AbstractPolyethyleneimine and carbon disulfide were employed to modify alkaline lignin (AL) in order to introduce −NH, −NH2 and −CSS-groups. Its adsorption performance towards Cu(II) was evaluated based on the residual concentration (Cr) against the discharge permissible concentration for Cu(II) (2.0 mg/L) established by the World Health Organization. The evaluation was operated under different conditions such as adjusting the solution pH, Cu(II) coexisting with K(I), Na(I), Ca(II) or Mg(II), changing the contact time (t) and initial concentration of Cu(II) (C0), as well as regeneration. When t and C0 equaled to 180 min and 50 mg/L, Cr was less than 2.0 mg/L at pH of 5.03 to 5.65. Under the situation of Cu (II) coexisting with 50 mg/L of K(I), Na(I), Ca(II) or Mg(II), Cr was as low as 1.96, 1.53, 1.97 or 1.55 mg/L (t = 180 min, C0 = 50 mg/L), respectively. Even the modified AL was regenerated and reused four times, Cr was closed to 2 mg/L for each time (t = 180 min, C0 = 51 mg/L). By comparing with other lignin-based materials according to the adsorption equilibrium time, the maximum adsorption capacity, and Cr, the modified AL exhibited higher application potential in copper-loaded water treatment.
1. IntroductionWater body loaded with heavy metals is an important environmental problem due to the heavy metals could be bio-accumulated and further cause ecological damage [1]. Although copper is important to human beings, it also injures health at high concentration [2]. After enriching via food chain, the excess Cu(II) could be assimilated into human body and trigger various diseases of gastrointestinal discomfort, liver and kidney damage [3, 4]. The discharge permissible concentration for Cu(II) in drinking water is 2.0 mg/L according to the World Health Organization (WHO) [5]. In China, the three-level discharged concentration of Cu(II) from wastewater is ruled as 2.0 mg/L (GB 8978-1996). However, the concentrations of Cu(II) in most industrial wastewater far exceed 2.0 mg/L. For example, the concentration of Cu(II) is approximately 50 mg/L discharged from electroplating industry [6] and 20 mg/L in washing water of circuit board production [6]. Therefore, it is necessary to control the contamination of Cu(II) in the aquatic environment. By far, various approaches have been developed for removing heavy metals from aqueous solution, such as coagulation flocculation [7], precipitation filtration [8], solvent extraction [9], reverse-osmosis [10] and adsorption [11]. Of these technologies, adsorption could be used as a promising method, attributing to the advantages of easy accessibility and cost effectiveness [12]. Currently, considerable research has focused on designing and fabricating a series of adsorbents with chemical activity and versatile functional groups by using biomass as raw materials due to their extensive sources, environmental friendliness and low cost [13].
Lignin is the second largest biomass after cellulose in the nature [14, 15]. As an industrial by-product, lignin is generated from pulping and bio-refinery on a large scale [16]. It was reported that approximately 70 million tons of lignin was produced from pulping worldwide in 2017 [17, 18]. Lignin can be exploited as a kind of adsorbent for removing heavy metals from aqueous solution [17, 19–21], but before the practical use lignin needs to be modified. For instance, Zhang et al. [22] used monochloroacetic acid and amine reagents as modifiers to make lignin carry oxygen- and nitrogen-containing functional groups including hydroxy (−OH), carboxyl (−COOH) and amine (−NH2). The modified lignin reduced 50 mg/L Cu(II) to 15 mg/L after 720 min [22]. Xu et al. [23] fabricated a kind of aminated lignin, in which amine substance was used to ornament lignin and consequently the nitrogen-containing groups of −NH and −NH2 were introduced; the aminated lignin reduced 50 mg/L Cu(II) to 8.5 mg/L after 360 min. Chakraborty and Tare [24] adopted xanthates to modify lignin with the functional groups of −CSS- and −COC, and then treated Cu(II)-loaded water (20 mg/L). After 720 min, approximately 16 mg/L of Cu(II) was detected in aqueous solution. Although these modified lignins could remove a certain percentage of Cu(II), all the residual Cu(II) still exceeded the discharge standard of 2.0 mg/L.
In our previous work, polyethyleneimine (PEI) and carbon disulfide (CS2) were applied as the reagents to modify alkaline lignin (named as AL-PEI-CS2), in which −NH, −NH2 and −CSS- groups functionalized the network of alkaline lignin [25]. The removal efficiency of Cu(II) was investigated, in which seven metal ions including Cu(II), Pb(II), Cd(II), Zn(II), Co(II), Ca(II) and Mg(II) coexisted in aqueous solution (pH = 6.0). All the initial concentrations for the seven ions were set at 2.0 mg/L. After 300 min, it was found that the residual concentration of Cu(II) was only 0.06 mg/L, far below the discharge permissible concentration (2 mg/L). However, the adsorption mechanism of AL-PEI-CS2 towards Cu(II) has not been studied systematically. In this work, the adsorption kinetics, adsorption isotherm, the binding interaction between AL-PEI-CS2 and Cu(II), as well as the reusing ability were investigated with the initial concentration of Cu(II) closed to that in real wastewater and the residual concentration of Cu(II) against WHO’s standard. This work aims at preparing a kind of modified alkaline lignin for treatment of Cu(II)-loaded wastewater and providing an insight into the treatment of wastewater contaminated by heavy metals by the biomass-based adsorbent.
2. Chemicals and Methods2.1. ChemicalsThe analytical grade of AL was brought from Bailaibo Technology Co. LTD (Beijing, China). PEI (branched, Mw = 70,000, 30 w/v aqueous solution) was obtained from Shanghai Xinrui Biotechnology Co., LTD (Shanghai, China). Other analytical grade reagents of sodium hydroxide (NaOH), hydrochloric acid (HCl), formaldehyde (HCHO), CS2 and ethanol were all provided by Chongqing Zinguang Chemical Co. LTD (Chongqing, China). Standard solutions (all in 1,000 mg/L) of copper nitrate (Cu(NO3)2), potassium nitrate (KNO3), calcium nitrate (Ca(NO3)2), sodium nitrate (NaNO3) and magnesium nitrate (Mg(NO3)2) were all supplied by the Guangzhou Analysis test center Keli Technology Development Co. LTD (Guangdong, China). Ultrapure water was prepared from a FAMO-10 equipment (resistivity = 18.25 mΩ-cm, Nanjing Quankun Biotechnology Co. LTD, China).
2.2. Preparation of AL-PEI-CS2 and CharacterizationThe modified alkaline lignin, named as AL-PEI-CS2, was prepared via the same method as described in our previous work [25]. AL was employed as the raw material and two reagents (PEI and CS2) as the modifiers in order to introduce nitrogen- and sulfur-containing functional groups. Briefly, three grams of AL were dissolved into 90 mL of ultrapure water in a 300-mL three-neck flask. The solution pH was adjusted at 13.0 via 0.1 mol/L of NaOH. The flask was placed on a magnetic stirring apparatus for agitating 30 min at room temperature. Ten milliliters of HCHO and 10 mL of PEI were dropwise added into the flask, in which the temperature of the apparatus was set at 90°C for sustaining 5 h and then cooled to 40°C. CS2 (15 mL) was added into the flask drop by drop. After 3 h, the precipitates in the flask were collected by centrifugation (TD-4M, Jinan Oulaibo Scientific Instrument Co., Ltd, China) at 5,000 rpm and 25°C. Ethanol and ultrapure water were used for washing the precipitates until the filtrate pH ranged from 6.0 to 7.0. The precipitates were dried via vacuum freeze dryer (FD-1A-50, Jiangsu Tianling Instrument Co., Ltd, China) at −60°C for 6 h. Finally, a kind of red-brown powders was obtained (AL-PEI-CS2) and stored in a centrifuge tube (Φ = 2.5 cm, Jinan Oulaibo Electronic Commerce Co., Ltd) at room temperature for further use.
