AbstractThe rising demand for cleaner energy among the nations has turned the focus from fossil fuel-based energy sources to renewables like biomass, solar, wind, etc. Biomass conversion to fuel has increased research in recent times due to its ease and availability throughout the year. Hydrothermal liquefaction is the process where biomass converts to a liquid product via complex reaction mechanisms. This review aims to summarize the hydrothermal liquefaction of algal biomass and the improvements in the bio-crude yield using heterogeneous and homogenous catalysts. Many references have been reviewed to provide the sources for the process and have been critically well structured. This review also provided information regarding the reaction pathway for algal biomass and the effects of process parameters like temperature, residence time, pH, etc. The focus of the review is on the effects of various catalysts based on their dosage whose results collected from various sources have been tabulated. The review briefly discusses the applications of products formed during hydrothermal liquefaction after post-processing.
Graphical Abstract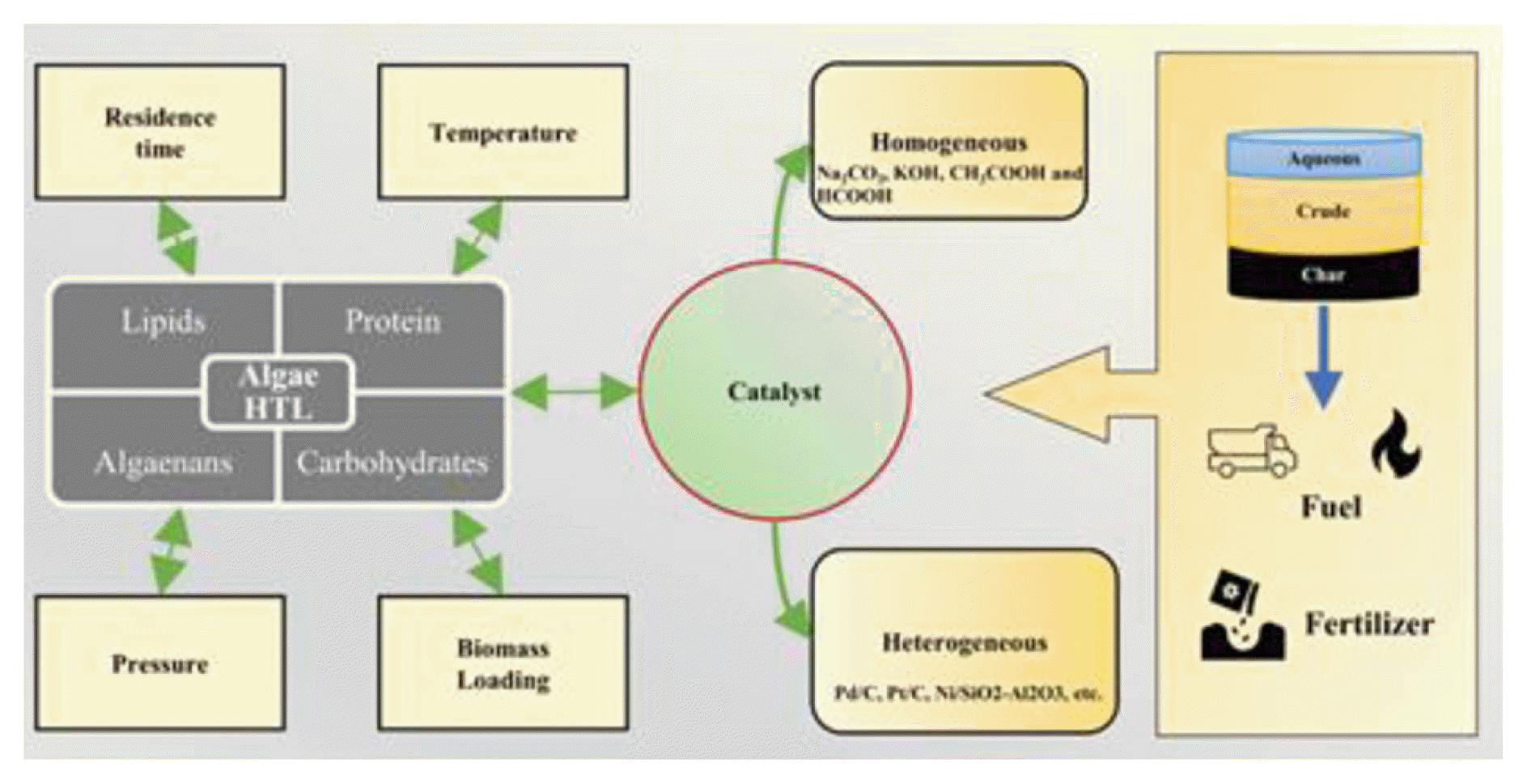
1. IntroductionFossil fuel plays a major role in our global energy needs. In recent times the world faces a difficult situation wherein the usage of fossil fuel is getting costlier day by day due to its non-renewable nature. In this respect, biofuel is considered one of the best alternative sources of renewable energy, to reduce the usage of fossil fuels [1] and also minimalize CO2 emissions and harmful gases [2–4]. Biomass is renewable organic material that comes from food crops (1st generation), agricultural and forest wastes (2nd generation), algae (3rd generation), and municipal wastes (4th generation). From the above, the first generation of biomass is not suitable for conversion as it would directly affect the food source, and biofuels made using 2nd and 4th generation biomass are less productive than 3rd generation biomass. There are multiple advantages to using algae as a feedstock for biofuel due to its ease of production [5–7]. Multiple studies have been performed to analyze the efficiency of algae as a rich source of biofuel, where different types of algae (micro and macro) were considered along with various homogenous and heterogeneous catalysts. Ali A.Jazie et al studied the effects of Hβ zeolite as a functional catalyst to improve the yield of F.vesiculosus algae which produced and yield of 38.47% [8]. Nguyen Thúy Lan Chi et al proposed a technique where the bio-char produced through thermochemical conversion can be utilized as a catalyst for the biofuel production from algal biomass, showing that the selection of catalyst in hydrothermal processing is important as the quality and property of the biofuel varies accordingly [9].
The ways through which the algal feedstock is converted into biofuel are classified into Thermal, Chemical, Thermochemical, and Biochemical [7, 10]. The thermochemical methods are further classified into the dry and wet processes. In Dry thermochemical conversion, the feedstock should be free from moisture or with a minimal amount of moisture. Whereas in wet thermochemical conversion, the feedstock can contain moisture or liquid [11].
1.1. Dry Thermochemical ConversionIt consists of several methods like pyrolysis, torrefaction, carbonization, liquefaction, and gasification, mostly the feedstock needs to be moisture free [11]. Carbonization is a slow process where the biomass is heated in the inert atmosphere to produce a carbon product, pyrolysis is a process where the biomass is burnt in an inert atmosphere mostly in nitrogen at around 300°C – 900°C [12], liquefaction is divided into direct and indirect, where the biomass is liquefied at around 160 to 280°C in the presence of glycerol [13], gasification is the process where the biomass is heated above 1000K under inert atmosphere to produce gaseous fuels [14].
1.1.1. PyrolysisThe conversion technique in which moisture-free biomass is heated in the presence of nitrogen, are classified into flash/fast pyrolysis, slow pyrolysis based on the heating rate, microwave-assisted pyrolysis based on the heating medium, and hydropyrolysis which takes place in the high-pressure hydrogen environment. Selection of the right kind of conversion method is critical to obtaining desired results [15]. Slow pyrolysis typically happens at a very low heating rate in the range of 5–10°C / min, this technique produces an estimate of 43% by volume of oil as investigated by Scott Grierson et al [16]. Fast pyrolysis in the contrast has a higher heating rate in the range of 100 – 200°C/s, and very little residence time during the reaction, higher heating rates upwards of 500°C were also studied by Xiaoling Miao et al to produce higher grade bio-oil, the product characterization showed that the bio-oil produced through fast pyrolysis contains lesser oxygen content compared with slow pyrolysis [17].
1.1.2. TorrefactionThe process of heating the biomass at lower temperatures compared with pyrolysis in the range of 200–300°C [18] in the atmospheric pressure in the presence of nitrogen to produce solid char as the main product, liquid, and gas as by-products. The conversion method is classified into two categories wet and dry torrefaction based on the temperature and residence time, both types have distinct advantages and disadvantages when used in the conversion of microalgae to solid fuel. Since dry torrefaction (200°C–300°C) is simply mild pyrolysis, there is a requirement for dry torrefaction that the moisture of the biomass is reduced to 10% to get high performance, in contrast, wet torrefaction (180°C–260°C) requires a complex reactor system to maintain high pressure which ultimately increases the cost of the overall process [19]. The primary advantage of both processes is the superior characteristics of the bio-char produced, and the bio-char produced can be filtered, dried, and can be used directly as fuel without further enhancement [20].
