AbstractWith the rapid development of printed circuit board (PCB) industry, the problem of the release of tetrabromobisphenol A (TBBPA) during PCB manufacturing processes was recognized and harmful to the receiving water body. In this study, TBBPA generation paths, fates in industrial wastewater in the PCB manufacturing processes were analyzed. Gas chromatograph-mass spectrometer (GC-MS) scanning results showed that the raw materials including solder mask ink, character ink and dry film mainly contained TBBPA. The PCB fabrication procedures, mainly including development, etching, stripping of inner layer manufacturing process, and pattern plating, tin-plating of outer layer manufacturing process, and development during the forming and surface treatment process would introduce TBBPA into wastewater. Flocculation and activated sludge process (ASP) that were commonly used for industrial wastewater treatment were selected to assess their removal capability for TBBPA in the wastewater, both which could remove more than 80% TBBPA. Low pH below 7, increased PAC and PAM dosages, and proper dosing during the slow-stirring stage could enhance the TBBPA removal of flocculation-sedimentation process. The ASP removes TBBPA mainly by the path of adsorption. High activated sludge concentration, low pH, medium-temperature and limited competitive adsorbates (NH4+, Cu2+) were beneficial to remove TBBPA by the ASP.
Graphical Abstract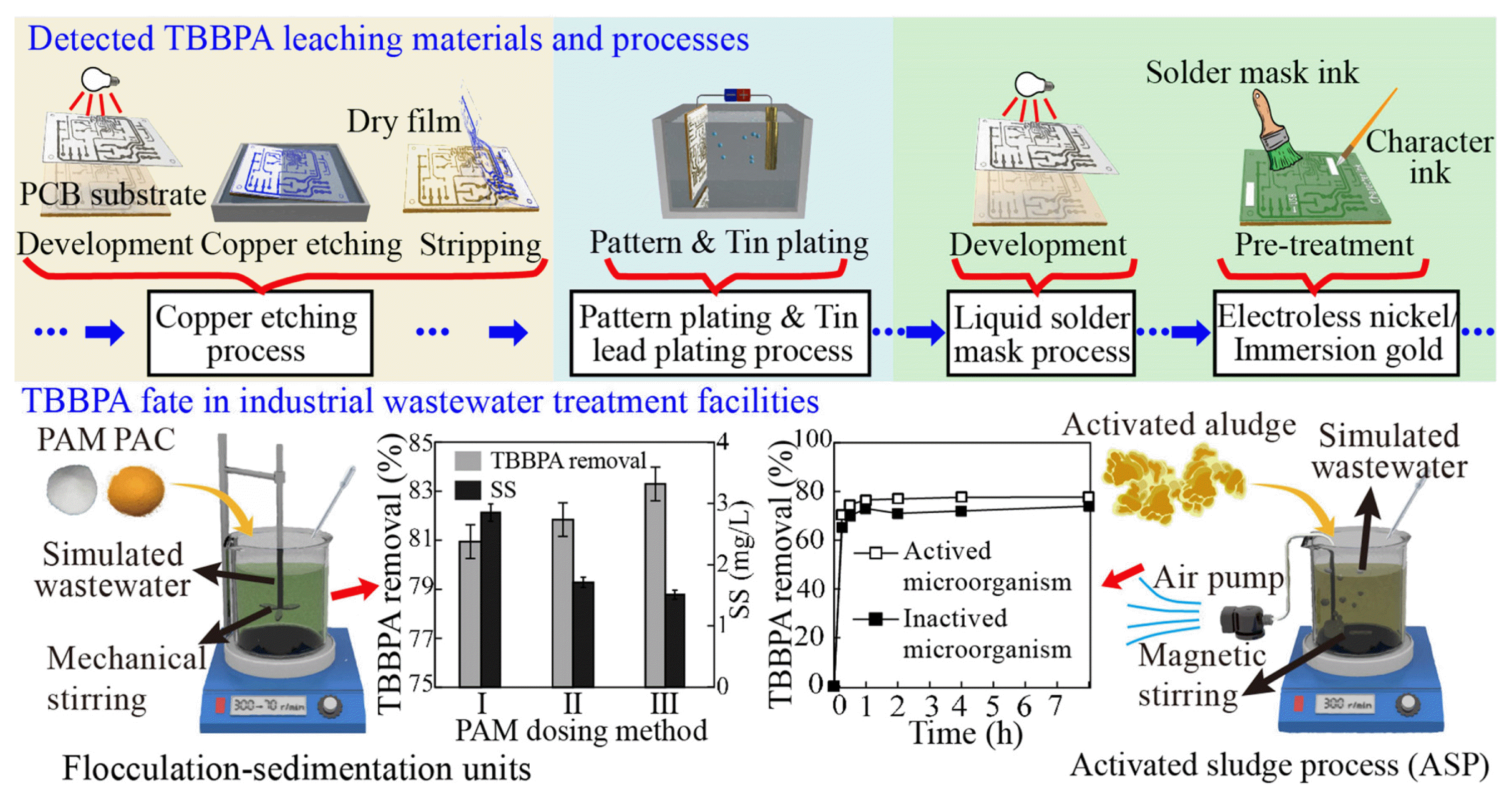
1. IntroductionWith the popularization and rapid upgrading of electronic equipment, the production demand of printed circuit boards (PCBs) is also growing dramatically. [1]. Tetrabromobisphenol A (TBBPA) is one of the most widely used brominated flame retardants (BFR) in the PCB industry due to its high-cost efficiency and wide compatibility with the boards [2]. However, TBBPA is a kind of endocrine disruption chemical (EDC), which is harmful to the environment and human health [3–5], and the leaking of TBBPA into the water bodies during the production process of PCB is always inevitable. Hence, exploring the actual generation paths of TBBPA to control its leakage in the PCB industrial process is highly necessary.
Many researchers have focused on the control and removal technologies of TBBPA in PCB industrial wastewater, especially on the new technology development. For example, microbial degradation [6], biochar adsorption [7], graphene-TiO2 photocatalysis [8], plasma oxidation [9], and Fenton oxidation [10] were proposed to degrade or remove TBBPA in water. However, due to the uncertainty of the TBBPA generation paths and the discharge behaviors during PCB manufacturing and treatment process, it is still difficult to control and remove TBBPA from real industrial wastewater effectively.
Fig. 1 shows a typical fabrication procedure of single-sided PCB [11]. Generally, the manufacturing process of PCB is mainly composed of four parts: exposure, development, etching, and plating [12,13]. TBBPA is mainly used in the PCB substrate as a brominated flame retardant to decrease the flammability of produced PCB. During the etching and plating processes, the PCB substrate was immersed in developer, etching solution and electroplating solution, respectively, and unreacted TBBPA was then probably dissolved into the aquatic solution and discharged to receiving wastewater. Under the condition of global sustainable development and reduction of carbon emissions, cleaner production, enacted by United Nations Environment Program (UNEP) at the end of the 1990s, has achieved a sympathetic response in environmental protection throughout the world [14,15]. Cleaner production emphasizes the importance of environmental protection in the whole lifecycle of the products [16], and highly requires the monitoring of the pollutants generation paths and fates in the industrial manufacturing, as well as their degradation and transformation in the wastewater treatment units.
