1. Introduction
The use of synthetic dyes is an inevitable part of society’s progress resulting in a massive industry with an annual production of about 7 × 105 tons per year [1, 2]. The effluent produced from large industry is a major cause of environmental pollution. Synthetic dyes are known to be carcinogenic, and although standards have been developed, the regulations of its discharge to the environment are seldom being monitored and implemented strictly, especially in developing countries [3].
Methylene blue is the most frequently used substance in dying cotton, wood, and silk. Methylene blue is a heterocyclic aromatic chemical compound (C16H18ClN3S3H2O) and is very toxic to humans and the environment [2, 4]. It is known to have several harmful effects to man including increased heart rate, vomiting, shock, Heinz body formation, cyanosis, jaundice, quadriplegia, and tissue necrosis [5–7]. When released to the environment even at a very low concentration (1 ppm), the presence of methylene blue could reduce the sunlight transmittance and inhibit the photosynthetic process, thus affecting the aquatic ecosystem [8]. Hence, treatment of effluents contaminated with this artificial dye must be of utmost concern.
Removal of methylene blue from contaminated wastewater has been performed through precipitation, flocculation, coagulation, flotation, ion exchange, membrane filtration, electrochemical degradation, ozonation, bioremediation, and adsorption [4, 9]. Precipitation, flocculation, coagulation, flotation are basic physico- chemical processes that generates large amounts of sludge resulting in high disposal costs. Also, employment of ion-exchange process is expensive, and does not accommodate wide range of dyes [2, 10]. Membrane filtration is a pressure-driven separation process that can mechanically and chemically sieve contaminants but is a costly and a high-maintenance process [11]. Electrochemical degradation uses electrodes to oxidize dyes, but stability is an issue along with the energy requirement [12].
On the other hand, biological treatments utilize algae, bacteria, fungi, and yeasts to disintegrate dyes but require large areas and less tractability in operation [11]. There are also treatment methods that utilize synergistic mechanisms of different dye removal methods such as biomimetic membranes [13] and utilization of binary or ternary oxide systems [14]. But adsorption remains the fundamental simple and effective method. Adsorption is the adhesion of dyes to a surface and activated carbon has been one of the most effective adsorbents in removing dye from contaminated wastewater, however commercial activated carbon is expensive, limiting the practicality of its use [15–17]. Activated carbon derived from coal and various carbon-rich biomass have varied adsorption capacities depending on raw materials used, history of preparation and treatment conditions, further study is still necessary to clearly establish the sorption mechanisms of these materials [18]. An alternative effective dye adsorbent is clay minerals due to their incredible textural properties, abundance, and inexpensiveness. However, clay particles are difficult to remove from the solution after adsorption, which makes the regeneration quite difficult, and when regenerated its adsorption capacity decreases significantly [19]. There is, therefore, a need to discover alternative materials that are cost-effective and abundant to address the limitations of the existing technologies in treating dye-contaminated wastewaters.
The utilization of abundant natural materials and certain waste products from industrial or agricultural operations as low-cost adsorbents for dyes is a sustainable approach. Cornhusk [7], corn cob [2], sawdust [3], rice husk [5], coconut shell fibers [11], banana pith [11] and industrial residues like fly ash, sludges, and furnace slags [20] are just a few of the reportedly used adsorbents for dye removal. Agricultural residues are cheap and available in very large quantities, making them a very good alternative for the expensive commercial activated carbon for wastewater treatment. Reutilization of agricultural wastes will help reduce the cost of its disposal, providing an additional market for the agricultural sector [21]. Rice husk is an agricultural waste produced in large quantities worldwide. The Philippines, being one of the world’s biggest rice consumers with an average Filipino consuming about 100 kg per year of rice, has ~ 2 million tons yearly generation of rice husk (RH) [22, 23]. The rice husk ash (RHA) derived from combustion of RH has very good adsorptive properties. RHA has been utilized as an effective and low-cost adsorbent for dyes and metals [5, 10], various organic and inorganic compounds, gold–thiourea complex refining, and air purifier in the cleaning of atmosphere contaminants [24].
Although proven to be very effective in removing pollutants from wastewater, the common disadvantage of adsorbents derived from these natural sources is its difficulty of separation from the wastewater stream. In addressing such problem, magnetic adsorbents have been developed usually synthesized by embedding magnetic particles such as oxides of Fe, Co, Ni, and Cu to the adsorbent base [20]. Due to its low cost and low toxicity, Fe3O4 nanoparticles are commonly used in the preparation of magnetic adsorbents [25] and hence were used in this study. Separation of the adsorbent from the wastewater stream, therefore, can be rapidly and easily achieved by application of an external magnetic field [20, 26].
In this study, the researchers obtained the RHA from the local markets of Davao City, Philippines. It is an agricultural waste which is a by-product of open-air burning of rice husks by local farmers, often left unused and sometimes collected and regarded only as cleanser for charred utensils. Its additional value as recyclable methylene blue adsorbent was investigated to exemplify direct utilization of agricultural residues locally. Also, this will serve as an upcycling route as a sustainable solution for managing solid wastes. The magnetic rice husk ash (MRHA) was synthesized by the addition of iron oxide to bare RHA. Its kinetics, isotherm, and optimal adsorption conditions in terms of contact time, initial dye concentrations, initial pH, and adsorbent dosage were determined. Recyclability of the magnetic rice husk ash was also evaluated based on its adsorption and desorption capacity for each cycle.
