Fabrication of Ti/Ir-Ru electrode by spin coating method for electrochemical removal of copper
Article information
Abstract
Recovery of valuable metals in the industrial wastewater and sludge has attracted an attention owing to limited metallic resources in the earth. In this study, we firstly fabricated Ti/Ir-Ru electrodes by spin coating technique for effective recovery of Cu in electrowinning process. Two different Ti/Ir-Ru electrodes were fabricated using 100 and 500 mM of precursors (i.e., Ir-Ru). SEM-EDX and AFM revealed that Ir and Ru were homogenously distributed on the surface of Ti plate by the spin coating, in particular the electrode prepared by 500 mM showed distinct boundary line between Ir-Ru layer and Ti substrate. XRD, XPS, and cyclic voltammetry also revealed that characteristics of IrO2, RuO2, and TiO2 and its electrocatalytic property increased as the concentration of coating precursor increased. Finally, we carried out Cu recovery experiments using two Ti/Ir-Ru as anodes in electrowinning process, showing that both anodes showed a complete removal of Cu (1 and 10 g/L) within 6 h reaction, but much higher kinetic rate constant was obtained by the anode prepared by 500 mM. The findings in this study can provide a fundamental knowledge for surface characteristics of Ti/Ir-Ru electrode prepared by spin coating method and its potential feasibility for effective electrowinning process.
1. Introduction
Generation of industrial wastewater and sludge containing various metals has continuously increased owing to a dramatic increase of metal usage during the industrialization and urbanization. In particular, metals such as copper, nickel, zinc, lead, cadmium, and chromium are highly contained in the industrial wastewater produced from electroplating, mining, smelting, battery and paint manufacturing, and printing industries [1, 2]. Because these highly concentrated metals can induce severe environmental pollutions and negatively influence human health causing carcinogenesis and some diseases when they are discharged to natural environments without appropriate treatment [3, 4].
Precipitation is one of most commonly used methods for removal of metals in industrial wastewater and sludge by forming metal hydroxides with CaOH2 or NaOH. However, secondary treatment processes are usually required to remove metals in the precipitated metal sludges, resulting in increase of operation cost largely [5]. Over the last few decades, the efforts on recovery of valuable metals in the industrial wastewater and sludge were to obtain metallic resources and reduce the amount of waste disposal. Several methods such as solvent extraction, ion exchange, membrane technology, ferrite process, and extraction separation have been developed to effectively recover metals from wastewater and toxic sludge [5, 6].
Electrowinning, a method for electrodeposition of metals onto the cathode, has been applied for metal recovery normally in acidic wastewater. Due to a relatively low cost, lead alloys are commonly used as an economic anode for electrowinning process [6]. However, the lead-based anodes revealed some drawbacks such as high energy consumption efficiency and low corrosion resistance [7]. In order to solve these problems, researchers have investigated various metals for development of effective anode substrate and commonly selected a titanium (Ti) substrate due to its corrosion resistance [8]. Recently, tantalum (Ta2O5), tin (SnO2), iridium (IrO2), ruthenium (RuO2) oxides and their combinations have been used for enhancement of electrocatalytic activity, stability, and energy efficiency of anodes [9]. Among the metal oxides, RuO2 showed the highest electrocatalytic activity with a relatively short life time [10], while IrO2 has slightly lower electrocatalytic activity and longer lifetime (almost 20 times) than that of RuO2 with an excellent stability in acidic media [11]. Therefore, a combination of IrO2 and RuO2 has been used for development of efficient anodes working on electrowinning process [12].
During fabrication of oxide coated electrode, a coating method of Ir and Ru oxides on Ti substrate can significantly influence the efficiency of electrowinning process because electrical properties of electrode are totally dependent on coating surface. Compared to other coating methods (e.g., brush-, spray-, dip-coating), spin coating technique using centrifugal force for spreading precursor solution on the surface of substrate could be an excellent alternative because the oxide films may form more uniformly and easier to control the surface layer by applying different precursor concentration, rotation speed, and time [13]. The development of Ti/Ir electrode by the spin coating has been reported, but researchers mainly focused on the kinetics study of formic acid oxidation showing that only the effect of IrO2 content and organic concentration (i.e., formic acid) on the oxidation efficiency of formic acid [14]. However, details for surface characteristics of Ti/Ir electrode characterized by physical-chemical surface analysis was not provided in the previous study. Therefore, a limited knowledge has been provided for development of Ti/Ir-Ru electrode by the spin coating technique to date, thus its surface characteristics and application to electrowinning process have not reported well.
