1. Introduction
Steroid estrogens (SEs), including estrone (E1), 17β-estradiol (E2), estriol (E3) and 17α-ethynylestradiol (EE2), have been widely detected in environmental waters [1] and gotten increasing focus in recent years due to their detrimental effects on both human and wildlife endocrine systems [2, 3]. As an active ingredient of oral contraceptives, EE2 has been extensively used and discharged into natural waters through the effluents of sewage treatment plants [4–6]. EE2 was documented to be not only more stable than natural estrogens (E1, E2 and E3) in the natural waters but also of the strongest estrogenicity among the SEs [7]. Therefore, knowledge on the behaviors and fate of EE2 in waters is essential for removal treatment and ecological risk assessment.
EE2 was reported to be degradable in pure aqueous solutions under the illumination of sunlight and simulated solar light due to its light absorption band overlapping with the solar emission spectrum in the range of 280 – 320 nm [8], but this progress was very slow even under the irradiation of a 250 W high pressure mercury lamp, in which the half-life of EE2 was determined to be decades to hundreds of hours [9]. Biodegradation of EE2 in natural environment was reported to be more time-consuming than its direct photodegradation, and the half-life of EE2 biodegradation was determined to hundreds of days under aerobic conditions [10, 11], and a longer lag stage could be expected under anaerobic conditions [12, 13]. However, our previous investigations indicated that although the amount of SEs discharged to Dianchi Lake (centered around 24°48′2″N, 102°40′17″E) by eight sewage treatment plants was 35.8 g per day, there are no significant changes in the concentration of SEs in the sediments and waters during 2009 – 2013 [4]. Thus, other removal pathways would accelerate the dissipation of EE2 from natural waters.
Photodegradation was reported to be an effective pathway for organic pollutants removing from not only waste waters but also natural aquatic systems [14–17]. Prior studies also found that EE2 could be photodegraded with a half-life of less than 2 days in the Quinsigamond Lake surface water [11], and the quick degradation was proposed to be related to indirect photodegradtion mediated by dissolved components. Ubiquitous occurrence of nitrate (μM - mM) in aquatic systems was always introduced by domestic and industrial wastewater discharge and fertilizer application [18]. Nitrate has been reported to be photoinductive towards organic contaminants, such as atrazine [19], trichloroethylene [20], and atenolol [21]. These photodegradations were attributed to the oxidation of the photogenerated reactive oxygen species (ROS) and other reactive photooxidants [22]. However, photochemical behaviors of EE2 induced by nitrate are unknown. Meanwhile, data regarding to the estrogenic potency of EE2 phototransformed products is scarce.
Herein, this work aimed to (1) study the contribution of nitrate photolysis to EE2 dissipation from natural waters; (2) explore the influence of water characteristics and components, mainly the pH value, HCO3−, Fe(III), Cl- and dissolved humic acid (DHA), on the induced photodegradation of EE2 by nitrate; (3) determine the main photodegradation products of EE2 and propose the possible pathways responsible for EE2 photodegradation in nitrate aqueous solutions; (4) evaluate the estrogenic potency variation of EE2 induced by the photolysis of nitrate. This study can extent our knowledge on the photochemical behaviors of SEs and provide information for ecological risk assessment.
2. Chemicals and Methods
2.1. Chemicals
EE2, pyridine, benzoic acid (BZA), terephthalic acid (TPA), ultra-pure NaCl, chromatographic grade isopropyl alcohol (IPA), trime-thylchlorosilane (TMCS) and N,O-bis (trimethylsilyl) trifluoroacetamide (BSTFA) were purchased from Sigma Aldrich with the highest purity available. Chromatographic grade acetonitrile, methanol, acetone, n-hexane and ethyl acetate were purchased from Merck Corporation. Dissolved humic acid (DHA) was extracted from the Dianchi Lake sediments by a conventional alkaline extraction method [23]. MCF-7 cells were purchased from Kunming Institute of Zoology, China. S1640 cell culture medium and bovine serum were purchased from GIBCO CO., Ltd. NaNO3, NaHCO3, Fe2(SO4)3 and other inorganic reagents used in this work were of analytical grade and purchased from Sinopharm Chemical Reagent Co., Ltd., China. Milli-Q water (electric resistance > 18.0 MΩ) was used throughout this work.
