AbstractThe present work deals with the modeling and optimization of photocatalytic degradation (UV/TiO2) of aqueous solution of Acid Red 114 (AR114) dye using Artificial Neural Networks (ANN) and RSM. Photocatalytic treatment of AR114 has been executed using suspension TiO2 catalyst for commercial applications exposed to ultraviolet irradiation in a shallow pond reactor. ANN optimization has been applied to for predicting the behavior of photocatalysis. The input parameters used for analysis of aqueous dye solution are - TiO2 dose, pH of the dye solution, initial dye concentration, UV light intensity, time and area/volume, and time whereas the outputs are evaluated in form of degradation and decolorization efficiency of AR114. The outcomes of ANN optimization have been experimentally validated. Results achieved establish ANN modeling as a good predictive model. Parameteric optimization using multi-parameter optimization has been employed with desirability function approach. Results obtained from RSM are in line as per the results of ANN modeling as well as experimental. First order kinetics is use to effectively express degradation and decolorization of AR114 dyes. Total organic carbon (TOC) removal and GC-MS study of the dye shows the total mineralization and formation of non-toxic intermediate products.
1. IntroductionEnvironmental safety and the improvement of environmental issues are the main concern for an actual development of life and for a sustainable development. Water is utilized in various human needs such as agriculture, industry, domestic, and energy production. Globally, domestic use represents only 15% of the total water consumption, while 25% is used in industrial activities. The major share of water constituting the remaining 60% is consumed in the agriculture activities. Associated to each of these activities, different types of pollutants are generated. Of all users, the industrial wastewater streams are posed a severe threat to human life, plants and animals and also to ecosystems of receiving water bodies. Industrial effluents from textile, pulp and paper, pharmaceutical and other chemical industries [1, 2] are the major source of ground and surface water pollution.
It has been predicted that approximately 15% of the dye is discharged as wastewater from dyeing industry [3]. The dye color is typically the first pollutant to be identified in the wastewater, which is produced due to the synthetic dyes in the industries. Considering both effluent compositions and volumes discharged, the wastewater produced by the textile industry is the most significant pollutant amongst the various industries. Specified the large diversity of dyes, fibers, finishing products and process aids in the application, the textile industries produce the wastewater with a large chemical diversity, quantity, and complexity. Because of increasing demands in textiles, synthetic organic dyes are extensively used in the dying process for textile fiber like polyester and cotton [4]. Besides the textile industry, the use of synthetic dyes is finding increased application in industries as petroleum, leather, paper, food products and plastics [5]. The dyes are used to color the products and the residuals are discharged into the environment, particularly aquatic environment. Synthetic dyes are difficult to biodegrade because of their complex aromatic nature. Thus, the removal of dye color from wastewater is a challenging problem. The wastewater containing textile dyes are toxic and carcinogenic. Synthetic dyes such as azo dyes (having one or more azo bonds) account for more than half of the usage of dying in the textile industry and hence are the major source of pollutants in the wastewater. Unfortunately, the dyes, mostly azo dyes with aromatic structures, are recalcitrant in nature. The azo dyes are difficult to degrade by aerobic digestion and steady in oxidizing agents [6]. Additionally, wastewater from the dying processes is at a high temperature and different pH, allowing it to hold a high amount of the color elements. These concerns have been directed to new and/or stringent rules regarding wastewater discharge from the dying industry and require more effective treatment methods.
Though problem of color removal/COD removal finds sufficient reference in recent research, no significant work has been on the dye degradation. Photo catalytic process for water treatment is generally preceded by decolorization that is removal of the dye color at the wavelength of maximum absorption. This requires study of degradation of dye color before photocatalysis is carried out. Most of the previous work is focuses on degradation efficiency in context of color removal and/or COD removal, thereby wrongly reflecting color removal as dye degradation [7, 8]. Depending on the method used for wastewater treatment, the dye may be existing in the wastewater in its existing or in its degraded form. This demands both dye degradation and color removal be studied independently to measure the true efficiency of water treatment method used.
Most of the techniques do not reduce contaminants but they transfer pollutants from one stage to another stage. Some techniques are selective but slow to moderate in degradation rate. Other alternative methods produce more harmful intermediates and some methods require more treatment time [9]. The inability of traditional wastewater treatment techniques to effectively destroy the bio-recalcitrant contaminants led to exploring new alternatives. The advanced oxidation processes (AOPs) has appeared as one of the most efficient technologies for the degradation of the contaminants/pollutants/toxicants [10]. AOPs are further classified as homogeneous and heterogeneous processes.
In the process of heterogeneous photocatalysis, the near UV irradiation is use to the photo-excitation of a semiconductor-based photocatalyst in the existence of oxygen. Beneath the given conditions, oxidizing agents, either bound hydroxyl radicals or generate free holes. Using heterogeneous photocatalysis, organic pollutants (textile dyes) could be totally mineralized, reacting with the oxidizing agents to produce CO2, H2O and a low concentration of organic acids. Instead of near UV region, this process could be carried out using the close to the solar band (λ < 380 nm) [1]. The semiconductor-based catalyst used in the analysis can either be in powder form suspended in the water or it may be fixed on a solid support.
Among the various semiconductors employed, the anatase phase of TiO2 (like WO3, TiO2, ZnO, Fe2O3, CdS, ZnS, V2O5, ZrO2, SnO2, CeO2, and Sb2O4) is known to be a good photocatalyst for the degradation of several pollutants due to its high photosensitivity and large bandgap. The large bandgap of TiO2of the order of 3.2 eV makes it difficult for charge carriers to be generated by thermal excitation. This instead requires absorption of the photon with energy ≥ 3.2 eV, which corresponds to UV light of wavelength < 380 nm.