In this study, we firstly used the spin coating technique to develop and characterize the Ti/Ir-Ru electrodes prepared by different concentrations of precursor solution. In order to characterize the surface of Ti/Ir-Ru, different surface analysis was carried out by using field emission scanning electron microscopy with energy dispersive X-ray spectroscopy (FESEM-EDX), atomic force microscopy (AFM), X-ray diffraction (XRD), and X-ray photoelectron spectroscopy (XPS). Cyclic voltammetry was also used to investigate electrochemical characterization of Ti/Ir-Ru electrodes. Finally, we performed the electrowinning of synthetic wastewater containing Cu in 0.5 M H2SO4 to highlight the potential feasibility of Ti/Ir-Ru as an effective anode for metal recovery by electrowinning process.
2. Experimental
2.1. Development of Ti/Ir-Ru Anodes
Ti/Ir-Ru anodes were prepared by a thermal decomposition of iridium and ruthenium chloride precursor mixtures. Ti substrates (10 × 10 × 0.04 cm3) were scrubbed by silicon carbide (SIC) paper to improve roughness of the surface, and washed with deionized water (DIW, 18.2 MΩ cm, ELGA PURELAB Classic system). Then, the Ti substrates were chemically etched in 10% boiling oxalic acid for 2 h to enhance adhesion force between the precursor solutions and substrate [15, 16] and washed with DIW several times to remove impurities on the surface. Fig. S1 shows the SEM (JSM-6060, JEOL) images of (a) bared, (b) scrubbed, and (c) etched Ti surfaces. It was observed that many scratches formed on the Ti substrates after scrubbing by SIC paper and the surface was significantly changed to porous structure by chemical etching process.
Precursor mixture containing iridium and ruthenium with 1:1 molar ratio was prepared by mixing of IrCl3·3H2O (99.8%, Alfa aesar) and RuCl3·xH2O (99.9%, Alfa aesar) in different concentrations (100 and 500 mM) with 2-propanol (≥99.5%, Sigma Aldrich) solution. An exact amount of homogeneous precursor mixtures was dropped onto the Ti substrate until forming thick layer and spread out precursor mixtures uniformly by centrifugal force at 1,000 rpm using spin coater. The substrates coated by the precursor were dried at 80°C for 10 min to evaporate solvent (i.e., 2-propanol). The back side was also coated by following the procedure described above. Finally, the Ti substrates coated both sides by Ir and Ru precursors were calcinated at 450°C for 10 min in air furnace atmosphere. We repeated the procedure 4 times more for each Ti/Ir-Ru anode to obtain a desired thickness of oxide films.
2.2. Surface and Electrochemical Characterization of Ti/Ir-Ru Anodes
For analysis of surface characteristics of Ti/Ir-Ru anodes, we washed each Ti/Ir-Ru anode with DIW several times to remove the residual impurities and cut them to make the size of 5 × 5 mm2. The surface morphology and elemental distribution of Ti/Ir-Ru anodes were identified by FESEM-EDX (SU8010_EX370, Hitachi). For FESEM-EDX analysis, the specimens were put on a carbon tape and coated by platinum for 30 s. AFM (LabRAM HR800, Horiba Jobin-Yvon) was applied to characterize the topography of Ti/Ir-Ru anodes at high resolution (~nm scale). The mineral phase and oxidation state of elements on the surface of Ti/Ir-Ru anodes were analyzed with XRD (Smartlab, Rigaku) and XPS (K-alpha, Thermo VG Scientific) [17], respectively. Cyclic voltammograms for Ti/Ir-Ru anodes were recorded by using a SP-150 potentiostat (Bio-Logic Science Instruments). The experiments were performed in the range of −0.1–1.5 V in 0.5 M H2SO4 solution containing 1 g/L of Cu. The stainless steel, Ag/AgCl, and the Ti/Ir-Ru anodes were used as a counter, reference, and working electrode in the cell, respectively.