1.2. Wet Thermochemical ConversionWet thermochemical conversion is classified into three methods viz. Hydrothermal carbonization, Hydrothermal liquefaction and Hydrothermal gasification. Hydrothermal carbonization mainly focuses on the production of solid products (bio-char) and this process is carried out at low to medium temperatures (180°C–275°C) and at lower pressure below 2MPa [21]. Hydrothermal liquefaction emphasizes the production of a liquid product termed bio-crude which is further converted into bio-oil after treatment, this process takes place at medium to high temperatures (280°C–370°C) and pressure ranging between 10–25MPa [22]. Hydrothermal gasification is also identified as supercritical water gasification (SCWG), in this process the primary product is combustible high calorific value gases. This gasification process is carried out at temperatures around 400°C–700°C and pressure 25MPa-30MPa [10].
1.2.1. Hydrothermal liquefactionHydrothermal liquefaction is a process where biomass either in the presence of a catalyst or without a catalyst is converted into bio-crude. In hydrothermal liquefaction the temperature is around 280°C – 370°C and the pressure around 10MPa – 25MPa [23]. The yield and efficiency of the process depend upon the processing conditions like temperature, pressure, biomass loading and residence time, the conversion of biomass to product also depends on the algal strain used, based on the information multiple attempts are made with various processes conditions to produce a liquid product. Hydrothermal liquefaction is where the depolymerization of algal feedstock takes place, which helps in the production of bio-crude [24]. The produced bio-crude has higher energy content than produced in pyrolysis along with lower energy consumption [10, 25]. The higher heating value is shown in Fig. S1, obtained through hydrothermal liquefaction is an important factor if the produced bio-crude is to be used as fuel. The proximate and ultimate analysis of algae species are given in Table 1 and Table 2, this data helps in the identification of suitable micro/macroalgae species for further processing.
2. Chemistry of the ProcessVery little information is known about the chemistry behind the hydrothermal liquefaction process, the basic process that happens is that during the process water will be at a subcritical condition and hence will involve in the decomposition of the biomass into bio-crude and other valuable products [40]. The main composition of microalgal species is carbohydrates, protein, and lipids [41]. These components undergo degradation in subcritical water to give a basic reaction mechanism of biomass decomposition, depolymerization (dehydration, decarboxylation, and deamination), and the recombination process. Even though hydrothermal liquefaction conversion is difficult to understand, only very few authors have identified the conversion pathways [10, 42]. The conversion pathway for hydrothermal liquefaction is shown in Fig. S2.
2.1. Mechanism of Carbohydrates in Hydrothermal LiquefactionCarbohydrates are complex sugars like cellulose, hemicellulose, polysaccharides and starch [43]. When these components undergo hydrothermal processing it rapidly forms glucose and a few other saccharides via hydrolysis, which further undergo degradation. In this regard, the hydrolysis rate depends upon the nature of polysaccharides like starch and hemicellulose break down quicker compared to cellulose [10].
2.1.1. CelluloseCellulose on the whole contains glucose units linked using beta-linked D-Glucose units, which help in the formation of stronger inter and intramolecular hydrogen bonds. Due to the nature of cellulose being linear chained and crystalline it does not undergo any significant deformation at low temperatures like starch [44]. Cellulose fiber is one of the most abundant organic polymers in the world, also a hydrophilic compound that does not dissolve in any organic solvents or water at low temperatures. They are complex carbohydrates that are essential in bio-oil formation and oil yield. These during the reaction process get hydrolyzed into simple sugars, based on the type of algae (high lipid and low lipid) used about 10–20% of the overall yield is obtained from carbohydrates. Based on the studies found that cellulose depolymerizes at temperatures below 400°C to form benzene, propionic acid, and 4-hydroxyphenyl alcohol [27] and alcohols and phenols [45]
2.1.2. HemicelluloseAs a heteropolymer consisting of various sugars like glucose, galactose, mannose and xylose. Due to the presence of a side group a less uniform structure of the hemicellulose. Around 30% of the biomass comprises hemicellulose and they vary according to the biomass type taken because the structure of the hemicellulose is branched they are weaker than cellulose and are easily degradable at low temperatures above 180°C and also hydrolysis in both acid and base catalyst[43]. The main products obtained through hydrothermal liquefaction of hemicellulose are furfurals, carboxylic acids, and aldehydes at higher temperatures around 300°C [46].
2.1.3. StarchStarch is made up of polysaccharides which consist of monomers like glucose connected with α-(1→6), β-(1→4) bonds. α-(1→4) is glucopyranoside which is a polymer and is soluble in cold water. β-(1→6) is a glucoside branch that is insoluble in cold water [47]. In the hydrothermal liquefaction process, It is usually referred to as amylum, as it contains two different molecules like amylose (helical), amylopectin (branched), and a liner structure. Though starch and cellulose have similar properties, starch is insoluble in cold water and is the most common type of carbohydrate available [48, 49]. During the processed starch first gets converted into small fragments of light molecular compounds through decarboxylation and hydrolysis, after which through cyclization and condensation the water-soluble organics get converted into new compounds and bio-char [48, 50].
2.2. Mechanism of ProteinProtein is the prime constituent of microbial biomass. Generally, protein consists of one or many peptide chains, which give rise to the chain of amino acids. The proteins are made up of amino acids that are grouped into 21 groups based on their side chain properties. All are linked through a peptide bond, which undergoes decarboxylation and deamination reaction. As the result of these reactions, the produced products are acids, amines and aldehydes [51].
A peptide bond is the one that links the amino acids primarily an amide bond between amino and carboxyl groups [52]. Nitrogen is the important molecule present in the protein due to its presence in amino groups. Thus it is the main contributor in terms of nitrogen content in both the aqueous phase and organic phase, the nitrogen molecules are hydrolyzed and present in the aqueous phase as amine compounds and those compounds are not in the form of pyrrole or pyrazine in the bio-crude [53]. Since the peptide bond in the protein is far more stable than the glucoside bonds present in starch and cellulose due to slow hydrolysis occurs. The yield of amino acids during hydrothermal liquefaction is very minimal compared to the conventional process due to the rapid hydrolysis of amino acids in the hydrothermal liquefaction (HTL) condition [10, 35].
2.3. Mechanism of LipidsLipids are fats present in organisms and they have similar chemical structures to the hydrocarbons used as fuels. And it has an aliphatic character, which is referred to chemically as triacylglycerides (TAG), a compound of fatty acids and glycerol [54]. Fats and oils are insoluble in ambient conditions, but triacylglycerides are easily hydrolyzed under subcritical conditions with or without the use of catalysts. As the result of hydrolysis, the triacylglycerides are converted into glycerol and fatty acids [55].
2.3.1. Glycerol and fatty acidsAs one of the main products formed during hydrolysis, it is an important compound used in bio-crude production. It can synthesize specialty chemicals that can be used for many propose. On hydrolysis, triacylglycerides degrade to produce acrolein, acetaldehyde, propionaldehyde, methanol, allyl alcohol, formaldehyde, ethanol and gas products, mainly CO2, CO, and H2. Fatty acids have higher thermal stability but they are converted into long-chain hydrocarbons, which have good properties for energy or fuel [10, 56]. The conversion pathway for proteins, lipids and carbohydrates is shown in Fig. 1.
2.3.2. Mechanism of AlgaenansAlgaenan’s conversion process is important in the conversion of microalgae in hydrothermal liquefaction. But not many studies are conducted on the conversion of algaenans. This is a macropolymer of hydrocarbon, which has the characteristic of resistance to chemical treatment than other microalgae fractions. Its resistance property makes it difficult to study and analyze. There is not much data available on the hydrothermal liquefaction of Algaenans [10].
2.5. H/C, O/C, N/C RatioTo identify the effectiveness of produced bio-crude as a potential fuel, the molar ratios of hydrogen, oxygen and carbon of bio-crude are plotted against the values of known liquid fuels in the Van-Krevelen plot. When compared with existing fossil fuel data, the obtained bio-crude is found to have a higher percentage of oxygen and hydrogen, which shows the need for a further purification process. The H/C vs O/C ratios of a few algal bio-crude is given in Fig. 2.
3. Non-Catalytic Hydrothermal LiquefactionIn non-catalytic hydrothermal liquefaction, water plays a major role in both catalysts as well as solvents. When the Catalyst is not used for the hydrothermal liquefaction process water is used as a catalyst [57, 58]. The properties of water under subcritical conditions are very important to understanding the degradation pathway along with hydrothermal processing of biomass, at subcritical and critical conditions of water, a gas such as CO2, and O2 are miscible. Organic compounds are dissolved in water in critical and subcritical conditions. Whereas the inorganic compounds which use solvent will decrease in critical conditions [59–63]. The bio-crude yield concerning various temperatures is given in Table 3.
