AbstractThis study contributes to the preliminary development of the active food packaging materials by using bio-based polymers under ultrasonic waves. For this alginate (Algi) polymer was reinforced with functionalized multiwalled carbon nanotubes (COOH-MWCNTs) dispersed in surfactants to form A-type of films. Similarly, to form a B-type of films, Algi polymer was blended with polyvinyl alcohol (PVA) in the presence of COOH-MWCNTs. The synthesized films were physiochemically characterized by FTIR, SEM, TEM, and mechanical testing. The antibacterial and antioxidant properties of prepared films were assessed by agar well diffusion method and ABTS/Ferrozine methods, respectively. Synthesized films showed prominent peaks of Algi, PVA, and of COOH-MWCNTs in FTIR analysis and dispersed nanofillers in SEM/TEM study. The Youngs Modulus of AD90 is 249.17 N/mm2, Bs90 is 216.80 N/mm2 and of Algi is 129.55 N/mm2. Staphylococcus aureus is the positive and Klebsiella pneumonia, Escherichia coli are the negative strains of bacteria that were successfully inhibited to grow by the prepared films. Synthesized materials also acted as strong antioxidants with no significant difference (P > 0.05) from ascorbic acid compared to Algi tested by ABTS and Ferrozine methods.
1. IntroductionIn the scientific community, there is an emerging interest in food quality assurance. Worldwide, about 1300 million tons of food are wasted per year, in which vegetables and fruits are mainly affected [1,2]. The microbial contamination, oxidation, respiration, and transpiration of fibrous materials even after harvesting are the key challenges that the food industry facing [2]. In this sense, the application of food packaging materials is being common that not only preserved the materials from climatic effects but also increased the storage, transport, and shelf life [3]. Thus, to date, the production and application of petroleum-based packaging materials are increasing alarmingly which protected the food on one side but raised the problem of contamination of food materials with plastic ingredients along with the non-degradable and disposal problems on the other side [4,5].
During the last two decades, the growing demand of consumers for natural products had led the researchers to focus on seeking the bio-based packaging materials obtained from renewable resources which keep the quality, safety, freshness, and shelf life [6]. The bio-sourced materials not only minimized the risks of degradability but also showed promising action due to the properties like oxygen barrier, water vapor barrier, low humidity, and high mechanical strength [3]. These films are called active films, as they contain active additives which inhibit the growth of microorganisms on food surfaces and prevent the deterioration of food from the environmental factor [7]. In this regard, the superiority of bio-based materials is the availability from replenishable agricultural resources, biodegradability, biocompatibility, ecological safety, and the possibility of preparing the chemical modified derivative for specific end-use [8]. Additionally, in the presence of bio-based packaging films, the aesthetic appearance, scares, and surface shines of food are improved by minimizing the development of physical damage [9, 10].
Alginate is a natural negative polysaccharide and is considered as a bioactive polymer obtained from brown seaweed having various amounts of β-D-mannuronic acid (M) and α-L-guluronic acid linked via glycosidic bonds [11–13]. It is a widely distributed polymer and showed remarkable properties like biocompatibility, low toxicity, biodegradability, and relatively low cost [14]. The backbone of alginate is flourished with plentiful −OH and −COOH functional groups. These functional groups are liable to interact with other materials, thus finding wide applications in the field of food industry and horticulture as a food coating material [14]. In the presence of alginate-based coating, the shelf life and quality of fruits/vegetables are improved by reducing oxidative rancidity, shrinkage, oil absorption, color, and flavor loss [15, 16]. However, a major drawback of alginate and alginate-based coating is hydrophilicity and water vapor transmission. The high swelling ability and fragile nature of alginate polymer make the poor moisture barrier of packaging films [17, 18].
The unprecedented opportunities in the field of nanotechnology open the unique way for natural polymers to withstand versatile industrial conditions and show multidimensional functions [19, 20]. In this way, natural polymers are reinforced with nanomaterials to modify the potential properties uniquely. The OH and COOH functional groups on alginate polymer can interact and accommodate the nanofillers and showed enhanced properties [21]. The multi-walled carbon nanotubes (MWCNTs) are nanofillers that have high mechanical strength, high stability, transparency, and excellent electrical, thermal, and photoluminescence properties [22]. The greater aspect ratio, lighter weight, greater surface area, and smaller size make the MWCNTs as promising materials for applications in industrial, environmental, and medical fields [20, 23]. In addition, MWCNTs are reported to show good potential in food packaging materials due to their antibacterial nature and non-toxic effect on foodstuff [24–27].
By utilizing the emerging concept of nanotechnology in composite synthesis, the drawback of the hydrophilic nature of alginate films is tackled by incorporating the COOH-MWCNTs in alginate-based substrates. By incorporating these nanofillers in alginate-based substrates, nanocomposites are aimed to show less disintegration and hydrophilicity due to the hydrophobic nature of MWCNTs and strong intermolecular interactions. For this purpose, the alginate and alginate-PVA substrates are reinforced with COOH-MWCNTs nanofillers to form A and B types of films, respectively. Polyvinyl alcohol is a water-soluble crystalline polymer, having potential to protect the encapsulating materials from various environmental factors. The PVA containing nanocomposites is reported to show the best performance as a coating agent, and active packaging materials [28]. To the best of our knowledge, no data was provided in the literature for the synthesis of nanocomposite films (A and B type) in a greener way (under ultrasonic waves). The prepared films were also the first time explored as an antibacterial and antioxidant film.
2. Materials and Methods2.1. MaterialsSodium alginate (from brown algae, purity: 98%, CAS number: 9005-38-3, molecular weight: 120,000–190,000 g/mol), M/G ratio: 1.56, polyvinyl alcohol (purity: 87–90 %, average molecular weight: 30,000–70,000 g/mol, CAS number: 9002-89-5, density: 1.2 g/cm3), sodium dodecyl sulphate (SDS), cetyltrimethylammonium bromide (CTAB), dimethylformamide (DMF), ABTS (514.62 g/mol), Ferrozine (Mw: 514.38 g/mol), potassium persulfate, ferrous sulphate (FeSO4.7H2O), potassium chloride, calcium chloride (dihydrated), sodium hydroxide and hydrochloric acid were purchased from Sigma-Aldrich (UK) and used without further purification. Functionalized multiwalled carbon nanotubes (COOH-MWCNTs) were kindly provided by National Centre for Physics (NCP), Islamabad, Pakistan. Nutrient agar (containing beef extract, a pancreatic digest of gelatine, and agar) was purchased from the bio-world (Karachi, Pakistan). Deionized water was used throughout the procedure, i.e., preparation of films, solutions preparation, antioxidant measurement, and antibacterial measurements.
2.2. Synthesis of FilmsNanocomposite films (Algi/COOH-MWCNTs, A-type) and Algi+PVA/COOH-MWCNTs, B type) were prepared by the simple solution casting method in the presence of ultrasonic waves [17]. In a typical procedure, a weighed quantity of sodium alginate (0.9 g) was firstly dissolved in distilled water (30 mL) by stirring for three hours at 40 °C. The nanofillers, COOH-MWCNTs (0.04 g) were separately dissolved in 30 mL of an aqueous solution having 0.05 g of surfactants (SDS or CTAB) or in 30 mL of non-aqueous solvent (DMF) without surfactants. To prepare A type of films, 2 mL of Algi solution was mixed with 2 mL of COOH-MWCNTs solution and sonicated for two hours at 40 °C. After completion of the required time, the blended solution mixture was poured into Petri plates and placed in a dust-free environment.
