AbstractDeveloped countries are increasing their demand for natural gas as it is an industrial requirement for fuel transportation. Most of modern society relies heavily on vehicles. However, the presence of CO2 gas has led to the categorization of sour natural gas which reduces the quality and price of natural gas. Therefore, the catalytic methanation technique was applied to convert carbon dioxide (CO2) to methane (CH4) gas and reduce the emissions of CO2 within the environment. In this study, samarium oxide supported on alumina doped with ruthenium and manganese was synthesized via wet impregnation. X-ray diffraction (XRD) analysis revealed samarium oxide, Sm2O3 and manganese oxide, MnO2 as an active species. The reduction temperature for active species was at a low reaction temperature, 268.2°C with medium basicity site as in Temperature Programme Reduction (TPR) and Temperature Programme Desorption (TPD) analyses. Field Emission Scanning Electron Microscopy (FESEM) analysis showed an agglomeration of particle size. The characterised potential catalyst of Ru/Mn/Sm (5:35:60)/Al2O3 (RMS 5:35:60) calcined at 1,000°C revealed 100% conversion of CO2 with 68.87% CH4 formation at the reaction temperature of 400°C. These results were verified by artificial neural network (ANN) with validation R2 of 0.99 indicating all modelling data are acceptable.
1. IntroductionEnergy demand for natural gas has increased from 2005 until present for household usage, transportation, electrical and industry. This is because natural gas is the cleanest option among fossil fuels and emits less CO2 into the atmosphere [1]. As part of the Kyoto Protocol, Malaysia’s natural gas must be treated in order to minimize the emission of CO2 gas into the air. The most promising technique to eradicate CO2 from natural gas is through catalytic methanation, which converts it into CH4 in the existence of H2 gas. The basic oxides for instance are oxides of rare earth metals which form a good catalyst for the reductive process in the natural gas conversion reaction [2]. The use of samarium as a promoter or support within the catalytic reaction has been widely investigated due to these interesting properties, i.e. reducibility and basicity. As investigated by Yamasaki et al. [3], the number of active surface nickel sites has increased as samarium was used as a promoter in the Ni-Zr catalyst, thus enhancing the performance of catalytic activity. However, no studies have been conducted on the possible optimization improvement of CO2 methanation over supported samarium oxide based as a heterogeneous basic catalyst.
During the experiment, the optimization process will be needed in order to identify the optimum parameter that gives higher CO2 conversion. Thus, modelling of variables in methanation reaction is needed to save time and cost and also give higher predictions of CO2 conversion. In recent years, artificial neural network (ANN) based modelling has grabbed many attentions in research area especially in an industry for developing empirical models for time series prediction. ANN is a process of information’s element, constituted by layers of neurons and an output as a result [4, 5]. Due to its high proficiency and unique features, the ANN method has given better predictions and its results depend on the number of variables entered [6].
ANN has been widely used in the industrial hydrogen production plants. A back-propagation feed forward ANN with three layers was used in predicting the optimum parameters of feed temperature, reformer pressure, steam to carbon ratio and carbon dioxide to methane ratio in the feed stream. From the results, the artificial neural network can be easily applied to analyze the performance of the entire hydrogen plant to achieve suitable operating conditions, with less time-consuming and high accuracy [7]. Recently, hydrogen production by catalytic dry reforming of methane through the use of artificial neural networks (ANN) has been reported by Alsaffar et al. [8]. They focused on the optimization of gas hourly space velocity (GHSV), the oxygen (O2) concentration in the feed, the reaction temperature and the CH4/CO2 ratio. The predicted hydrogen yield and the CH4 conversions by the optimized ANN model were in close agreement with the observed values obtained from the experimental runs.
In the area of CO2 methanation, Ngo and Lim [9] developed the physics-informed neural networks (PINNs) to solve the catalytic CO2 methanation in isothermal fixed bed (IFB) model. When only predicting the concentration outside the training domain, PINN showed excellent extrapolation performance with an accuracy of 88.1%. These results indicate that PINN can be used for solution and system identification in a fixed bed model with chemical reaction kinetics. Meanwhile, the study by Esfandyari et al. [10] also found ANN to be a powerful and accurate tool for building up empirical models for prediction in Fischer-Tropsch. Four variables such as operating temperature, operating pressure, time and CO/H2 ratio have been studied to predict the CH4, CO2 and CO compositions. Therefore, the uniqueness of current study is application of supported samarium oxide-based catalyst for CO2 methanation and its process optimization using an ANN to achieve exact optimal parameters and the prediction of experimental performance. Additionally, the physicochemical characteristic of Ru/Mn/Sm/Al2O3 (RMS) catalyst was also examined using several characterization techniques.
2. Experimental2.1. Material and ReagentsIn this research, all metals salts used for catalyst preparation were of high purity and were purchased from Sigma Aldrich. A samarium (III) nitrate hexahydrate (Sm(NO3)3.6H2O) was used as based catalysts while, manganese nitrate tetrahydrate (MnNO3.4H2O) and ruthenium(III) chloride (RuCl3) were used as dopant and co-dopant. Then, aluminium oxide bead, γ-Al2O3 with 3 mm was used as a support material of catalyst.
2.2. Preparation and Catalytic Activity of Ru/Mn/Sm/Al2O3 (RMS) CatalystThe RMS catalyst was prepared by the aqueous incipient wetness impregnation method [11, 12]. A 5 g of Sm(NO3)3.6H2O was weighed in a beaker and dissolved with 10 mL of distilled water. The mixed catalyst solution was prepared by adding the appropriate amount of dopant MnNO3 (0.141 g) and co-dopant RuCl3 (0.289 g) into the based solution to get the desired ratio of RMS (5:35:60). The catalyst was then labelled as RMS (5:35:60) to indicate that there is 60 wt% of Sm, 35 wt% of Mn and 5 wt% of Ru. Then, the mixture catalyst was stirred continuously for 30 min before being immersed with 7 g of 3 mm alumina beads for 1 h. The alumina beads were left in the desiccator overnight, and then aged at 80–90°C overnight in an oven before calcination in the furnace occurred at 400°C for 5 h using a 10°C/min ramp rate. The same procedure was repeated for other Sm loadings of 50–85 wt% and calcination temperatures of 700–1,100°C.
Meanwhile, the catalytic activity testing was performed under atmospheric pressure in a fixed micro-reactor and analysed online by use of a Fourier Transform Infrared from ambient temperatures of up to 400°C of reaction temperature at a ramp rate of 5°C/min. Glass tube made of Pyrex glass with a diameter of 10 mm and a length of 520 mm was used as the catalyst holder and placed in an isothermal furnace. The gaseous mixture of CO2 and H2 with a ratio of 1:4 was launched into the reactor system. The formation of CH4 was measured via a Gas Chromatography Flame Ionisation Detector system equipped with an Ultra 1 capillary column (nominal column 25.0 m × 200 μm × 0.11 μm).
