AbstractThis study presented an original study on the F− removal by Fe and Zr doped Gismondine-dominated Zeolite. Various modified zeolites are prepared by systematically adjusting the synthesis variables, namely pH, mass ratio of zeolite to modify agent (mZeolite:m(Fe+Zr)), mass ratio of Fe to Zr (Fe:Zr) to investigate their effects on the F− adsorption. The performance of prepared Fe-Zr-Zeolite on F− removal was examined through both statistic adsorption and dynamic adsorption. Results indicated that when the modified pH was 7, the Fe:Zr ratio was 1:1 and mZeolite:m(Fe+Zr) was 1:2, the modified natural zeolites which was named Fe-Zr-Zeolite showed the best removal efficiency on F−. Fe-Zr-Zeolite could remove over 80% when the initial concentration was less than 20 mg/L and F− adsorption followed pseudo-second-order adsorption kinetic and Langmuir adsorption isotherm, indicating that F− adsorption by Fe-Zr-Zeolite was chemically dominated. Besides, Fe-Zr-Zeolite had better removal efficiency of F− under acidic conditions than that under alkaline conditions, and the competing anions, typically, Cl−, NO3− and SO42− had negligible effect on F− removal by Fe-Zr-Zeolite. The dynamic adsorption test demonstrated that in order to maintain the high removal efficiency of F−, the filling thickness of Fe-Zr-Zeolite should be at least 30 cm and the small the flow rate is, the higher the F− removal efficiency will be.
1. IntroductionAs a semi-essential trace element for human beings, fluorine at low levels (< 1.5 mg/L) is beneficial to human health [1–3]. However, with the rapid development of industry and agriculture, a large amount of high concentration fluorine-containing wastewaters that come from electroplating, metal processing and other industries, as well as phosphate fertilizers manufacturing were produced [4, 5]. These wastewaters post great threats to human health due to fluorine is an accumulated animal poison and can cause adverse effects such as dental and skeletal fluorosis, osteoporosis, and arthritis, etc. at excessive concentrations [6–8]. Actually, fluoride pollution is doing a disservice to the people in developing countries, where drinking water is usually groundwater without pretreatment [9, 10]. It is estimated that about 200 million people globally are suffering from the adverse effects of water pollution caused by fluoride [11, 12]. Therefore, developing techniques and synthesizing materials that are economically viable and environmentally compatible for defluoridation is highly desired.
Till now, various methods have been reported for this purpose, out of which adsorption is the most widely used [13–15]. The adsorption process depends significantly on the development of high-quality adsorbent materials with optimum efficiency and the required sorption capability. According to the former studies, activated alumina is the most widely used adsorbent for defluoridation [16–18]. However, activated alumina is highly pH-sensitive and performed well at pH=5~ 6.5, this is unacceptable for field applications [17, 19]. Recently, nanosized metal oxides have emerged as new-generation adsorbents for water defluoridation [20–22]. Among them, ZrO2-based materials with both acid and base active sites on the surface have high electrical affinity for fluoride and have been used as promising adsorbents for defluoridation [23, 24]. Nonetheless, the intrinsic features of F− such as small atomic weight and halogen-specific structure led to its weak selectivity and low capacity against ZrO2. Therefore, numerous attempts are endeavored to develop ZrO2-derived materials for defluoridation [25–28]. For instance, Zhang et al. [15] synthesized a kind of carbon hybrid membrane made of amyloid fibril/ZrO2 nanoparticles that could remove over 99.5% of F− at both low and high (5–200 mg/L) concentrations and showed high selectivity for F− against different competitive ions. In another study, He et al. [27] successfully prepared Zr-MOFs with a specific surface area of 740.28 m2/g and the maximum adsorption capacity of F− reached 102.40 mg/g at pH 7.0 at the initial F− concentration of 200 mg/L. However, above mentioned ZrO2-derived materials usually have a relatively high cost and regeneration poses a complex problem for water defluoridation [29–31].
Natural zeolites are highly porous hydrated alumina silicate materials having three-dimensional crystal structures. They have been intensively studied for the removal of pollutants, such as heavy metal ions, ammonium, inorganic anions, phenols, pesticides [32–35]. High ion-exchange capacity and relatively high specific surface areas, and more importantly, their relatively low cost make zeolites attractive adsorbents. However, the adsorption of anionic reactive dyes using natural zeolite is very limited due to the surface of the zeolite and the dye molecules having negative charges [36–38]. The properties of zeolites vary by the silica to aluminum ratio (Si/Al ratio), and low-silica zeolites with a Si/Al ratio of less than 2 have excellent ion exchange capacity and are usually used to remove hardness, heavy metals, and ammonium in water [39–41]. While high-silica zeolites provide favorable characteristics for organic micro-pollutants (OMP) adsorption in aqueous solutions [34, 42–44].
However, considering the weak physical interactions between F− ions and natural zeolites, innovative methods for the modification of natural zeolites are necessary. Since the adsorption ability of zeolites is related to its surface characteristics such as surface functional groups, acidity and basicity, surface charge, hydrophilicity, and porosity, herein, we synthesized a series of Fe- and Zr-modified zeolite through a facile method. The surface characteristics of the modified zeolites were analyzed. The mechanism of F− sorption was established by tests on the variation of pH, adsorbent dose, time, initial F− ion concentration, and temperature. To our best knowledge, this is the first time to report the Fe and Zr co-doped zeolite for high efficiency F− removal.
2. Materials and Methods2.1. MaterialsZirconyl chloride octahydrate (ZrOCl2·8H2O, 98%), anhydrous ferric chloride (FeCl3, 98%), sodium hydroxide (NaOH, 96%), and sodium citrate (Na3C6H5OH·nH2O, 98%) were bought from Macklin Chemical Reagent Co., Ltd. Sodium chloride (NaCl, 99.95%), sodium sulphate (Na2SO4, 99%), and sodium nitrate (NaNO3) were bought from Sinopharm Chemical Reagent Co., Ltd. Sodium fluoride (NaF, 99%) was received from Aladdin Chemical Reagent Co., Ltd., Shanghai. All the chemicals were used as received without purification. Deionized (DI) water was used to prepare all the solutions throughout the experiment. The natural zeolites with different particle sizes were firstly sieved to pass through 40 mesh sieves, and then washed by DI water thoroughly. After dried at 105 °C for 24 h, the zeolites were collected and sealed for further use. The element analysis indicated that O (54.34%), Al (9.21%), Si (33.77%), and Ca (2.68%) are the dominant elements in the natural zeolites.
