AbstractThe presence of antibiotics in aquatic environments has become a serious concern since they develop the antibiotic/multi-drug-resistant bacteria which further affect to living beings. The study intended to assess the freshly synthesized ferrate (VI) in the degradation of an important emerging micro-pollutant i.e., sulfamethazine (SMZ). Moreover, the real matrix implications are extensively conducted for implication of ferrate (VI) technology as safer and viable options. Batch reactor studies enabled the molar ratio of ferrate (VI) to sulfamethazine is 2:1 with overall rate constant 6,128 mM−2.min−1. Percentage elimination of sulfamethazine was observed Ca. 80% at initial sulfamethazine concentration 0.02 mM and ferrate (VI) dose 0.1 mM. Presence of several co-ions NaCl, Na2HPO4, NaNO3, oxalic acid and NaNO2 showed insignificant effect on elimination of sulfamethazine; whereas the efficiency of ferrate (VI) was lowered due to glycine and EDTA. Mineralization of sulfamethazine is significantly increased at lower pH value (pH 5.0). Further, the removal of sulfamethazine in the real water matrix showed that the elimination efficiency of sulfamethazine is almost unaffected as compared to the distilled water treatment. This implied that ferrate (VI) is a viable and greener option for treatment of emerging water pollutants to enhance the efficiency of existing wastewater treatment plants.
1. IntroductionThe presence of antibiotics in the aquatic environment has become a special concern due to their potential adverse effects in human and other living organisms. Even trace amounts of antibiotics present in aqueous media possibly will develop antibiotic/multi-drug-resistant bacteria which is a serious threat to modern medicine [1, 2]. Sulfonamides are the most common antibiotics which are widely used in poultry breeding and livestock for treating various diseases in order to increase the animal products. The extensive usage of sulfonamides by humans or animals caused them to enter into the aquatic environment since these antibiotics are only partially metabolized [3]. Moreover, the wastewater released from pharmaceutical manufacturing industries causes additional environmental concerns [4]. It has been reported that the soil fertilized with manure containing sulfonamide served as a long term source of antibiotic into aquatic environments [5]. Upon entering the soil the antibiotic enters the trophic chain through surface and ground water which significantly enhances the tolerance of bacteria towards the antibiotics [6, 7].
Sulfamethazine [4-amino-N-(4,6-dimethylpyrimidin-2-yl)benzene sulfonamide] is a sulfonamide with sulfanilamide and para-amino groups and it is a commonly prescribed as sulfonamide drug. These along with other sulfonamides are often detected in aquatic environments including the drinking water, surface water, groundwater, and wastewater treatment plant effluent. Due to its low cost and efficient antibiotic impact, sulfamethazine is commonly used as a feed additive in the dairy processing industry and also utilized for the treatment and prevention of influenza [8, 9]. Hence, the extensive uses of it, the level of sulfamethazine exposure in aquatic ecosystems is significantly increased and reports have shown the concentration of sulfamethazine varies from ng.L−1 to μg.L−1 [10]. Sulfamethazine is strongly hydrophilic substance and it is poorly metabolized by microorganisms [11]; further, it is reported that sulfonamide antibiotics possibly inhibits the development of several Gram-negative as well as most of Gram-positive bacteria [12, 13].
The exposure of sulfamethazine in wastewater treatment plant effluent or even in treated drinking water indicates that it is not effectively removed by conventional water and wastewater treatment [14]. Moreover, due to the formation of large volumes of sludge the current water treatment plants triggered substantial contamination to the human environment [15]. Therefore, various treatment methods are developed to eliminate efficiently the sulfamethazine from wastewater, including physical adsorption [16, 17], ozonation [18, 19], electrochemical oxidation [20], Fenton processes [11, 21–23], biological processes [24], photocatalysis [25, 26], etc. It is indicated that the chlorine, hypochlorous, and ozone compounds are often employed for the removal of sulfamethazine since these are efficient and easily accessible to use. However, use of these oxidants might be inappropriate due to its tendency to form potentially harmful by-products such as bromates and trihalomethanes as a result of ozonation and chlorination [27].
On the other hand, the ferrate (VI) is an environmentally benign and, possibly, an emerging oxidant for water purification since it possesses good selectivity and stability in presence of other compounds in the water matrix [28–30]. In addition, the ferrate (VI) oxidation minimizes the possibility of toxic by-products in oxidation processes [31]. The redox potential of ferrate (VI) is exceptionally high under acidic conditions (2.20 V) which is well compatible with that of ozone (2.0 V), whereas at extreme basic pH (pH 14) it becomes a slightly weaker oxidant (0.7 V). Ferrate (VI) in aqueous solution undergoes spontaneous decomposition and is reduced to Fe (III) which is a non-toxic by-product and considered as environmentally safe chemical for coagulation [32, 33]. Ferrate (VI) is a diprotic acid that exists in four distinct species viz., FeO42−, HFeO4−, H2FeO4 and H3FeO4+ [34]. The stable species of ferrate (VI) in acidic and alkaline mediums are HFeO4− and FeO42−, respectively; however, the strength and oxidizing ability of HFeO4− get reduces on increasing the pH of the reaction media [35, 36]. Ferrate (VI) have been successfully employed for various water purification processes including degradation or oxidation of organic compounds, removal of toxic inorganic compounds, removal of dissolve suspended/colloidal particulate materials, attenuation of phosphate in sewage treatment and removal of microorganisms from water, etc. [37–39]. Recent studies have shown that ferrate (VI) is utilized for degradation of micro-pollutants in aqueous media and the results are found to be promising [31, 40]. Therefore, the aim of this research is to investigate the efficiency of freshly synthesized ferrate (VI) in oxidative removal of sulfa-drug in aqueous solutions. The impact of various operational variables such as molar ratios of ferrate (VI) to sulfamethazine and pH are extensively investigated. Moreover, the mineralization of sulfamethazine in the ferrate (VI) treatment is obtained. Further, the degradation of sulfamethazine in the real water samples is conducted for possible implications of ferrate (VI) in the wastewater treatment strategies.
