AbstractSkim is a major by-product of the virgin coconut oil (VCO) industry consisting of nutrients and is currently discharged to the environment as waste. Hence, this research aimed to utilize skim as a medium for Botryococcus braunii and Chlorella vulgaris growth. The effect of organic carbon resourced from glucose and glycerol on biomass was also investigated at certain concentrations with intervals 100:0 to 0:100 (%). Results indicated that Botryococcus braunii and Chlorella vulgaris cultivated under 20% VCO mill effluent gave the highest biomass production, which accounts for 1.69 g/L and 5.34 g/L; 89.40% and 86.70% COD reduction efficiency. Whereas, mixotrophic condition showed a positive trend for Botryococcus braunii at 80:20 (glucose:glycerol) with 5.60 g/L biomass production and 97.64% COD removal efficiency. Vice versa, the addition of glucose:glycerol gave an inhibitory impact on Chlorella vulgaris as the biomass production was merely 1.66 g/L with COD efficiency 94.03%.
1. IntroductionThe 2020–2024 strategic plan of the Indonesian Ministry of Agriculture reported that Indonesia is estimated to produce coconut circa 2,980,926 tons in 2021 [1]. Meanwhile, Burton J [2] mentioned that coconut has the largest plantation area in Indonesia, larger than rubber and oil palm, which is around 26% of the total plantation area and is worth 17.13 million tons per year as shown in Fig. S2. Therefore, Indonesia is categorized as the largest coconut producing country in the world.
As the world leader of coconut production, Indonesia has various coconut-based products encompassing clothing, animal feed, beauty creams and strong fibers. For example, coconut oil is extracted, processed, and marketed for culinary, medicinal, and cosmetic uses, or also known as virgin coconut oil (VCO). VCO can be produced using traditional enzymatic (fermentation), inducement, and heating [3, 4]. During the process, coconut milk also generates a large amount of skim, which is mainly composed of water. Skim is usually discarded because it contains a small amount of oil. Furthermore, skim can cause environmental pollution if it discharged directly to the environment because skim still contains a fairly high COD (Chemical Oxygen Demand), BOD (Biological Oxygen Demand), Total Solids (TS), oil and grease. According to Nur MMA et al. [5], skim contains 4,916.67 ppm COD, 983.33 ppm BOD, 0.7 ppm phosphate, 104.16 ppm nitrogen and 0.5 ppm lipid. Meanwhile, Tripetchkul S et al. [6], reported that skim wastewater has pollutant characteristics as shown in Table S1. Therefore, it is a defiance to discover a suitable technology that is environmentally-friendly and economically-feasible to conduct [7, 8]. However, despite high pollutant loaded in skim, the wastewater has abundant micronutrient that is beneficial and can still be utilized as nutrient for microalgae growth. This is also supported by previous research that wastewaters derived from industrial, municipal, agricultural resources (e.g., virgin coconut oil) have been tested for microalgae growth and managed as nutrient removal [9].
Microalgae are photosynthetic microorganisms that have the ability to use sunlight and carbon dioxide to produce biomass, which is rich in carbohydrates, lipids, and proteins. They have substantial advantages compared to other oil crops, which accounts for higher growth rate, less cultivation area requirement, higher ability of carbon capture and storage as well as self-adaptive to low-quality medium such as wastewater [10]. Microalgae are also reported having a prominent role as a phycoremediator to reduce the levels of COD (Chemical Oxygen Demand), nitrogen and phosphorus contained in the waste and more environmentally sustainable for not producing additional solid waste [11, 12]. Despite that the biomass produced and nutrients removed under wastewater medium are still inefficient owing to carbon source limitation and photo deficiency for autotrophic dominated growth of microalgae at high cell concentration [13] since most of microalgae are categorized as photoautotrophic, in which inorganic carbon and illumination serves as energy source. Albeit some microalgae live heterotrophically by consuming organic substrates as sole carbon source, which happens to yield a higher biomass [14], this cultivation requires high costs and leads to major environmental concern due to metabolism waste of carbon dioxide. Therefore, mixotrophic cultivation is proposed as a more suitable way to enhance growth rate and biomass production [15].
Mixotrophic is a cultivation in which heterotrophic and autotrophic processes are carried out at the same time. In this study, mixotrophic cultivation was employed due wastewater, such as skim, dairy waste, brine, etc. degrades water quality and needs to be processed further through industrial applications [16–18], yet microalgae cultivated in the wastewater have the difficulty to perform photosynthesis because of the limitation of light entering the turbid waste. Thereof, carbon sources are constantly fed in the medium. Saccharide and acetate are generally used as carbon sources for microalgae cultivation. Among them, the most commonly used carbon source is carbohydrate in the form of glucose [19]. However, the high cost of glucose leads to an infective and inefficient way to conduct. To overcome this problem, the addition of glycerol at various concentration ratios is expected to reduce production costs since glycerol is produced as a waste in the biodiesel production. With the abundance of biodiesel industry, the plant is brimmed with glycerol. Subsequently, the manufacturers must discover solutions to treat the waste. One breakthrough of utilizing glycerol is to be employed as a potential nutrient for mixotrophic cultivation.
