AbstractManaged aquifer recharge (MAR), an intentional storage of excess water to an aquifer, is becoming a promising water resource management tool to cope with the worldwide water shortage. Bioclogging is a commonly encountered operational issue that lowers hydraulic conductivity and overall performance in MAR. The current study investigates the impact of carbon and nitrogen in recharge water on bioclogging in MAR. For this investigation, continuous-flow columns packed with sand grains were operated with influents having 0 (C1), 5 (C2), and 100 mg/L (C3) of glucose with or without introduction of nitrate. Hydraulic conductivity was analyzed to evaluate bioclogging in the systems. In C1 and C2, hydraulic conductivity was not significantly changed overall. However, hydraulic conductivity in C3 was decreased by 28.5% after three weeks of operation, which appears to be attributed to generation of fermentation bacteria. Introduction of nitrogen to C3 led to a further decrease in hydraulic conductivity by 25.7% compared to before it was added, most likely due to stimulation of denitrifying bacteria. These findings indicate that high carbon contents and introduction of additional nitrogen in recharge water cause serious bioclogging in MAR, suggesting the necessity for controlling quality of recharge water.
1. IntroductionGlobal access of freshwater resources on earth is approaching the limits of sustainability due to less constant water supply and increasing global population [1–4]. Thus, water reclamation and appropriate reuse has become a critical issue world-wide [2]. Recently, managed aquifer recharge (MAR), the purposeful recharge of water to an aquifer, is receiving great attention as a vital management tool in the sustainable use of the world’s water resources [5–8]. MAR provides safe storage of water from a variety of sources, including stream, stormwater runoff, municipal or industrial wastewater, and irrigation flow, against evaporation and secondary contamination in times of water surplus to meet demand in times of shortage [5, 9–11]. Moreover, the MAR potentially improves the quality of water via physical and biogeochemical processes during infiltration and subsurface transport, and it can also act as a barrier to prevent saline instruction [12–15]. MAR takes on various forms depending on the sources of recharge water, infiltration techniques, end use of recovered water, and scale and complexity, with aquifer storage and recovery (ASR) and infiltration basin type being the most common [6, 16, 17].
Clogging is commonly encountered in MAR [2, 18–20]. This can result from natural physicochemical processes caused by filtration of suspended solids in recharge water [2, 21] or biological reactions in porous soil media caused by bacterial cell accumulation and extracellular polysaccharide production [2, 19, 22]. While microbially-induced clogging, termed bioclogging, may increase the efficiency of natural filtration as retention time increases [23], it is generally problematic in MAR by decreasing the porosity and hydraulic conductivity of a saturated porous medium and limiting the capacity of the MAR [24–28]. Therefore, understanding microbial stimulation and factors affecting it in MAR could lead to better operation and efficiency of the MAR.
There are earlier studies reported microbially-induced clogging in porous media [29–31]. They focused mainly on the influences of growth, thickness, transport, and morphology of biofilm on hydraulic conductivity in porous media [29–31]. However, despite the fact that nutrients such as carbon and nitrogen in recharge water can be utilized for microbial growth and can thereby contribute to bioclogging in the MAR, their effects on hydraulic conductivity are not fully understood.
The current study evaluated the impacts of nutrients in recharge water on bioclogging in a sand grain MAR. In particular, we investigated microbial stimulation in a MAR by high contents of carbon and nitrogen in recharge water. For this study, we operated a continuous-flow permeameter packed with sand grains and analyzed changes in hydraulic conductivity depending on the quantity of glucose and additional introduction of nitrogen in recharge water. Based on the findings from the present study, we discuss the contributions of the quality of recharge water to bioclogging in the MAR.
2. Materials and Methods2.1. Configuration and Operational Conditions of the Continuous-Flow ColumnsThe configuration of the constant-head permeameter is shown in Fig. 1. Experiments were conducted in three cylindrical acrylic columns with 10 cm lengths and 7 cm diameters. The columns were packed with 0.07–0.87 mm diameter graded Ottawa sand (silica classified #C109 ASTM #C778), supported by stainless steel mesh placed inside the column end caps. Tap water was used as the source of influent; it was dechlorinated by adding granular activated carbon (200 mg/L) and sparging with air for 2 h prior to use. After dechlorination, tap water was again sparging with nitrogen gas to remove dissolved oxygen (DO) for simulating conditions of low dissolved oxygen in groundwater. Characteristics of tap water were as follows: 6.7 ± 0.02 of pH, 217 ± 6.1 μS/cm of electrical conductivity (EC), 0.1 ± 0.05 of DO, 0.17 ± 0.06 of total nitrogen, and 0.6 ± 0.08 NTU of turbidity. Local soil provided the inoculum in the columns. Approximately 100 g of soil was collected from the premises of Kangwon National University, Chuncheon, Korea and mixed with 300 mL of tap water to simulate microbial growth in a natural soil environment. The mixture was shaken for 10–15 min in an incubator at 150 rpm (30ºC) and 0.5 mL of supernatant was added as inoculum to each column.
