1. Introduction
In the contemporary world, scarcity has become a hot issue. In particular, many areas of the globe currently suffer from a lack of access to clean water and the need for potable and usable water has resulted in the development and increased use of water treatment technologies. According to forecasts produced by von Grebmer [1], by 2050, 52% of the global population, which is estimated as 4.8 billion people, will have a lack of access to clean water, and this water stress will have significant effects like an increase in the operational costs for water, energy, and food supply. The wastewater that is currently discharged into the environment is the byproduct of both domestic and industrial processes. At present, domestic wastewater is accountable for the highest volumetric ratio in terms of total discharge. As such, as the population increases with the development of humanity, so too does the unit water consumption per capita and the unit pollution loads per capita. Furthermore, discharge standards that are designated according to the total daily mass of pollutants required to protect the quality of receiving waterbody necessitates the treatment of wastewater with high removal efficiencies.
Several types of wastewater treatment techniques are currently in use. These can be broadly classified as physical, chemical and biological. Physical treatment is generally employed as the primary treatment mechanism and is used to help and protect the biological treatment units in which the secondary treatment processes take place. Biological wastewater treatment utilizes both conventional approaches, such as activated sludge systems and rotating biological contactors, and innovative applications like activated sludge systems with modifications and membrane bioreactors (MBR).
The membrane bioreactor utilizes a treatment technology that combines the activated sludge and membrane filtration processes. The membrane can be basically defined as a selective wall that can separate into two different phases [2]. While pollutants cannot pass across through the membrane, treated water can be collected from behind it. Biological treatment in the MBR tank can be realized by the activated sludge via organic matter removal during the life cycles of the microorganisms that exist within it. MBRs have many advantages. The lower HRT value results in a smaller tank volume and, thus, entails that the tank can be placed in a smaller area [3, 4]. In addition to this, high Mixed Liquor Suspended Solid (MLSS) values help MBR systems to tolerate shock loadings [5, 6]. Moreover, there is no need for the use of clarifiers in the treatment plant because solids are separated from the mixed liquor by means of filtration [7, 8].
The biggest advantage of MBRs is that effluent can find several beneficial usage areas, like irrigation. It is perhaps due to these advantages that the use of MBRs has increased in recent years. According to existing studies in this domain, the global use of MBRs has grown at a rate of between 10 and 15%, and the total value of the global MBR market, which is 1.81 billion USD in 2016, is expected to witness positive growth in the near future and will be 8.27 billion USD in 2025 [9, 10].
The current market research indicates that MBR technology is increasingly being viewed as a modern and reliable technology; however, uncertainty remains as to the relatively high risks and costs associated with the use of MBRs versus conventional technologies [11]. Furthermore, specific concerns have been raised regarding the fouling problem that is associated with the operation of MBRs. One of the fundamental limitations of all membrane processes is that they result in membrane fouling. Rejected pollutants and microorganisms in the mixed liquor tend to attach to the surface of the filtration membrane. Fouling can result in flux reduction under the constant transmembrane pressure (TMP) or TMP increase under the constant flux, and these changes result in an increase in the unit cost of wastewater treatment [2]. Strategies that are specifically designed to prevent fouling as an output of the MBR operation can be broadly grouped into five different approaches: 1) Physically treating the feed, 2) Physically and chemically cleaning the membrane, 3) Reducing the flux, 4) Increasing the aeration rate, and 5) Making chemical or biochemical modifications to the mixed liquor [2]. Since all of these operation strategies increase the unit treatment cost, researchers have invested a significant amount of effort in identifying alternative methods of preventing fouling that minimizes this cost. Studies on the fouling problem have gradually increased in recent years and generally involve the modification of membrane material or module configuration. However, many people view these studies as insufficient because the modifications proposed are costly and have largely failed to stand the test of time during long-term MBR operations. The reason for this insufficiency is that the creation of biofilm, and the gradual increase of this biofilm, is a totally natural process. Membrane biofouling in the MBR is generally associated with extracellular polymeric substances (EPS) and soluble microbial products (SMP) [12–15]. EPS is a structural material of microbial aggregations, such as biofilm, floc and activated sludge liquor and a term used for macromolecules like carbohydrates, proteins, lipids, and other polymeric substances on the surfaces of the cells or in the gap between the cells [16]. EPS, with its unstable and heterogeneous structure, can create a highly hydrated gel, and this gel structure forms a barrier against the permeate flow during the membrane filtration process [17]. Besides, SMP is used for the definition of cellular compounds created during cell decay, synthesis, etc. [18].
The key aspect of biofilm creation that involves a totally natural process is quorum sensing (QS). QS is the communication between species using signalization. QS microorganisms show several types of group behaviors including EPS and SMP secretions and biofilm creation. Microorganisms in communication with each other start to accumulate in the biofilm and create a bio-cake that has less porosity. In order to prevent this situation, signalization mechanisms have to be interrupted, and this interruption mechanism is known as quorum quenching (QQ). Although the application of QQ in MBRs is a relatively new topic in the area of anti-biofouling MBR studies, there are some examples of the use of QQ within MBRs. For the purposes of this review, existing QQ MBR studies were compiled by evaluating their advantages and disadvantages, and informative explanations on the future potential of this new vision were formulated.