2. Experimental
2.1. Materials
White RHA was purchased from the local market of Davao City (Bankerohan). Isopropyl alcohol (≥ 99.8% isopropanol, Scharlab S.L., Spain) was used for RHA washing. Iron(III) chloride hexahydrate (≥ 99.0% FeCl3·6H2O) and iron(II) sulfate heptahydrate (≥ 99.0% FeSO4·7H2O) from HiMedia Laboratories Pvt. Ltd. (India), were used to attribute magnetic properties to RHA. Methylene blue (MB, C16H18N3CIN3S•xH2O, x = 2 – 3), MW = 319.85, λmax = 664 nm) supplied by Scharlau Chemie S.A. (European Union) was used for preparing synthetic dye solutions for adsorption experiments. Ammonium hydroxide (25% NH3 in H2O, NH4OH, Scharlau, Spain) and hydrochloric acid (37% HCl, RCI Labscan Limited, Thailand) were used to adjust the pH of MB solution. All chemicals were used as received.
2.2. Preparation of Rice Husk Ash (RHA)
RHA was sieved (60-mesh screen), washed with distilled water, and dried at 80°C for 12 h. Afterwards, RHA was stirred in isopropanol for 2 h (room temperature), washed with distilled water until the colorless filtrate was obtained, and then dried at 80°C for 12 h.
2.3. Synthesis of Magnetic rice husk ash (MRHA)
RHA was functionalized with Fe3O4 as described in an earlier study with minor modification [27]. Briefly, 2:1 molar proportion of FeCl3·6H2O and FeSO4·7H2O respectively were stirred in distilled water (40 mL) for 1 h. NH3·H2O (10 mL) was added drop by drop to the solution and kept at 60°C for 2 h in an oven. RHA (2 g) was added to the solution, mixed for 1h and oven-heated at 80°C for 2 h. The resulting magnetic RHA particles were collected using an external magnet, rinsed with distilled water (thrice), and dried at 90°C for 12 h.
2.4. Characterization of the RHA and MRHA
Morphological property of the as-grown samples were analyzed using a scanning electron microscope (SEM, Hitachi SU150, Japan) equipped with energy dispersive X-ray (EDX). Fourier-transform infrared (FTIR) spectra of samples were recorded with KBr pellet using a Shimadzu 8400 spectrophotometer. Porosity and surface area of RHA and MRHA was determined by N2 adsorption – desorption at 77 K with a Brunauer-Emmett-Teller (BET) analyzer (BEL: BELSORP-MINI, Japan).
2.5. Adsorption Experiments
The adsorptive properties of MRHA were investigated at varied adsorption times, initial dye concentrations, initial pH, and adsorbent dosage to determine its kinetics, isotherm, and optimal adsorption conditions. Every batch adsorption run was done at room temperature. MRHA were dispersed in a certain volume of dye solution (V) and agitated (150 rpm) at a specified time (t). Synthetic MB solutions were prepared by dissolving the dye powder in distilled water (DW) at desired initial concentration (Co). Samples were taken after the MRHA were magnetically separated (within 20 s) from the solution to determine the concentration at time t(Ct) or until equilibrium dye concentration (Ce) was reached. Dye removal efficiency (% RE, Eq. (1)) [2, 3] and equilibrium adsorption capacities (Eq. (2)) [4] were determined considering the mass of MRHA (m) dispersed into the dye solution.
Adsorption kinetic studies were conducted by using dye solutions with Co = 40 mg L−1 and then measuring the dye uptake (qt) of MRHA at varied t until equilibrium was attained (Eq. (3)) [2]. Adsorption isotherm was carried out at Co = 5–200 mg L−1, whereas the investigation of the effect of MRHA loading at S/L = 5–20 g L−1 with Co = 40 mg L−1. Adsorption at different initial pHs was done using dye solutions with Co = 40 mg L−1 and initial pHs were adjusted to the desired value by using pure NH4OH and HCl (0.1 M) solutions.
2.6. Recyclability of MRHA
The reusability of MRHA retaining its removal efficiency was investigated through cycled adsorption/desorption runs in MB dye solutions (Co = 40 mg L−1, pH 7) performed at room temperature with S/L = 10 g L−1 and t = 10 min. After every adsorption run, MRHA was separated from the solution by an external magnet and washed with DW. It was then dispersed in a 0.1 M NaOH, agitated (150 rpm) for 10 min. It was then washed with DW several times and dried. Regenerated MRHA with known mass was then used for subsequent adsorption experiments. Desorption efficiency (% DE, Eq. (4)) [17] was also determined in every cycle considering the amount of MB adsorbed onto MRHA (mads) relative to the amount of desorbed MB in the solution (mdes).
2.7. Analytical Methods
Concentrations of collected liquid samples were determined with the aid of UV-Visible spectrophotometer (Shimadzu UV-VIS: UV-MINI1240, Japan). The absorbance of different dye solutions with known concentrations (0.05–10 mg L−1) was measured using UV-vis. A calibration graph relating the dye solution to its concentration was generated and used for determining concentration of other samples. A separate calibration curve was also prepared for a varied concentration of MB in 0.1M NaOH solution for the desorption samples.