For the preparation of B type of films, a similar method was followed with slight modifications i.e., 2 mL of Algi solution was mixed in 2 mL of COOH-MWCNTs solution along with 10 mL of aqueous solution (10 % w/v) of PVA and sonicated under ultrasonic waves for two hours at 40 °C. Thus, the nanocomposite films As90 (Algi/MWCNTs, SDS), Ac90 (Algi/MWCNTs, CTAB) AD90 (Algi/MWCNTs, DMF), Bs90 (Algi+PVA/MWCNTs, SDS), Bc90 (Algi+PVA/MWCNTs, CTAB), and BD90 (Algi+PVA/MWCNTs, DMF) were formulated by greener method. The films without nanofillers (COOH-MWCNTs) coded as Algi and Algi+PVA were also prepared by a similar method except nanofillers were not added in the polymeric matrix. The blended solutions of prepared films were poured into the Petri plates and placed in a dust-free environment for 3–4 weeks. The obtained films were washed with ethanol and dehydrated in the oven at 40 °C for 5 hours. Dried films of nanocomposites (~0.050 mm) and of starting materials Algi (0.03 mm) and Algi+PVA (0.045 mm) were sealed in polyethylene bags for further use.
2.3. Characterization TechniquesThe synthesized materials were analyzed by various instrumental techniques to know the physicochemical properties of films. The SEM (Joel JSM-6510LV) and TEM (JEOL JEM 1200 EXII) images were used to visualize the surface morphology of synthesized films working at 20.0 KV and 100.0 KV, respectively. The presence of various functional groups and types of bonds in prepared films was determined by FTIR spectroscopy (Perkin Elmer spectrum 100 series spectrometer). The tensile strength and percent elongation at break was measured by using Universal Testing Machine (100–500 KN) at −70 °C to 300 °C. The testing machine operated at the maximum force (100 KN) and speed (1.0 mm/min) on the strips (20.00 mm x 15.00 mm x 0.05 mm) of nanocomposites.
2.4. Antibacterial MeasurementThe antibacterial measurement of synthesized films was done by using a reported paper disc diffusion or agar well diffusion method [29]. Cultures of gram-positive (Staphylococcus aureus) and gram-negative (Klebsiella pneumonia and Escherichia coli) bacteria were obtained from the Combined Military Hospital (CMH) Muzaffarabad. In this method, first of all, 10 % inoculum suspension (1 mL) of each strain was cultivated in Petri plates having agar nutrient solution (15 mL, 2.8 % (w/v)) for 24 hours at 37 °C. On complete cultivation, the sterilized paper discs (Whatman No. 1) of 6 mm diameter impregnated with sample solution (50 μL/mL) were placed on a culture medium. The reference discs dipped in the solution of Algi films and Algi+PVA films were also placed along with sample discs for comparison. A disc impregnated with standard antibiotic (Ampicillin, 10 μg) was also placed in a culture medium which acts as a control to assess the antibacterial behavior of prepared films. The discs containing plates were sealed and further incubated for 24 hours at 37 °C. The inhibition tendency of the samples to the growth of bacteria was measured with a ruler in mm and recorded in % inhibition on comparing with control (ampicillin).
2.5. Antioxidant measurementThe synthesized films were subjected to antioxidant measurement by a reported ABTS [30] and Ferrozine [31] methods. In the typical procedure of ABTS assay, 25 mL of ABTS (2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (7 mM) solution and 25 mL of potassium persulfate (2.7 mM) solution were mixed and incubated for 24 hours in dark to generate the ABTS• free radicle. On generating the radicle, the solution turned into bluish-green color which gives the absorbance data near to about 2. The control solution for the ABTS assay was prepared by mixing 1 mL of ABTS solution in 2 mL of distilled water. The absorbance data of the control solution was recorded with respect to distilled water kept in the reference compartment. Now the aqueous solution of synthesized samples (10–50 μL) was prepared by adding the sample solution in 1 mL of ABTS solution and the total volume of sample solution was adjusted 3 mL by distilled water. The sample solution was examined after the incubation period of two minutes in dark. On adding the sample solution to the ABTS solution, the color of the ABTS solution changed due to the consumption of free radicles by the sample solution. This consumption of radicles was measured by taking the UV absorbance data of the sample solution at 405 nm. The protocol applied for the preparation of the sample solution is represented in Table 1.
The antioxidizing ability of synthesized films was also assessed by the Ferrozine method. Just like to ABTS method, in this method, first of all, the Ferrozine-Fe+2 complex having red color was generated by mixing ferrozine solution (1 mL, 6.15 mg/50 mL of D.I water) with ferrous sulfate (218 mg/50 mL of D.I water) solution. A mixture containing 1mL of ferrozine solution, 1 mL of freshly prepared FeSO4 solution, and 1 mL of distilled water was used as a control solution. The concentration of Ferrozine-Fe+2 complex in the control solution was measured by taking UV absorbance at 517 nm and was measured with reference to distilled water placed in the reference compartment. The aqueous solutions of the sample having various concentrations (10 μL, 20 μL, 30 μL, 40 μL, 50 μL) were mixed with ferrozine-ferrous sulfate (1mL each) solution and the volume is adjusted to 3000 μL with distilled water (Table 2). The saturation of ferrozine-Fe+2 complex was generated by incubating the sample solution in dark for ten minutes. Now the absorbance of the sample solution was measured at 517 nm.
The percent scavenging activity (% SA) of samples using the Ferrozine method and ABTS method was calculated by using absorbance data and represented by the following Eq. 1 [30, 32].
Where Ao is the absorbance of the control and Ai is the absorbance of prepared samples.
The IC50 values of each concentration of the sample were calculated from the % SA values by using the trend line equation. The IC50 value of the standard oxidant (ascorbic acid) was also measured and compared with the prepared antioxidants.
3. Results and Discussion3.1. Characterization TechniquesThe synthesized nanocomposites (A and B types) are characterized by FTIR spectroscopy in comparison with the starting materials Algi and Algi+PVA (Fig. 1a and b). The spectral results indicated that the stretching vibrations that appeared at 1647 cm−1 and 1560 cm−1 in Algi are attributed to asymmetric and symmetric vibrations of sodium carboxylate ions, respectively. On the addition of COOH-MWCNTs and COOH-MWCNTs/PVA in the Algi polymer, Ac90, AD90 and Bs90, Bc90, and BD90 type of nanocomposites are formulated. Spectral results confirmed that the formulated nanocomposites contain the intermolecular interaction between nanofillers (COOH-MWCNTs) and substrates (Algi, Algi+PVA). The disappearance of carboxylate ions in the nanocomposites indicates interaction between nanoparticles and substrate. The somewhat broader band of the O-H group in pristine Algi appeared at ~3000 cm−1 to 3700 cm−1 is changed to a sharper and intense peak in nanocomposites (in both A and B types of nanocomposites). The C-O stretching frequency in Algi appeared at 1081 cm−1 is corresponds to the C-O bond of the pyranose ring. In nanocomposites each spectrum contains two or three different types of peaks that appeared in the C-O stretching region, thus indicating that nanocomposites have different types of C-O bonds. These bonds may be of ethereal C-O bond, alcoholic C-O bond, and carboxylic C-O bond. The presence of COOH-MWCNTs in nanocomposites is confirmed by the appearance of a C=O stretching vibration at about 1718-1705 cm−1. The relatively lower stretching vibrations of C=O functional groups are due to the conjugations, created by the pi-electrons of the graphene sheet, which indicate the interaction of COOH-MWCNTs with the substrate. When PVA along with COOH-MWCNTs are added then the interaction between carboxylate ions of Algi, OH group of PVA, and COOH group of MWCNTs occurred and as a result, nanocomposites are formed [33]. In all types of nanocomposites, a medium-sized peak at 2925 cm−1 is attributed to aliphatic C-H stretching vibrations.