Three columns (C1, C2 and C3) were operated under water-saturated conditions at constant room temperature. Using an adjustable peristaltic pump, the influent was fed at a flow rate of 0.94 mL/min, which approximates the flow of velocity of typical groundwater in sandy aquifers [28]. Empty bed contact time for all columns was calculated to be 57.7 h. The contents of glucose and nitrate in the influents for C1, C2, and C3 during the experiments are summarized in Table 1. Column 1 (C1) was fed with tap water alone as a control. Columns 2 (C2) and 3 (C3) were fed with 5 mg/L and 100 mg/L of glucose, respectively, which simulate recharge water that contains relatively low and high contents of nutrients. Until day 84, all columns were operated with glucose only and then 20 mg N/L of nitrate was introduced until the end of operations (day 112). All influents were refreshed every 2 d to prevent degradation of glucose before they were applied to the sand grain columns. All experiments were performed in triplicate and the average values are reported in the results.
2.2. Measurement of Hydraulic ConductivityHydraulic conductivity (K;cm/s) was analyzed based on Darcy’s Law (1) on days 1, 21, 42, 63, 86, 105, and 118:
where Q represents the rate of flow or volume of water per unit time (cm3/s), L is the length of the sand column (cm), h is the constant head difference (cm), A represents the cross-sectional area (cm2) and t denotes time (s).
2.3. Biochemical AnalysisFor chemical analyses, 50 mL of sample was collected from the influent and effluent ports of each column on day 1, 21, 42, 63, 86, 105, and 118, and chemical oxygen demand (COD), glucose, and nitrate (only on day 86, 105, and 118) were measured. COD was determined based on standard methods for water and wastewater [32]. Glucose concentrations were analyzed using the phenol-sulfuric method [33]. For nitrate analysis, approximately 20 mL of sample was passed through a 0.45 μm syringe filter (Smart Pore; Woongki Science Co. Ltd., South Korea) and stored at 4ºC prior to ion chromatography analysis (DX-100, AS14 column; Dionex Corp, USA). The numbers of bacteria were measured by cell counting using a Neubauer chamber on days 21, 84, and 118.
2.4. Statistical AnalysisAll data were analyzed using Microsoft Excel 2016 and graphed using Microsoft Excel 2016 and Sigma Plot 10. To evaluate the statistical significance of the results, p-values were analyzed based on the t-test using the method in a study of Welch [34].
3. Results and DiscussionK values obtained during the bioclogging test in columns packed with sand grains under continuous-flow condition are illustrated in Fig. 2. The K values in C1 (0 mg/L of glucose) and C2 (5 mg/L of glucose) did not show significant changes overall. On the contrary, the K values in C3 (100 mg/L of glucose) were substantially changed by time and the introduction of nitrate. Effects of carbon (glucose) and nitrogen (nitrate) on hydraulic conductivity are discussed in detail below.
3.1. Impact of Glucose on Hydraulic ConductivityThe effect of glucose on bioclogging was evaluated based on the K values during operations of C1, C2, and C3 with no introduction of nitrate. The K values in C1 were statistically constant (p-value < 0.05) until day 63 with an average value of 0.0227 ± 2.16 × 10−4 cm/s. While overall K values in C2 (on average, 0.0221 ± 3.45 × 10−4 cm/s) were slightly smaller than those in C1, they also remained statistically unchanged (p-value < 0.05) until day 63. However, the K values in C3 significantly decreased from 0.0235 ± 2.52 × 10−4 cm/S to 0.0168 ± 4.16 × 10−4 cm/s during the first 21 days and then they were not notably changed until day 63. This change in the K values in C3 corresponds to the earlier reports about bioclogging [35–39]. For example, Jeong et al [39] likewise reported that hydraulic conductivity sharply decreased early in the operation of a sandy column under continuous flow and then no further significant drop in hydraulic conductivity was discovered. They considered that in the early stages of bioclogging, aerobic and anerobic microorganisms multiplied rapidly to form colonies and occupied the pore channel, causing blocking water flow and a sharp decrease in hydrodynamic dispersion. However, when microbial colonies developed to a certain level, water flow transformed from uniform to non-uniform flow, resulting in no more significant decrease in hydraulic conductivity.
