Application of multivariate statistics towards the geochemical evaluation of fluoride enrichment in groundwater at Shilabati river bank, West Bengal, India
Article information
Abstract
To obtain insightful knowledge of geochemical process controlling fluoride enrichment in groundwater of the villages near Shilabati river bank, West Bengal, India, multivariate statistical techniques were applied to a subgroup of the dataset generated from major ion analysis of groundwater samples. Water quality analysis of major ion chemistry revealed elevated levels of fluoride concentration in groundwater. Factor analysis (FA) of fifteen hydrochemical parameters demonstrated that fluoride occurrence was due to the weathering and dissolution of fluoride-bearing minerals in the aquifer. A strong positive loading (> 0.75) of fluoride with pH and bicarbonate for FA indicates an alkaline dominated environment responsible for leaching of fluoride from the source material. Mineralogical analysis of soli sediment exhibits the presence of fluoride-bearing minerals in underground geology. Hierarchical cluster analysis (HCA) was carried out to isolate the sampling sites according to groundwater quality. With HCA the sampling sites were isolated into three clusters. The occurrence of abundant fluoride in the higher elevated area of the observed three different clusters revealed that there was more contact opportunity of recharging water with the minerals present in the aquifer during infiltration through the vadose zone.
1. Introduction
There is no existence of life on the earth without water. Groundwater is the most valuable source of water for various purposes and indispensable because of its inherent value. But increased urbanization and agricultural expansions result in overexploitation of groundwater resources in an unsustainable declining trend [1]. The major problem with groundwater is that once contaminated, it is very much difficult to restore its quality again [2]. The quality of groundwater in an area is mainly influenced by natural processes as well as anthropogenic activities [3]. Groundwater fluoride enrichment is one of such process which is associated with natural phenomena such as prolonged water-rock interaction, weathering and leaching of fluoride-bearing minerals or due to anthropogenic activities such as the excess application of pesticides and phosphatic fertilizers in the agricultural field, industrial activity such as aluminium smelting [4, 5]. Potential sources of fluoride in groundwater incorporate different minerals present in rocks and soils for example fluorite, apatite, micas, muscovite, amphiboles which are ordinarily found in gneiss terrane [6]. Groundwater chemistry enhancing the fluoride concentration includes pH, the concentration of HCO3−, Ca2+ and Na+ ions [7]. Other than groundwater chemistry the elevated evapotranspiration in the arid and semi-arid region also accelerate the fluoride concentration in groundwater [8].
Fluoride has both positive and negative health impacts on the human body when it is consumed by drinking water. Daily ingestion of about 1 mg/L of fluoride in drinking water is beneficial for mineralization of bone and formation of enamel to reduce the incidence of dental caries [9, 10]. Consumption of excess fluoride for a prolonged period causes incurable crippling of dental and skeletal fluorosis and additionally another detrimental health effect like retardation of normal growth, mobility loss, DNA structure change and even death when reaches to abnormal dose (about 250 mg/L) [11, 12].
It has been reported that more than 200 million people around the world are under the threat of fluorosis (Disease caused by excess intake of fluoride) due to the intake of drinking water containing fluoride concentration which exceeds the present WHO permissible limit of 1.5 mg/L [10, 13]. In India 20 states are under the threat of endemic fluorosis which includes 65% of the rural population [14]. Taking into account the expanding reports on fluoride contamination in groundwater a Rapid Assessment Survey was conducted by the Fluoride Task Force, Govt. of West Bengal. From the survey, it was finally found that predominance of elevated fluoride concentration in 43 blocks of 7 districts namely Bankura, Birbhum, Purulia, Malda, South 24-Parganas, North and South Dinajpur [15]. The present study has been conducted in the villages near Shilabati river bank of Bankura district where groundwater is the only source for drinking as well as irrigation purpose. From the socio-economic point of view, the study area is backward. Due to less availability of fresh drinking water and unavailability of centralized drinking water treatment facility people compelled to take fluoride contaminated water for drinking purpose.
The application multivariate statistical techniques, such as factor analysis (FA), cluster analysis helps to consolidate a large number of original data with great efficiency so that they can be readily interpreted to obtain information of environmental geochemical origin, for the identification of the possible factors or sources influencing water quality and offers a valuable tool for water resource management [16, 17]. These techniques have been applied by several researchers to understand the dominant factors controlling fluoride geochemistry and also the spatiotemporal variation of fluoride in groundwater [18–20].
The primary objective of the present research work is to evaluate the characteristic of fluoride rich groundwater using FA for explaining the hydrogeochemical process of fluoride enrichment and also to delineate the spatial variation of fluoride in the study area using cluster analysis.
2. Description of Study Area
2.1. Location and Hydrometeorology
The study area that has been taken is a part of Silabati River Basin in Simlapal block of Bankura district of West Bengal State. It is geographically extended from 22°59′34″ North to 22°54′37.00″ North latitude and 86°58′51″ East to 87°4′60″ East longitudes. The entire study area is about 114 km2.
The climatic condition of the area under study is tropical with an average annual rainfall of 1,400 mm. About 80% of the total precipitation is received during July to October. The maximum temperature reaches 46°C during summer and lowers to minimum 8°C during winter.