2.2. Zeolite ModificationTo prepare the Fe and Zr modified zeolites, the pre-treated zeolites as aforementioned were mixed with ZrOCl2·8H2O and FeCl3 at a design mass ratio. The mixture was then transferred to 50 mL of DI water and the pH of the slurry was adjusted to 5, 7 or 10 using 10 mol/L of NaOH and 5 mol/L of HCl. After stirring for 3 h, the slurry was dried in an oven at 105 °C for 24 h. The harvested sample was then calcined at 400 °C at a heating rate of 3 °C/min for 2 h. The final products were ground into uniform powder for adsorption tests. The abovementioned factors (e.g., pH value of the mixture, the mass ratio of raw zeolite to modify agents (mZeolite:m(Fe+Zr)), and mass ratio of Fe to Zr (Fe:Zr)) that may affect the modification of zeolite was optimized through response surface methodology (RSM) [45]. In this study, the variables are pH (X1), mZeolite:m(Fe+Zr) (X2), and Fe:Zr mass ratio (X3) and the response was the F− removal by the modified zeolites (See Table S1). The experimental runs designed by RSM with different variables and response are given in Table 1.
2.3. Material CharacterizationTo determine the specific surface area and pore size of the samples, N2 adsorption and desorption isotherm was performed using ASAP 2460 (Micromeritics instrument, USA) surface area and pore size analyzer (Quantachrome Instruments). The crystallinity of the natural zeolite and Fe-Zr-modified was evaluated by X-ray diffraction (XRD) using 51XMX1121 (Bruker, Germany) equipped with a Cu Kα radiation source at 40 mA and 40 kV. The surface morphologies of the samples were analyzed by Scanning Electron Microscopy - Energy Dispersive Spectrometer (SEM-EDS) (Sigma 500, Hitachi). The surface functional groups of the zeolite before and after modification were determined using Fourier transform infrared (FT-IR) spectrometer (Nicolet 8700, Thermal Fisher Scientific).
2.4. Adsorption TestIn this study, the adsorption performance of F− ions by natural zeolite and Fe-Zr-zeolite was examined using both statistic adsorption and dynamic column adsorption tests. In a typical statistic adsorption test, 0.5 g zeolite or Fe-Zr-zeolite was added into 100 mL of F− solution at 10 mg/L. The mixture was then stirred at 250 rpm and 5 mL of the sample was taken out at designed intervals using a syringe and filtered through 0.45 μm filter to determine the residual F− concentration. In the dynamic adsorption test, the zeolites were firstly filled in a plexiglass column with an inner diameter of 20 mm, the F− solution (10 mg/L NaF) flows out of the zeolite layer from top to bottom at a certain rate, then 5 mL of effluent was taken and filtered to determine the F− concentration. The concentration of F− was determined using fluoride ion selective electrode method. Typically, adding 10 mL of TISAB·II solution to the F− solution that has been taken out, and then the mixture was diluted to 50 mL and the potential was measured using an ion-selective electrode (PF-202, Leici, Shanghai). The TISAB·II solution in this study was prepared by adding 57 mL of glacial acid, 58 g of sodium chloride and 12 g of sodium citrate into 500 mL DI water at room temperature. The pH of the solution was then adjusted to 5.0–5.5 using 6 mol/L of NaOH and then the solution was cooled down to room temperature for standby. The adsorption capacity of F− by each natural zeolite and modified zeolite was calculated using Eq. (1):
Where, q is the adsorption capacity of F− (mg/g), V is the solution volume (100 mL), m is the dosage of adsorbent (0.5 g), and C0 and Ce (mg/L) are the initial and equilibrium concentration of F−, respectively.
3. Results and discussion3.1. Optimization of Modification Conditions of Natural ZeolitesTo determine the optimal modification parameters for natural zeolites, the RSM analysis was employed and 17 runs were performed according to the RSM design. As shown in Table 1, F− removal rate reached 98.2% when the pH value of the mixture was 7, the mass ratio of mZeolite:m(Fe+Zr) was set to be 1:2, and the mass ratio of Fe to Zr was 1:1. The interaction effects between each factor on F− removal were also studied and the results are given in Fig. 1. As shown in Fig. 1a, it can clearly see that under the condition of controlling the Fe:Zr ratio unchanged, the removal efficiency of F− decreased with the increase of pH of the mixture. In addition, the removal rate of F− increased with the decrease of mZeolite:m(Fe+Zr), indicating that the loading amount of Fe and Zr was significant to improve the removal of F−. However, when the mZeolite:m(Fe+Zr) decreased to a certain extent, the removal rate of F− basically did not increase obviously. Thus, it is reasonable to conclude that the loading of Fe and Zr can occupy the active sites on the surface of zeolites and too much loading of Fe and Zr will inhibit the adsorption of F−. As can be seen from Fig. 1b, when the mZeolite:m(Fe+Zr) ratio is too high or too low, the removal efficiency of F− is not as high as that of mZeolite:m(Fe+Zr) ratio is 1:1. On the other hand, the lower the Fe:Zr ratio is, the lower the F− removal efficiency will be, indicating that the content of Fe should not be too high. From Fig. 1c, when mZeolite:m(Fe+Zr) ratio was kept constant, the effect of pH and Fe: Zr ratio on the removal efficiency of F− was not as obvious as that in Fig. 1a and Fig. 1b. Therefore, it can be determined that the modified pH should be controlled at 7, and the Fe:Zr ratio at 1:1 and mZeolite:m(Fe+Zr) at 1:2. The modified natural zeolites which prepared under these conditions are named Fe-Zr-Zeolite, and was used as the ideal sample for characterization and best adsorbent for the rest experiment. The ANOVA results summarized in Table 2 demonstrated that the model is significant due to the model p-value<0.001 and the lack of fit was 0.011. Therefore, the RSM design was effective and rational.
