1. Introduction
Conversion of iron into rust is an oxidative natural reaction that occurs with time on the iron surface, it depend on different environmental conditions like moisture, pH and temperature [1]. The oxidized Fe-hydroxide phase (FeOOH) is known as rust. Every nation’s economy is getting negatively impacted by the corrosion/rusting of a vast amount of iron. It affects the normal working of any iron-based device and degrades its performance [2]. Failures of iron-based underground pipes are regularly reported due to corrosion, which requires lots of maintenance consequently causing a remarkable loss at social as well as at economic levels [3]. Waste shavings from rusted iron mainly comprise Fe0, Fe2+, and Fe3+ states of iron [4]. The utilization of iron rust and its conversion into a valuable material with desired properties is a costly and difficult process. Nano-sized catalysts are gaining prime focus because of their specific structural, physical, and chemical properties. Nano ferrites are the special ones, they have significant applications in various fields and their magnetic property makes them easy to separate.
A constant increase in metal demand poses pressure on their primary sources which are getting depleted due to their finite amount in nature. Hence, the usage of metallic/metal-containing wastes for the preparation of metallic nanoparticles is an economically efficient approach. Now days, several low-cost secondary sources of iron such as rust, iron ore tailing, etc. are being explored for the synthesis of iron nanoparticles. The preparation of efficient nanoparticles from iron rust is gaining attention these days [5]. A few studies have been reported on the use of iron rust for the synthesis of iron nanoparticle [6]. Abid and Kadhim [7] prepared iron oxide nanoparticle by mixing of iron rust with red chili at 300 °C for 90 min and applied them for antibacterial activity. Iron rust can be transformed in Fe2O3 nanoparticles through its calcination [8].
Some other iron rich wastes which are generally considered hazardous waste are reported to be used for the recovery of iron and preparation of iron nanoparticles. Mhamane et al. [9] prepared α-Fe2O3 nanoparticles using rusted iron wire waste as an iron precursor and used them as a high-performing anode for Lithium-ion batteries. Similarly, acid mine drainage (AMD) is also being used as a source of iron for the synthesis of iron nanoparticles. AMD is generally rich in various metals therefore it is considered as significant environmental hazard [10]. Iron ore tailing is a waste generated from the processing of iron ore, it contains about 25% of the iron. This iron-bearing waste is also being utilized as a low-cost source of iron for nanoparticle synthesis [11]. Besides iron, some other metal-containing solid wastes have also been used to prepare bimetallic nanoparticles or nanocomposites. Behura et al. [12] recovered iron from iron ore tailing and cobalt from lithium-ion batteries then both the recovered metals were used for the synthesis of CoFe2O4 nanoparticles for photo/sono catalytic degradation of dye. Several researchers have also used laterite as a source of iron. Dissanayake et al. [13] prepared hematite nanoparticles from laterite by hydrolyzing it with urea, prepared nanoparticles were applied for adsorption. Another study was carried out by Dissanayake et al. [14] on the synthesis of iron oxide nanoparticles using laterite as an iron source and applied it for adsorption. Synthesis of iron nanoparticles via green route using plant extract is an environmentally convenient, easy, and sustainable process as phytochemicals are biodegradable and non-lethal. Several plants, food and agricultural waste extracts have been explored till now as a potent reducing and stabilizing agent for the synthesis of iron nanoparticles like extract of red peanut skin [15], green tea [16], Musa coocinea peel [17], Cleistocalyx operculatus leaves [18], Ziziphora clinopodioides leaves and leave of green and black tea [19].
Several inorganic, organic, and biological pollutants are released from different industries and domestic activities that create environmental hazards. Water pollution is a worldwide problem, that leads to > 14,000 casualties every day. Various kinds of dyes, pesticides and phenols are among the most readily found organic water pollutants [20, 21]. Phenol and its derivatives like PNP are priority water pollutants as they cause acute toxicity and also act as bio-recalcitrant [22]. Phenolic compounds are present in water as intermediates [23]. Phenols are mainly discharged from industries like pesticide, paint, plastic, leather, paper and pulp, car wash, oil refineries, steel industries, and herbicide manufacturing industries [24–26]. They are known to pose serious health issues even at very low concentrations in water. The permissible concentration of phenol in water owing to the toxic and carcinogenic behavior of phenols is set to be 1 μg/L for drinking water and 100 μg/L for wastewater by United State Environmental Protection Agency (USEPA) [27]. A number of methods have been used till now to eradicate these pollutants from the water like adsorption [28], biological degradation [29], and membrane filtration [30]. In comparison with other removal techniques, the advanced oxidation process (AOP) is more effective, it generates highly reactive °OH radicals with a high oxidation potential. These radicals degrade toxic, non-biodegradable water pollutants into simpler non-toxic compounds or CO2 and H2O [31, 32].
The excessive use of iron worldwide leads to the production of useless waste iron scrap and rust which get disposed of in the garbage yards, where it poses serious environmental threats. Some researchers have used this waste iron rust/scrap for the preparation of iron nanoparticles. The green route of synthesis ensures cost-effective, easy, and non-toxic synthesis of nanoparticles, along with it green synthesis allows the usage of plant-based waste which is generated in abundance and is essential to manage. In this work, iron nanoparticles are synthesized by iron recovered from waste iron rust instead of using any commercially available iron salt and the synthesis was done via an easy green route using extract of dead Jatropha leaves for the first time. This work reports the development of a valuable material by using two social wastes. The material developed was highly efficient and better than previously reported green synthesized iron nanoparticles in terms of surface area. The method is easy, environmentally harmless, and economically efficient as an iron precursor (iron rust) and reducing agent (dead Jatropha leaves) used in this study both were wastes to the society and were obtained freely. The synthesized nanoparticles were successfully applied to remove phenols and PNPs from their aqueous solutions by catalytic degradation process. The study also includes the regeneration of the nanoparticles.
2. Material and Methods
2.1. Materials
Iron rust was collected from the corroding iron utilities from the surroundings. Phenol, PNP, hydrogen peroxide (H2O2), gallic acid (GA), sodium bicarbonate (NaHCO3), Folin-Ciocalteu, sodium hydroxide (NaOH), and hydrochloric acid (HCl) used in this study were purchased from ThermoFisher. Other chemicals and reagents used in the study were of analytical grades and were utilized without any further purification. Dried leaves of Jatropha were hand-picked from the Babasaheb Bhimrao Ambedkar University campus, Lucknow, India.
2.2. Preparation of Iron Rust Leachate
The collected rust was cleaned manually by removing big gravel and other particles. Then it was washed with distilled water to remove dirt and impurities the iron from the water was separated through a magnet. Iron rust was then dried and grounded to form a uniform powder. For leaching, the rusted iron powder was added in the 3M HCl with a solid to liquid ratio of 1:10. The mixture was then subjected to proper mixing on a magnetic stirrer for 2h at 80°C. After leaching, the leachate was filtered upon cooling and then the filtrate was used as an iron precursor to synthesize iron nanoparticles.