The morphology of AL and AL-PEI-CS2 (coated with Au powders) was analyzed through scanning electron microscope energy (VEGAIIXMU, Germany) coupled with dispersive spectroscopy (OXFORD, England) (SEM-EDS). Fourier transform infrared spectroscopy (FT-IR) (Nicolet NEXUS 750, USA) was operated under the condition of resolution 4 cm−1 and scans number 16. X-ray photoelectron spectroscopy (XPS) spectra was run by using Mg and Kα as X-ray sources (1253.6 eV protons) (Thermo Scientific K-Alpha, USA). All the binding energies of XPS spectra were calibrated based on C 1s at 284.6 eV. Zeta potential (Malvern Zetasizer Nano S90, England) was conducted by adding 10 mg of AL-PEI-CS2 into 10 mL of aqueous solutions (pH = 1.0, 3.0, 4.0, 5.0, 6.0 and 9.0); after ultrasonic for 1 h and standing for 24 h, the samples of the solutions were measured. The specific surface areas were analyzed at 77 K via N2 adsorption-desorption isotherms (ASAP2460, McMuratik Instrument Co., Ltd, USA).
2.3. Adsorption Performance Evaluation2.3.1. Monitoring the residual concentration of Cu(II) at different solution pHThe initial concentration of Cu(II) was 50 mg/L. The values of solution pH varied from 1.0 to 6.0 adjusting by 0.1 mol/L of HCl or NaOH. Fifty milliliters of Cu(II) solution and 0.0500 g of AL-PEI-CS2 were put into a 250-mL conical flask, and then covered with parafilm (Huimin Tengjie Plastic Industry Co. Ltd, China). The flasks were placed on a shaker (HYQ45, Wuhan Huicheng Biological Technology CO. Ltd, China) at 25°C and 140 rpm. After 180 min, 5 mL of sample was withdrawn (Finnpipette™ F1, Thermo Fisher Scientific Co., Ltd, USA) and passed through 0.45 μm nylon membrane filter (Guangzhou Tongpu Experimental Instrument Co., Ltd, China). The filtrate was collected for monitoring the residual concentration of Cu(II) via the inductively coupled plasma optical emission spectrometry (ICP-OES) (Thermo, USA). Three parallel tests were carried out and error bars were used to analyze the data depicting 95% confidence intervals. The control experiments were performed in the absence of AL-PEI-CS2 to determine if the loss in Cu(II) happened through other pathways (e.g., adsorption on the flask wall, evaporation, or sorbed by the filter paper).
2.3.2. Monitoring the residual concentration of Cu(II) at the presence of cationsFour cations of K(I), Na(I), Ca(II) and Mg(II) were used. As for each cation, 2.5 or 5 mL of standard solution (1,000 mg/L) was mixed with Cu(II) solution (2.5 mL, 1,000 mg/L) in a 250-mL conical flask. The mixed aqueous solution was diluted to be 50 mL with adjusted pH as 5.03, in which C0 of Cu(II) was obtained as 50 mg/L. The value of C0 for each cation was 50 and 100 mg/L, respectively. Then 0.0500 g of AL-PEI-CS2 was added into the flask for the adsorption experiments. Three parallel tests were carried out and error bars were used to analyze the data depicting 95% confidence intervals. Other operations were the same as those mentioned in Section 2.3.1.
2.3.3. Monitoring the residual concentration of Cu(II) under different contact times and initial concentrationsFifty milliliters of Cu(II) solutions (5, 10, 15, 20, 30 and 50 mg/L) and 0.0500 g of AL-PEI-CS2 were put into a 250-mL conical flask, respectively. All the solution pH values were set at 5.03. The flasks were placed on a shaker at 25°C and 140 rpm. At certain intervals, 5 mL of the sample was withdrawn. Three parallel tests were carried out and error bars were used to analyze the data depicting 95% confidence intervals. Other operations were the same as those mentioned in Section 2.3.1.
Adsorption kinetics and isotherm were shown in Text S1 in the Supplementary Materials (SI).
2.3.4. Monitoring the residual concentration of Cu(II) during the regeneration processFifty milliliters of Cu(II) solution (C0 = 51 mg/L, pH = 5.03) and 0.0500 g of AL-PEI-CS2 were added into a 250-mL conical flask and then covered with parafilm. The flask was placed into a shaker at 25°C and 140 rpm. After 180 min, all the things in the flask were poured out and passed through filter paper (Eco Safety Technology Co., Ltd, China) for collecting Cu(II)-loaded AL-PEI-CS2 powder. Ultrapure water was used to wash Cu(II)-loaded AL-PEI-CS2 powder until no detection of Cu(II) in filtrate. Cu(II)-loaded AL-PEI-CS2 powder was dried in air for 12 h at ambient temperature (~25°C) and then used for monitoring the residual concentration of Cu(II) during the regeneration process and adsorption mechanism, respectively.
For monitoring the residual concentration of Cu(II) during the regeneration process, the Cu(II)-loaded AL-PEI-CS2 powder was mixed with 50 mL of HCl solution (0.2 mol/L) in a 250-mL conical flask under 140 rpm at 25°C for regeneration. After 180 min, all the things in the flask were poured out and passed through filter paper for collecting the regenerated powder. Ultrapure water was used to wash the regenerated powder until filtrate pH was in the range of 5.0 to 6.0. Then the regenerated powder (0.0500 g) was mixed with 50 mL of Cu(II) solution (C0 = 51 mg/L, pH = 5.03) in a-250 mL conical flask. The conical flask was covered with parafilm and shaken at 25°C and 140 rpm. After 180 min, the sample was withdrawn with 5 mL pipette and filtered through 0.45 μm nylon membrane. The filtrate was used for the analysis of residual Cu(II) concentration. The number for the adsorption-desorption cycle was four. Three parallel tests were carried out and error bars were used to analyze the data depicting 95% confidence intervals.
where
is the equilibrium adsorption capacity of Cu(II) for fresh AL-PEI-CS2;
is the equilibrium adsorption capacity for regenerated AL-PEI-CS2 at each cycle.
2.4. Adsorption MechanismCu(II)-loaded AL-PEI-CS2 powder (AL-PEI-CS2-Cu(II) complexes) was analyzed by SEM-EDS, FT-IR and XPS. The powder was ground and squashed with KBr pellets for FT-IR spectra with resolution being 4 cm−1 and scans number being 16. XPS used Mg and Kα as X-ray sources (1,253.6 eV protons). The experimental steps can be seen from Section 2.3.4.
2.5. CalculationThe adsorption capacity (qt) and removal efficiency (%) of Cu(II) were calculated by the following equations:
where C0 (mg/L) is initial concentration of Cu(II); Cr (mg/L) is the residual concentration of Cu(II) at time t; V (L) and M (g) are the volumes of Cu(II) solution and the mass of AL-PEI-CS2, respectively.