Parameters affecting the photocatalytic degradation of organic dyes have been studied such as initial dye concentration, photocatalyst dose, initial pH, light irradiation and reaction time [11–14]. The photocatalysis of organic dye degradation also depends on the geometry of the reactor i.e. area to volume ratio. This parameter has not been considered so far for the modelling of photocatalysis of organic dye degradation. The complexity of the processes limits the conventional mathematical modeling techniques [15] and necessitates the use of multivariate correlation [16] to solve these complex quandaries.
Artificial neural networks (ANNs) are efficient modeling and optimization tool capable of effectively solving the nonlinear systems, especially when of more than one process parameters are involved. ANN is capable to solve the system without requiring previous information of the interactions of process variables ([17]). Kasiri et al [18] applied ANN for the degradation of Acid Red 14 dye by the photo-Fenton process and found that the initial concentration of the dye and initial pH to be more influential parameters in the degradation process. Khataee [19] applied ANN for the degradation of Basic Red 46 by heterogeneous photocatalysis and found that the reaction time, pH, initial dye concentration and UV light intensity have the importance of 26.75, 27.03, 30.75 and 15.47%, respectively. Khataee and Zarei [20] and Zarei et al [21] developed an ANN model with feedforward backpropagation for the photodegradation of Direct Yellow 12 and Basic Red 46 dyes using photoelectron-Fenton process and found a reasonable predictive performance. To choose the model of neural network different approaches have been evolved, most commonly being the hit and trial method. The number of the input layer and output layer neurons come from the inputs and outputs used. For hidden layer hit and trial method of a testing different number of neurons in one or two hidden layers and evaluating each topology on basis of MSE we identified an ANN model with 6:7:2:2 structures. From literature, only single work [22] could be identified with using ANN model with two hidden layers having a large number of neurons as 24 and 15, respectively in hidden layer 1 and hidden layer 2. In many cases, a large number of neurons in hidden layer may cause of over train the network. Every model has the significant contribution of input parameters by which it has developed. So far the influence of parameters for developing the model or percentage importance of parameters for model development has not been studied.
Previous studies in this field have primarily focused on traditional one factor at a time experiments. Analyzing one factor individually not only makes the process time consuming and tedious but is marred by the inability to capture the effect of interaction between different the parameters. Since photocatalytic degradation analysis requires study of more than one factor, conventional optimization techniques are insufficient to study the complex interactions involved. Response surface methodology (RSM) in systematic optimization is a suitable technique capable of addressing the complexity of more than one influencing factors. It makes possible to estimate linear interaction and quadratic effects of the input process parameters and to establish a mathematical relationship for the prediction of the responses. RSM has been used to optimize process parameters for various processes [23–26]. Chao and Zoh [27], Khataee et al [28], and Khataee et al [29], have also applied RSM methodology to model and optimize UV/TiO2, photo-Fenton, and visible light/TiO2 processes.
In the present work, both ANN coupled with RSM have been used for optimizing the photocatalysis process. The proposed study was undertaken with the objectives: (1) Degradation of Acid Red 114 by the photocatalytic process using UV/TiO2 and optimizing the parameters like pH, catalyst loading, initial concentration of dye, area to volume ratio, UV light intensity and time. (2) Modeling and optimization of the process parameters by artificial neural network and response surface methodology (3) comparison of the results obtained by ANN, RSM, and experiments (4) Reaction kinetics and total mineralization study by total organic carbon (TOC) analyzer. (5) Identification of relative contribution of the parameters for decolorization and degradation
2. Materials and Methods2.1. Photo Degradation of AR114The photocatalytic degradation studies of AR114 (Sigma Aldrich) were conducted using a shallow pond slurry reactor as discussed in our previous article [11]. A batch type slurry pond reactor was fabricated for performing the experiments. A Borosilicate glass vessel of 250 mL volume was used as the shallow pond slurry reactor and was irradiated with UV rays. The photo-reactor was fitted with 8 UV black tubes of 36 W each (make of Philips Inc.), fitted in parallel on the top of the reactor. The UV lamps emit radiation in the range of 300–400 nm, with the peak intensity at 350 nm. This reactor is placed on a magnetic stirrer to possess the slurry in the reactor well mixed so that the TiO2 (Degussa P25) remains suspended and the concentration of the pollutant within the reactor could be assumed to be constant at any time.
The adsorption/desorption of dye on the catalyst was allowed for 30 min in dark condition. An aliquot of 5 mL was taken from the reactor with the help of a syringe. After 30 min, UV lights were switched on. The door of the UV chamber was kept closed so that no UV rays come out. After the regular time interval of 30 min, 5 mL sample is withdrawn from the reactor with the help of a syringe and diluted by 1:1 ratio each time. This process of photocatalysis was allowed to continue for the next 180 min. With an objective to filter the suspended TiO2 particles from the dye sample, centrifugation is done at the high speed of 15,000 rpm for 10 min. While centrifugation is done it has to be ensured that the level of the sample in each centrifuge tube is same. As a result, the TiO2 particles get settled at the bottom of the tube.
A computer-based UV spectrophotometer was used for determining the concentration of samples. The decolorization and degradation efficiency were measured in terms of absorbance using spectrophotometer at wavelengths 522 and 373 nm, respectively. The conversion percent of dye has been obtained at different intervals. The degradation and decolorization (Y) are given by Eq. (1)
Where Co is the initial concentration of dye and Ct is the concentration of dye at time t.
Using the set-up described above degradation and decolorization of dye has been determined. A series of experiments have been conducted to achieve optimized values of all the input parameters considered- initial concentration, pH and catalyst loading, UV light intensity, area to volume ratio and time.