2. Quorum Sensing
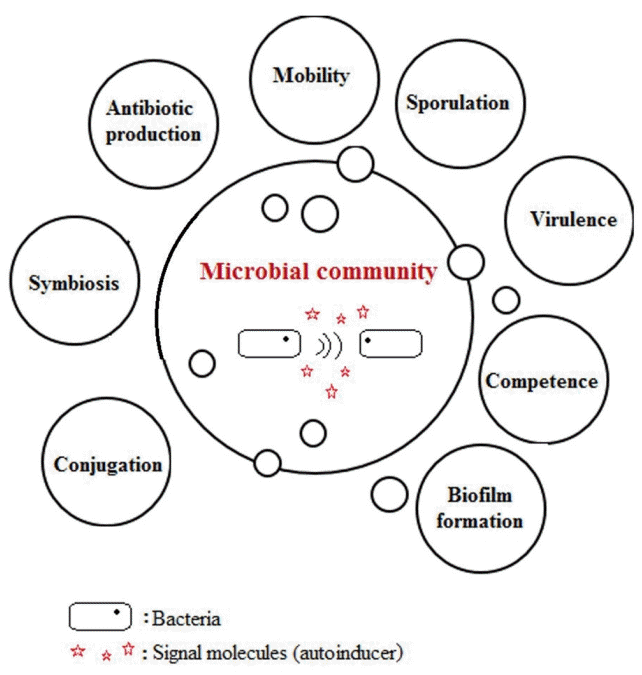
Bacteria communicate to one another by producing signal molecules, called ‘Autoinducers (AIs)’ to coordinate their group behaviors. Using these QS signal-response systems, bacteria regulate gene expression in response and synchronize particular behaviors such as bioluminescence, antibiotic production, virulence, biofilm formation, the production of SMP and EPS [19, 20]. These quorum systems are potential targets for biofouling control in MBR because bacteria control the expression of biofilm formation via QS networks. The discovery that bacteria are able to communicate with one another changed our general perception of many single, simple organisms inhabiting our world. However, the concept that bacteria produce pheromones and communicate with one another was met with considerable skepticism by many and disinterest by others in the early 1990s [21]. A schematic drawing of microbial group behaviors via QS, illustrated using data from Greenberg [22], is given in Fig. 1.
Although several QS systems are known, N-Acyl-HSL (AHLs) are the most common signaling molecules used by Gram-negative bacteria and peptide-based signaling systems are used by Gram-positive bacteria [23, 24]. Additionally, both Gram-negative and Gram-positive bacteria produce autoinducer-2 (AI-2), which is a member of a family of signaling molecules used in QS that can facilitate interspecies cell-to-cell signaling.
The first observed QS system based on AHL autoinducer and its cognate regulatory circuit was that of the bioluminescent marine bacterium Vibrio fischeri (V. Fischeri), which was examined as a model for QS in most Gram-negative bacteria. At low cell densities in natural seawater, V. fischeri is non-luminescent. However, when it is proliferated to high cell densities in the laboratory, a V. fischeri culture bioluminesces with a blue-green light [25]. While the squid host uses the bacterial-produced light for counter illumination to mask its shadow as an anti-predation tactic, the bacteria profits from the nutrient-rich squid and grows to a high cell density that is unachievable in seawater. Two regulatory proteins, called LuxI and LuxR, are essential for QS control of bioluminescence in V. fischeri. LuxI is the AI synthase enzyme in V. fischeri, and it catalyzes the formation of the AHL. Then, AHL molecules freely diffuse out of the cell membrane and accumulate. At high AHL concentrations (high cell density), QS signaling molecule produced by LuxI is bound by LuxR and this AHL-LuxR complex activates transcription of the LuxICDABE operon. In the absence of an AHL signal, LuxR is inactive. At low cell densities, the AHL signals produced by LuxI diffuse passively out of the cell following a concentration gradient. Thus, the lux operon is not expressed. Different types of AHL-based QS molecules are produced by different bacteria. AHLs are composed of homoserine lactone (HSL) rings that carry acyl chains that differ from C4 to C18 in length [26]. A large number of other Gram-negative bacteria possess LuxIR-type proteins and communicate with AHL signals for intra-species QS [21].
While Gram-negative bacteria use AHLs as auto-inducers, Gram-positive bacteria mostly use secreted peptides as autoinducers for QS. Peptides are encoded in genes and are made as a larger precursor protein (pro-peptides) inside the cell. These are then further processed to small linear or cyclic peptides during secretion. According to Parsek and Greenberg [24]:
“In contrast to AHL-based signaling, peptide signals are not detected inside the cell. In some cases, a membrane-bound sensor protein belonging to the two-component signal transduction family interacts with the peptide. The peptide-bound sensor then activates an associated response regulator, which modulates expression of quorum-sensing-regulated genes. (p. 1)”
The AI-2 quorum-sensing system was first described in Vibrio harveyi and has been implicated in interspecies communication [27]. In all AI-2-producing bacteria, the precursor for the AI-2 signal is 4, 5-dihydroxy-2, 3-pentanedione (DPD), the product of the reaction catalyzed by the LuxS enzyme. Homologs of luxS exist in hundreds of Gram-negative and Gram-positive bacteria, consistent with a role for AI-2 in interspecies communication that allows other luxS-encoding bacteria in a particular environment to contribute to the overall cell-density information [28, 29]. QS signals and QS-controlled behaviors in some bacteria are listed in Table 1, which is adapted and updated from Dobretsov et al. [30].