From a comprehensive survey on the wastewater characterization and pollutants generation, and experimental analyses in an actual typical PCB manufacturing process, the generation and distribution of TBBPA were investigated. Traditional wastewater treatment units, mainly including coagulation-sedimentation and activated sludge process, were operated on a lab scale to explore their treatment performance for TBBPA, as well as the influential factors. Moreover, aiming to effectively control TBBPA from sources to treatment facilities in the PCB manufacturing processes, a comprehensive TBBPA control strategy was proposed for treatment process optimization. The obtained results are helpful for TBBPA generation and control in the PCB manufacture.
2. Materials and Methods2.1. Analytical MethodsGas Chromatograph with Mass Spectrometer (GC-MS, Agilent7890A/GC-5975C MS) was used to qualitatively detect the presence or generation of TBPPA [17] in the raw materials and manufacturing processes. Ultra-Performance Liquid Chromatography (UPLC, Waters H-Class) was used to quantitatively examine the TBBPA removals by flocculation-sedimentation and activated sludge processes in industrial wastewater [18]. The details were given in supporting information. Moreover, pH was tested using a pH meter (FE28-Standard, Mettler Toledo). The suspended solids (SS) of the sludge were tested by weighing method [19].
2.2. Sampling for TBBPA Generation Investigation2.2.1. Taking sample with raw materialsTBBPA that may be existed in the raw materials for PCB manufacturing was detected by using GC-MS, such as PCB substrate, dry film, character inks, solder mask inks, and prepreg. The solid materials were pre-treated for TBBPA leaching. Firstly, 3 g solid sample with its original shape and structure was placed in a 200 mL conical flask with 50 mL dichloromethane for extraction. After ultrasonic treatment for 10 min, the conical flask was sealed by parafilm and reset for 24 h. Subsequently, the extracting solution was transferred to a 50 mL centrifuge tube, and then centrifuged at 8000 r/min for 15 min. Afterward, the supernatant containing TBBPA was transferred into a 5 mL nitrogen blowpipe and concentrated by a pressure blowing concentrator (DCY-12S, Qingdao Haike Instrument Co., Ltd.) before being transferred to a GC-MS analyzer.
TBBPA in the liquid raw material, predominantly brown oxide solution, was also extracted. In brief, more than 50 mL brown oxide solution was sampled and placed in a 250 mL separating funnel, and about 1 g sodium chloride and 5 mL dichloromethane were added. This separating funnel was then shaken for 5 min, and the extracted solution was dehydrated by using anhydrous sodium sulfate, which was then transferred into a 10 mL nitrogen blowpipe. The extraction was repeated twice, and the extracting solution was concentrated by nitrogen blowing. Finally, the sampling solutions were analyzed by the GC-MS to evaluate the presence of TBBPA.
2.2.2. Sampling during PCB manufacturing processSimulated wastewater was synthesized for TBBPA tracing experiment. The simulated wastewater characteristics were based on six indicators of the real wastewater in the corresponding PCB manufacturing processes: pH, COD, NO2−-N, NH4+-N, NO3−-N, and TP, as listed in Table S1 and S2. Accurately 50 mL simulated wastewater was added to a 200 mL conical flask, and the raw materials were used in the corresponding simulated manufacturing process. The specific manufacturing processes and soaking times were concisely based on the real PCB manufactory processes given in Table S3, where the dosage of PCB substrate was 3 g, while the content of dry film, character inks, solder mask inks was kept at 1 g, respectively. After that, the soaking solution was transferred to a 50 mL centrifuge tube for centrifugation at 8000 r/min for 15 min. Then the supernatant was poured out into a 250 mL separating funnel for TBBPA extraction according to the extraction method described previously. For each simulation process, a control sample that only added PCB substrate was used to evaluate whether the PCB substrate itself would produce TBBPA during the manufacturing processes. All the obtained samples were qualitatively analyzed by using GC-MS.
2.3. TBBPA Fate Simulations in Traditional Industrial Wastewater Treatment UnitsTypical wastewater treatment processes were simulated to study the TBBPA fates during the traditional wastewater treatment units. Two main processes, flocculation-sedimentation units and ASP were studied after a survey of the PCB manufacturing factory. The wastewater was collected after each process, and the detailed sampling processes were listed in Fig. 2.
2.3.1. Flocculation-sedimentation unitsPolyaluminum chloride (PAC, ≥27.0, Tianjin Dingshengxin Chemical Co., Ltd., China) was used as coagulant, and polyacrylamide (PAM, ≥88.0, Beijing Keephway Technology Co., Ltd., China) was the coagulant aid. A flocculation test mixer (TA6-4, Wuhan Hengling Technology Co., Ltd., China) was equipped and the PAC and PAM were dosed instantly. Influences on TBBPA removal by flocculation-sedimentation by pH, PAC dosage, PAM dosage, and dosing method of PAM were also analyzed. The waste-water used herein was the mixture of domestic water, tap water and methanol solution of TBBPA. The ratio between domestic water and tap water was 1:1. The TBBPA concentration was set at 1 mg/L according to the literature [2,20]. The testing operation condition parameters included the pH ranging from 7.5 to 8.0, PAC dosage of 300 mg/L, and PAM dosage of 3 mg/L. Three different PAM dosing strategies were used to evaluate TBBPA removal efficiency by the flocculation-sedimentation method: PAM was dosed at (a) the first 30 s, (b) after 6 min reaction, and (c) after reaction completion of 15 min. Five pH values, 3.0, 5.0, 7.0, 9.0 and 11.0, were employed to examine the effect of pH on the TBBPA removal efficiency. The effect of PAC dosages was carried out by using the PAC dosage of 0, 100, 200, 300, 400, 500, and 600 mg/L, separately, where the pH was kept from 7.5 to 8.0 and without PAM addition. Different PAM dosages at 0, 0.1, 0.3, 0.5, 1, and 3 mg/L were also used to investigate the effects of PAM dosage on the TBBPA removal efficiency. The stirring modes of the coagulation mainly included 300 r/min for 30 s, 150 r/min for 6 min, 70 r/min for 15min, followed by sedimentation for 10 min. After sedimentation, the supernatant was collected and analyzed for TBBPA concentration.
2.3.2. Activated sludge process (ASP)Five identical setups were used for assessing the TBBPA removal performance by ASP, where the activated sludge was collected from the aerobic biological tank from the actual PCB industrial wastewater plant. The wastewater used was the mixture of activated sludge and the TBBPA dissolved in the methanol solution with an initial TBBPA content of 1 mg/L, as well as in the flocculation-sedimentation experiments. The volume of the reactors was 1 L, and the concentration of dissolved oxygen was controlled at around 4 mg/L. Magnetic stirring was used to stir the suspension to keep it fully mixed. The reaction time was 8 h, which is similar to the hydraulic retention time of the actual aeration tank in the PCB wastewater treatment plant. The bioreactor supernatant was sampled with a time interval of 0, 0.25, 0.5, 1, 2, 4, and 8 h for TBBPA concentration measurement.