3. Results and Discussion
3.1. Characterization
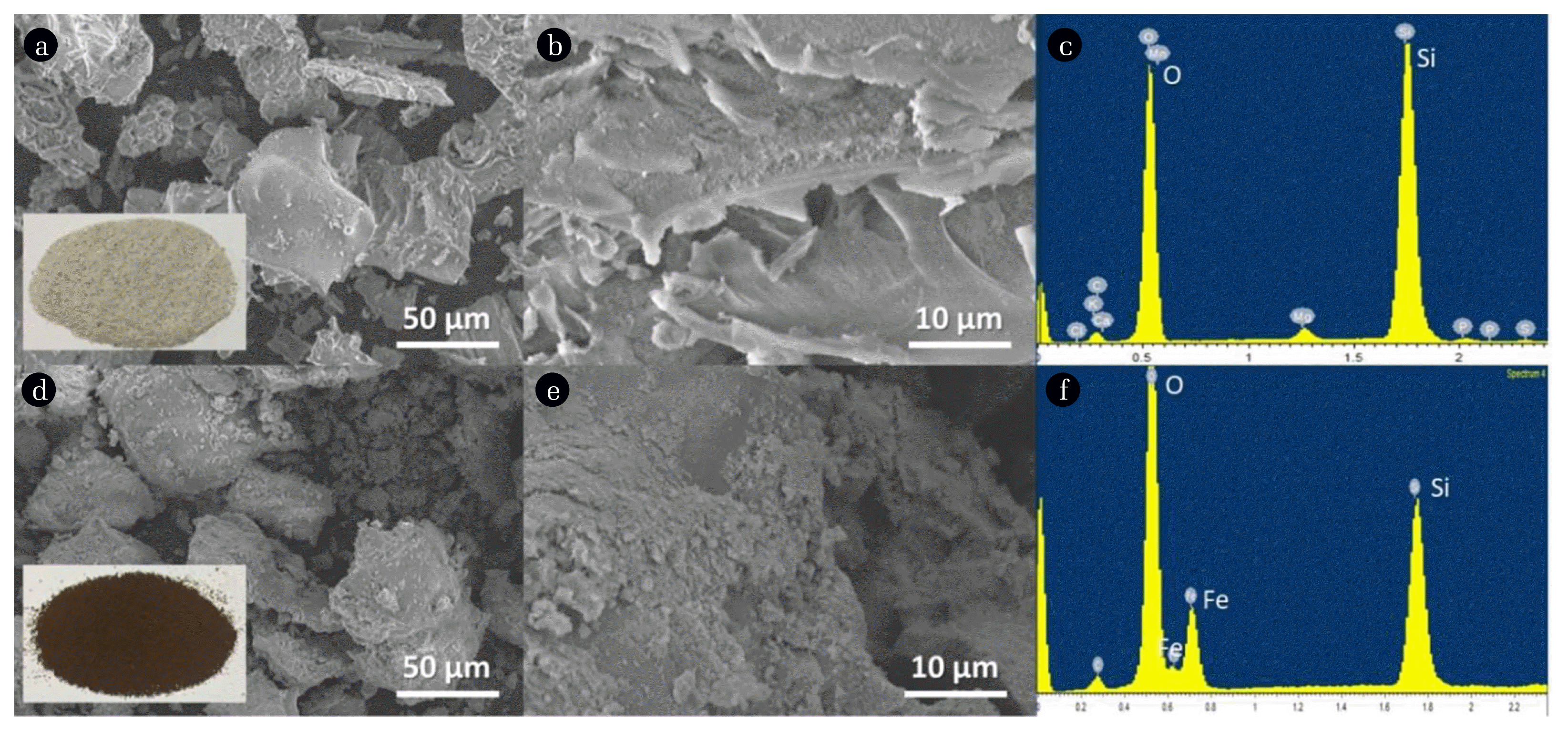
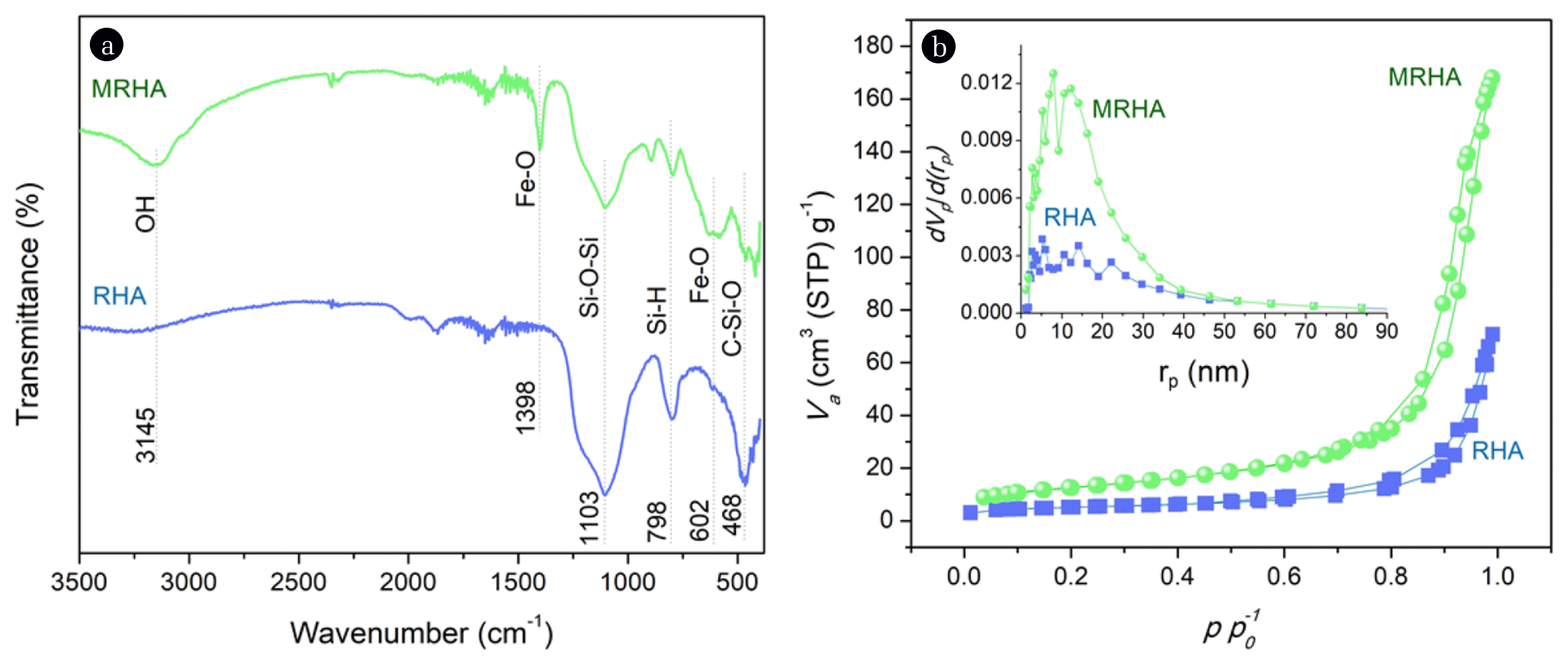
RHA (Fig. 1(a) inset) used in this study has predominantly white color signifying that it contains a high amount of silica resulting from the combustion of rice husks in the air [28]. Its SEM images (Fig. 1(b), (c)) revealed irregular shapes while the blackened MRHA (Fig. 1(d) inset) structurally resembles RHA but has more uniform morphology basing on its SEM images (Fig. 1(d), (e)). Also, RHA is confirmed to be predominantly composed of silica (Fig. 1(c)) and upon functionalization presence of Fe and O elements are observed in MRHA (Fig. 1(f)). FTIR spectrum (Fig. 2(a)) showed that only the MRHA has peaks at 602 and 1398 cm−1 which are related to the Fe-O bending and stretching vibration respectively [29, 30]. Both for RHA and MRHA the peaks observed at 468, 798, 1103, 3145 cm−1 indicate the silica functional groups of C–Si–O, Si-H, Si–O-Si and -OH stretching respectively [29, 31, 32]. The N2 adsorption-desorption isotherms of RHA and MRHA showed Type-IV isotherm with H3-type hysteresis (Fig. 2(b)) indicating the presence of mesopores supported by the BJH pore size distribution [33, 34]. Pore size between 2 to 50 nm is classified as mesoporous according to IUPAC [35]. MRHA has a higher surface area and mesopore volume (Table S1) compared to RHA possibly caused by the presence of ferrite nanoparticles which is known to have a large surface area and pore volume [36]. Primarily, the higher mesopore volume of MRHA could be due to ferrite particles growing uniformly along the silica framework creating more mesopores [37] and functionalized iron oxide also has reportedly high inherent mesopore volume [29, 30, 37].
3.2. MB Adsorption Mechanism in MRHA