2.1. The Role of Quorum Sensing in Biofilm Formation
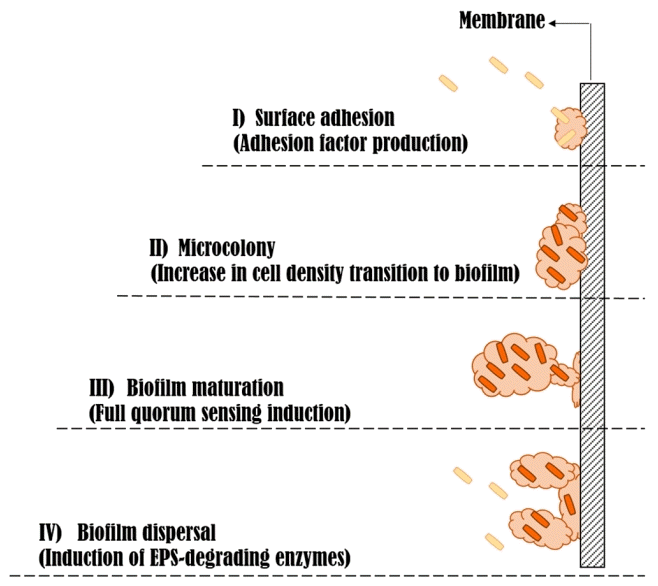
Surfaces are important microbial habitats that generally provide enormous access to nutrients, protection from predation and environmental stresses, and a means for cells to remain in a favorable habitat without being washed away. Biofilm formation occurs when bacterial cells grow on surfaces. Bacterial cells that attach to a surface combine through an adhesive matrix secreted by the cells. The matrix is composed of a variety of polysaccharides and proteins. Biofilms utilize nutrients more easily for microbial growth and help prevent the detachment of cells on dynamic surfaces, such as in flowing systems. Biofilms may contain only one or two species or, more commonly, many species of bacteria. As the organisms adhere to a surface, they keep signaling to one another, and ultimately an expression of genes-related biofilm is initiated [48, 49] (Fig. 2). For example, the major intracellular signaling molecules are AHLs in Pseudomonas aeruginosa. As these lactones accumulate according to population density, they are released and subsequently recognized by adjacent cells. The signaling lactones then control the expression of genes that contribute to biofilm formation:
At least four reasons have been proposed for the formation of biofilms. First, biofilms are a means of microbial self-defense that increase survival. Second, biofilm formation allows cells to remain in a favorable niche. Third, biofilms form because they allow bacterial cells to live in close association with each other. Finally, biofilms seem to be the typical way bacterial cells grow in nature. The biofilm may be the “default” mode of growth for prokaryotes in natural environments, the latter of which differ dramatically in nutrient levels from the rich liquid culture media used in the laboratory [50].
3. Detection of Signal Molecules
The methods by which qualitative and quantitative determination of AHL signal molecules have been made in the previous QQ MBR studies were also examined within the scope of this review. As previously mentioned, the first QQ MBR study was carried by Yeon et al. [51]. They opted to use Agrobacterium tumafaciens (A. tumefaciens) A136 as a biosensor for AHL detection, based on the works of Fuqua and Winans in 1996 and Kawaguchi et al. in 2008 [52, 53].