2.3. CharacterisationX-ray Diffraction (XRD) analysis was performed using a Crystalloflex D5000 Diffractometer with CuKα radiation (λ = 1.54060A). The sample was placed into the sample holder with diameter of 10–15 mm and approximate depth of 1 mm in a 40 × 33 × 2 mm glass plate. X-ray Photoelectron Spectroscopy (XPS) analysis was performed via Kratos AXIS Ultra DLD using energy of 15.0 kV. The samples were tested at the electron take-off angle which is normal to the surface working at 20 eV. Electron Spin Resonance (ESR) analysis was conducted using the ThermoFlex3500 model and was operated with a center field of 336 mT, a modulation amplitude of 1.00 × 100, a scan time equal to 4 min with 3 number of scans, and a microwave power of 0.998 mW. Meanwhile, H2-Temperature Programmed Reduction (H2-TPR) and CO2-Temperature Programmed Desorption (CO2-TPD) analyses were accomplished by using Micromeritics Autochem 2920. The measurement was carried out by ramping the catalyst sample from room temperature to 1,000°C with a total gas flow of 20 mL/min at 10°C/min. For the Field Emission Scanning Electron Microscopy-Energy Dispersive X-Ray (FESEM-EDX) study, a 15 kV Zeiss Supra 35 VP FESEM coupled with EDX analyzer was used to scan the sample under 25 kV. Lastly, surface area analysis was measured by Nitrogen sorption analysis using a Micromeritics ASAP 2010 at −196°C. Prior to the measurement, the calcined catalysts were degassed at 120°C overnight.
2.4. Statistical Analysis2.4.1. Artificial Neural Network (ANN)ANN’s most common technique, known as feed forward MLP artificial neural network (MLP-FF-ANN), is one of the techniques that relies on memory less and thus the reflection in response to the result is much faster than the feedback type [13]. In this study, MLP-FF-ANN generated by JMP version 10 (SAS, USA) was used for simulation because of its flexibility and ease of use when compared to the Statsoft Statistica Release 7.0 software [14]. This network type consists of a system of multi-level interconnected “neurons” or nodes that are formed in three layers, namely input layers (independent variables), hidden layers (one or more), and output layers (dependent variables) [15, 16]. The input variables were calcination temperature (°C), based ratio (%) and catalyst dosage (g), while the output was CO2 conversion (%). The parameters were chosen based on our previous study [11, 17], as they could give great influence on the performance and physicochemical properties of the prepared catalyst. The model was trained by using the Levenberg Marquardt Learning Algorithm [14, 18]. The respected R2 and RMSE were computed using Eq. (1) and (2), respectively:
The predicted value is signified by where ti the measured value of the experimental samples is, tdi signifies the predicted value, and N represents the total of samples. The range calculation for R2 is in between 0.0–1.0. The lowermost value of R2 is considered weak, whilst the highest value (near to 1.0) indicates suitability to be chosen as the best predictor [18]. Next, the efficiency model (EM) by Nash and Sutcliffe [19] was used to test the fit between measured and modelled data using the Eq. (3):
Where, E refers to coefficient of efficiency, Om represents the measured value, Qp indicates the predicted value and Qmea_avg denotes an arithmetic average measured value. If modelled values perfectly match the measured ones, then E is equal to 1.
3. Result and Discussion3.1. Characterization3.1.1. XRD analysisXRD analysis was performed to observe the presence of active oxide phases on the prepared RMS (5:35:60) catalyst in different calcination temperatures of 400, 700, 900, 1,000 and 1,100°C. Calcination temperature would influence both the crystal phase and crystallite size of the catalyst. Some processes including change in structure, arrangement of crystal lattice and active phase formation could also occur during sintering [11, 20]. The XRD diffractograms of RMS (5:35:60) catalysts calcined at different temperatures as shown in Fig. 1.
As shown in Fig. 1, the phases for RMS (5:35:60) catalysts were highly amorphous and most of them were dominated by alumina support, Al2O3 at 2θ values of 66.86°, 45.79°, 37.73°, and 39.42°. The amorphous phases may be due to the presence of the lanthanide element, samarium in the catalyst and may have hindered the crystallinity of the catalyst phase. RMS (5:35:60) catalyst heated at 400°C and 700°C shows similar diffractograms with the presence of alumina cubic peaks only. Meanwhile, RMS (5:35:60) catalysts heated at 900 to 1,100°C showed the existence of tetragonal RuO2 at 2θ values of 28.08°, 35.09°, and 54.53° besides cubic Sm2O3 peaks at 27.88°, 32.31°, and 45.36° in addition to the cubic of Al2O3. Interestingly, the presence of new small peaks of MnO2 with tetragonal phase at 2θ values of 36.99°, 56.40°, and 42.82° were observed for the catalyst calcined at 1,000 and 1,100°C. However, the appearance of low MnO2 intensity peak in the diffractogram was in accordance with research conducted by Xu et al. [21] who also observed a broad peak of MnO2 which indicated the amorphous nature of the product. Therefore, 900°C of calcination temperature is not sufficient to increase the crystallinity and size of the MnO2 species thus, it was not observable in the diffractogram. Besides that, a few peaks on the diffractogram that overlapped each other were also observed. These overlapping peaks occurred due to very similar crystallographic characters from the PDF XRD profile.
3.1.2. X-ray Photoelectron Spectroscopy (XPS) analysisTo determine the oxidation state of elements in the catalyst, the relative contents of each state over the catalyst surface were deconvulated using XPS software based on the binding energy. The relative surface charge was adjusted against the C 1s line (284.50 eV) as a standard. Fig. 2 shows the deconvulation of XPS spectra for O 1s, Al 2p, Mn 2p and Sm 3d. Oxygen constitutes a high percentage in the surface composition, which was 25.42%. The O 1s photoemission spectrum exhibited single-lobed envelopes. The deconvulation produced two peaks, which suggests the presence of two different elements. The strongest peak had an Eb value of 531.50 eV and was assigned to Al2O3 or oxygen of the Sm3+-O groups. The presence of a weak peak at an Eb value of 529.96 eV was assigned as oxygen of Sm2+-O groups. This is related to the oxygen in the crystal lattice of Sm2O3 [22]. Aluminium constituted a high percentage in the surface composition, which was 65.04%, confirming the Al2O3 as a support. Deconvulation of Al 2p produced two peaks that indicated Al3+ in different chemical environments. The Eb values of 73.40 and 74.47 eV, were credited to the presence of Al3+ in Al2O3 and Al(OH)3, respectively. Meanwhile, Mn 2p consisted of two doublets which were Mn 2p1/2 and 2p3/2 which are equal to 11.5 eV. In this study, the high binding energy in the range of 642 – 647 eV was ascribed to the presence of Mn4+ from the MnO2 species [23] and was aligned with the observation obtained from the XRD analysis in Fig. 1.
