiO2 P25 nanoparticles genotoxicity on lung, blood, and liver cells in lung overload and non-overload conditions after repeated respiratory exposure in rats. Toxicol. Sci. 2017;156:527–537.
http://dx.doi.org/10.1093/toxsci/kfx006
61. Wahab R, Siddiqui MA, Saquib Q, Dwivedi S, Ahmad J, Musarrat J, Al-Khedhairy AA, Shin H-S, et al. ZnO nanoparticles induced oxidative stress and apoptosis in HepG2 and MCF-7 cancer cells and their antibacterial activity. Colloids Surf. B. 2014;117:267–276.
http://dx.doi.org/10.1016/j.colsurfb.2014.02.038
62. Pfuhler S, Downs TR, Allemang AJ, Shan Y, Crosby ME. Mutagenesis. 2017;32:5–12.
http://dx.doi.org/10.1093/mutage/gew064
63. Nemmar A, Yuvaraju P, Beegam S, Yasin J, Kazzam EE, Ali BH. Toxicity of nanoparticles. Int. J. Nanomed. 2016;11:919.
http://dx.doi.org/10.2147/IJN.S92278
64. Elje E, Mariussen E, Moriones OH, et al. Hepato (Geno) toxicity assessment of nanoparticles in a hepg2 liver spheroid model. Nanomaterials. 2020;10:545.
https://doi.org/10.3390%2Fnano10030545
65. Li Y, Bhalli JA, Ding W, et al. Cytotoxicity and genotoxicity assessment of silver nanoparticles in mouse. Nanotoxicology. 2014;8:36–45.
https://doi.org/10.3109/17435390.2013.855827
66. Bin-Jumah MN, Al-Abdan M, Al-Basher G, Alarifi S. Molecular mechanism of cytotoxicity, genotoxicity, and anticancer potential of green gold nanoparticles on human liver normal and cancerous cells. Dose-Response. 2020;18:1559325820912154.
https://doi.org/10.1177%2F1559325820912154
67. Conway GE, Shah U-K, Llewellyn S, et al. Adaptation of the in vitro micronucleus assay for genotoxicity testing using 3D liver models supporting longer-term exposure durations. Mutagenesis. 2020;35:319–330.
https://doi.org/10.1093/mutage/geaa018
68. Llewellyn SV, Conway GE, Shah U-K, et al. Advanced 3D liver models for in vitro genotoxicity testing following long-term nanomaterial exposure. JoVE. 2020. e61141.
https://doi.org/10.3791/61141
69. Liu H-L, Yang H-L, Lin B-C, et al. Toxic effect comparison of three typical sterilization nanoparticles on oxidative stress and immune inflammation response in rats. Toxicol. Res. 2015;4:486–493.
https://doi.org/10.1039/C4TX00154K
70. Gaiser BK, Hirn S, Kermanizadeh A, et al. Effects of silver nanoparticles on the liver and hepatocytes in vitro. Toxicol. Sci. 2013;131:537–547.
https://doi.org/10.1093/toxsci/kfs306
71. Manna P, Ghosh M, Ghosh J, Das J, Sil PC. Contribution of nano-copper particles to in vivo liver dysfunction and cellular damage: Role of IκBα/NF-κB, MAPKs and mitochondrial signal. Nanotoxicology. 2012;6:1–21.
https://doi.org/10.3109/17435390.2011.552124
72. Zhu B, Li Y, Lin Z, Zhao M, Xu T, Wang C, Deng N. Silver nanoparticles induce HePG-2 cells apoptosis through ROS-mediated signaling pathways. Nanoscale. Res. Lett. 2016;11:1–8.
http://dx.doi.org/10.1186/s11671-016-1419-4
Table 1Mode of action of different metallic nanoparticles in inducing oxidative stress
Table 2Mode of action of different metallic nanoparticles in inducing histopathological alterations
Table 3Mode of action of different metallic nanoparticles in inducing genotoxicity
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||