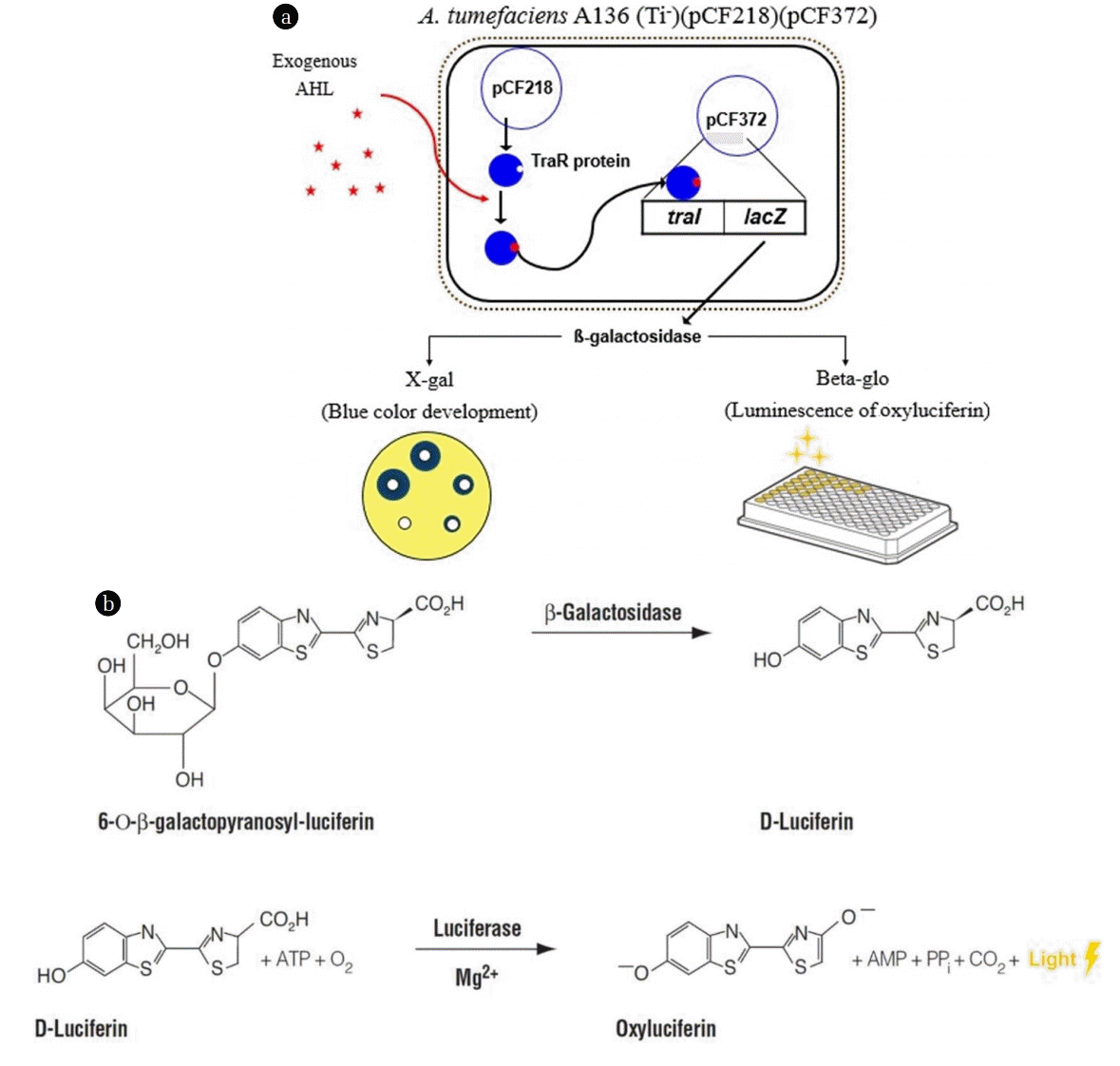
A. tumefaciens (Ti-)(pCF218)(pCF372) is a genetically modified microorganism for the detection of exogenous AHL signal molecules. This microorganism has the three following genetic characteristics: 1) Knockout of Ti plasmid (Ti-); 2) pCF 218, which codes for traR; and 3) pCF 372, which contains traI-lacZ fusion, which is under traR regulation. A. tumefaciens A136 cannot produce the AHL autoinducers because the Ti plasmid on which regulatory components of the A. tumefaciens QS system are located has been genetically removed. Instead, when exogenous AHL diffuses into A. tumefaciens A136, it makes a complex with TraR protein from the pCF 218. TraR is an AHL-responsive transcription factor that recognizes N-3-(oxooctanoyl)-L-HSL (AHL of A. tumefaciens) as well as a wide range of related AHLs. This AHL-TraR complex activates traI-lacZ on pCF 372 and induces the production of beta-galactosidase, which degrades X-gal and develops a blue color. The blue colors spread on an agar plate covered X-gal as shown in Fig. 3(a), and the diameters of these zones are in direct proportion to the AHL concentrations. By using the calibration equation between the diameters and known AHL concentrations, the concentration of AHL in a sample can be calculated.
The concentrations of AHL molecules are also measured via luminescence method using the same reporter strain of A. tumefaciens A136 [54]. The luminescence method was adopted for the measurement of AHLs in MBR for wastewater treatment by Oh et al. [55]. The reporter strain (A136) and the AHLs sample are mixed and loaded on the microwell plate. The microwell plate is placed on the incubator to keep the temperature at 30°C for 1.5 h, and then the Beta-GloÒ Assay System is added to the solution for the luminescent reaction with b-galactosidase produced by the reporter strain. After 40 min, oxyluciferin is synthesized, resulting in luminescence (Fig. 3(b)). The intensity of luminescence is in direct proportion to the AHL concentration and can be measured by a luminometer. The amounts of AHLs are calculated using relationship equations based on the calibration curve derived from standard samples of AHLs.
The luminometer method is preferred over the agar plate method because the former requires shorter experiment time, leading to the analyses of multiple samples on the same day. The schematic drawing of these two methods for AHL determination is presented in Fig. 3. Although the ultra-sensitive detection of N-Acyl HSL type QS molecules can be realized by bioassay strains like Agrobacterium [56, 57], newly developed techniques have been quite attractive and successful for the signal molecule detection. Ligand-insensitive LuxP mutant fluorescence resonance energy transfer protein sensors could be developed for the detection and quantification of AI-2 QS molecules [58]. Furthermore, Culhane and his colleagues had studied on the development of a new method to create laser-fabricated plasmonic nanostructures for surface- enhanced Raman spectroscopy of bacteria QS molecules. Silver nanostructures were employed in the detection of N-butyryl- L-HSL via Raman signals [59].