The peak of samarium formed two doublet structures of 3d5/2 and 3d3/2 equal to 28 eV as a result of spin-orbit splitting and their satellites in rare-earth compounds as also explained by Chung et al. [24]. The first doublet corresponded to Sm 3d5/2 at a binding energy of 1,082.33 eV that was attributed to Sm3+ ion under its oxidized form in metal oxide, Sm2O3. The observation of satellite Sm2O3 shouldered at 1,085.73 eV was associated with a strong charge-transfer effect, which leads to an increase in the number of unpaired 4f electrons by more than one of the samarium oxides [25]. It was also reported that Sm was in a trivalent state when adsorbed onto transitional metals due to the close binding energy at 1,082.33 and 1,085.73 eV [26, 27]. This is supported with XRD where Sm2O3 was observed in the diffractograms.
3.1.3. Electron Spin Resonance (ESR) analysisThe ESR spectra of the RMS (5:35:60) catalyst calcined at various temperatures are shown in Fig. 3. From the spectra, all of the catalysts showed a g value of 2.12 with different intensities. The shift from the value was caused by the electron spin-orbit interaction in atomic orbital. The ‘wing’ that forms from both sides at the g value of 2.12 was attributed to Mn4+ as suggested by Chakradhar et al. [28]. This result correlates well with the result obtained from the XRD and XPS analysis in Fig. 1 and 2 whereby the manganese species that exists in this catalyst were MnO2 (Mn4+). This result is similar to Phan et al. [29] who identified that MnO2 will exhibit a symmetrical center which is caused by a paramagnetic property at a magnetic field about 300 mT. From the ESR spectra, at calcination temperatures of 1,000°C and 1,100°C, both peaks overlapped and exhibited the highest peak intensity as compared to 900°C. It can be clearly seen in XRD diffractograms that the presence of MnO2 peaks in the catalyst calcined at 1000 and above. However, the peak of MnO2 was not detected in XRD analysis calcined at 900°C which can be explained by the lower signal ESR peak due to lower intensity and crystallinity of the MnO2 species. Therefore, the paramagnetic properties of the catalysts were said to be higher at 1,000°C and 1,100°C which could contribute to the maximum catalytic activity of CO2 conversion.
3.1.4. H2-Temperature Programmed Reduction (H2-TPR) analysisA H2-TPR analysis was performed to provide information on the reducibility of various chemical species contained in the catalyst, the degree of interaction between metal-support, and to determine the reduction temperature. The H2-TPR profile of RMS (5:35:60) was depicted in Fig. 4. The H2 consumption of the profile provides a cursory measure level of coordination and availability of the surface capping oxygen ions in the catalyst [30].
At calcination temperatures of 900, 1,000 and 1,100°C, four reduction peaks were detected at 268.2, 433.5, 594.2, and 976.7°C, respectively. The first reduction peak at 268.2°C was owing to the reduction temperature of RuO2 reduction to metal Ru where the H2 consumption was 0.12 cm3/g STP [31]. It can be observed that only a tiny amount of H2 was consumed on the catalyst. As observed by Peluso et al. [32], the second reduction peak at 433.5°C of H2 consumption, 0.02 cm3/g STP belonged to the reduction of Mn4+ to Mn2+. On the other hand, the reduction peak at 594.2°C was due to the reduction of the Mn-O-Al species with a total H2 consumption, of 0.02 cm3/g STP [33]. The last reduction peak at 976.7°C was consigned as the bulk oxygen reduction from SmO2 to Sm2O3 where the amount of H2 consumption was recorded as 0.13 cm3/g STP. The reduction peaks at 1,100°C showed a shift to higher temperatures, implying stronger interactions between active species and alumina support which led to a decrease in reducibility. The outflow of H2 to the catalyst surface increased the H2 adsorption area and improved the hydrogenation activity. The H2-TPR profile demonstrated that γ-Al2O3 was also an inert carrier, so no reduction peak of γ-Al2O3 was observed [34].
3.1.5. CO2-Temperature Programmed Desorption (CO2-TPD) analysisA CO2-TPD analysis was done to study the basicity of the catalyst surface and determine the CO2 and H2 sorption profiles on its surface. Lin et al. [35] found that supported noble metal catalysts have strong metal-support interactions, which affects the performance of active species, and favors the adsorption through electron-deficient state of metal nanoparticles. The difference in desorption profile was caused by the coordination of CO2 to metal oxides with different energies. The CO2-TPD profiles were depicted in Fig. 5.
The peak at the lowest temperature of 117.6°C was due to desorption of low chemisorption or physical adsorption of CO2 on the catalyst surface [36]. Nevertheless, the peaks at 210.6°C and 279.4°C could be ascribed to the inorganic carboxylate desorbed on the surface of the catalyst as suggested by Cox [37]. In addition, the TPD profile’s peak area of CO2 desorbed from the surface is used to determine the catalyst’s total basicity. Therefore, the broad peak areas at 210.6°C and 279.4°C indicated higher basicity surface of catalysts due to many CO2 gas desorbed on the basic sites of the catalyst. Aside from that, the amount of CO2 adsorbed on the catalyst at 1,000°C was also the highest at 210.6°C and 279.4°C with 48.62 cm3/g and 34.00 cm3/g at STP respectively. As a result, the catalytic activity of the catalyst will be higher and more active at reaction temperatures above 200°C.
3.1.6. Field Emission Scanning Electron Microscopy (FESEM) analysisFESEM analysis was used to study the morphology of the RMS (5:35:60) catalyst supported on alumina. Fig. 6 displays the FESEM micrographs at 50000X magnification of the RMS (5:35:60) catalyst calcined at 400, 700, 900, 1,000 and 1,100°C for 5 h. The micrograph for different calcination temperatures showed no significant changes of morphology. As can be observed, the particles became more agglomerated with increasing calcination temperatures. The micrograph also showed that when the calcination temperature was raised to 1,000°C, the catalyst had more pores and this structure could help in the CO2 methanation activity. From the morphology of the catalyst, it revealed more agglomeration occurred by providing higher surface areas for the active species to absorb the gasses which contributed to higher catalytic activity. At a calcination temperature of 400°C, the catalyst surface was tightly packed, which results in a small surface area, which limits the active sites of the methanation reaction and thus reduces the catalytic activity performance. At a calcination temperature of 1,000°C, micrographs showed that small spherical particles were agglomerated on the catalyst surface which can provide the highest catalytic activity.