2. Materials and Methods2.1. MaterialsHexane, sulfamethazine (SMZ) and diethyl ether are obtained from Sigma Aldrich Co., USA. GF/C Whatman filter paper is obtained from Whatman, USA. Glass filtration with fritted funnel, iron (III) nitrate nonahydrate, KOH, HCl and oxalic acid are obtained from Merck India Ltd., India. Glycine, Na2B4O7.10H2O, NaCl, syringe filter (0.22 μm) are purchased from Himedia India Ltd India. NaNO2, NaNO3 and EDTA are obtained from Loba Chemicals, India. Purified sodium hypochlorite is obtained from Thermo Fisher Scientific India Pvt. Ltd. The water purification system Sartorius is used for purification of water (Arium Mini Plus UV Lab, Sartopore 2150, Sterile Plus, Germany). The real water sample is collected from Theihai Lui, located at the outskirts of Aizawl city, Mizoram (India). UV-Vis spectrophotometer (Shimadzu, UV-1800, Japan) is used for absorbance measurements. TOC (Total Organic Carbon; TOC-VCPH/CPN, Shimadzu) analyzer is employed for measuring the NPOC (Non-Purgeable Organic Carbon) values.
2.2. Methods2.2.1. Preparation of potassium ferrate(VI)Potassium ferrate (VI) was synthesized by the method described previously [41] and the purity of ferrate (VI) was obtained by the known method [42]. In brief: 300 mL chilled sodium hypochlorite solution (12–15%) was saturated with the excess of potassium hydroxide and filtered with a GF/C filter paper to obtain a clear yellow highly alkaline sodium hypochlorite solution. Further, 20 g of pulverized ferric nitrate was gradually added to the cold alkaline sodium hypochlorite solution. Once the ferric nitrate was fully added, the mixture was stirred constantly for another 30 min. Cold conditions and strong alkaline environment favored the oxidation or conversion of iron (III) to iron (VI). A rapid change in color from yellow brown to dark purple occurred which indicates the formation of ferrate (VI). Further, about 50 g of potassium hydroxide pellets was slowly added to this solution and kept the mixture temperature < 10°C. The solution mixture was kept in the refrigerator for Ca. 40 min. The resultant slurry having dark purple color was filtered through GF/C filter paper (pore size of approximately 10–15 μm) using a glass filtration assembly. Filtrate was discarded and solid was washed with 100 mL of 3 M potassium hydroxide chilled cold solution. This allows the ferrate (VI) to get dissolved and filtrate is collected in the filtration flask. The filtrate was taken in a flask and then added 100 mL of chilled saturated solution of potassium hydroxide. The potassium ferrate is precipitated easily and is filtered again using GF/C filter paper. The filtrate was eliminated, and the solid was again flushed with a cold 3 M potassium hydroxide solution (50 mL) and the filtrate was collected. The ferrate (VI) was again precipitated using chilled cold saturated potassium hydroxide solution. Similarly, re-precipitation was performed at least two more times to eliminate any remaining impurities and thereby to improve the purity of ferrate (VI). Lastly, the solid was washed with (10 mL) of n-hexane followed by diethyl ether (10 mL). The end product was carefully collected; it was nearly black in color. The purity of ferrate (VI) is > 90%. The potassium ferrate (VI) are stored in a vacuum desiccator contained with excess of potassium hydroxide pellets. However, the freshly prepared ferrate (VI) was preferred for experimentation.
2.2.2. Batch reactor methodA stock solution of sulfamethazine (0.5 mM) is prepared by dissolving an appropriate amount of sulfamethazine in purified water. The successive dilution of stock solution provides required experimental concentrations (0.02 to 0.2 mM) of sulfamethazine. The pH of each solution is controlled by using 0.1 M of NaOH or HCl solutions by a drop wise addition. In a reactor vessel, batch reactor operations are carried out varying the concentration of sulfamethazine from 0.02 to 0.2 mM and the solution pH is fixed. On the other hand, the batch reactions are carried out varying pH values from pH 5.0 to 8.0 at a fixed concentration of sulfamethazine for pH dependent studies. A constant amount of solid potassium ferrate (equivalent to 0.1 mM) was added in reaction mixture and continuously stirred. The decomposition of ferrate (VI) in a reactor implies the degradation of the pollutant; i.e., sulfamethazine. The absorbance of the solution mixture is calculated and reported using a UV-Vis Spectrophotometer at a regular time interval of 1 min for a total time period of 20 min at wavelength 510 nm. The absorbance of self- decomposition of ferrate (VI) at the same pH and solution is also taken at 510 nm to nullify the blank corrections by comparing the absorbance in presence of sulfamethazine. After the treated sample is detained for another 2 h, reaction mixture is filtered using a syringe filter. One portion of the filtered sample is used for TOC (total organic carbon) measurements, and the other part is used for sulfamethazine measurements using HPLC (High Performance Liquid Chromatography).
The HPLC instrument has a C18 column (4.6 × 250 mm), Waters 515 HPLC pump and Waters 2489 UV-Vis detector. The sample volume pumped into the column is 20 μL. Acetonitrile and water are the mobile phases used, all of which are HPLC grade, in a proportion of 45:55. The wave length set is 275 nm and the flow rate of mobile phase is set 1 mL.min−1. An appropriate volume of sulfamethazine is dissolved in water (HPLC grade) to prepare sulfamethazine standard solutions, and the area of the peak obtained is used to achieve the exact percentage elimination of sulfamethazine. The results are then represented as a function of concentration and pH in terms of percentage removal of sulfamethazine. Total organic carbon (TOC) calculation was used to determine sulfamethazine mineralization, which is then recorded in terms of percentage elimination using the initial TOC values of SMZ. Furthermore, the disparity in TOC values between the initial and treated samples are used to measure the amount of sulfamethazine eliminated, and the results are shown as a function of pH and initial pollutant concentration.
The degradation of sulfamethazine by ferrate (VI) is also performed in presence of various co-existing ions such as NaCl, C2H5NO2, NaNO3, C2H2O4, NaNO2, Na2HPO4 and EDTA. The concentrations of sulfamethazine, ferrate (VI), and co-existing ions are taken in a fixed molar ratio of 0.1: 0.2: 0.5 and at pH 6.0. The samples are stirred for 2 h then filtered and subjected for sulfamethazine measurements using HPLC.
3. Results and Discussion3.1. Effect of Ferrate (VI) ConcentrationSulfamethazine solution having concentrations from 0.02 mM to 0.2 mM is treated with ferrate (0.1 mM) at pH 6.0. The concentration of sulfamethazine is varied to obtain varied ferrate (VI) to SMZ molar ratios (i.e., from 0.1:0.02 to 0.1:0.2). The time dependent variation in ferrate (VI) concentration in presence of sulfamethazine is continuously recorded using UV-visible spectrophotometer. The results are graphically presented as in Fig. 1. It is noticed that the reduction of ferrate (VI) is considerably increased at higher concentrations of sulfamethazine. This indicated that the rate of degradation of sulfamethazine is favored at higher concentration of pollutants. Furthermore, it is noted that the degradation of ferrate (VI) is reasonably faster during the initial period of reaction, but it reaches almost a constant value in the later stages of time, i.e., after 10 min of interaction. This result reveals that the bulk of sulfamethazine is degraded within the first stage of interaction. Thus, ferrate (VI) is observed to be an effective oxidant in the degradation of sulfamethazine in aqueous medium.