2.2. Solution Preparation
EE2 working solution was prepared by adding 5.00 ± 0.05 mg EE2 in 1 L Milli-Q water followed by continuously stirred for 24 h at room temperature. The supernatant was collected by passing the solution through 0.45 μm glass fibre filters (GF/F, Millipore Corp.) which were prebaked at 450°C for 5 h. The filtrate was stored in dark reagent bottles at 4°C and used within 5 days. NaNO3 aqueous stock solution (5.0 mM) was prepared freshly every day and stored in dark glass bottles.
DHA stock solution was obtained by dissolving 500 mg purified humic acid powder into 300 mL NaOH solution (0.05 M). The solution was oscillated for 24 h followed by passing through the prebaked 0.45 μm GF/F. The pH of the filtrate was then adjusted to 7.8 ± 0.1 using 1 M NaOH and 1 M H2SO4. The prepared DHA stock solution was stored in dark glass bottles and kept at 4°C for use within 3 weeks, and no significant TOC content variation was found during the storage period.
2.3. Photodegradation Experiments
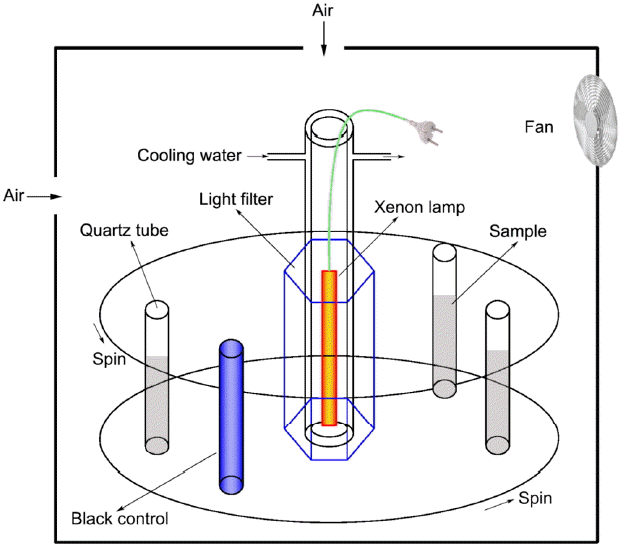
All the photodegradations were performed on an XPA-7 merry-go-round photochemical reactor (Fig. 1) with 50 mL cylindrical quartz tubes (Φ = 15 mm) and magnetic stirrers (150 rmp). A 300 W medium pressure Hg lamp was used as a light source which was equipped with 290 nm light cut-off filters. The light intensity at the surface of the irradiated solutions was measured to be 3.71 mW m−2 at 365 nm and 21.6 mW m−2 for λ > 420 nm, respectively. The lamp was cooled down by recirculating cooling water and turned on preliminarily for 30 min for stabilization.
To explore the photochemical behaviors of EE2 induced by nitrate, batch experiments were conducted as follows: (i) dark controls in Milli-Q water and nitrate aqueous solutions, (ii) photo-lyzing EE2 in Milli-Q water, (iii) photolyzing EE2 in aqueous solutions with different concentration of nitrate and pH conditions, (iv) photolyzing different concentration of EE2 in 2 mM nitrate aqueous solutions, (v) photolyzing EE2 in 2 mM nitrate solutions with i-PrOH, BZA and TPA to detect the mechanisms responsible for nitrate mediating EE2 photodegradation, (vi) photolyzing EE2 in 2 mM nitrate solutions to identify the photodegradation products and the changes of EE2 estrogenic potency. Unless otherwise specified, the initial EE2 concentration and pH value of all the tested solutions were controlled at 1.05 mg·L−1 and 7.8 ± 0.1. Sample tubes were fixed to allow air flow in and maintained at 25 ± 0.5°C in a recirculating cooling water bath. Aliquots of 500 μL sample of each experimental system was withdrawn at desired time intervals and analyzed the EE2 residual using a high performance liquid chromatography (HPLC). All the experiments were carried out in triplicate.