3. Results and Discussion3.1. Characterization of the Modified Alkaline LigninAs presented in Fig. S1(a), the color of the raw AL was black while changed into yellow-brown after modification. SEM pictures exhibited that AL was composed of large blocks whereas the particles of AL-PEI-CS2 were relatively small and incompact (Fig. S1(b)). According to N2 absorption-desorption isotherm (Fig. S1(c)), the BET specific area of AL-PEI-CS2 was calculated to be 1.656 m2/g, almost 16 times higher than that of raw AL (0.102 m2/g). FT-IR spectra displays that AL contained the functional groups of −OH (3,431 cm−1) [19] and −OCH/H-C-H (2,935/2,840 cm−1) [26] as well as aromatic ring skeleton (1,592/1,509 cm−1) [27] (Fig. 1(a)). As for AL-PEI-CS2, four new peaks corresponding to −NH2 (1,662 cm−1) [28], C-N (1,366 cm−1) [29], C=S (1,081 cm−1) [30] and C-S (855 cm−1) [31] were recorded. In addition, the stretching vibration of −NH can also be discovered at 3,431 cm−1 for AL-PEI-CS2 [19]. Besides FT-IR, the technology of XPS was adopted. Fig. 1(b) shows full scan survey XPS spectra for AL and AL-PEI-CS2. Different from the three peaks of C 1s, O 1s and S 2p carried by AL, one new peak of N 1s was detected in AL-PEI-CS2 (Fig. 1(b)). As for the discovery of S 2p in AL, it was due to the usage of sodium sulfide (Na2S) during its preparation [20]. As depicted in Fig. 1(c), the spectra of O 1s were determined as C-O (532.6 eV) [32] and O-H (531.7 eV) [32]. According to Fig. 1(d), N 1s spectra corresponded to N-CS2 (403.9 eV) [33, 34] and −NH/-NH2 (399.5 eV) [19]. Fig. 1(e) was the binding energy of S 2p, which belonged to C=S (168.6 eV) [33] and C-S (163.2 eV) [33]. Considering the results from FT-IR and XPS, the nitrogen-, sulfur- and oxygen-containing groups of the modified alkaline lignin were determined as −OH, −NH, −NH2, and −S-C=S- (−CSS-), which would serve as the adsorption sites for removal of metal ions from aqueous solution.
3.2. Evaluation on Adsorption Performance of the Modified Alkaline Llignin3.2.1. Residual concentration of Cu(II) at different solution pHAs for effluent discharged from mine wastewater, electroplating wastewater and washing water of circuit board production, the pH value generally ranges from 2.0 to 5.0 [35]. Therefore, the solution pH in this study was set at 1.0 to 6.0. As seen from zeta potential curve (Fig. 2(a)), surface of the modified alkaline lignin (AL-PEI-CS2) would be positively charged at solution pH < 3.1 while negatively charged at solution pH > 3.1 (pHpzc = 3.1). Fig. 2(b) exhibits the dissociation forms of copper at different solution pH values, in which Cu2+ ions exist as the main species accompanied by a little amount of CuOH+ ions when pH value is less than 6.0. It can be assumed that electrostatic attraction would occur between AL-PEI-CS2 and Cu(II) when the solution pH varies from 3.1 to 6.0. The higher solution pH is, the stronger the electrostatic attraction happens. The adsorption ability of AL-PEI-CS2 towards Cu(II) is supposed to follow the sequence (in pH) of 6.0 > 5.0 > 4.0 > 3.0 > 1.0. In order to verify such a sequence, the adsorption experiments for Cu(II) over AL-PEI-CS2 at different pH values were conducted.
As displayed in the insert of Fig. 2(a), the adsorption capacity (qt) and the removal efficiency was 45 mg/g and 92% for Cu(II) at pH of 1.30 and then increased to 49 mg/g and 98% at pH of 5.65 when the initial concentration (C0 ) of Cu(II) was 50 mg/L and the contact time was 180 min, respectively. The possible reason for this result was the excess protons (H+) resulted in the protonation of the functional groups carried by AL-PEI-CS2 at lower pH, and consequently the electronic attraction between Cu2+ ions and AL-PEI-CS2 weakened, whereas the deprotonation occurred to improve the adsorption at a higher pH value [36, 37].
In order to verify the change in solution pH before and after adsorption, the values of pHinitial were set as 1.30, 3.15, 4.14, 5.03 and 5.65. When pHinitial equaled to 1.30, the value of pHfinal was measured to be 1.33 (insert in Fig. 2(b)). As shown in Fig. 2(a), at the condition of pH lower than pHpzc (3.1), the surface of AL-PEI-CS2 is charged positively, which means that H+ pronated the functional groups of −OH, −NH, −NH2 and −CSS- [36, 37] and consequently the amount of protons in solution decreased. This might explain why the value of pHfinal (1.33) was higher than that of pHinitial (1.30). When pHinitial was set at 3.15, 4.14, 5.03 and 5.65, the values of pHfinal ascended to 4.65, 5.35, 5.85 and 6.01. The possible reason is that at the condition of pH higher than pHpzc (3.1) the negatively charged AL-PEI-CS2 would interact with proton via electronic attraction, and therefore, result in the increase in pHfinal. Interestingly, the difference between pHfinal and pHinitial was 1.5, 1.21, 0.82 and 0.36 (insert in Fig. 2(b)), suggesting more protons retained in solution at higher pHinitial. The decreasing trend might attribute to increasing CuOH+ amounts at relatively higher initial solution pH (Fig. 2(b)). CuOH+ might compete with proton and interact with AL-PEI-CS2.
The residual concentration of Cu(II) (Cr) was measured to be 4.55, 3.95, 2.8, 1.2 and 1.0 mg/L corresponded to solution pH of 1.30, 3.15, 4.14, 5.03 and 5.65. It was discovered that when solution pH was limited in the range of 5.03 to 5.65, the residual concentration of Cu(II) in the effluent can meet with the standard of 2 mg/L regulated by WHO and Chinese government. Ge et al. [19] adopted a kind of amino and sulfonic ornamented lignin (ASL) as the adsorbent, in which the removal efficiency of Cu(II) was 60%, 61%, 62%, 63% and 70% when the value of solution pH was 3.0, 4.0, 5.0, 6.0 and 7.0 (Fig. 2(c)), respectively. Accordingly, the Cr value of Cu(II) at 90 min was 20, 19.5, 19, 18.5 and 15 mg/L with C0 of 50 mg/L (Fig. 2(c)) [19]. Todorciuc et al. [38] employed the raw lignin derived from wheat straw to adsorb Cu(II) (C0 = 38.6 mg/L) (Fig. 2(c)). The residual concentration was 36.1, 28.6, 26.6 and 3.6 mg/L with solution pH being 2.0, 3.0, 4.0 and 6.0 as well as t being 180 min (Fig. 2(c)) [38], respectively. Xu et al. [23] used a kind of aminated lignin (AL) to remove Cu(II) from water. The value of Cr for Cu(II) at 150 min was detected to be approximately 70, 68, 65, 57, 45 and 34 mg/L corresponded to the solution pH of 3.0, 3.5, 4.0, 4.5, 5.0 and 5.5 as C0 of Cu(II) was 100 mg/L (Fig. 2(c)), respectively. As seen from these results, it can be concluded that the removal ability of AL-PEI-CS2 for Cu(II) is ideal at a wide pH range from 1.0 to 6.0, especially at pH of 5.0 and 6.0.