4. Enzymatic Quorum Quenching
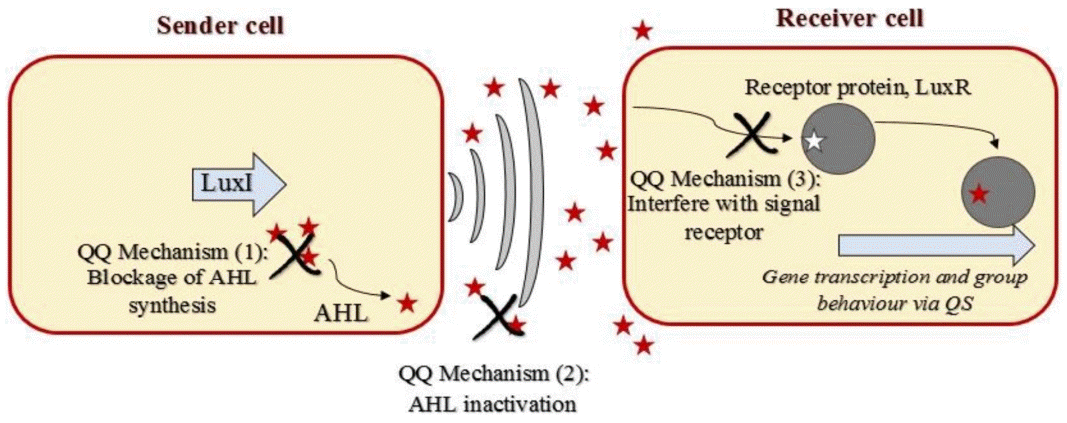
The most common approaches to control bacterial growth in a given environment involve the use of antibiotics; however, it is a well-known fact that bacteria species can develop resistance to these anti-bacterial agents [60, 61]. In addition, the main aim of biofouling prevention during membrane bioreactor operation is not to kill or deactivate all bacteria because a high level of death and deactivation may reduce the efficiency with which pollutants are removed. It is widely recognized that N-acyl Homoserine Lactone (AHL) - mediated QS plays the main role in biofilm creation. As such, some researchers have suggested the use of a new paradigm “QQ” to control the group behaviors of bacteria without affecting their lifecycles [62]. Because the number of Gram-negative bacteria is higher than the number of Gram-positive bacteria in the activated sludge, AHL-mediated QQ has been investigated. There are three main AHL-mediated QQ mechanisms (schematically shown in Fig. 4): 1) Blocking AHL synthesis in the sender cell, 2) Deactivating AHL via enzymatic destruction, and 3) Interfering with the signal receptor in the receiving cell [63].
As mentioned above, the first mechanism involves blocking AHL synthesis. The inhibition of AHL synthesis by blocking LuxI-type synthase proteins is possible and, since AHL is synthesized from S-adenosylmethionine, the analogues of this amino acid can be used to block AHL synthesis [64, 65]. Additional research has found that erythromycin can block AHL synthesis at the ribosomal level through the use of an unknown mechanism [66].
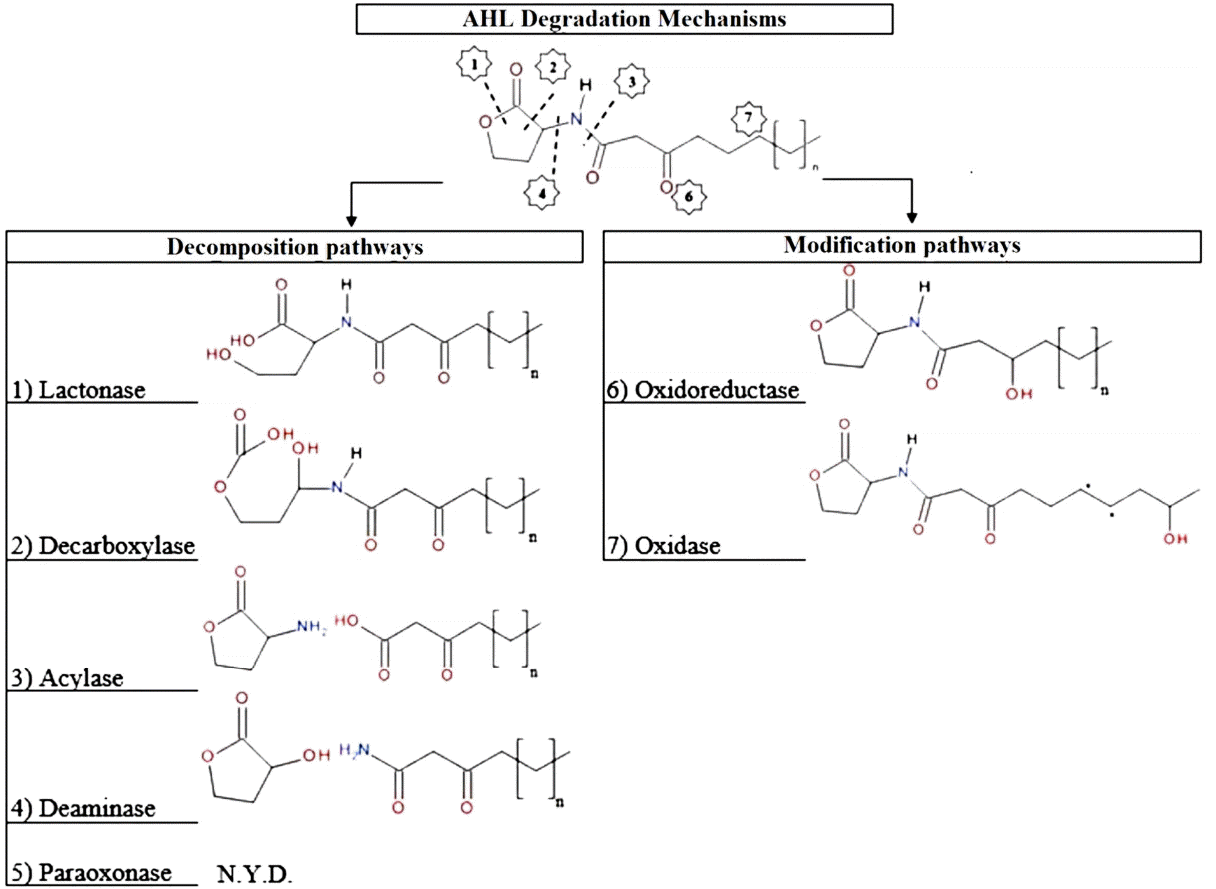
The second mechanism, AHL degradation, is the most well-known mechanism. Enzymatic destruction of AHL molecules can be realized by different types of enzymes, and this prevents AHL accumulating in the environment. Seven different AHL degradation enzymes have been described in the literature. While some of them prefer the decomposition pathway, others use the modification pathway (Fig. 5). The name of QQ enzymes and the final products that result from AHL degradation are presented in Fig. 6.
While lactonases open the homoserine (HSL) ring by disruption of the bond at the left of the double-bonded oxygen [67–69], decarboxylases open this ring by disrupting the bond at the right of the double bonded oxygen [70]. At present, no studies have investigated the mechanism of decarboxylase with a certain bacteria species. In addition, acylases, which has synonyms in the literature like aminase and amidohydrolysis, cleave the acyl side