Many studies also have reported the increasing in microalgae biomass by mixotrophic and utilization of glycerol during cultivation as followed: Chlorella vulgaris in real centrate wastewater [9], Chlorella in dairy wastes [20], C. protothecoides in dairy wastes [11, 12], Chlorella sp. in palm oil mill effluent [21], Phaeodactylum tricornitum and Chlorella vulgaris with glycerol addition as carbon source [19, 22]. However, the study of cultivating microalgae in VCO mill effluent has not been further probed with the addition of glucose:glycerol at various concentrations. Therefore, the purpose of this research was to investigate the potency of VCO mill effluent as well as glycerol:glucose used as the carbon sources in the medium for Chlorella vulgaris and Botryococcus braunii. This paper is subdivided into the following sections: Title, Authors and Affiliation, Abstract, Introduction, Materials and Methods, Results and Discussion, Acknowledgments, Conflict of Interest Statement, Author Contributions, Nomenclature, References and Supplementary Materials.
2. Materials and Methods2.1. MaterialsCultures of Chlorella vulgaris and Botryococcus braunii were selected for this study due these algal have the capacity to grow rapidly on carbon and/or light sources. The isolated algal strain was obtained from Balai Besar Pengembangan Budidaya Air Payau (BBPBAP) Jepara, Central Java, Indonesia and grown phototropically using Walne fertilizer in open raceway ponds with the capacity of 1–20 ton.
Meanwhile, wastewater was supplied by small and medium-sized enterprises called Klentik Putih located in Yogyakarta, Indonesia. Chemicals such as ammonium sulfate, glucose, glycerol, ferric chloride, potassium bicarbonate, urea and triple superphosphate were obtained from CV. Chemix Pratama located in Indonesia.
2.2. MethodsThe methodology of this study is depicted in Fig. S4 and described as followed:
2.2.1. VCO mill effluent preparationIn this study, wastewater was filtered and sterilized at 50°C for 10 min. Thus, then the COD, N and P substances were analysed at Penguji dan Kalibrasi laboratory in Balai Besar Teknik Kesehatan Lingkungan dan Pengendalian Penyakit Penguji dan Kalibarasi (BBTKLPP) Yogyakarta, Indonesia as shown in Table S2. Meanwhile, the initial COD content was analysed using volumetric approach followed SNI 19-4234-1989 method [23].
2.2.2. Microalgae cultivationA total of 2-L sample was prepared for each wastewater concentration consisting of 20% of microalgae strains (1 g/L) and wastewater: water (20:60% (0.4:1.2 g/L); 40:40% (0.8:0.8 g/L); 60:20% (1.2:0.4 g/L); 80:0% (1.6:0 g/L) for 14 days with the addition of synthetic nutrients encompassing Walne fertilizer. The highest growth rate showed by optical density (OD) was then cultured under mixotrophic condition by adding carbon sources (glucose:glycerol) at 0:100 – 100:0 (mol/mol) (100:0% (15:0 g/L), 80:20% (12:1.527 g/L), 60:40% (9:3.054 g/L), 40:60% (6:4.581 g/L), 20:80% (3:6.108 g/L) and 0:100% (0:7.636 g/L)). During the cultivation, cultures were maintained at 28–30°C and pH 6.8–7.2, which was adjusted using 0.1 N HCl and NaOH, respectively, and observed periodically using a pH meter AZ Instrument 8686. The light intensity was set at 6,000–7,000 lux and cultivation salinity was managed at 3 ppt. Microalgae was gently mixed by sparging aerators for 24 h. Overall, the experimental set up is shown in Fig. S3.
2.2.3. Measurement of specific growth rateThe effect of VCO mill effluent and synthetic carbon added was evaluated in the relation to the growth rate of the algal biomass within days. To analyze the growth rate, a sample of microalgae in growth medium was put in the cuvette and measured using spectrophotometer Optima SP-300 at 680 nm. The specific growth rate (μ) was calculated in conformity within Eq. (1):
where OD1 and OD0 are the optical density on day 1 (t1) and day 0 (t0) accordingly.
2.2.4. Biomass production analysisGrowth rate was determined by optical density (OD) at a wavelength of 680 nm [24]. This relationship was obtained by preliminary research conducted on control media by preparing 5 Erlenmeyer flasks containing 200 mL strains. The optical density of each flask was measured using spectrophotometer every day. On the first day, the first flask was then harvested, dried and weighted to obtain the biomass. Afterwards, the same procedure was conducted for other flasks on the next day. The relationship between OD and biomass produced in the control medium is connected by linear equation.
2.2.5. Chemical oxygen demand (COD) analysisThe COD analysis was undertaken followed SNI 19-4234-1989 method [23]. Firstly, standardize the KMnO4 solution by adding 0.01 N H2C2O4, 4N H2SO4 and a small amount of phenolphthalein into the Erlenmeyer flask. The mixture was then placed underneath a calibrated burette containing the titrant and heated up until 70–80°C at a stipulated time. Afterwards, a small amount of titrant was then added to the analyte until the color changed indicated the end point of titration. Eventually, the titrant volume was recorded (b mL), therefore, the normality of KMnO4 can be determined followed Eq. (2):
Secondly, 10 mL of VCO mill effluent was pipetted into the flask and mixed with 0.01 N H2C2O4, 4N H2SO4 and phenolphthalein at 70–80°C. The mixture was then titrated until the end of titration volume (a mL). In sum, COD was measured according to Eq. (3) [25].