3.2. Characterization of Nature Zeolites and Fe-Zr-ZeoliteXRD test was conducted to identify the main components and crystalline structure of the nature zeolites and Fe-Zr-Zeolite. As shown in Fig. 2a, the peaks around 12.2° 21.1, and 26.7° were identified as Gismondine (PDF#20–0452) [46, 47], this is in line with the EDS analysis in Fig. 2d. The main peaks located around 20.8, 26.6, 50.1, and 59.9° were attributed to (−1 1 0), (0 −1 1), (−1 −1 2), and (−1 −2 1) of silicon oxide ((PDF#77–1060) [34, 38]. For Fe-Zr-Zeolite, it can be found that the main peaks are almost the same as that of natural zeolite. However, no Fe and Zr compounds were identified. The main reason could be the intensity of Gismondine and SiO2 were so strong that the peaks of other compounds were obliterated. From the SEM images of the samples (Fig. 2b and 2c), we can find that natural zeolites show irregular sheet structure. After modification by FeCl3 and ZrO2, the microstructure of zeolite changes to amorphous solid with different diameters. The element mapping results given in Fig. 2e for Fe-Zr-zeolite confirmed that Zr and Fe were successfully loaded on the surface of natural zeolite.
N2 adsorption-desorption analyses were further performed on natural zeolites and Fe-Zr-Zeolite to investigate the variation of pore structure. As shown in Fig. 3a, all the samples show type II adsorption-desorption isotherm with an H1 hysteresis loop, indicating the mesoporous structure of them and free single multilayer reversible adsorption process on non-porous solid surface or macroporous solid [20, 31]. This is further proved by the pore size distribution of natural zeolites and Fe-Zr-zeolite as presented in Fig. 3b. The average pore sizes of them are 12.2 and 10.78 nm, respectively. In the meanwhile, the specific surface area of natural zeolites and Fe-Zr-zeolite are 2.499 and 8.464 m2/g. To explore the functional groups in zeolite before and after modification, FTIR analysis was performed. As shown in Fig. 3c, the peaks from 800~500 cm−1 were probably the vibrations of Si-O and Al-O tetrahedra [46]. The peak at about 1050 cm−1 may be attributed to the asymmetric stretching vibration of Si-O and Al-O [48]. The small peaks at about 3600–3700 cm−1 correspond to the −OH stretching [49, 50]. No other characteristic peaks were found in the FTIR patterns indicates that doping of Fe and Zr had no effect on the functional groups of natural zeolites.
3.3. F− Removal by Fe-Zr-Zeolite3.3.1. Effect of initial concentration of FIn this study, the effect of the initial concentration of F− on the adsorption performance of Fe-Zr-Zeolite was firstly evaluated at 2, 5, 8, 10, 15, and 20 mg/L. As shown in Fig. 4a, Overall, when the F− concentration does not exceed 15 mg/L, the initial concentration has little effect on the removal efficiency of F− and the removal efficiencies are about 80%. However, when the initial concentration was 20 mg/L, the removal rate of F− decreased to 52.7%. This is reasonable because the adsorbent cannot provide enough active sites for fluoride adsorption when increasing the initial F− concentration. This indicates that Fe-Zr-Zeolite can effectively remove fluoride from water only when the initial F− concentration is below 20 mg/L.
3.3.2. The effect of solution pHConsidering that pH can significantly affect the removal efficiency of pollutants in aqueous solutions. Typically, when the solution pH was less than the point of zero charge pH (pHpzc), extra H+ or H3O+ ions will exist in the solution and may occupy the active sites of Fe-Zr-Zeolite, resulting in a positively charged adsorbent [22, 51]. In this case, negatively charged pollutants tend to be adsorbed. On the contrary, the negatively charged surface of Fe-Zr-Zeolite will trap positively charged pollutants when pH > pHpzc. When the pH was <3.5, the formation of the weak acid HF increased. Therefore, it is necessary to address the effect of pH on the adsorption of F−. In this study, the initial solution pH was set to be 2, 4, 6, 8, and 10. As shown in Fig. 4b, Fe-Zr-Zeolite had a better removal performance of F− under acidic conditions than that under alkaline conditions. When pH is 2, the removal rate of F− is about 60.21%. When pH increased to 4 and 6, the removal rate of F− also increased from 77.95% to 83.91. The adsorbent surface had a weak attraction to HF in the solution, resulting in a decreased adsorption capacity. When pH=8, the removal rate of F− was suddenly decreased to 5.42%. When pH continued to increase to 10, the removal rate of F− was only 1.63%. The main reason could be that when the pH was >10, the abundant OH− competed with the F− ions for the active sites of the adsorbent surface, leading to the decrease in the adsorption capacity [26, 52].
3.3.3. The effect of competing anionsIt is well known that other anions, typically, Cl−, NO3− and SO42− often exist in water and the existence of these anions may affect the removal rate of F− by adsorbent. Thus, the equilibrium F− adsorption in the presence of Cl−, NO3− and SO42− was studied. The competing anions at different concentration were prepared in 10 mg/L of F− solution and treated with 50 mg Fe-Zr-Zeolite. As shown in Fig. 4c, the removal efficiency of F− was lower than 80% in all concentrations of competing anions. Since the F− removal efficiency was over 80% when the initial concentration was 10 mg/L (Fig. 4a), it is confirmed that these anions can reduce the F− removal by Fe-Zr-Zeolite slightly. In general, when the concentration of competitive ions increased to 50 mg/L, which is 5 times that of the F− concentration, the removal efficiency of F− was almost the same as that 20 mg/L, indicating that the binding of these ions to Fe-Zr-Zeolite was very limited and would not occupy too many active sites on Fe-Zr-Zeolite. Therefore, it is reasonable to conclude that the effect of competing anions on F− removal was negligible.