2.3. Electrowinning of Copper
A rectangular batch reactor (90 × 120 × 170 cm3) (Fig. S2) was used for the removal and recovery of Cu in synthetic wastewater in this study [18]. A commercial stainless steel (SUS 304) and Ti/Ir-Ru electrodes developed in this study were used as a cathode and anode (10 × 7 × 0.04 cm3), respectively. The distance between electrodes was designed to be 1 cm. 1.5 L of synthetic wastewater prepared by CuSO4·5H2O (99%, Samchun) in 0.5 M H2SO4 solution was used for recovery of Cu by the electrowinning process. Two different Cu concentrations (1 and 10 g/L) were used to simulate highly concentrated industrial wastewater and leached liquor from metal sludge in acidic media.
All electrowinning experiments were carried out at constant imposed voltage (2.4 V) by using a DC Power Supply (EX30-60) in mixing condition at 200 rpm at room temperature (24 ± 1.5°C). In order to monitor the removal kinetics of Cu, we collected 4 mL of aqueous sample at each sampling time and analyzed the Cu concentration by inductively coupled plasma-optical emission spectrometry (ICP-OES, Thermo). The amount of Cu deposited on the stainless steel cathode was also measured after finishing the experiments to compare with the concentration of Cu removed in aqueous phase.
3. Results and Discussion
3.1. Morphological Characteristics of Ti/Ir-Ru Anodes
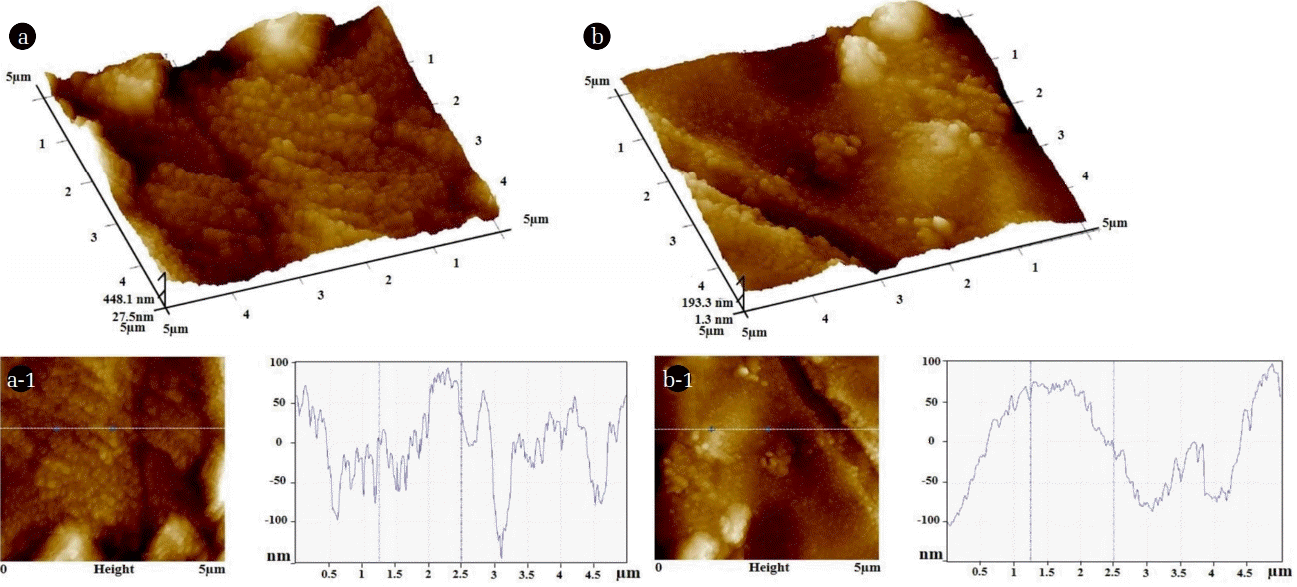
Fig. 1 shows the SEM images of Ti/Ir-Ru anodes coated with 100 (Fig. 1 (a)) and 500 mM (Fig. 1 (b)) precursor solutions. The scratches and porous structure on the surface of Ti substrates created during the pretreatment processes were not observed owing to formation of metal oxide film. It should be noted that each anode prepared by 100 and 500 mM of precursor solutions displayed different surface morphology. We observed a distribution of needle shaped nanoparticles on the surface of Ti/Ir-Ru anode prepared by 100 mM precursor (Fig. 1 (a-1)), which forms wave patterns on the Ti substrate. In contrast, a relatively uniformed flat area and cracked-mud structure was obtained from the Ti/Ir-Ru anode prepared at 500 mM precursor (Fig. 1 (b-1)), which can be typically observed when the metal oxide films formed by the thermal decomposition method. The results indicate that an optimal precursor concentration should be considered during the development of Ti/Ir-Ru electrodes by the spin coating technique. The formation of cracks may form during the solvent evaporation and cooling process of the electrodes [19].