2.2. Geology and Hydrology of the Study Area
Geomorphologically Bankura district is divided into three parts. Hilly hard rock dominates the western part while the central part is dominated by a mixed formation of both alluvial and rocky segments. The eastern part is a sedimentary alluvial plain, where the present study has been conducted. In this part, the water table lies between 1.5 m to 15 m below the ground level in the unconsolidated sandy sediments of recent alluvium which mostly contains laterite and fine loamy soil. The groundwater yield is good and used for agriculture with the help of shallow and deep tube wells.
3. Materials and Methods
3.1. Sample Collection
Groundwater samples were collected from 58 different location of the study area and the coordinates of sampling locations were noted by using a handled GPS set (Trimble Juno SA). To obtain a representative sample, the groundwater was collected after 5 min initiation of pumping procedure in two different set of sampling bottles, one 500 mL of the sample without any preservatives and one 250 mL of sample with HCl as a preservative for analysis of cations. For further chemical analysis collected samples were stored in an incubator at 4°C.
3.2. Sample Analysis
Onsite determination of pH, Temperature, Total Dissolved Solids (TDS), Electrical Conductivity (EC) were conducted during sampling using a Multi-Parameter PCSTestr 35 (Eutech) and Oxidation and reduction potential using an ORPTestr 10 (Eutech). The major cations (Mg2+, Ca2+, Na+, K+) were analyzed using atomic absorption spectrometer (AAS, Perkin Elmer 900T) by following the standard method (EPA 7000B). The accuracy of the AAS results was confirmed by maintaining the R2 value of the calibration curve 0.99. The concentration of major anions (SO42−, HCO3−, Cl−) was estimated by following standard test method [21]. As per the standard method sulphate (SO42−) was estimated by UV-Visible spectrophotometer (Shimadzu, UV1800) with the photometric accuracy of ± 0.002 Å. Titrimetric method was used to determine the alkalinity of unacidified samples. Chloride concentration was determined by argentometric method where relative standard deviation was maintained within 4.2% and a relative error of 1.7%. Fluoride in the groundwater samples was determined potentiometrically using ion selective electrode (Orion 4 Star, Thermo scientific) by following the standard method (ASTM D 1179). This standard test method can be used to detect the fluoride concentration in water in between the concentration range of 0–19,990 ppm. Sodium fluoride (AR Grade, Merck) was used to prepare standard (0.1–10 mg/L) and stock solutions (100 mg/L). During analysis, the interference of other ions was restricted by adding a buffer solution (TISAB III, Orion) at 1:10 ratio of the samples. Before analysis, the fluoride meter was calibrated for a slope of −59 ± 2.0.
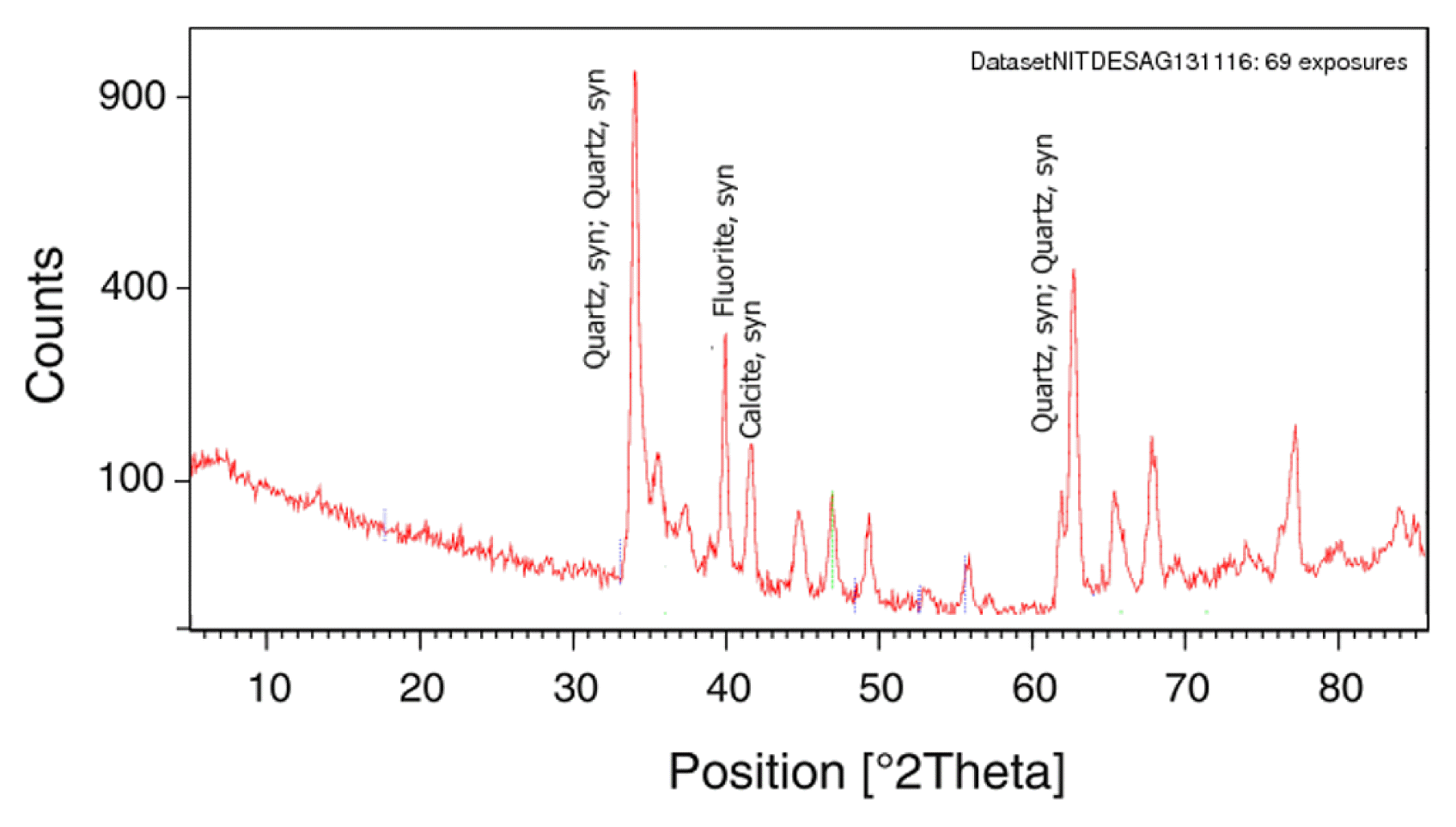
3.3. XRD Analysis