3. Results and Discussion3.1. TBBPA Release Simulation in PCB Manufacturing Process3.1.1. TBBPA in the major raw materialsThe major raw materials used in the factory was listed in Table S4, among which, the etching solution, reducing agent, stripping solution, etc., mainly composed of simple mineral that was free of TBBPA. The PCB substrates, including dry film, character ink, solder mask ink, prepreg, and brown oxide solution containing complex compositions, were selected to assess their contribution toward TBBPA discharge. As displayed in Fig. 3a, the total ion chromatography of TBBPA (50 mg/L) dichloromethane solution showed the retention time of TBBPA was 18.108 min. Fig. 3b–d displayed the occurrences of the peaks of different areas near 18.108 min, and that of the solder mask ink was the largest one, followed by character ink and dry film, which meant the occurrences of TBBPA content in these materials. Meanwhile, the corresponding peaks in the brown oxide solution (Fig. 3e), prepreg (Fig. 3f), PCB substrate (Fig. 3g) were undetectable, indicating there was little or even no TBBPA in these materials. Thus, it is recognized that controlling the utilization of solder mask ink, character ink and dry film to avoid the possible emission directly from the raw materials is feasible to control TBBPA in the industrial wastewater.
3.1.2. TBBPA leaching behaviors during PCB manufacturingAs there was obvious TBBPA in raw materials, it was inevitable to leach TBBPA from raw materials into generated wastewater during PCB manufacturing, which needs to be clarified. The main PCB manufacturing processes were mainly divided into three parts (Fig. S1): inner layer manufacturing, outer layer manufacturing, and forming and surface treatment. The inner layer manufacturing is mainly composed of board cutting, lamination, development, exposure, etching, board lay-up and pressing processes, among which, the development step aims to remove the unreacted dry film after lye exposure, and the etching step uses acid etching solution to remove the exposed copper after development, and the stripping step wash away the dry film that protecting the copper surface to reveal the required circuit pattern. These processes would generate a large amount of wastewater, and hence it is necessary to trace the TBBPA leaching behavior during inner surface manufacturing. Similarly, the pattern plating step and tin-plating step in the outer layer manufacturing process, the development step and the electroless nickel/immersion gold step in the final forming and surface treatment process are related closely to the utilization of the TBBPA-containing materials. They were chosen to measure their TBBPA emission behaviors, and seven control groups were selected with the same operational process.
The TBBPA detection results were displayed in Fig. 4, and for the experimental groups, Fig. 4(a~f) showed the peaks appearing at 18.108 min. It means there was TBBPA releasing in the development and stripping steps during inner layer manufacturing, pattern plating and tin-plating steps during outer layer manufacturing, development and electroless nickel/immersion gold steps. Considering the peak area, the TBBPA emission followed a descending order of stripping, development (inner layer manufacturing), etching, pattern plating, development (forming and surface treatment), tinplating, and electroless nickel/immersion gold. Hence, more attention should be paid to those steps with TBBPA leaching. According to the control groups, it was found from Fig. 4 (h~m) that the peaks appear at 18.108 min, indicating the release of TBBPA. As there was only simulated wastewater and PCB substrate in these groups, the TBBPA appearance may be traced to the extraction in the PCB substrate. Moreover, the peak area at 18.108 min of Fig. 4k was larger than that in Fig. 4d, and then it was also suggested that the TBBPA releasing from PCB substrate in the pattern plating step took a very large proportion of the TBBPA in the industrial wastewater. In consequence, it is also concerned in well management of the waste PCB substrates during every manufacturing process.
3.2. TBBPA Fate Simulation in Flocculation-sedimentation ProcessThe detailed wastewater treatment facilities in the PCB factory are shown in Fig. S2. The whole water treatment process could be fractionated into primary physicochemical treatment, secondary biochemical treatment, and advanced physicochemical treatment. It is reasonable to note that the flocculation-sedimentation process and ASP were the main units for PCB waste-water treatment, which might be capable of removing TBBPA by means of adsorption or biodegradation [21,22]. Hence, the main concerns were focused on exploring TBBPA removal efficiency in these two processes, which helped to clarify the fates of TBBPA in the wastewater treatment facilities and their contribution in TBBPA control, and thus guide effective control strategies for TBBPA discharging into environments. Experimental tests were carried out to study the fate of TBBPA in the flocculation-sedimentation process and ASP, which were summarized in Table S5. Firstly, the TBBPA removal capability of the flocculation-sedimentation process was as follows.
3.2.1. Removal performanceTBBPA removal efficiency in the flocculation-sedimentation process was assessed by using PAC and PAM as their wide application as coagulants [23], and the initial TBBPA concentration was 1 mg/L. Fig. 5 illustrated the TBBPA removal efficiency by using three PAM dosing methods, which simulated the micro-flocculation stage, growth stage and the steady stage in the coagulation process [24]. The method I represents the coagulant dosing at the beginning, II means coagulant dosing during the middle stage with high-speed stirring, and III stands for dosing at the final 15 min, during the coagulation process. The TBBPA removal efficiency was 80.9%, 81.8% and 83.3%, at Method I, II and III, respectively. Overall, PAM was more suitable for adding at the slow stir stage to get a high TBBPA removal efficiency, which may be due to the less interference during flocculation. Meanwhile, it could be deemed that the TBBPA removal capacity of flocculation and sedimentation was about 80%.
3.2.2. Influencing factorsDuring the flocculation process, the pH, PAC and PAM dosages are crucial to impact the TBBPA removal efficiency [25]. Fig. 6a showed the removal efficiency of TBBPA under different pH when the TBBPA concentration of 1 mg/L and PAC dosage of 3 mg/L. When the pH was kept at 3.0, the TBBPA removal efficiency was 92%, and the SS in the effluent was 74 mg/L. With the increase of pH, the TBBPA removal efficiency decreased slightly when the pH reached 7. This removal efficiency dropped sharply from 87% to 52% once the pH continuously increased from 7 to 9. This may be due to the pKa1 of TBBPA being 7.5 [26], and when the pH was lower than 7.0, TBBPA was easy to leach from water. While the pH increased up to 7.0, TBBPA tended to dissolve in water, which then resulted in low TBBPA removal efficiency that was hard to be captured by the formed flocs. Furthermore, when the pH was 7.0, the SS in the effluent reached the lowest level of 1 mg/L, so the neutral pH was the optimized condition for TBBPA removal in wastewater by the flocculation-sedimentation method.