SEM images of the Ti/Ir-Ru anodes coated with (a) 100 and (b) 500 mM precursor solutions and their enlarged images (a-1 and b-1).
Fig. S3 shows the cross-sectional SEM images for Ti/Ir-Ru anodes prepared by 100 (Fig. S3(a)) and 500 mM (Fig. S3(b)) of precursor solutions. For the sample of 100 mM, the boundary layer between metal oxide film and Ti substrate was not clearly observed owing to the insufficient amount of metal loading on the surface of Ti substrate. In contrast, we observed a clear formation of metal oxide film for the sample of 500 mM with an approximate thickness of 3 μm. Normally, the thickness of oxide film higher than 2 μm has been considered as an appropriate electrode [19], indicating that the Ir-Ru precursor solution higher than 100 mM may be needed to create a proper metal oxide film on the surface of Ti substrate.
The presence of main elements (i.e., Ti, Ir, and Ru) on the surface of Ti/Ir-Ru anodes was determined by EDX mapping as shown in Fig. 2. We observed a large amount of Ti on the surface of Ti/Ir-Ru anode prepared by 100 mM precursor, resulting in a relatively small amount of Ir and Ru with a poorly distributed condition (Fig. 2(a)), while the Ti/Ir-Ru anode prepared by 500 mM precursor revealed a relatively small amount of Ti with large amount and well distributed Ir and Ru on the surface. In particular, almost no Ti was detected in the areas of cracked-mud, indicating that these areas possess a thick Ir-Ru oxide layer as shown in the SEM images. AFM images for the sample of 100 mM displayed that lateral fluctuations of surface features can be detected in almost every 250 nm distance with 30–200 nm of topography deviation (Fig. 3(a) and (a-1)). In comparison, we did not observe a significant change in topography for the sample of 500 mM (Fig. 3(b) and 3(b-1)), which show a relatively flat surface than that of 100 mM sample as concluded from the SEM results. All morphological information from SEM-EDX and AFM revealed that the Ti/Ir-Ru electrode prepared by 500 mM precursor solution may have a high potential to be used for efficient anode of electrowinning process rather than that of 100 mM.