The sediment samples collected from the most contaminated area were subjected to mineralogical analysis using X-ray diffractometry (XRD). Before analysis sediment samples were powdered and kept in a hot air oven for 24 h to expel the moisture content. To obtain a smooth and flat surface oven dried sample was placed into the sample holder. After that, the sample was inserted into the XRD machine to obtain the diffractograph of individual samples. The peak position and intensity of mineral structure were shown by the diffractogram. For identification of peak OriginPro 8 software was used. The peak of the diffractograph showed the presence of quartz, fluorite, and calcite in the soil sample (Fig. 2).
3.4. Quality Assurance and Quality Control
To maintain quality assurance and quality control analytical grade (Merck) chemicals were used for the standard preparation and also to conduct all desired experiments. To assure the quality of AAS result, a blank sample was run after 25 runs of samples to verify baseline stability. After analysis of every ten samples, the sensitivity of the instrument was verified using a known standard concentration to confirm the AAS reading. To cross-check the accuracy of major ion analysis, inorganic charge balance was conducted which was within ± 5% of the variance.
4. Results and Discussion
4.1. General Hydrogeochemistry
The physicochemical parameters along with descriptive statistics of collected groundwater samples are presented in Table 1. During extensive sampling, it was observed that pH of the groundwater sample varies in a wide range from 5.9 to 8.82 and 6.13 to 9.25 during pre- and post-monsoon, respectively, indicating slightly acidic to highly alkaline in nature. The higher pH in post-monsoon may be attributed to more carbonate dissolution. TDS varies from 73 mg/L to 790 mg/L and 60 mg/L to 1,330 mg/L during preand post-monsoon, respectively. Higher TDS during post-monsoon reveals more weathering and dissolution with the recharging rainfall water. The EC values were found to lie in the range of 126.8 to 1,240 μS/cm in the dry season and 5.02 μS/cm to 1,450 μS/cm in the wet season. The higher standard deviation of TDS and EC values indicate the spatial variation of leaching and dissolution process in the study area. The oxidation and reduction potential (ORP) values were found to lie between −70 mV to 63 mV and −133.11 mV to 51.47 mV during pre- and post-monsoon, respectively. The prevalence of oxidation and reducing conditions during pre- as well as post- monsoon indicate the existence of mixed type aquifer system in the study area. However, reduction conditions were predominant than oxidizing conditions. This could be due to predominance of alkaline ionized water along with the presence ferrous and sulphate ion that functioned as reducing agent in groundwater [22, 23]. The fluoride concentrations were varying from 0.25 mg/L to 17.81 mg/L and 0.08 mg/L to 18.5 mg/L during pre- and post-monsoon, respectively. The sample with higher fluoride concentration was observed to have mostly negative ORP showing more tendencies to reduce the minerals present in the study area. It was also found that Ca2+, Na+, Mg2+ and K+ were dominant cations and HCO3−, Cl− and SO42− were major anions in the groundwater sample.