3.2.2. Residual concentration of Cu(II) at the presence of cationsThe influences of K(I), Na(I), Ca(II) and Mg(II) on the removal of Cu(II) by AL-PEI-CS2 were conducted as these cations naturally exist in the aqueous environment. As displayed in Fig. S2, the dominant speciation of K(I), Na(I), Ca(II) and Mg(II) is K+, Na+, Ca2+ and Mg2+ when solution pH is less than 8.0, respectively. These cations might compete with Cu(II) during the adsorption process. In this research, the initial concentrations of K(I), Na(I), Ca(II) and Mg(II) were set at 0, 50 and 100 mg/L since these ions exist in the natural aqueous environment with the concentration of 0.2 to 167.4 mg/L [39]. Fig. S3 shows that the removal capacity of AL-PEI-CS2 towards Cu(II) was not obviously affected by K(I), Ca(II), Na(I) and Mg(II). Even coexisted with the four cations, all the residual concentrations of Cu(II) almost meet with the standard of 2.0 mg/L regulated by WHO and Chinese government (Fig. 3(a)).
Fig. 3(a) compares the Cu(II) Cr between AL-PEI-CS2 and nanotubes synthesized by Liu et al. [40]. It was found that when the initial concentrations of these cations, the initial concentration of Cu(II) and the contact time were close, the Cu(II) Cr for nanotubes were almost 20 to 30 times higher than those for AL-PEI-CS2 and far beyond the discharged standard of 2 mg/L. Similar phenomenon was also observed for hydrogel prepared by Zhang et al. [41] (Fig. 3(b)).
As for the three adsorbents (AL-PEI-CS2, nanotubes and hydrogel), the monovalent cations including Na(I) and K(I) slightly affected the value of Cr while the influences of bivalent cations such as Ca(II) and Mg(II) were higher. The hydration free energy follows the order of K+ (−1,095 kJ/mol) > Na+ (−1,097 kJ/mol) > Ca2+ (−1,588 kJ/mol) > Mg2+ (−1,900 kJ/mol) [42, 43]. Cheng et al. [44] pointed out that higher hydration free energy tends to keep metal ions in aqueous solution, that is, prevents the transfer of the ions from bulk solution to the adsorbent surface. Therefore, Ca(II) and Mg(II) are easier to attach on the surfaces of AL-PEI-CS2, nanotubes and hydrogel than Na(I) and K(I), and consequently, competition of bivalent cations for adsorption would be more fierce.
3.2.3. Residual concentration of Cu(II) under different contact times and initial concentrations
Fig. 4(a) shows that all the values of adsorption capacity (qt) increased significantly from 0 to 60 min and then slightly from 60 to 120 min. After 120 min, the values of qt kept almost unchanged even the contact time was prolonged to 180 min. The equilibrium time was determined as 180 min. According to the insert in Fig. 4(a), the adsorption efficiencies of Cu(II) over AL-PEI-CS2 reached 93% (C0 = 5 mg/L), 95% (C0 = 10 mg/L), 95% (C0 = 15 mg/L), 95% (C0 = 20 mg/L), 94% (C0 = 30 mg/L), and 96% (C0 = 50 mg/L) at 180 min, respectively. The values of Cr was observed to be as low as 1.72, 0.98, 1.15, 1.77, 1.76 and 1.86 mg/L corresponding to C0 of 5, 10, 15, 20, 30 and 50 mg/L at the contact time of 20, 40, 90, 90, 180 and 180 min (Fig. 4(b)), respectively. These results demonstrated that all the Cu(II) Cr can meet with the standard of 2 mg/L within the initial concentration range of 0 to 50 mg/L. As for lower C0 (5 and 10 mg/L), even at the initial stage (0 to 40 min) the values of Cr were less than 2 mg/L. It was noted here that at the equilibrium time of 180 min, the Cu(II) Cr was finally determined to be 0.32, 0.45, 0.65, 0.94, 1.76 and 1.86 mg/L when the initial concentration was 5, 10, 15, 20, 30 and 50 mg/L, respectively.
Descriptions on the adsorption kinetics and isotherm of Cu(II) onto AL-PEI-CS2 were given in Text S1. As displayed in Text S1, it showed that experimental data were well fitted by pseudo-second-order kinetic, suggesting the adsorption of Cu(II) over AL-PEI-CS2 was chemisorption. Boyd’s film-diffusion model proved the adsorption rate of Cu(II) onto AL-PEI-CS2 was determined by film diffusion. The maximum adsorption capacity (qm) of Cu(II) was obtained as 58 mg/g by using the Langmuir isothermal model. Table 1 compares the adsorption capacity for different lignin-based adsorbents towards Cu(II) according to qm values and the contact time to reach equilibrium, while Table 2 according to the residual concentration of Cu(II) at equilibrium. As seen from in Table 1, the value of qm for AL-PEI-CS2 was higher than adsorbents VI to XII by approximately 1.04, 1.27, 1.36, 3.25, 6.34, 6.87 and 207 times, respectively. It was observed that qm of AL-PEI-CS2 was lower than adsorbents II to IV, whereas the contact time to reach equilibrium for adsorbents II to IV was 4 to 16 times longer than that of AL-PEI-CS2. Although adsorbent V presented a shorter equilibrium time and a larger qm compared to AL-PEI-CS2, the value of Cr was as high as 25 mg/L (Table 2). Under similar initial concentrations of Cu(II), all the values of Cr for adsorbents II to XII were higher than that of AL-PEI-CS2 (Table 2). These results demonstrated that comprehensively considering the values of qm, equilibrium time, and Cr, AL-PEI-CS2 exhibited a relatively ideal application potential.
3.2.4. Residual concentration of Cu(II) during the regeneration processIn order to evaluate the reusing ability of AL-PEI-CS2, a four-adsorption-desorption-cycle was designed. As presented in Fig. S5, the values of qe was 49.9, 49.1, 48.9, 49.0 and 48.8 mg/g for fresh AL-PEI-CS2 and the regenerated AL-PEI-CS2 at cycles 1 to 4, respectively. Compared to fresh AL-PEI-CS2, the decrease ratio in the qe value corresponding to cycles 1 to 4 was only 1.6%, 2.0%, 1.8% and 2.2%, respectively. Excitingly, the Cu(II) Cr was as low as 1.9, 2.1, 2.0 and 2.2 mg/L for cycles 1 to 4, basically meeting with the maximum acceptable limit (2 mg/L) regulated by WHO and Chinese government. These results illustrated that the reusing ability of AL-PEI-CS2 is acceptable.