Where COD denotes chemical oxygen demand concentration (ppm); Nk denotes the normality of KMnO4 (N); Vk = a denotes the amount of KMnO4 to standardize the mixture of H2C2O4, H2SO4 and phenolphthalein (mL); Vo denotes the volume of H2C2O4 (mL); No denotes the normality of H2C2O4 (N); and b denotes the amount of KMnO4 needed to titrate VCO Mill Effluent (mL).
Meanwhile, the efficiency of COD removal was determined in accordance with Eq. (4):
Where COD0 and COD1 denotes initial and final COD concentrations (ppm).
3. Result and Discussion3.1. Effect of VCO Mill Effluent on Biomass and Growth rate of Botryococcus Braunii and Chlorella VulgarisIn this study, the comparison between control medium (0% addition of VCO mill effluent) and mixotrophic cultivation was observed to study its effect on growth rate and biomass at various wastewater: water concentrations (20:60% (0.4:1.2 g/L); 40:40% (0.8:0.8 g/L); 60:20% (1.2:0.4 g/L); 80:0% (1.6:0 g/L). However, to obtain the relationship between biomass produced (dry weight) and optical density, a calibration curve is behooved. Based on Section 2.2.4, the standard curved of Botryococcus braunii and Chlorella vulgaris are shown in Fig. S1.
The mixotrophic growth of Botryococcus braunii and Chlorella vulgaris was analysed by supplementing nutrients to fulfill C, N, O and P needs in the waste medium. A pretreatment procedure was performed to reduce the starting microbial contained in the VCO mill effluent in order to evade deleterious competition with algal growth by mild heating process. Our initial work showed that at 20% waste concentration, both microalgae attained the highest biomass with concentration of 1.69 g/L and 5.34 g/L consecutively in accordance with the calibration curve shown in Fig. 1.
The concentration of 20% did not have a toxic effect on the biomass of Botryococcus braunii and Chlorella vulgaris. Therefore, it can be utilized by microalgae as nutrients to increase the amount of biomass up to two weeks. The nutrients provided by VCO mill effluent are shown in Table S2.
On average, Botryococcus braunii and Chlorella vulgaris were able to optimally grow on the 4th (20%), 5th (40% and 60%), and 10th day (80%) accordingly. However, the addition of VCO waste at concentration of 40–100% had an inhibitory effect on the growth of microalgae. This was due microalgae did not have the ability to digest excessive nutrients in VCO waste, thereby leading to the possibility of producing toxic metabolites [26, 27]. Likewise, microbial load in the effluent also had a role to render growth rate as they competed to consume the nutrients [20]. Based on Table S3, the highest growth rate was obtained at 20% effluent concentration in accordance with Fig. 1.
3.2. Effect of Botryococcus Braunii and Chlorella Vulgaris on COD Decrease in VCO Mill EffluentDuring 14 days of cultivation, both microalgae were able to reduce COD levels in VCO waste with initial COD content was 49,090 mg/L. This proves that the content of VCO waste, especially organic compounds, can be utilised as nutrients. Yang C et al. [28] stated that organic compounds can also be employed by microalgae as an energy source and carbon sources while reducing the need for additional nutrients for microalgae to produce biomass. Thereof, by utilizing the waste, microalgae can be cultivated with sufficient nutritional needs and reduce the cost of production of microalgae at once. Based on the result, the highest COD reduction obtained for Botryococcus braunii and Chlorella vulgaris was at 20% VCO waste concentration with COD removal efficiency is 89.40% and 86.70% consecutively as shown in Table 1.
3.3. Effect of Glucose:Glycerol on Biomass of Botryococcus Braunii and Chlorella Vulgaris
Fig. 2 shows the effect of different glucose:glycerol concentrations on the growth of Botryococcus braunii and Chlorella vulgaris at 20% VCO Mill Effluent and control (0% VCO Mill Effluent). The ratio of glucose:glycerol used was 100:0% (15:0 g/L), 80:20% (12:1.527 g/L), 60:40% (9:3.054 g/L), 40:60% (6:4.581 g/L), 20:80% (3:6.108 g/L) and 0:100% (0:7.636 g/L) respectively.
During the experiment, mixotrophic cultivation produced dark colored biomass. This indicated high biomass was produced in the cultivation condition. Botryococcus braunii and Chlorella vulgaris had the capacity to grow rapidly on carbon and/or light sources. The cell densities of the mixotrophic cultures demonstrated that the effects of light and CO2 with glucose were equally strong. Microalgae produced the highest concentration of biomass under mixotrophic conditions compared to heterotrophic and autotropic conditions. Therefore, mixotrophic conditions are an alternative as a medium for growing microalgae in wastewater with high turbidity and difficult to enter light [29].