The data from bacteria counting (Fig. 3) confirms that the variation in hydraulic conductivity between C2 and C3 was mainly attributed to microorganism stimulation in sand grains. Bacterial counts in C1 and C2 at day 21 accounted for 1.43 × 104 ± 1404 and 1.57 × 104 ± 1501 cells/mL, respectively; whereas the value for C3 was considerably higher, 5.97 × 104 ± 3113 cells/mL. It is speculated that this greater microbial stimulation in C3 is associated with fermentation. As Fig. 4 demonstrates, C3 showed a significant glucose removal (approximately 40%); however, no COD removal was observed in C3. Moreover, while C1 and C2 commonly showed fairly similar pH values between influents (on average, 6.5 ± 0.2 in C1 and 6.7 ± 0.1 in C2) and effluents (on average, 6.6 ± 0.2 in C1 and 6.5 ± 0.1 in C2), only C3 presented a substantial drop in pH between influents (on average, 6.6 ± 0.1) and effluents (on average, 3.1 ± 0.3). Given that electron acceptors such as dissolved oxygen and nitrate rarely exist in the sand grains, these results suggest that fermentation was the main cause for glucose removal and fermentation by-products such as organic acids and alcohols attributed to the no COD removal and decrease in pH in C3.
The above results of the K values in C1, C2, and C3 until day 63 indicate that the content of glucose highly impacts hydraulic properties by stimulating microorganisms. When recharge water contains low carbon concentration (C2), hydraulic conductivity was not substantially worsened. On the other hand, recharge water that possesses high carbon content (C3) leads to a significant decrease in hydraulic conductivity in the system. This observance suggests that control for organic matter in recharge water is necessary for sustainable operation of MAR.
3.2. Impact of Nitrate on Hydraulic ConductivityHydraulic conductivity in C1, C2, and C3 before and after introduction of nitrate is compared to investigate the effect of nitrogen on bioclogging. In C1 and C2, slight decreases in K values were observed after introduction of nitrate, on average, from 0.0228 ± 2.16 × 10−4 cm/s to 0.0218 ± 3.09 × 10−4 cm/s and from 0.0221 ± 3.44 × 10−4 cm/s to 0.0209 ± 3.16 × 10−4 cm/s, respectively (Fig. 2). Results of bacterial counts before and after introduction of nitrate in C1 and C2 were also minimally changed (Fig. 3). For example, before nitrate was introduced, the average bacterial counts in C1 and C2 accounted for 1.43 × 104 ± 1404 and 1.57 × 104 ± 1501 cells/L at day 21, respectively, which slightly increased to 1.57×104 ± 1285 and 1.67 × 104 ± 1137 cells/L at day 84, respectively, with introduction of nitrate.
Unlike in C1 and C2, the K values in C3 before and after introduction of nitrate were fairly varied. Before introduction of nitrate, the average K value in C3 was 0.0179 ± 3.73 × 10−3 cm/s, which decreased to 0.0133 ± 1.11 × 10−3 cm/s after introduction of nitrate (Fig. 2). Data of bacterial counts in C3 supports that this decline is attributed to microbial reproduction. Before nitrate was introduced, the result of bacterial counts in C3 was 5.97 × 104 ± 3113 cells/mL at day 21; with introduction of nitrate, this value increased to 8.15 × 105 ± 3139 cells/mL at day 84 (Fig. 3). It appears that this increase in bacterial counts is relevant to stimulation of denitrifying bacteria that utilizes nitrogen as an electron acceptor. Fig. 4 presents that introduction of nitrate led to COD removal 57% in C3, which did not occur before nitrate was introduced. Furthermore, a decrease in pH between influents (on average, 6.6 ± 0.1) and effluents (on average, 6.4 ± 0.2) was no longer observed in C3 with introduction of nitrate. These results suggest that fermentation did not occur after nitrate was introduced. This is most likely because the introduced nitrate served as an electron acceptor. In addition, the finding that 44% of the introduced nitrate was removed only in C3 (Fig. 5) also supports that denitrifying bacteria were stimulated.
The findings from the above analyses demonstrate that nitrogen can be a factor affecting bioclogging in MAR. While introduction of nitrate was not able to stimulate significant microbial growth under conditions of insufficient organic content, nitrate boosts microbial reproduction, particularly that of denitrifying bacteria, when sufficient organic matter is provided. This suggests that control of both electron donors (i.e., organic matter) and acceptors (i.e., nitrogen) in recharge water can provide a more effective measure for avoiding excessive bioclogging in the MAR.