2.3. Preparation of Leaf Extract and Its Analysis
The leaf extract was prepared according to the description in Rawat and Singh. [33]. In brief, the collected leaves of Jatropha were washed, dried, ground into a fine powder, and sieved through 250 μm mesh. Then the leaf powder (6 g) was added to distilled water (100 mL) and stirred for 1h at 80°C then filtered after cooling of the mixture. The prepared extract was then subjected to the qualitative determination of phytochemicals by standard methods described in the literature [34–37]. Total polyphenolic content (TPC) of the Jatropha leaf extract was estimated by the Folin-Ciocalteu method [38]. For the determination of TPC, a 0.5 mL volume of the prepared extract was mixed in deionized water (6 mL) and Folin-Ciocalteu reagent (0.5 mL). A 1.5 mL of sodium bicarbonate was added after 2 min to it and then the mixture was make-up to 10 mL with deionized water. It was kept for 2 h at 25°C then its absorbance was measured at 760 nm by UV-Visible spectrophotometer. The TPC was calculated using the calibration curve of GA solution prepared in the range of 15 to 500 μg/L the TPC was expressed in μg GA equivalents per gram of dry weight (μg GAE/g dw)
2.4. Synthesis of Iron Nanoparticles
Previously several methods have been used for the synthesis of iron nanoparticles like chemical reduction by sodium borohydride (NaBH4) under mechanical stirring or ultrasonication in an inert atmosphere [39], Laser fragmentation of iron plates into ligand-free iron nanoparticles in different liquids [40], chemical co-precipitation of Fe2+ and Fe3+ solutions in a definite ratio for magnetite preparation [41] as Lakhanpal et al. [42] did in their study to coat magnetite nanoparticle over sand particles. The organic green synthesis method of iron nanoparticle synthesis generally use water as a solvent and it is free of toxic chemicals, requiring less time, energy, and cost for synthesis [43]. Therefore, for green synthesis of iron nanoparticles from iron rust leachate was done by prepared extract of Jatropha. A 50 mL of leachate was taken in a 500 mL beaker and then it was diluted by adding 50 mL of distilled water, then a 100 mL plant extract was added to it drop by drop with vigorous stirring, and the pH of the solution was maintained to 6 by using 1 M NaOH solution. The color of the solution turns black due to the formation of iron nanoparticles. After that, the mixture was ultrasonicated for the proper dispersion and homogenization of the nanoparticles. The nanoparticles prepared this way were separated from the suspension by centrifugation after washing with distilled water and ethanol. Washed nanoparticles were then dried and homogenized into powder form for characterization and catalytic degradation of phenol and PNP. Iron nanoparticles prepared using iron rust and Jatropha leaf extract were termed as IRNPs@Ja.
2.5. Characterization of Prepared Nanoparticles
The particle size and external morphology of the prepared nanoparticles were analyzed by scanning electron microscope (SEM) using model no. JSM 4490, JEOL, Japan and field emission-scanning electron microscope (FE-SEM) (JEOL JSM 7610f model). Elemental analysis of the nanoparticles was done using energy dispersive X-ray spectroscopy (EDS) equipped with SEM. The crystalline structure of nanoparticles was analyzed through powder X-ray diffraction (pXRD) with Cu Kα (λ = 1.5406 Å) (Model: D8 Advance Eco, Make: Bruker, Germany). Information about the surface structure of the nanoparticle with the help of vibration in the bonds was analyzed by Fourier transform infrared spectroscopy (FTIR) analysis. The IR spectrum was scanned in the range of 400 to 4,000 cm−1 using NICOLET 6700, Thermo Fischer Scientific, USA by making a KBr pellet of the solid nanoparticles powder. Surface area and pore volume information were determined by analyzing nanoparticles with Brunauer-Emmett Teller (BET) instrument (Autosorb-1C, Quantachrome, USA). The zero-point charge (pHZPC) of the nanoparticles was determined through the pH drift method described by Sahu et al. [44] with few modifications. A 50 mL solution of 0.01 M NaCl solution was taken in 5 different beakers and the pH of each solution in the beaker was adjusted to 2, 4, 6, 8 and 10 followed by adding 0.2 g iron nanoparticles to each beaker and then kept for 48 h. A similar experiment was also performed without adding iron nanoparticles as a control. The pH of the solution was measured after 48 h and the change in pH was plotted on the graph and the intersecting point of the final pH of IRNPs@Ja and control was referred to as the pHZPC of the nanoparticles.
2.6. Batch Experiments
An Erlenmeyer flask of 250 mL capacity was used as the reactor for all the Fenton-like catalytic reactions. A typical experimental run consisted addition of a predetermined amount of prepared nanoparticles and H2O2 in a 50 mL aqueous solution of phenol and PNP having an initial concentration of 10 mg/L individually and solution pH was maintained 3. The solution was then shaken for a definite time and the remaining pollutant concentration was analyzed through a UV-Visible spectrophotometer after removing iron nanoparticles by centrifugation. The analysis was done at 510 nm for phenol and at 317 nm for PNP. Phenol analysis was done after the development of color using 4-aminoanti pyrene and potassium ferric cyanide. The optimization of the degradation process was done by varying the concentration of iron nanoparticles, concentration of H2O2, initial concentration of the pollutants, pH of the medium and operating temperature. All the experiments were performed in triplicates. The degradation percentage of phenol and PNP was estimated by using Eq. (1)
Where C0 is initial concentration and Ct is residual concentrations at different time intervals of the pollutants.
2.7. Study of Degradation Kinetics
The linear equations of pseudo-first-order and pseudo-second-order kinetics to analyse the degradation rate of phenol and PNP are represented below in Eq. (2) and (3) [45]:
Where C0 is initial concentration and Ct is residual concentrations at different time intervals of the pollutants. k1 and k2 are rate constants for pseudo-first-order and pseudo-second-order kinetics, respectively.
2.8. Chemical Oxygen Demand (COD) Removal
The degradation of phenol and PNP was also tested by the COD removal with time by titrimetric method. COD reactor (IIC 501 A Icon instrument company, India) was used for the digestion of sample using oxidant K2Cr2O7, in acidic pH created by H2SO4 and Ag2SO4 (with water in small quantity) at 165°C for 2 h [46]. The COD removal percentage was calculated from the following expression:
Where, COD0 represents the initial COD value and CODt represents COD value at different time intervals.