Based on Fig. 3, both microalgae reached the highest turbidity at the same time, on the 14th day with biomass produced was 5.60 g/L for Botryococcus braunii and 1.66 g/L for Chlorella vulgaris at 60:40% carbon synthetic in 20% effluent concentration. Meanwhile, [29] reported that the highest biomass productivity was at 80:20% (glucose:glycerol) for Chlorella vulgaris and [30] was at 5 g/L glycerol for Chlorella vulgaris, Botryococcus braunii and Scenedesmus sp. Both afore-mentioned experiments, however, did not utilise wastewater. However, an intriguing finding is obtained in this study since Chlorella vulgaris yielded a lower biomass production compared to without waste. This is because the addition of glucose and glycerol exerted inhibitory at high concentrations as the algae were not able to consume the carbon source completely. This finding is in line with [31] that excessive glucose and glycerol did not give a positive impact on Chlorella vulgaris. Other than that, some metal in the skim may act as micronutrient, whilst the remaining has the possibility of toxicity metal influences towards Chlorella vulgaris since it could lead to cell break off [32].
The result also showed that the highest biomass concentration was obtained at a higher initial ratio of glucose compared to glycerol. Nonetheless, glucose is consumed more rapidly if the glucose concentration is initially low. There is a significant difference in the biomass concentration at 3 different initial glucose concentrations, for example, if the initial glucose concentration is 5 g/L, microalgae will consume the overall substrate within 24 h, 15 g/L within 48 h, and vice versa at 30 g/L microalgae are not able to consume completely albeit with an extended consumption time [29]. In addition, glucose consumption is very low in the lag phase in the mixotrophic cultivation and will increase in the exponential phase. It is intriguing to observe that the rate of glucose consumption decreases as the maximum cell concentration is almost reached, which may indicate spoilage. This is also in line with this experiment where at 100% and 80% glucose, the optical density of each alga was still low as shown in Fig. 3. Meanwhile, an excessive glycerol was inhibitive to the cell growth while the microalgae can solely bear less than 40% of glycerol concentration. Therefore, by combining carbon sources from glucose and glycerol, the nutrients provided can be consumed as a whole by microalgae and one of ways out to reduce production costs.
3.4. Effect of Glucose:Glycerol as Carbon Sources for Botryococcus Braunii and Chlorella Vulgaris on COD Decrease in VCO Mill EffluentLight and organic carbons contained in the waste are used during algae growth, which is called as mixotrophic cultivation [33]. The process of assimilation of organic carbon in the waste medium by microalgae occurs during the process of cellular respiration. Organic compounds are used as electron donors while oxygen is consumed as final electron acceptors. This respiration has two main functions, namely providing exclusive energy for maintenance and biosynthesis in dark conditions. In this mixotrophic growth, cells utilised organic carbon as energy sources (CO2) which were provided by glucose, glycerol, and organic carbon from VCO mill effluent alternately, therefore, chemical oxygen demand in the wastewater can be decreased [34]. The COD content in VCO mill effluent was measured after two weeks cultivated in a batch reactor as shown in Table 2 representing COD concentration and COD removal efficiency in each glucose:glycerol concentration.
Based on the result, the highest COD removal efficiency was found in the ratio of 80:20% for Botryococcus braunii and Chlorella vulgaris with the final COD concentration was 120 mg/L and 304 mg/L or equal to 97.64% and 94.03% consecutively. This is because in these conditions, the composition between microalgae and nutrient content are ideal. According to [35], the addition of nutrients is an ideal condition because the nutrient needed by microalgae is sufficient, therefore, the microalgae growth can be boosted up. Istirokhatun T et al. [36] reported that the higher the number of microalgae cells, the lower the COD concentration obtained. Other than that, aeration is also one of the factors that accelerates the reduction of wastewater pollutants. Prior study, Fadla B et al. [37] stated that aeration provides the same function as stirring. With the aeration, the supply of O2 sunlight can be evenly distributed. Oxygen is needed to increase in decomposition process of organic matter in the waste.
3.5. Comparison of Previous Study
Table 3 shows several previous researches which were conducted using various wastewaters in different microalge species. It can be clearly seen that microalgae can efficiently remove COD contents and absorb nutrients taking a role as a phycoremediator. However, the highest COD reduction was obtained at 20% VCO (virgin coconut oil) mill effluent with the addition of 80:20 (glucose:glycerol) and resulted 97.64% COD removal efficiency using Botryococcus braunii.
4. ConclusionsResearch was undertaken by cultivating Botryococcus braunii and Chlorella vulgaris in different virgin coconut oil (VCO) mill effluent and carbon synthetic concentrations. VCO mill effluent was potential to be utilised as medium for Botryococcus braunii growth producing 5.30 g/L biomass and 89.40% COD reduction efficiency. Meanwhile, the addition of 80:20 (glucose:glycerol) increased the result which accounts for 5.60 g/L biomass production and 97.64% COD removal efficiency. However, the addition of glucose:glycerol gave an inhibitory impact as the biomass production was merely 1.66 g/L compared to only in 20% VCO mill effluent, 5.34 g/L with COD efficiency 94.03%. Further study is recommended to conduct research in terms of finding other strains that can survive in VCO mill effluent with mixotrophic condition with more carbon source options.