3.1.7. Energy Dispersive X-ray (EDX) analysisThe composition of the element that was present on the catalyst surface was confirmed by EDX analysis. The EDX analysis revealed a low atomic ratio of Sm, Mn, and Ru with below than 1.00%. This is most likely due to the absorption of alkaline metal, Sm, Ru and Mn in highly porous and high surface area alumina beads. This is in agreement with the FESEM analysis which showed many pores on the surface. However, when the calcination temperature was raised to 1,000°C, the atomic ratio of each element increased which are Sm (0.72%), Mn (0.79%), except for Ru (0.37%). This is supported by the XRD where the peak of the RuO2 species was very small in intensity. Previous research conducted by Zielinska et al., reported that the content of elements increased with the increasing calcination temperature [38]. However, when the catalyst was calcined at 1100°C, the element composition of Sm and Mn were slightly decreased with 0.49% and 0.64%, respectively except for the Ru element which showed an increase with 0.54%. This observation was corroborated by XRD analysis and again showed the appearance of the Mn and Ru in the catalyst samples.
3.1.8. Nitrogen Adsorption (NA) analysisThe pore structure and surface area analyses were measured by nitrogen adsorption analysis with the Brunauer–Emmett–Teller (BET) method. It was found that the BET surface area of the RMS (5:35:60) catalysts decreased with each increment of calcination temperature. The reduction of surface area among the catalysts might be due to agglomeration of metal oxide particles as shown by the FESEM analysis. At a 400°C calcination temperature, the BET surface area was higher by 155.03 m2/g with a 89.66 nm average pore diameter, indicating a smaller particle size. This was verified by a H2-TPR analysis (Fig. 3) in which the H2 consumption showed a reduction at lower reduction temperature due to an increase in the particle dispersion [39]. When the calcination temperature was increased until 1000°C, the BET surface area showed only 41.36 m2/g with an average pore diameter of 207.20 nm. Increasing the calcination temperature increases the pore size. This is due to the fact that large pores can lead to sintering, resulting in a decrease in pore volume and the formation of dense solid. Although, higher calcination temperatures reduce BET surface area, but have the advantage of ensuring the presence of a stable structure on the catalyst.
3.2. Catalytic Activity3.2.1. Effect of calcination temperatureThe screening of monometallic and bimetallic catalysts has been discussed in our previous study [40]. The lower CO2 conversion for monometallic and bimetallic oxides has steered to the trimetallic oxide catalyst. However, the higher reaction temperature was not compatible with the requirement of industry to achieve a low reaction temperature. Thus, the effect of calcination temperatures on the potential catalyst of RMS (5:35:60) with respect to the catalytic performance using FTIR analysis was investigated. At 400, 700, 800 and 900°C, the CO2 conversion were 94, 96, 89 and 91%, respectively. Meanwhile, at 1000°C calcination temperature, the CO2 conversion showed a 100% CO2 conversion, followed by 1,100°C, with a 98% which was slightly decreased at a maximum 400°C of reaction temperature. From the diffractograms in Fig. 1, the noticeable peaks of MnO2, RuO2 and Sm2O3 showed that these species contributed in determining higher catalytic activity (100%) of RMS (5:35:60) calcined at 1,000°C.
3.2.2. Effect of metal oxide based loadingsThe next parameter step was to optimise the RMS (5:35:60) catalyst for various samarium loadings to observe its effect on the performance of catalyst. The ratios of 50–85 wt% of samarium oxide catalysts were selected. Low performance (< 30%) was observed for all the prepared catalysts (50–85 wt%) at 200°C reaction temperature except for the 60 wt% based loading which able to convert 65% of CO2 at the same reaction temperature. It was clearly showed that an appropriate amount of samarium could increase the activity of the catalyst. As RMS (5:35:60) catalyst gives highest CO2 conversion at 200 and 400°C reaction temperature (100%), it can be concluded that 60 wt% is the optimum loading for the samarium content. When the Sm content was increased beyond the optimum loading (60 wt%), the CO2 conversion decreased. The excessive loading of Sm may block the active species on the catalyst surface as reported by He et al. [41]. Therefore, the trend for based loading is as follows: 50 < 55 < 85 < 80 < 75 < 70 < 65 < 60 wt%.
3.2.3. Effect of catalyst dosageThe parameters for various catalyst dosages were also investigated to find an optimum value for CO2 conversion. The CO2 conversion began to increase drastically at a reaction temperature of 200°C for each dosage. At a reaction temperature of 400°C with 3 g of catalyst or more, a complete CO2 conversion (100%) was observed. Almost complete conversion of 98.53% occurred for 5 g of catalysts. This observation is mainly due to the increase in the number of active sites on the catalyst surface which are affected by the increase in the catalyst dosage. This finding was consistent with Su et al. [42], who found that when the catalyst dosage is increased, it would also increase the catalytic activity. Therefore, the catalyst dosage trend for CO2 conversion was 3 < 5 < 7 g.
3.3. Methane Detection by Gas ChromatographyThe potential catalysts selected from the screening process were further studied for the production of methane by GC analysis. No methane was produced at the initial temperature of 100°C for RMS (5:35:60) catalyst calcined at 1,000°C. However, when the reaction temperature was raised to 300, 350, and 400°C, about 31.11, 48.91 and 68.87% of methane production was detected. This indicates that the CH4 content increases with increasing reaction temperatures. This might due to exothermic reactions which are required to form CH4 at high reaction temperatures. Other than CH4, the by-product of CH3OH and H2O were also formed from the reaction. Due to the high methane formation of RMS (5:35:60) calcined at 1,000°C, compared to the others, it was assigned as a potential catalyst among other studied samarium catalysts.
3.4. Reusability TestReusability test for the catalytic activity of RMS (5:35:60) catalyst was conducted by using the same catalyst several times until it deactivated through decreasing in catalytic activity of CO2 conversion. The trend of reusability test for RMS (5:35:60) catalyst showed that after 5 times of reused catalyst, the CO2 conversion started to decrease around 60%. All tested catalysts from 2 to 5 times showed a similar pattern with substantially increasing CO2 conversion at 250°C till the highest examined reaction temperature, as shown in the Fig. S1. According to a study conducted by Argyle and Bartholomew, carbon deposition could occur when the catalyst was run repeatedly numerous times [43]. This is because carbon can chemisorb aggressively as a monolayer or physically adsorb in a multilayer, thus blocking the reactant from reaching metal surface sites.
3.5. Statistical Analysis of Ru/Mn/Sm/Al2O33.5.1. Artificial neural networkIn this study, a neural network technique was applied to develop the best CO2 conversion prediction model using three hidden nodes. The ANN model was trained using the Levenberg Marquardt Learning Algorithm. The factors were calcination temperature, °C (900–1,100°C), based ratio, % (60–80%), and catalyst dosage, g (3–7 g). The parameters were selected based on preliminary results. Table 1 shows the experimental and predicted values for CO2 conversion.
In general, the percentages of CO2 conversion obtained were high. The efficiency test of modelling was also was performed to validate the quality of the model between predicted and measured values. Based on the findings, almost all prediction values for CO2 conversion were near to 1. According to Nash and Sutcliffe [19] and Grunwald and Frede [44], the closer the model efficiency is to 1, the more accurate the model. Table 2 summarizes the statistical parameters of the selected ANNs: Number of hidden nodes, R2, RMSE and sum frequency. Selection of the network for prediction of CO2 conversion was carried out with a trial-and-error technique. The optimal number of hidden nodes is three. The training and validation of R2 and RMSE values are 0.99/0.32 and 0.99/0.22, respectively.