Fig. 6b demonstrated the effect of PAC dosage on the removal of TBBPA and SS. With the TBBPA concentration of 1 mg/L, and the pH of from 7.5 to 8.0, the PAC dosage from 0 to 600 mg/L was used. When there was without PAC added, only more than 6% TBBPA was removed, where the SS in the effluent was over 82 mg/L. Once the PAC was added, the removal efficiency of TBBPA increased to 36%, and the SS content decreased sharply to 7 mg/L. With the increased dosage of PAC, the TBBPA removal efficiency gradually increased to over 95%. Fig. 6c displayed the changes of sludge volume (SV) during the treatment by different PAC dosages, and when the PAC dosage increased from 100 to 300 mg/L, the settling rate of sludge increased slightly. However, when the PAC dosage was increased to larger than 300 mg/L, the formed flocs settled rapidly within 5 min, and almost 95% of flocs could be settled in 10 min. However, the continuous increase of PAC dosage to 600 mg/L resulted in a decelerated flocs settling velocity that may take 30 min, indicating the re-stabilization of formed flocs [27].
The effect of PAM dosage was displayed in Fig. 6d. As one common flocculation aid, PAM helps PAC to promote flocculation and enhance its adsorption capability. With the increase of PAM dosage, the TBBPA removal efficiency gradually improved, while the SS concentration in the effluent reduced simultaneously. When the PAM dosage was more than 0.5 mg/L, the TBBPA removal efficiency reached a steady level of as high as 86%, and the SS was kept below 1 mg/L in the effluent. As displayed in Fig. 6e, the formed flocs could be settled within 5 min when the PAM dosage was 3 mg/L, and it took more than 10 min when there was no PAM dosed.
Thus, taking the costs and co-existing pollutants removals into consideration, the optimized condition parameters for TBBPA by flocculation included a neutral pH of around 7.0, a PAC dosage of 300 mg/L, and a PAM dosage of 0.5 mg/L, under which the flocculation-sedimentation method could remove over 80% TBBPA. If the costs and other factors are not taken into account, the maximum TBBPA removal efficiency by the flocculation-sedimentation method was up to 95%.
3.3. TBBPA Fate Simulation in ASP3.3.1. Removal performanceASP is a wide applied wastewater treatment process [28,29], and has a certain capability in TBBPA removal, which should be clarified to realize its importance in TBBPA control in the existing industrial wastewater treatment plant, as well as to give a guide to TBBPA control in real manufactory. Fig. 7a showed the removal efficiency of TBBPA by ASP. Under a condition of the initial TBBPA concentration of 1 mg/L, a reaction temperature of 25°C, and a sludge concentration of 400±50 mg/L, the activated sludge system reached a steady-state operation within 1 h, which could remove about 78% TBBPA in water. When the sludge was inactivated by 20 mg/L sodium azide, which could devitalize the sludge and maintain its structure characteristic, the inactivated sludge system showed a low TBBPA removal efficiency of around 74%. Hence, it was believed that TBBPA was mainly removed by the adsorption of activated sludge [30,31]. Meanwhile, the marginally higher removal efficiency of the activated sludge system indicated the degradation of the biological sludge played a relatively limited role in TBBPA removal.
Furthermore, real industrial wastewater containing 1 mg/L TBBPA was treated by ASP with different concentrations, as exhibited in Fig. 7b. It took over 3 h to reach steady state for removing TBBPA when the sludge content was 230 mg/L, while that took 15 min for the sludge content of 1150 mg/L. With an activated sludge content of 230 mg/L, the removal efficiency of TBBPA was 67%, which increased to over 90.5% when the sludge content increased to 1150 mg/L. These suggested that a high sludge concentration could not only accelerate the adsorption rate but also enhance the removal capacity. Fig. 7c furtherly illustrates the positive relationship between the TBBPA in effluent and the TBBPA loading rate, and their linear correlation coefficient R2 was 0.98. This result indicated that if the TBBPA discharged standard was lower than 0.3 mg/L, the TBBPA loading rate should be controlled below 0.34 kg/(kgSS·d).
3.3.2. Influencing factorsThe effect of pH on TBBPA removal efficiency was exhibited in Fig. 8a. When the pH was 3, 5, and 7, the TBBPA removal efficiency was 85.5%, 84%, and 85%, respectively. Once the pH changed to alkali of around 9, the removal efficiency slightly decreased to 82%, and the further pH increased to 11 resulting in a slightly decreasing TBBPA removal efficiency of 78%. It could thus be concluded that the alkaline environment inhibited the TBBPA removal efficiency, and the major reason was the same as discussed in section 3.2.2. As the basic control principle of activated sludge was similar to that of flocculation, the alkaline environment improved the solubility of TBBPA in the liquid phase, and consequently, the adsorption of TBBPA by activated sludge was then decreased [26].
Fig. 8b shows that the TBBPA removal efficiency at a temperature of 10°C, 25°C, and 40°C was 81%, 85%, and 82%, respectively, indicating that the changes of temperature insignificantly affected the TBBPA removal performance in activated sludge process. The real wastewater often contains a variety of co-existing substances, and the effects of co-existing NH4+ and Cu2+ on the TBBPA removals were carried out. As listed in Fig. 8c, the TBBPA removal efficiency was 81% when there was no NH4+ added. When the NH4+ addition increased to 40 mg/L, the TBBPA removal gradually decreased to 61%. Similarly, a low Cu2+ concentration of 1 mg/L could also reduce the TBBPA removal efficiency from 81% to 65% (Fig. 8d). There may be two reasons for the decreased removal efficiency. Firstly, the co-existed NH4+ and Cu2+ ions may inhibit microbial activities and thus reduce the biodegradation of the ASP. More importantly, the co-existed ions would compete with TBBPA for adsorption sites [32], and reduce the TBBPA adsorption capacity of the ASP.
3.4. ImplicationThe flocculation-sedimentation and activated sludge methods showed a comparative capacity of over 80% in TBBPA control, and it was feasible to furtherly enhance the TBBPA removal degree by increasing the regent dosage or sludge loading, optimizing the temperature or acid-base environment. Since most existing waste-water treatment facilities have equipped with both the flocculation- sedimentation process and ASP, it seems easy to control the TBBPA releasing into the environment in PCB industry. However, more attention should be focused on the TBBPA release from the raw material which left over after manufacturing. Because the raw materials like solder mask ink, character ink, dry film, and PCB substrate would release TBBPA into the environment. It makes sense to recycle these materials or use TBBPA-free materials. Furthermore, the most important point is the evolution of flame retardants because it is difficult to control the PCBs that are already processed and sold to the market. These PCB substrates would constantly release TBBPA to the environment without monitoring and finally harm human health.