Nomenclatureμ Growth rate (day−1) a Volume of KMnO4 to standardize waste (mL) CODo Initial chemical oxygen demand concentration (mg/L) COD1 Chemical oxygen demand concentration at time (mg/L) Nk Normality of KMnO4 (N) No Normality of H2C2O4 (N) OD0 Optical density/absorbance at initial point (−) OD1 Optical density/absorbance at time t (−) t0 Initial cultivation time (D) t1 Cultivation time (D) b, Vk Volume of KMnO4 to standardize the mixture of H2C2O4, H2SO4 and phenolphthalein (mL) Vo Volume of H2C2O4 (mL) V Volume (mL) AcknowledgmentsThe authors would like to thank to Ministry of Education, Culture, Research and Technology of Indonesia for providing financial assistance for this research.
NotesAuthor Contributions A.A.S (researcher/B.Eng.) and A.C.A (researcher/B.Eng.) carried out the experiments and curated the data. R.N.D (researcher/M.Eng) conducted formal analysis and wrote the manuscript draft. M.M.AN (researcher/Ph.D) and M (researcher/Ph.D) supervised the study, reviewed and edited the manuscript. References1. Indonesian Ministry of Agriculture. Rencana Startegis Kementerian Pertanian 2020–2024. 2020. Jakarta: p. 133–151.
2. Burton J. The World Leaders in Coconut Production. [cited 2 December 2021]. Available from: https://www.worldatlas.com/articles/the-world-leaders-in-coconut-production.html
3. Nour AH, Mohammed FS, Yunus R, et al. Demulsification of virgin coconut oil by centrifugation method: a feasibility study. Int J Chem Technol. 2009;1:59–64.
https://doi.org/10.3923/ijct.2009.59.64
4. Hariyani S. Pengaruh waktu pengadukan terhadap kualitas virgin coconut oil (VCO) [dissertation]. Fakultas Matematika dan Ilmu Pengetahuan Alam. Semarang: Universitas Negeri Semarang; 2006.
5. Nur MMA, Irawan MA, Hadiyanto . Utilization of coconut milk skim effluent (CMSE) as medium growth for Spirulina platensis
. Procedia Environ Sci. 2015;23:72–77.
https://doi.org/10.1016/j.proenv.2015.01.011
6. Tripetchkul S, Kusuwanwichid S, Koonsrisuk S, et al. Utilization of wastewater originated from naturally fermented virgin coconut oil manufacturing process for bioextract production: Physico-chemical and microbial evolution. Bioresour Technol. 2010;101:6345–6353.
https://doi:10.1016/j.biortech.2010.03.056
7. Hashem MA, Payel S, Hasan M, et al. Green Preservation of Goatskin to Deplete Chloride from Tannery Wastewater. HighTech Innov J. 2021;2(2)99–107.
http://dx.doi.org/10.28991/HIJ-2021-02-02-03
8. Mirra R, Ribarov C, Valchev D, et al. Towards Energy Efficient Onsite Wastewater Treatment. Civ Eng J. 2020;6(7)1218–1226.
http://dx.doi.org/10.28991/cej-2020-03091542
9. Ren H, Tuo J, Addy MM, et al. Cultivation of Chlorella vulgaris in a pilot-scale photobioreactor using real centrate wastewater with waste glycerol for improving microalgae biomass production and wastewater nutrients removal. Bioresour Technol. 2017;245(Pt A)1130–1138.
https://doi.org/10.1016/j.biortech.2017.09.040
10. Pradana YS, Dewi RN, Livia KD, et al. Advancing biodiesel production from microalgae Spirulina sp. by a simultaneous extraction–transesterification process using palm oil as a co-solvent of methanol. Open Chem. 2020;18:833–842.
https://doi.org/10.1515/chem-2020-0133
11. Setiaji B, Setyopratiwi A, Cahyandaru N. Exploiting a benefit of coconut milk skim in coconut oil process as nata de coco substrate. Indones J Chem. 2002;2(3)167–172.
https://doi.org/10.22146/ijc.21912
12. Patel AK, Joun J, Sim SJ. A sustainable mixotrophic microalgae cultivation from dairy wastes for carbon credit, bioremediation and lucrative biofuels. Bioresour Technol. 2020;313:123681.
https://doi.org/10.1016/j.biortech.2020.123681
13. Ma X, Zhou W, Fu Z, et al. Effect of wastewater-borne bacteria on algal growth and nutrients removal in wastewater-based algae cultivation system. Bioresour Technol. 2014;167:8–13.
https://doi.org/10.1016/j.biortech.2014.05.087
14. Mohan SV, Rohit MV, Chiranjeevi P, et al. Heterotrophic microalgae cultivation to synergize biodiesel production with waste remediation: progress and perspectives. Bioresour Technol. 2015;184:169–178.
https://doi.org/10.1016/j.biortech.2014.10.056
15. Min M, Hu B, Zhou W, et al. Mutual influence of light and CO2 on carbon sequestration via cultivating mixotrophic alga Auxenochlorella protothecoides UMN280 in an organic carbon-rich wastewater. J Appl Phycol. 2012;241099–241105.
https://doi.org/10.1007/s10811-011-9739-3
16. Panagopoulos A. Techno-economic assessment of Minimal Liquid Discharge (MLD) treatment systems for saline wastewater (brine) management and treatment. Process Saf Environ Prot. 2021;146:656–669.