chain from the HSL ring [65, 71] and create fatty acid and HSL. Even though there are no examples of studies that have been carried out with a certain bacteria species, deaminase can also cleave the acyl side chain from the HSL ring, but only with a different point. Deaminases create final products as an HSL chain with OH− and a side chain with NH2 [70]. The last example of the QQ enzymes that carry degradation pathway is paraoxonase. Paraoxonase is an enzyme that is sourced from mammalian cells, and information about its mechanism has not yet been exactly determined; however, research has proven that it can both disrupt the chain and cleave the side chain of AHL [72–75]. On the other hand, two enzymes can carry the modification pathway: oxidoreductase and oxidase. While oxidoreductase can convert the oxygen that is double bonded to the side chain of AHL to OH− [76, 77], oxidase modifies the last bond of the side chain [77]. Species that use these QQ enzymes are listed in Table 2.
The last AHL-mediated QQ mechanism involves interfering with the signal receptor [116, 117]. AHL analogues that compete with receptors have been tested in order to prevent microorganism from receiving QS signals. The analogues can be produced by using side chains of AHL. These compounds interact with LuxR, the receptor protein responsible for AHL, and result in the elimination of AHL [118].
5. Biofouling Prevention via Quorum Quenching in MBR
As mentioned, biofilms are unwanted in the MBR process for wastewater treatment because they significantly decrease the permeate flux during membrane filtration and removal of biofilms by chemical treatments may not be sufficient via the tolerance of microorganisms. Since the relationship between QS signal and membrane biofouling was proved, different QQ applications including enzymatic QQ, bacterial QQ, and fungal QQ have been studied in MBR processes for biofouling control. Within the scope of this review, examples of QQ MBR applications that have been developed for the purposes of preventing the biofouling of the filtration membranes located in these MBRs were examined and compiled. A list of the studies examined is presented in Table 3. These studies were listed according to application methods, such as QQ media type. Grouping the research in this way also resulted in them being generally listed chronologically because application methods have changed over time, continuously improving existing methods. In Table 3, QQ applications in MBR were compared to each other in terms of QQ enzyme, QQ microorganism, QQ media, and advantages and disadvantages of QQ method.