To use as the food packaging materials in the food industry, the nanocomposites films should be strong enough to handle, wear, and use repeatedly. For this purpose, Youngs Modulus (N/mm2), elongation at break (mm), stress at peak (N/mm2), and strain at peak (%) of prepared films were recorded (Fig. 1c and d). From the obtained results, it is known that the film of Algi has poor mechanical integrity. While the mechanical strength of nanocomposites was great enough due to the presence of reinforcing materials. For instance, the film of Algi showed Youngs Modulus 129.55 N/mm2, while after reinforcing with COOH-MWCNTs, the AD90 and Bs90 nanocomposites indicated the values in 249.17 N/mm2, 216.80 N/mm2 range, respectively. Similarly, encouraging results in elongation at break, stress at peak and strain at the peak were obtained in nanocomposite films compared to the starting Algi. The pristine Algi breaks at 1.25 mm, whereas nanocomposite films (AD90, Bs90) break at 3.29 mm and 35.70 mm, respectively. The strain at peak (%) and stress at peak (N/mm2) for Algi peak is 3.82 % and 1.57 N/mm2. While in AD90 and Bs90 films, the strain lies in the range of 15.83 %, 141.55 %, and stress at 9.84 N/mm2,18.30 N/mm2, respectively. Our results also coincide with the work of other researchers who claimed that in the presence of carbon-based nanofillers, the mechanical properties of nanocomposite increased [34].
The surface morphology of prepared nanocomposites was analyzed by SEM and TEM techniques and results are included in Fig. 2. The prepared nanocomposites were also morphologically compared with the starting materials, i.e., Algi and Algi+PVA. From these results, it is indicated that pristine Algi and Algi+PVA showed somewhat smooth surfaces, while the surfaces of nanocomposites were different due to the presence of dispersed and interacted nanofillers with the substrate matrices. The embedded nanoparticles in the matrix molecules physiochemically interacted with the substrate to form stable nanocomposites. The surface analysis also indicates that the type of films having no surfactants did not appear different than the films containing any surfactants, which led us to conclude the little effect of dispersing media in morphological properties.
3.2. Antibacterial Measurement of NanocompositesTo know the bactericidal effect of synthesized materials, the films were subjected to antibacterial assay against the positive strain (Staphylococcus aureus) and negative strains (Klebsiella pneumonia, Escherichia coli) of bacteria (Fig. 3). From the experimental data, it is obtained that both types of films (A and B types) showed bactericidal effects against all strains of bacteria. However, the films which did not contain nanofillers COOH-MWCNTs (i.e., Algi and Algi+PVA) exhibited no inhibitory effect to any strain of bacterium. The smaller size, large surface area, and interactive nature of COOH-MWCNTs make the nanocomposites lethal to bacteria and prevent their growth by damaging the bacterial cell wall [32]. Dispersed COOH-MWCNTs being more interactive have the greater ability to physiochemically interact with bacterial cells, induce the release of DNA and RNA, oxidation of membrane, and reduction of the bacterial membrane potential [35, 36]. The high bactericidal performance of MWCNTs embedded in the polymeric matrix coincides with other studies, in which embedded nanoparticles showed a greater lethal effect on bacterial growth compared to the untreated MWCNTs [37–39].
3.2.1. Effect of various concentration ratios of the matrix to nanofillers on antibacterial activityThe prepared A and B nanocomposite films are further categorized into various forms based on the concentration ratio of substrate to nanofillers. For instance, in all types of A90 (As90, Ac90, AD90) and B90 (Bs90, Bc90, BD90) films, the concentration ratio of substrate to nanofillers is 90:1. Whereas, in A50 (As50, Ac50, AD50), B50 (Bs50, Bc50, BD50) and A10 (As10, Ac10, AD10), B10 (Bs10, Bc10, BD10) types of films, the concentration ratio is 50:1 and 10:1, respectively. The antibacterial study of all these materials was done and found that the bactericidal effect of films having a lower concentration ratio (50:1 and 10:1) is not as much appreciated as in A90 and B90 types of films (Fig. 3a and b). The film As90 showed the % inhibition of 55±0.5 % in K. pneumonia, 54±0.1 % in E. coli, and 53±0.1 % in S. aureus. Similarly, Ac90 and AD90 films showed the % inhibition of 50±0.5 %, 63±0.6 % in K. pneumonia 42±0.2 %, 37±0.5 % in E. coli, and 59±0.4 %, 45±0.5 % in S. aureus, respectively. The B90 types of films like Bs90 and Bc90 films showed the lethal effect of 48±0.1 % and 50±1 % in K. pneumonia, 49±1 % and 45±0.5 % in E. coli, 50±1 % and 51±0.1 % in S. aureus, respectively. Whereas, the BD90 film showed, 53±0.1 %, 52±1 %, and 50±1 % inhibition in K. pneumonia, E. coli, and S. aureus strains of bacteria, respectively. The films having a lower concentration ratio of substrate to nanofillers showed lesser values of % inhibition (Fig. 3c). The smaller values of antibacterial effect may be attributed to the agglomeration of carbon nanotubes in the lower concentration of Algi and Algi+PVA substrate matrices. In the lower concentration of substrate matrix, the strong intermolecular forces among MWCNTs become dominant and agglomerated into bundles [24]. The agglomerated MWCNTs did not efficiently interact with the bacterial cell wall to prevent their growth [40]. On the other hand, the dispersed form of MWCNTs interacted with the bacterial wall and oxidized the membrane. As in the A90 and B90 types of films, the concentration of matrix is sufficient for the complete dispersion of nanofillers. Therefore, encouraging results of bacterial inhibition are obtained in A90 and B90 films. Thus, the salient feature of the current study is the best performance of nanocomposites having only trace amounts of nanofillers (1 %) in substrate matrix (90 %) due to the greater probability of dispersion.
3.2.2. Effect of surfactants on the antibacterial activityThe nanocomposites having SDS surfactant are represented as As and Bs, the CTAB surfactant is represented as Ac, Bc, and without surfactant is represented as AD and BD. On the comparative study of surfactant-containing films in the lethal effect to bacteria, no distinctive difference was noted in A90 and B90 types of films. In some strains of bacteria, SDS-containing films showed a maximum lethal effect, while in other films, CTAB containing films showed better results. Similarly, non-surfactant-containing films (AD90 and BD90) also showed a bactericidal effect in the same way as surfactants containing films. However, the films having a lower concentration of substrates to nanofillers showed no or low inhibitory effect on the strains of bacteria. For instance, AD50, BD50, AD10, BD10, and As50 exhibited no inhibition to any strain of bacterium, which may be attributed to the lesser dispersion of MWCNTs in a lower concentration of substrate matrix. Whereas the Ac50, Bc50, Ac10 and Bc10 types of films showed the lethal effect to positive strain as well as negative strains of bacteria. It may be due to the stronger lethal effect of quaternary ammonium ion of CTAB on the bacterial cell and greater is the antibacterial activity [41].