4. ConclusionsInvestigation of the factory revealed that the solder mask ink, character ink and dry film, and the PCB substrate would release TBBPA in the PCB manufacturing processes, so it is important to strictly manage the waste of these materials. Besides, the main processes discharging TBBPA were the stripping, development (inner layer manufacturing), etching, pattern plating, development (forming and surface treatment), and tin-plating steps. Both the flocculation-sedimentation and the activated sludge methods showed a TBBPA removal rate of about 80% in different conditions. When pH = 7.0, PAC dosage = 300 mg/L, PAM dosage = 0.5 mg/L, dosing during the slow stir stage, the flocculation-sedimentation method reached the balance between economy and removal efficiency for TBBPA. What is more, the optimum condition of the activated sludge method was higher sludge concentration, lower pH below 7, medium temperature around room temperature, and minimal content of NH4+ and Cu2+. These results showed that the traditional wastewater treatment facilities could handle the TBBPA in waste-water well. Although the authors had tried to simulate the real TBBPA release at the lab scale, there may still be differences from field experiments. Besides, TBBPA in raw materials requires more attention. Because the abuse of these materials in the factory would not only enhance the treatment loads, and some of these materials like PCB substrates would come into people’s daily lives and continuously damage the environment and human health. Hence, more comprehensive investigations containing more raw materials and manufacturing processes would be carried out. And TBBPA release from discarded PCB productions in the natural environment is also a matter of concern. The complete and permanent solution may be the new environment-friendly flame retardants.
AcknowledgmentsThis research was supported by Shenzhen Sustainable Development Project (KCXFZ20201221173413037), Shenzhen Science and Technology Funding Project [Grant No. JCYJ20170816102318538, JCYJ20180306172001505, JCYJ20200109112825061], National Natural Science Foundation, China [Grant No. 52070053, 51808165], and Guangdong Natural Science Foundation, China [Grant No. 2018A 030313348].
NotesAuthor Contributions H.D. (Engineer): Conceptualization, Data curation, Methodology. Q.S. (Research Assistant): Validation, Formal analysis, Writing–original draft. P.L. (Ph.D.): Investigation, Writing–review & editing. D.X. (Assistant Professor): Methodology, Resources. W.D. (Professor): Methodology, Resources, Supervision. Z.D. (Assistant Professor): Methodology, Resources, Supervision. F.S. (Associate Professor): Conceptualization, Methodology, Project administration, Supervision, Writing–review & editing. References1. Leng J, Ruan G, Song Y, et al. A loosely-coupled deep reinforcement learning approach for order acceptance decision of mass-individualized printed circuit board manufacturing in industry 4.0. J. Clean Prod. 2021;280:124405.
https://doi.org/10.1016/j.jclepro.2020.124405
2. Zhou X, Guo J, Zhang W, Zhou P, Deng J, Lin K. Tetrabromobisphenol A contamination and emission in printed circuit board production and implications for human exposure. J. Hazard. Mater. 2014;273:27–35.
https://doi.org/10.1016/j.jhazmat.2014.03.003
3. Huang W, Yin H, Yang Y, Jin L, Lu G, Dang Z. Influence of the co-exposure of microplastics and tetrabromobisphenol A on human gut: Simulation in vitro with human cell Caco-2 and gut microbiota. Sci. Total Environ. 2021;778:146264.
https://doi.org/10.1016/j.scitotenv.2021.146264
4. Wang S, Ji C, Li F, et al. Tetrabromobisphenol A induced reproductive endocrine-disrupting effects in mussel Mytilus galloprovincialis. J. Hazard. Mater. 2021;416:126228.
https://doi.org/10.1016/j.jhazmat.2021.126228
5. Włuka A, Woźniak A, Woźniak E, Michałowicz J. Tetrabromobisphenol A, terabromobisphenol S and other bromophenolic flame retardants cause cytotoxic effects and induce oxidative stress in human peripheral blood mononuclear cells (in vitro study). Chemosphere. 2020;261:127705.
https://doi.org/10.1016/j.chemosphere.2020.127705
6. Peng X, Wang Z, Wei D, Huang Q, Jia X. Biodegradation of tetrabromobisphenol A in the sewage sludge process. J. Environ. Sci. 2017;61:39–48.
https://doi.org/10.1016/j.jes.2017.02.023
7. Li T, He Y, Peng X. Efficient removal of tetrabromobisphenol A (TBBPA) using sewage sludge-derived biochar: Adsorptive effect and mechanism. Chemosphere. 2020;251:126370.
https://doi.org/10.1016/j.chemosphere.2020.126370
8. Cao M, Wang P, Ao Y, Wang C, Hou J, Qian J. Photocatalytic degradation of tetrabromobisphenol A by a magnetically separable graphene-TiO2 composite photocatalyst: Mechanism and intermediates analysis. Chem. Eng. J. 2015;264:113–124.
https://doi.org/10.1016/j.cej.2014.10.011
9. Wang Q, Wang T, Qu G, et al. High-efficient removal of tetrabromobisphenol A in aqueous by dielectric barrier discharge: Performance and degradation pathways. Sep. Purif. Technol. 2020;240:116615.
https://doi.org/10.1016/j.seppur.2020.116615
10. Xiang M, Huang M, Li H, et al. Nanoscale zero-valent iron/co-balt@mesoporous hydrated silica core–shell particles as a highly active heterogeneous Fenton catalyst for the degradation of tetrabromobisphenol A. Chem. Eng. J. 2021;417:129208.
https://doi.org/10.1016/j.cej.2021.129208
11. Khandpur RS. Printed circuit boards: design, fabrication, assembly and testing. Tata McGraw-Hill Education; 2006. p. 5–6.
12. Phung TH, Jeong J, Gafurov AN, et al. Hybrid fabrication of LED matrix display on multilayer flexible printed circuit board. Flex. Print. Electron. 2021;6:24001.
https://doi.org/10.1088/2058-8585/abf5c7
13. Zhu L, Xu YP, Sun QF. Analysis on the Copper Metabolism in Printed Circuit Board Industry. AMM. 2011;71–78:2860–2866.
https://doi.org/10.4028/www.scientific.net/AMM.71-78.2860
14. de Melo Duarte Borges T, Devós Ganga GM, Filho MG, Delai I, Santa-Eulalia LA. Development and validation of a cleaner production measurement scale. J Clean Prod. 2021;299:126907
https://doi.org/10.1016/j.jclepro.2021.126907
15. Sakr D, Abo Sena A. Cleaner production status in the Middle East and North Africa region with special focus on Egypt. J. Clean Prod. 2017;141:1074–1086.
https://doi.org/10.1016/j.jclepro.2016.09.160
16. Chung I; UNEP D. Environmental agreements and cleaner production: Questions and answers. Paris, France: United Nations Environment Programme, Division of Technology, Industry, and Economics; 2006. p. 3–4.
17. Zhu A, Liu P, Gong Y, Li M, Su J, Liu G. Residual levels and risk assessment of tetrabromobisphenol A in Baiyang Lake and Fuhe river, China. Ecotox. Environ. Safe. 2020;200:110770.
https://doi.org/10.1016/j.ecoenv.2020.110770
18. Wang X, Hu X, Zhang H, Chang F, Luo Y. Photolysis Kinetics, Mechanisms, and Pathways of Tetrabromobisphenol A in Water under Simulated Solar Light Irradiation. Environ. Sci. Technol. 2015;49:6683–6690.
https://doi.org/10.1021/acs.est.5b00382
19. Clescerl LS, Greenberg AE, Eaton AD. Standard Methods for the Examination of Water and Wastewater. 20th ed.American Public Health Association; American Water Works Association; Water Environment Federation: 1999. p. 9240–9250.