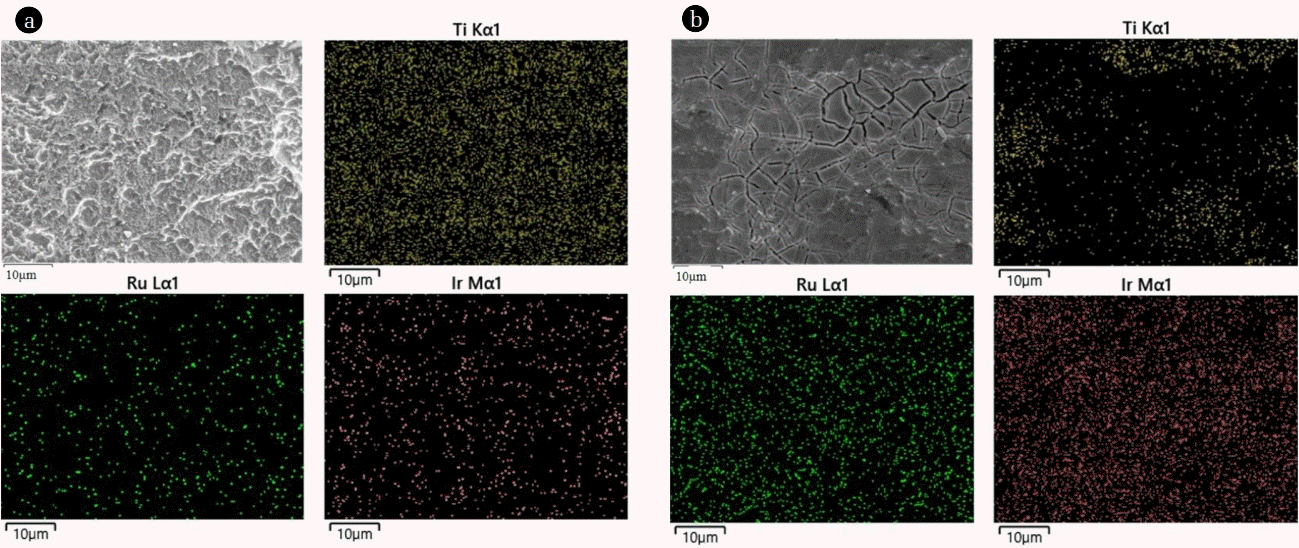
FE-SEM images and corresponding results of EDX mapping for the Ti/Ir-Ru anodes coated with (a) 100 and (b) 500 mM precursor solutions.
3.2. X-ray Based Analysis (XRD and XPS) for Ti/Ir-Ru Anodes
Crystallinity and mineral phase on the surface of each Ti/Ir-Ru were investigated by XRD in the range of 10–80° 2θ (Fig. S4). We observed the presence of several peaks corresponding to IrO2, RuO2, TiO2, and Ti on the surface of both Ti/Ir-Ru anodes. The peaks for both metal oxides (i.e., Ir and Ru) were observed in the identical 2θ values with rutile type of TiO2 because the ionic radii of Ru4+, Ir4+, and Ti4+ are almost same as 0.076, 0.077 and 0.075 nm, respectively [12]. It is noteworthy that the peak intensity for IrO2 and RuO2 increased as concentration of precursor solution increased from 100 to 500 mM, leading to the decrease in peak intensity of Ti. The results was in good agreement with what we obtained from EDX analysis, implying that the Ir-Ru metal oxide films covered well on the surface of Ti substrate [12].
The XPS analysis was also conducted to investigate the oxidation states of Ir and Ru on the surface of Ti/Ir-Ru anode prepared by 500 mM precursor solution (Fig. S5). The narrow region spectra for Ru(3p5/2) (Fig. S5(a)) and Ir (4f7/2) revealed the presence of zerovalent metals (Ru(0) and Ir(0)) and oxides forms (RuO2 and IrO2), but the most dominant form was each metal oxides which was corresponding to the results of XRD.
3.3. Cyclic Voltammetry Measurement for Ti/Ir-Ru Anodes
Cyclic voltammetry experiments were performed to investigate the electrochemical characterization of each Ti/Ir-Ru anode (Fig. S6). A cathodic peak was observed at approximately −0.1 V potential due to hydrogen evolution (Eq. (1)) and Cu (Eq. (2)) deposition, while the anodic peak was observed at approximately 1.5 V potential owing to oxygen evolution (Eq. (3)).
In addition, slight peaks were detected at 0.5 and 0.6 V for 100 and 500 mM coated electrodes, respectively. We obtained slightly irreversible peaks during the cyclic voltammetry experiment, which is attributed to the transition of Ru and Ir from (III) to (IV) species [20, 21]. Overall increase in current density was observed in the sample of 500 mM, indicating that the 500 mM coated Ti/Ir-Ru anode possess larger electrochemically active surface area than that of 100 mM sample.