Summery Statistic of Hydrochemical Parameters of the Groundwater Sample Collected from the Study Area
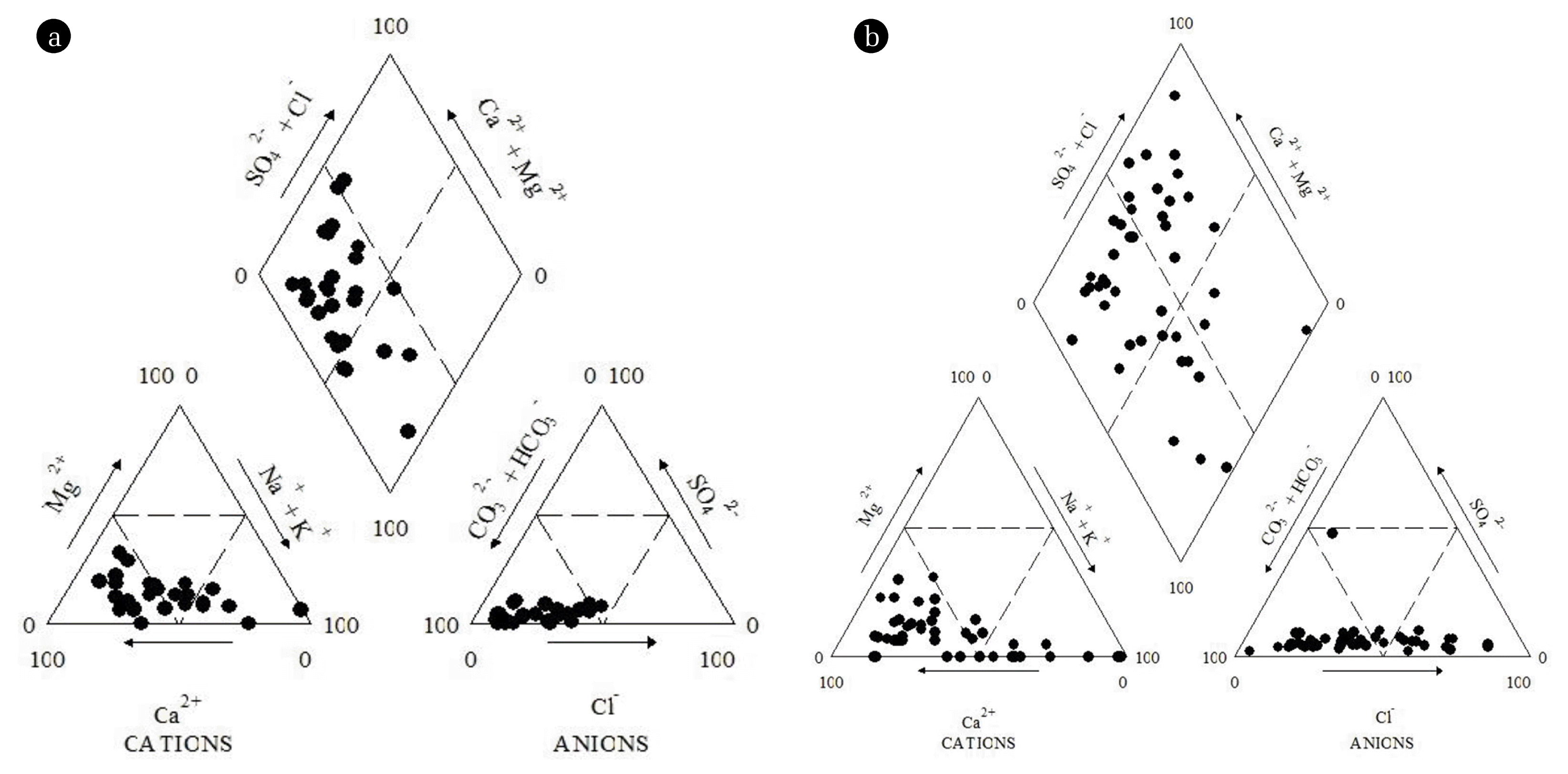
Using the major cations and anions, Piper trilinear diagrams were plotted for the classification of the groundwaters and the seasonal variations in hydrological facies [24]. From the diagram it was observed that majority of the groundwater samples plotted in the anion field contain HCO3− as major anion and the samples plotted in cation field contain Na+, Ca2+ as major cation. The analysis the central diamond field of piper plot suggested Na-Ca-HCO3 type of the groundwater for both the seasons. The high HCO3− type water in both the seasons indicate an alkaline environment is promoting the dissolution of F− enriched minerals due to precipitation of calcite, under weaker fluorite solubility limit [25]. Piper plots also show the prevalent heterogeneity in groundwater quality which may be due to the presence of different aquifers at different depths or due to different geochemical processes influencing the groundwater chemistry in the study area.