2.9. Determination of Dissolved Iron Concentration in the Reaction Mixture
The determination of total iron, Fe2+, and Fe3+ ions concentration in the reaction mixture during the degradation process was done using the colorimetric method as described by Roy and Moholkar [47]. The concentration of iron was assessed by its reaction with 1,10-phenanthroline, in acidic pH that gives an orange-red complex which can be measured by UV-Visible spectrophotometer at 510 nm wavelength. The oxidation degree of Fe2+, and Fe3+ or vice-versa was estimated by the determination of total iron concentration and Fe2+ concentrations in an aqueous solution. For estimation of total iron concentration, 50 mL of deionized water, 2 mL HCl (concentrated), and 1 mL of 1.45 M hydroxylamine were mixed in the 5 mL sample withdrawn from the degradation reaction mixture. It was boiled till volume reduced to 15–20 mL of total volume and then 4 mL of 5 mM phenanthroline solution and 10 mL of ammonium acetate buffer was added to it and the final volume was made up to 100 mL with deionized water. The absorbance of this solution was then recorded at 510 nm. For the determination of Fe2+ concentration, a 5 mL sample withdrawn from the reaction mixture was acidified with 2 mL concentration HCl followed by the addition of 20 mL phenanthroline solution and 10 mL ammonium acetate buffer then it was diluted to 100 mL and its absorbance was recorded at 510 nm. The concentration of Fe3+ was estimated by subtracting the concentration of Fe2+ from the total iron concentration. The determination of iron in the reaction solution was done after the elimination of solid catalyst from it.
3. Results and Discussion
3.1. Analysis of Phytochemicals and TPC in Plant Extract
Table S1 shows various phytochemical compounds present in Jatropha leaf extract. TPC in leaf extract was found to be GAE 4587.6 ± 44 μg/g dw.
3.2. Characterization of the Iron Nanoparticles
3.2.1. UV-Visible analysis
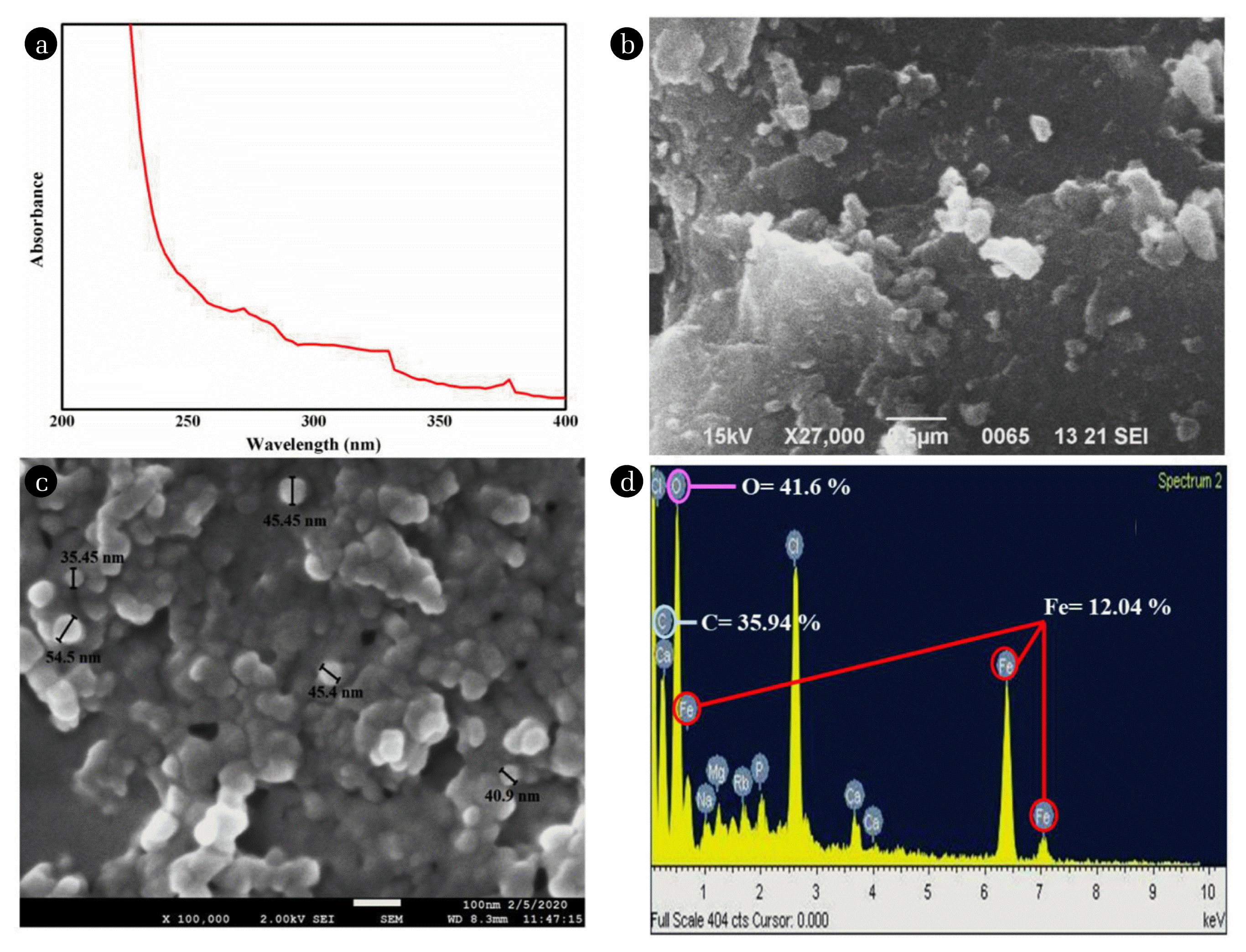
The color transformation of the solution from brown to intense black indicates the synthesis of iron nanoparticles and the black color appears due to the surface plasmon resonance of the iron nanoparticles [48]. The UV-visible spectrum of the synthesized iron nanoparticles shows the absorption of light in the range of 250–350 nm (Fig. 1(a)) which confirms the formation of iron oxide nanoparticles due to their surface plasmon resonance. Similar observations were also reported by Sundaram et al. [49]; Madubuonu et al. [50]; Behera et al. [51].
3.2.2. SEM and FE-SEM analysis
The morphological details and particle size of the prepared iron nanoparticles were obtained from SEM and FE-SEM analysis (Fig. 1(b) and (c)). The SEM image shows a high agglomeration of nanoparticles. Synthesized nanoparticles can be seen in big clumps. The FE-SEM images show better morphological features of the nanoparticles. It shows that the synthesized IRNPs@Ja had irregular shapes; however, a few of them are roughly round in shape with their particle size ranging from 35.45 to 54.5 nm. Elemental analysis of the nanoparticles was also done using EDS which shows the presence of carbon, iron and oxygen as the main element in nanoparticles (Fig. 1(d)).