3.2.3. Effect of type of films on antibacterial activityOn the comparative study of A90 with B90 types of films, it is obtained that the A90 types showed a greater lethal effect compared to B90 films (Fig. 4d). In A90 films the COOH-MWCNTs are dispersed and embedded in the alginate matrix and showed greater capacity to interact with the bacterial cell wall. Whereas, in B90 films COOH-MWCNTs are dispersed in the Algi+PVA matrix. The COOH functional groups of MWCNTs interacted with COOH and OH groups of polymers and embedded them in the matrix. The probability of interaction of COOH-MWCNTs with the functional groups of Algi+PVA is greater compared to the functional groups of Algi substrate. As a result, reactive sites of nanofillers are comparatively less available (compared with A90 nanocomposites) for interaction with the bacterial cell wall. The prepared nanocomposite films were also compared with starting materials and found that Algi and Algi+PVA films showed no inhibitory effect due to the absence of nanofillers, whereas the nanocomposites showed bactericidal effect with greater % inhibition. Thus, by incorporating reinforcing materials in substrate matrix, not only the physicochemical properties of nanocomposites are improved, but their lethal effect also enhanced.
3.2.4. Statistical analysisThe Zones of inhibition in mm of prepared films were comparatively analyzed with standard ampicillin by applying one-way ANOVA. The obtained results are included in Table 3 and Fig. 4a, b, and c. Table 3 indicated that the films which showed the significant difference from the standard ampicillin are indicated by (*), more significant is indicated by (**) and highly significant is indicated by (***).
3.3. Antioxidant study3.3.1. Antioxidant measurement by ABTS methodThe synthesized nanocomposite films were subjected to antioxidant study by the ABTS and Ferrozine methods. In both types of methods, the nanomaterials were also comparatively analyzed with pristine Algi, Algi+PVA, and standard ascorbic acid. In the ABTS method, the ability of synthesized nanocomposites to scavenge the free radicle was quantified by adding the solution of the sample in the solution of ABTS. It was noted that on the addition of the sample solution, the color of the ABTS solution was changed. The consumption of free radicles of ABTS was further confirmed by taking the UV absorbance of the solution. The absorption data indicated that on the addition of sample solution in the solution of ABTS, UV absorbance decreased, which confirm the scavenging ability of synthesized films. The unsaturation in the graphene sheet (carbon of graphene is sp2 hybridized) of MWCNTs and reactive centers (free OH and COOH groups) on substrates, makes the nanocomposites more scavengers than the starting materials. It was also observed from the experimental data that the values of % SA were largely affected by the concentration of the sample and the reaction time. Grater amount of scavenger scavenged greater number of radicles and vice versa. Similarly, prolonged time of contact between ABTS solution and sample solution, increased the values of % SA. The results of the current study are compared with the results of another study of pristine alginate polymer [30]. On comparatively analyzing, a significant difference was observed with appreciating results of synthesized nanocomposites to scavenge the free radicle of ABTS solution. The superiority of prepared nanocomposites is due to the reason that in pristine alginate only COO− groups are involved in the delocalization of ABTS-free radicles [30]. Whereas, in nanocomposites, along with the free functional groups of mannuronic acid (M) and guluronic acid (G) (M/G ratio is 1.56) units, the unreacted COOH groups of MWCNTs are also involved in scavenging the free radicle of ABTS.
Additionally, the presence of unsaturation in the graphene sheet of COOH-MWCNTs further increased the % SA value which is due to the greater probability of delocalization of free electrons. However, in pristine Algi, as no unsaturation/conjugation is present, so only the COO− group is responsible for such retention, hence a decrease % SA was observed.
On the addition of PVA polymer in Algi polymer along with COOH-MWCNTs, a relative decrease in %SA was obtained (Fig. 5a). It may be attributed to the fact that in addition to PVA, some of the COOH groups of Algi as well as of COOH-MWCNTs are consumed by the interaction with PVA, and as a result lesser number of carboxylate groups are available for the accommodation of free radicle of ABTS.
Antioxidant results are represented by IC50 (Half maximal Inhibitory Concentration) values which indicate the concentration of films that scavenge 50 % of radicles. These values are obtained by trendline equation obtained from the graph of percent scavenging activity and found inversely proportional to the % SA. From the experimental data, it was revealed that synthesized nanocomposites have lower IC50 values (higher scavenging ability) than ascorbic acid. The IC50 of 31±0.5 M, 28±0.5 M, 23±0.7 M are exhibited by As90, Ac90, and AD90 films, while Bs90, Bc90 and BD90 films showed the IC50 values in the range of 60±0.7 M, 54±0.5 M, 59±0.6 M, respectively. The nanocomposite films have lesser values of IC50 compared to starting film Algi (176±0.5 M) and standard ascorbic acid (169±0.5 M). The greater values of IC50 (smaller values of % SA) in the starting film and standard ascorbic acid are due to the least tendency to accommodate the free radicle generated by the ABTS solution.
3.3.2. Antioxidant measurement by Ferrozine methodThe antioxidant property of synthesized films was also assessed via a metal chelating ability in ferrozine assay (Fig. 5b). It was noted that on the addition of sample solution, the decrease in the concentration of ferrozine was measured, which is attributed to chelation with ferrous ions. The nanocomposites of Algi and Algi+PVA with COOH-MWCNTs can accommodate the Fe+2 ions and give the decreasing trend in absorbance.
In the comparative study of pristine Algi with that of nanocomposites, pristine polymers (Algi and Algi+PVA) have a lesser tendency to accommodate and retain the Fe+2 ions. The fragile, soft, and water-soluble nature of pristine Algi is unable to retain the Fe+2 ions and as a result, the up-taking and releasing rate of Fe+2 ions are in equilibrium. It is also known that, in the presence of PVA polymer along with MWCNTs (B-type), nanocomposites showed lesser scavenging ability to ferrous ions in comparison with scavenging ability Algi/COOH-MWCNTs composites. The PVA polymer interlinked with the COOH and OH groups of Algi along with the COOH groups of MWCNTs thus reducing the possibility to scavenge the Fe+2 ions. Thus, the ferrozine scavenging activity of synthesized materials is in the following order.
No prominent contribution of surfactants in the scavenging ability of Fe+2 ions was observed. In some results, the cationic surfactant-containing films showed better results than anionic surfactant-containing films and vice versa, as a result, no conclusion can be made for the participation of surfactants in the scavenging process. The obtained IC50 values of synthesized materials which are based on the % SA of the synthesized were calculated and found that synthetic nanomaterials have lower IC50 (greater % SA) values than standard ascorbic acid. Experiments indicated that As90, Ac90 and AD90 films have 65±1 M, 58±0.5 M, and 64±0.1 M IC50 values compared to standard ascorbic acid having IC50 values 169±0.5 M. In the case of Bs90, Bc90, and BD90 types of films, the IC50 values lie in the range of 87±0.5 M, 92±1 M, 96±1 M, respectively. While the starting material (Algi) contains 225±1 M IC50 values. This greater amount of IC50 values in Algi polymer is due to the least tendency to accommodate and stabilize the metal ions. Lee and money analyzed the antioxidant property of alginate-based composites and found that these composites can accept divalent ions [42]. In contrast to their findings, the current study finds very good results in scavenging the Fe+2 ions by forming an egg-box model due to the uniform dispersion of nanoparticles, stability of films, and somewhat hydrophobic domains in films.
3.3.3. Statistical analysisThe obtained %SA of synthesized films was further explored by statistical one-way ANOVA in ABTS and Ferrozine methods (Fig. 5c and d). In the ABTS method, on comparing the % SA of synthesized films with ascorbic acid, it is obtained that the synthesized nanocomposite films A90 and B90 showed no significant difference having ‘p’ values 0.0873 (R2 = 0.938) and 0.0818 (R2 = 0.967), respectively. On the other hand, the starting film (Algi) showed a significant difference of 0.0001 with R2 = 0.998.