2.2. Modeling by Artificial Neural NetworkThe concept of ANN is based on massive parallelism of the human brain making it capable of establishing a relationship between a set of inputs and outputs. Of all ANN Multilayer Feedforward networks are dominant in problem-solving and optimization. It is a fully connected network which generally uses back propagation learning algorithm for the training of network. The basic architecture of the MLF network is as shown in Fig. 1(a).
It comprises an input layer of neurons representing the inputs to the problem and set of output layer neurons representing the output variables. In between the input and output are hidden layers with a variable number of neurons. Depending on the complexity of the problem number of hidden layers may vary from one to any number as required. Further, each hidden layer can have the same or a different number of neurons. Once all layers of the network are defined every neuron in one layer is connected to every neuron in the next layer using a weighted connection. This is called a fully connected network. Ns in hidden and the output layer are governed by a neuron output function and a bias function. The role of the training algorithm used is to change the weights of connections between different layers so as to achieve the desired output.
In our problem, a four-layered feedforward network has been used with two hidden layers. To limit the output between 0 and 1 log-sig has been used as the output function and back-propagation learning algorithm has been used for network training.
2.3. Multi Response Optimization Using RSMRSM is based on the statistical design of experiments [30] evaluating the effects of various process parameters, and identifying optimum conditions for desirable responses [31, 32]. RSM covers a number of statistical techniques including Box-Behnken design (BBD) for model building and the use of the model. BBD helps identify the interactive effects of various input process parameters on the outputs (responses). The number of experimental and/or simulation runs required to evaluate the probable inter-parametric effects on photocatalytic degradation and decolorization of dyes too are optimized using RSM.
Consider a process or system to be examined using RSM with a response E, where E depends on the input parameters p1, p2 … pn. The correlation between the output (response) and the input process parameters is designated by function ‘k’ as:
Where ‘k’ is the real response function and is unknown, ɛ is the residual error. A second-order polynomial, eq. (3), has been used through nonlinear regression to fit the experimental/simulated data and to identify the significant model terms. Ruminating all the linear terms, linear-by-linear interaction terms, and square terms, the quadratic response model can be represented as [33]:
Where m0 is constant, mi is the slope accounting for the linear effect of the input factor pi, mij is the linearly mapped interaction effect between the input factors pi and pj, and mii is the quadratic effect of input factor pi [31].
These designs are preferred for the fitting of the second order models. In general, the second order model is sufficed and provides a good prediction throughout the region of interest. To choose the best model suitable to the problem under consideration generally sequential F-test accompanied by other competence measures are used [34]. The F-test is the most commonly employed method for comparing statistical models that have to fit a data set, used to recognize the model that best fits the population from which the data has been sampled. The least squares method is also a popularly used method to evaluate the parameters in approximating the polynomials. The response surface analysis is then performed using the fitted surface. If the fitted surface is a good approximation of the real response function, the analysis of the fitted surface will be approximately comparable to the analysis of the real system.
In the proposed study multi-response process optimization with desirability function approach has been applied to optimize the photocatalytic process parameters. The desirability, di, lies between 0 and 1, signifying the closeness of a response to its ideal value [33]. Overall desirability De is achieved by the combined effect of individual desirability functions using:
Where d1 and d2 are the individual desirability values for the first and second response, respectively.
RSM based BBD has been used to the simulated data using the Design-Expert software version 11 (STAT-EASE Inc., Minneapolis, US). The simulated data has been analyzed by the regression analysis to fit the equations developed and also for the assessment of the statistical significance of the equations.
3. Result and Discussion3.1. Modeling and Optimization3.1.1. Artificial Neural Network Model DevelopmentA four-layered feed-forward neural network (6:7:2:2) has been used in the proposed work for modeling of Y1 and Y2. The network is trained using a supervised learning algorithm back-propagation. The range of input and output values used in this analysis has been listed in Table 1.
ANN model gives the flexibility to vary the number of hidden layers. To choose the optimized network configuration, initially, one hidden layer was used and the number of neurons in the hidden layer was varied from 2 to 14. Each topology was evaluated for reduction in mean square error (MSE) between expected and achieved outputs. Increasing hidden layer neurons beyond 14 resulted in an increase in MSE. Next, another hidden layer was added with 2 neurons and again each topology with neurons of first layer varying from 2 to 14 results was evaluated for MSE. The lowest value of MSE for both the outputs has been achieved for configuration of (6:7:2:2) as can be seen in MSE variations with hidden neurons in Fig. 1(b). Each topology was carried out twice to remove arbitrary connections if any due to random initialization of weights in the network.
In this work, the proposed network has been first trained on the set of data values of input with known output using Levenberg-Marquardt (LM) algorithm. A data set of 145 values from the experimental data was used for ANN training. To test the results a set of 23 values has been used. The results (Fig. 2) show the regression analysis for predicted results using ANN and experimental values achieved for Y1 and Y2. In Fig. 2 analysis of experimental and predicted values of Y1 and Y2 show a good approximation of output using ANN with R2 values of 0.9924 and 0.9983 Y1 and Y2, respectively, which establishes the ability of proposed ANN model to effectively predict the outputs.
The weight matrix of the developed ANN model has been used to evaluate the relative contribution of the various input parameters towards the output parameters. The expected relative importance of each input parameter on the output is computed based on an equation derived from the partitioning of connection weights of ANN used:
Where Ij is the measure of the relative contribution of the jth input factor on the output. Here subscripts k, l, m, and n represent the input layer, first hidden layer, the second hidden layer, and output layer neurons, respectively. Ni is the number of neurons in the input layer; Nh1 and Nh2 are the numbers of neurons in the first and second hidden layers, respectively. The relative importance of input parameters on outputs, Y1, and Y2 as evaluated using Eq. (5) have been shown in Fig. 3. All parameters showed strong effects on the dye degradation and decolorization efficiencies, the [TiO2]o was most effective than other parameters. A/V is the second largest influence parameter for the degradation and decolorization of AR114.