2.4. Estrogenic Potency Variation Detection
MCF-7 cell proliferation toxicity experiments were conducted using a previously reported method with some modifications [24]. Briefly, 800 μL photodegraded samples were sterilized and then diluted twice by using cell culture medium. Desired amount of starved MCF-7 cells was added with the diluted samples (200 μL) and incubated at 30°C for 24 h, which was then colored and recorded the optical density at 580 nm by a plate reader (LT-4000MS, Labtech). Different concentration of EE2 standard solutions and controls were also subjected to the same estrogenic potency detection procedures to obtain a reference dose response curve.
2.5. Photoproducts Identification
Irradiated EE2 solutions were subjected to a solid phase extraction with the Oasis HLB cartridges (Milford, MA, USA) for obtaining the phototransformed products using our previously developed method [4]. Prior to GC-MS detection, the obtained analytes were derivatized by adding 50 μL BSTFA + 2% TMCS and 50 μL pyridine, and then incubated at 70°C for 30 min. The derivatized samples were separated by an Agilent DB-5 MS capillary column (30 m × 0.25 mm × 0.25 mm) using a GC (Thermo Fisher Scientific, USA) equipped with an auto-sampler Triplus AS and a DSQ quadrupole mass spectrometer. Ultra high purity helium carrier gas was maintained at a constant rate of 1 mL min−1. Injector temperature was held at 280°C, and the injection volume was 1.0 μL in a splitless mode. The column temperature for separating the targets was programmed as previously used [4]. The energy of the ionizing electrons was kept at 70 eV, and the mass spectra were operated in a full scan mode (m/z, 50 – 600).
2.6. Procedures of EE2 Quantification
EE2 was quantified by a HPLC (Agilent Technologies 1260) equipped with a C18 reversed phase column (Waters Symmetry, 5 μm, 4.6 mm × 250 mm) and a fluorescence detector (excitation at 236 nm, emission at 310 nm). The mobile phase was 60:40 (v:v) of acetonitrile and Milli-Q water containing 0.1% trifluoroacetic acid. EE2 was eluted from the column by the mobile phase at a constant flow rate of 1.0 mL min−1, and the retention time of EE2 was 4.9~5.1 min. The quantification limit of EE2 (LOQ) was 0.03 mg·L−1, and the relative standard deviations (RSDs) for all samples were within 5%.
3. Results and discussion
3.1. Photodegradation of EE2 in Nitrate Solutions
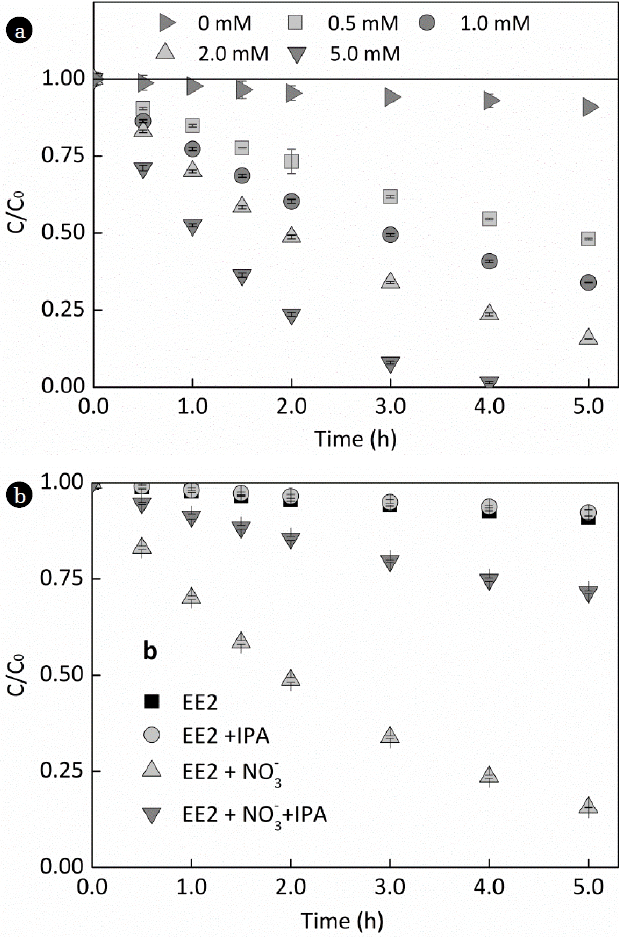
Results of EE2 photodegradation in 0 – 5 mM nitrate solutions are shown in Fig. 2a. EE2 was removed about 9%, 52%, 66%, 85% and 100% from 0, 0.5, 1.0, 2.0, and 5.0 mM nitrate solutions, respectively. Compared to the photodegradations in pure aqueous solutions, nitrate could effectively accelerate the photodegardation of EE2. It was reported that nitrate could produce HO • under illumination, and the yield of HO • was increased linearly with the concentration of nitrate [18]. Thus, the removal of EE2 in nitrate solutions would be aroused by the photogenerated HO • which can react with most organic chemicals at a diffusion-controlled rate [25]. The