Typically, MB adsorption onto nanoparticles involves diffusion to the adsorbent surface, migration on the adsorbent pores and its interstitial lattices, and monolayer buildup of adsorbate [38]. However, there are several factors that affect the inherent process in the MB uptake of MRHA that warrants further investigation.
3.2.1. Kinetics of MB adsorption
The adsorption of MB onto MRHA increased rapidly as contact time increased (Fig. 3(a)). This is significantly fast as it reached ~ 100% removal after just 10 min. But when contact time is > 7 min increase in qt is sluggish. Removal efficiency increases with time but as equilibrium adsorption approaches the change is no longer significant due to the saturation of active sites on the adsorbent surface [29, 38]. To further elucidate the MRHA’s adsorptive properties, non-linear Lagergren (Eq. (5)) and pseudo-second-rate equations (Eq. (6)) were used where k1 and k2 are the respective rate constants [39, 40].
In considering rates of adsorption, it is assumed that MB diffusion through the bulk solution onto the adsorbent particle was eliminated by vigorous shaking. Results (Fig. 3(b)) show that in the first few min, MB and adsorbent interaction follow the pseudo-first-order model but the whole range of contact time can be effectively described by the pseudo-second-order rate equation. Derived kinetic constants (Table S2) of the non-linear regression analysis showed that the correlation coefficient of the pseudo-second-order is slightly greater than that of Lagergren pseudo-first-order equation. This indicates that the adsorption process follows a chemisorption nature where the rate is limited by the number of active sites in MRHA’s surface [38, 39, 41]. Hence, the MRHA’s high surface area (43.5 m2 g−1) is a contributing factor to the fast adsorption rate.