3.4. Dynamic adsorption testIn the dynamic adsorption test, different filling thickness and flow rates mean that the contact time of F− and Fe-Zr-Zeolite is different. In the actual wastewater treatment process, the longer the contact time, the higher the pollutant removal efficiency. Therefore, in dynamic adsorption tests, we tested the effects of different filling thickness and solution flow rate on the removal of F−. As shown in Fig. 5a, when the flow rate is set to 2 mL/min and the filling thickness is 10 cm, the removal efficiency of F− in the first 270 min was close to 100%. From 270 min, the removal rate of F− decreased gradually, and after 600 min of continuous adsorption, the removal rate of F− was only 12%. The main reason for this phenomenon is the insufficient thickness, which leads to the gradual filling of the active sites of Fe-Zr-Zeolite filled inside, thus losing the ability to adsorb F−. In contrast, when the filling thickness is 20 cm, the removal rate of F− is higher than 97% within 480 min. Since then, the removal rate decreased gradually, and there was only about 40% at 600 min. When the filling thickness was 30 cm, the removal rate of F− remained above 98% within 600 min of the continuous test.
Similarly, the slower the flow rate, the longer the time required for the packed layer to saturate. Therefore, as shown in Fig. 5b, when the filling thickness is 10 cm and the flow rate is 2 mL/min, the removal efficiency of F− remained above 98% for the first 270 min. Then it gradually decreased to 12% at about 600 min. When the flow rate was increased to 4 mL/min, the F−-removal rate was more than 99% in the first 210 min, and then gradually decreased. After 600 min of continuous reaction, this value decreased to about 2%. When the flow rate increased to 4 mL/min, the F− removal rate was more than 98% in the first 90 min, and then gradually decreased to less than 2% at 600 min. Consequently, in order to maintain the high removal efficiency of F−, the filling thickness should be at least 30 cm and the small the flow rate is, the higher the F− removal efficiency will be.
3.5 The regeneration of saturated Fe-Zr-ZeoliteTo regenerate the saturated Fe-Zr-Zeolite, glacial acetic acid rinsing was used to remove the adsorbed F−. The saturated Fe-Zr-Zeolite was firstly stirred in 0.1 mol/L glacial acetic acid for a period of 10 min, and then washed using DI water three times. After being dried in an oven, the regenerated Fe-Zr-Zeolite was refilled in a plexiglass column for the dynamic F− adsorption experiment. The adsorption ability of regenerated Fe-Zr-Zeolite was evaluated by the decrease of F− concentration in the effluent. As shown in Fig. 6a, after the first cycle of regeneration, the F− removal efficiency kept at 91.6% after 80 min adsorption. After that, the F− removal efficiency decreased gradually, and only 24.1% and 7.6% left after 120 and 140 min, respectively. For the second regeneration cycle, it can clearly see that the F− removal efficiency was significantly lower than that in the first cycle. The F− removal efficiency was only 42.4% at 80 min, which is less than half of the first regeneration cycle. As a result, the used Fe-Zr-Zeolite tends to be saturated after multiple uses.
3.6 Adsorption kinetics and isotherm analysesTo investigate the adsorption process, the adsorption kinetics and isotherm were analyzed. The adsorption kinetics analysis of F− by Fe-Zr-Zeolite was investigated through the pseudo-first-order model [53] (Eq. 2) and the pseudo-second-order model [54] (Eq. 3), respectively. Langmuir [55] (Eq. 4) and Freundlich [56] (Eq. 5) adsorption isotherms were employed to simulate the adsorption process of F−.
In the above equations, qe (mg/g) and qt (mg/g) are the quantity of contaminants removed at equilibrium and time t, respectively. a1 (min−1) and a2 (g/mg/min) represent the reaction constant of the pseudo-first order and the pseudo-second order model, respectively. Q (mg/g) represents the maximum adsorbed quantity of contaminants, K (L/mg), k (L/g) are Langmuir and Freundlich isotherm constants, respectively. 1/Z indicates the heterogeneity of the adsorbent’s surface. Usually, the smaller 1/Z is, the more heterogeneous the surface will be. As shown in Fig. S1a and Table S2, the R2 for pseudo-second-order adsorption was all higher than 0.9999, which is much higher than that of pseudo-first-order adsorption (0.6095). In this case, the adsorption of F− by Fe-Zr-Zeolite was dominated by chemical adsorption. According to the adsorption isotherm analysis, which is shown in Fig. S1b and Table S3. The R2 for Langmuir adsorption isotherm was 0.9906, which further proved that the adsorption was chemically dominated [31, 51].
4. ConclusionsIn this work, Fe-Zr-Zeolite was synthesized through the calcination of natural zeolites and Fe and Zr salts, and the synthesis parameters were optimized using Response Surface Methodology. The performance of prepared Fe-Zr-Zeolite on F− removal was examined through both statistic adsorption and dynamic adsorption. Results indicated that when the modified pH should be controlled was 7, the Fe:Zr ratio was 1:1 and mZeolite:m(Fe+Zr) was 1:2, the modified natural zeolite which was named Fe-Zr-Zeolite showed the best removal efficiency on F−. With the initial concentration less than 20 mg/L, Fe-Zr-Zeolite could remove over 80% of F− and the initial concentration had little effect on the removal efficiency of F−. The kinetics and isotherms analysis revealed that F− adsorption by Fe-Zr-Zeolite was chemically dominated. Besides, Fe-Zr-Zeolite had a better removal effect on F− under acidic conditions than under alkaline conditions. Furthermore, the competing anions, typically, Cl−, NO3− and SO42− had negligible effect on F− removal by Fe-Zr-Zeolite. The dynamic adsorption tests revealed that in order to maintain the high removal efficiency of F−, the filling thickness of Fe-Zr-Zeolite should be at least 30 cm and the small the flow rate is, the higher the F− removal efficiency will be. The regeneration process demonstrated that after regenerating by glacial acetic acid, the F− removal efficiency kept at 91.6% after 80 min adsorption.