3.1.2. Statistical Analysis using BBDTo validate the findings of ANN modeling three-level BBD has been applied. BBD evaluates the photocatalysis process parameters which affect the degradation and decolorization efficiency. A range of values of the input parameters- [TiO2]o, [AR114]0, [pH]o, A/V, LI, and t has been used for the optimization.
Table 1 shows the input parameters used along with values and ranges. The 3-factor levels for BBD used can be interpreted as −1 (low), 0 (central point or middle), and 1 (high) ([35]). The simulated data has been analyzed by regression analysis to fit the equations developed and also for the assessment of the statistical significance of the equations. The input parameters used in the present work are given in Table 1. The results of the Y1 and Y2 for photo-degradation of AR114 have been analyzed conferring to the design matrix as proposed by BBD.
As established from results above [TiO2]o, [AR114]o, LI, [TiO2]o * [pH]o, [pH]o * A/V are the highly significant parameters for degradation of AR114. On the other hand [AR114]o, LI, (LI)2, [AR114]o * [pH]0, [AR114]o * LI and [pH]o * LI are the highly significant parameters for decolorization of AR114. Findings of ANOVA are fall in line with the results from previous analysis by 37[] which reported that pH and initial dye concentration are the most significant process parameters for the photocatalytic degradation of methylene blue.
A manual regression method has been used to fit the second order polynomial eq. (3) to the simulated data and to recognize the significant model terms. The model summary statistic shows the regression coefficient (R2) to be the maximum for the quadratic model for the Y1 (R2= 0.9311) and Y2 (R2= 0.9872). For the Y1 and Y2 adequate precision ratios of 11.048 and 26.503, respectively, have been obtained. For a model to efficiently a problem precision ratio of greater than 4 is required. With precision ratio higher than 4 a model effectively explore the design space [36]. The findings of the analysis of variance (ANOVA) for the Y1 and Y2 have been tabulated in Table 2. The F-value of 13.02 and 74.37 Y1 and Y2, respectively establish the significance of the used model. Removal of insignificant terms from ANOVA, reduced quadratic model is obtained which summarizes the outcomes for each response and shows the significant model terms.
As observed from results data points on normal probability plot and a dot diagram of these residuals lie sufficiently nearby to the straight line which justifies the assumptions used in the investigation. Therefore, the models developed are sufficient and the minimum residuals have been obtained from the prediction of each response. The 3D response surface plots of two independent variables, while keeping the other constant, adequately give information on the individual and interaction effects of the independent variables on the responses. Fig. 5(a) and (b) show the 3D response surface plots for photocatalytic decolorization and degradation of AR114, respectively. From Fig. 4(a) and (b), it is clear that the maximum decolorization has been in the light intensity range of 11.18 to 12.00 Wm−2. Results also indicate that the degradation of AR114 increases with an increase in A/V of the reactor (Fig. 4(c) and (d)) i.e. from 0.20 to 0.26 cm−1.
The accuracy of the developed models has been validated by carrying out the confirmatory experimental runs under the given test conditions. The simulation ranges used are as defined earlier. The optimized process parameters at an initial dye concentration of 80 mg L−1 are found to be [TiO2]o = 1.2 g L−1, [pH]o = 3.6, A/V = 0.25 cm−1, LI = 10.22 Wm−2 and T = 150 min.
Comparative analysis of degradation and decolorization efficiency attained by BBD was found to be 1 each, from ANN model, these were 0.99 and 0.985, respectively and from experimental runs 0.988 and 0.968, respectively, using optimum process parameters have been tabulated in Table S1. Results in Table S1 clearly show that the optimization of process parameters for the photocatalytic decolorization and degradation of AR114 can be carried out accurately and satisfactorily using the said method. Using this method fewer simulation runs are required in comparison to other techniques reported in the literature.
3.1.3. Reaction kinetics of degradation and decolorization of AR114The reaction kinetics of degradation and decolorization of AR114 were studied using optimized conditions as shown in Table S1. Results obtained from the UV light irradiation and fitting the first order reaction kinetics (ln Co/C = k′t, where, Co is the initial dye concentration, C is the concentration at any given time t) has been plotted in Fig. 5(a). The reaction rates were found to be 0.253 and 0.0542 min−1 for Y1 and Y2, respectively.
3.1.4. TOC Removal versus degradation and decolorizationTotal Organic Carbon (TOC) is highly significant in terms of identifying the pollutants. TOC is a very superficial, non -vague measurement of all organics contaminates present in a wastewater. Also, low amount of TOC could ensure the lack of possibly toxic organic contaminants in wastewater. In continuation of this, Fig. 5(b) shows the TOC removal versus photo-decolorization and degradation of AR114 on optimized conditions as shown in Table S1. Results show that the Photo-degradation and decolorization of AR114 continuously increasing with respect to time and it is totally decolorized and degraded after 150 min of UV irradiation. But in the case of TOC removal, it gets slow after 30 min of UV light irradiation. But after 150 min UV light irradiation, TOC removal was also observed 98%. This will be due to the conversion of intermediate products; later these products have also been degraded.