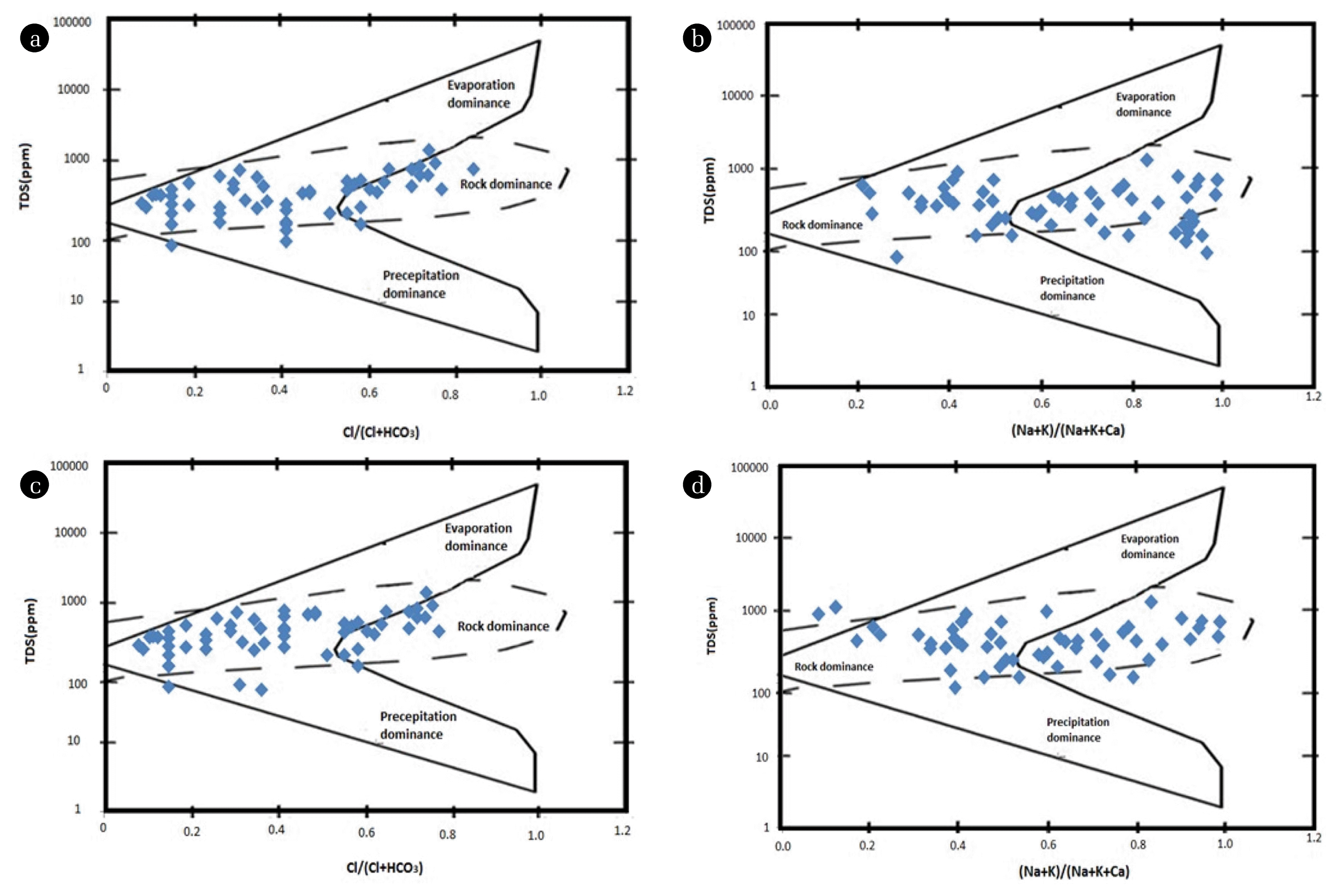
To understand the controlling mechanism of the groundwater chemistry, Gibbs diagram was plotted to know the source of major ions. This diagram represents the ratio of [Cl/(Cl + HCO3)] and [(Na + K)/(Na + K + Ca)] as a function of TDS for the assessment of working sources of dissolved ions in groundwater such as evaporation dominance, rock dominance and precipitation dominance [26].
Gibb’s Ratio I of groundwater samples ranges from 0.1 to 0.8 and whereas Gibb’s Ratio II ranges from 0.2 to 0.9 for both the seasons, respectively. Gibbs plot demonstrates that rocks are the origin of major ions present in the groundwater samples. So, ions bearing minerals control the leaching mechanism.
4.2. Factor Analysis
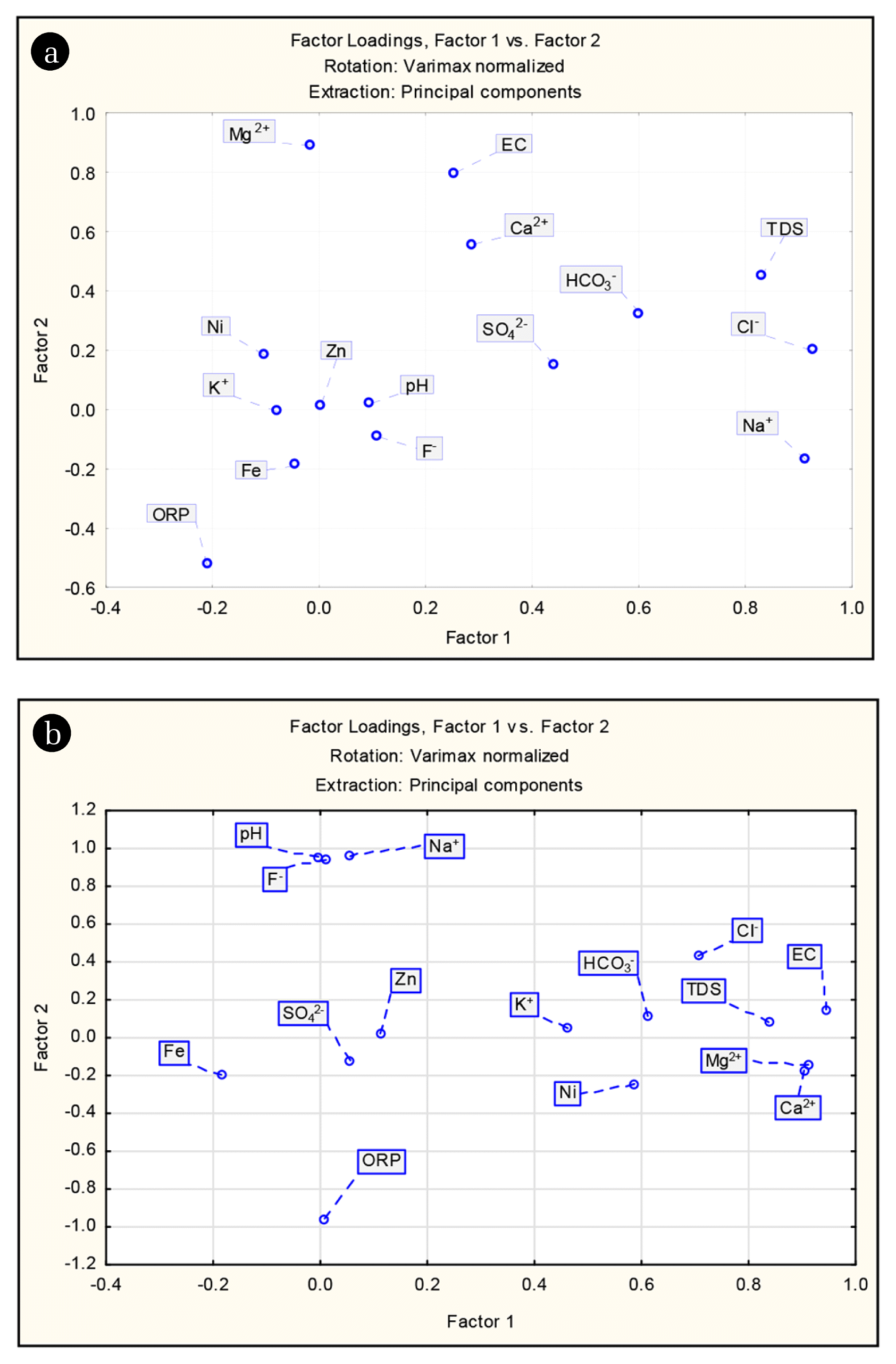
FA was performed on fifteen physico-chemical parameters determined in 116 groundwater samples. The major components of the four principal factors which aggregately clarified 63.82% and 70.07% variation of the data during pre- and post-monsoon, respectively were deduced in Table 2. After completion of FA, an attempt was undertaken to interpret the principal factors. Factor loading greater than 0.75 considered as strong loading, factor loading between 0.75–0.5 considered as moderate loading while factor loading less than 0.5 as weak loading [27]. Varimax rotation technique was applied for a simple and easier interpretation where factors were arranged in decreasing order of importance [28]. The factor with the highest data variance commonly explains the most important process or blend of procedures which control the hydrochemistry [29].