3.2. Kinetics of SMZ DegradationThe kinetics of sulfamethazine degradation by ferrate (VI) are studied using time dependency observations i.e., the change in ferrate (VI) concentration with time at pH 6.0. Eq. (1) shows the rate expression of reduction of ferrate(VI) in presence of sulfamethazine (SMZ) [43]:
where kapp is the rate constant. The above equation is further simplified as:
where, k = kapp [SMZ]n
The time dependence data is utilized for the pseudo-first order and pseudo-second order kinetics and the results are fitted well with the pseudo-2nd-order rate equation (Cf. Table 1; Fig. 2(a)). This specifically implies that the reaction follows pseudo-2nd-order kinetics with respect to each concentration of sulfamethazine, and the value of ‘m’ is inferred as 2. Further, the rate constant values of pseudo-1st-order or pseudo-2nd-order kinetics at pH 6.0 along with the regression coefficient are shown in Table 1. The values obtained for pseudo-2nd-order rate constant were meaningfully upsurges as the sulfamethazine concentration is raised up from 0.02 to 0.2 mM which is probably because of potential of ferrate (VI) to quickly oxidize the pollutant in aqueous medium.
Further, the pseudo-2nd-order rate constant values obtained at various concentrations of sulfamethazine at pH 6.0 are used to calculate the value of ‘n’ along with an apparent value of ‘kapp’. Fig. 2(b) shows an empirical plot of sulfamethazine concentrations against the calculated value of ‘k’. The value of k is saturated at the higher concentration of reactant i.e., sulfamethazine concentration of 0.2 mM since a slight deviation in linear relationship was observed. Overall, the results showed that at pH 6.0, a reasonably good linear relationship is attained between the concentration of sulfamethazine and k values. This indicates that the value of ‘n’ is ‘1’. Hence, the stoichiometric ratio of ferrate (VI) to sulfamethazine is 2:1. Furthermore, the apparent or overall rate constant kapp value is calculated as 6,128 mM−2.min−1. Relatively large kapp value indicated that the ferrate (VI) is efficient in the degradation of sulfamethazine in aqueous medium [31].
In addition, an increase in concentration of SMZ from 0.02 to 0.2 mM causes a reduction in the removal of efficiency of sulfamethazine as shown in Fig. 3. A reduction in percentage elimination of sulfamethazine is found to be from 79.97 to 15.05%, respectively with the increase in sulfamethazine concentration from 0.02 to 0.2 mM. This reduction in the percentage elimination is attributed to the assumption that a comparatively higher amount of ferrate (VI) is available at lower concentration of sulfamethazine to degrade the smaller number of its molecules [31]. Moreover, an apparent increase in the content of sulfamethazine elimination is therefore achieved by increasing the sulfamethazine concentration. However, the content of sulfamethazine removed is almost identical at the pollutant concentrations > 0.06 mM employing the constant dose of ferrate (VI) i.e., 0.2 mM.
3.3. Effect of pHIt is necessary to study the pH dependent degradation of sulfamethazine as this explains the process involved in ferrate (VI)-mediated pollutant degradation [44]. Moreover, it is important to optimize the optimal pH for degradation of pollutants in aqueous media. The pH dependence studies are conducted using the ferrate (VI) and SMZ molar ratio of 2:1. Therefore, a fixed dose of ferrate (VI) concentration (0.2 mM) is employed for degrading the sulfamethazine having fixed concentration of 0.1 mM and solution pH is varied from pH 5.0 to 8.0. The degradation efficacy of ferrate (VI) in presence of sulfamethazine at various pH is shown as in Fig. 4(a). It is observed that the degradation of ferrate (VI) in presence of sulfamethazine is greatly favored as the acidity of the solution is increased. The degradation of ferrate (VI) is relatively slower at pH 8.0; however, at pH 5.0, the concentration of ferrate(VI) is reduced from 0.2 to 0.14 mM within 20 min of contact.
The degradation of sulfamethazine is further investigated by obtaining the percentage removal of sulfamethazine using the HPLC measurements. It is observed that the sulfamethazine removal efficiency is increased from 41.22% to 51.37% while the solution pH is decreased from 8.0 to 5.0 as shown in Fig. 4(b). This is mainly because the ferrate(VI) exists as HFeO4− species at neutral and acidic medium while the FeO42− species is formed in the basic medium [45]. The spin density of HFeO4− is larger than the unprotonated species; hence, increases its oxidizing capability in the degradation of sulfamethazine [46]. Thus, higher percentage removal is observed at lower pH. It is further assumed that the degradation of sulfamethazine occurs through the cleavage of C–S and S–N bonds in sulfamethazine due to hydroxylation by ferrate (VI). Moreover, degradation is likely to occur with the oxidation of aniline moiety (NH2 substituent) of sulfamethazine by ferrate (VI) species of HFeO4− [47].
3.4. Effect of Co-existing IonsThe oxidative removal of sulfamethazine is also assessed in presence of several co-existing ions using ferrate(VI) at a dose of 0.2 mM and pH 6.0. Co-ions viz., oxalic acid, glycine, NaCl, EDTA, NaNO2, Na2HPO4 and NaNO3 are used. The concentration of each of the selected ions is employed as 0.5 mM, while that of pollutant is 0.1 mM. Thus, the actual molar ratio of ferrate (VI) to sulfamethazine to co-ion is 2:1:5. Depending upon the nature of co-ion, the degradation or removal of sulfamethazine is varied. As shown in Fig. 5, the effect of presence of electrolytes such as NaCl, Na2HPO4, NaNO3, oxalic acid and NaNO2 on the degradation of sulfamethazine using ferrate (VI) is very less/or insignificant. Whereas the decomposition of sulfamethazine is greatly affected in presence of glycine and EDTA. The percentage elimination of sulfamethazine in presence of EDTA and glycine is found to be 14.53% and 15.86% which are comparatively low as compared to that of the blank i.e., 49.37% removal in absence co-ions. The less removal of sulfamethazine in presence of EDTA and glycine is possibly due to competitive oxidation of sulfamethazine. The ferrate (VI) degraded the organic impurity preferentially. On the other hand, the radical scavenging is less possible since EDTA is efficient in scavenging the h+ in the semiconductors specially the TiO2 [48, 49]. Moreover, previously, it was reported that the reductive pathways of ferrate (VI) is demonstrated as [43, 50]:
Therefore, the in-situ hydroxyl radical generated takes part in the degradation of pollutants in the aqueous medium.