https://doi.org/10.1016/j.psep.2020.12.007
17. Panagopoulos A. Study and evaluation of the characteristics of saline wastewater (brine) produced by desalination and industrial plants. Environ Sci Pollut Res. 2021;1–14.
https://doi.org/10.1007/s11356-021-17694-x
18. Panagopoulos A. Techno-economic assessment of zero liquid discharge (ZLD) systems for sustainable treatment, minimization and valorization of seawater brine. J Environ Manage. 2022. 306:114488.
https://doi.org/10.1016/j.jenvman.2022.114488
19. Choi HJ, Lee SY. Advances in microalgal biomass/bioenergy production with agricultural by-products: Analysis with various growth rate models. Environ Eng Res. 2019;24(2)271–278.
https://doi.org/10.4491/eer.2018.193
20. Gramegna G, Scortica A, Scafati V, et al. Exploring the potential of microalgae in the recycling of dairy wastes. Bioresour Technol. 2020;12:100604.
https://doi.org/10.1016/j.biteb.2020.100604
21. Muria SR, Chairul , Naomi DC. Pemanfaatan mikroalga Chlorella sp. untuk pengolahan palm oil mill effluent [POME] secara fed batch. Jurnal Sains dan Teknologi (JST). 2020;19(1)7–12.
https://doi.org/10.31258/jst.v19.n1.p7-12
22. Morais KCC, Ribeiro RLL, Santos KR, et al.
Phaeodactylum tricornutum microalgae growth rate in heterotrophic and mixotrophic conditions. Therm Eng. 2009;8:84–89.
https://doi.org/10.5380/reterm.v8i1.61887
23. Rahim Y. Analisis Kandungan Aluminium (Al), Sulfida, BOD, COD, Total Padatan Tersuspensi (TSS) dan pH dari Air Sungai Kapal Keruk di Desa Karang Anyer Kec. Secanggang Kab. Langkat [dissertation]. Fakultas Matematika dan Ilmu Pengetahuan Alam. Medan: Universitas Sumatera Utara; 2010.
24. Sim SJ, An JY, Kim BW. Two-phase extraction culture of Botryococcus braunii producing long-chain unsaturated hydrocarbons. Biotechnol Lett. 2001;23:201–205.
https://doi.org/10.1023/A:1005667522375
25. Rahmat TA, Dias RD, Soetrisnanto D. Kultivasi Botryococcus braunii memanfaatkan air dadih (whey) tahu sebagai potensi biodiesel. J Kim Teknol. 2013;2(4)72–83.
http://ejournal-s1.undip.ac.id/index.php/jtki
26. Anita AF, Juwita R. Pemanfaatan air limbah pabrik pupuk kadar amonia tinggi sebagai media kultur mikroalga untuk perolehan sumber minyak nabati sebagai bahan bakar biodiesel. 2011;
27. Suminto , Hirayama K. Effects of bacterial coexistence on the growth of a marine diatom Chaetoceros gracilis
. Asian Fish Sci. 1996;62(1)40–43.
https://doi.org/10.2331/fishsci.62.40
28. Yang C, Hua Q, Shimizu K. Energetics and carbon metabolism during growth of microalgal cells under photoautotrophic, mixotrophic and cyclic light-autotrophic/dark-heterotrophic conditions. Biochem Eng J. 2000;6(2)87–102.
https://doi.org/10.1016/S1369-703X(00)00080-2
29. Arroyo TH, Wei W, Ruan R, et al. Mixotrophic cultivation of Chlorella vulgaris and its potential application for the oil accumulation from non-sugar materials. Biomass Bioenerg. 2011;35(5)2245–2253.
https://doi.org/10.1016/j.biombioe.2011.02.036
30. Choi H, Yu S. Influence of crude glycerol on the biomass and lipid content of microalgae. Biotechnol Biotechnol Equip. 2015;29(3)506–513.
https://doi.org/10.1080/13102818.2015.1013988
31. Liang Y, Sarkany N, Cui Y. Biomass and lipid productivities of Chlorella vulgaris under autotrophic, heterotrophic and mixotrophic growth conditions. Biotechnol Lett. 2009;31:1043–1049.
https://doi.org/10.1007/s10529-009-9975-7
32. Buaisha M, Balku S, Yaman SO. Heavy Metal Removal Investigation in Conventional Activated Sludge Systems. Civ Eng J. 2020. 63
http://dx.doi.org/10.28991/cej-2020-03091484
33. Singh SK, Bansal A, Jha MK, et al. An integrated approach to remove Cr(VI) using immobilized Chlorella minutissima grown in nutrient rich sewage wastewater. Bioresour Technol. 2012;104:257–65.
https://doi.org/10.1016/j.biortech.2011.11.044
34. Garcia OP, Escalante FME, de-Bashan LE, et al. Heterotrophic cultures of microalgae: Metabolism and potential products. Water Res. 2011;45(1)11–36.
https://doi.org/10.1016/j.watres.2010.08.037
35. Mahdi MZ, Titisari YN, Hadiyanto H. Evaluasi pertumbuhan mikroalga dalam medium POME: variasi jenis mikroalga, medium dan waktu penambahan nutrient. Jurnal Teknologi Kimia dan Industri. 2012;1(1)284–291.