3.3. Quality of Recharge Water and Its Impact on Bioclogging in MARThe current study investigated effects of nutrients in recharge water on bioclogging in a sand grain MAR. The results demonstrate that high contents of carbon and additional introduction of nitrogen resulted in significant stimulation of fermentation and denitrifying bacteria, leading to serious bioclogging in a sand grain MAR. As discussed in the introduction, sources for recharge water include not only highly treated water such as effluents from water treatment facilities but also less or none treated water including stormwater runoff and irrigation flow. The present study demonstrates that employment of untreated water to MAR can adversely affect sustainability of MAR. However, reliable guidelines for quality of recharge water to prevent bioclogging in MAR have not been developed yet. Most earlier attempts have been based on limited research data [40], and others have recommended treating recharge water to a level of drinking water quality [5]. Given that most MAR operations utilize reclaimed water, achieving such costly high-quality recharge water may be inefficient. Hence, further evaluation for relations between various water quality parameters and bioclogging in MAR systems is necessary, which will aid in developing efficient guidelines for sustainable operation of MAR.
4. ConclusionsThe effects of glucose and additional nitrate introduction on bioclogging to continuous-flow columns packed with sand grains were evaluated based on hydraulic conductivity (K value). In C1 and C2, having influents with 0 and 5 mg/L of glucose, respectively, hydraulic conductivity was relatively unchanged during overall experiments. However, in C3, having influents with 100 mg/L of glucose, hydraulic conductivity decreased by 28.5% in the first three weeks, which was associated with growth of fermentation bacteria. Moreover, introduction of nitrate to C3 caused a further decrease in hydraulic conductivity by 25.7%, which is possibly attributed to reproduction of denitrifying bacteria. These results show that nutrients in recharge water are factors affecting bioclogging in MAR, suggesting that it is necessary to control the quality of recharge water for better and sustainable operation of MAR.
AcknowledgmentsThis work was supported by the Korea Ministry of Environment (The SEM project: 2018002450001) and partially supported by 2017 Research Grant from Kangwon National University.
NotesAuthor Contributions H.E. (Post-doctoral researcher) planned the study and wrote the manuscript. S.F. (Post-doctoral researcher) and A.G. (Post-doctoral researcher) conducted experiments. H.S. (Senior researcher), Y.K.. (Senior researcher), Y.S.K. (Professor), and S.P.J. (Professor) performed data analysis. S.-E.O. (Professor) planned the study and revised the manuscript. References2. Rinck-Pfeiffer S, Ragusa S, Sztajnbok P, et al. Interrelationships between biological, chemical, and physical processes as an analog to clogging in aquifer storage and recovery (ASR) wells. Water Res. 2000;34:2110–2118.
3. Kivaisi AK. The potential for constructed wetlands for wastewater treatment and reuse in developing countries: A review. Ecol Eng. 2001;16:545–560.
4. Bruch I, Fritsche J, Bänninger D, et al. Improving the treatment efficiency of constructed wetlands with zeolite-containing filter sands. Bioresour Technol. 2011;102:937–941.
5. Bouwer H. Artificial recharge of groundwater: hydrogeology and engineering. Hydrogeol J. 2002;10:121–142.
7. Bouwer H, Pyne R, Brown J, et al. Design, operation, and maintenance for sustainable underground storage facilities. Denver: AWWA Research Foundation; 2008. p. 1–5.
8. Page D, Bekele E, Vanderzalm J, et al. 2018 Managed aquifer recharge (MAR) in sustinable urban water management. Water. 2018;10:239.
9. Ma L, Spalding RF. Effects of artificial recharge on ground water quality and aquifer storage recovery. J Am Water Resour Assoc. 1997;33:561–572.
10. Drewes JE. Ground water replenishment with recycled water quality improvements during managed aquifer recharge. Ground Water. 2009;47:502–505.
11. Page D, Miotlinski K, Dillon P, et al. Water quality requirements for sustaining aquifer storage and recovery operations in low permeability fractured rock aquifer. J Environ Manage. 2011;92:2410–2418.
12. Fox P, Houston S, Westerhoff P. Advances in Soil Aquifer Treatment Research for Sustainable Water Reuse. Denver: AWWA Research Foundation; 2006. p. 70–72.
13. Shammas M. The effectiveness of artificial recharge in combating seawater intrusion in Salalah coastal aquifer, Oman. Environ Geology. 2008;55:191–204.
14. Daher W, Pistre S, Kneppers , et al. Karst and artificial recharge: Theoretical and practical problems: A preliminary approach to artificial recharge assessment. J Hydrol. 2011;408:189–202.
15. Schmidt CM, Fisher AT, Racz AJ, et al. Linking denitrification and infiltration rates during managed groundwater recharge. Environ Sci Techol. 2011;45:9634–9640.
16. Kimrey JO. Artificial recharge of groundwater and its role in water management. Desalination. 1989;72:135–147.
17. Smith L. Clogging mechanisms in managed aquifer recharge: a case study at Mining Area C [thesis]. Perth: The University of Western Australia; 2014.
18. Alfredo P. Integrated modelling of clogging processes in artificial groundwater recharge [dissertation]. Barcelona: Technical University of Catalonia; 2000.