3.2.2. MB adsorption isotherm
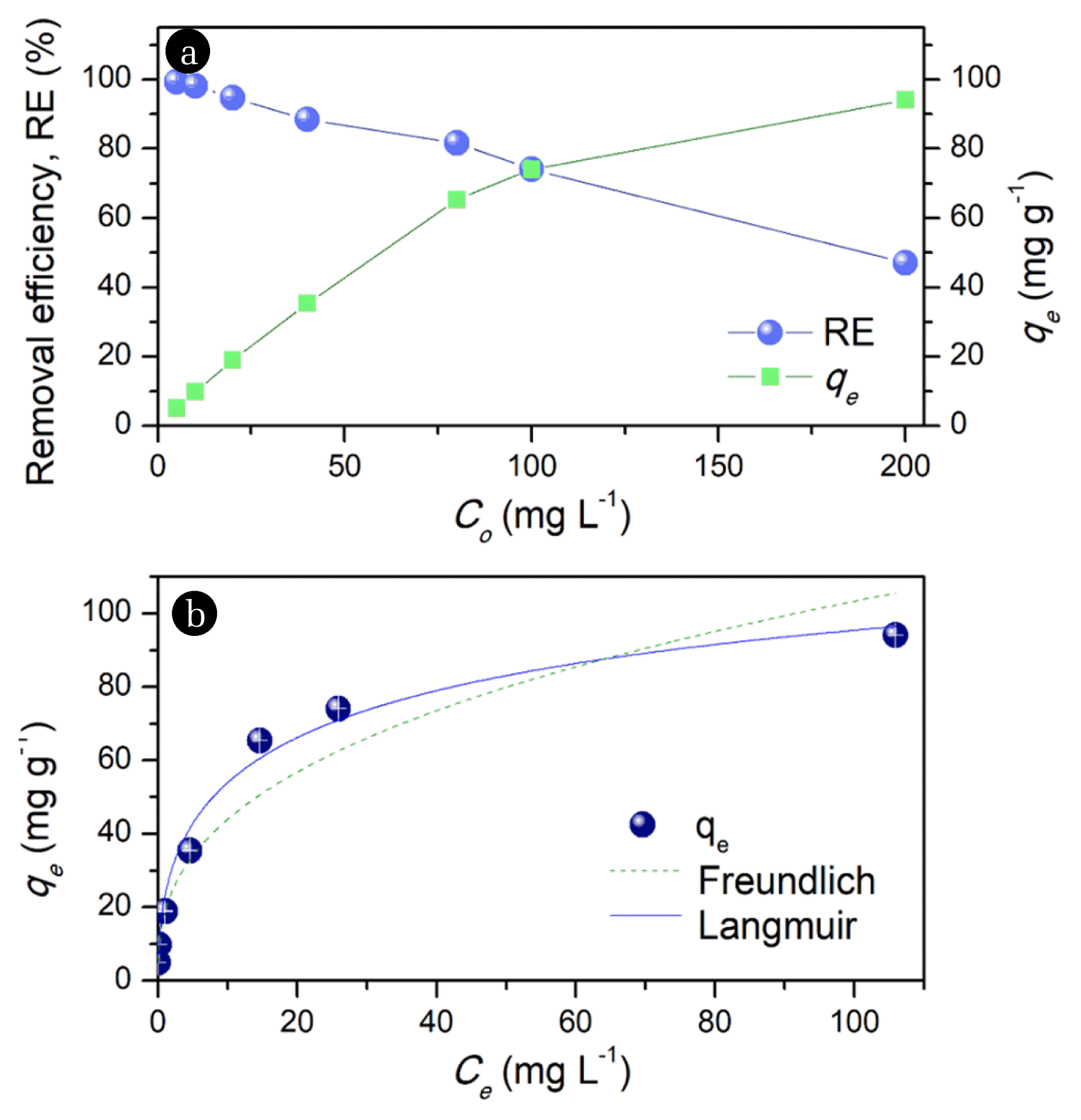
The removal efficiency of the MRHA particles steadily declines while qe significantly improved as the initial MB concentration increased (Fig. 4(a)). This means that the adsorbent has limited and fixed sites for the uptake of MB molecules. To understand the uptake mechanism of MB molecules in MRHA, equilibrium adsorption capacity at various initial concentrations were fitted with Langmuir (Eq. (7)) and Freundlich models (Eq. (8)) [19, 40]. These isotherms relate the amount of MB adsorbed (qe) to the equilibrium MB concentration in the solution (Ce) at a given temperature. Particularly, the linearized Langmuir equation gives the maximum monolayer capacity (qm) relative to the constant for the energy of adsorption (kL) needed for the process. On the other hand, the Freundlich adsorption model has an empirical relative adsorption capacity indicator (kF) in relation to the adsorption intensity (n) of the system.