Then, the equation for CO2 conversion calculation was developed based on the ANN analysis. The equation can be expressed as in Eq. (4)–(6):
Firstly, the calculation of three hidden nodes was performed:
Lastly, the prediction model of CO2 conversion was calculated using Eq. (7):
where, H1, H2 and H3 represent the hidden nodes.
4. ConclusionsThe catalyst of RMS (5:35:60) calcined at 1,000°C gave 100% CO2 conversion with 68.87% of CH4 formation at a 400°C reaction temperature. The high CO2 conversion obtained was due to existence of active species on the catalyst surface. Other than that, the predicted value obtained from the ANN is almost identical with the experimental data by R2 values of CO2 conversion at 0.99. This showed that the models provide a reliable outcome and the equation generated from the ANN can be used to predict the CO2 conversion for other value in range of studied parameter. The optimum conditions from the ANN also revealed the RMSE value for training and validation are 0.32 and 0.22, respectively which indication of accuracy of model.
AcknowledgmentThe authors thank to Universiti Sultan Zainal Abidin, and Universiti Teknologi Malaysia for GUP grant vote 13H34.
NomenclatureCO2 carbon dioxide CH4 methane H2 hydrogen CO Carbon monoxide XRD X-ray diffraction Sm2O3 samarium (III) oxide MnO2 manganese (IV) oxide Sm samarium Mn manganese Ru ruthenium Al aluminium RMS Ru/Mn/Sm/Al2O3
MnNO3 Manganese (III) nitrate RuCl3 Ruthenium (III) chloride Al2O3 Aluminium oxide CH3OH methanol TPR Temperature Programme Reduction TPD Temperature Programme Desorption FESEM Field Emission Scanning Electron Microscopy EDX Energy Dispersive X-ray XPS X-ray photoelectron spectroscopy GC Gas chromoatography ESR Electron Spin Resonance FTIR Fourier Transform Infrared ANN artificial neural network MLP-ANN Multi-layer perception artificial Neural Netwok NotesAuthor Contributions S.J.M.R. (Ph.D) conducted the experiment in catalytic activity, A.A. (Ph.D) doing statistical analysis using ANN, S.T. (Ph.D) doing the characterisation catalyst, W.A.W.A.B. (Ph.D) doing the proofread and content arrangement, A.Z.A.H. (Ph.D) doing the characterisation catalyst, W.N.A.W.M. (Ph.D) doing the characterisation catalyst, A.F.A. (MSc. Student) doing analysis and statistical analysis using ANN, N.Z. (MSc. student) doing analysis and statistical analysis using ANN and S.M.R. (MSc. student) formatted and revise the manuscript. References1. Rosid SJM, Toemen S, Asif Iqbal MM, Bakar WAWA, Mokhtar WNAW, Aziz MMA. Overview performance of lanthanide oxide catalysts in methanation reaction for natural gas production. Environ Sci Pollut Res. 2019;26:36124–36140.
https://doi.org/10.1007/s11356-019-06607-8
2. Wang SG, Liao XY, Cao DB, et al. Factors controlling the interaction of CO2 with transition metal surfaces. J Phys Chem C. 2007;111:16934–16940.
https://doi.org/10.1021/jp074570y
3. Yamasaki M, Komori M, Akiyama E, et al. CO2 methanation catalysts prepared from amorphous Ni-Zr-Sm and Ni-Zr-misch metal alloy precursors. Mater Sci Eng A. 1999;267(2)220–226.
https://doi.org/10.1016/S0921-5093(99)00095-7
4. Le Dimet FX, Souopgui I, Ngodock HE. Sensitivity analysis applied to a variational data assimilation of a simulated pollution transport problem. Int J Numer Methods Fluids. 2017;83(5)465–482.
https://doi.org/10.1002/fld.4274
5. Yusof KMKK, Azid A, Jamalani MA. Determination of significant variables to particulate matter (pm10) variations in northern region, Malaysia during haze episodes (2006–2015). J Fundam Appl Sci. 2018;10(1S)300–312.
6. Azid A, Juahir H, Toriman ME, et al. Prediction of the level of air pollution using Principal Component Analysis and Artificial Neural Network Techniques: a case study in Malaysia. Water Air Soil Pollut. 2014;225(8)2063.
https://doi.org/10.1007/s11270-014-2063-1
7. Zamaniyan A, Joda F, Behroozsarand A, Ebrahimi H. Application of artificial neural networks (ANN) for modelling of industrial hydrogen plant. Int J Hydrog Energy. 2013;38:6289–6297.
https://doi.org/10.1016/j.ijhydene.2013.02.136
8. Alsaffar MA, Mageed AK, Abdel Ghany MAR, Ayodele BV, Mustapa SI. Elucidating the non-linear effect of process parameters on hydrogen production by catalytic methane reforming: an artificial intelligence approach. IOP Conf Ser: Mater Sci Eng. 2020;99:1012078.
https://doi.org/10.1088/1757-899X/991/1/012078
9. Ngo SI, Lim Y-I. Solution and Parameter Identification of a Fixed-Bed Reactor Model for Catalytic CO2 Methanation Using Physics-Informed Neural Networks. Catalysts. 2021;11:1304.
https://doi.org/10.3390/catal11111304
10. Esfandyari M, Amiri M, Koolivand-Salooki M. Neural Network prediction of the Fischer-Tropsch synthesis of natural gas with Co (lll)/Al2O3 catalyst. Chem Eng Res Bull. 2015;17:25–33.
https://doi.org/10.3329/cerb.v17i1.22915
11. Toemen S, Sulaiman SF, Rosid SJM, et al. Effectiveness of Ru/Mg/Ce Supported on Alumina Catalyst for Direct Conversion of Syngas to Methane: Tailoring Activity and Physicochemical Studies. Arab J Sci Eng. 2021;
https://doi.org/10.1007/s13369-021-06300-4
12. Asif Iqbal MM, Toemen S, Bakar WAWA, Razak FIA, Rosid SJM, Azelee NIW. Catalytic methanation over nanoparticle heterostructure of Ru/Fe/Ce/g-Al2O3 catalyst: Performance and characterization. Renew Energy. 2020;162:513–524.
https://doi.org/10.1016/j.renene.2020.06.093
13. Azid A, Juahir H, Latif MT, Zain SM, Osman MR. Feed-Forward Artificial Neural Network Model for Air Pollutant Index Prediction in the Southern Region of Peninsular Malaysia. J Environ Prot Sci. 2013;4:1–10.
https://doi.org/10.4236/jep.2013.412A1001
14. Azaman F, Azid A, Juahir H, et al. Application of artificial neural network and response surface methodology for modelling of hydrogen production using nickel loaded zeolite. J Teknol. 2015;77(1)109–118. .