3.1.5. Determination of dye degradation products/intermediates in UV/TiO2 processThe intermediate products formed during the photo-degradation of AR114 under UV light irradiation at optimized conditions as shown in Table 2 were examined by GC/MS. It has been tried to recognize the major aromatic compounds resultant from AR114 degradation. The identified intermediate products are shown in Table S2. As it has been shown in Table S2, seven compounds were effectively noticed. It should be noted that some more chromatographic peaks have also been found but could not be completely recognized.
A possible photo-degradation reaction pathway for Acid Red 114 based on GC/MS study has been proposed as shown in Fig. 6. The AR114 dye (poly-aromatic) experienced an asymmetric cleavage by oxidation in presence of hydroxyl radical to form aromatic compounds, aniline derivative, and sulphur based aromatic compounds and intermediates. Further, as the aromatic rings of intermediate compounds break into different cleavage compounds followed by organic acids like carboxylic and aliphatic acids. These non-toxic acids further oxidized in presence of hydroxyl radical and produce carbon dioxide and water.
4. ConclusionsThe present study shows the efficacy of photocatalytic degradation and decolorization of AR114 employing the UV/TiO2. Modeling and optimization results using ANN and RSM are presented for the photocatalytic treatment of AR114. Multi-response optimization by desirability function approach was used to optimize the photocatalytic process variables.
The MSE is found minimum with seven and two neurons for first and second hidden layer, respectively for the decolorization and degradation of AR114 using the Levenberg-Marquardt (LM) algorithm with four-layered learning algorithm back-propagation in ANN network. The relative importance of the input parameters on decolorization and degradation efficiencies show the TiO2 dose as the most effective input parameter in comparison to other input operating parameters followed by area to volume ratio. For the photocatalytic treatment of AR114 dye, the optimum conditions were evaluated. The predicted values for Model F using ANOVA are 74.37 and 13.02 for decolorization and degradation efficiency of AR114, respectively implying that the developed model is significant. The first order kinetics was obtained for decolorization and degradation of AR114. The first order rate constants were found to be 0.0547 min−1 and 0.0253 min−1 for the decolorization and degradation, respectively of AR114. The mineralization of the dye has also been confirmed by removal of TOC as well as GC-MS analysis under the optimum conditions.
NotesAuthor Contributions A.G. (Assistant Professor) conducted all the experiments and wrote the manuscript. G.K. (Assistant Professor) conducted the coding for Artificial Neural Network and wrote the manuscript. V.K.S. (Associate Professor) supervised all experiments closely and made corrections in the manuscript. P.K.B. (Professor) supervised all experiments closely and made corrections in the manuscript. S.U. (Associate Professor) conducted the coding for Artificial Neural Network and wrote the manuscript. References1. Malato S, Blanco J, Vidal A, Richter C. Photocatalysis with solar energy at a pilot-plant scale: An overview. Appl Catal B Environ. 2002;37:1–15.
2. Das S, Srivastava VC. Recent advances in fabrication of photocatalytic micro-reactor. Mater Sci Forum. 2016;855:156–167.
3. Yadav A, Mukherji S, Garg A. Removal of chemical oxygen demand and color from simulated textile wastewater using a combination of chemical/physicochemical processes. Ind Eng Chem Res. 2013;52:10063–10071.
4. Mane VS, Mall ID, Srivastava VC. Use of bagasse fly ash as an adsorbent for the removal of brilliant green dye from aqueous solution. Dyes Pigm. 2007;73:269–278.
5. Aye T, Mehrvar M, Anderson WA. Effects of photocatalysis on the biodegradability of Cibacron Brilliant Yellow 3G-P (Reactive Yellow 2). J Environ Sci Health Part A. 2004;39:113–126.
6. Chen C-Y, Chang J-C, Chen A-H. Competitive biosorption of azo dyes from aqueous solution on the templated crosslinked-chitosan nanoparticles. J Hazard Mater. 2011;185:430–441.
7. Hama Aziz KH. Application of different advanced oxidation processes for the removal of chloroacetic acids using a planar falling film reactor. Chemosphere. 2019;228:377–383.
8. Hama Aziz KH, Mahyar A, Miessner H, et al. Application of a planar falling film reactor for decomposition and mineralization of methylene blue in the aqueous media via ozonation, Fenton, photocatalysis and non-thermal plasma: A comparative study. Proc Saf Environ Protect. 2018;113:319–329.
9. Sharma J, Mishra IM, Kumar V. Mechanistic study of photo-oxidation of bisphenol-A (BPA) with hydrogen peroxide (H2O2) and sodium persulfate (SPS). J Environ Manage. 2016;166:12–22.
10. Sharma J, Mishra IM, Kumar V. Degradation and mineralization of bisphenol A (BPA) in aqueous solution using advanced oxidation processes: UV/H2O2 and UV/S2O8
− oxidation systems. J Environ Manage. 2015;156:266–275.
11. Garg A, Sangal VK, Bajpai PK. Decolorization and degradation of Reactive Black 5 dye by photocatalysis: Modeling, optimization and kinetic study. Desal Water Treat. 2016;57:18003–18015.
12. Chatterjee D, Patnam VR, Sikdar A, Joshi P, Misra R, Rao NN. Kinetics of the decoloration of reactive dyes over visible light-irradiated TiO2 semiconductor photocatalyst. J Hazard Mater. 2008;156:435–441.
13. Kansal SK, Kaur N, Singh S. Photocatalytic degradation of two commercial reactive dyes in aqueous phase using nanophotocatalysts. Nanoscale Res Lett. 2009;4:709–716.
14. Mahadwad OK, Parikh PA, Jasra RV, Patil C. Photocatalytic degradation of reactive black-5 dye using TiO2 impregnated ZSM-5. Bull Mater Sci. 2011;34:551–556.