3.4. Electrowinning Process Using Ti/Ir-Ru Anodes for Recovery of Cu
Fig. 4 shows the removal efficiencies of Cu ((a) 1 and (b) 10 g/L) by electrowinning process working with different Ti/Ir-Ru anodes and a stainless steel cathode in 0.5 M H2SO4 solution. During the electrowinning of Cu, it may be expected the redox reactions at each cathode (Eq. (1) and (2), net cathodic reaction: Cu2+ + 2H+ + 4e− = Cu(0) + H2) and anode (Eq. (3)). All electrowinning experiments were carried out at a constant cell voltage (2.4 V), because the voltage lower than 2.4 V may significantly oxidize metallic Cu(0) to Cu2O [22]. For the case of 1 g/L Cu, a complete Cu removal was obtained within 6 h reaction in the experiments using both Ti/Ir-Ru anodes and their removal kinetics were almost similar due probably to a relatively low initial concentration of Cu with high enough potential. Compared to that of 100 mM case, we observed the enhanced Cu removal for the case of 10 g/L Cu by using the 500 mM coated Ti/Ir-Ru anode, showing the complete removal within 5 h reaction. The differences in Cu concentration from 0 to 3 h reaction were varied in the level of thousand mg/L between both cases, which is considered as a significant enhancement in 500 mM case. In addition, the initial blue color of synthetic Cu wastewater changed to be transparent after finishing the electrowinning (Fig. 4 (a) and (b) insets), indicating the complete removal of aqueous Cu. The results indicate that the initial concentration of Cu can influence the total removal efficiency significantly [23] and Ti/Ir-Ru electrode can be used as an anode for efficient removal of highly concentrated Cu in the wastewater owing to the enhanced physical and electrochemical properties as shown above.

The efficiency of Cu removal during electrowinning process using a stainless steel cathode and Ti/Ir-Ru anodes in the (a) 1 g/L and (b) 10 g/L Cu solutions.
The removal kinetics of Cu in this study can be described as two distinct stages from initial time to 2 h (Fig. 5(a)) and after 2 h (Fig. 5(b)), which were followed by zero-order (Eq. (4)) and first-order kinetics (Eq. (5)), respectively [22].

The linear plots of (a) zero- and (b) first-order kinetics of Cu recovery by electrowinning process.
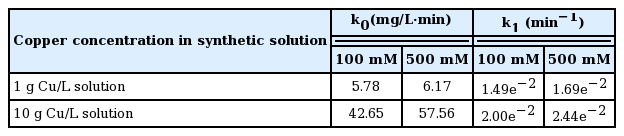
where [Cu2+]t is the Cu concentration at time t, [Cu2+]i is the initial Cu concentration, k0 is the zero-order rate constant, and k1 is the first-order rate constant, respectively. A good linearity for all kinetic analysis was obtained in this study, which is in agreement with previous study [22]. The change in kinetic orders may be due to the requirement of induction time for the Cu reduction at initial period [24]. After finishing the induction period, the removal kinetics was followed by first-order, which highlights the importance of aqueous Cu concentration after 2 h reaction. Table 1 shows the k0 and k1 values for each experiment, showing that all the k values for 500 mM coated Ti/Ir-Ru anode were higher than that of 100 mM case.
Fig. 6 shows the images of Ti/Ir-Ru anodes before and after the Cu removal (10 g/L) in the synthetic wastewater. Almost no change was observed from the initial Ti/Ir-Ru anodes (a: 100 mM and b: 500 mM) after the electrowinning (a-1: 100 mM and b-1: 500 mM), while a significant change was observed in the stainless steel cathode, resulting in the attachment of dark powders on the surface (Fig. 6(c-1)) after the electrowinning comparable to that of the initial Ti/Ir-Ru anode (Fig. 6(c)). The formation of dark powder may be attributed the oxidation of inner metallic Cu(0) to Cu2O through the direct reaction by oxygens during the electrowinning process. The oxygens could be formed on the surface of anode and transferred to the cathode owing to an aggressive stirring in this study [22]. Indeed, we observed the presence of Cu(0) foil (Fig. 6(d)) inside of CuO2 powders (Fig. 6(e)). In addition, weight of Cu recovered by electrowinning process was measured after the experiments, confirming the recovery efficiency higher than 100% in all the experiments (Table S1). The Cu weight higher than we injected into the reactor may be induced by the presence of CuO2 powders, which increase the weight of Cu in total. The results showed that the highly concentrated Cu could be effectively removed and recovered by electrowinning process working with a well fabricated Ti/Ir-Ru anode by the spin coating technique.