https://doi.org/10.11113/jt.v77.4265
15. Isiyaka HA, Juahir H, Toriman ME, et al. Spatial assessment of air pollution index using environ metric modeling techniques. Adv Environ Biol. 2014;8(24)244–256. .
https://doi.org/10.11113/jt.v75.3977
16. Azid A, Juahir H, Toriman ME, et al. Selection of the most significant variables of air pollutants using sensitivity analysis. J Test Eval. 2016;44(1)376–384. .
https://doi.org/10.1520/JTE20140325
17. Rosid SJM, Bakar WAWA, Ali R. Characterization and modelling optimization on methanation activity using Box-Behnken design through cerium doped catalysts. J Clean Prod. 2018;170:278–287.
https://doi.org/10.1016/j.jclepro.2017.09.073
18. Azid A, Rani NLA, Samsudin MS, et al. Air quality modelling using chemometric techniques. J Fundam Appl Sci. 2017;9(2S)443–466. .
https://doi.org/10.4314/jfas.v9i2s.30
19. Nash JE, Sutcliffe JV. River flow forecasting through conceptual models, Part I - A discussion of principles. J Hydrol. 1970;10:282–290.
https://doi.org/10.1016/0022-1694(70)90255-6
20. Rosid SJM, Bakar WAWA, Ali R. Physicochemical study of supported cobalt–lanthanum oxide-based catalysts for CO2/H2 methanation reaction. Clean Techn Environ Policy. 2015;17:257–264.
https://doi.org/10.1007/s10098-014-0766-z
21. Xu B, Wei J, Yu Y, Li J, Zhu Q. Size Limit of Support Particles in an Oxide-Supported Metal Catalyst: Nanoparticles Ni/ZrO2 for Utilization of Natural Gas. J Phys Chem, B. 2003;107:5203–5207.
https://doi.org/10.1021/JP030127L
22. Nguyen TD, Mrabet O, Do TO. Controlled self-assembly of Sm2O3 nanoparticles into nanorods: Simple and large-scale synthesis using bulk Sm2O3 powders. J Phys Chem C. 2008;112:15226–15235.
https://doi.org/10.1021/jp804030m
23. Toemen S, Bakar WAWA, Ali R. Effect of ceria and strontia over Ru/Mn/Al2O3 catalyst: Catalytic methanation, physicochemical and mechanistic studies. J CO2 Util. 2016;13:38–49.
https://doi.org/10.1016/j.jcou.2015.11.005
24. Chung J, Park JH, Park JG, et al. Photoemission study of rare earth ditelluride compounds (ReTe2: Re = La, Pr, Sm, and Gd). J Korean Phys Soc. 2001;38(6)744–749. .
https://arxiv.org/abs/cond-mat/9910443v1
25. Suzuki C, Kawai J, Takahashi M, Vlaicu AM, Adachi H, Mukoyama T. The electronic structure of rare earth oxides in the creation of the core hole. Chem Phys. 2000;253:27–40.
https://doi.org/10.1016/S0301-0104(99)00380-8
26. Nguyen TD, Dinh CT, Do TO. Monodisperse samarium and cerium orthovanadate nanocrystals and metal oxidation states on the nanocrystals surface. Langmuir. 2009;25(18)11142–11148. .
https://doi.org/10.1021/la901387q
27. Bao X, Jiang Z, Zhou W, Tan D, Zhai R. Evidence for perimeter sites over SmOx-modified Rh(100) surface by CO chemisorption. Surf Sci. 2004;565:269–278.
https://doi.org/10.1016/j.susc.2004.07.021
28. Chakradhar PS, Murali A, Rao JL. A study of electron paramagnetic resonance and optical absorption in calcium manganese phosphate glasses containing praseodymium. J Mater Sci. 2000;35:353–359.
https://doi.org/10.1023/A:1004713805467
29. Phan TL, Zhang P, Tran HD, Yu SC. Electron spin resonance study of Mn-doped metal oxides annealed at different temperatures. J Korean Phys Soc. 2010;57(5)1270–1276. .
https://doi.org/10.3938/jkps.57.1270
30. Liu J, Liu J, Zhao Z, Wei Y, Song W. Fe-Beta@CeO2 Core-Shell Catalyst with Tunable Shell Thickness for Selective Catalytic Reduction of NOx with NH3
. AIChE J. 2017;63(10)4430–4441.
https://doi.org/10.1002/aic.15743
31. Li YW, He D, Zhu Z, Zhu Q, Xu B. Properties of Sm2O3-ZrO2 composite oxides and their catalytic performance in isosynthesis. Appl Catal A-Gen. 2007;319:119–127.
https://doi.org/10.1016/j.apcata.2006.11.020
32. Peluso MA, Hernandez WY, Dominguez MI, Thomas HJ, Centeno MA, Sambeth JE. CO oxidation: Effect of Ce and Au addition on MnOx catalysts. Lat Am Appl Res. 2012;42:351–358.
http://hdl.handle.net/10261/98974
33. Gandia LM, Vicenta MA, Gil A. Preparation and characterization of manganese oxide catalysts supported on alumina and zirconia-pillared clays. Appl Catal A-Gen. 2000;196:281–292.
https://doi.org/10.1016/S0926-860X(99)00479-2
34. Djebaili K, Mekhalif Z, Boumaza A, Djelloul . XPS, FTIR, EDX, and XRD analysis of Al2O3 scales grown on PM2000 alloy. J Spectrosc. 2015;1–16.
https://doi.org/10.1155/2015/868109
35. Lin WW, Cheng HY, Ming J, Yu YC, Zhao FY. Deactivation of Ni/TiO2 catalyst in the hydrogenation of nitrobenzene in water and improvement in its stability by coating a layer of hydrophobic carbon. J Catal. 2012;291:149–154.
https://doi.org/10.1016/j.jcat.2012.04.020
36. Meng F, Zhong P, Li Z, Lui X, Zheng H. Surface structure and catalytic performance of Ni-Fe catalyst for Low-tempertature CO hydrogenation. J Chem. 2014;1–7.
https://doi.org/10.1155/2014/534842
37. Cox PA. The element on Earth-Inorganic Chemistry in the Environment. Oxford; Oxford University Press; 1995. p. 147–158.