3.3. Adsorption MechanismSEM-EDS exhibits the uptake of Cu(II) by AL-PEI-CS2 (Fig. S6). FT-IR spectra of AL-PEI-CS2 and AL-PEI-CS2-Cu(II) complexes were displayed in Fig. 5(a). Compared to AL-PEI-CS2, the peaks centered at 1,662 (−NH2) and 855 cm−1 (C-S) weakened for AL-PEI-CS2-Cu(II) complexes, indicating that the groups of −NH2 and −CSS- were responsible for the uptake of Cu(II). XPS technology was employed to further confirm the adsorption mechanism. Fig. 5(b) presents that one peak of Cu 2p for AL-PEI-CS2-Cu(II) complexes was recorded. The characteristic peaks of Cu 2p centered at 951.8 and 931.9 eV were assigned to Cu 2p1/2 and Cu 2p3/2 (Fig. 5(c)) [52], respectively. The high-resolution spectra for S 2p displayed that the binding energy of C=S and C-S groups in AL-PEI-CS2 shifted from 163.2 eV to 162.8 eV and 168.6 eV to 167.2 eV in AL-PEI-CS2-Cu(II) complexes (Fig. 5(d)). A new peak of 168.7 eV emerged in AL-PEI-CS2-Cu(II) complexes, which was attributed to S-Cu. These results demonstrated that S atom of the −CSS- group in AL-PEI-CS2 accounted for binding with Cu(II) [53]. The binding energies of N 1s for −N-CS2 (403.9 eV) and −NH/-NH2 groups (399.5 eV) carried by AL-PEI-CS2 were observed to be shifted to 400.9 eV and 399.2 eV in AL-PEI-CS2-Cu(II) complexes (Fig. 5(e)), respectively, which may be due to the groups of C-N and −NH/−NH2 interacted with Cu(II) [19]. As shown in Fig. 5(f), the peaks at 532.6 and 531.7 eV (correspondingly assigned to the functional groups of C-O and −OH) moved to 532.4 and 531.2 eV. Moreover, a new peak at 529.9 eV was assigned to O-Cu. These results indicated that the oxygen containing functional groups participated in the adsorption process. Combined FT-IR with XPS spectra, it can be concluded that the groups of −OH, −NH2, −NH, C-N, and −CSS-carried by AL-PEI-CS2 served as the adsorption sites.
In order to explain the advantage of AL-PEI-CS2 over other lignin-based adsorbents presented in Table 2, the functional groups of these materials were given. An obvious difference lies in the existence of −CSS- group for AL-PEI-CS2. In our previous study [37], a kind of modified alkaline lignin (FLAL) was prepared, in which alkaline lignin was used as raw material, and carbon disulfide (CS2) was employed as the modifier. The functional groups of −CSS-carried by FLAL was proved to be the primary adsorption site for Pb(II) and Cu(II). According to the traditional theory [43, 44], the adsorption ability of FLAL towards Pb(II) should be higher than Cu(II) as the hydration radius of Pb(II) (4.01 Å) is less than Cu(II) (4.19 Å). It is well known that lower hydration radius is beneficial to adsorption. In contrast, FLAL exhibited better removal performance for Cu(II) than for Pb(II). The reason was that the bond energy between the functional group of −CSS- and Cu(II) was lower than that of Pb(II) based on DFT calculations. Thus, we pointed out that not only the physio-chemical property of sorbate but also the functional group of adsorbent determines the adsorption performance. On the basis of our previous research, we hypothesized that it might be the existence of the −CSS- group that makes AL-PEI-CS2 work well with the residual concentration of Cu(II) meeting with the standard of 2 mg/L under variously operating conditions.
4. ConclusionsIn this work, the adsorbent AL-PEI-CS2 was used to remove copper from aqueous solution and its performance was evaluated based on the residual concentration (Cr) of Cu(II) under various operating conditions including solution pH, coexisting cations, contact time, and the initial concentrations of Cu(II). When the solution pH ranged from 5.03 to 5.65, the values of Cr were measured to be 1.2 and 1.0 mg/L, lower than the discharged standard of 2.0 mg/L regulated by WHO and Chinese government. Under the situation of Cu (II) coexisting with K(I), Na(I), Ca(II) and Mg(II), the values of Cr were determined to be 1.96 (or 2.06), 1.53 (2.18), 1.97 (1.71) and 1.55 (2.53) mg/L when the initial concentrations of the four cations were 50 (or 100) mg/L, respectively. At the contact time of 180 min, the values of Cr were 0.32, 0.45, 0.65, 0.94, 1.76 and 1.86 mg/L corresponding to the initial copper concentrations of 5, 10, 15, 20, 30 and 50 mg/L, respectively. Even AL-PEI-CS2 was regenerated for four times, the values of Cr were as low as 1.9, 2.1, 2.0 and 2.2 mg/L for cycles 1, 2, 3 and 4 at the initial concentration of Cu(II) of 51 mg/L and contact time of 180 min. Compared to other lignin-based adsorbents based on qm, equilibrium time and Cr at equilibrium, AL-PEI-CS2 exhibited higher application potential to the treatment of Cu(II)-loaded wastewater. Adsorption mechanism revealed that −OH, −NH2, −NH, C-N and −CSS- contributed to the uptake of copper ions.
AcknowledgmentsKey R&D Program of Shaanxi Province (2019SF-253) and the Natural Science Foundation of Chongqing (cstc2020jcyj-msxmX0763) supported this work. We would also like to thank the Instrument Analysis Center of Xi’an Jiaotong University for its support. It is also a great pleasure to obtain valuable comments from editor and anonymous.
NotesAuthor Contributions C.L.Z (Associate Professor) contributed to conceptualization, review, editing and funding acquisition. Q.R.W (Ph.D. student) conducted conceptualization, investigation, formal analysis and original draft. Y.J.D (Senior Engineer) was responsible for data curation. B.Z (Professor) offered funding acquisition. Y.Y.Z (Ph.D. Assistant Professor) collected resources. T.C.Z (Professor) was in charge of sources. References1. Schwarzenbach RP, Escher BI, Fenner K, et al. The challenge of micropollutants in aquatic systems. Science. 2006;313:1072–1077.
2. Yao ZY, Qi JH, Wang LH. Equilibrium, kinetic and thermodynamic studies on the biosorption of Cu(II) onto chestnut shell. J Hazard Mater. 2010;174:137–143.
3. Zhang H, Omer AM, Hu ZH, et al. Fabrication of magnetic bentonite/carboxymethyl chitosan/sodium alginate hydrogel beads for Cu (II) adsorption. Int J Biol Macromol. 2019;135:490–500.
4. Karim Z, Claudpierre S, Grahn M, et al. Nanocellulose based functional membranes for water cleaning: Tailoring of mechanical properties, porosity and metal ion capture. J Membr Sci. 2016;514:418–428.
5. Jung KW, Lee SY, Choi JW, et al. A facile one-pot hydrothermal synthesis of hydroxyapatite/biochar nanocomposites: Adsorption behavior and mechanisms for the removal of copper(II) from aqueous media. Chem Eng J. 2019;369:529–541.
6. China Sewage Treatment Engineering Network. Treatment technology of copper(II)-containing wastewater [Internet]. Carlton: China Sewage Treatment Engineering Network; c2019. [cited February 2021]. Available from: https://www.dowater.com/jishu/2019-11-07/1097697.html
7. Pang FM, Kumar P, Teng TT, et al. Removal of lead, zinc and iron by coagulation-flocculation. J Taiwan Inst Chem Eng. 2011;42:809–815.
8. Esalah J, Husein MM. Removal of heavy metals from aqueous solutions by precipitation-filtration using novel organo-phosphorus ligands. Sep Sci Technol. 2008;43:3461–3475.
9. Silva JE, Paiva AP, Soares D, et al. Solvent extraction applied to the recovery of heavy metals from galvanic sludge. J Hazard Mater. 2005;120:113–118.