During pre-monsoon, factor 1 accounted for 29.47% of the variance and having substantial loadings on Na+, K+, SO42−, TDS, and EC. It represents the primary dissolved load of groundwater is due to dissolution of parent rocks like charnockites and gneisses as well as anthropogenic activities such as agriculture which influences the groundwater environment. The high loadings of TDS and EC are strongly influenced by evaporative processes. Factor 2 which explains 14.24% of data variance was mainly influenced by pH, HCO3−, F− and ORP. Strong loadings for F− and pH ruminate an alkaline dominated environment which promotes the fluoride enrichment due to desorption of F− ions in aquifer materials thus supplementing the dissolution of fluoride-bearing minerals. Generally, the concentration of fluoride not depends upon water soluble components but a significant correlation exists between pH and F−. The solubility of fluoride is very low in the pH range of 5–6.5 [30]. But at higher pH, the hydroxyl ion can supplant the replaceable F− ion of the fluoride-bearing minerals (biotite/muscovite) [31, 32]. Geochemically, F− and OH− ions have the same negative charge and possess nearly the same ionic radius, 1.33 Å for F− and 1.40 Å for OH− [33, 34]. Therefore, facilitating the replacement of OH− and F− ions and thus enhancing the fluoride concentration of in groundwater. The hydroxyl ion supplants fluoride ion from muscovite and biotite is demonstrated as follows:
Muscovite
Biotite
The high positive loading for HCO3− also supports the fact that groundwater in the study area belongs to HCO3− type. Fluoride exists as a simple negative ion (F−) in Na – HCO3 type groundwater which is highly active. Though fluoride combines with sodium to form water-soluble salt but the presence of sodium in groundwater decreases the sedimentation of calcium fluoride hence increases the fluoride concentration as below.
The interaction of groundwater with aquifer sediments containing fluorite (CaF2) and calcite (CaCO3) develops equilibrium reactions with both the solid phases by the following equations [35].