38. Zielinska B, Mijowska E, Kalenczuk RJ. Synthesis and characterization of K-Ta mixed oxides for hydrogen generation in photocatalysis. Int J Photoenergy. 2012;1–7.
https://doi.org/10.1155/2012/525727
39. Pojanavaraphan C, Luengnaruemitchai A, Gulari E. Catalytic activity of Au-Cu/CeO2-ZrO2 catalysts in steam reforming of methanol. Appl Catal A-Gen. 2013;456:135–143.
https://doi.org/10.1016/j.apcata.2013.02.010
40. Rosid SJM, Abu Bakar WAW, Ali R. Methanation Reaction over Samarium Oxide Based Catalysts. Mal J Fund Appl Sci. 2013;9(1)28–34. .
https://doi.org/10.11113/mjfas.v9n1.78
41. He HQ, Zhang L, Wu H, Li CZ, Jig SP. Synthesis and characterization of doped La9ASi6026.5 (A= Ca,Sr,Ba) oxy apatite electrolyte by water-based gel-casting route. Int J Hydrog Energy. 2011;36(11)6862–6874.
42. Su P, Chu O, Wang L. Studies on catalytic activity of nanostructure Mn2O3 prepared by solvent-thermal method on degrading crystal violet. Mod Appl Sci. 2010;4(5)125–129. .
https://doi.org/10.5539/mas.v4n5p125