3.5. Mineralization of SulfamethazineA total organic carbon analyzer is used to evaluate the mineralization of sulfamethazine by ferrate(VI). In this study, 0.1 mM of sulfamethazine solution is treated with 0.2 mM of ferrate (VI). The reaction time was provided at 2 h. The experiments are performed at various pH, i.e., from pH 5.0 to 8.0. Once the degradation reaction is completed, the solution is filtered and then taken for total organic carbon analysis along with that of untreated samples (i.e., the blank samples). The values obtained in this analysis are further used for the calculation of percentage mineralization of sulfamethazine. The results are graphically illustrated in Fig. 6(a). It is clear from the figure that decreasing the pH of reaction mixture favored the mineralization of sulfamethazine. More quantitatively, decreasing the solution pH from pH 8.0 to 5.0 has caused the percentage mineralization of sulfamethazine from 8.4 to 22.78% using ferrate (VI) to sulfamethazine molar ratio of 2:1. The actual amount of sulfamethazine mineralized with respect to solution pH is also illustrated in Fig. 6(b). Figure shows that the oxidation of sulfamethazine by ferrate (VI) is favored at lower pH values. This confirms that the reactivity of ferrate (VI) in aqueous solution is higher at lower pH. These results are comparable to the HPLC results, i.e., the percentage removal of sulfamethazine is slightly affected with the change in pH of the solution. However, the TOC data showed that, although partial mineralization of sulfamethazine is achieved a significant percentage of sulfamethazine is mineralized using a small amount of ferrate (VI).
3.6. Treatment of Real Water TreatmentThe applicability of ferrate (VI) in water purification is measured based on its effectiveness and performance in a real water matrix. Thus, this study aims to conduct the oxidative degradation of sulfamethazine using real water samples. The real water sample is collected from Theihai Lui, located outskirts of Aizawl City, Mizoram Province (India). A photometer multi-parameter was used to analyze various water parameters and results are shown in Table S1 (Supplementary Material). The common anions and various elements present in real water samples are also obtained and given in Table S1. The real water sample is contained with a relatively high amount of Ca, Fe and Mn as compared to the other elements which are present at low level. Moreover, the TOC analysis shows that the real water contains low values of inorganic carbon and high value of non-purgeable organic carbon. The real water collected is utilized to prepare sulfamethazine solution at various concentrations (0.02 to 0.2 mM) and pH 6.0. The oxidative degradation is then performed by using a constant ferrate(VI) concentration of 0.1 mM. After 2 h, the treated samples are filtered and then analyzed by HPLC. The percentage removal is obtained and compared to the result obtained with distilled water as shown in Fig. 7. Thus, the results showed that the removal of sulfamethazine by ferrate (VI) is not significantly affected in actual water samples. This implies that ferrate (VI) is efficient and possessed with adequate suitability in the removal of sulfamethazine in real water treatment.
4. ConclusionsHigh purity ferrate (VI) was synthesized and effectively employed for degradation of sulfamethazine in batch studies. Ferrate (VI) enabled a very fast degradation of sulfamethazine which slowed down with lapse of time and apparent reaction was completed within 10 min of interaction. The removal of ferrate (VI) in presence of sulfamethazine has followed pseudo-second order kinetics. The molar ratio of ferrate (VI) to sulfamethazine is estimated as 2:1 with an apparent rate constant of 6,128 mM−2 min−1. A significantly high percentage elimination of sulfamethazine was observed using the HPLC measurements and about 80% of sulfamethazine was removed by ferrate (VI) at the initial pollutant concentration of 0.02 mM. The presence of co-ions viz., NaCl, Na2HPO4, NaNO3, oxalic acid and NaNO2 showed insignificant effect on the degradation of sulfamethazine using ferrate (VI); whereas a marked decrease in removal efficiency was recorded in presence of glycine and EDTA. Decreasing the pH of solution from pH 8.0 to 5.0 greatly favored the percentage mineralization of sulfamethazine using the ferrate (VI) treatment. The oxidative degradation of sulfamethazine in a real water matrix sample showed no significant difference in sulfamethazine removal using the ferrate (VI) treatment. This implied that ferrate (VI) is a fairly selective and useful greener option for the efficient removal of emerging water pollutants from aqueous media.
AcknowledgmentsOne of the author DT acknowledges the CSIR, New Delhi providing the financial assistance in the form of Extra Mural Research Grant vide No. 24(354)/18-EMR-II.
References1. Koch N, Islam NF, Sonowal S, Prasad R, Sarma H. Environmental Antibiotics and Resistance Genes as Emerging Contaminants: Methods of Detection and Bioremediation. Curr Res Microb Sci. 2021;2:100027.
2. Schaenzer AJ, Wright GD. Antibiotic Resistance by Enzymatic Modification of Antibiotic Targets. Trends Mol Med. 2020;26:768–782.
3. Hu L, Flanders PM, Miller PL, Strathmann TJ. Oxidation of Sulfamethoxazole and Related Antimicrobial Agents by TiO2 Photocatalysis. Water Res. 2007;41:2612–2626.
4. Larsson DGJ. Pollution from Drug Manufacturing: Review and Perspectives. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130571.
5. Yu J, Kiwi J, Wang T, Pulgarin C, Rtimi S. Evidence for a Dual Mechanism in the TiO2/CuxO Photocatalyst during the Degradation of Sulfamethazine under Solar or Visible Light: Critical Issues. J Photochem Photobio A: Chem. 2019;375:270–279.
6. Carvalho IT, Santos L. Antibiotics in the Aquatic Environments: A Review of the European Scenario. Environ Inter. 2016;94:736–757.
7. Yi X, Lin C, Ong EJL, Wang M, Zhou Z. Occurrence and Distribution of Trace Levels of Antibiotics in Surface Waters and Soils Driven by Non-Point Source Pollution and Anthropogenic Pressure. Chemosphere. 2019;216:213–223.
8. Lin C-C, Wu M-S. Feasibility of Using UV/H2O2 Process to Degrade Sulfamethazine in Aqueous Solutions in a Large Photoreactor. J Photochem Photobio A: Chem. 2018;367:446–451.
9. Zhao H, Cao Z, Liu X, et al. Seasonal Variation Flux Estimation and Source Analysis of Dissolved Emerging Organic Contaminants in the Yangtze Estuary China. Mar Pollut Bull. 2017;125:208–215.
10. Wen D, Wu Z, Tang Y, Li M, Qiang Z. Accelerated Degradation of Sulfamethazine in Water by VUV/UV Photo-Fenton Process: Impact of Sulfamethazine Concentration on Reaction Mechanism. J Hazard Mater. 2018;344:1181–1187.