3.2.3. FTIR analysis
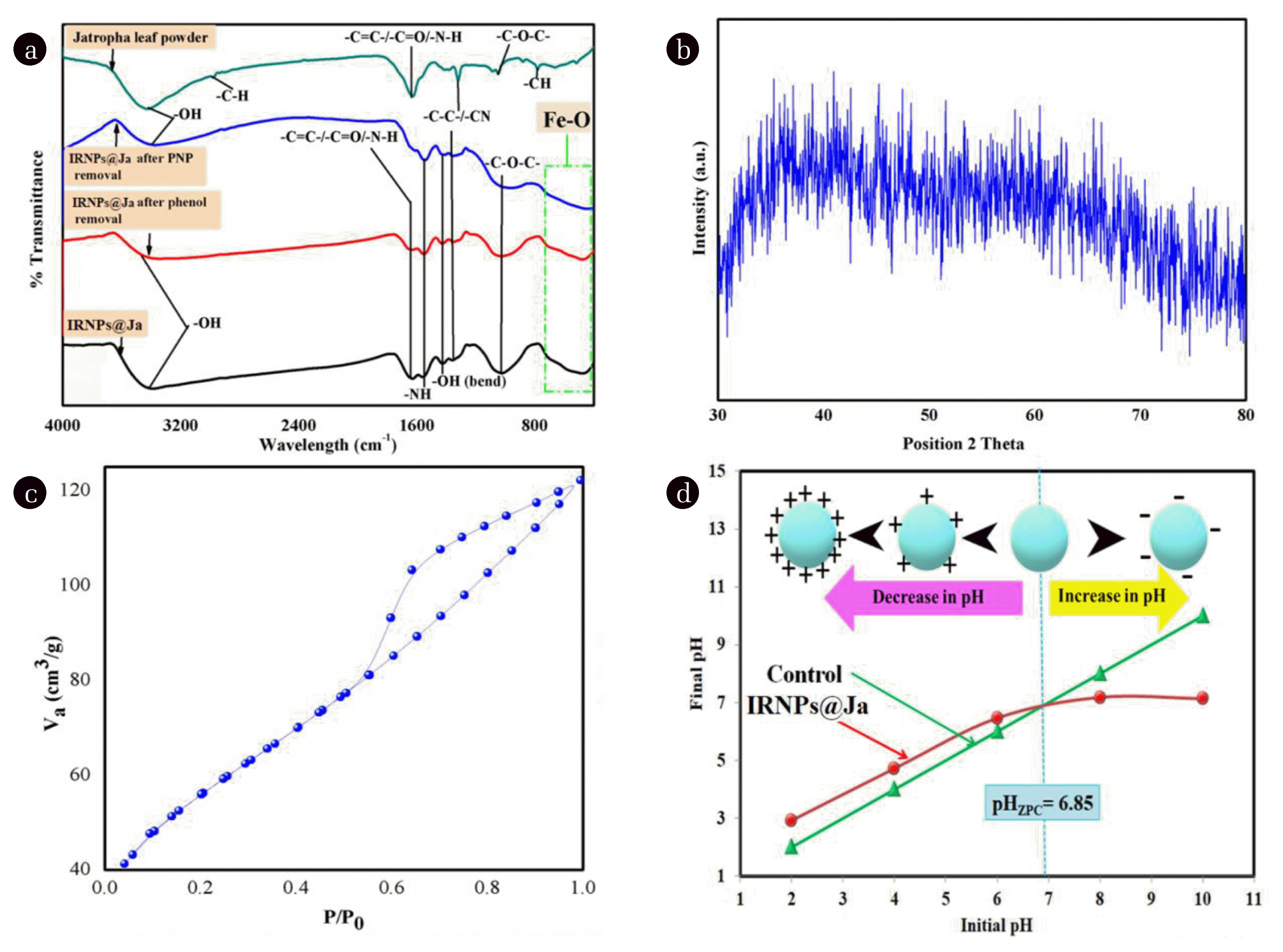
The FTIR analysis was used to have information about functional groups of Jatropha leaf powder and those present on the surface of iron nanoparticles before and after removal of phenol and PNP the results of FTIR analysis are shown in Fig. 2(a). A peak at 3,400 cm−1 represent in all the graphs, it corresponds to the vibration of the −OH group, present in carboxylic groups, phenols and alcohols. The peaks at 1631 cm−1 and 1050 cm−1 represent stretching in −C=C/−C=O bond and −C-O-C-functional groups, respectively, on the surface of leaf powder. Some other functional groups C-C bond and −NH2 are also present in leaf powder. Whereas IRNPs@Ja before and after removal of both the pollutants showed almost similar peaks with some variations in their intensity that might be due to some changes in the surface of the nanoparticles in the catalytic degradation process. The nanoparticles show peaks representatives of −NH group bending in primary and secondary amines and stretching in C-N bond. A distinctive broad peak at ~500 cm−1 which is absent in leaf powder is representative of stretching in metal oxide bond which confirms the presence of Fe-O group in the synthesized nanoparticles.
3.2.4. XRD analysis
Fig. 2(b) shows no prominent peaks which indicate the amorphous behavior of the nanoparticles. Several other authors have also reported the amorphous nature of green synthesized iron nanoparticles [52]. Moreover, absence of any characteristic peak for iron may also result from the encapsulation of the iron nanoparticles by the organic phytochemicals of the plant extracts, as previously reported by authors [53].
3.2.5. BET analysis
BET provides precise information about the specific surface area of the nanoparticles by multilayer adsorption of nitrogen gas. It involves the estimation of total surface area and area of pores thus providing specific surface area information. The nitrogen adsorption-desorption plot is shown in Fig. 2c which represents an occurrence of nitrogen adsorption-desorption curve type IV indicative of spherical mesopores in the nanoparticles [54]. The BET surface area of IRNPs@Ja was recorded 190.2 m2/g, it is much higher than most of the green synthesized iron nanoparticles except work done by Vinayagam et al [55] who reported 190.8 m2/g surface area of iron oxide nanoparticles prepared by leaf extract of the Spondias dulcis through green synthesis. A comparison of IRNPs@Ja surface area with some of the previously green synthesized iron nanoparticles is presented in Table S2. The pore volume was 0.1895 cm3/g and the average pore radius was 1.992 nm.
3.2.6. pHZPC analysis
pHZPC is defined as the pH at which the electrical charge over the surface becomes neutral. It governs the behavior of nanoparticles at different pH values. The pHZPC of IRNPs@Ja was found to be 6.72 as shown in Fig. 2(d). The surface of the nanoparticles tends to acquire a positive charge when solution pH is below the value of pHZPC. However, when solution pH is more than pHZPC then surface of nanoparticle will hold negative charges.