Yeon and his colleagues published the first QQ MBR study in 2009. They directly added an enzyme (Acylase) in the MBR to inhibit QS by decomposing signal molecules (AHLs) [51]. They confirmed that the addition of acylase retarded the TMP rise compared with that of the control reactor by regulating EPS concentration. In this study, a QQ mechanism efficiency of 57% was achieved, and a huge contribution to the literature was realized. However, they observed free enzyme (Acylase) had been rapidly inactivated [102]. Then, they prepared magnetic enzyme carriers (MECs) on which free acylase enzymes were immobilized. By using MECs, not only higher QQ activities were prolonged, but also the immobilization media were easily separated from activated sludge to be reused [102]. Kim et al. [119] immobilized a QQ enzyme (Acylase) onto a nanofiltration membrane and visualized the spatial distribution of cells and polysaccharides on the surface of control and immobilized membranes. They concluded that QQ inhibits polysaccharides and thus mitigates biofilm formation on the acylase immobilized membrane. At the end of these studies that could result in a significant delay of the TMP increase reflecting slower biofilm formation on the membrane surface, it was also surprisingly observed that the inhibition of biofouling by enzymatic QQ was reversible and the following filtration performance of the membrane returned to the original state if QQ enzyme application interrupted. In the light of these results, it can be mentioned that continuous QQ enzyme addition is indispensable for a continuous biofouling control.
QQ enzymes are commercially available; however, the cost of the enzyme is too high to be applied to MBR for wastewater treatment. Following enzymatic QQ, a lot of bacterial QQ studies have been conducted to seek an economically feasible way [112, 120]. It is obvious that the activity of whole cell catalysts cannot as high as that of isolated enzymes; however, there are three reasons for the preference of bacterial QQ. These reasons can be listed as follows: i) more readily and less expensively preparation, ii) more stability for long-term application as the QQ enzymes inside cells are protected from the external environment (activated sludge), and lastly iii) easier handling. Within this regard, researchers have developed various immobilization media that can interfere with QS continuously. These immobilization media provided protection of immobilized QQ bacteria against other microorganisms in activated sludge and a microporous structure for efficient mass transfer of dissolved oxygen and nutrients. Oh et al. isolated and identified QQ bacteria Rhodococcus sp. BH4 in a real MBR plant and prepared a polymeric microbial vessel containing these QQ bacteria [120]. The polymeric microbial vessel was stimulated as a small tube containing 10–12 hollow fiber membranes. In this study, biofouling was prevented with a success rate of 50%. The polymeric microbial vessel showed reproducible QQ efficiency, delaying TMP rise-up substantially. The location of the vessel in MBR had a direct effect on the QQ efficiency because the vessel is fixed and thus decomposition of signal molecules are very diffusion limited. The QQ vessel was more efficient when it was placed in a membrane tank instead of bio-reactor in MBR [121]. F/M ratio for QQ bacteria in the vessel could be relatively low due to mass transfer limitation. Cheong et al. [122] substituted a ceramic vessel for a polymeric one. The ceramic microbial vessel contained a few lumens through which inner mode feeding was possible. They increased the F/M ratio for QQ bacteria in the ceramic vessel with an inner mode feeding, resulting in a higher QQ efficiency. In addition, the rotation microbial carrier frame (RMCF) was composed of a polycarbonate frame and four cubbyholes that were covered with a microfiltration flat sheet membrane and the usage of RMCF resulted in a higher anti-biofouling effect during MBR operation when compared to the anti-biofouling effect obtained with the usage of normal QQ-vessels [123].
Since vessels have no mobility, moving beads are generally preferred as QQ media in MBR. The advantage of moving QQ beads is the extra physical washing of biocakes deposited on membrane due to their continuous collision to each other. While Jiang et al. immobilized acylase enzymes into globular beads [19], Kim et al. entrapped Rhodococcus sp. BH4 into beads [101] using a natural polymer (sodium alginate). With the combination of biological QQ effect and physical washing effect, 10 times longer time passed to reach the same TMP compared to an MBR without QQ beads (a very high QQ efficiency of 87%). Jiang et al. achieved efficient biofouling prevention and energy saving, whereas the high cost of enzyme still remained an issue with this application [19]. However, moving microbial beads offered high efficiency and long-term life span.
The only disadvantage of alginate beads is that they are subject to biodegradation in the bioreactor during the MBR operation, leading to short life span. To reinforce alginate beads, Lee et al. [124] immobilized acylase into magnetically-separable mesoporous silica, Kim et al. [125] tried to coat the alginate beads with a synthetic polymer (polysulfone), which prevented QQ bacteria from leaking outside the macrocapsules. Another approach to overcome the disadvantage of alginate beads was to mix a natural polymer, sodium alginate with a synthetic polymer, polyvinylalcohol [126, 127]. Furthermore, there are various studies that are innovative and mention about new QQ MBR applications. First one of these innovative applications is the fungal QQ study carried out with to success the energy saving through fungal-to-bacterial QQ and researchers could decrease the rate of TMP rise-up even for lower aeration intensities [126]. After some results showed that the QQ bacteria located at the periphery of a bead can degrade signal molecules more than the QQ bacteria located inside of the bead have come to light, it was needed to create new immobilization media designs. In 2016, another QQ study with QQ-hollow cylinder was also presented to the readers and this newly developed immobilization medium could increase the surface area and eliminate the disadvantage of hollow fibers used as an immobilization medium [129]. QQ-hollow cylinder presented higher QQ activity than did the QQ beads. Furthermore, the shape of the QQ-hollow cylinder provided more physical contact between media and biofilm and this led to a higher efficiency of physical washing. The final step for the immobilization medium design and development is small QQ-sheets that showed 2.5-fold higher biological QQ activity when compared to the QQ activity of QQ beads [129]. Besides, immobilization of QQ enzymes on the membrane surface has been a hot issue and stability problems for enzyme immobilization for QQ applications have started to be recently solved [130]. All the existing QQ media have their own advantages and disadvantages and offer some elements of superiority to others. In this regard, the development of QQ MBR studies is expected to enhance continuously together with innovative QQ media and new QQ microorganisms.