Results (Table S2) have higher correlation for the Langmuir adsorption model as compared to Freundlich isotherm (Fig. 4(b)). This entails that the adsorption process follows a second order reaction involving an adsorbent capable of binding monolayer of MB molecules on MRHA’s homogeneous and localized sites [42, 43]. Moreover, the qm of the MRHA (150.53 mg g−1) and the energy of adsorption (kL) are significantly comparable to reported values ([Table 1 [2, 4, 7, 15, 38, 39, 42, 44, 45])) for MB uptake. Also, the equilibrium parameter (RL= 0.03, Eq. (9)) of the Langmuir isotherm considering the highest initial MB concentration (Co) indicates a favorable (0 < RL < 1) adsorption process of the system employed [46].
3.3. Effect of Dosage
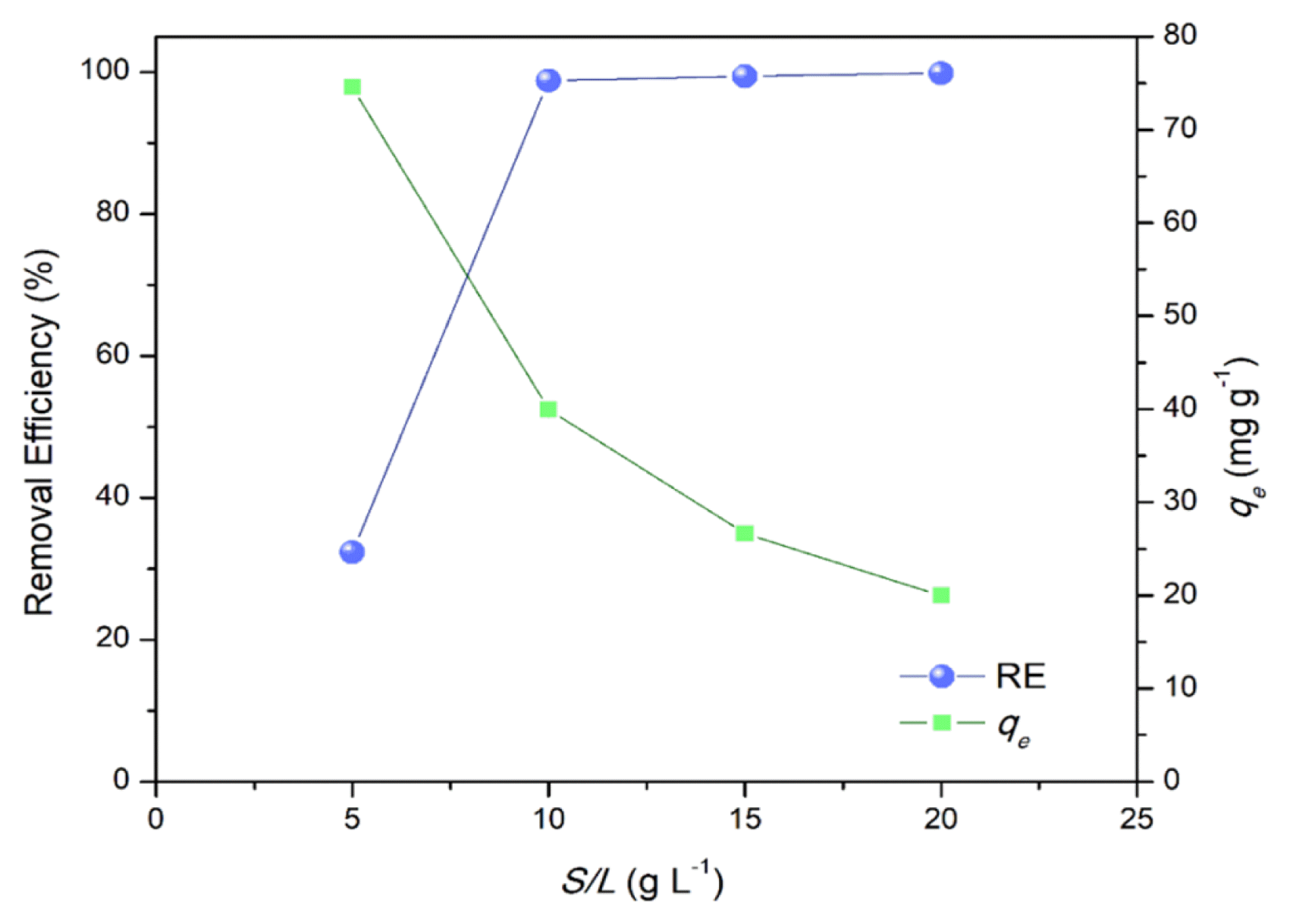
Increasing the adsorbent dosage (S/L) entails an increase in the number of active sites per unit volume of solution, which leads to an increase in the MB removal efficiency (Fig. 5). There is more MRHA surface area for the MB molecules to interact with a high S/L. Hence, a rapid increase in percentage removal as the dosage increased but it became negligible at a higher adsorbent dose (> 10 g L−1), whereas the qe steadily declined. Therefore, for quantitative removal of MB reaching ~ 100% RE an S/L = 10 g L−1 can be considered optimal for the adsorbent system employed. This is comparable to other studies were 100% removal of MB (Co 100 mg L−1) is achieve at S/L = 10 to 50 at a contact time of t < 60 min [5, 17, 47].