11. Tang J, Wang J. MOF-Derived Three-Dimensional Flower-like FeCu@C Composite as an Efficient Fenton-like Catalyst for Sulfamethazine Degradation. Chem Eng J. 2019;375:122007.
12. Wan Z, Wang J. Ce-Fe-Reduced Graphene Oxide Nanocomposite as an Efficient Catalyst for Sulfamethazine Degradation in Aqueous Solution. Environ Sci Pollut Res. 2016;23:18542–18551.
13. Liu Y, Wang J. Degradation of Sulfamethazine by Gamma Irradiation in the Presence of Hydrogen Peroxide. J Hazard Mater. 2013;250–251:99–105.
14. Garoma T, Umamaheshwar SK, Mumper A. Removal of Sulfadiazine Sulfamethizole Sulfamethoxazole and Sulfathiazole from Aqueous Solution by Ozonation. Chemosphere. 2010;79:814–820.
15. Gracia-Lor E, Sancho JV, Serrano R, Hernández F. Occurrence and Removal of Pharmaceuticals in Wastewater Treatment Plants at the Spanish Mediterranean Area of Valencia. Chemosphere. 2012;87:453–462.
16. Wei X, Zhang Z, Qin L, Dai J. Template-Free Preparation of Yeast-Derived Three-Dimensional Hierarchical Porous Carbon for Highly Efficient Sulfamethazine Adsorption from Water. J Taiwan Inst Chem Eng. 2019;95:532–540.
17. Zhuang S, Liu Y, Wang J. Covalent Organic Frameworks as Efficient Adsorbent for Sulfamerazine Removal from Aqueous Solution. J Hazard Mater. 2020;383:121126.
18. Bai Z, Yang Q, Wang J. Catalytic Ozonation of Sulfamethazine Using Ce01Fe09OOH as Catalyst: Mineralization and Catalytic Mechanisms. Chem Eng J. 2016;300:169–176.
19. Sui Q, Huang J, Lu S, et al. Removal of Pharmaceutical and Personal Care Products by Sequential Ultraviolet and Ozonation Process in a Full-Scale Wastewater Treatment Plant. Front Environ Sci Eng. 2014;8:62–68.
20. Yu N, Lu X, Song F, Yao Y, Han E. Electrocatalytic Degradation of Sulfamethazine on IrO2-RuO2 Composite Electrodes: Influencing Factors Kinetics and Modeling. J Environ Chem Eng. 2021;9:105301.
21. Wan Z, Wang J. Degradation of Sulfamethazine Using Fe3O4-Mn3O4/Reduced Graphene Oxide Hybrid as Fenton-like Catalyst. J Hazard Mater. 2017;324:653–664.
22. Foteinis S, Monteagudo JM, Durán A, Chatzisymeon E. Environmental Sustainability of the Solar Photo-Fenton Process for Wastewater Treatment and Pharmaceuticals Mineralization at Semi-Industrial Scale. Sci Total Environ. 2018;612:605–612.
23. Sopaj F, Oturan N, Pinson J, Podvorica FI, Oturan MA. Effect of Cathode Material on Electro-Fenton Process Efficiency for Electrocatalytic Mineralization of the Antibiotic Sulfamethazine. Chem Eng J. 2020;384:123249.
24. Yang C-W, Hsiao W-C, Chang B-V. Biodegradation of Sulfonamide Antibiotics in Sludge. Chemosphere. 2016;150:559–565.
25. Zhou C, Zeng Z, Zeng G, et al. Visible-Light-Driven Photocatalytic Degradation of Sulfamethazine by Surface Engineering of Carbon Nitride: Properties Degradation Pathway and Mechanisms. J Hazard Mater. 2019;380:120815.
26. Fukahori S, Fujiwara T. Photocatalytic Decomposition Behavior and Reaction Pathway of Sulfamethazine Antibiotic Using TiO2
. J Environ Manage. 2015;157:103–110.
27. Krasner SW, Westerhoff P, Chen B, Rittmann BE, Amy G. Occurrence of Disinfection Byproducts in United States Wastewater Treatment Plant Effluents. Environ Sci Technol. 2009;43:8320–8325.
28. Sun X, Zhang Q, Liang H, Ying L, Xiangxu M, Sharma VK. Ferrate(VI) as a Greener Oxidant: Electrochemical Generation and Treatment of Phenol. J Hazard Mater. 2016;319:130–136.
29. Sharma VK, Chen L, Zboril R. Review on High Valent FeVI (Ferrate): A Sustainable Green Oxidant in Organic Chemistry and Transformation of Pharmaceuticals. ACS Sust Chem Eng. 2016;4:18–34.
30. Han Q, Dong W, Wang H, Liu T, Tian Y, Song X. Degradation of Tetrabromobisphenol A by Ferrate(VI) Oxidation: Performance Inorganic and Organic Products Pathway and Toxicity Control. Chemosphere. 2018;198:92–102.
31. Sailo L, Tiwari D, Lee S-M. Degradation of Some Micro-Pollutants from Aqueous Solutions Using Ferrate (VI): Physico-Chemical Studies. Sep Sci Technol. 2017;52:2756–2766.
32. Ghernaout D, Naceur MW. Ferrate(VI): In Situ Generation and Water Treatment - A Review. Desalin Water Treat. 2011;30:319–332.
33. Sharma VK. Oxidative Transformations of Environmental Pharmaceuticals by Cl2 ClO2 O3 and Fe(VI): Kinetics Assessment. Chemosphere. 2008;73:1379–1386.
34. Zajíček P, Kolář M, Prucek R, et al. Oxidative Degradation of Triazine- and Sulfonylurea-Based Herbicides Using Fe(VI): The Case Study of Atrazine and Iodosulfuron with Kinetics and Degradation Products. Sep Purif Technol. 2015;156:1041–1046.
35. Sharma VK. Disinfection Performance of Fe(VI) in Water and Wastewater: A Review. Water Sci Technol. 2007;55:225–232.
36. Liu Y, Wang L, Huang Z, et al. Oxidation of Odor Compound Indole in Aqueous Solution with Ferrate (VI): Kinetics Pathway and the Variation of Assimilable Organic Carbon. Chem Eng J. 2018;331:31–38.
37. Lee Y, Yoon J, von Gunten U. Kinetics of the Oxidation of Phenols and Phenolic Endocrine Disruptors during Water Treatment with Ferrate (Fe(VI)). Environ Sci Technol. 2005;39:8978–8984.