3.3. Catalytic Degradation of Phenol and PNP
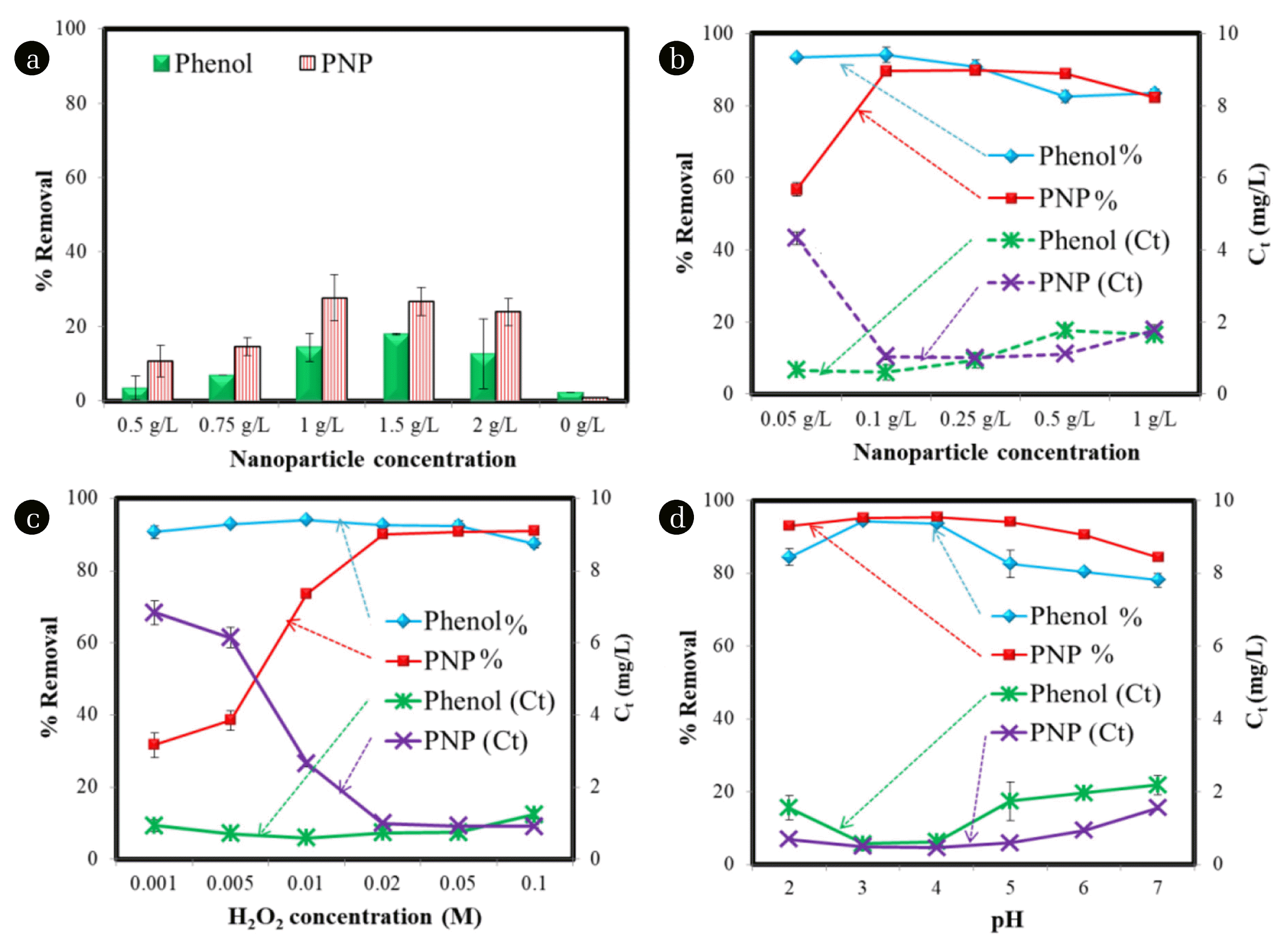
A 100 mL aqueous solution of phenol and PNP (C0 = 10 mg/L) was taken with the IRNPs@Ja nanoparticles concentration varying from 0.5 g/L to 2 g/L without H2O2 for 120 min at neutral pH as control experiments. It determines the removal of phenol and PNP by adsorption on the surface of nanoparticles. Another control was also run with H2O2 (0.05 M) without adding IRNPs@Ja to assess the removal of the pollutants by reaction with H2O2. Fig. 3(a) shows negligible removal of both the pollutants using H2O2 only (with 0 g/L IRNPs@Ja), while experiments with nanoparticles only showed a maximum removal of phenol 17.82% ± 0.2 and maximum removal of PNP 27.72% ± 6.16 in 120 min.
3.3.1. Effect of nanoparticles amount
The amount of catalyst is a major player in the catalytic degradation mechanism it provides active sites for the generation of reactive •OH radicals. Degradation process was increased with an increasing an amount of catalyst, it happened due to increase in number of active sites. This experiment was run with initial concentration 10 mg/L of phenol and PNP, H2O2 concentration 0.02 M, pH 3, temperature 25°C, and IRNPs@Ja concentration 0.05 g/L to 1.0 g/L. Fig. 3(b) shows the maximum 93% removal of phenol was observed at 0.1 g/L and the removal of PNP was observed to be 79.9% at 0.25 g/L, while at 0.1 g/L the removal or PNP was almost similar to that of 0.25 g/L. Furthermore, an enhancing amount of IRNPs@Ja leads to decrease in the removal percentage, it may occur due to the clumping of nanoparticles. It also may occur due to the scavenging effect of excessive metals ions on °OH radicals present in solution. Excessive metal ions are capable of reacting with °OH radicals and transforming them into OH− ions [56]. A similar effect of catalyst concentration was also reported in a previous study [57]. The catalyst concentration used in our study is lesser in comparison with many previously synthesized heterogeneous catalysts which were prepared chemically or physically that is due to the higher surface area of IRNPs@Ja. A comparison of the IRNPs@Ja based on the surface area, catalyst amount used and reaction time to degrade different pollutants with some previously reported heterogeneous Fenton catalyst prepared and modified via different methods has been shown in Table 1.
3.3.2. Effect of H2O2 concentration
The concentration of H2O2 directly influences the efficiency of Fenton-like degradation process as it governs the number of reactive °OH radicals generated. The effect of H2O2 concentration was studied in the range of 0.001 M to 0.1 M concentration of H2O2, with IRNPs@Ja concentration 0.1 g/L, phenol and PNP concentration 10 mg/L, pH 3 and temperature 25°C. Concentration of H2O2 0.01 M was found optimum for the phenol removal, while 0.02 M concentration of H2O2 was found optimum for PNP removal (Fig. 3(c)). On further increasing the concentration of H2O2, the removal of phenol decreased slightly while the PNP removal was remained almost similar. A concentration of H2O2 < 0.01 M and < 0.02 M was insufficient for the effective removal of phenol and PNP, respectively. Removal of the pollutants was decreased with an increasing H2O2 concentration. Increasing H2O2 concentration results in a higher number of °OH radicals which may decrease degradation process due to collision of these highly reactive radicals with each other and H2O2 molecules, these reactions are represented in Eq. (5), (6) and (7) [65, 66]. Xu et al. [67] reported a similar degradation trend for phenol by Fenton reaction using 0.5 g/L concentration of Fe2O3 coated palygorskite clay as a heterogeneous catalyst. The degradation of phenol by palygorskite clay was increased with an increasing concentration of H2O2 from 10 to 30 mM and then decreased due to oxidation of Fe2+ into Fe3+ when presence of OH° radicals was excess in amount and a similar observation of H2O2 concentration effect on Fenton-like degradation of methyl orange was also reported by Perrotti et al. [68].