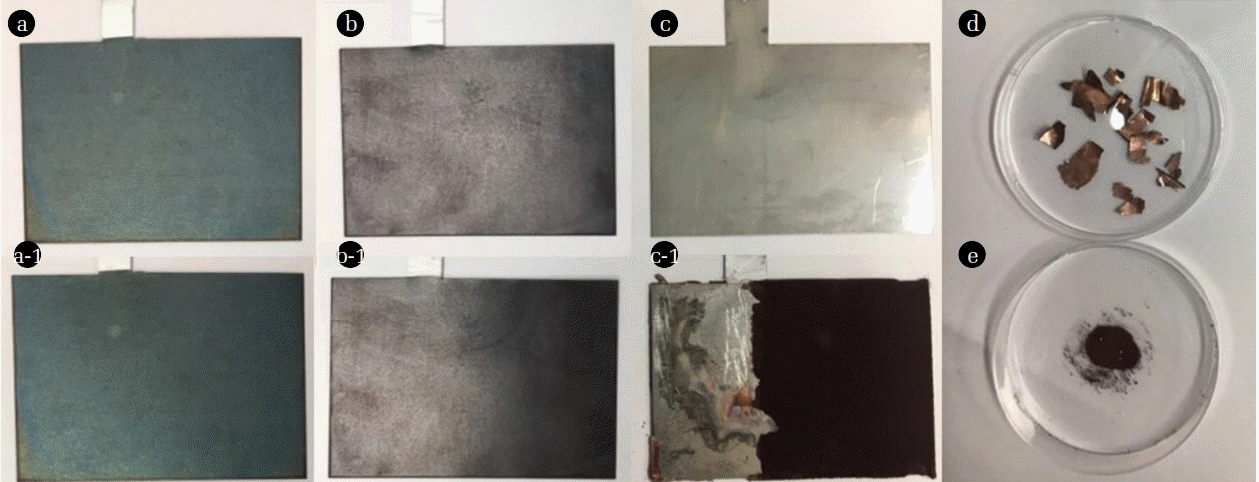
The images of Ti/Ir-Ru anodes coated by 100 mM precursor (a) before and (a-1) after the electrowinning of Cu. (b), (b-1), (c), and (c-1) describe Ti/Ir-Ru anodes coated by 500 mM precursor and stainless steel cathodes before and after the electrowinning of Cu, respectively. The recovered Cu after the electrowinning as a (d) Cu(0) foil and (e) CuO2 powder.
4. Conclusions
In this study, the spin coating technique was successfully employed to fabricate Ti/Ir-Ru electrodes for different concentrations of precursor that can be used for removal and recovery of valuable metals in wastewater. The results from various surface analysis showed that Ti/Ir-Ru anode surface prepared by 500 mM precursor solution possessed a cracked-mud structure and the metal oxide films of Ir and Ru were well deposited on the Ti substrate. Cyclic voltammetry revealed an overall increase in current density in the sample of 500 mM, implying that it can be favored for the electrowinning process. Indeed, we observed the enhanced Cu removal by using the Ti/Ir-Ru anode prepared by 500 mM precursor solution in the case of 10 g/L of Cu. Finally, the amount of Cu deposited on the cathode was easily recovered by scrubbing the electrode surface. The results obtained from this study can provide the fundamental knowledge of surface characteristics of Ti/Ir-Ru electrode fabricated by spin coating technique and its feasibility to be used as an effective anode for removal and recovery of heavy metals by the electrowinning process.
Supplementary Information
Acknowledgments
This subject is supported by Korea Ministry of Environment (MOE) as “Technologies for the Risk Assessment and Management Program (2017000140006)” and the Korea Institute of Energy Technology Evaluation and Planning (KETEP) and the Ministry of Trade, Industry and Energy (MOTIE, 20174010201490).