43. Argyle MD, Bartholomew CH. Heterogeneous catalyst deactivation and regeneration: A review. Catalysts. 2015;5:145–269.
https://doi.org/10.3390/catal5010145
44. Grunwald S, Frede H-G. Using the modified agricultural non-point source pollution model in German Watershed. Catena. 1999;37:319–328.
https://doi.org/10.1016/S0341-8162(99)00024-7
Fig. 1XRD Diffractograms of RMS (5:35:60) catalyst calcined at (a) 400°C, (b) 700°C, (c) 900°C, (d) 1,000°C and (e) 1,100°C for 5 h. 
Fig. 2Binding energy for elements of O 1s, Al 2p, Mn 2p, Sm 3d over RMS (5:35:60) catalyst calcined at 1,000°C for 5 h. 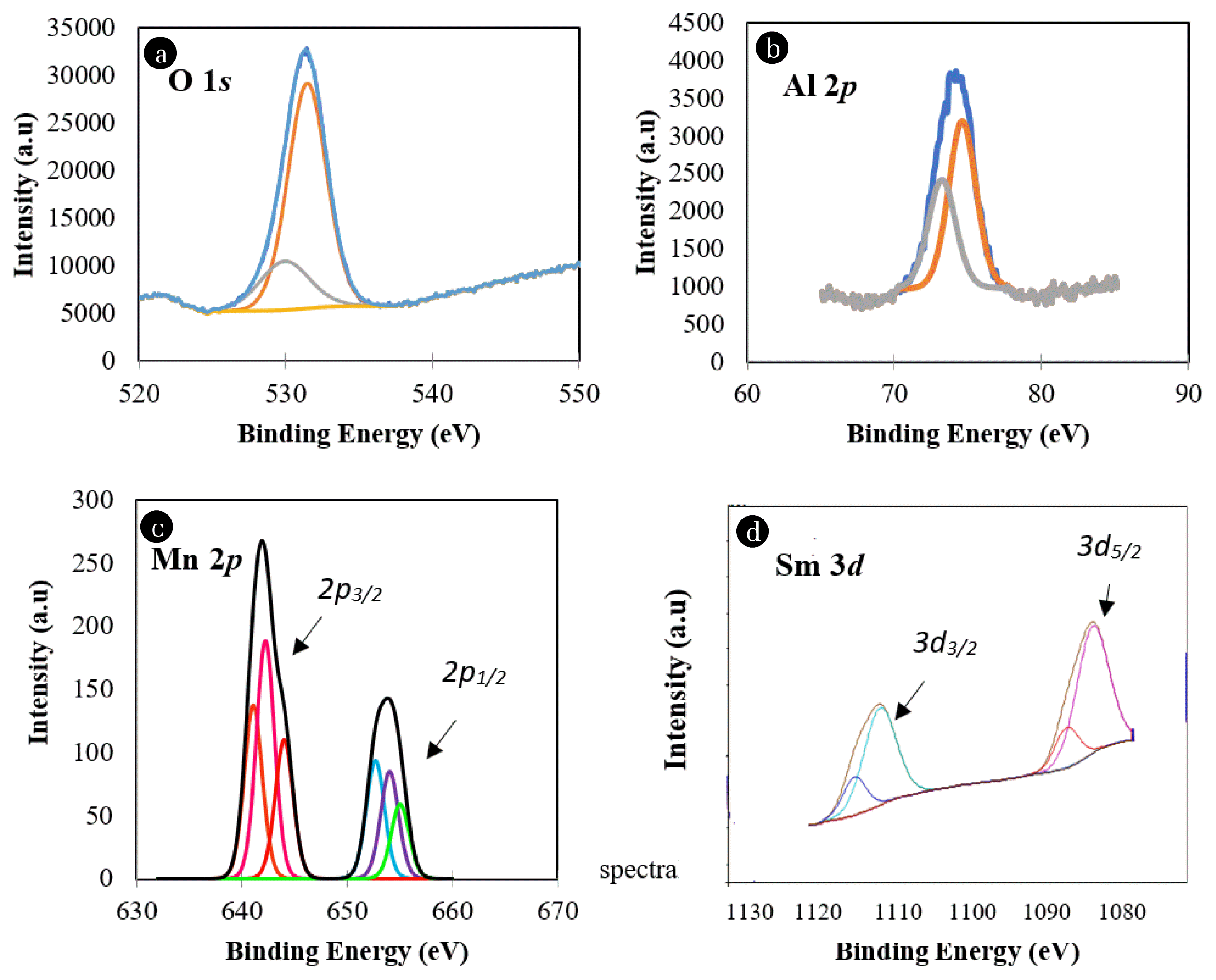
Fig. 3Overlay of ESR spectra over RMS (5:35:60) catalyst calcined at 900, 1,000, and 1,100°C for 5 h. 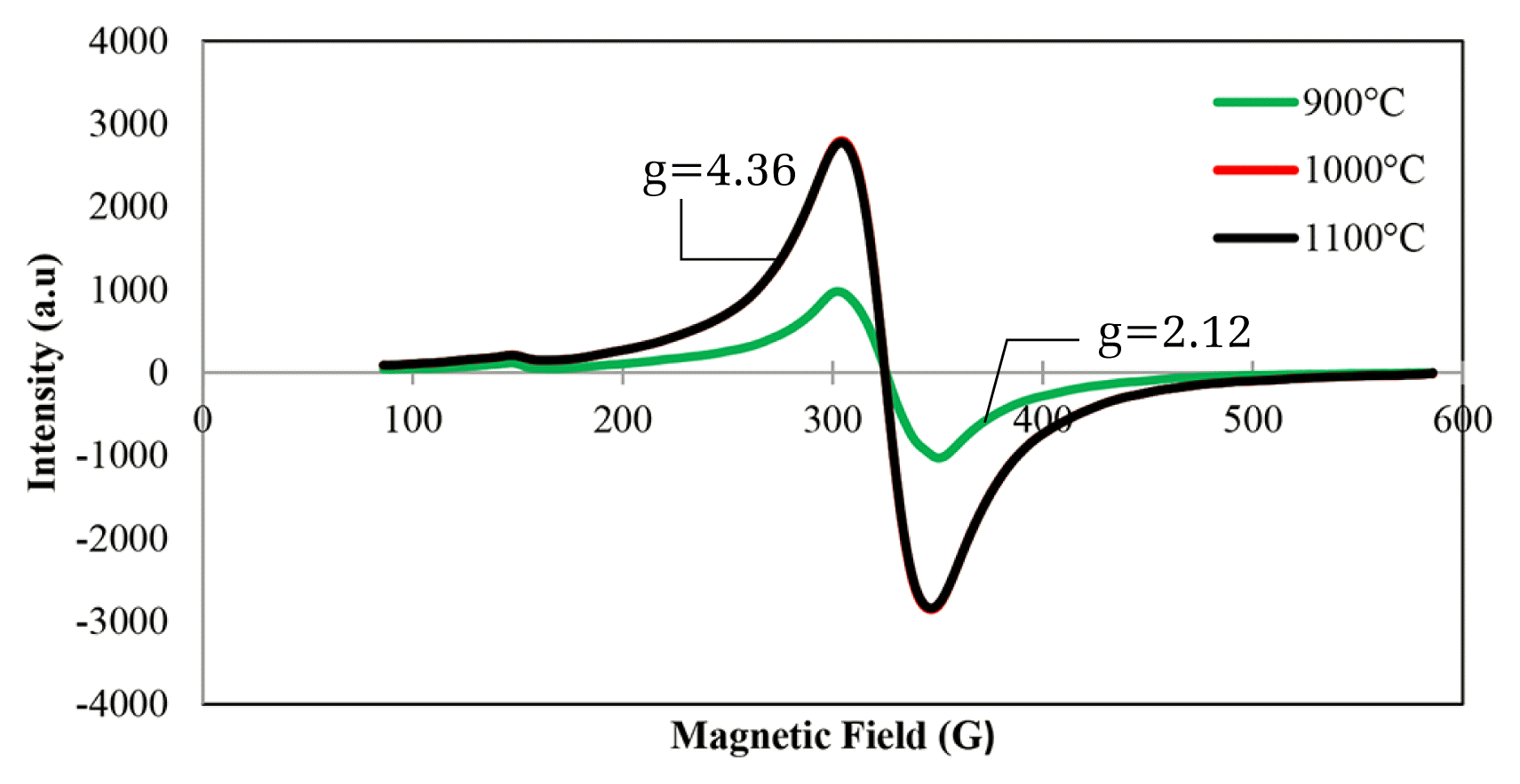
Fig. 6FESEM micrographs of RMS (5:35:60) catalyst calcined at (a) 400°C, (b) 700°C, (c) 900°C, (d) 1,000°C and e) 1,100°C for 5 h at 50000x magnification. 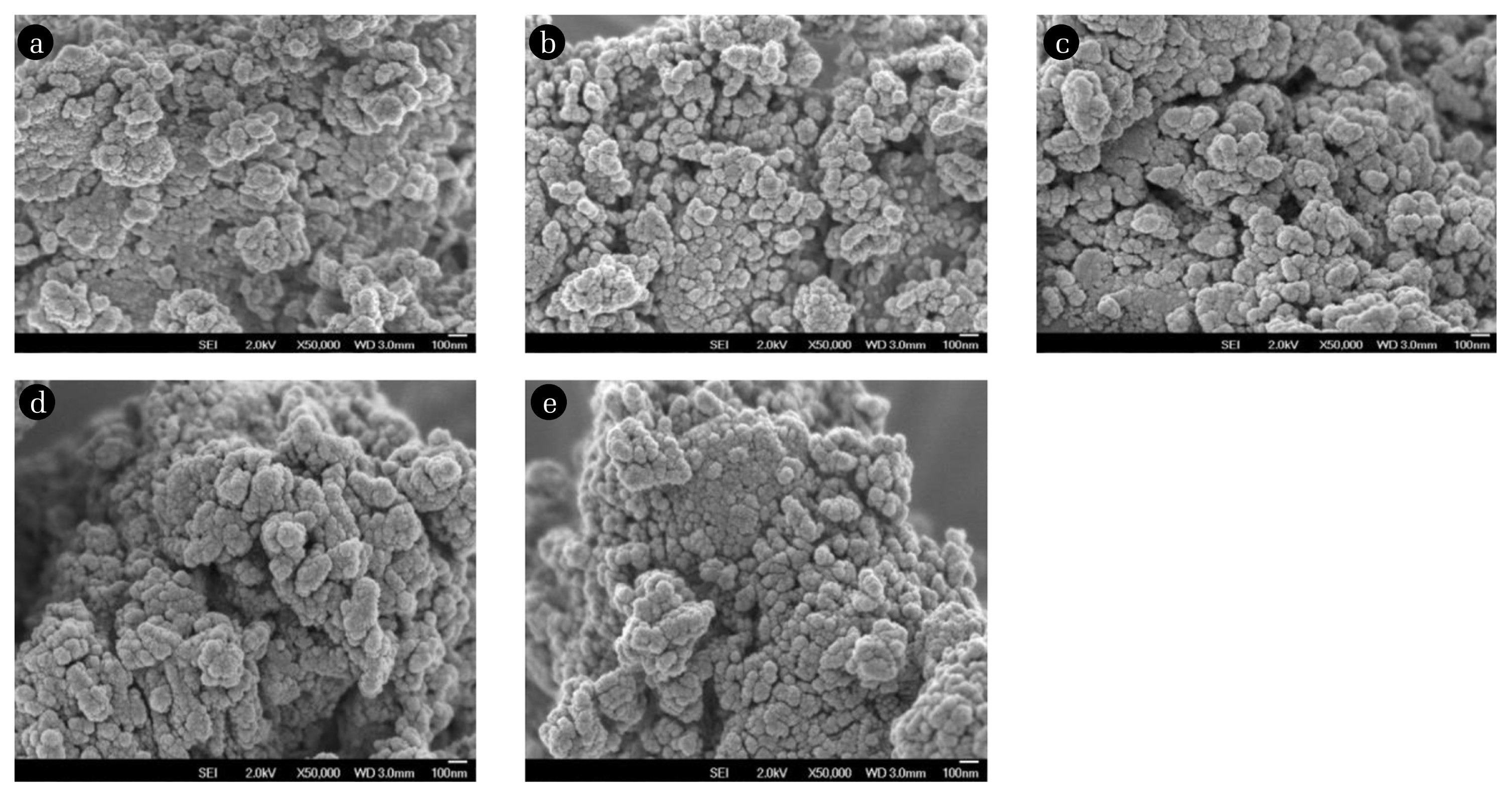
Table 1Experiment Design for CO2 Conversion and ANN Results for Samarium Catalyst |
|
||||||||||||||||||||||||||||||||||||||||