10. Mohsen-Nia M, Montazeri P, Modarress H, et al. Removal of Cu2+ and Ni2+ from wastewater with a chelating agent and reverse osmosis processes. Desalination. 2017;217:276–281.
11. Lo SF, Wang SY, Tsai MJ, et al. Adsorption capacity and removal efficiency of heavy metal ions by Moso and Ma bamboo activated carbons. Chem Eng Res Des. 2012;90:1397–1406.
12. Bailey SE, Olin TJ, Bricka RM, et al. A review of potentially low-cost sorbents for heavy metals. Water Res. 1999;33:2469–2479.
13. Khin MM, Nair AS, Babu VJ, et al. A review on nanomaterials for environmental remediation. Energy Environ Sci. 2012;5:8075–8109.
14. Klapiszewski L, Siwinska-Stefanska K, Kolodynska D. Development of lignin based multifunctional hybrid materials for Cu(II) and Cd(II) removal from the aqueous system. Chem Eng J. 2017;330:518–530.
15. Vanholme R, Demedts B, Morreel K, et al. Lignin biosynthesis and structure. Plant Physiol. 2010;153:895–905.
16. Ragauskas AJ, Beckham GT, Biddy MJ, et al. Lignin valorization: improving lignin processing in the biorefinery. Science. 2014;344:709.
17. Supanchaiyamat N, Jetsrisuparb K, Knijnenburg JTN, et al. Lignin materials for adsorption: Current trend, perspectives and opportunities. Bioresource Technol. 2019;272:570–581.
18. Thakur VK, Thakur MK, Raghavan P, et al. Progress in green polymer composites from lignin for multifunctional applications: A review. ACS Sustainable Chem Eng. 2014;2:1072–1092.
19. Ge Y, Li Z, Kong Y, et al. Heavy metal ions retention by bi-functionalized lignin: Synthesis, applications, and adsorption mechanisms. J Ind Eng Chem. 2014;20:4429–4436.
20. Ge YY, Li ZL. Application of lignin and its derivatives in adsorption of heavy metal ions in water: A review. ACS Sustainable Chem Eng. 2018;6:7181–7192.
21. Merdy P, Guillon E, Aplincourt M, et al. Copper sorption on a straw lignin: Experiments and EPR characterization. J Colloid Interface Sci. 2002;245:24–31.
22. Zhang YC, Ni SZ, Wang XJ, et al. Ultrafast adsorption of heavy metal ions onto functionalized lignin-based hybrid magnetic nanoparticles. Chem Eng J. 2019;372:82–91.
23. Xu J, Zhu SY, Liu P, et al. Adsorption of Cu(II) ions in aqueous solution by aminated lignin from enzymatic hydrolysis residue. RSC Adv. 2017;7:44751–44758.
24. Chakraborty S, Tare V. Role of various parameters in synthesis of insoluble agrobased xanthates for removal of copper from wastewater. Bioresour Technol. 2006;97:2407–2413.
25. Wang QR, Zheng CL, Shen ZX, et al. Polyethyleneimine and carbon disulfide co-modified alkaline lignin for removal of Pb2+ ions from water. Chem Eng J. 2019;359:265–274.
26. Jin C, Zhang XY, Xin JN, et al. Clickable synthesis of 1,2,4-triazole modified lignin-based adsorbent for the selective removal of Cd(II). ACS Sustainable Chem Eng. 2017;5:4086–4093.
27. Liu Y, Hu TJ, Wu ZP, et al. Study on biodegradation process of lignin by FT-IR and DSC. Environ Sci Pollut R. 2014;21:14004–14013.
28. Deng SB, Ting YP. Characterization of PEI-modified biomass and biosorption of Cu(II), Pb(II) and Ni(II). Water Res. 2005;39:2167–2177.
29. Liu C, Jin RN, Ouyang XK, et al. Adsorption behavior of carboxylated cellulose nanocrystal-polyethyleneimine composite for removal of Cr(VI) ions. Appl Surf Sci. 2017;408:77–87.
30. Desseyn HO, Fabretti AC, Forghieri F, et al. Isotopic infrared study of some nickel (II) and copper (II) complexes containing heterocyclic dithiocarbamate ligands. Spectrochim Acta, Part A. 1985;41:1105–1108.
31. Matlock MM, Howerton BS, Henke KR, et al. A pyridine-thiol ligand with multiple bonding sites for heavy metal precipitation. J Hazard Mater. 2001;82:55–63.
32. Lou ZN, Xiao X, Huang MN, et al. Acrylic acid-functionalized metal-organic frameworks for Sc(III) selective adsorption. ACS Appl Mater Inter. 2019;11:11772–11781.
33. Jin C, Zhang XY, Xin JN, et al. Thiol-ene synthesis of cysteine-functionalized lignin for the enhanced adsorption of Cu(II) and Pb(II). Ind Eng Chem Res. 2018;57:7872–7880.
34. Battistoni C, Giuliani AM, Paparazz E, et al. Platinum complexes of the methyl-esters of dithiocarbazic acid and 3-phenyldithiocarbazic acid. J Chem Soc Dalton Trans. 1984;7:1293–1299.
35. Luo K, Zhang JG. Status quo of the disposal of acidic mining waste water. Res Environ Eng. 2005;19:45–49. (in Chinese)
36. Zhang WL, Fu R, Wang L, et al. Rapid removal of ammonia nitrogen in low-concentration from wastewater by amorphous sodium titanate nano-particles. Sci Total Environ. 2019;668:815–824.
37. Wang QR, Zheng CL, Cui W, et al. Adsorption of Pb2+ and Cu2+ ions on the CS2-modified alkaline lignin. Chem Eng J. 2020;391.
38. Todorciuc T, Bulgari L, Popa VI. Adsorption of Cu(II) from aqueous solution on wheat straw lignin: Equilibrium and kinetic studies. Cellul Chem Technol. 2015;49:439–447.
39. Zhou JX, Ding YJ, Zeng GX, et al. Major ion chemistry of surface water in the upper reach of Shule River basin and the possible controls. Environ Sci. 2014;35:1–10. (In Chinese)
40. Liu W, Wang T, Borthwick AGL, et al. Adsorption of Pb2+, Cd2+, Cu2+ and Cr3+ onto titanate nanotubes: Competition and effect of inorganic ions. Sci Total Environ. 2013;456:171–180.
41. Zhang W, Song JY, He QL, et al. Novel pectin based composite hydrogel derived from grapefruit peel for enhanced Cu(II) removal. J Hazard Mater. 2020;384:121445.
42. Hofer TS, Hunenberger PH. Absolute proton hydration free energy, surface potential of water, and redox potential of the hydrogen electrode from first principles: QM/MM MD free-energy simulations of sodium and potassium hydration. J Chem Phys. 2018;148:1–29.
43. Noyes RM. Thermodynamics of ion hydration as a measure of effective dielectric properties of water. J Am Chem Soc. 1962;84:513–522.
44. Cheng TW, Lee ML, Ko MS, et al. The heavy metal adsorption characteristics on metakaolin-based geopolymer. Appl Clay Sci. 2012;56:90–96.
45. Chen FG, Shahabadi SIS, Zhou D, et al. Facile preparation of cross-linked lignin for efficient adsorption of dyes and heavy metal ions. React Funct Polym. 2019;143:1–6.