In an analysis of synthesized materials by the Ferrozine method, all films like A90, B90, and Algi showed a significant difference compared with standard ascorbic acid. A90 film has p-value 0.0006 (R2 = 0.998), B90 has 0.0005 (R2 = 0.999) and Algi has 0.0001(R2 = 0.998). Results concluded that synthesized materials showed high antioxidant activity and have the potential to use as antioxidant films at the industrial level.
4. ConclusionsAntibacterial and antioxidant alginate-based nanocomposite films were successfully prepared by solution casting method under ultrasonic waves. The nanocomposite films showed better physicochemical, antibacterial, and antioxidant properties compared to pristine polymers. Nanocomposites of A-type have a better potential to inhibit the growth of bacteria compared to B type of nanocomposites. The synthesized nanocomposites also showed the property to scavenge the free radicles of ABTS and Fe+2 ions of Ferrozine oxidants, respectively. On the comparative study of nanocomposite films with the pristine polymeric films, it is known that due to the presence of dispersed COOH-MWCNTs nanofillers, the nanocomposite films are the best antioxidant materials, in which A type of nanocomposites has a greater ability to scavenge than the B type of nanocomposites. From this study, it is suggested that A-type (As90, Ac90, AD90) and B-type (Bs90, Bc90, BD90) films have good potential to use as antibacterial and antioxidant packaging films in the food industry. The obtained results also suggest that the alginate-based surface coating with desired penetration power can be made by altering the degree of crosslinking. Moreover, by mixing other materials in synthesized nanocomposites, the prepared films can act as multi-function food packaging films which can perform as active, intelligent, and green packaging films. After analysis the food safety, the prepared films can be used as a food covering material.
AcknowledgmentsThe author(s) gratefully acknowledge Dr. Muhammad Farooq, Department of Chemistry, the University of Peshawar for mechanical testing, SEM, and TEM characterizations.
NotesConflicts of interest/Competing interests The author(s) declared no potential conflicts of interest concerning the research, authorship, and/or publication of this article. References1. Nair MS, Tomar M, Punia S, Kukula-Koch W, Kumar M. Enhancing the functionality of chitosan-and alginate-based active edible coatings/films for the preservation of fruits and vegetables: A review. Int J Biol Macromol. 2020;
http//10.1016/j.ijbiomac.2020.07.083
2. Castro-Yobal MA, Contreras-Oliva A, Saucedo-Rivalcoba V, Rivera-Armenta JL, Hernández-Ramírez G, Salinas-Ruiz J, Herrera-Corredor A. Evaluation of physicochemical properties of film-based alginate for food packing applications. E-Polym. 2021;21:82–95.
https://doi.org/10.1515/epoly-2021-0011
3. Garavand F, Rouhi M, Razavi SH, Cacciotti I, Mohammadi R. Improving the integrity of natural biopolymer films used in food packaging by crosslinking approach: A review. Int J Biol Macromol. 2017;104:687–707.
https://doi.org/10.1016/j.ijbiomac.2017.06.093
4. Cazón P, Velazquez G, Ramírez JA, Vázquez M. Polysaccharide-based films and coatings for food packaging: A review. Food Hydrocoll. 2017;68:136–148.
https://doi.org/10.1016/j.foodhyd.2016.09.009
5. Aydin F, Kahve HI, Ardic M. Lipid-based edible films. J sci eng Res. 2017;4:86–92.
https://www.researchgate.net/publication/320373382
6. Tahir HE, Xiaobo Z, Mahunu GK, Arslan M, Abdalhai M, Zhihua L. Recent developments in gum edible coating applications for fruits and vegetables preservation: A review. Carbohydr Polym. 2019;224:115141.
https://doi.org/10.1016/j.carbpol.2019.115141
7. Saputra E, Kismiyati HP, AAA , MAA . An edible film characteristic of chitosan made from shrimp waste as a plasticizer. J Nat Sci Res. 2015;5:118–124.
http://www.iiste.org
8. Prashanth KH, Tharanathan RN. Chitin/chitosan: modifications and their unlimited application potential: an overview. Trends Food Sci Technol. 2007;18:117–131.
https://doi.org/10.1016/j.tifs.2006.10.022
9. Ncama K, Magwaza LS, Mditshwa A, Tesfay SZ. Plant-based edible coatings for managing postharvest quality of fresh horticultural produce: A review. Food Packag Shelf Life. 2018;16:157–167.
https://doi.org/10.1016/j.fpsl.2018.03.011
10. Murmu SB, Mishra HN. The effect of edible coating based on Arabic gum, sodium caseinate and essential oil of cinnamon and lemon grass on guava. Food chem. 2018;245:820–828.
https://doi.org/10.1016/j.foodchem.2017.11.104
11. Rahmani B, Hosseini H, Khani M, Farhoodi M, Honarvar Z, Feizollahi E, Shokri B, Shojaee-Aliabadi S. Development and characterisation of chitosan or alginate-coated low density polyethylene films containing Satureja hortensis extract. Int J Biol Macromol. 2017;105:121–130.
https://doi.org/10.1016/j.ijbiomac.2017.07.002
12. Mahcene Z, Khelil A, Hasni S, Akman PK, Bozkurt F, Birech K, Goudjil MB, Tornuk F. Development and characterization of sodium alginate based Active edible films incorporated with essential oils of some medicinal plants. Int J Biol Macromol. 2020;145:124–132.
https://doi.org/10.1016/j.ijbiomac.2019.12.093
13. Xu L, Zhang B, Qin Y, Li F, Yang S, Lu P, Wang L, Fan J. Preparation and characterization of antifungal coating films composed of sodium alginate and cyclolipopeptides produced by Bacillus subtilis. Int J Biol Macromol. 2020;143:602–609.
https://doi.org/10.1016/j.ijbiomac.2019.12.051
14. Parreidt T, Müller K, Schmid M. Alginate-Based Edible Films and Coatings for Food Packaging Applications. Foods. 2018;7:170.
http//doi:10.3390/foods7100170
15. Racmayani N, Husni A. Effect of Different Formulations on Characteristic of Biobased Alginate Edible Films as Biodegradable Packaging. E3S Web of Conferences (14703003) EDP Sciences. 2020;
https://doi.org/10.1051/e3sconf/202014703003
16. Chaudhary S, Kumar S, Kumar V, Sharma R. Chitosan nano emulsions as advanced edible coatings for fruits and vegetables: Composition, fabrication, and developments in last decade. Int J Biol Macromol. 2020;152:154–170.
https://doi.org/10.1016/j.ijbiomac.2020.02.276
17. Bibi A, Akhtar T, Akhtar K, Farooq M, Shahzad MI. Alginate-chitosan/MWCNTs nanocomposite: A novel approach for sustained release of Ibuprofen. J Polym Res. 2020;27:1–6.
https://doi.org/10.1007/s10965-020-02342-8
18. Jost V, Kobsik K, Schmid M, Noller K. Influence of plasticizer on the barrier, mechanical and grease resistance properties of alginate cast films. Carbohydr Polym. 2014;110:309–319.
https://doi.org/10.1016/j.carbpol.2014.03.096
19. Wang L, Hu C, Shao L. The antimicrobial activity of nanoparticles: present situation and prospects for the future. Int J Nanomed. 2017;12:1227–1249.
https://doi.org/10.2147/IJN.S121956
20. Teixeira-Santos R, Gomes M, Mergulhão FJ. Carbon Nanotube-Based Antimicrobial and Antifouling Surfaces Engineered Antimicrobial Surfaces. Springer Singapore; Singapore: 2020. p. 65–93.