19. Pavelic P, Dillon PJ, Barry KE, et al. Water quality effects on clogging rates during reclaimed water ASR in a carbonate aquifer. J Hydrol. 2007;334:1–16.
20. Kim J-W, Choi H, Pachepsky YA. Biofilm morphology as related to the porous media clogging. Water Res. 2010;44:1193–1201.
21. Pavelic P, Dillon Pj, Barry KE, et al. Well clogging effects determined from mass balances and hydraulic response at a stormwater ASR site. In : Proceedings of the Third International Sympsium on Artificial Recharge of Groundwater; 21–25 September 1998; Amsterdam. p. 61–66.
22. Pell M, Nyberg F. Infiltration of Wastewater in a Newly Started Pilot Sand-Filter System: II. Development and Distribution of the Bacterial Populations. J Environ Qual. 1989;18:457–462.
23. Van Cuyk S, Siegrist R, Logan A, et al. Hydraulic and purification behaviors and their interactions during wastewater treatment in soil infiltration systems. Water Res. 2001;35:953–964.
24. Taylor SW, Milly PCD, Jaffé PR. Biofilm growth and the related changes in the physical properties of a porous medium: 2. Permeability. Water Resour Res. 1990;26:2161–2169.
25. Clement TP, Hooker BS, Skeen RS. Macroscopic Models for Predicting Changes in Saturated Porous Media Properties Caused by Microbial Growth. Ground Water. 1996;34:934–942.
26. Baveye P, Vandevivere P, Hoyle BL, et al. Environmental impact and mechanisms of the biological clogging of saturated soils and aquifer materials. Crit Rev Environ Sci Techol. 1998;28:123–191.
27. Soleimani S, Van Geel PJ, Isgor OB, et al. Modeling of biological clogging in unsaturated porous media. J Contam Hydrol. 2009;106:39–50.
28. Seifert D, Engesgaard P. Sand box experiments with bioclogging of porous media: Hydraulic conductivity reductions. J Contam Hydrol. 2012;136–137:1–9.
29. Cunningham AB, Characklis WG, Abedeen F, et al. Influence of biofilm accumulation on porous-media hydrodynamics. Environ Sci Technol. 1991;25:1305–1311.
30. Wu J, Gui S, Stahl P, Zhang R. Experimental study on the reduction of soil hydraulic conductivity by enhanced biomass growth. Soil Sci. 1997;162:741–748.
31. Stoodley P, Dodds I, De Beer D, et al. Flowing biofilms as a transport mechanism for biomass through porous media under laminar and turbulent conditions in a laboratory reactor system. Biofouling. 2005;21:161–168.
32. APHA. Standards Methods for the examination of water and wastewater. 20th edWashington DC: American Public Health Association; 1998. p. 5.14–5.19.
33. DuBois M, Gilles KA, Hamilton JK, et al. 1956 Colorimetric Method for Determination of Sugars and Related Substances. Anal Chem. 1956;28:350–356.
34. Welch BL. The generalization of ‘Student’s’ problem when several different population variances are involved. Biometrika. 1947;34:28–35.
35. Engesgaard P, Seifert D, Herrera P. Bioclogging in porous media: Tracer studies. Riverbank Filtration Hydrology - Impacts on System Capacity and Water Quality. Hubbs SA, editorNATO Sci Ser IV Earth Environ SciDordrecht: Springer; 2006. p. 155–178.
37. Physics of bacterial near-surface motility using flagella and type IV pili: implications for biofilm formation. Microbio Res. 2012;163:619–629.
38. Zhong X, Wu Y. Bioclogging in porous media under continous-flow condition. Eviron Earth Sci. 2013;68:2417–2425.
39. Jeong HY, Jun SC, Cheon JY, et al. A review on clogging mechanisms and managements in aquifer storage and recovery (ASR) applications. Geosci J. 2018;22:667–679.