46. Mohan D, Pittman CU, Steele PH. Single, binary and multi-component adsorption of copper and cadmium from aqueous solutions on Kraft lignin-a biosorbent. J Colloid Interf Sci. 2006;97:489–504.
47. Liu X, Zhu H, Qin C, et al. Adsorption of heavy metal ion from aqueous single metal solution by aminated epoxy-lignin. BioResources. 2013;8:2257–2269.
48. An L, Si CL, Bae JH, et al. One-step silanization and amination of lignin and its adsorption of Congo red and Cu(II) ions in aqueous solution. Int J Biol Macromol. 2020;159:222–230.
49. Shen XP, Xi YJ, Wang QW, et al. Enhanced heavy metal adsorption ability of lignocellulosic hydrogel adsorbents by the structural support effect of lignin. Cellulose. 2019;26:4005–4019.
50. Sciban M, Klasnja M. Study of the adsorption of copper(II) ions from water onto wood sawdust, pulp and lignin. Adsorpt Sci Technol. 2004;22:195–206.
51. Duan YQ, Freyburger A, Kunz W, et al. Lignin/chitin films and their adsorption characteristics for heavy metal ions. ACS Sustain Chem Eng. 2018;6:6965–6973.
Fig. 1(a) FT-IR spectra of AL and AL-PEI-CS2; (b) full scan survey XPS spectra of AL and AL-PEI-CS2; high resolution XPS spectra of AL-PEI-CS2: (c) O 1s, (d) N 1s, and (e) S 2p. 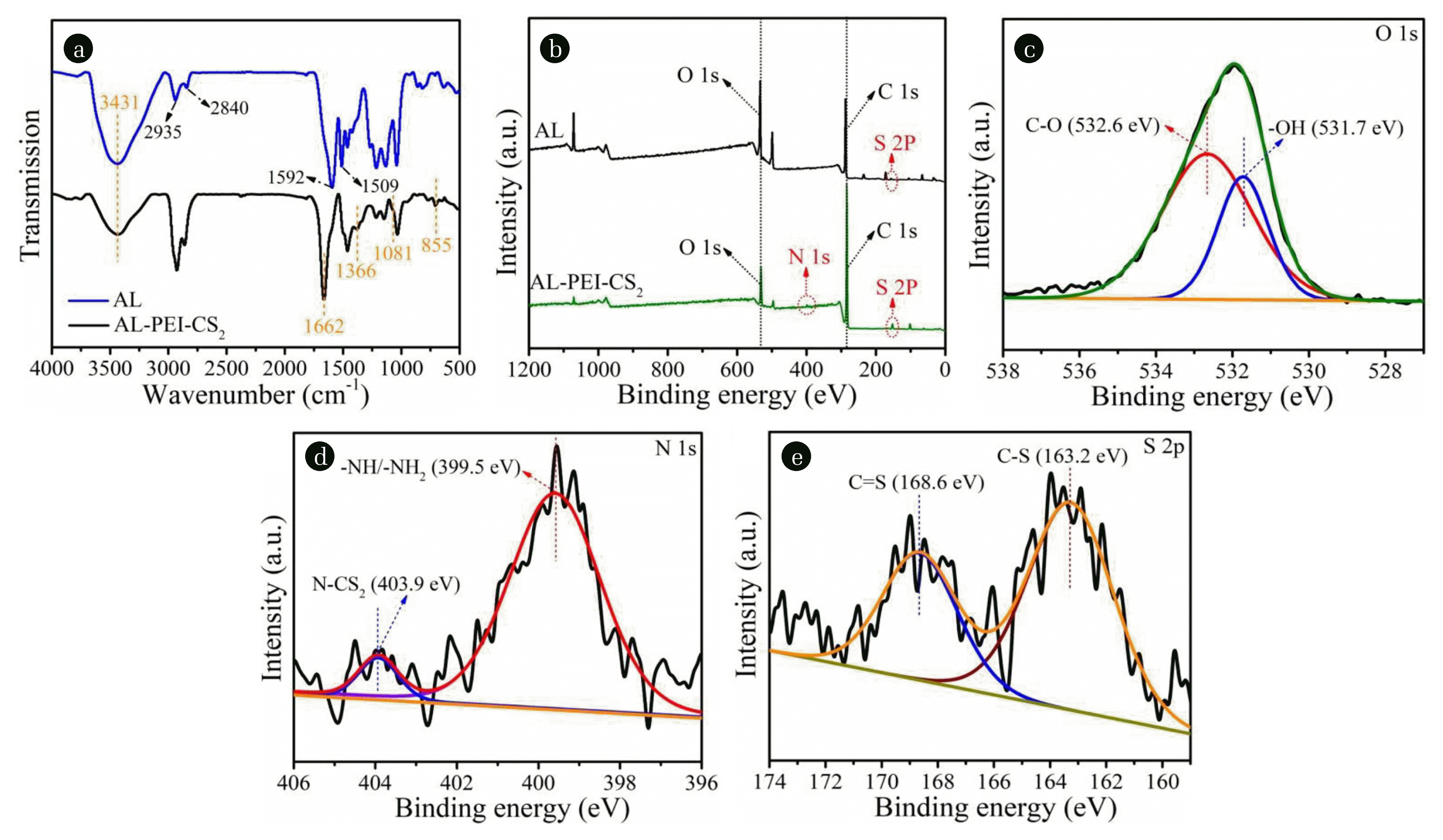