Lastly, it is necessary to check the QQ effect on the system performance of MBR and energy and cost saving of QQ applications in MBRs. QQ MBR studies to date showed that there is no negative effect on the system performance of MBR while EPS secretion is inhibited without affecting the bacterial growth and treatment level. Effluent quality was checked by looking to the chemical oxygen demand (COD) and total nitrogen (TN) in various studies and no significant difference in effluent quality between conventional (control) and QQ MBRs [19, 23, 55, 102, 112, 123]. Besides, biofouling in an MBR process is directly associated with energy consumption via TMP increase. When TMP increases to obtain a stable permeate volume during the process, it results in more physical and chemical membrane cleaning, a higher aeration rate and higher suction pump rates. In the study carried out by Jahangir et al. indicated that the application of QQ-vessel in an MBR could decrease the aeration intensity for membrane aeration (the reduction of the aeration rate by 0.5–1.0 L/min) that can be pointed out as one of the main contributors of total energy consumption during an MBR operation [121]. In addition, the reduction in the energy need for the membrane aeration and permeate suction pumps were determined and evaluated by Köse-Mutlu et al. for the use of QQ beads, QQ vessels and RMCF and it was mentioned that these QQ applications resulted in important savings in operating costs and the most feasible one was found as RMCF especially for long-term operations [123]. These are the most powerful arguments of the QQ MBR technology.
6. Chanllenges in Quorum Quenching
Although QS inhibition is a promising mechanism to prevent microbial communication, it is better for researchers to be aware of the challenges of QQ. Firstly, there is a variation in the number of LuxI and LuxR homologs between different strains of the same species that were determined for several bacteria. Burkholderia mallei, Burkholderia pseudomallei, and Rhizobium etli, in which the number of LuxR homolog ranges from two to nine, can be given as examples for these species [131]. This situation can result in a challenge for the design of receptor-binding antagonists. Moreover, some of the LuxR homologs are orphans. The existence of these orphans comes up with some questions regarding the possible roles of these orphan LuxR homologs [132]. In addition to these, there is a possibility of the development of a resistance against the QS inhibition. Compensatory mutation leads to the re-organization of social independence with the playing a role as a QS-proficient (a cooperator) instead of QS-deficient (a cheater). It was mentioned in the literature that this mutation is a possible mechanism of bacteria to overcome the QS inhibition [133, 134]. One of the explanations for this situation is the binding of a signaling molecule to another QS regulator [135]. A study showed that the mutation in Qrr sRNA resulted to the failure of Qrr/hapR binding, where hapR is the gene encoding LuxR homolog in V. cholera and prevents different genes activities; however, a mutant possessing only one of these four Qrr sRNAs can activate the QS-related activities [136]. This results in a challenge for receptor-binding antagonists to play against the QS system of V. cholera. Four strategies to reduce the risk of QQ resistance development that were mentioned in the literature can be listed as follows [137–139]: the use of QQ enzymes that target a broad range of AHLs like lactonase and acylase, the use of different QQ compounds against bacteria, the combination of the QQ approach with other treatments like antibiotics, to create a synergistic effect, and finally targeting the virulence factors.
Another challenge has been the correct detection of QS. As mentioned in this review, there are a few basic biochemistry experiments that can be used by the researchers who aim to have information about the QS molecules. When the first QS signal was discovered in the early 90s, the mass spectrophotometry could not measure up and researchers tended to use and develop some traditional methods based on the light. The challenge is that AHL reporter systems are tended to false positives when used to detect QS inhibitors [140, 141]. It can be basically said that an inhibitor may be interacting with the reporter gene instead of the QS gene. If the researchers analyze a sample, they should take care to confirm that the found molecules are really involved in QS.
7. Conclusions
MBR, which are involved in water recovery and reuse, are currently plagued by a range of biofouling problems. One of the most common causes of biofouling is the development of a thickened biofilm layer that results from bacterial adhesion. For this reason, the studies described in this literature review have attempted to develop detailed insights into the QS system and to apply the QQ system as a means of preventing biofouling in MBRs. The results of these studies indicate that extra energy consumption caused by the biofouling problem can be significantly reduced.
If bio-product and biocompatible materials are described as two important elements, while bio-products can involve bacteria and enzymes, the biocompatible material can refer to the immobilization media. For an effective QQ-MBR operation, bio-product and biocompatible material are of equal importance. When the future of this study subject is considered, two essential windows can be opened: 1) there can be the need for new bacteria species and new enzymes originated from these bacteria species or other creatures, and 2) there can be a new open multi-disciplinary research area on biocompatible innovative materials used in immobilization media.