15. Soleymani AR, Saien J, Bayat H. Artificial neural networks developed for prediction of dye decolorization efficiency with UV/K2S2O8 process. Chem Eng J. 2011;170:29–35.
16. Aleboyeh A, Kasiri MB, Olya ME, Aleboyeh H. Prediction of azo dye decolorization by UV/H2O2 using artificial neural networks. Dyes Pigm. 2008;77:288–294.
17. Dutta S, Parsons SA, Bhattacharjee C, Bandhyopadhyay S, Datta S. Development of an artificial neural network model for adsorption and photocatalysis of reactive dye on TiO2 surface. Expert Syst Appl. 2010;37:8634–8638.
18. Kasiri MB, Aleboyeh H, Aleboyeh A. Modeling and optimization of heterogeneous photo-fenton process with response surface methodology and artificial neural networks. Environ Sci Technol. 2008;42:7970–7975.
19. Khataee AR. Photocatalytic removal of C.I. Basic Red 46 on immobilized TiO2 nanoparticles: Artificial neural network modelling. Environ Technol. 2009;30:1155–1168.
20. Khataee AR, Zarei M. Photocatalysis of a dye solution using immobilized ZnO nanoparticles combined with photoelectrochemical process. Desalination. 2011;273:453–460.
21. Zarei M, Khataee AR, Ordikhani-Seyedlar R, Fathinia M. Photoelectro-Fenton combined with photocatalytic process for degradation of an azo dye using supported TiO2 nanoparticles and carbon nanotube cathode: Neural network modeling. Electrochim Acta. 2010;55:7259–7265.
22. Piuleac CG, Curteanu S, Rodrigo MA, Sáez C, Fernández FJ. Optimization methodology based on neural networks and genetic algorithms applied to electro-coagulation processes. Cent Eur J Chem. 2013;11:1213–1224.
23. Kalil SJ, Maugeri F, Rodrigues MI. Response surface analysis and simulation as a tool for bioprocess design and optimization. Process Biochem. 2000;35:539–550.
24. Mannan S, Fakhru’l-Razi A, Alam MZ. Optimization of process parameters for the bioconversion of activated sludge by Penicillium corylophilum, using response surface methodology. J Environ Sci. 2007;19:23–28.
25. Kumar A, Prasad B, Mishra IM. Process parametric study for ethene carboxylic acid removal onto powder activated carbon using Box-Behnken design. Chem Eng Technol. 2007;30:932–937.
26. Agnihotri S, Kaur P, Sangal VK, Garg A, Verma A. Parametric study for the treatment of simulated cetirizine wastewater using electrochemical methods: Optimization and cost analysis. J Electrochem Soc. 2018;165:E556–E562.
27. Cho I-H, Zoh K-D. Photocatalytic degradation of azo dye (Reactive Red 120) in TiO2/UV system: Optimization and modeling using a response surface methodology (RSM) based on the central composite design. Dyes Pigm. 2007;75:533–543.
28. Khataee AR, Zarei M, Moradkhannejhad L. Application of response surface methodology for optimization of azo dye removal by oxalate catalyzed photoelectro-Fenton process using carbon nanotube-PTFE cathode. Desalination. 2010;258:112–119.
29. Khataee AR, Zarei M, Moradkhannejhad L, Nourie S, Vahid B. Nitrogen doping of commercial TiO2 nanoparticles for enhanced photocatalytic degradation of dye under visible light: Central composite design approach. Adv Chem Lett. 2013;1:24–31.
30. Vaez M, Zarringhalam Moghaddam A, Alijani S. Optimization and modeling of photocatalytic degradation of azo dye using a response surface methodology(RSM) based on the central composite design with immobilized titania nanoparticles. Ind Eng Chem Res. 2012;51:4199–4207.
31. Box GEP, Hunter JS. Multi-factor experimental designs for exploring response surfaces. Annal Math Stat. 1957;28:195–241.
32. Box GEP, Behnken DW. Some new three level designs for the study of quantitative variables. Technometrics. 1960;2:455–475.
33. Sangal VK, Kumar V, Mishra IM. Process parametric optimization of a divided wall distillation column. Chem Eng Commun. 2014;201:72–87.
34. Muthukumar M, Mohan D, Rajendran M. Optimization of mix proportions of mineral aggregates using Box Behnken design of experiments. Cem Concr Comp. 2003;25:751–758.
35. Evans M. Optimization of manufacturing processes: A response surface approach. London: Maney Materials Science; 2003.
36. Sangal VK, Kumar V, Mishra IM. Optimization of structural and operational variables for the energy efficiency of a divided wall distillation column. Comput Chem Eng. 2012;40:33–40.
37. Sahoo C, Gupta AK. Optimization of photocatalytic degradation of methyl blue using silver ion doped titanium dioxide by combination of experimental design and response surface approach. J Hazard Mater. 2012;215–216:302–310.
Fig. 2Comparison of the experimental results with those predicted values via ANN modelling for the test set (a) Y2 and (b) Y1. 