3.3.3. Effect of pH
The Fenton-like catalytic degradation process is strongly influenced by the pH of the reaction solution, it influences the generation of reactive °OH radicals [69]. The pH must be less than 3 to protect the iron catalyst from getting precipitated. The effect of different reaction pH on degradation process was carried out in pH range of 2 to 7 with initial pollutant concentration 10 mg/L, IRNPs@Ja concentration 0.1 g/L, H2O2 concentration 0.01 M for phenol and 0.02 M for PNP at 25°C and the results of this study are shown in the Fig. 3d. The maximum removal of both the pollutant was found at pH 3, while on further increasing pH of the reaction solution, the removal percentage was decreased slowly. However, the decrease in removal efficiency was not much in the range of 3–6 suggesting the application of synthesized iron nanoparticles for the degradation of organic pollutants in a wider pH range. At higher pH formation of the ferric-hydroxyl complex (Fe(OH2) or Fe(OH)3) may take place which negatively affects the generation of °OH radicals and a decrease in the removal percentage [70]. The decomposition of H2O2 and degradation potential of °OH radicals is also interrupted at higher pH causing a decrease in removal efficiency [71]. The degradation process was also decreased at pH 2 which can occur because of [Fe(H2O)6]+2 complex formation by Fe2+ that slow down the generation of °OH radicals [72]. The equations [8] and [9] shows how the solubility of Fe2+ and Fe3+ depends on the pH of the solution that affects the generation of OH° radicals in the degradation process:
3.3.4. Effect of Initial pollutant concentration
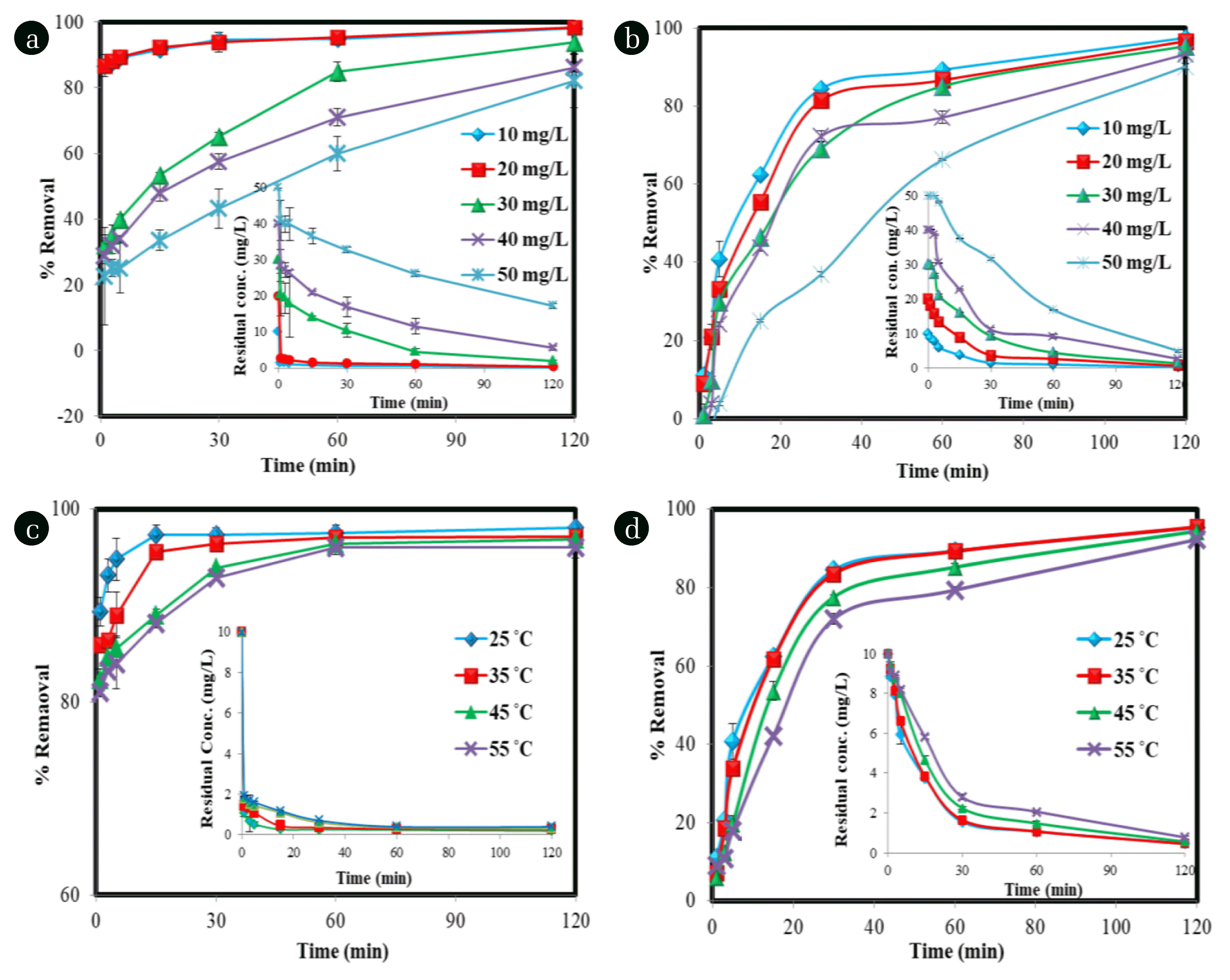
The initial pollutant concentration effect was tested within the range of 10 mg/L to 50 mg/L. Fig. 4(a) and (b) represented that removal of both phenol and PNP was decreased when their initial concentration was increased. An increasing concentration of pollutants causes enhancement in the number of pollutant molecules in reaction solution which overcomes the numbers of degrading agents (OH° radical) due to the limited amount of catalyst and H2O2 [73]. Hence, after consumption of all that reactive radicals, the excess pollutant molecules remain undegraded and a decrease in removal of both the pollutants was noticed.