photodegradation of EE2 followed the pseudo-first order kinetics in both pure aqueous solutions and nitrate solutions when the removal rate of EE2 lower than 50%, and the photodegradation rate constants were determined to be 0.0193, 0.1629, 0.2540, 0.3587, and 1.1854 h−1, respectively. Due to the fluctuation of the EE2 and nitrate concentration, the photodegradation of EE2 in nitrate solutions did not follow the pseudo-first order kinetics when its removal rate higher than 50%. Compared to the photodegradation of the organic pollutants shown in Table 1, EE2 is a relative photo recalcitrant chemical, and the photodegradation of EE2 always varied with different waters affected by other aquatic components.
Production of HO• generated by the photolysis of 2 mM nitrate was determined by using TPA as molecular probe [33] and calculated by eq. (1) and (2). The concentration of HO• was determined to be 8.5 × 10–14 M, which was significantly higher than that photogenerated by dissolved humic substances in natural waters [34]. Oxidation of EE2 by HO• was confirmed by a well-established kinetics competition method using BZA and calculated by eq. (3) and (4) [35], which demonstrated that EE2 could react with HO • at a diffusion-controlled reaction rate of 1.14 × 10−10 M−1 s−1. The reactions between EE2 and HO • were also corroborated by using IPA (100 mM) as a selective ROS scavenger. As is shown in Fig. 2b, the removal rate of EE2 in 2 mM nitrate was decreased by 55% upon addition of IPA. Thus, HO• was the main contributor to EE2 photodegradation in nitrate containing waters.
where the kTPA,HO•, kBZA,HO• and kEE2,HO• are the reaction rate constant between HO • and TPA, BZA or EE2, respectively; kobs is the observed degradation rate constant of EE2 in nitrate solutions; [2-hTPA], [TPA], [BZA] and [EE2] are the concentration of 2-hTPA, TPA, BZA and EE2, respectively; the yield of 2-hTPA is 0.35; [HO •]SS is the steady state concentration of HO •.
3.2. Influence of EE2 Concentration
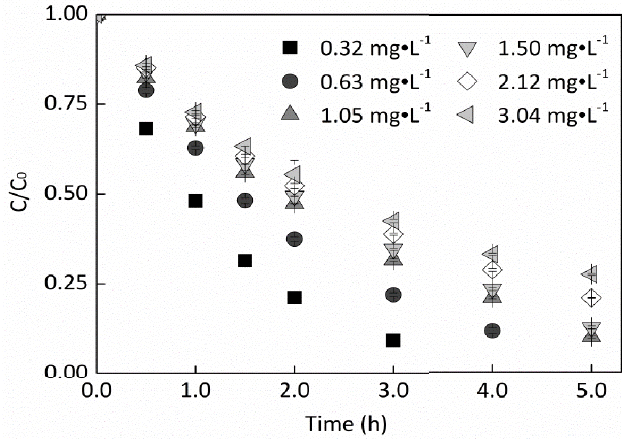
For exploring the influence of EE2 concentration on the photodegardation mediated by nitrate, different concentration of EE2 was illuminated in 2.0 mM nitrate solutions. Fig. 3 shows that the photodegradation of EE2 was significantly inhibited when its concentration increased from 0.32 mg·L−1 to 1.05 mg·L−1 and slightly inhibited when its concentration higher than 1.05 mg·L−1. Generally speaking, the removal rate of EE2 over an irradiation period of 3 h decreased from 91% to 58% when its concentration increased from 0.32 to 3.04 mg·L−1. This phenomenon corroborated the results reported by Zhan and his coworkers [30]. We previously found that the photodegradation rate of pure aqueous EE2 would decrease to a stable value when the concentration of EE2 increased [23], but a similar trend was not found in nitrate solutions. Furthermore, EE2 in the environment typically ranges from ng L−1 to μg·L−1. Therefore, the photodegradation rate of EE2 in natural waters with comparable nitrate concentration would be much faster than that determined here.