38. Jiang JQ, Yin Q, Zhou JL, Pearce P. Occurrence and Treatment Trials of Endocrine Disrupting Chemicals (EDCs) in Wastewaters. Chemosphere. 2005;61:544–550.
39. Sharma VK, Li XZ, Graham N, Doong R. Ferrate(VI) Oxidation of Endocrine Disruptors and Antimicrobials in Water. J Water Supply: Res Technol Aqua. 2008;57:419–426.
40. Yang B, Ying G-G, Zhao J-L, Liu S, Zhou L-J, Chen F. Removal of Selected Endocrine Disrupting Chemicals (EDCs) and Pharmaceuticals and Personal Care Products (PPCPs) during Ferrate(VI) Treatment of Secondary Wastewater Effluents. Water Res. 2012;46:2194–2204.
41. Tiwari D, Kim H-U, Choi B-J, et al. Ferrate(VI): A Green Chemical for the Oxidation of Cyanide in Aqueous/Waste Solutions. J Environ Sci Health Part A. 2007;42:803–810.
42. Tiwari D, Sailo L, Pachuau L. Remediation of Aquatic Environment Contaminated with the Iminodiacetic Acid Metal Complexes Using Ferrate(VI). Sep Purif Technol. 2014;132:77–83.
43. Pachuau L, Lee SM, Tiwari D. Ferrate(VI) in Wastewater Treatment Contaminated with Metal(II)-Iminodiacetic Acid Complexed Species. Chem Eng J. 2013;230:141–148.
44. Tiwari D, Kim H-U, Lee S-M, Yang J-K, Kim H. Ferrate(VI) for waste water treatment : oxidation of cyanide in aqueous medium. Environ Eng Res. 2006;11:318–324.
45. Shiota Y, Kihara N, Kamachi T, Yoshizawa KA. Theoretical Study of Reactivity and Regioselectivity in the Hydroxylation of Adamantane by Ferrate(VI). J Org Chem. 2003;68:3958–3965.
46. Sharma VK. Oxidation of Nitrogen-Containing Pollutants by Novel Ferrate(VI) Technology: A Review. J Environ Sci Health Part A. 2010;45:645–667.
47. Sun S, Liu Y, Ma J, et al. Transformation of Substituted Anilines by Ferrate(VI): Kinetics Pathways and Effect of Dissolved Organic Matter. Chem Eng J. 2018;332:245–252.
48. Jia Y, Liu J, Liu S, et al. Magnetically separable Au-TiO2/nanocube ZnFe2O4 composite for chlortetracycline removal in wastewater under visible light. J Ind Eng Chem. 2017;47:303–314.
Fig. 1Degradation of ferrate (VI) in presence of various concentration of sulfamethazine at pH 6.0. 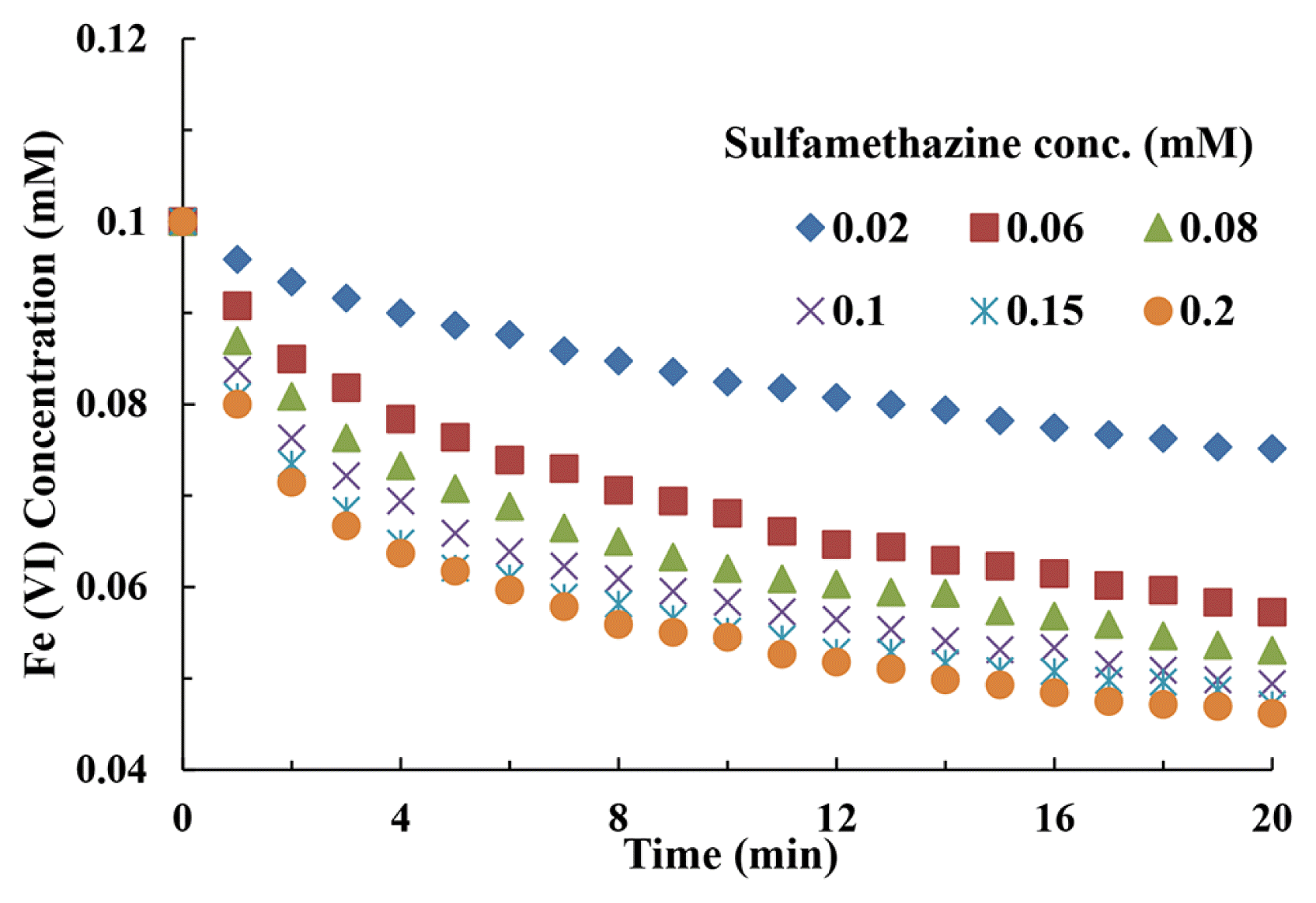
Fig. 2(a) Plot of 1/(a–x) vs. time ‘t’ for the degradation of ferrate (VI) in presence of sulfamethazine at pH 6.0 [Ferrate (VI)]: 0.10 mM; [SMZ]: 0.10 mM and ‘a’ and ‘x’ are initial and final concentration of sulfamethazine at time ‘0’ and ‘t’ min, respectively; and (b) Variation of k (pseudo-2nd-order rate constant) vs sulfamethazine concentration at pH 6.0. 