20. Yu Y, Yu Z, Chen H, et al. Tetrabromobisphenol A: Disposition, kinetics and toxicity in animals and humans. Environ. Pollut. 2019;253:909–917.
https://doi.org/10.1016/j.envpol.2019.07.067
21. Potvin CM, Long Z, Zhou H. Removal of tetrabromobisphenol A by conventional activated sludge, submerged membrane and membrane aerated biofilm reactors. Chemosphere. 2012;89:1183–1188.
https://doi.org/10.1016/j.chemosphere.2012.07.011
22. Malkoske T, Tang Y, Xu W, Yu S, Wang H. A review of the environmental distribution, fate, and control of tetrabromobisphenol A released from sources. Sci Total Environ. 2016;569–570:1608–1617.
https://doi.org/10.1016/j.scitotenv.2016.06.062
23. Guo K, Gao B, Wang J, Pan J, Yue Q, Xu X. Flocculation behaviors of a novel papermaking sludge-based flocculant in practical printing and dyeing wastewater treatment. Front. Env. Sci. Eng. 2021;15:270–280.
https://doi.org/10.1007/s11783-021-1390-x
24. Wang Z, Nan J, Ji X, Yang Y. Effect of the micro-flocculation stage on the flocculation/sedimentation process: The role of shear rate. Sci. Total Environ. 2018;633:1183–1191.
https://doi.org/10.1016/j.scitotenv.2018.03.286
25. Liu Y, Li C, Lou Z, Zhou C, Yang K, Xu X. Antimony removal from textile wastewater by combining PFS&PAC coagulation: Enhanced Sb(V) removal with presence of dispersive dye. Sep. Purif. Technol. 2021;275:119037.
https://doi.org/10.1016/j.seppur.2021.119037