3.3. Influence of pH
The pKa of EE2 was reported to be 10.5 [23], and different photo-degradation rates of EE2 could be expected under different pH conditions. Thus, photodegradation of 1.05 mg·L−1 EE2 in 2 mM nitrate solutions under different pH conditions were performed to study the influence of pH on the photodegradation kinetics of EE2.
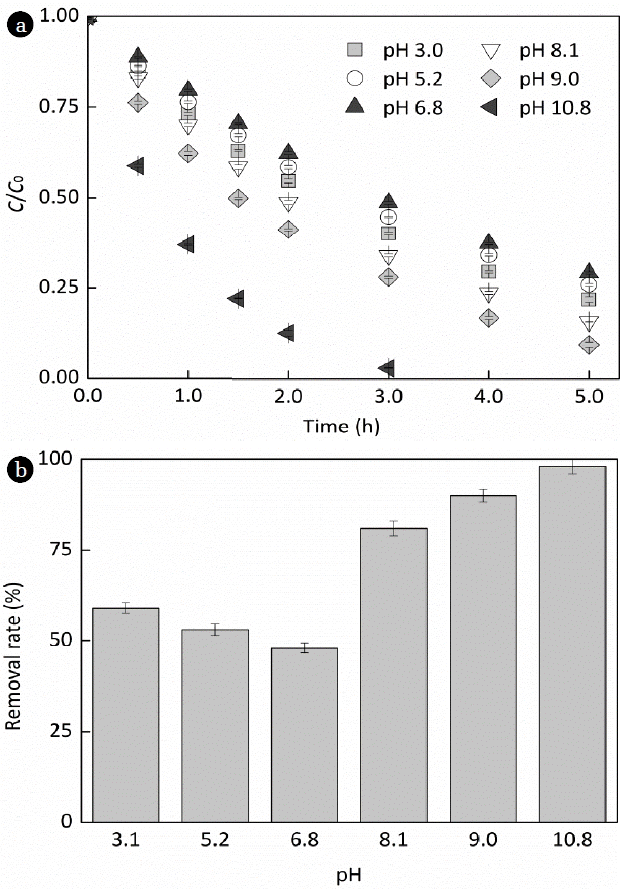
Increasing the solution pH from 3.1 to 10.8, EE2 photodegradation rate was first decreased slightly and then increased dramatically, as shown in Fig. 4a. Removal rate of EE2 over an irradiation period of 3 h ranged from 48% to 98% (Fig. 4b), and the photodegradation rate of EE2 in alkaline solutions was always higher than that in acidic solutions. The higher degradation rate of EE2 in alkaline solutions could be typically attributed to the increased light absorption capacity [23] and the promoted yield of HO• [29]. Furthermore, the delocalization of the negative charge on the phenolic hydroxyl group could increase the electron density on the benzene ring, which would be advantageous of HO • attacking.