https://doi.org/10.1007/978-981-15-4630-3-4
21. Al-Jumaili A, Alancherry S, Bazaka K, Jacob MV. Review on the antimicrobial properties of carbon nanostructures. Materials. 2017;10:1066.
https://doi.org/10.3390/ma10091066
22. Bibi A, Rehman SU, Yaseen A. Alginate-nanoparticles composites: kinds, reactions and applications. Mater Res Exp. 2019;6:092001.
https://doi.org/10.1088/2053-1591/ab2016
23. Iqbal SS, Bahadar A, Hossain N, Gull N, Ahmad T, Ehsan N, Khan SU, Riaz T. Synergetic influence of F-MWCNTS on polyvinylpyrolidone sodium alginate composite membrane for reverse osmosis. J Environ Chem Eng. 2021;10608.
https://doi.org/10.1016/J.JECE.2021.106085
24. Vagos MR, Gomes M, Moreira JM, Soares OS, Pereira MF, Mergulhão FJ. Carbon nanotube/poly (Dimethylsiloxane) composite materials to reduce bacterial adhesion. Antibiotics. 2020;9:434.
https://doi.org/10.3390/antibiotics9080434
25. Mitura K, Wyre K, Zarzycki PK. Bioactive food packaging with nanodiamond particles manufactured by detonation and plasma-chemical methods. Food packg. 2017. 7295:328.
https://doi.org/10.1016/B978-0-12-804302-8.00009-1
26. Khaneghah AM, Hashemi SMB, Limbo S. Antimicrobial agents and packaging systems in antimicrobial active food packaging: An overview of approaches and interactions. Food Bioprod Process. 2018;111:1–19.
https://doi.org/10.1016/j.fbp.2018.05.001
27. Mitura K, Kornacka J, Kopczyńska E, Kalisz J, Czerwińska E, Affeltowicz M, Kaczorowski W, Kolesińska B, Frączyk J, Bakalova T, Svobodová L. Active carbon-based nanomaterials in food packaging. Coatings. 2021;11:161.
https://doi.org/10.3390/coatings11020161
28. Levi S, Rac V, Manojlovi V, Raki V, Bugarski B, Flock T, Krzyczmonik KE, Nedovi V. Limonene encapsulation in alginate/poly (vinyl alcohol). Procedia Food Sci. 2011;1:1816–1820.
https://doi.org/10.1016/j.profoo.2011.09.266
29. Valgas C, Souza SM, Smânia EFA, Smânia A. Screening methods to determine antibacterial activity of natural products. Braz J Microbiol. 2007;38:369–380.
https://doi.org/10.1590/S1517-83822007000200034
30. Falkeborg M, Cheong LZ, Gianfico C, Sztukiel KM, Kristensen K, Glasius M, Xu X, Guo Z. Alginate oligosaccharides: Enzymatic preparation and antioxidant property evaluation. Food Chem. 2014;164:185–194.
https://doi.org/10.1016/j.foodchem.2014.05.053
31. Chew YL, Goh JK, Lim YY. Assessment of in vitro antioxidant capacity and polyphenolic composition of selected medicinal herbs from Leguminosae family in Peninsular Malaysia. Food chem. 2009;116:13–18.
https://doi.org/10.1016/j.foodchem.2009.01.091
32. Alizadeh A, Razmjou A, Ghaedi M, Jannesar R. Nanoporous solid-state membranes modified with multi-wall carbon nanotubes with anti-biofouling property. Int J Nanomedicine. 2019;14:1669–1685.
https://doi.org/10.2147/IJN.S189728
33. Pavia DL, Lampman GM, Kriz GS. Introduction to spectroscopy: A guide for students of organic chemistry. Philadelphia: W.B. Saunders Co; 1979.
34. Bibi S, Price GJ, Yasin T, Nawaz M. Eco-friendly synthesis, and catalytic application of chitosan/gold/carbon nanotube nanocomposite films. RSC Adv. 2016;6:60180–60186.
https://doi.org/10.1039/C6RA11618C
35. Chen H, Wang B, Gao D, Guan M, Zheng L, Ouyang H, Feng W. Broad-spectrum antibacterial activity of carbon nanotubes to human gut bacteria. Small. 2013;9:2735–2746.
https://doi.org/10.1002/smll.201202792
36. Liu S, Ng AK, Xu R, Wei J, Tan CM, Yang Y, Chen Y. Antibacterial action of dispersed single-walled carbon nanotubes on Escherichia coli and Bacillus subtilis investigated by atomic force microscopy. Nanoscale. 2010;2:2744–2750.
https://doi.org/10.1039/c0nr00441c
37. Aslan S, Loebick CZ, Kang S, Elimelech M, Pfefferle L, Tassel P. Antimicrobial biomaterials based on carbon nanotubes dispersed in poly (lactic-co-glycolic acid). Nanoscale. 2010;2:1789–1794.
https://doi.org/10.1039/c0nr00329h
38. Sah U, Sharma K, Chaudhri N, Sankar M, Gopinath P. Antimicrobial photodynamic therapy: single-walled carbon nanotube Porphyrin conjugate for visible light mediated in activation of Staphylococcus aureus. Colloids Surf B Bio interfaces. 2018;162:108–117.
https://doi.org/10.1016/j.colsurfb.2017.11.046
39. Teixeira-Santos R, Gomes M, Gomes LC, Mergulhão FJ. Antimicrobial and anti-adhesive properties of carbon nanotube-based surfaces for medical applications: a systematic review. Iscience. 2020;102001.
https://doi.org/10.1016/j.isci.2020.102001
40. Vagos MR, Moreira JM, Soares OS, Pereira MF, Mergulhão FJ. Incorporation of carbon nanotubes in polydimethylsiloxane to control Escherichia coli adhesion. Polym Compos. 2019;40(S2)E1697–E1704.
https://doi.org/10.1002/pc.25125
41. Falk NA. Surfactants as antimicrobials: a brief overview of microbial interfacial chemistry and surfactant antimicrobial activity. J Surfactants Deterg. 2019;22:1119–1127.