26. Castro M, Lindqvist D. Liposome-mediated delivery of challenging chemicals to aid environmental assessment of Bioaccumulative (B) and Toxic (T) properties. Sci Rep. 2020;10:9725.
https://doi.org/10.1038/s41598-020-66694-3
27. Kristianto H, Tanuarto MY, Prasetyo S, Sugih AK. Magnetically assisted coagulation using iron oxide nanoparticles-Leucaena leucocephala seeds’ extract to treat synthetic Congo red wastewater. Int. J. Environ. Sci. Technol. 2020;17:3561–3570.
https://doi.org/10.1007/s13762-020-02721-0
28. Ling J, Germain E, Murphy R, Saroj D. Designing a Sustainability Assessment Framework for Selecting Sustainable Wastewater Treatment Technologies in Corporate Asset Decisions. Sustainability. 2021;13:3831.
https://doi.org/10.3390/su13073831
29. Ariesyady HD, Mayanda MR, Ito T. The diversity of active microbial groups in an activated sludge process treating painting process wastewater. E3S Web Conf. 2020;148:1002.
https://doi.org/10.1051/e3sconf/202014801002
30. Saber AN, Zhang H, Islam A, Yang M. Occurrence, fates, and carcinogenic risks of substituted polycyclic aromatic hydrocarbons in two coking wastewater treatment systems. Sci. Total Environ. 2021;789:147808.
https://doi.org/10.1016/j.scitotenv.2021.147808
31. Zhang L, Zhang M, You S, Ma D, Zhao J, Chen Z. Effect of Fe3+ on the sludge properties and microbial community structure in a lab-scale A2O process. Sci. Total Environ. 2021;780:146505.
https://doi.org/10.1016/j.scitotenv.2021.146505
32. Tong F, Gu X, Gu C, Ji R, Tan Y, Xie J. Insights into tetrabromobisphenol A adsorption onto soils: Effects of soil components and environmental factors. Sci. Total Environ. 2015;536:582–588.