3.4. Influence of Coexistent Substances
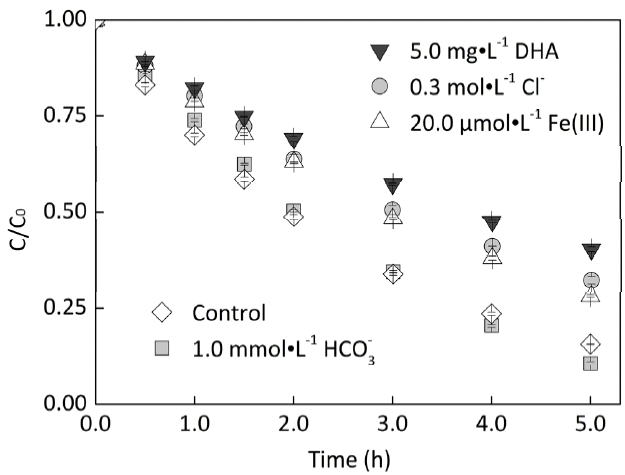
In order to explore the influence of natural water components on EE2 photodegradation, EE2 was photodegraded in 2 mM nitrate solutions in the presence of 5.0 mg·L−1 DHA, 0.3 M Cl−, 20 μM Fe(III), and 1.0 mM HCO3−, respectively. As shown in Fig. 5, Cl−, Fe(III) and DHA inhibited the photodegradation of EE2. The mechanisms responsible for Cl− inhibiting EE2 photodegradation were proposed as the reactions (5) – (8) [36]. Although iron was reported to be positive in inducing some organic contaminants degradation in dissolved humic substances containing solutions [37], it inhibited the photodegradation of EE2 mediated by nitrate. The inhibition effects of Fe(III) and DHA could be attributed to the roles of light screening and HO• scavenging [38, 39]. It was reported that HCO3− could inhibit HO• oxidizing organic pollutants through the reaction (9) [40], but no significant influence of HCO3− on EE2 photodegradation was observed in this work. The possible reasons could be drawn as follows: (i) • CO3− can selectively react with electron-rich compound, including steroid estrogens [41]; (ii) the reaction between HO • and HCO3− can disturb the solvent-cage effect and increase the possibility of HO • reacting with EE2 [42].
3.5. Photodegradation Products Identification
To further shed light on the depletion of EE2 in the irradiated nitrate solutions, the degradation products of EE2 were determined by GC-MS, and the detection results are detailed in table 2.
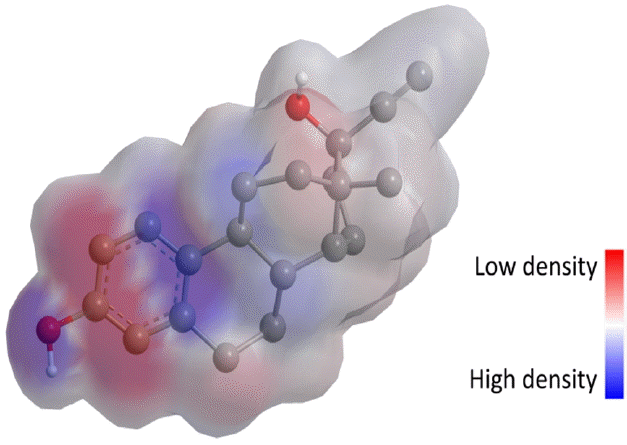
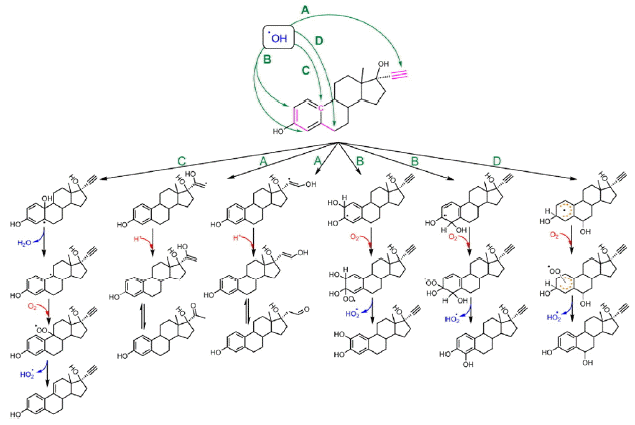
HO • is an electrophilic reagent with an electron affinity of 569.3 KJ, and it can react with organic compounds by the following three competitive pathways: (i) addition to unsaturated carbon, (ii) hydrogen abstraction from saturated carbon, (iii) electron abstraction from aromatic ring, carbon-carbon double bond or carboxylate [43]. HO• addition to electron rich structures is typically faster than hydrogen and electron abstraction when it reacts with organic chemicals [44]. Therefore, HO• is more likely to attack the π-electron system in EE2. According to the GC-MS detection information (Table 2) and surface charge distribution of EE2 (Fig. 6) modeled by MM2 force filed, the formation pathways regarding to the detected products were proposed in Fig. 7. The proposed mechanisms are in line with the results reported by Mazellier and his coauthors [8] and corroborate the pathways for HO• oxidizing microcystin-LR [45].