Fig. 3Removal efficiency of sulfamethazine as a function of sulfamethazine concentration at pH 6.0 and [Fe(VI)] = 0.1 mM. 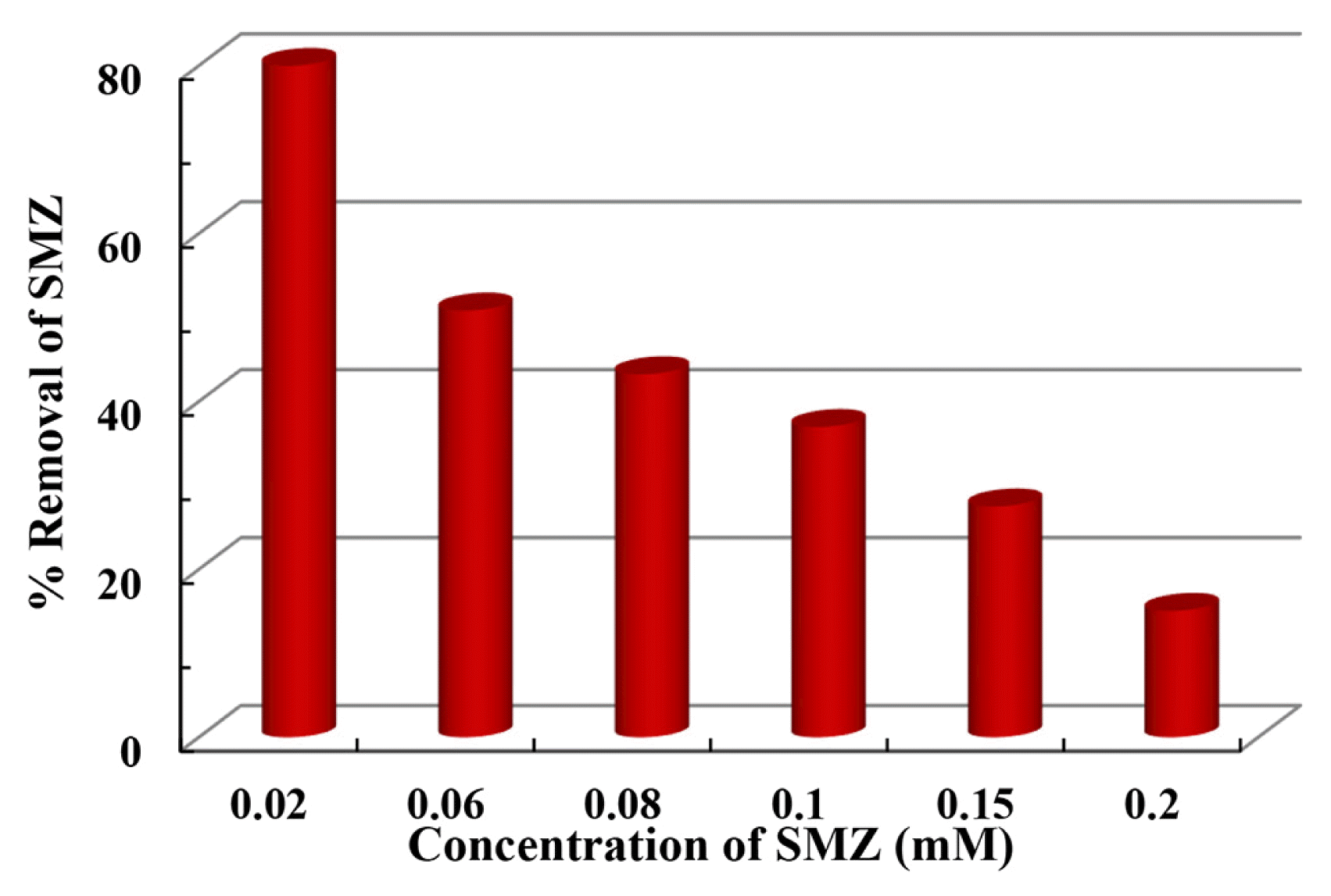
Fig. 4(a) Degradation of ferrate (VI) concentration (0.2 mM) as a function of pH at constant concentration of SMZ (0.1 mM); and (b) Percentage removal of sulfamethazine as a function of pH ([SMZ] = 0.1 mM, [Fe(VI)] = 0.2 mM). 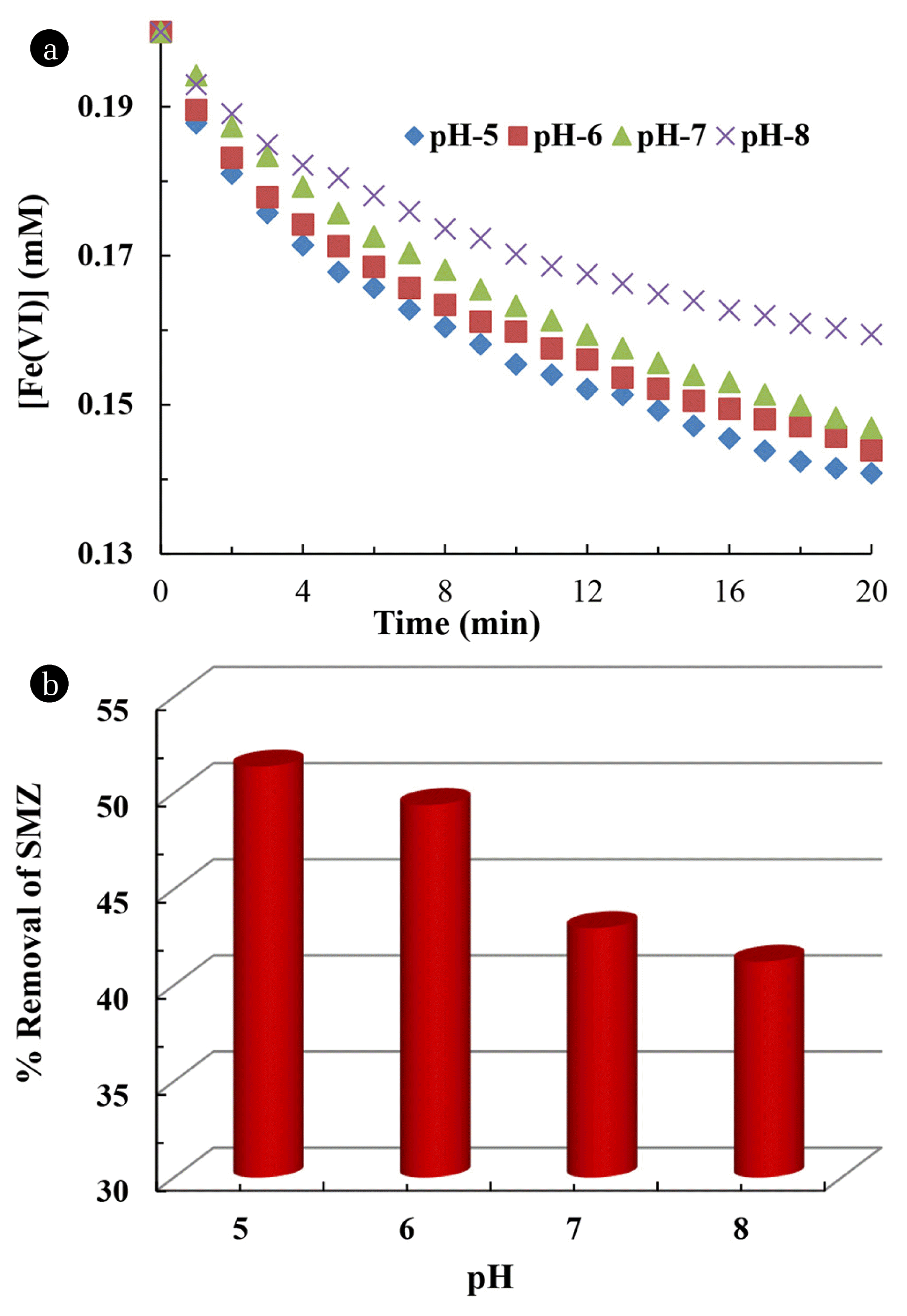
Fig. 5Oxidative degradation of sulfamethazine in presence of co-existing ions [Concentration of SMZ: 0.1 mM; Concentration of co-existing ions: 0.5 mM; pH: 6.0]. 