https://doi.org/10.1016/j.scitotenv.2015.07.063
Fig. 2Illustration of TBBPA removal tests by flocculation-sedimentation units (a) and activated sludge process (b). 
Fig. 3TBBPA tracing in raw materials: the total ion chromatography of TBBPA (50 mg/L) dichloromethane solution (a), dry film (b), character ink (c), solder mask ink (d), brown oxide solution (e), prepreg (f) and PCB substrate (g). 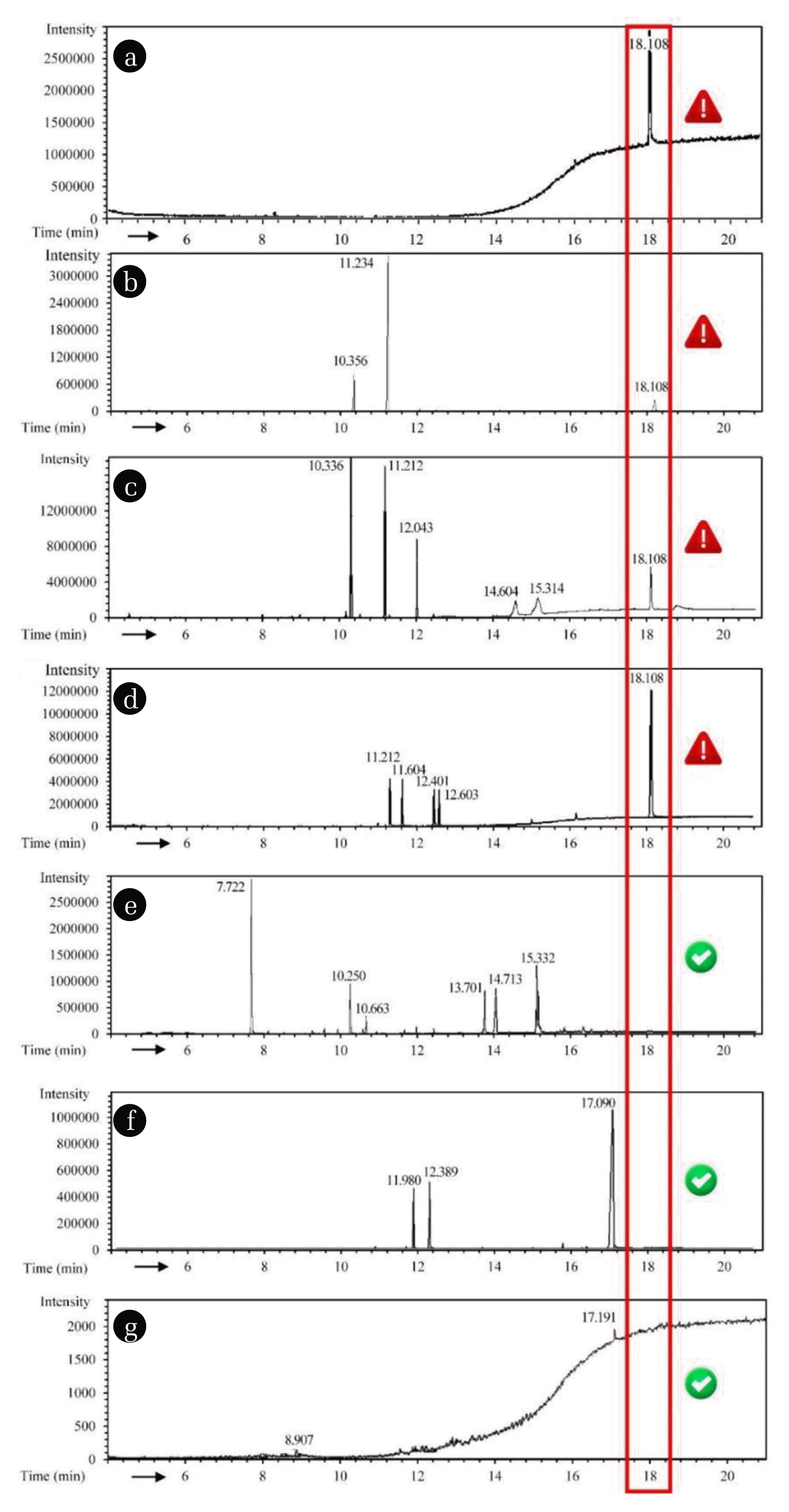
Fig. 4TBBPA leaching behaviors during: Inner layer manufacturing: development (a), etching (b), stripping (c); Outer layer manufacturing: pattern plating (d), tin-plating (e); Forming and surface treatment: development (f), electroless nickel/immersion gold (g); (h)~(n) are the control groups of (a)~(g), respectively, soaked in manufacturing wastewater without TBBPA-containing materials. 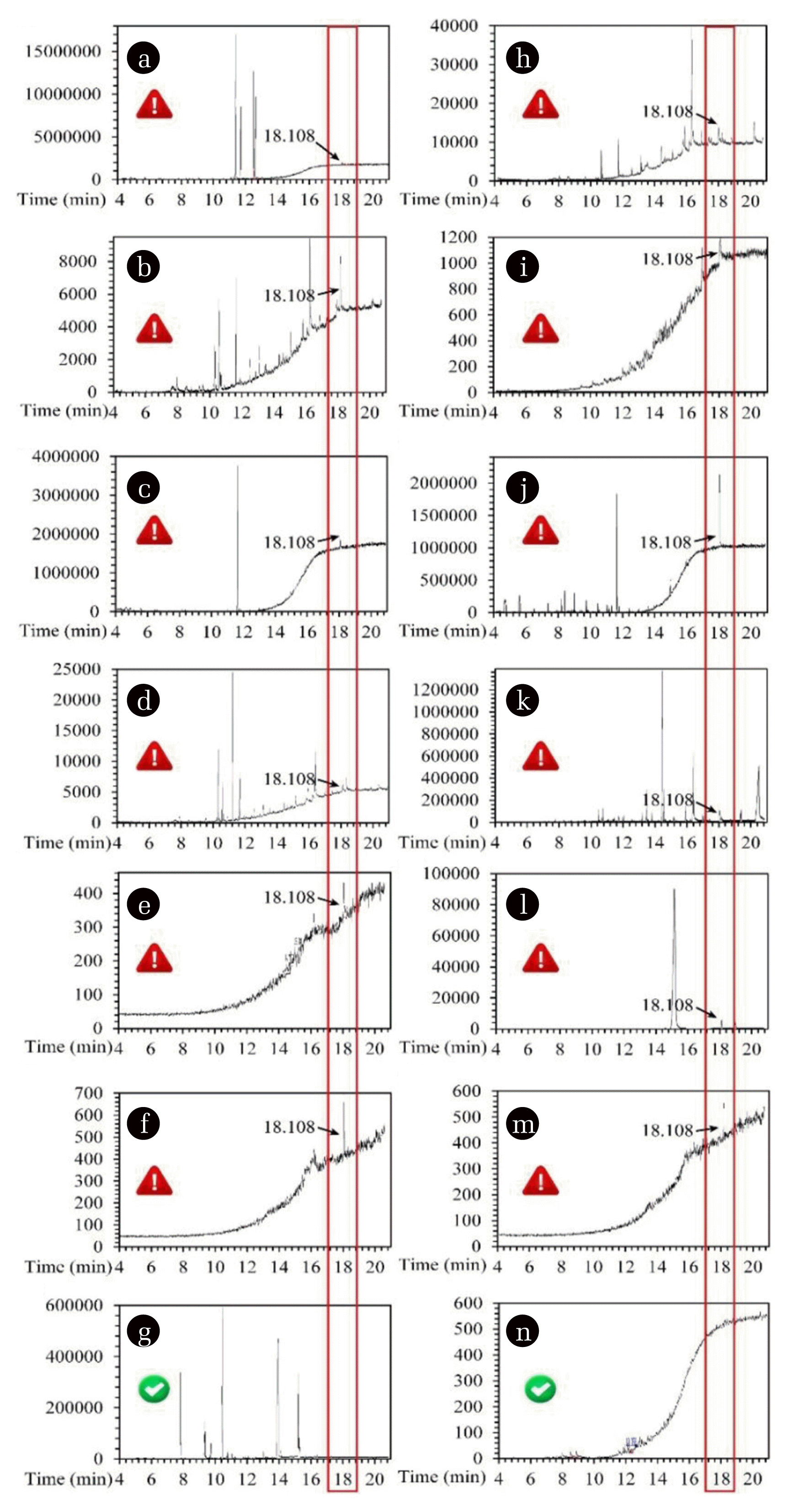
Fig. 5TBBPA removal efficiency in the flocculation-sedimentation process, where I represents dosing in the first 30 s, II represents dosing at the middle 6 min, and III represents the last 15 min. 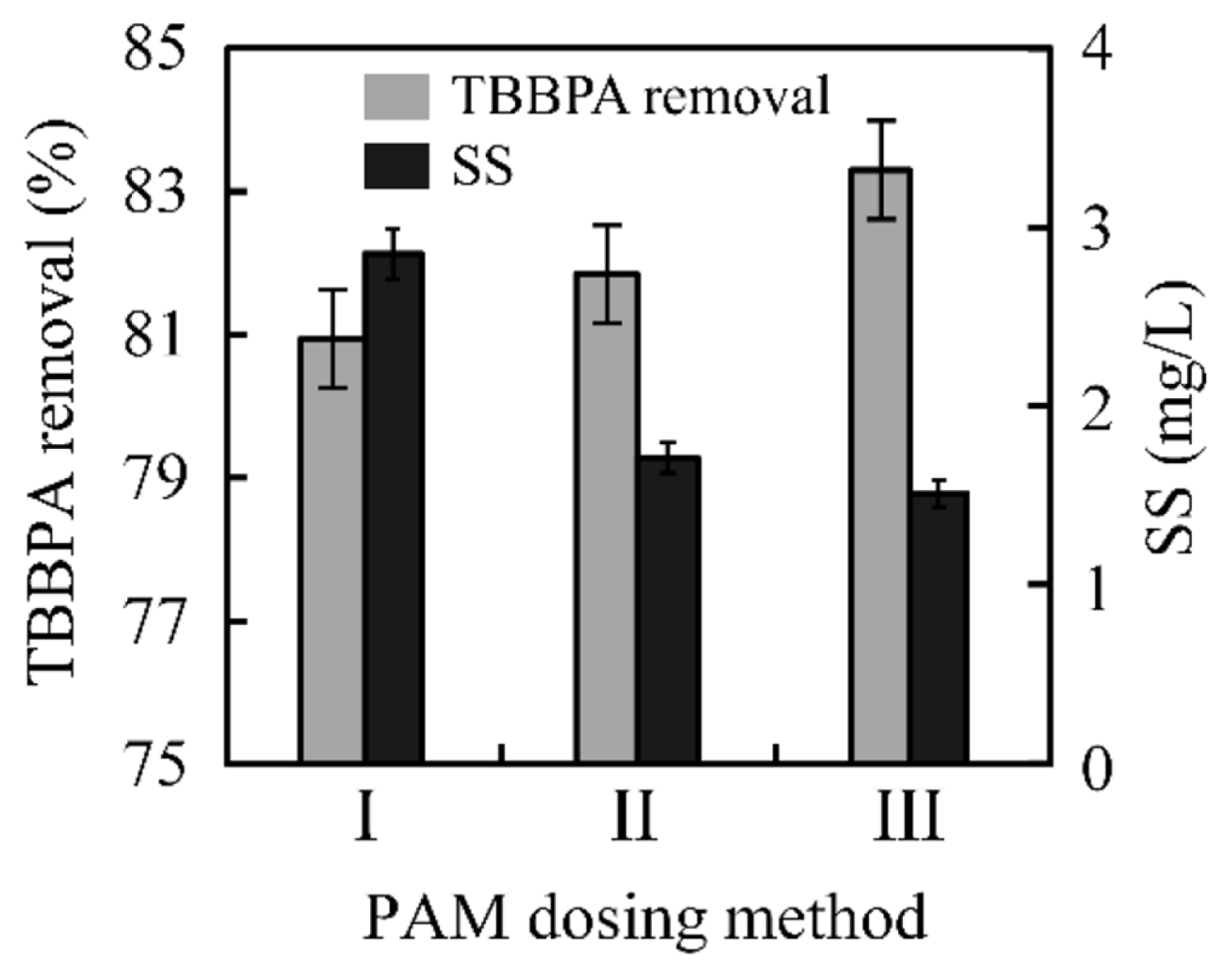
Fig. 6Influence factors to TBBPA removal in the flocculation-sedimentation process: TBBPA removal and SS in effluent vs. pH (a), PAC dosage (b), PAM dosage (d); SV during flocculation & sedimentation vs. PAC dosage (c), PAM dosage (e). 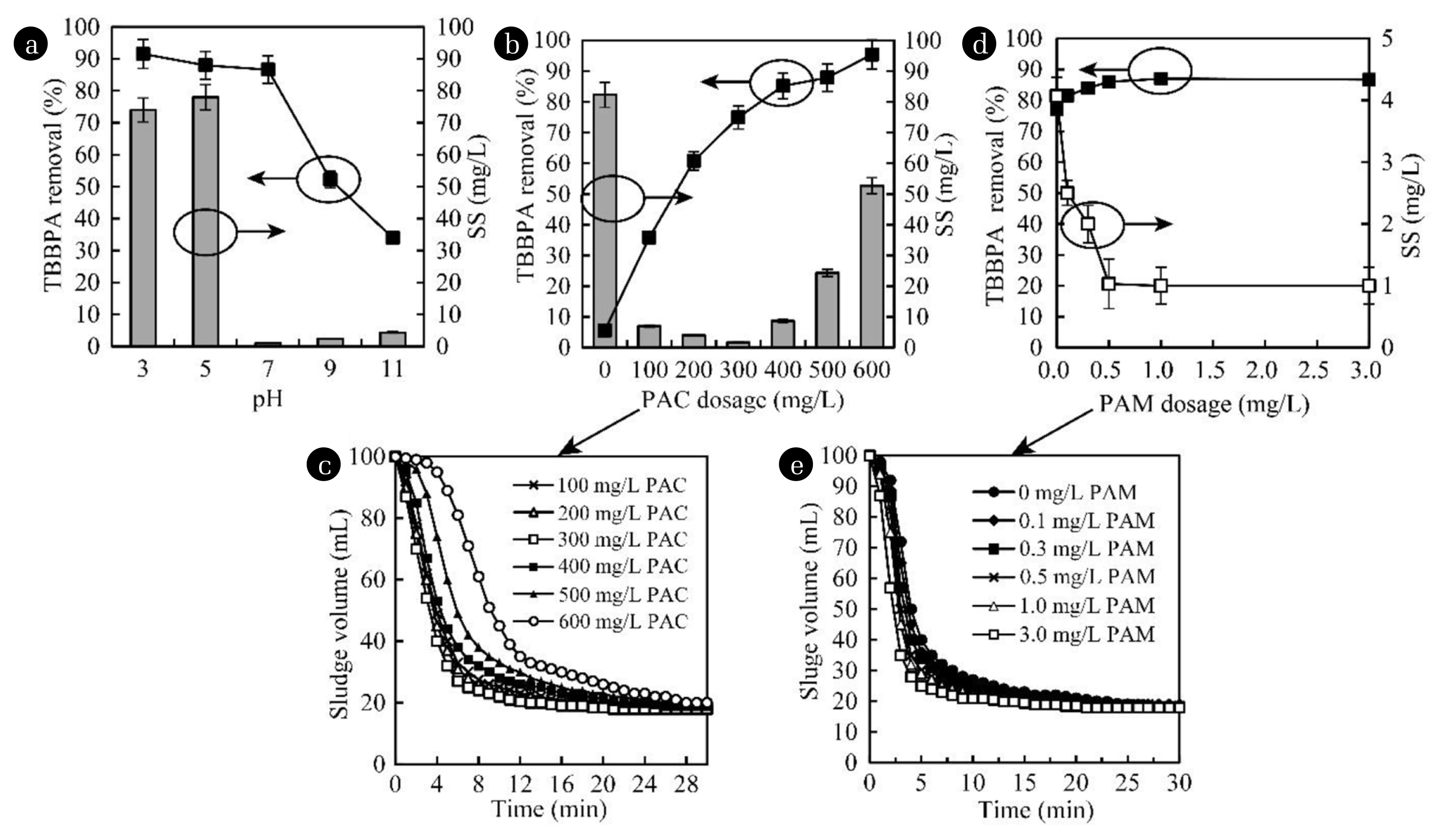
|
|
||||||||||||||||||||||||||||||||||||||||