Fig. 2(a) Zeta potential of AL-PEI-CS2 (insert: the effect of solution pH on the adsorption performance); (b) speciation of Cu(II) at different pH values [37] (insert: the changes in pH values before and after adsorption); and (c) the residual concentration (Cr) of Cu(II) treated via ASL [19], wheat lignin [38] and AL [23] at different solution pH values. 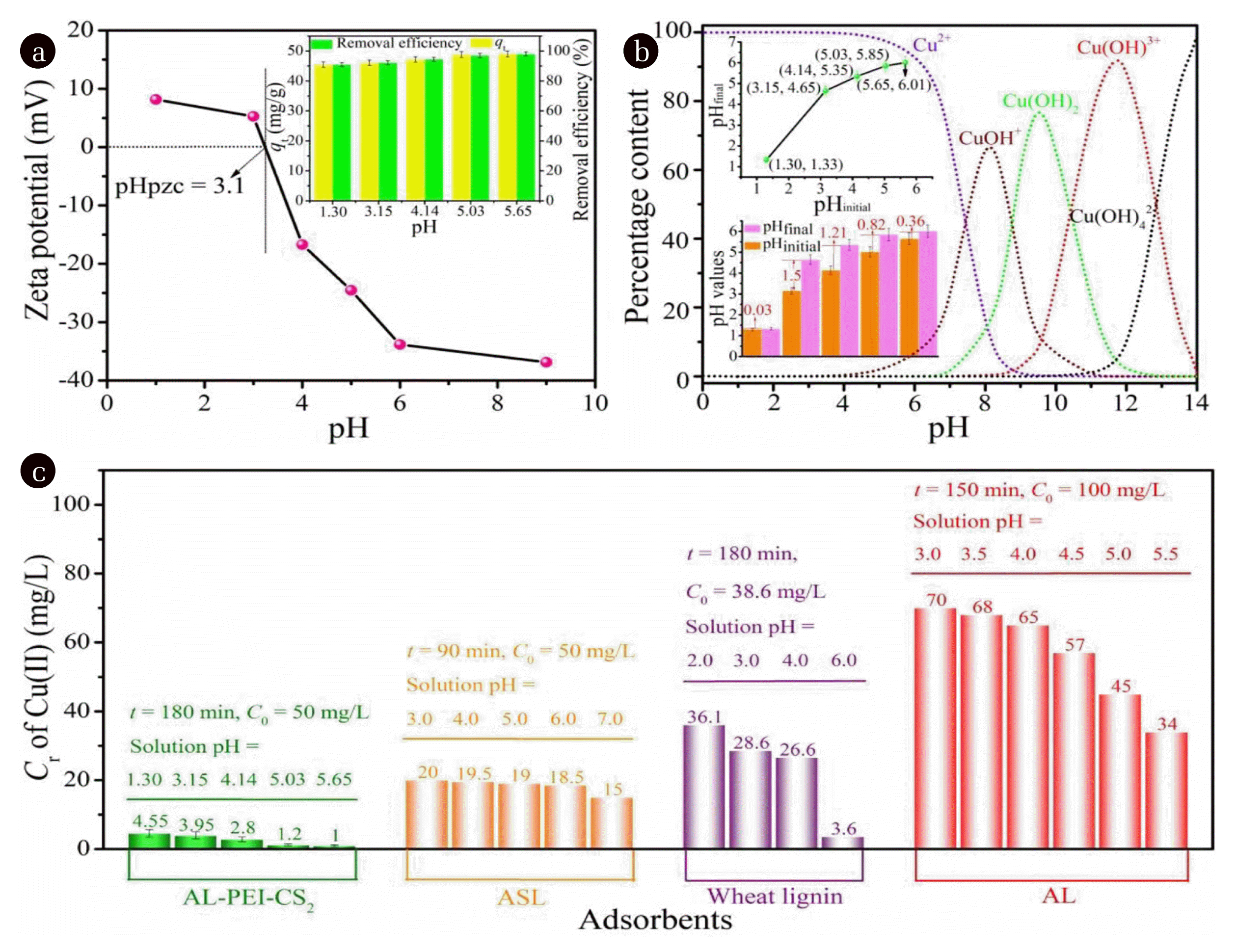
Fig. 4(a) The influence of contact time on the adsorption of Cu(II) over AL-PEI-CS2 under different initial concentrations (insert: the removal efficiency of Cu(II) at 60, 120 and 180 min); and (b) the values of Cr at different contact times when C0 of Cu(II) ranged from 0 to 50 mg/L. 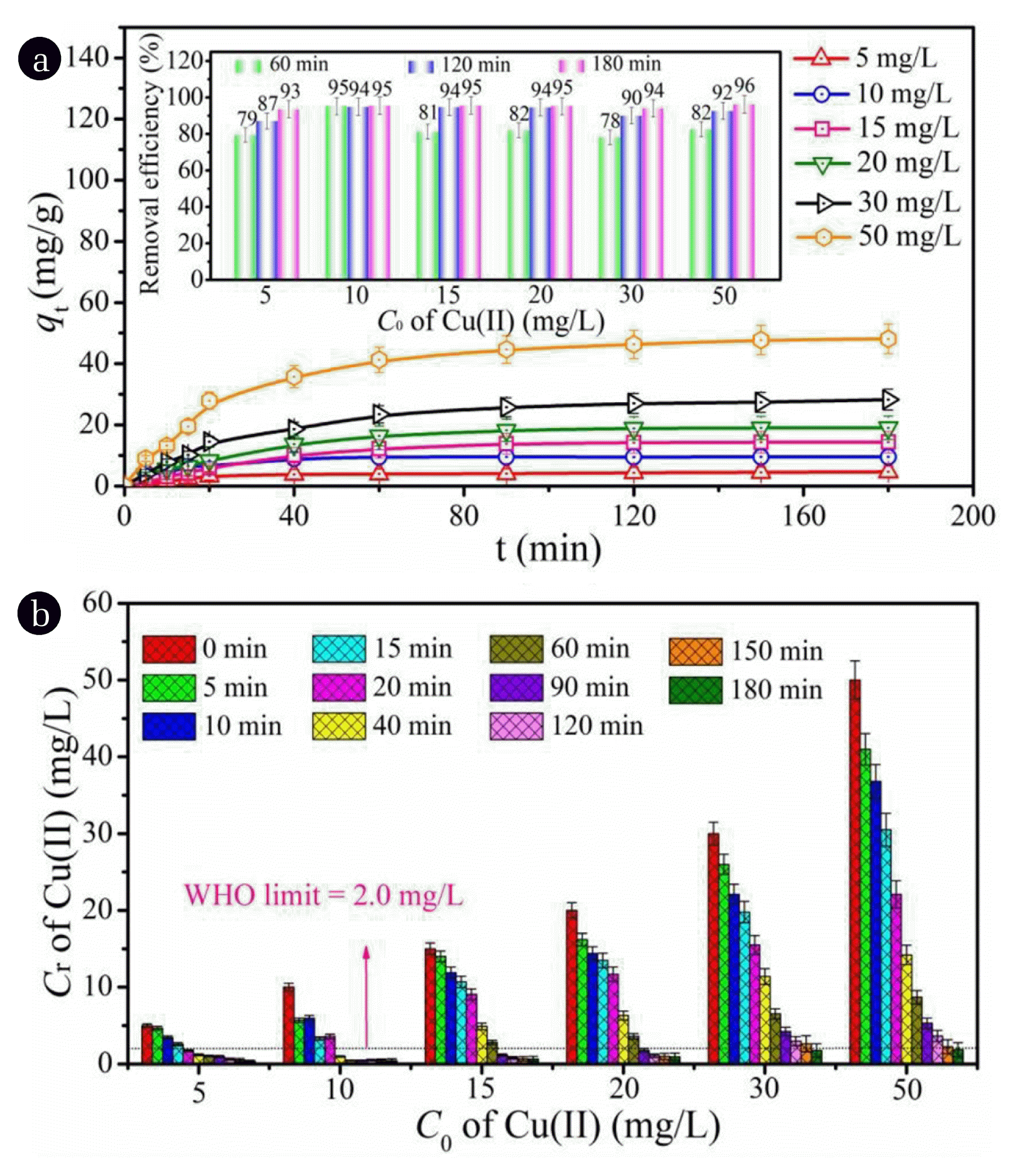
Fig. 5(a) FT-IR spectra of AL-PEI-CS2 and AL-PEI-CS2-Cu(II) complexes; (b) full scan survey XPS spectra of AL-PEI-CS2/AL-PEI-CS2-Cu(II) complexes; (c) high resolution spectra of Cu 2p for AL-PEI-CS2-Cu(II) complexes; high resolution spectra of S 2p (d), N 1s (e) and O 1s (f) of AL-PEI-CS2/AL-PEI-CS2-Cu(II) complexes (i: AL-PEI-CS2 and ii: AL-PEI-CS2-Cu(II) complexes). 
Table 1The Adsorption Capacity of Different Lignin-based Adsorbents towards Cu(II) According to qm and the Time to Reach Equilibrium.
Table 2The Adsorption Capacity of Different Lignin-based Adsorbents towards Cu(II) According to the Residual Concentration of Cu(II) at Equilibrium |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||