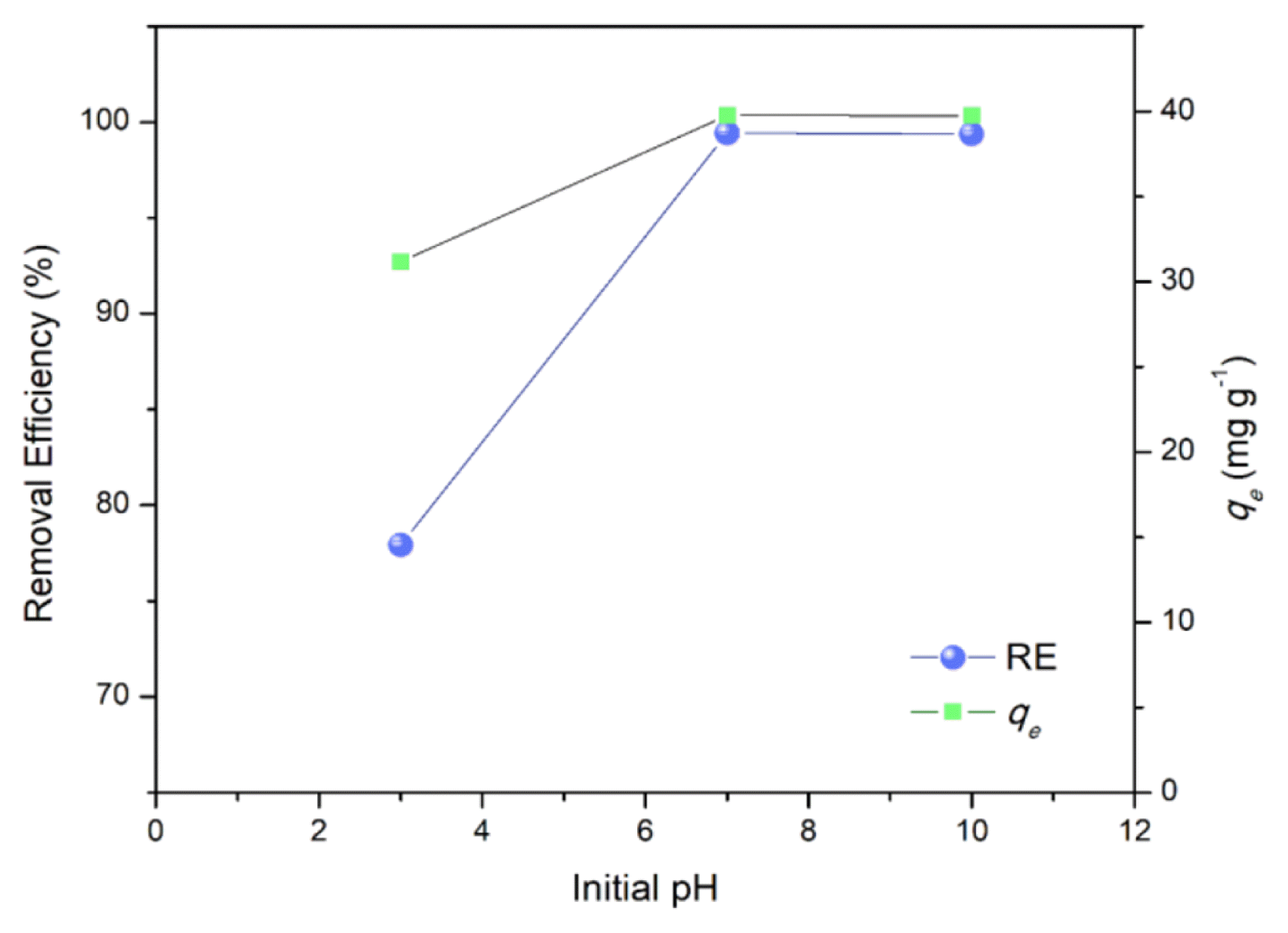
3.4. Effect of pH
The pH of the solution appears to be a key factor affecting the adsorption characteristics of MB onto the MRHA particles (Fig. 6). Increasing the initial pH did not primarily contribute to a significant increase in both removal efficiency and qe but a decrease in pH significantly declined the adsorption performance. Initial pH of the aqueous solution affects the dye adsorption process by changing the adsorbent’s surface charge and its ionization behavior [45]. Hence, the point of zero charge (pHpzc) of the MRHA was determined by mass titration method [48] to better understand the mechanism of MB uptake of MRHA. The pHpzc of MRHA is determined to be 3.8. As the pH increase MRHA surface become more negatively charged, so the adsorption of MB cationic molecules increases when pH > pHpzc [27]. However, at pH < 7, qe plateaued which means no further significant change in the electrostatic interactions between the MB molecules and the MRHA brought by saturation of adsorption sites at the given conditions [49].