3.3.5. Effect of reaction time
The effect of reaction time on the degradation process was studied in the range of 0 to 120 minutes. Fig. 4(a) and (b) represents that the degradation of phenol was highly appreciable at 10 mg/L concentration more than 90% of phenol was removed rapidly in 5 min, while at 50 mg/L most of the phenol was removed in 30 min. For PNP also, there was a rapid removal observed in the initial 30 min and then removal was increased at a slower rate. At the initial stage, the surface of IRNPs@Ja was vacant to react with H2O2 and produce a maximum number of °OH radicals. As the increasing reaction time the surface of IRNPs@Ja become occupied with adsorbed H2O2, pollutant molecules, oxidized iron species, or degradation by-products which lessen the production of °OH radicals. Moreover, in the initial stage of the degradation process, there was the maximum number of pollutant molecules which implies a driving force toward catalyst that enhances the interaction of pollutant and °OH radical while when most of the pollutant molecules get degraded with an increasing time, driving force was decreased. Present study shows the fast degradation process as compared to the other studies (Table 1), around 90% removal of phenol was achieved 5 min and in 30 min an appreciable amount of both phenol and PNP was removed, the higher surface area of the IRNPs@Ja provides a large number of active sites for the generation of OH° in a short period using its low concentration that removes pollutants in a short time.
3.3.6. Effect of operating temperature
Degradation reaction was studied at different operating temperatures (25°C, 35°C, 45°C, and 55°C) for both phenol and PNP, results are shown in Fig. 4(c) and (d). The degradation processes for both the pollutants were decreased at higher temperature in initial time while in 60 min the degradation of the pollutants at all temperatures was almost similar. A decreased in removal of phenol and PNP may occur due to decomposition of H2O2 at higher temperature, a similar result was also reported by Wang, [74].
3.4. Degradation Kinetics
The degradation kinetic data of both phenol and PNP was analyzed by pseudo-first-order and pseudo-second-order kinetics. Fig. 5(a) and (b) show the linear plots of pseudo-first-order kinetic models, while the linear plots of pseudo-second-order are presented in Fig. S1. The k1, k2, and R2 values are listed in Table 2. According to the R2 value, the degradation kinetics of both the pollutants fitted best with the pseudo-first-order kinetic.
3.5. COD Removal
COD is a measure of oxygen required to oxidize organic matter present in water, it indirectly indicates the amount of organic matter in water [75]. Alterations in COD value during the degradation process were tested with initial pollutant concentration 10 mg/L; catalyst concentration 0.1 g/L; H2O2 0.01 M for phenol and 0.02 M for PNP; pH 3; temperature 25°C and reaction time 120 min. Fig. 5(c) shows the result of the COD removal test which shows a continuous decrease in the value of COD with the time that represents a reduction in the oxidizable organic content in the reaction mixture due to the breakdown of both the pollutants, as the organic pollutants breakdown with time resulting COD value was decreased. The COD value for phenol was decreased from 336 mg/L to 56 mg/L, while it decreased from 384 mg/L to 64 mg/L for PNP. The percentage removal of COD from 0 to 120 min was 83.6% and 83.3% for phenol and PNP, respectively. Remaining COD value in solution is due to the presence of intermediates or simpler compounds formed due to the breakdown of phenol and PNP.
3.6. Determination of Dissolved Iron Concentration in the Reaction Mixture
The concentration of dissolved iron in the reaction mixture was tested during the degradation process with different process parameters such as initial pollutant concentration 10 mg/L; catalyst concentration 0.1 g/L; H2O2 0.01 M for phenol and 0.02 M for PNP; pH 3; temperature 25°C and reaction time 120 min. Fig. 5(d) shows the results obtained from this study, it represents total iron concentration was increased from 1.9 to 2.4 mg/L in phenol and 2.8 to 3.7 mg/L in PNP with time. However, a maximum amount of total iron was recorded initially and then there was a slow increase in the concentration of total iron. The Fe2+ concentration for both the pollutants was found highest at initial time and then it was decreased continuously with time due to oxidation of Fe2+ to Fe3+. Therefore, Fe3+ concentration showed an opposite trend, its concentration was increased with an increasing time. The concentration of iron in a process of PNP degradation was found more than phenol degradation process because the H2O2 concentration used in PNP removal was higher than that used in phenol removal which caused more corrosion of catalyst in the process of PNP removal. Results represent that iron present in a reaction solution was majorly in the form of Fe3+. Furthermore, the higher concentration of Fe3+ and lower concentration of Fe2+ in a reaction is due to the heterogeneous Fenton reaction. This reaction takes place on the surface of the catalyst and the Fe2+ oxidized on the surface get leached in the reaction solution in the form of Fe3+. The dissolved iron concentration was also tested without the addition of H2O2 in the reaction solutions under optimum experimental conditions and the results show the presence of total iron, and Fe2+ concentration was < 0.1 mg/L in a solution for both the pollutants which is because without the addition of H2O2 the corrosion of iron from catalyst was very less.
This study showed that under similar conditions, the degradation of PNP was lower than of phenol which is due to the presence of the nitro group on nitrophenols, which is electron-withdrawing in nature and it resists the oxidation of nitrophenols chemically or biologically and also resist its hydrolysis. A similar degradation trend for both phenol and nitro phenol was also reported in other studies [76, 77]. The degradation of PNP was found comparatively lower than phenol by photo-degradation using NiFe2O4/MWNT [76] and by sono and sono-enzyme degradation [77]. The concentration of total iron and Fe3+ was found more in the PNP degradation system than phenol. A higher amount of Fe3+ could reduce the efficiency of the Fenton degradation due to the accumulation of Fe3+ and formation of Ferric complexes with the carboxylated species generated in the degradation process [78], it may be the another reason behind a lower degradation of PNP in comparison with phenol.
3.7. Regeneration and Recycling of the Catalyst
To examine the degradation capability of the used catalyst, it was recycled for five cycles for phenol and PNP degradation under the following conditions: C0 = 10 mg/L, pH 3, Temperature 25°C, and catalyst dose which was optimized in the aforementioned study. The used catalyst was removed from the reaction mixture through centrifugation then washed with distilled water and dried for overnight. Dried catalyst stored and used in the next cycle. As shown in Fig. 5(e) the removal of both the pollutants decreased (62.44 ± 3.4 % for phenol and 54.89 ± 5.9 % for PNP). The decrease in degradation efficiency of the catalyst may result from the corrosion of its surface to form an iron oxide layer on it and adsorption of pollutants as well as degradation by-products over the catalyst surface as reported by other authors [79]. However, the decrease in degradation efficiency in the first three cycles was not that much affected which allows the application of the prepared catalyst for the water treatment process successfully for three cycles.