https://doi.org/10.1002/jsde.12293
42. Lee K, Mooney D. Alginate: properties and biomedical applications. Pro Polym Sci. 2012;37:106–126.
https://doi.org/10.1016/j.progpolymsci.2011.06.003
Fig. 1a) FTIR results of A-type, b) FTIR results in B-type of films, c) Tensile results of A-types, d) tensile results of B-type of films. 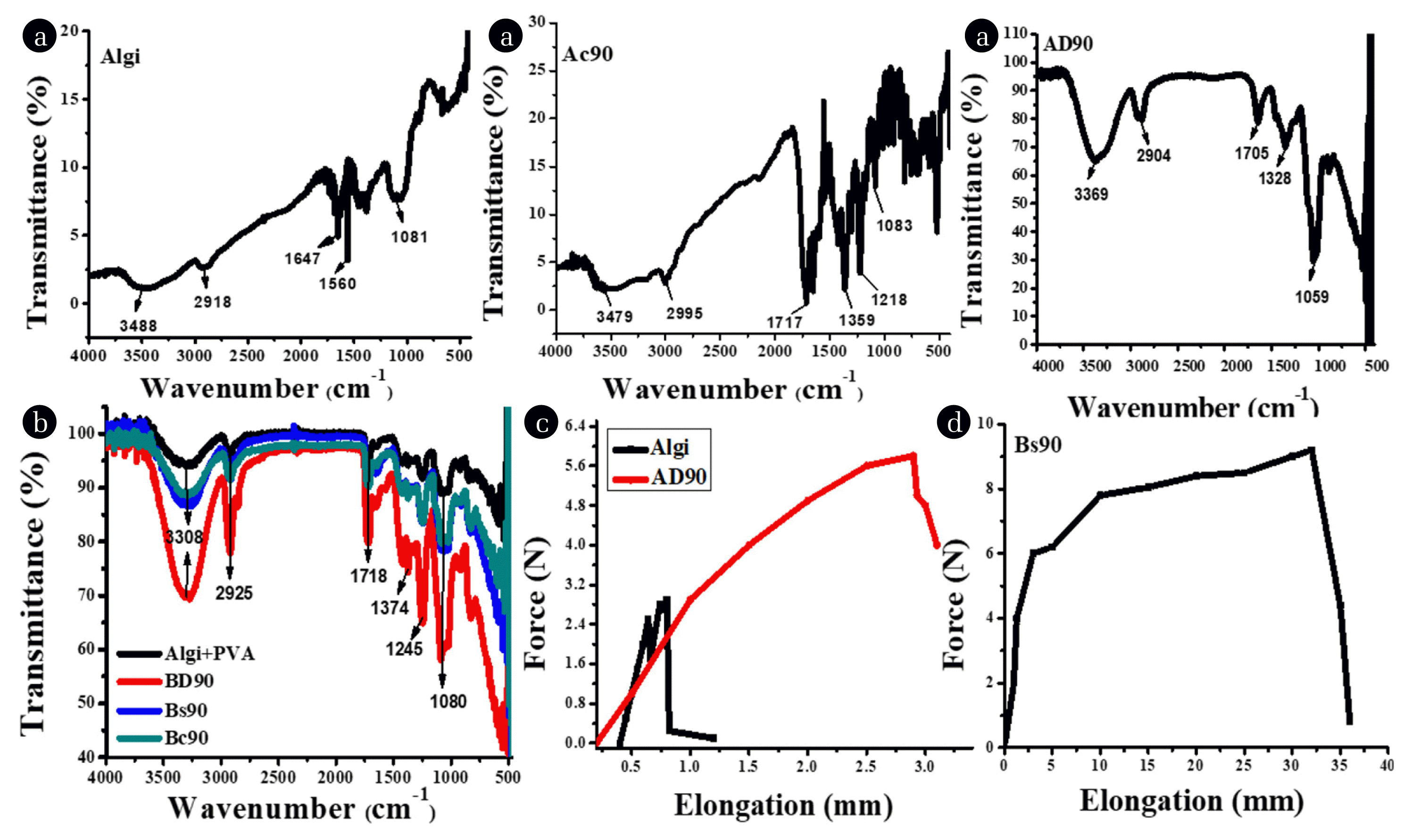
Fig. 2a) SEM results of A-type of films, b) SEM results of B-type of films, c) TEM results of B-type of films, d) Photographic images of synthesized nanocomposites. 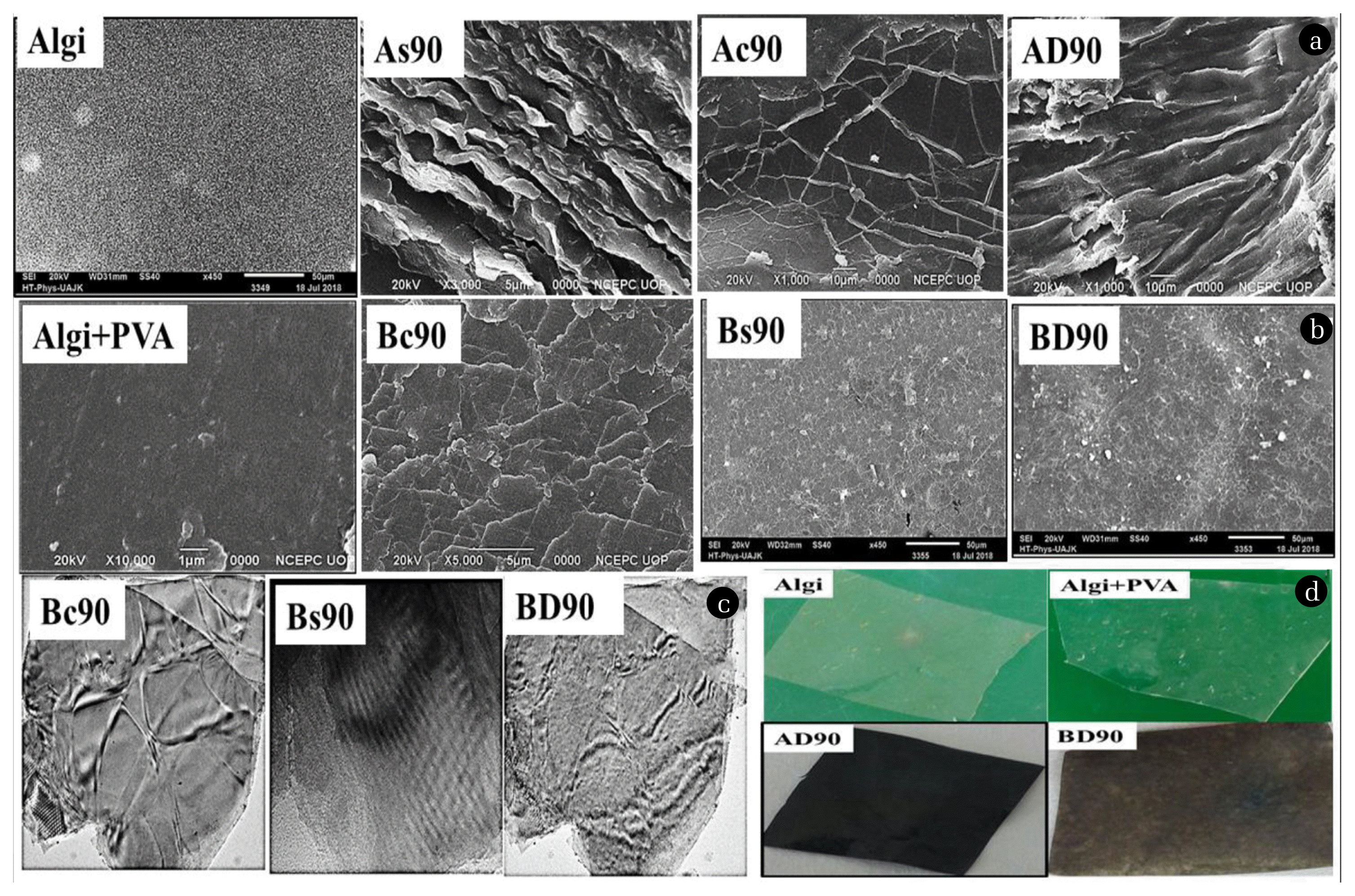
Fig. 3Antibacterial results of a) B-type of films, b) A-type of films, c) A and B types of films having a lower concentration ratio of substrate to nanofillers. 