AcknowledgmentsThis research was funded by the National Natural Science Foundation of China (No.52170096).
NotesAuthor Contributions B. Yang (Ph.D student) and CM. Jia (Master Student) conceived and designed the experimental investigations. B. Yang (Ph.D student) and Q.Q. Huo (Master student) performed the experiments and analyzed the data. B.X., Quan (Ph.D student) helped to plot figures and discussed the results., B. Yang (Ph.D student), G.R. Sun, (Master Student) and P.D. Su (Assistant professor) wrote the manuscript, C.H. Zhang (Professor) and P.D. Su (Assistant professor) revised the manuscript and supervised the whole experimental process. Supporting information The adsorption kinetics and isotherms of F− adsorption by Fe-Zr-Zeolite, levels of variables used in the experimental design, adsorption kinetic parameters of pseudo-first-order and pseudo-second-order adsorption model, adsorption isotherm parameters of Langmuir and Frendlich are summarized in Supporting information. Reference1. WHO. 2011 Guidelines for drinking-water quality. 4th ed[Internet]World Health Organization; c2011. (cited 31 July 2011. Available from: https://apps.who.int/iris/handle/10665/44584
2. Li J, Zhang H, Zhang J, Xiao Q, Du X, Qi T. Efficient removal of fluoride by complexation extraction: mechanism and thermodynamics. Environ Sci Technol. 2019;53(15)9102–9108.
https://doi.org/10.1021/acs.est.9b02369
3. Wang G, Yan T, Shen J, Zhang J, Zhang D. Capacitive Removal of Fluoride Ions via Creating Multiple Capture Sites in a Modulatory Heterostructure. Environ Sci Technol. 2021;55(17)11979–11986.
https://doi.org/10.1021/acs.est.1c03228
4. Pekel JF, Cottam A, Gorelick N, Belward AS. High-resolution mapping of global surface water and its long-term changes. Nature. 2016;540(7633)418–422.
https://doi.org/10.1038/nature20584
5. Ali S, Thakur SK, Sarkar A, Shekhar S. Worldwide contamination of water by fluoride. Environ Chem Lett. 2016;14(3)291–315.
https://doi.org/10.1007/s10311-016-0563-5
6. Ando M, Tadano M, Yamamoto S, et al. Health effects of fluoride pollution caused by coal burning. Sci Total Environ. 2001;271(1–3)107–116.
https://doi.org/10.1016/S0048-9697(00)00836-6
7. Camargo JA. Fluoride toxicity to aquatic organisms: a review. Chemosphere. 2003;50(3)251–264.
https://doi.org/10.1016/S0045-6535(02)00498-8
8. Alkurdi SS, Al-Juboor RA, Bundschuh J, Hamawand I. Bone char as a green sorbent for removing health threatening fluoride from drinking water. Environ Int. 2019;127:704–719.
https://doi.org/10.1016/j.envint.2019.03.065
9. Barathi M, Kumar ASK, Rajesh N. Impact of fluoride in potable water–An outlook on the existing defluoridation strategies and the road ahead. Coordin Chem Rev. 2019;387:121–128.
https://doi.org/10.1016/j.ccr.2019.02.006
10. Lacson CFZ, Lu MC, Huang YH. Fluoride-containing water: A global perspective and a pursuit to sustainable water defluoridation management-An overview. J Clean Prod. 2021;280:124236.
https://doi.org/10.1016/j.jclepro.2020.124236
11. Amini M, Mueller KIM, Abbaspour KC, et al. Statistical modeling of global geogenic fluoride contamination in groundwaters. Environ Sci Technol. 2008;42(10)3662–3668.
https://doi.org/10.1021/es071958y
12. Jagtap S, Yenkie MK, Labhsetwar N, Rayalu S. Fluoride in drinking water and defluoridation of water. Chem Rev. 2012;112(4)2454–2466.
https://doi.org/10.1021/cr2002855
13. Kang D, Yu X, Ge M. Morphology-dependent properties and adsorption performance of CeO2 for fluoride removal. Chem Eng J. 2017;330:36–43.
https://doi.org/10.1016/j.cej.2017.07.140
14. Khandare D, Mukherjee S. A review of metal oxide nanomaterials for fluoride decontamination from water environment. Materials Today: Proceedings. 2019;18:1146–1155.
https://doi.org/10.1016/j.matpr.2019.06.575
15. Zhang Q, Bolisetty S, Cao Y, et al. Selective and efficient removal of fluoride from water: in situ engineered amyloid fibril/ZrO2 hybrid membranes. Angew ChemInt Edit. 2019;131(18)6073–6077.
https://doi.org/10.1002/ange.201901596
16. Ghorai S, Pant KK. Investigations on the column performance of fluoride adsorption by activated alumina in a fixed-bed. Chem Eng J. 2004;98(1–2)165–173.
https://doi.org/10.1016/j.cej.2003
17. Cheng J, Meng X, Jing C, Hao J. La3+-modified activated alumina for fluoride removal from water. J Hazard Mater. 2014;278:343–349.
https://doi.org/10.1016/j.jhazmat.2014.06.008
18. Craig L, Stillings LL, Decker DL. Assessing changes in the physico-chemical properties and fluoride adsorption capacity of activated alumina under varied conditions. Appl Geochem. 2017;76:112–123.
https://doi.org/10.1016/j.apgeochem.2016.11.011
19. Maliyekkal SM, Shukla S, Philip L, Nambi IM. Enhanced fluoride removal from drinking water by magnesia-amended activated alumina granules. Chem Eng J. 2008;140(1–3)183–192.
https://doi.org/10.1016/j.cej.2007.09.049
20. Wan S, Lin J, Tao W, Yang Y, Li Y, He F. Enhanced fluoride removal from water by nanoporous biochar-supported magnesium oxide. Ind Eng Chem Res. 2019;58(23)9988–9996.
https://doi.org/10.1021/acs.iecr.9b01368
21. Kong L, Tian Y, Pang Z, et al. Needle-like Mg-La bimetal oxide nanocomposites derived from periclase and lanthanum for cost-effective phosphate and fluoride removal: characterization, performance and mechanism. Chem Eng J. 2020;382:122963.