4. Catalytic Hydrothermal LiquefactionIt is widely demonstrated that the presence of a catalyst would promote the conversion of biomass into bio-crude which leads to higher yield and quality product. In hydrothermal liquefaction, different types of catalysts are used [48, 59, 71]. There are two types of catalysts that are being used in hydrothermal liquefaction, homogeneous catalysts and heterogeneous catalysts. The most widely used catalyst in hydrothermal liquefaction such as alkaline solutions are K2CO3, Na2CO3, LiOH, NaOH and KOH. The key role of catalysts is to decompose the macromolecules(cellulose and hemicellulose) present into small fragmented molecules that are unstable, reactive, and repolymerize into oil-based compounds [72]. For different species of microalgae, the bio-crude yield varies. It is due to the different lipid content present in the algae. For spirulina, the yield value is significantly increased for the catalyst NaOH. For the other catalyst spirulina bio-crude yield is lower and similar to each other. In the case of nanochloropsis, the bio-crude yield is maximum for the catalyst of KOH and the bio-crude yield for other catalysts is lower [73–76]. The bio-crude yield of four algal biomass in presence of an organic and inorganic catalyst is given in Fig. S3.
4.1. Homogenous CatalystUsually, hydrothermal liquefaction uses catalysts that are water-soluble at room temperature (e.g., Na2CO3, KOH, CH3COOH and HCOOH). As a result, they often encourage algae to liquefy by improving the hydrothermal process. Normally Na2CO3 is the most used homogenous catalyst in the hydrothermal liquefaction process [77, 78]. Because it will increase the bio-crude and solid residue yield among the homogenous catalyst. A carbonate, hydroxide and carboxylic acid homogenous catalyst does not appear to be very common in hydrothermal reactions. Due to their low susceptibility to catalyze the decarboxylation of fatty acids, aromatization and isomerization coupled with their non-recyclable nature [79]. The homogenous catalyst is used during the reaction to some degree, and because its recovery cost is high, it is difficult to select it for cyclic use [80–82]. Some of the commercially available homogenous catalyst ad their effectiveness on the bio-crude yield is summarized in Table 4.
4.2. Heterogeneous CatalystThe heterogeneous catalyst has the advantage in the separation of liquefied products from solid residue and it is reused after proper treatment [86]. The heterogeneous catalysts are widely used due to their higher catalytic efficiency, low corrosion and higher bio-crude recovery. One of the most widespread disadvantages of homogenous catalysts is the damages that occur due to chemical reaction, which is not present in heterogeneous catalysts. Heterogeneous catalysts have been extensively studied for hydrothermal liquefaction, such as supported metal catalysts (Pt, Ni, Pd, Ru, and others) [79, 87]. In algae hydrothermal liquefaction, metal catalysts have a complex effect on bio-crude yields, but all catalysts do not increase yields significantly. Examples of materials with good catalytic desulfurization activity include Pd/C, Pt/C, Ni/SiO2-Al2O3, and CoMo/*-Al2O3 [38, 77, 88]. Table 5. provides the summary of heterogeneous catalyst and their yield.
5. Effects of Process Parameters on the Bio-Crude Yield5.1. TemperatureThe temperature of the hydrothermal process is crucial in the bio-crude formation and their yield, the addition of catalysts not only increases yield but also the characteristics of the bio-crude obtained [65, 88]. An increase in temperature usually leads to quicker depolymerization thus producing higher bio-crude yield along with gaseous products, but this also depends on the other process conditions like catalyst dosage, residence time, etc., thus bio-crude yield may vary at the same temperature due to variation in other process conditions or type of catalyst [94, 95]. One of the major reasons that hydrothermal liquefaction yield of algal biomass is higher compared to other sources is the presence of peptide bonds, during lower temperatures the peptide bonds are stable thus resulting in lower bio-crude yield. But at higher temperatures in the range of 300°C – 400°C the peptide bonds are less stable and rapidly hydrolyses to produce higher bio-crude yield [63, 96]. The yield differences due to temperature are shown in Fig. 3. which clearly shows that yield of bio-crude is increased with an increase in temperature.
5.2. PressureHydrothermal liquefaction is also influenced by pressure when it comes to decomposition and hydrolysis. An increase in pressure is used during the hydrothermal liquefaction process where higher enthalpies are not required in phase change [97, 98]. To identify the effect of pressure in the process during the experiments all the other parameters are kept constant while the pressure is varied, this showed that there is a significant increase in bio-oil as the pressure increases [99]. Pressures above the critical pressure can be maintained to control the rate of hydrolysis and biomass dissolution, which can increase the productivity of bio-crude through improved thermodynamics [100]. Catalytic run for the same conditions shows a decrease in the amount of oil produced, this is due to the blocking of catalyst active site by high-density solvent when the pressure is increased [101]. This emphasizes that selection of catalyst is crucial during high-pressure liquefaction.
5.3. Residence TimeResidence time is termed as the time required for the hydrothermal reaction to occur after the desired temperature or pressure is reached, this excludes heating and cooling time [102, 103]. This residence time is very crucial in obtaining higher quality bio-crude and other useful products. Lack of residence time may result in incomplete conversion, while excessive residence time can result in the decomposition of desirable products [39, 48, 104]. Small residence times are preferred over longer residence times during the hydrothermal liquefaction at higher temperatures as the decomposition and hydrolysis of components are rapid. The addition of a catalyst at the time would improve the conversion as it provides space for the reaction to occur much quicker than that process without it under the same process conditions [105, 106].
5.4. pHThe reaction medium pH is very important during the hydrothermal process, as the reaction pathway depends on pH, researchers have identified that hydrothermal liquefaction of biomass produces a higher yield in alkaline and acidic conditions depending upon the type of catalysts and solvents [107, 108]. The compounds like levulinic acids and 5-(Hydroxymethyl) furfural (5-HMF) do not decompose in acidic conditions, The biomass produces carboxylic acids function groups such as acetic acids and lactic acids, due to the self-degradation of H2O to H+ and OH− at very high temperatures where the pH does not affect the conversion [65, 109]. During the process, if the reaction condition is weakly alkaline as the reaction progresses the pH changes to acidic [109–112]. The products obtained during hydrothermal liquefaction are primarily liquid (bio-crude & aqueous), and the pH condition of the reaction medium is very important [113–116]. When the bio-crude yields are compared with the pH of the solution it is found that to ensure higher yield at a lower temperature the reaction medium is to be acidic but at the same time to produce higher yields at medium to higher temperatures the reaction medium is to be alkaline as given in Fig. S4. But it is very important to consider the fact that there will be a difference in yield at same process condition but with different catalysts as they tend to affect the reaction medium, so selection of catalyst is very important during hydrothermal process.
5.5. Biomass LoadingBiomass loading is crucial in determining the process and the yield to be obtained, during the hydrothermal liquefaction process the ratio of biomass to water will be lower compared with carbonization to facilitate hydrolysis to produce higher amounts of liquid products [117]. Maintaining the optimum loading is very important and it is reported that when biomass loading is increased the bio-crude yield will also increase but beyond a certain point, the yield will reduce due to degradation of biomass through cyclic formation, polymerization, and condensation reactions [118, 119]. Bita Motavaf and Phillip E. Savage (2021) reported that with biomass loading of 10–20% the yield of the bio-oil increased and reached the maximum of 30% at the critical temperature [120]. Gargi Goswami et al (2022) investigated the catalytic conversion of lipid-enriched microalgae and found out that the maximum yield of 28% was obtained at the biomass loading of 8.86% and corresponding catalyst dosage [121]. Christopher Jazrawi et al (2013) studied the continuous hydrothermal liquefaction of algae and found that at 350°C and loading of 10% produced a yield of 41.7 wt% [122]. Peter J.Valdez et al (2012) discovered that when the biomass loading for the hydrothermal liquefaction of Nannochloropsis sp. from 5–35 wt% showed an increased bio-crude yield of 36% to 46% [123]. Thus, multiple studies have reported that when the biomass loading is increased the bio-crude yield also increases but that depends on the biomass and the process conditions.