Fig. 4Comparison of the zone of inhibition of synthesized materials with ampicillin by one-way ANOVA in a) K. pneumonia, b) E. coli and in c) S. aureus), d) the % inhibition of synthesized films. 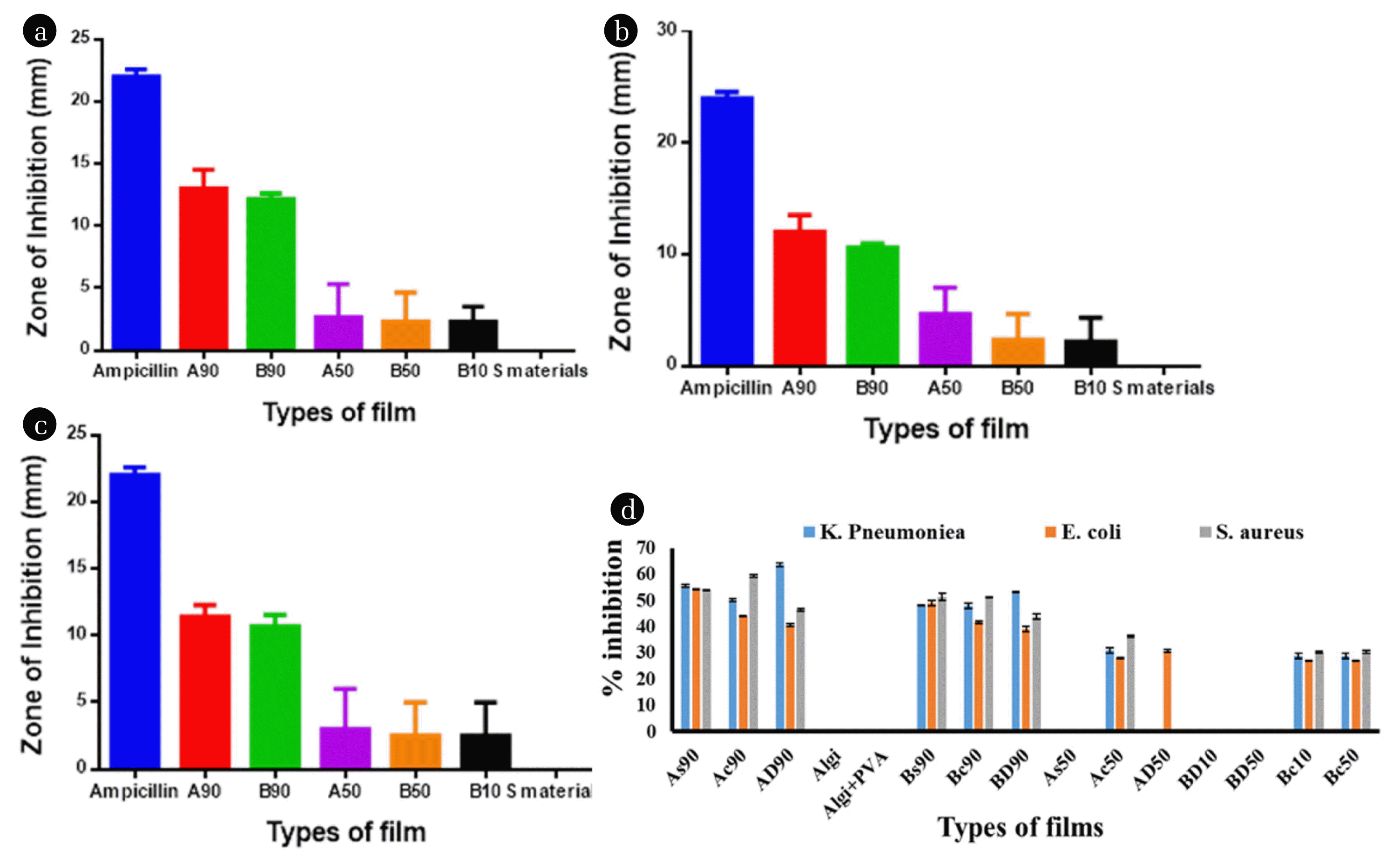
Fig. 5IC50 values of antioxidants by a) ABTS method, b) Ferrozine method, c) Comparison of %SA values of synthesized materials with ascorbic acid by ANOVA tested in c) ABTS method and d) Ferrozine method. 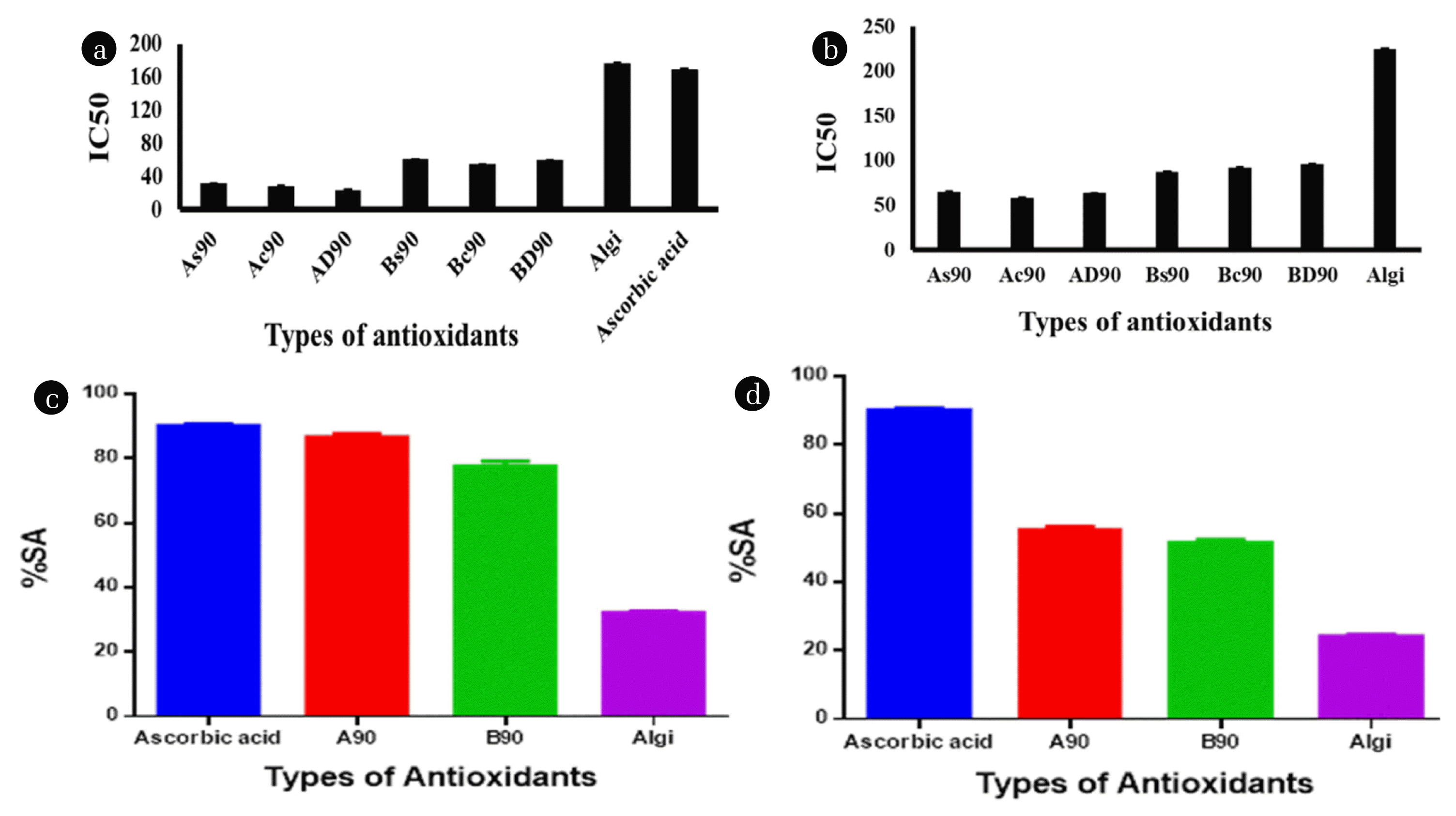
Table 1Protocol Applied for the Preparation of Sample Solution
Table 2Protocol Applied for the Preparation of Sample Solution Table 3R2 and P-Values of Synthesized Films after Statistical Analysis |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||