https://doi.org/10.1016/j.cej.2019.122963
22. Chaudhary M, Maiti A. Fe–Al–Mn@ chitosan based metal oxides blended cellulose acetate mixed matrix membrane for fluoride decontamination from water: Removal mechanisms and anti-bacterial behavior. J Membrane Sci. 2020;611:118372.
https://doi.org/10.1016/j.memsci.2020.118372
23. Yu Z, Xu C, Yuan K, et al. Characterization and adsorption mechanism of ZrO2 mesoporous fibers for health-hazardous fluoride removal. J Hazard Mater. 2018;346:82–92.
https://doi.org/10.1016/j.jhazmat.2017.12.024
24. Wang Q, Chen P, Zeng X, et al. Synthesis of (ZrO2-Al2O3)/GO nanocomposite by sonochemical method and the mechanism analysis of its high defluoridation. J Hazard Mater. 2020;381:120954.
https://doi.org/10.1016/j.jhazmat.2019.120954
25. Wang J, Xu W, Chen L, et al. Excellent fluoride removal performance by CeO2–ZrO2 nanocages in water environment. Chem Eng J. 2013;231:198–205.
https://doi.org/10.1016/j.cej.2013.07.022
26. Zhang CH, Tan SH, Niu XM, Su PD. Treatment of geothermal water with high fluoride content by electrocoagulation. Desalin Water Treat. 2015;54(8)2223–2227.
https://doi.org/10.1080/19443994.2014.900727
27. He J, Cai X, Chen K, et al. Performance of a novelly-defined zirconium metal-organic frameworks adsorption membrane in fluoride removal. J Colloid Interf Sci. 2016;48:162–172.
https://doi.org/10.1016/j.jcis.2016.08.074
28. Zhang Y, Su P, Weathersby D, et al. Synthesis of γ-Fe2O3-ZnO-biochar nanocomposites for Rhodamine B removal. Appl Surf Sci. 2020;501:144217.
https://doi.org/10.1016/j.apsusc.2019.144217
29. Xia Y, Zhang C, Wang JX, Wang D, Zeng XF, Chen JF. Synthesis of transparent aqueous ZrO2 nanodispersion with a controllable crystalline phase without modification for a high-refractive-index nanocomposite film. Langmuir. 2018;34(23)6806–6813.
https://doi.org/10.1021/acs.langmuir.8b00160
30. Yao X, Chen L, Cao J, et al. Enhancing the deNOx performance of MnOx/CeO2-ZrO2 nanorod catalyst for low-temperature NH3-SCR by TiO2 modification. Chem Eng J. 2019;369:46–56.
https://doi.org/10.1016/j.cej.2019.03.052
31. Su PD, Gao XY, Zhang JK, et al. Enhancing the adsorption function of biochar by mechanochemical graphitization for organic pollutant removal. Front Environ Sci Eng. 2021;15(6)130.
https://doi.org/10.1007/s11783-021-1418-2
32. Zhang J, Wang L, Shao Y, Wang Y, Gates BC, Xiao FS. A Pd@zeolite catalyst for nitroarene hydrogenation with high product selectivity by sterically controlled adsorption in the zeolite micropores. Angew ChemInt Edit. 2017;129(33)9879–9883.
https://doi.org/10.1002/ange.201703938
33. Li Z, Wang L, Meng J, et al. Zeolite-supported nanoscale zero-valent iron: new findings on simultaneous adsorption of Cd (II), Pb (II), and As (III) in aqueous solution and soil. J Hazard Mater. 2018;344:1–11.
https://doi.org/10.1016/j.jhazmat.2017.09.036
34. Jiang N, Shang R, Heijman SG, Rietveld LC. High-silica zeolites for adsorption of organic micro-pollutants in water treatment: A review. Water Res. 2018;144:145–161.
https://doi.org/10.1016/j.watres.2018.07.017
35. Su PD, Zhang J, Tang J, Zhang C. Preparation of nitric acid modified powder activated carbon to remove trace amount of Ni (II) in aqueous solution. Water Sci Technol. 2019;80(1)86–97.
https://doi.org/10.2166/wst.2019.248
36. Benkli YE, Can MF, Turan MUSTAFA, Celik MS. Modification of organo-zeolite surface for the removal of reactive azo dyes in fixed-bed reactors. Water Res. 2005;39(2–3)487–493.
https://doi.org/10.1016/j.watres.2004.10.008
37. Petrov AW, Ferri D, Krocher O, Van Bokhoven JA. Design of stable palladium-based zeolite catalysts for complete methane oxidation by postsynthesis zeolite modification. ACS Catal. 2019;9(3)2303–2312.
https://doi.org/10.1021/acscatal.8b04486
38. Jin Z, Wang L, Zuidema E, et al. Hydrophobic zeolite modification for in situ peroxide formation in methane oxidation to methanol. Science. 2020;367(6474)193–197.
https://doi.org/10.1126/science.aaw1108
39. Oliveira JA, Cunha FA, Ruotolo LA. Synthesis of zeolite from sugarcane bagasse fly ash and its application as a low-cost adsorbent to remove heavy metals. J Clean Prod. 2019;229:956–963.
https://doi.org/10.1016/j.jclepro.2019.05.069
40. Su P, Zhang J, Xiao K, et al. C3N4 modified with single layer ZIF67 nanoparticles for efficient photocatalytic degradation of organic pollutants under visible light. Chinese J Catal. 2020;41(12)1894–1905.
https://doi.org/10.1016/S1872-2067(20)63620-8
41. Sellaoui L, Hessou EP, Badawi M, et al. Trapping of Ag+, Cu2+, and Co2+ by faujasite zeolite Y: New interpretations of the adsorption mechanism via DFT and statistical modeling investigation. Chem Eng J. 2021;420:127712.