In order to relate fluorite and calcite when both the solute species are in contact with water the combined mass law equation can be explained by the Eq. (3) [35]. From these equations, it can be inferred that the change in HCO3− will lead to a corresponding alteration in F− [35]. The HCO3− acts as a sink for Ca2+ to form CaCO3 and thus makes favourable condition for the dissolution of F− from CaF2 into the groundwater [36].
On the other hand, negative loading of ORP indicates a reduction condition prevails in the aquifer which further enhances the enrichment process.
Factor 3 which represents 10.91% of the aggregate fluctuation in the hydrochemistry, shows positive loadings for Ca2+, Mg2+, and Cl−. This factor can likewise be named as the hardness factor. High loadings of Ca2+ and Mg2+ suggests weathering of carbonate minerals such as calcite and dolomite as the source of these two cations, particularly in recharge areas. The mobilization of Clfrom anthropogenic sources such as fertilizer may account for its strong loading. The dominating procedures represented by Factor 4, reflects the similar origin of Fe, Ni, and Zn in groundwater. High loading of Fe also points to reductive hydrolytic processes in the aquifer of the study area.
During post-monsoon, Factor 1 explains 32.27% of total variance and contains high loadings on TDS, EC, Ca2+, Mg2+, K+, Cl−, and HCO3−. It once again represents the weathering of different minerals such as halite, dolomite, gypsum, etc. from the underlying geology. Factor 2 explains 26.64% of total variance and has strong positive loadings on pH, Na+ and F− and negative loading with ORP. High loading of HCO3− and pH reflects an alkaline dominated environment promoting the F− enrichment due to the ion exchange process. Factor 3 explains 10% of the total variance and has the significant positive weight of SO42− and HCO3− which suggests the weathering of carbonate, sulfide minerals. Factor 4 accounts for 8.7% of the total variance, contains high loadings for Fe, Ni, and Zn indicating the similar source of these metals in the groundwater.
Comparing the results of FA for two seasons significant differences in factor weight has been observed for pH, F−, Ca2+, Mg2+, Na+, and HCO3−. The factor loadings were more during post-monsoon which revealed that recharging rainfall water enhanced the different hydro-geochemical processes such as weathering, dissolution, ion-exchange, etc. influencing the overall groundwater chemistry as well as fluoride enrichment in the study area. Finally, the result of FA for both the season provides better confidence in data interpretation.
First two factor loadings which explain 43.71% and 58.92% of total variance for pre- and post-monsoon respectively were plotted and illustrated in Fig. 5. These Scatter plots represent the possible relationship among the variables and their influence in the overall data structure. In order to comprehend the basic information structure in the present study and the significant factors based on the eigenvalue scree plot was also used as shown in Fig. S1.
4.3. Clusters Analysis
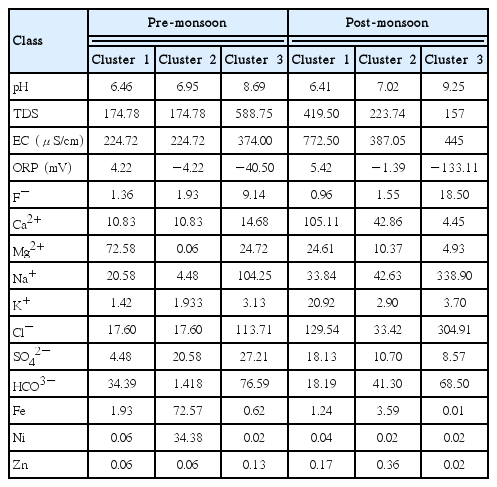
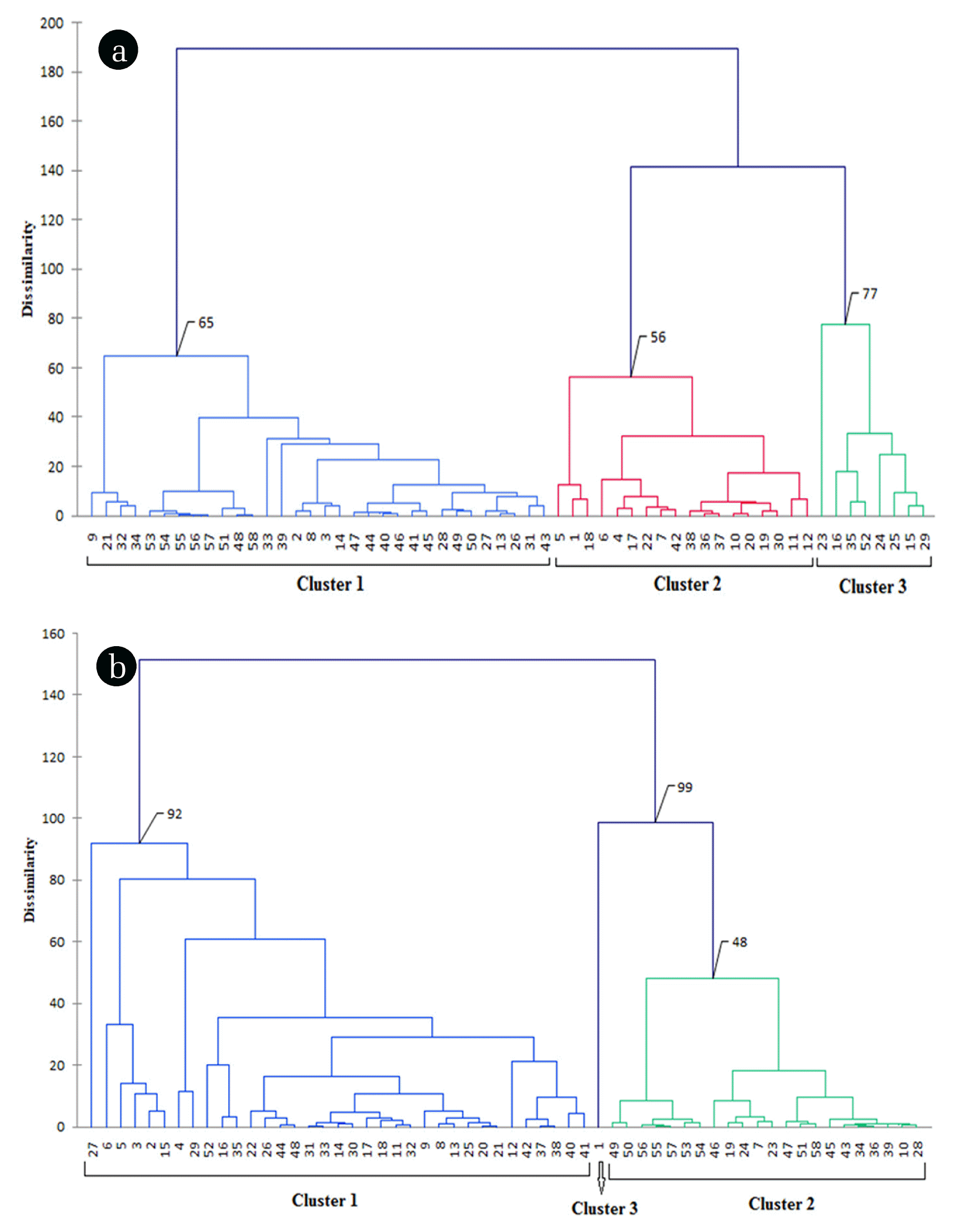