5.6. Catalyst DosageWhen considering catalytic hydrothermal liquefaction, the dosage of the catalyst is very important. The yield will not be optimum if there is too little or too much catalyst in the system [124]. Wenjia Wang et al investigated the catalytic hydrothermal liquefaction of Nannochloropsis sp. using metal-based TiO2 catalyst, the results showed that the yield of bio-crude increased from 30% to 42 % when Ni-based TiO2 is used, the results also showed a decrease in the yield of 29.06% if Fe was used, all the other process variable sare constant [89]. These two catalysts Na2CO3 and HCOOH are homogenous catalysts, which produces lower yield when compared with heterogeneous catalyst. For heterogeneous catalysts, Ni/Al2O3 and Co/Al2O3 are taken for the hydrothermal liquefaction process for chlorella vulgaris. When Ni/Al2O3 is used as the catalyst for the reaction the bio-oil yield is around 30% at a temperature of 350°C and for Co/Al2O3 bio-oil yield is around 38.7% at a temperature of 350°C [125].
5.7. Bio-Crude YieldThe yields of bio-crude and other end products produced by hydrothermal liquefaction of microalgae with and without catalyst are shown in Tables 3, 4 & 5. Peigao Duan and Phillip E. Savage while investigating the hydrothermal liquefaction of Nannochloropsis. sp using heterogenous catalyst found that bio-crude yield increased from 35% (No Catalyst) to around 65% (with Pd/C) [7]. With the detailed investigation of different kinds of literature, it was found that in the aspect of bio-crude yield is not only based on triglycerides but also includes carbohydrates, proteins and fibers [126]. Because hydrothermal liquefaction has such a high potential for conversion of different types of biomacromolecules into bio-crude, it applies to a wide range of microalgae and wet biomass. Though catalysts are different based on their chemical nature, structure, etc., it was found that few of them produced the same yields which are interesting but still in-depth discussion must be done to verfy the same [127, 128]. In few instances the bio-crude yield is low compared with non-catalytic conditions as the presence of catalyst at certain conditions will enable hydrocracking which reduces the molecular size and produce more volatile compounds rather than heavy liquid compounds [129, 130].
6. ApplicationThe algae biomass is converted into bio-crude, aqueous phase, and bio-char. Every end product has its own and different applications. The aqueous phase can be recycled for algal growth since it contains more amount of micro and macronutrient required for algal growth. And it is also used for agricultural and irrigation propose[131, 132]. The bio-char application is identified using the physicochemical characterization of hydrothermal liquefaction bio-char [133, 134]. This bio-char contains high nitrogen and carbon content along with inorganic metals like Mg, Ca, P, and K allowing the bio-char to be utilized in the soil in agricultural practices[135]. It will improve soil respiration, soil enzyme activity, seed germination, and soil respiration. The bio-char consists of surface-active moieties which help the bio-char act as a good absorbent for removing heavy metals and pollutants from wastewater. The refining and upgrading required for the possible ratio for blending with crude fuel are found using the physicochemical characterization of bio-oil. De-polymerization of the biomass of microalgae gives oxygen content which gives numerous oxygenated compounds such as furans, alcohols, phenols ketones, aldehydes, and other oxygenated compounds. High content of oxygen in the bio-crude will have various problems such as poor miscibility with fossil fuels, rendering side reactions of bio-crude low thermal stability, and highly reactive nature [119].
Low ratio H/C has a disadvantage in the fuel properties not allowing the direct application of bio-crude as a transport fuel for the vehicle. While these properties are not suitable for biofuels, it is clever to produce biofuels with the same properties as conventional fuels and also confirm the minimal emission of engines. It is important to carry out a standard crude oil refining process of the bio-crude before being utilized for blending commercial uses [136, 137].
7. ConclusionHydrothermal liquefaction of microalgae seems to be an emerging technology for bio-oil production. Compared with pyrolysis this method has quite a few advantages like no pre-removal of moisture is needed for the biomass, much less energy required and at lower temperatures, the process is not risky, and the yield obtained though a little less is still a great advantage over pyrolysis, the disadvantage is the amount of hydrogen and oxygen in the final product that reduces the calorific value. This can be seen in the elemental analysis of the produced bio-crude, to rectify this drawback the use of external agents like catalysts are used. At earlier stages, homogenous catalysts (inorganic & organic) were used but the recent development of heterogeneous catalysts was found to be more attractive in some cases but they are still in the developmental stage and cost more than homogenous catalysts. The presence of catalysts not only improves the bio-crude yield but also reduces the by-product formation and parallel reactions that would produce undesirable products. The end products can be specifically produced by changing the catalysts.
In algae, hydrothermal liquefaction, Ni/TiO2, Pt/Al2O3, ZrO2/SO4 & Ru/C produced higher yields compared with other heterogeneous catalysts, on the other hand, sodium-based homogeneous catalysts produced higher yields. Based on a comprehensive understanding of the catalyst mechanism, the development of catalysts with higher activity and stability, longer lifespan, and lower cost remains important. In addition, collecting and purifying water-insoluble and water-soluble bio-crudes separately seems to be a better approach than treating them comprehensively.
AcknowledgmentsThe authors express their acknowledgments towards Kongu Engineering College for their facility support for this research. The authors would like to thank all the researchers who performed the experiments that provide the base of this review.
NotesFuture Prospective Though the hydrothermal liquefaction process produces higher-grade bio-crude at a much lesser cost than other conventional processes there is a long way to be more effective and economical. The hydrothermal process is still at large a lab-scale process with very little output that will not be sufficient if it is converted into industrial scale, so a lot of research and development is needed in this area to streamline the process and convert it into a large scale process that can compensate the demand in the future [40, 48]. Author Contributions S.S. (Ph.D.) & S.R.R (Assistant professor) wrote the manuscript and devised the methodology. C.D.V. (Professor) conceptualized and supervised the writing of manuscript. M.S. (Assistant professor) conducted the formal anaslysis and review of the manuscript. K.C.S. (Bachelor’s), D.C. (Bachelor’s) & G.A.R. (Bachelor’s) equally contributed to the data collection and curation of the manuscript. Reference1. Park JY, Jung S, Na YG, et al. Biodiesel production from the black soldier fly larvae grown on food waste and its fuel property characterization as a potential transportation fuel. Environ. Eng. Res. 2022;27:200704.
https://doi.org/10.4491/eer.2020.704
2. Yu G, Zhang Y, Schideman L, Funk T, Wang Z. Distributions of carbon and nitrogen in the products from hydrothermal liquefaction of low-lipid microalgae. Energ. Environ. Sci. 2011;4:4587.
https://doi.org/10.1039/c1ee01541a
3. Lopez Barreiro D, Zamalloa C, Boon N, et al. Influence of strain-specific parameters on hydrothermal liquefaction of microalgae. Bioresource Technol. 2013;146:463–471.
https://doi.org/10.1016/j.biortech.2013.07.123
4. Palomino A, Godoy-Silva RD, Raikova S, Chuck CJ. The storage stability of biocrude obtained by the hydrothermal liquefaction of microalgae. Renew. Energ. 2020;145:1720–1729.
https://doi.org/10.1016/j.renene.2019.07.084
5. Shuping Z, Yulong W, Mingde Y, et al. Production and characterization of bio-oil from hydrothermal liquefaction of microalgae Dunaliella tertiolecta cake. Energy. 2010;35:5406–5411.
https://doi.org/10.1016/j.energy.2010.07.013
6. Yu G, Zhang Y, Schideman L, Funk T, Wang Z. Hydrothermal liquefaction of low lipid content microalgae into bio-crude oil. TASABE. 2011;54:239–246.
https://doi.org/10.13031/2013.36241
7. Duan P, Savage PE. Hydrothermal liquefaction of a microalga with heterogeneous catalysts. Ind. Eng. Chem. Res. 2011;50:52–61.
https://doi.org/10.1021/ie100758s
8. Jazie AA, Haydary J, Abed SA, Al-Dawody MF. Hydrothermal liquefaction of Fucus vesiculosus algae catalyzed by Hβ zeolite catalyst for Biocrude oil production. Algal Res. 2022;61:102596.
https://doi.org/10.1016/j.algal.2021.102596
9. Chi NTL, Anto S, Ahamed TS, et al. A review on biochar production techniques and biochar based catalyst for biofuel production from algae. Fuel. 2021;287:119411.
https://doi.org/10.1016/j.fuel.2020.119411
10. López Barreiro D, Prins W, Ronsse F, Brilman W. Hydrothermal liquefaction (HTL) of microalgae for biofuel production: State of the art review and future prospects. Biomass Bioenerg. 2013;53:113–127.
https://doi.org/10.1016/j.biombioe.2012.12.029
11. Zhang L, Xu CC, Champagne P. Overview of recent advances in thermo-chemical conversion of biomass. Energ. Convers. Manage. 2010;51:969–982.
https://doi.org/10.1016/j.enconman.2009.11.038
12. Lee Y, Park J, Ryu C, et al. Comparison of biochar properties from biomass residues produced by slow pyrolysis at 500°C. Bioresource. Technol. 2013;148:196–201.