Fig. 3Relative contribution of the parameters for (a) Decolorization and (b) Degradation efficiencies, respectively. 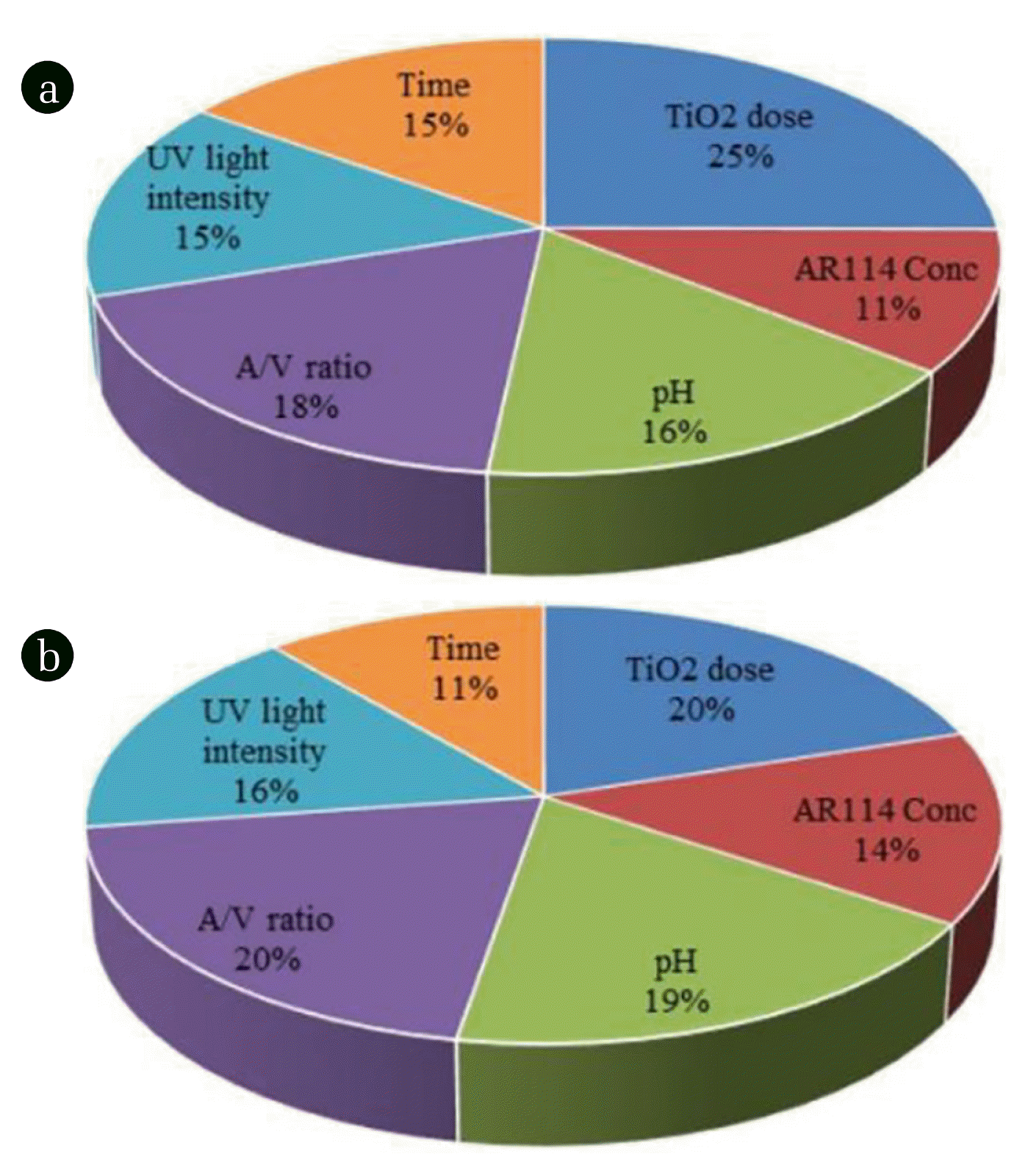
Fig. 43D plots from BBD for AR114 (a) LI Vs [TiO2]o for the Y2, (b) LI Vs [AR114]o for the Y2, (c) A/V Vs [TiO2]o for the Y1, (d) A/V ratio Vs [pH]o for Y1. 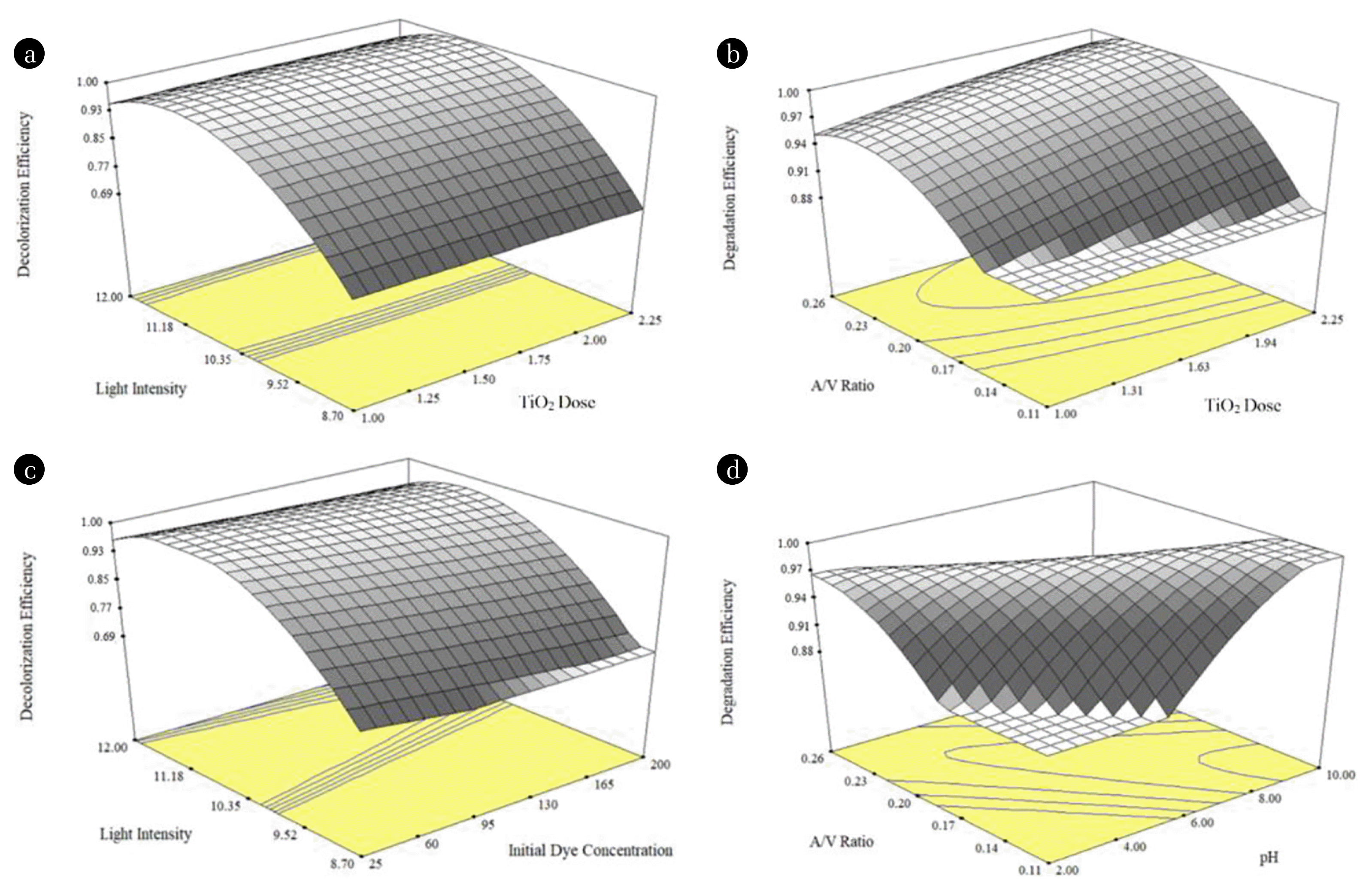