The hierarchical cluster analysis (HCA) was performed on fifteen geochemical parameters for an adequate partitioning of sampling sites based on the groundwater quality. In clustering Ward’s method was used as an amalgamation rule and Euclidean distance as dissimilarity measure. Finally, the result of the cluster analysis is shown in dendrogram or tree diagram. The dendrogram represents three statistically significant clusters of 58 sampling sites. The sites of an individual cluster represent similarity in the geochemical process as well as have a similar origin. Out of the three clusters, the first cluster consists of 27 sampling sites during pre-monsoon and 35 sampling sites during post-monsoon. The second cluster contains 23 sites during pre- and 22 sites during post-monsoon, respectively. The third one contains 8 sampling sites during pre- and only one sampling site during post-monsoon.
The mean values of chemical constituents for each cluster were shown in Table 3. It was found that Cluster 1 samples contain lowest fluoride concentration associated with slightly acidic pH and positive ORP value along with low HCO3− concentration. Cluster 2 samples contain fluoride concentration above permissible limit associated with normal pH, negative ORP value and moderate HCO3− concentration. Cluster 3 samples contain highest fluoride concentration along with alkaline pH, highly negative ORP value and high HCO3− concentration. This table once again validates the result of FA.
Fig. 7 represents the geographical location of all sampling sites according to their clusters. The sites of Cluster 1 are mainly lying in the middle and southwestern portion of the study area, the sites of Cluster 2 lying in the southeastern portion of the study area and the sites of Cluster 3 mostly appear in north and northwestern part of the study area. It was also found from the cluster analysis of sampling site that fluoride contamination was not uniform throughout the area under study and significant change in fluoride contamination was also observed during pre- and post-monsoon, respectively. Contamination level is high in the elevated region where recharging rainwater gets more contact opportunity with the minerals when it infiltrates through the vadose zone. From this elevated region, fluoride migrates to the low-lying areas and contaminates the groundwater.
5. Conclusions
An elevated level of fluoride concentration was found in the study area which made most of the groundwater samples unsuitable for drinking. FA of major water quality parameters revealed that fluoride contamination was strongly associated with pH, ORP, and the concentration HCO3− and Na+ ions. It was found from the study that some common geochemical process controlling the overall groundwater quality also promote the fluoride enrichment process. Among them, predominant processes were weathering and leaching of fluoride-bearing minerals, anion exchange between F− and OH− ions, evapotranspiration which concentrate the fluoride ions. From HCA, an un-uniform level of fluoride concentration was observed throughout the area under study which might be due to aquifer heterogeneity or variation in contact opportunity of the recharging rain water with the mineral during infiltration through the vadose zone.
Supplementary Materials
Acknowledgments
We would like to acknowledge National Institute of Technology Durgapur for providing infrastructure. We are thankful to all faculty members, staff members and student of the Department of Earth and Environmental Studies, NIT Durgapur for the endless support. We are also thankful to the villagers, lived in the study area, for their valuable help during sampling.