https://doi.org/10.1016/j.biortech.2013.08.135
13. Zhang S, Yang X, Zhang H, et al. Liquefaction of Biomass and Upgrading of Bio-Oil: A Review. Molecules. 2019;24:2250.
https://doi.org/10.3390/molecules24122250
14. Zhang J, Zhang X. The thermochemical conversion of biomass into biofuels. Verma D, Fortunati E, Jain S, Zhang X, editorsBiomass, Biopolymer-Based Materials, and Bioenergy. Woodhead Publishing; 2019. p. 327–368.
https://doi.org/10.1016/B978-0-08-102426-3.00015-1
15. Sekar M, Mathimani T, Alagumalai A, et al. A review on the pyrolysis of algal biomass for biochar and bio-oil – Bottlenecks and scope. Fuel. 2021;283:119190.
https://doi.org/10.1016/j.fuel.2020.119190
16. Grierson S, Strezov V, Ellem G, McGregor R, Herbertson J. Thermal characterisation of microalgae under slow pyrolysis conditions. J. Anal. Appl. Pyrol. 2009;85:118–123.
https://doi.org/10.1016/j.jaap.2008.10.003
17. Miao X, Wu Q, Yang C. Fast pyrolysis of microalgae to produce renewable fuels. J. Anal. Appl. Pyrol. 2004;71:855–863.
https://doi.org/10.1016/j.jaap.2003.11.004
18. Phusunti N, Phetwarotai W, Tekasakul S. Effects of torrefaction on physical properties, chemical composition and reactivity of microalgae. Korean J. Chem. Eng. 2017;35:503–510.
https://doi.org/10.1007/s11814-017-0297-5
19. Gan YY, Ong HC, Show PL, et al. Torrefaction of microalgal biochar as potential coal fuel and application as bio-adsorbent. Energ. Convers. Manage. 2018;165:152–162.
https://doi.org/10.1016/j.enconman.2018.03.046
20. Akbari M, Oyedun AO, Kumar A. Techno-economic assessment of wet and dry torrefaction of biomass feedstock. Energy. 2020;207:118287.
https://doi.org/10.1016/j.energy.2020.118287
21. Aragón-Briceño CI, Pozarlik AK, Bramer EA, et al. Hydrothermal carbonization of wet biomass from nitrogen and phosphorus approach: A review. Renew. Energ. 2021;171:401–415.
https://doi.org/10.1016/j.renene.2021.02.109
22. Guo Y, Yeh T, Song W, Xu D, Wang S. A review of bio-oil production from hydrothermal liquefaction of algae. Renew. Sust. Energ. Rev. 2015;48:776–790.
https://doi.org/10.1016/j.rser.2015.04.049
23. Islam M, Park J-H. A short review on hydrothermal liquefaction of livestock manure and a chance for Korea to advance swine manure to bio-oil technology. J. Mater. Cycles Waste. 2016;20:1–9.
https://doi.org/10.1007/s10163-016-0566-0
24. Akhtar J, Saidina Amin NA. A review on process conditions for optimum bio-oil yield in hydrothermal liquefaction of biomass. Renew. Sust. Energ. Rev. 2011;15:1615–1624.
https://doi.org/10.1016/j.rser.2010.11.054
25. Chiaramonti D, Prussi M, Buffi M, Rizzo AM, Pari L. Review and experimental study on pyrolysis and hydrothermal liquefaction of microalgae for biofuel production. Appl. Energ. 2016;185:963–972.
https://doi.org/10.1016/j.apenergy.2015.12.001
26. Martinez-Fernandez JS, Chen S. Sequential Hydrothermal Liquefaction characterization and nutrient recovery assessment. Algal Research. 2017;25:274–284.
https://doi.org/10.1016/j.algal.2017.05.022
27. Toor SS, Reddy H, Deng S, et al. Hydrothermal liquefaction of Spirulina and Nannochloropsis salina under subcritical and supercritical water conditions. Bioresour. Technol. 2013;131:413–419.
https://doi.org/10.1016/j.biortech.2012.12.144
28. Dandamudi KPR, Luboowa KM, Laideson M, et al. Hydrothermal liquefaction of Cyanidioschyzon merolae and Salicornia bigelovii Torr.: The interaction effect on product distribution and chemistry. Fuel. 2020;277:118146.
https://doi.org/10.1016/j.fuel.2020.118146
29. Biswas B, Fernandes AC, Kumar J, Muraleedharan UD, Bhaskar T. Valorization of Sargassum tenerrimum: value addition using hydrothermal liquefaction. Fuel. 2018;222:394–401.
https://doi.org/10.1016/j.fuel.2018.02.153
30. Shan Y-Q, Yin L-X, Djandja OS, Wang Z-C, Duan P-G. Supercritical water gasification of waste water produced from hydrothermal liquefaction of microalgae over Ru catalyst for production of H2 rich gas fuel. Fuel. 2021;292:120288.
https://doi.org/10.1016/j.fuel.2021.120288
31. Han Y, Hoekman SK, Cui Z, Jena U, Das P. Hydrothermal liquefaction of marine microalgae biomass using co-solvents. Algal Research. 2019;38:101421.
https://doi.org/10.1016/j.algal.2019.101421
32. Chen Y, Zhao N, Wu Y, et al. Distributions of organic compounds to the products from hydrothermal liquefaction of microalgae. Environ. Prog. Sustain. 2017;36:259–268.
https://doi.org/10.1002/ep.12490
33. Gai C, Zhang Y, Chen W-T, et al. Characterization of aqueous phase from the hydrothermal liquefaction of Chlorella pyrenoidosa. Bioresource Technol. 2015;184:328–335.
https://doi.org/10.1016/j.biortech.2014.10.118
34. Tian C, Liu Z, Zhang Y, et al. Hydrothermal liquefaction of harvested high-ash low-lipid algal biomass from Dianchi Lake: effects of operational parameters and relations of products. Bioresource Technol. 2015;184:336–343.
https://doi.org/10.1016/j.biortech.2014.10.093
35. Gai C, Zhang Y, Chen W-T, Zhang P, Dong Y. An investigation of reaction pathways of hydrothermal liquefaction using Chlorella pyrenoidosa and Spirulina platensis. Energ. Convers. Manage. 2015;96:330–339.
https://doi.org/10.1016/j.enconman.2015.02.056
36. Guo B, Yang B, Silve A, et al. Hydrothermal liquefaction of residual microalgae biomass after pulsed electric field-assisted valuables extraction. Algal research. 2019;43:101650.
https://doi.org/10.1016/j.algal.2019.101650
37. Biller P, Ross A. Potential yields and properties of oil from the hydrothermal liquefaction of microalgae with different biochemical content. Bioresource Technol. 2011;102:215–225.
https://doi.org/10.1016/j.biortech.2010.06.028
38. Mathimani T, Mallick N. A review on the hydrothermal processing of microalgal biomass to bio-oil-Knowledge gaps and recent advances. J. Clean. Prod. 2019;217:69–84.
https://doi.org/10.1016/j.jclepro.2019.01.129
39. Eboibi B, Lewis D, Ashman P, Chinnasamy S. Effect of operating conditions on yield and quality of biocrude during hydrothermal liquefaction of halophytic microalga Tetraselmis sp. Bioresource Technol. 2014;170:20–29.
https://doi.org/10.1016/j.biortech.2014.07.083
40. Hao B, Xu D, Jiang G, et al. Chemical reactions in the hydrothermal liquefaction of biomass and in the catalytic hydrogenation upgrading of biocrude. ACS Sym. Ser. 2021;23:1562–1583.
https://doi.org/10.1039/D0GC02893B
41. Peterson AA, Vogel F, Lachance RP, et al. Thermochemical biofuel production in hydrothermal media: a review of sub-and supercritical water technologies. Energ. Environ. Sci. 2008;1:32–65.
https://doi.org/10.1039/B810100K
42. Toor SS, Rosendahl L, Rudolf A. Hydrothermal liquefaction of biomass: a review of subcritical water technologies. Energy. 2011;36:2328–2342.
https://doi.org/10.1016/j.energy.2011.03.013
43. Gollakota A, Kishore N, Gu S. A review on hydrothermal liquefaction of biomass. Renew. Sust. Energ. Rev. 2018;81:1378–1392.
https://doi.org/10.1016/j.rser.2017.05.178
44. Delmer DP, Amor Y. Cellulose biosynthesis. The plant cell. 1995;7:987.
https://dx.doi.org/10.1105%2Ftpc.7.7.987
45. Srokol Z, Bouche A-G, van Estrik A, et al. Hydrothermal upgrading of biomass to biofuel; studies on some monosaccharide model compounds. Carbohyd. Res. 2004;339:1717–1726.