https://doi.org/10.1016/j.cej.2020.127712
42. Burton A. Recent trends in the synthesis of high-silica zeolites. Catal Rev. 2018;60(1)132–175.
https://doi.org/10.1080/01614940.2017.1389112
43. Jiang N, Erdős M, Moultos OA, et al. The adsorption mechanisms of organic micropollutants on high-silica zeolites causing S-shaped adsorption isotherms: An experimental and Monte Carlo simulation study. Chem Eng J. 2020;389:123968.
https://doi.org/10.1016/j.cej.2019.123968
44. Doekhi-Bennani Y, Leilabady NM, Fu M, Rietveld LC, van der Hoek JP, Heijman SG. Simultaneous removal of ammonium ions and sulfamethoxazole by ozone regenerated high silica zeolites. Water Res. 2021;188:116472.
https://doi.org/10.1016/j.watres.2020.116472
45. Su P, Li Y, Zhang J, Li Y. Characterization and chemical fixation of stainless steel pickling residue using sodium sulfide hydrate. Environ Sci Pollut R. 2019;26(10)10240–10250.
https://doi.org/10.1007/s11356-019-04500-y
46. Król M, Mozgawa W, Jastrzębski W, Barczyk K. Application of IR spectra in the studies of zeolites from D4R and D6R structural groups. Micropor Mesopor Mat. 2012;156:181–188.
https://doi.org/10.1016/j.micromeso.2012.02.040
47. Liu W, Aldahri T, Xu C, Li C, Rohani S. Synthesis of sole gismondine-type zeolite from blast furnace slag during CO2 mineralization process. J Environ Chem Eng. 2021;9(1)104652.
https://doi.org/10.1016/j.jece.2020.104652
48. Liu Y, Yan C, Zhao J, et al. Synthesis of zeolite P1 from fly ash under solvent-free conditions for ammonium removal from water. J Clean Prod. 2018;202:11–22.
https://doi.org/10.1016/j.jclepro.2018.08.128
49. Zhao X, Cao D, Su PD, Guan X. Efficient recovery of Sb (V) by hydrated electron reduction followed by cathodic deposition in a photoelectrochemical process. Chem Eng J. 2020;395:124153.
https://doi.org/10.1016/j.cej.2020.124153
50. Yang B, Jiang S, Zhang C, et al. Recovery of iron from iron-rich pickling sludge for preparing P-doped polyferric chloride coagulant. Chemosphere. 2021. 131216:1–9.
https://doi.org/10.1016/j.chemosphere.2021.131216