Fig. 6(a) Percentage removal of sulfamethazine; and (b) extent removal of sulfamethazine as a function of pH at [SMZ] = 0.1 mM; [Fe(VI)]: 0.2 mM. 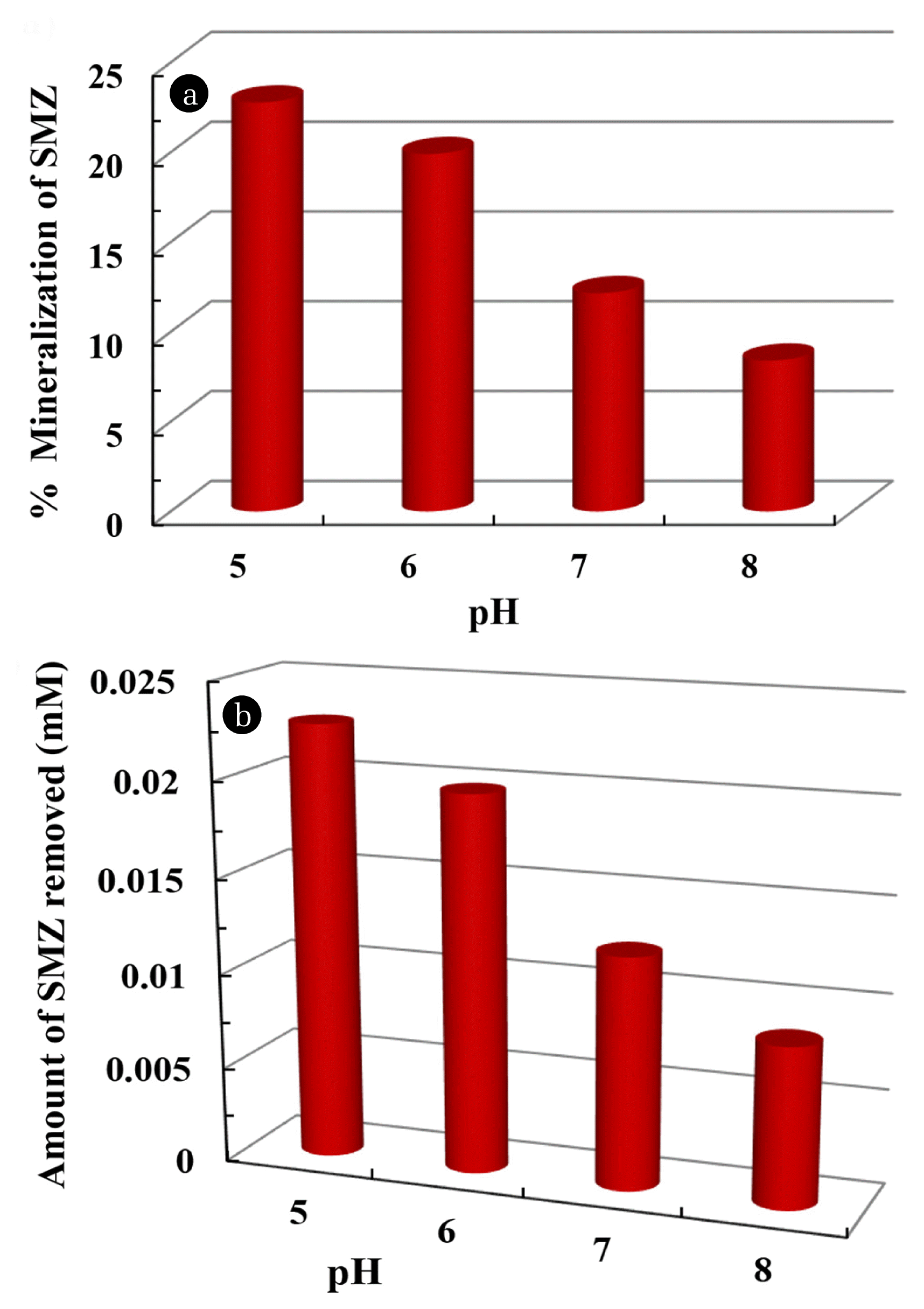
Fig. 7Percentage removal of sulfamethazine in real and distilled water as a function of pH [SMZ] = 0.1 mM; [Fe(VI)] = 0.1 mM. 
Table 1Values of Pseudo-2nd-order Rate Constant Obtained in the Degradation of Ferrate (VI) in Presence of SMZ at pH 6.0 |
|
||||||||||||||||||||||||||||||||||||