https://doi.org/10.1016/j.carres.2004.04.018
46. Cao L, Zhang C, Chen H, et al. Hydrothermal liquefaction of agricultural and forestry wastes: state-of-the-art review and future prospects. Bioresource Technol. 2017;245:1184–1193.
https://doi.org/10.1016/j.biortech.2017.08.196
47. Nagamori M, Funazukuri T. Glucose production by hydrolysis of starch under hydrothermal conditions. J. Chem. Technol. Biot. 2004;79:229–233.
https://doi.org/10.1002/jctb.976
48. Hu T, Gong M, Feng S, Xu C, Bassi A. A review of recent developments of pre-treatment technologies and hydrothermal liquefaction of microalgae for bio-crude oil production. Renew. Sust. Energ. Rev. 2019;101:476–492.
https://doi.org/10.1016/j.rser.2018.11.037
49. Valdez PJ, Dickinson JG, Savage PE. Characterization of Product Fractions from Hydrothermal Liquefaction of Nannochloropsis sp. and the Influence of Solvents. Energy & Fuels. 2011;25:3235–3243.
https://doi.org/10.1021/ef2004046
50. Bi Z, Zhang J, Peterson E, et al. Biocrude from pretreated sorghum bagasse through catalytic hydrothermal liquefaction. Fuel. 2017;188:112–120.
https://doi.org/10.1016/j.fuel.2016.10.039
51. Rogalinski T, Herrmann S, Brunner G. Production of amino acids from bovine serum albumin by continuous sub-critical water hydrolysis. J. Supercrit. Fluid. 2005;36:49–58.
https://doi.org/10.1016/j.supflu.2005.03.001
52. Brunner G. Near critical and supercritical water. Part I. Hydrolytic and hydrothermal processes. J. Supercrit. Fluid. 2009;47:373–381.
https://doi.org/10.1016/j.supflu.2008.09.002
53. Hao B, Xu D, Jiang G, et al. Chemical reactions in hydrothermal liquefaction of biomass and in catalytic hydrogenation upgrading of biocrude. Green Chem. 2021;23:1562–1583.
https://doi.org/10.1039/D0GC02893B
54. Toor S, Rosendahl L, Rudolf A. Hydrothermal liquefaction of biomass: A review of subcritical water technologies. Energy. 2011;36:2328–2342.
https://doi.org/10.1016/j.energy.2011.03.013
55. Holliday RL, King JW, List GR. Hydrolysis of vegetable oils in sub-and supercritical water. Ind. Eng. Chem. 1997;36:932–935.
https://doi.org/10.1021/ie960668f
56. Lehr V, Sarlea M, Ott L, Vogel H. Catalytic dehydration of biomass-derived polyols in sub- and supercritical water. Catal. Today. 2007;121:121–129.
https://doi.org/10.1016/j.cattod.2006.11.014
57. Jindal M, Jha M. Hydrothermal liquefaction of wood: A critical review. Rev. Chem. Eng. 2016;32:459–488.
https://doi.org/10.1515/revce-2015-0055
58. Gu X, Martinez-Fernandez JS, Pang N, Fu X, Chen S. Recent development of hydrothermal liquefaction for algal biorefinery. Renew. Sust. Energ. Rev. 2020;121:109707.
https://doi.org/10.1016/j.rser.2020.109707
59. Chunyan T, Li B, Liu Z, Zhang Y, Lu H. ChemInform Abstract: Hydrothermal Liquefaction for Algal Biorefinery: A Critical Review. Renew. Sust. Energ. Rev. 2014;38:933–950.
https://doi.org/10.1016/j.rser.2014.07.030
60. Yang J-H, Shin H-Y, Ryu Y-J, Lee C-G. Hydrothermal liquefaction of Chlorella vulgaris: Effect of reaction temperature and time on energy recovery and nutrient recovery. J. Ind. Eng. Chem. 2018;68:267–273.
https://doi.org/10.1016/j.jiec.2018.07.053
61. Wądrzyk M, Janus R, Vos MP, Brilman DWF. Effect of process conditions on bio-oil obtained through continuous hydrothermal liquefaction of Scenedesmus sp. microalgae. J. Anal. Appl. Pyrol. 2018;134:415–426.
https://doi.org/10.1016/j.jaap.2018.07.008
62. López Barreiro D, Riede S, Hornung U, Kruse A, Prins W. Hydrothermal liquefaction of microalgae: Effect on the product yields of the addition of an organic solvent to separate the aqueous phase and the biocrude oil. Algal Res. 2015;12:206–212.
https://doi.org/10.1016/j.algal.2015.08.025
63. Kandasamy S, Zhang B, He Z, et al. Effect of low-temperature catalytic hydrothermal liquefaction of Spirulina platensis. Energy. 2020;190:116236.
https://doi.org/10.1016/j.energy.2019.116236
64. Yoo G, Park MS, Yang J-W, Choi M. Lipid content in microalgae determines the quality of biocrude and Energy Return On Investment of hydrothermal liquefaction. Appl. Energ. 2015;156:354–361.
https://doi.org/10.1016/j.apenergy.2015.07.020
65. Shakya R, Whelen J, Adhikari S, Mahadevan R, Neupane S. Effect of temperature and Na2CO3 catalyst on hydrothermal liquefaction of algae. Algal Res. 2015;12:80–90.
https://doi.org/10.1016/j.algal.2015.08.006
66. Biller P, Sharma BK, Kunwar B, Ross AB. Hydroprocessing of bio-crude from continuous hydrothermal liquefaction of microalgae. ACS SYM SER. 2015;159:197–205.
https://doi.org/10.1016/j.fuel.2015.06.077
67. Eboibi BE-O, Lewis DM, Ashman PJ, Chinnasamy S. Hydrothermal liquefaction of microalgae for biocrude production: Improving the biocrude properties with vacuum distillation. Bioresource Technol. 2014;174:212–221.
https://doi.org/10.1016/j.biortech.2014.10.029
68. Barreiro DL, Zamalloa C, Boon N, et al. Influence of strain-specific parameters on hydrothermal liquefaction of microalgae. Bioresource Technol. 2013;146:463–471.
https://doi.org/10.1016/j.biortech.2013.07.123
69. He Z, Xu D, Liu L, et al. Product characterization of multi-temperature steps of hydrothermal liquefaction of Chlorella microalgae. Algal Res. 2018;33:8–15.
https://doi.org/10.1016/j.algal.2018.04.013
70. Anastasakis K, Ross AB. Hydrothermal liquefaction of the brown macro-alga Laminaria Saccharina: Effect of reaction conditions on product distribution and composition. Bioresource Technol. 2011;102:4876–4883.
https://doi.org/10.1016/j.biortech.2011.01.031
71. Kiran Kumar P, Vijaya Krishna S, Verma K, et al. Bio oil production from microalgae via hydrothermal liquefaction technology under subcritical water conditions. J. Microbiol. Methods. 2018;153:108–117.
https://doi.org/10.1016/j.mimet.2018.09.014
72. Demirbas A. Effect of lignin content on aqueous liquefaction products of biomass. Energ. Convers. Manage. 2000;41:1601–1607.
https://doi.org/10.1016/S0196-8904(00)00013-3
73. ShankhaKoley S, Khadase M, Mathimani T, Raheman H, Mallick N. Catalytic and non-catalytic hydrothermal processing of Scenedesmus obliquus biomass for bio-crude production – A sustainable energy perspective. Energ. Convers. Manage. 2018;163:111–121.
https://doi.org/10.1016/j.enconman.2018.02.052
74. Muppaneni T, Reddy HK, Selvaratnam T, et al. Hydrothermal liquefaction of Cyanidioschyzon merolae and the influence of catalysts on products. Bioresour Technol. 2017;223:91–97.
https://doi.org/10.1016/j.biortech.2016.10.022
75. Zhang J, Qian C, Yan K, Song J, Jiang B. Hydrothermal Liquefaction of Enteromorpha prolifera for Bio-Crude Production: An Experimental Study on Aqueous Phase Recirculation. T. ASABE. 2019;62:1113–1119.
https://doi.org/10.13031/trans.13418
76. Leng L, Zhang W, Peng H, et al. Nitrogen in bio-oil produced from hydrothermal liquefaction of biomass: A review. Chem. Eng. J. 2020;401:126030.
https://doi.org/10.1016/j.cej.2020.126030
77. Nazari L, Yuan Z, Souzanchi S, Ray MB, Xu CC. Hydrothermal liquefaction of woody biomass in hot-compressed water: catalyst screening and comprehensive characterization of bio-crude oils. Fuel. 2015;162:74–83.