40. Pérez-Paricio A, Carrera J. EU Project on Artificial Recharge of Groundwater, Research Program on Environment and Climate. 1999. Contract ENV-CT95-0071European Commonwealth; Brussels:
Fig. 1Schematic diagram (a) and a picture of continuous-flow columns (b). The values for L, h, and Q are 10 cm, 7 cm, and 0.94 mL/min, respectively. 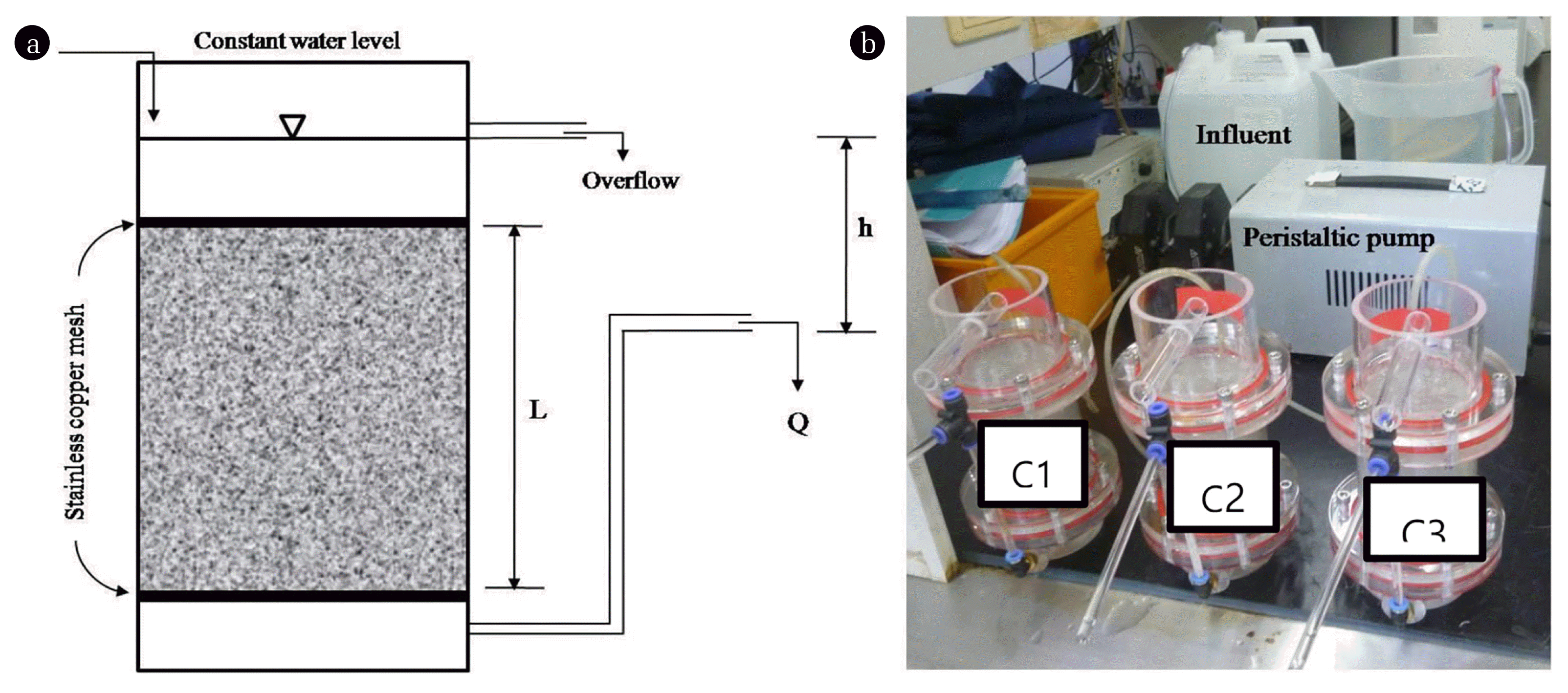