51. Li Y, Su P, Li Y, Wen K, Bi G, Cox M. Adsorption-desorption and degradation of insecticides clothianidin and thiamethoxam in agricultural soils. Chemosphere. 2018;207:708–714.
https://doi.org/10.1016/j.chemosphere.2018.05.139
52. Sharma M, Mondal D, Singh N, et al. Seaweed-derived nontoxic functionalized graphene sheets as sustainable materials for the efficient removal of fluoride from high fluoride containing drinking water. ACS Sustain Chem Eng. 2017;5(4)3488–3498.
https://doi.org/10.1021/acssuschemeng.7b00198
53. Kalam S, Abu-Khamsin SA, Kamal MS, Patil S. Surfactant Adsorption Isotherms: A Review. ACS omega. 2021;6(48)32342–32348.
https://doi.org/10.1021/acsomega.1c04661
54. Ho YS, McKay G. Sorption of dye from aqueous solution by peat. Chem Eng J. 1998;70:115–124.
https://doi.org/10.1016/S0923-0467(98)00076-1
55. Langmuir I. The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc. 1918;40:1361–1403.
http://doi/pdf/10.1021/ja02242a004
56. Freundlich HMF. Über die adsorption in lösungen Z. Phys Chem A. 1906;385–470.
https://doi.org/10.1515/zpch-1907-5723
Fig. 13D response surface for interactive effects of pH (X1), mZeolite:m(Fe+Zr) (X2), and Fe:Zr mass ratio (X3) on the removal of F−. 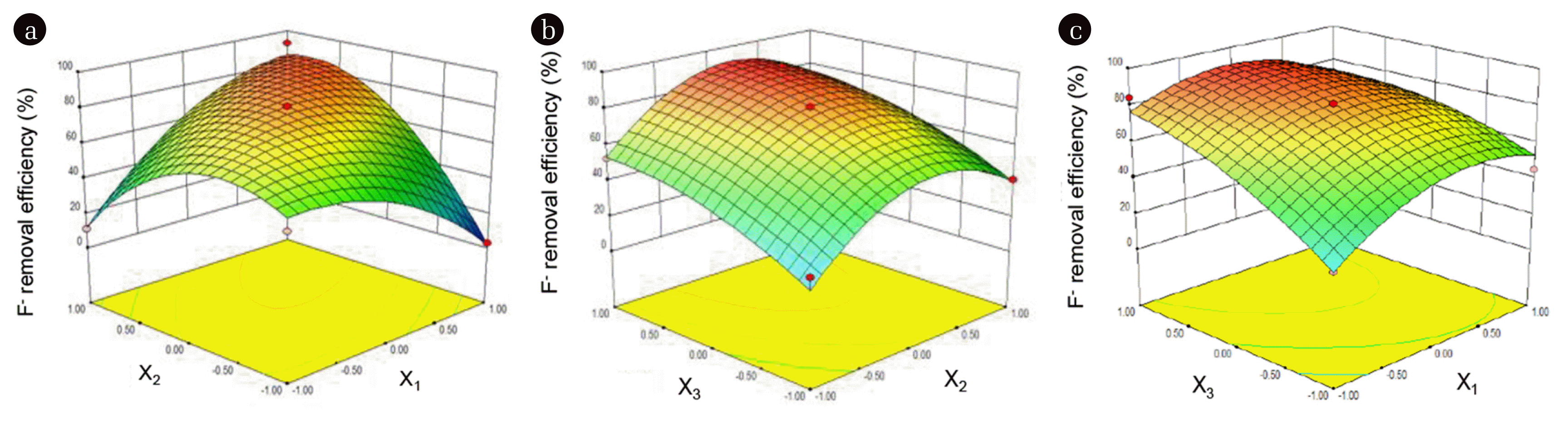
Fig. 2(a) the XRD patterns of natural zeolite and Fe-Zr-Zeolite. (b) and (c) are SEM images of natural zeolite and Fe-Zr-Zeolite, respectively. (d) and (f) are element of natural zeolite and Fe-Zr-Zeolite 
Fig. 3N2 adsorption isotherms (a) and pore width (b) of natural zeolite and Fe-Zr-Zeolite, (c) FTIR plots of natural zeolite and Fe-Zr-Zeolite 
Fig. 4The effects of initial concentration of F− (a), pH value (b) and competing anions on the removal of F−. 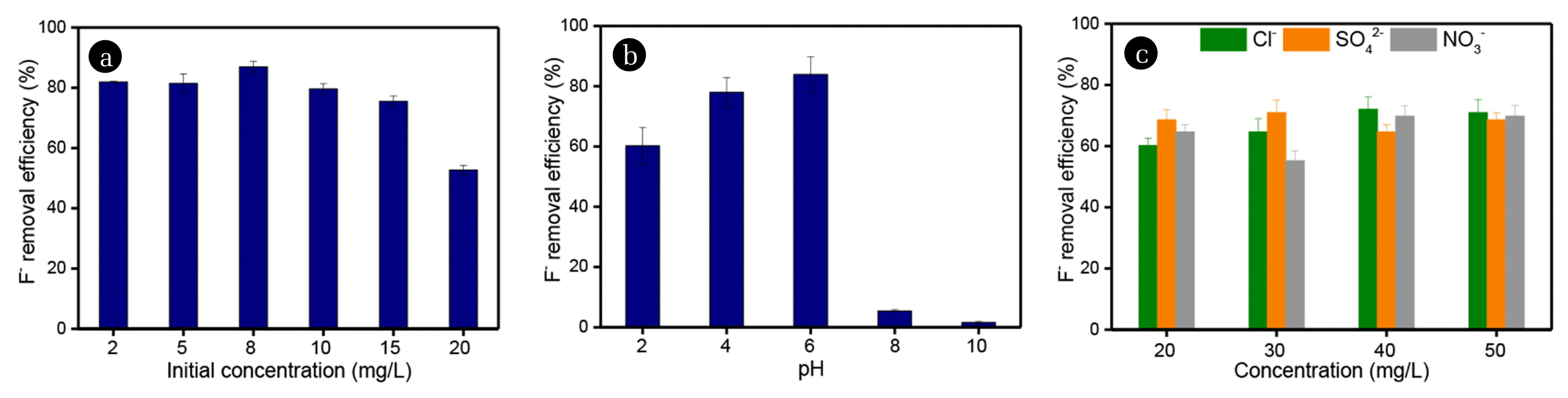
Fig. 6(a)The regeneration of saturated Fe-Zr-Zeolite and its performance on F− removal in different regeneration cycles. (b) The XRD patterns of raw Fe-Zr-Zeolite and regenerated Fe-Zr-Zeolite 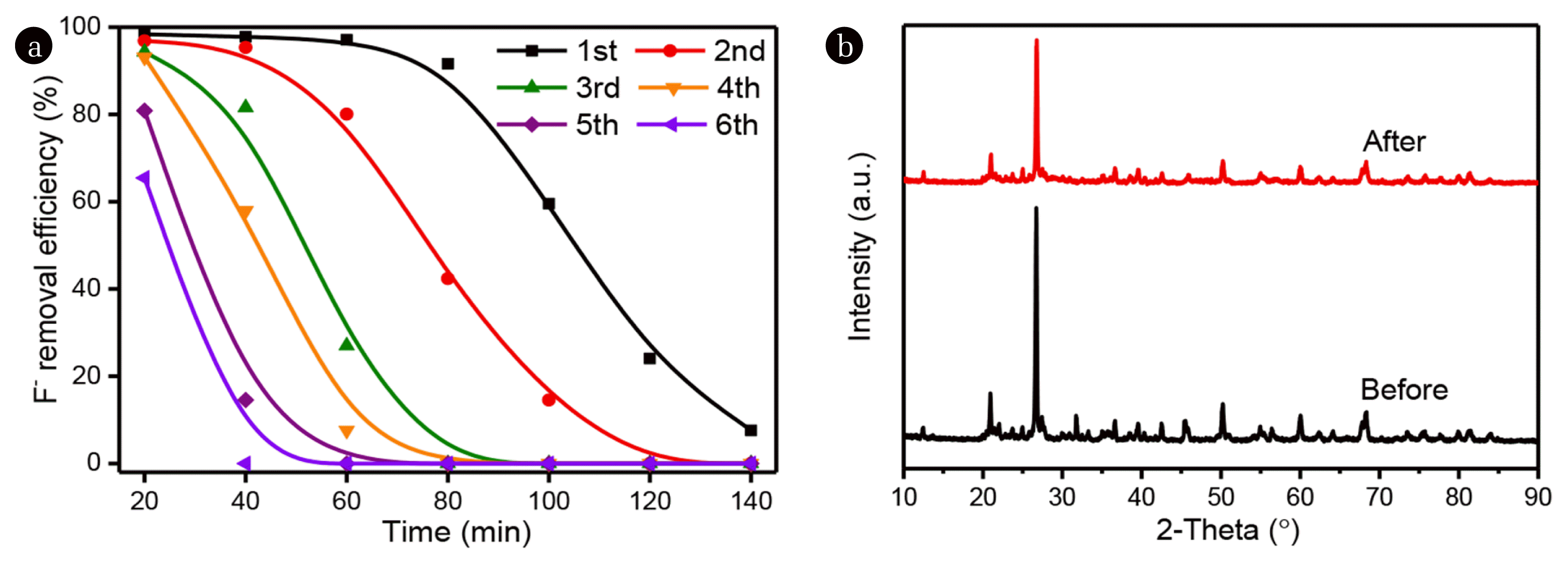
Table 1Experimental Runs and F− Removal Rate Table 2ANOVA Analysis on the RSM Results
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||