3.8. Mechanism
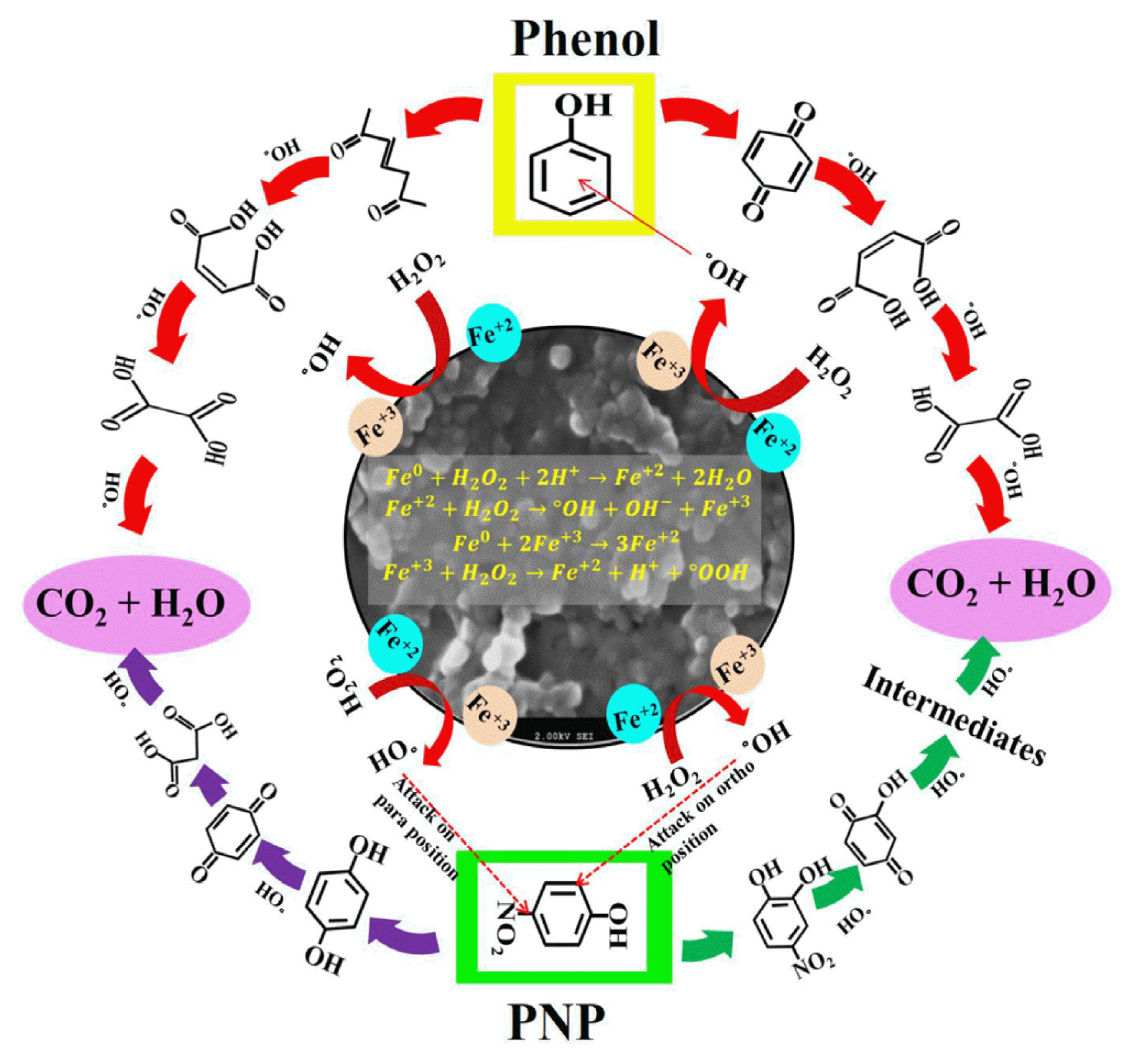
AOP is a promising mean for water treatment it includes the addition of oxidants like O2, O3 and H2O2 followed by the addition of a catalyst homogenous or heterogeneous which generates highly reactive radicals °O2−, °OOH, and °OH. Oxidant H2O2 is reported to overcome all the other oxidants due to its high oxidation potential of 2.8 eV (°OH), 1.8 eV (°OOH), and −2.4 eV (°O2−) [80]. The generation of °OH radicals in a heterogeneous Fenton-like process using nano zerovalent iron (nZVI) takes place by reaction of nZVI and H2O2. In the first step, nZVI is oxidized into Fe2+ and in the second step Fe2+ reacts with H2O2, in this process Fe+2 further get oxidized into Fe+3 which again react with another molecule of H2O2 by which generation of °OOH radical takes place (Eq. (10) to (13)) [27, 81] and iron again get reduced that can participate again in the generation of °OH radical. In the determination of dissolved iron both Fe2+ and Fe3+ were found and the reduction in Fe2+ concentration due to its oxidation into Fe3+ by H2O2 was noticed which increased Fe3+ concentration in the reaction solution.
The control experiments showed negligible removal of phenol and PNP, while a little amount of pollutants was reported to be absorbed on the surface of nanoparticles. It is confirming that the removal in all the other studies was the result of catalytic degradation. The degradation of phenol may take place in two stages i) the hydroxylation of the aromatic ring may occur which form dihydroxybenzenes like catechol and hydroquinone, it degraded into simpler chemical forms and then get mineralized, ii) the phenol may form carboxylic acids by ring-opening and then get mineralized [82]. The PNP molecule exhibits two major groups −OH and −NO2 the generated °OH radicals tend to attack ortho or para positions of the −OH groups and it forms 4-nitrocatechol by an attack on ortho position resulting it can get mineralized and form oxidized intermediated 4-nitrocatechol or 4-nitropyrogallol. PNP can also get denitrated by °OH radical, attacks on para position resulting in the formation of hydroquinone which forms simpler compounds or CO2 and H2O [83]. A diagram related to the proposed mechanism is shown in Fig. 6.
4. Conclusions
This study concluded that iron nanoparticles were synthesized successfully from easily available iron rust as waste material. This method allows the recovery of iron from iron rust and its conversion in a high value added product without use of any expensive chemical. The synthesized nanoparticles were economic and environmentally convenient. Nanoparticles were applied for the removal of toxic water pollutants such as phenol and PNP from their aqueous solutions effectively. Very fast degradation of phenol and PNP by synthesized nanoparticle was achieved which might be due to its high reactivity and high specific surface area of 190.2 m2/g. The catalytic activity of the nanoparticles was observed high in the range of pH 3–5 and it was very less affected by the process of nanoparticles recycling in the first three cycles. Based on the above results, this study concluded that the iron rust derived iron nanoparticles (synthesized via green method) were very effective catalyst to degrade toxic pollutants even with its low amount. Furthermore, iron nanoparticle can work as catalyst for the degradation of organic pollutants under wider pH range. Therefore, these synthesized iron nanoparticles have wider applicability for the degradation of toxic pollutants occurs in wastewater discharged from various industries with wider pH range.