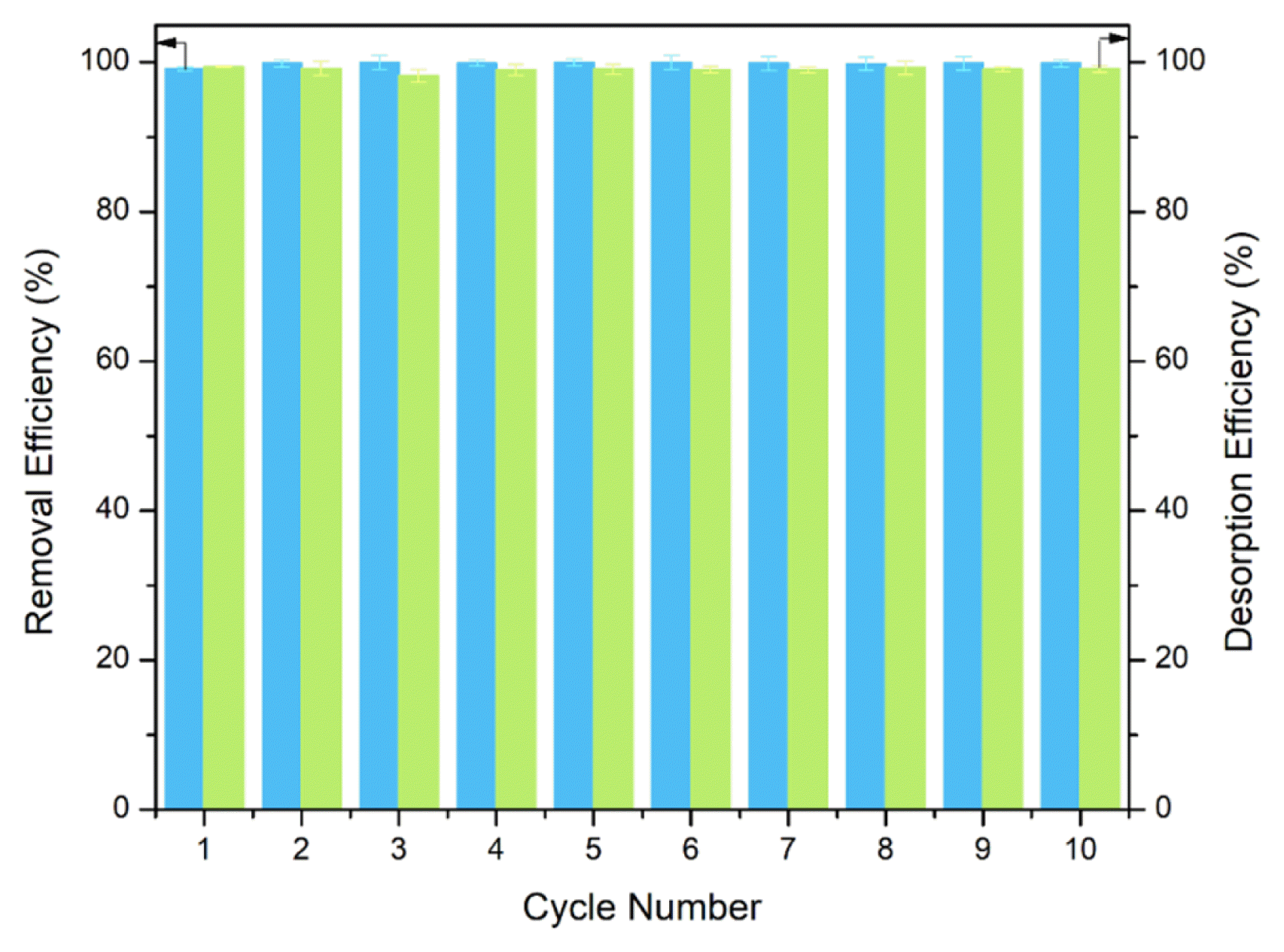
3.5. Recyclability of Magnetic RHA
An effective adsorbent is characterized not only by its high adsorption performance but also through its long-term reusability. MRHA’s recyclability was examined through repeated adsorption and desorption cycles. The apparent optimal conditions from the parameters tested were used as follows: Co = 40 mg L−1, pH = 7, and S/L ratio = 10 g L−1. As can be observed there is only negligible loss in the adsorption capacity of the composite adsorbent throughout the ten cycles (Fig. 7). This directly means that no significant disruption in the structure of the adsorbent hence maintaining initial dye removal efficiency. The reusability of the MRHA while maintaining its adsorption ability as well as its easy handling renders it feasible for more economical and efficient wastewater treatment. Given that handling of MRHA for its use and regeneration can be conveniently done with the use of an external magnet (Fig. S1).
4. Conclusions
MRHA’s MB uptake process is best described by a Langmuir-type behavior which follows a pseudo-second-order rate of adsorption. Isotherm experiments reveal that MRHA can capture MB molecules up to 150.53 mg g−1, which is comparable to reported values for RHA adsorbents in literature. MRHA can also be more efficiently used at pH 7 and S/L = 10 g L−1 providing ~ 100% RE in just 10 min at room temperature. Furthermore, the reusability of MRHA and its consistent performance in cycled MB adsorption/desorption runs establish its potential as an effective and economical adsorbent for real wastewater applications. The RHA that is regarded as agricultural waste and used only as a cleanser for charred utensils was proven to be a recyclable and efficient adsorbent for methylene blue. However, a continuous MB removal system utilizing the MRHA will be more beneficial for actual applications and should be delved on in the future. But the challenge remains on developing scale-up technology for magnetic adsorbents appropriate to industrial wastewater plants.