Fig. 2Changes of hydraulic conductivity in continuous-flow columns. Error bars represent the standard deviations from triplicate experiments. 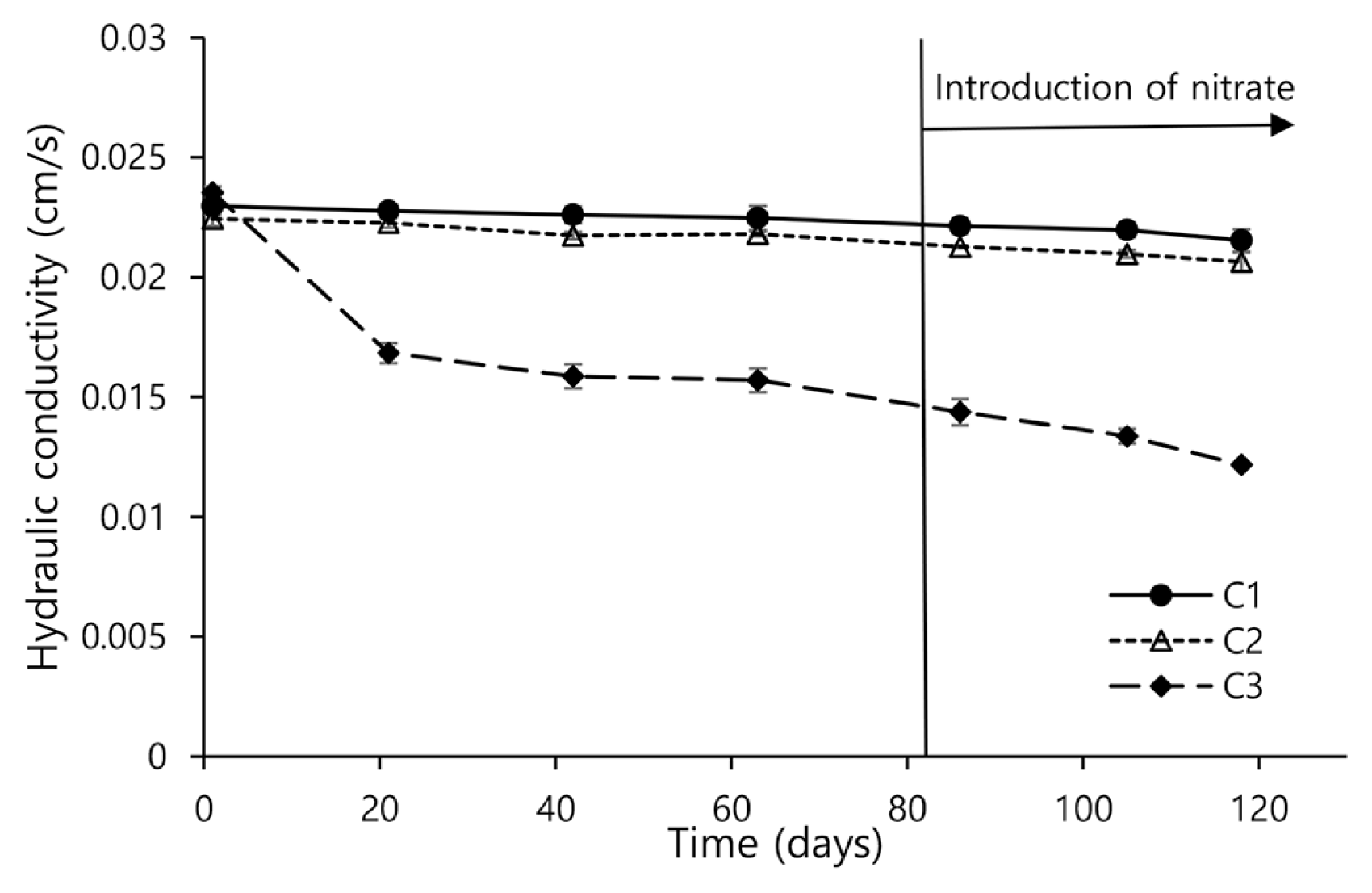
Fig. 3Bacterial counts in sand grains of continuous-flow columns. Error bars represent the standard deviations from triplicate experiments. 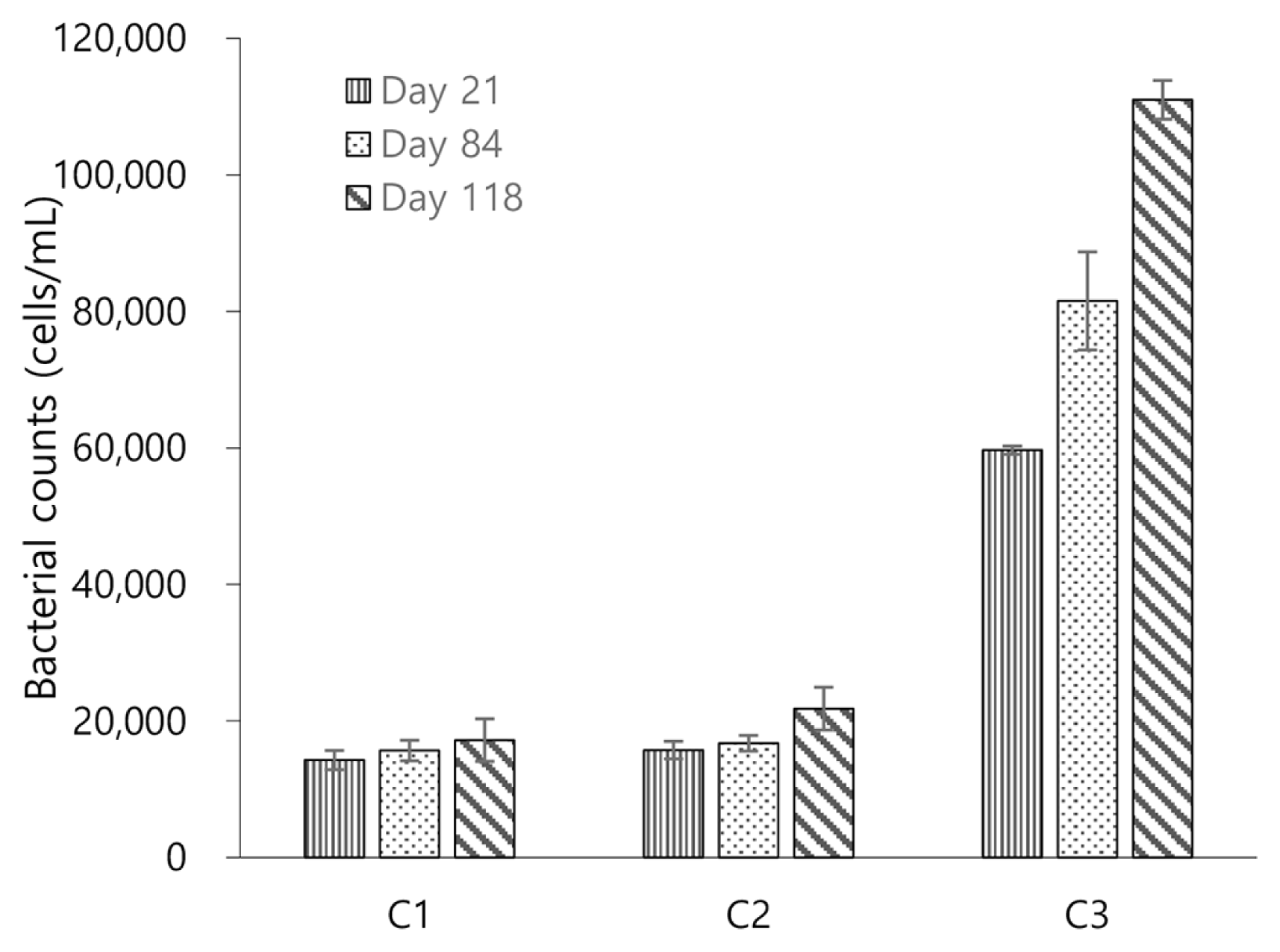