3.6. Estrogenicity Variation of Irradiated EE2-nitrate Solutions
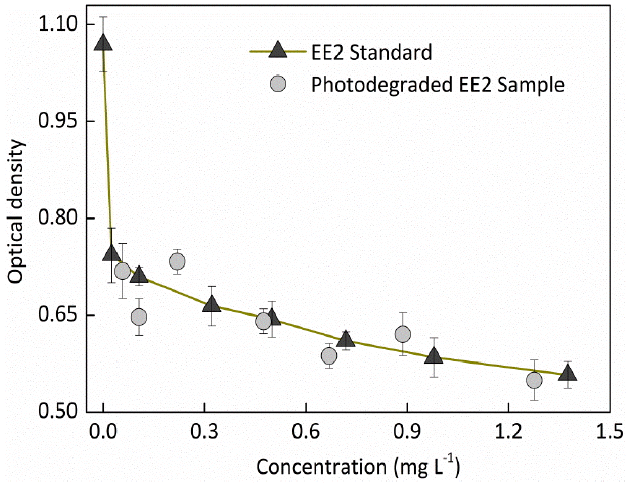
GC-MS usually cannot detect the volatiles and unstable species such as organic peroxides which may be decomposed on the column or the GC port. Furthermore, some products at a concentration lower than the limit of detection of GC-MS could also exhibit hazards to human health and ecosystem. Therefore, in vitro estrogenicity tests are essential to assess the changes in estrogenic potency of the irradiated EE2-nitrate solutions. Different EE2 concentration solutions, irradiated for 5 h previously, were subjected to the estrogenic potency determination, and the results are shown in Fig. 8.
The OD580 was decreased with the increased concentration of EE2 standard sample, i.e., EE2 can inhibit the proliferation of MCF-7 cell. A good agreement between the optical density of the photodegraded EE2 solutions and that of the same concentration of standard EE2 solutions was found in our study, which indicates that the photogenerated intermediates of EE2 were not estrogenic, that is to say, EE2 completely lost estrogenicity when it was photodegraded in nitrate solutions. This result corroborated the previous studies which identified the phototransformed intermediates and the lost estrogenic potency of 17β-estradiol and EE2 in aqueous solutions [26]. Therefore, EE2 photodegradation mediated by the photolysis of nitrate is an effective way for removing EE2 from natural waters and reducing the ecotoxicity towards human and ecosystems.
4. Conclusions and environmental implications
Since the irradiation intensity and time used in this study were similar to the sunlight exposed by natural waters, the results of EE2 photodegradation can well simulate the photodegradation of EE2 induced by nitrate in natural waters. Although EE2 is a relative refractory chemical towards direct photolysis, it can be rapidly phototransformed into chemicals without estrogenic potency in nitrate solutions, which was mainly aroused by the oxidization of the photogenerated HO•. The photodegradation of EE2 in nitrate solutions could be affected by not only the concentration of nitrate and itself but also the aquatic characteristics, including pH condition and other dissolved substances. Generally speaking, compared to EE2 biodegradation, in which the half-life of EE2 varied from 5 to 108 days [11], photodegradation mediated by nitrate is one of the fast and effective pathways for EE2 removal from sunlit natural waters.
Although the concentration of EE2 used here exceeded “natural” levels by three to six orders of magnitude, this laboratory work can be reasonably extrapolated to know the photochemical behaviors of SEs in natural waters. Photodegradation could be incorporated into seweage treatment plants to minimize the hazards of SEs to ecosystem because the effluents of sewage treatment plants were determined to be one of the main sources of estrogens in the environment.