https://doi.org/10.1016/j.fuel.2015.08.055
78. Shakya R, Adhikari S, Mahadevan R, et al. Influence of biochemical composition during hydrothermal liquefaction of algae on product yields and fuel properties. Bioresour Technol. 2017;243:1112–1120.
https://doi.org/10.1016/j.biortech.2017.07.046
79. Xu D, Lin G, Guo S, et al. Catalytic hydrothermal liquefaction of algae and upgrading of biocrude: A critical review. Renew. Sust. Energ. Rev. 2018;97:103–118.
https://doi.org/10.1016/j.rser.2018.08.042
80. Möller M, Nilges P, Harnisch F, Schröder U. Subcritical Water as Reaction Environment: Fundamentals of Hydrothermal Biomass Transformation. Chemsuschem. 2011;4:566–79.
https://doi.org/10.1002/cssc.201000341
81. Hu Y, Gong M, Xu C, Bassi A. Investigation of an alternative cell disruption approach for improving hydrothermal liquefaction of microalgae. Fuel. 2017;197:138–144.
https://doi.org/10.1016/j.fuel.2017.02.022
82. Yang W, Li X, Liu S, Feng L. Direct hydrothermal liquefaction of undried macroalgae Enteromorpha prolifera using acid catalysts. Energ. Convers. Manage. 2014;87:938–945.
https://doi.org/10.1016/j.enconman.2014.08.004
83. Sharma N, Jaiswal KK, Kumar V, et al. Effect of catalyst and temperature on the quality and productivity of HTL bio-oil from microalgae: A review. Renew. Energ. 2021;174:810–822.
https://doi.org/10.1016/j.renene.2021.04.147
84. Hupfauf B, Süß M, Dumfort A, Fuessl-Le H. Cultivation of microalgae in municipal wastewater and conversion by hydrothermal carbonization: a review. ChemBioEng Reviews. 2016;3:186–200.
https://doi.org/10.1002/cben.201600008
85. Nagappan S, Bhosale RR, Nguyen DD, et al. Catalytic hydrothermal liquefaction of biomass into bio-oils and other value-added products–A review. Fuel. 2021;285:119053.
https://doi.org/10.1016/j.fuel.2020.119053
86. Yang J, Hong C, Xing Y, et al. Research progress and hot spots of hydrothermal liquefaction for bio-oil production based on bibliometric analysis. Environ. Sci. Pollut. R. 2021;28:7621–7635.
https://doi.org/10.1007/s11356-020-11942-2
87. Duan P, Savage P. Hydrothermal Liquefaction of a Microalga with Heterogeneous Catalysts. Ind. Eng. Chem. Res. 2011;50:52–56.
https://doi.org/10.1021/ie100758s
88. López Barreiro D, Gómez B, Ronsse F, et al. Heterogeneous catalytic upgrading of biocrude oil produced by hydrothermal liquefaction of microalgae: State of the art and own experiments. Fuel Process Technol. 2016;148:117.
https://doi.org/10.1016/j.fuproc.2016.02.034
89. Wang W, Xu Y, Wang X, et al. Hydrothermal liquefaction of microalgae over transition metal supported TiO2 catalyst. Bioresour. Technol. 2018;250:474–480.
https://doi.org/10.1016/j.biortech.2017.11.051
90. Xu D, Guo S, Liu L, et al. Heterogeneous catalytic effects on the characteristics of water-soluble and water-insoluble biocrudes in chlorella hydrothermal liquefaction. Appl. Energ. 2019;243:165–174.
https://doi.org/10.1016/j.apenergy.2019.03.180
91. Chen Y, Wu Y, Ding R, et al. Catalytic hydrothermal liquefaction of D. tertiolecta for the production of bio-oil over different acid/base catalysts. AICHE J. 2015;61:1118–1128.
https://doi.org/10.1002/aic.14740
92. Djandja OS, Wang Z, Chen L, et al. Progress in Hydrothermal Liquefaction of Algal Biomass and Hydrothermal Upgrading of the Subsequent Crude Bio-Oil: A Mini Review. Energ. Fuel. 2020;34:11723–11751.
https://doi.org/10.1021/acs.energyfuels.0c01973
93. Jiao J-L, Wang F, Duan P-G, Xu Y-P, Yan W-H. Catalytic hydrothermal gasification of microalgae for producing hydrogen and methane-rich gas. Energ. Source. Part A. 2017;39:851–860.
https://doi.org/10.1080/15567036.2016.1270375
94. Ji C, He Z, Wang Q, et al. Effect of operating conditions on direct liquefaction of low-lipid microalgae in ethanol-water co-solvent for bio-oil production. Energ. Convers. Manage. 2016;141:155–162.
https://doi.org/10.1016/j.enconman.2016.07.024
95. Jayaseelan A, Kannappan Panchamoorthy G, Sivaraman Jayachandran S, et al. Effects of Process Parameters on Hydrothermal Liquefaction of Microalgae Biomass Grown in Municipal Wastewater. Petrol Chem+. 2019;59:194–200.
https://doi.org/10.1134/S0965544119020026
96. Alimoradi S, Stohr H, Stagg-Williams S, Sturm B. Effect of temperature on toxicity and biodegradability of dissolved organic nitrogen formed during hydrothermal liquefaction of biomass. Chemosphere. 2020;238:124573.
https://doi.org/10.1016/j.chemosphere.2019.124573
97. Motavaf B, Savage P. Effect of Process Variables on Food Waste Valorization via Hydrothermal Liquefaction. ACS ES&T Engineering. 2021;1:363–374.
https://doi.org/10.1021/acsestengg.0c00115
98. Prapaiwatcharapan K, Sunphorka S, Kuchonthara P, Kangvansaichol K, Hinchiranan N. Single-and two-step hydrothermal liquefaction of microalgae in a semi-continuous reactor: Effect of the operating parameters. Bioresource Technol. 2015;191:426–432.
https://doi.org/10.1016/j.biortech.2015.04.027
99. Basar IA, Liu H, Carrere H, Trably E, Eskicioglu C. A Review on Key Design and Operational Parameters to Optimize and Develop Hydrothermal Liquefaction of Biomass for Biorefinery Applications. Green. Chem. 2021;23:1404–1446.
https://doi.org/10.1039/D0GC04092D
100. Chan YH, Yusup S, Quitain AT, et al. Effect of process parameters on hydrothermal liquefaction of oil palm biomass for bio-oil production and its life cycle assessment. Energy Convers. Manag. 2015;104:180–188.
https://doi.org/10.1016/j.enconman.2015.03.075
101. Jindal M, Jha M. Effect of process parameters on hydrothermal liquefaction of waste furniture sawdust for bio-oil production. RSC Adv. 2016;6:41772–41780.
https://doi.org/10.1039/C6RA02868C
102. Jiang J, Savage P. Metals and Other Elements in Biocrude from Fast and Isothermal Hydrothermal Liquefaction of Microalgae. Energ. Fuel. 2017;32:4118–4126.
https://doi.org/10.1021/acs.energyfuels.7b03144
103. Faeth J, Savage P. Effects of processing conditions on biocrude yields from fast hydrothermal liquefaction of microalgae. Bioresource Technol. 2016;206:290–293.
https://doi.org/10.1016/j.biortech.2016.01.115
104. Durak H, Genel S. Catalytic Hydrothermal Liquefaction of Lactuca scariola with a heterogeneous catalyst: the investigation of temperature, reaction time and synergistic effect of catalysts. Bioresource Technol. 2020;309:123375.
https://doi.org/10.1016/j.biortech.2020.123375
105. Hietala D, Godwin C, Cardinale B, Savage P. The independent and coupled effects of feedstock characteristics and reaction conditions on biocrude production by hydrothermal liquefaction. Appl. Energ. 2019;235:714–728.
https://doi.org/10.1016/j.apenergy.2018.10.120
106. Valdez P, Nelson M, Wang H, Lin X, Savage P. Hydrothermal liquefaction of Nannochloropsis sp.: Systematic study of process variables and analysis of the product fractions. Biomass Bioenerg. 2012;46:317–331.
https://doi.org/10.1016/j.biombioe.2012.08.009
107. Zhang Y, Yin Y, Funk T, Riskowski G. Effects of feedstock pH, initial CO addition, and total solids content on the thermochemical conversion process of swine manure. T. ASAE. 2001;44:697–701.
https://doi.org/10.13031/2013.6108
108. Yin S. Hydrothermal liquefaction of cellulose to bio-oil under acidic, neutral and alkaline conditions. Appl. Energ. 2012;92:234–239.