http://ejournal-s1.undip.ac.id/index.php/jtki
36. Istirokhatun T, Aulia M, Sudarno . Potensi Chlorella sp. untuk menyisihkan COD dan nitrat dalam limbah cair tahu. Jurnal Presipitasi: Media Komunikasi dan Pengembangan Teknik Lingkungan. 2017;14(2)88–96.
https://doi.org/10.14710/presipitasi.v14i2.88-96
37. Fadla B, Syarif FR, Yelmira Z. Pemanfaatan simbiosis mikroalga Chlorella sp dan Agrobost untuk menurunkan kadar polutan limbah cair sagu. J Agric Sci Technol. 2019;18(1)
https://doi.org/10.31258/sagu.v18i1.7863
38. Arinto DJ, Paramastri HP, Soetrisnanto D. Potensi air dadih (whey) tahu sebagai nutrien dalam kultivasi Chlorella Sp. untuk bahan baku pembuatan biodisel. Jurnal Teknologi Kimia dan Industri. 2013;2(4)233–242.
http://ejournal-s1.undip.ac.id/index.php/jtki
Fig. 1Biomass production of (a) Botryococcus braunii and (b) Chlorella vulgaris at various virgin coconut oil mill effluent concentration (VCOME). 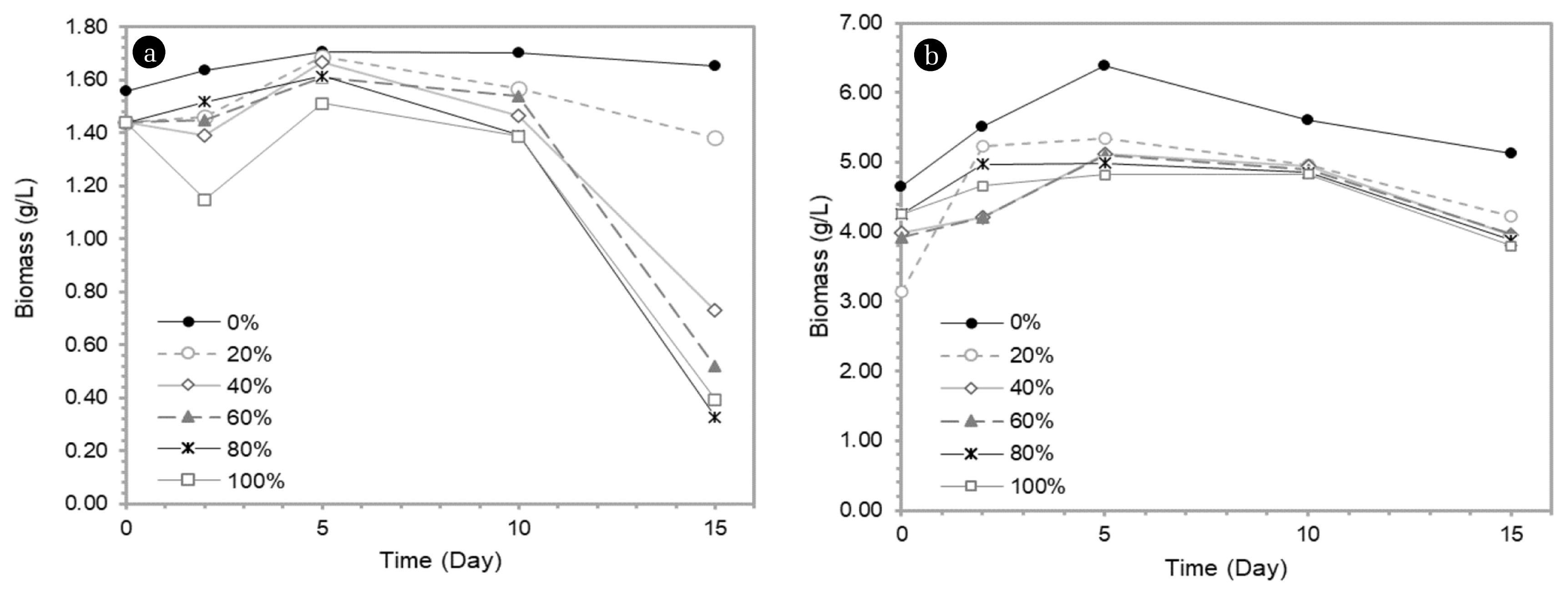
Fig. 2Biomass production of (a) Botryococcus braunii and (b) Chlorella vulgaris at various carbon sources concentrations. 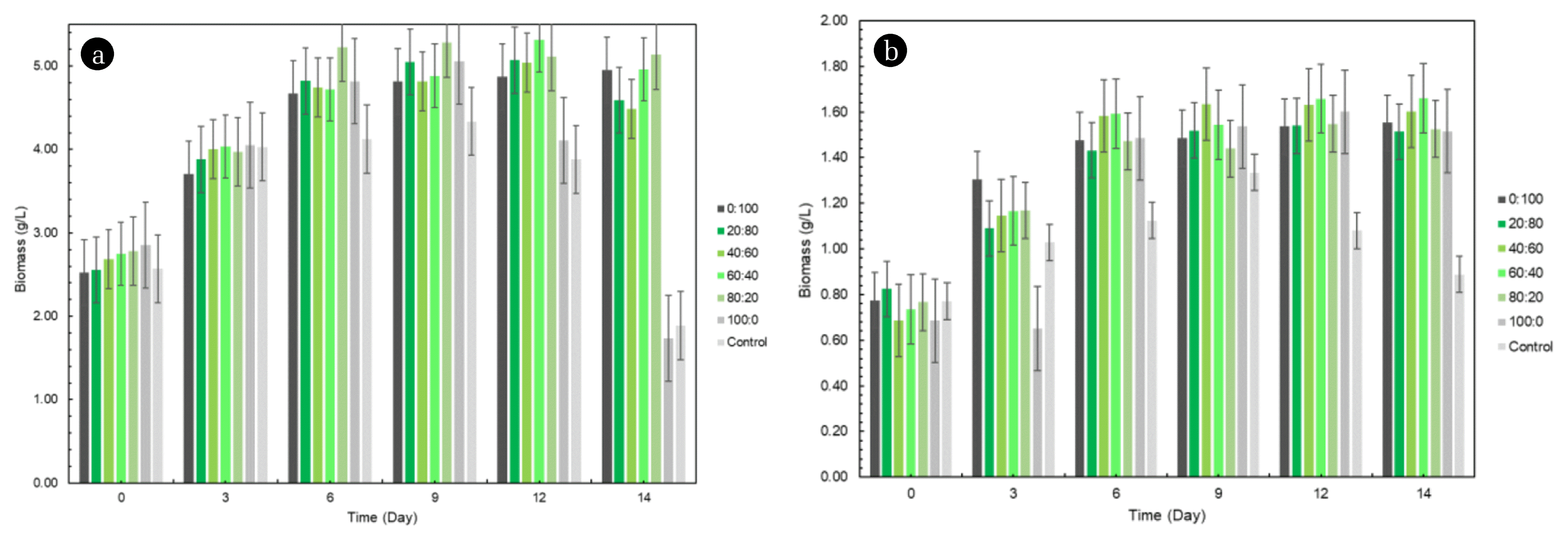
Fig. 3Biomass production and optical density comparison of (a) Botryococcus braunii and (b) Chlorella vulgaris at various carbon sources concentrations 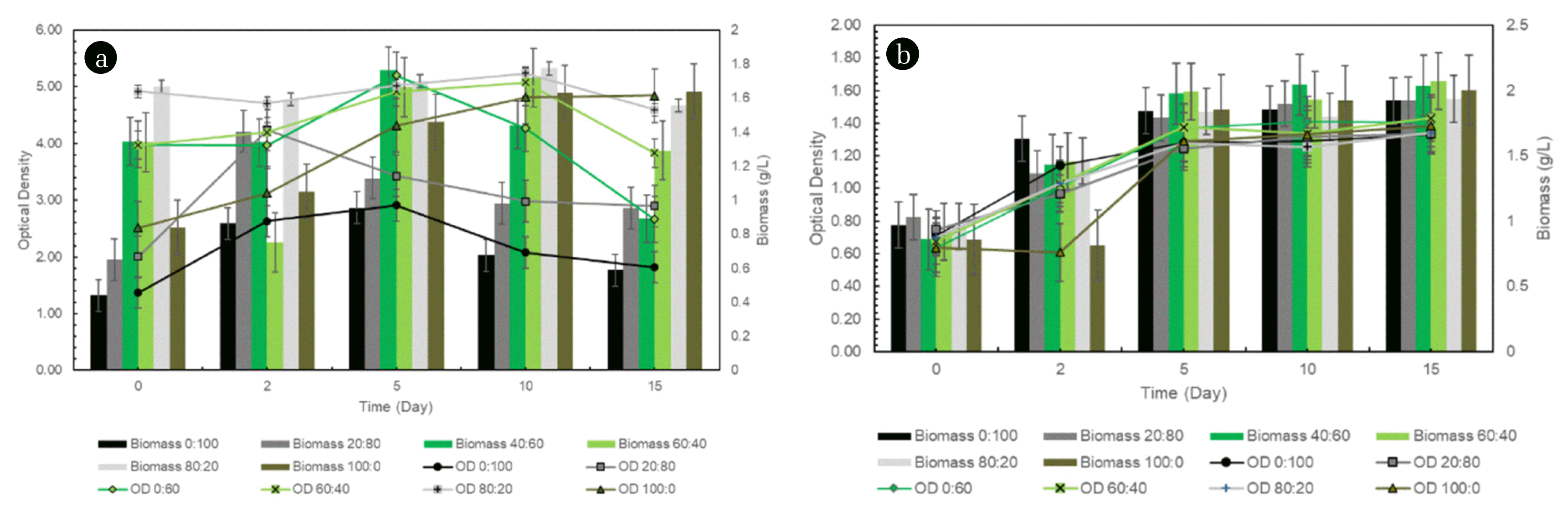
Table 1COD Removal Efficiency of Botryococcus Braunii and Chlorella Vulgaris at Various Virgin Coconut Oil Mill Effluent Concentration (VCOME) Table 2COD Result at Various Glucose:Glycerol Concentrations Table 3Comparison of COD Decrease from Various Microalgae in the Wastewater
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||