Fig. 5(a) Reaction kinetics of AR114 (b) Dye degradation, decolorization, and TOC removal (Reaction conditions: [Dye]o = 80 mg L−1, [TiO2]o = 1.2 g L−1, [pH]o = 3.6, A/V = 0.25 cm−1, LI = 10.22 Wm−2) 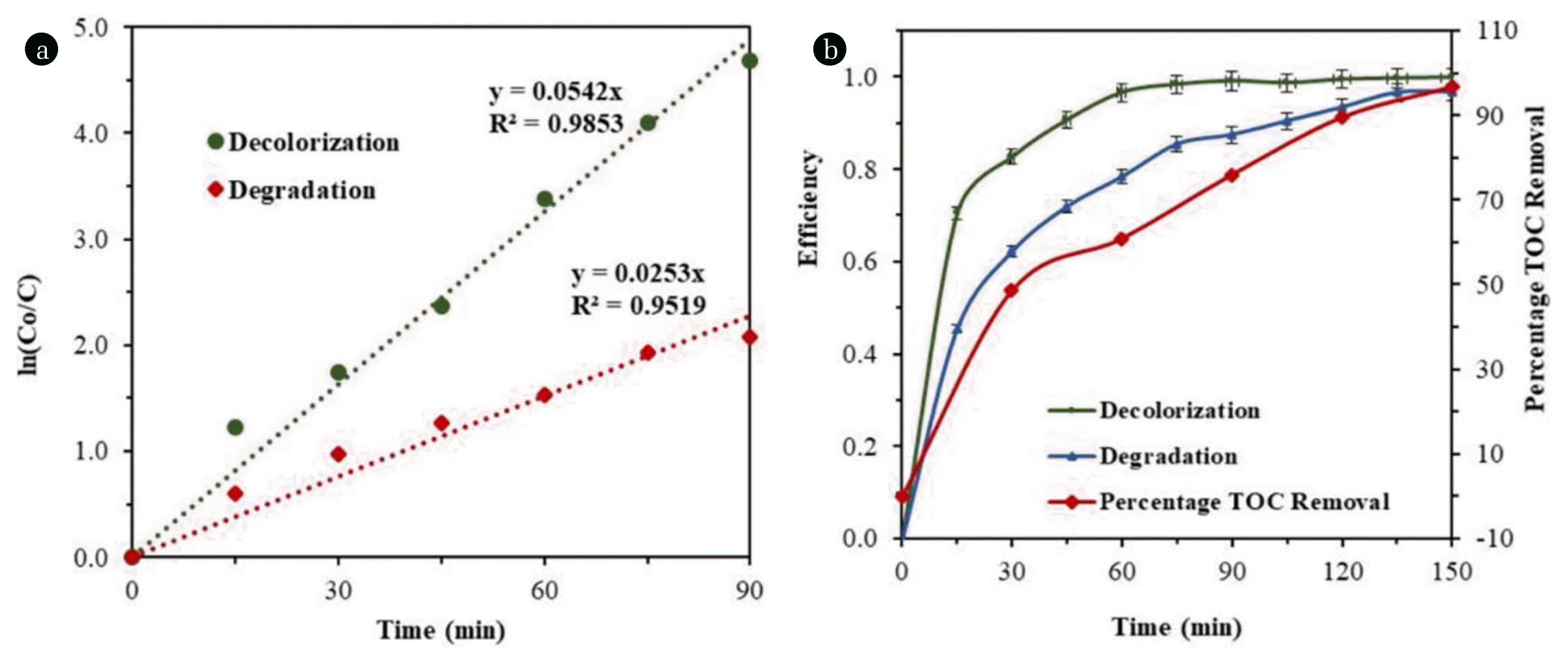
Table 1Results of 3 Level BBD for Chosen Variables
Table 2Results of ANOVA Model for Y1 and Y2
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||