https://doi.org/10.1016/j.apenergy.2011.10.041
109. León M, Gomis A, García Á. Hydrothermal liquefaction (HTL) of animal by-products: Influence of operating conditions. Waste Manage. 2019;99:49–59.
https://doi.org/10.1016/j.wasman.2019.08.022
110. Biller P, Ross AB, Skill SC, et al. Nutrient recycling of aqueous phase for microalgae cultivation from the hydrothermal liquefaction process. Algal res. 2012;1:70–76.
https://doi.org/10.1016/j.algal.2012.02.002
111. Gai C, Zhang Y, Chen WT, et al. Characterization of aqueous phase from the hydrothermal liquefaction of Chlorella pyrenoidosa. Bioresour Technol. 2015;184:328–335.
https://doi.org/10.1016/j.biortech.2014.10.118
112. Han Y, Hoekman K, Jena U, Das P. Use of Co-Solvents in Hydrothermal Liquefaction (HTL) of Microalgae. Energies. 2019;13:124.
https://doi.org/10.3390/en13010124
113. Madsen R, Biller P, Jensen M, et al. Predicting the Chemical Composition of Aqueous Phase from Hydrothermal Liquefaction of Model Compounds and Biomasses. Energ. Fuel. 2016;30:10470–10483.
https://doi.org/10.1021/acs.energyfuels.6b02007
114. Ji-lu Z, Zhu M-Q, Wu H-t. Alkaline hydrothermal liquefaction of swine carcasses to bio-oil. Waste Manage. 2015;43:230–238.
https://doi.org/10.1016/j.wasman.2015.05.010
115. Shuping Z, wu Y, Yang M-D, Chun L, Tong J. Production and characterization of bio-oil from hydrothermal liquefaction of microalgae Dunaliella Tertiolecta cake. Energy. 2010;35:5406–5411.
https://doi.org/10.1016/j.energy.2010.07.013
116. Déniel M, Haarlemmer G, Roubaud A, Weiss-Hortala E, Fages J. Optimisation of bio-oil production by hydrothermal liquefaction of agro-industrial residues: Blackcurrant pomace (Ribes nigrum L.) as an example. Biomass Bioenerg. 2016;95:273–285.
https://doi.org/10.1016/j.biombioe.2016.10.012
117. Obeid R, Lewis D, Smith N, Hall T, Eyk P. Reaction kinetics and characterisation of species in renewable crude from hydrothermal liquefaction of monomers to represent organic fractions of biomass feedstocks. Chem. Eng. J. 2020;389:124397.
https://doi.org/10.1016/j.cej.2020.124397
118. Panneerselvam Sundar R, Kannappan Panchamoorthy G, Jayaseelan A, Kirubanandam Grace P. Hydrothermal Liquefaction of Scenedesmus abundans biomass spent for sorption of petroleum residues from wastewater and studies on recycling of post hydrothermal liquefaction wastewater. Bioresour. Technol. 2019;283:36–44.
https://doi.org/10.1016/j.biortech.2019.03.077
119. Sintamarean IM, Pedersen TH, Zhao X, Kruse A, Rosendahl LA. Application of algae as cosubstrate to enhance the process-ability of willow wood for continuous hydrothermal liquefaction. Ind. Eng. Chem. Res. 2017;56:4562–4571.
https://doi.org/10.1021/acs.iecr.7b00327
120. Motavaf B, Savage PE. Effect of Process Variables on Food Waste Valorization via Hydrothermal Liquefaction. ACS ES&T Engineering. 2021;1:363–374.
https://doi.org/10.1021/acsestengg.0c00115
121. Goswami G, Kumar R, Sinha A, et al. ALGLIQOL: A two stage integrated process towards synthesis of renewable transportation fuel via catalytic hydrothermal liquefaction of lipid enriched microalgae biomass and distillation. Energ. Convers. Manage. 2022;263:115696.
https://doi.org/10.1016/j.enconman.2022.115696
122. Jazrawi C, Biller P, Ross AB, et al. Pilot plant testing of continuous hydrothermal liquefaction of microalgae. Algal Res. 2013;2:268–277.
https://doi.org/10.1016/j.algal.2013.04.006
123. Valdez PJ, Nelson MC, Wang HY, Lin XN, Savage PE. Hydrothermal liquefaction of Nannochloropsis sp.: Systematic study of process variables and analysis of the product fractions. Biomass Bioenerg. 2012;46:317–331.
https://doi.org/10.1016/j.biombioe.2012.08.009
124. Thiruvenkadam S, Izhar S, Yoshida H, Danquah M, Harun R. Process application of Subcritical Water Extraction (SWE) for algal bio-products and biofuels production. Appl. Energ. 2015;154:815–828.
https://doi.org/10.1016/j.apenergy.2015.05.076
125. Duan P. Upgrading of Crude Duckweed Bio-Oil in Subcritical Water. Energ. Fuel. 2013;27:4729–4738.
https://doi.org/10.1021/ef4009168
126. Kandasamy S, Devarayan K, Bhuvanendran N, et al. Accelerating the production of bio-oil from hydrothermal liquefaction of microalgae via recycled biochar-supported catalysts. J. Environ. Chem. Eng. 2021;9:105321.
https://doi.org/10.1016/j.jece.2021.105321
127. Sharma N, Jaiswal K, Kumar V, et al. Effect of catalyst and temperature on the quality and productivity of HTL bio-oil from microalgae: A review. Renew. Energ. 2021;174:810–822.
https://doi.org/10.1016/j.renene.2021.04.147
128. Zhang B, Lin Q, Zhang Q, et al. Catalytic hydrothermal liquefaction of Euglena sp. microalgae over zeolite catalysts for the production of bio-oil. RSC Adv. 2017;7:8944–8951.
https://doi.org/10.1039/C6RA28747F
129. Ren R, Han X, Zhang H, et al. High yield bio-oil production by hydrothermal liquefaction of a hydrocarbon-rich microalgae and biocrude upgrading. Carbon Resour. Convers. 2018;1:153–159.
https://doi.org/10.1016/j.crcon.2018.07.008
130. Raikova S, Smith-Bädorf H, Bransgrove R, et al. Assessing hydrothermal liquefaction for the production of bio-oil and enhanced metal recovery from microalgae cultivated on acid mine drainage. Fuel Process Technol. 2016;142:219–227.
https://doi.org/10.1016/j.fuproc.2015.10.017
131. Makut B, Goswami G, Das D. Evaluation of bio-crude oil through hydrothermal liquefaction of microalgae-bacteria consortium grown in open pond using wastewater. Biomass Convers. Biorefin. 2020;12:2567–2581.
https://doi.org/10.1007/s13399-020-00795-x
132. Dandamudi KPR, Murdock T, Lammers PJ, Deng S, Fini EH. Production of functionalized carbon from synergistic hydrothermal liquefaction of microalgae and swine manure. Resour. Conserv. Recy. 2021;170:105564.
https://doi.org/10.1016/j.resconrec.2021.105564
133. Zheng C, Wang Q, Dai Y, et al. Performance of a modified alkaline lignin towards copper adsorption under different conditions. Environ. Eng. Res. 2022;27:210097–0.
https://doi.org/10.4491/eer.2021.097
134. Deng H, Li YF, Tao SQ, et al. Efficient adsorption capability of banana and cassava biochar for malachite green: Removal process and mechanism exploration. Environ. Eng. Res. 2022;27:200575–0.
https://doi.org/10.4491/eer.2020.575
135. Pedersen T, Rosendahl L. Production of fuel range oxygenates by supercritical hydrothermal liquefaction of lignocellulosic model systems. Biomass Bioenerg. 2015;83:206–215.
https://doi.org/10.1016/j.biombioe.2015.09.014
136. Déniel M, Haarlemmer G, Roubaud A, Weiss-Hortala E, Fages J. Modelling and Predictive Study of Hydrothermal Liquefaction: Application to Food Processing Residues. Waste Biomass Valori. 2017;8:2087–2107.
https://doi.org/10.1007/s12649-016-9726-7
137. Erkelens M, Ball AS, Lewis DM. The application of activated carbon for the treatment and reuse of the aqueous phase derived from the hydrothermal liquefaction of a halophytic Tetraselmis sp. Bioresource Technol. 2015;182:378–382.
https://doi.org/10.1016/j.biortech.2015.01.129
Table 1Proximity Analysis of Microalgae Species
Table 2Ultimate Analysis of Microalgae Species
Table 3Ultimate Analysis and Bio-Oil Yield without Catalyst
Table 4Ultimate Analysis and Bio-Oil Yield using Homogenous Catalysts
Table 5Ultimate Analysis and Bio-Oil Yield using Heterogeneous Catalyst
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||