Fig. 4(a) Average concentrations of glucose and (b) COD in influents and effluents of continuous-flow columns. Error bars represent the standard deviations. The number of sample collection for data of influent, effluent without addition of nitrate, and effluent with addition of nitrate were 7, 4, and 3, respectively. 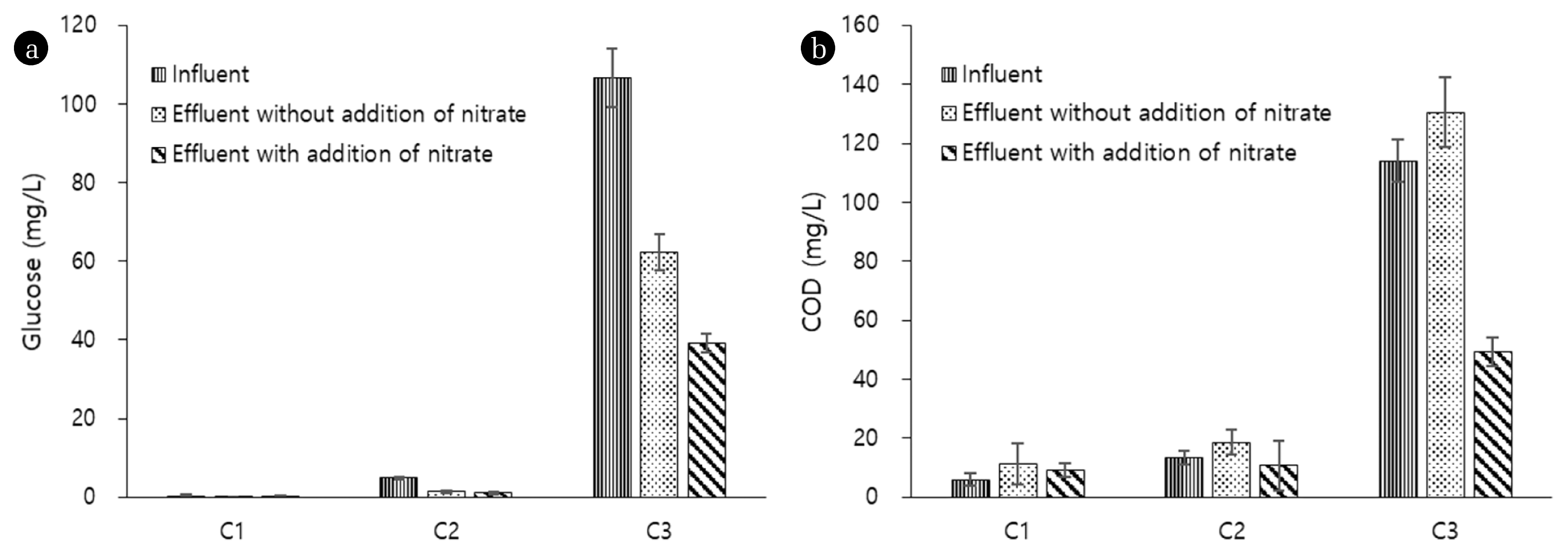
|
|
||||||||||||||||||||||||||||||||||||||