Fabrication of reusable polymer nanocomposite films made of thermoplastic polyurethane and modified BiVO4 for photodegradation of Malachite Green
Article information
Abstract
Semiconducting BiVO4, is successfully prepared by solid-state route and attempts are taken to dope vanadium sites by titanium ions to produce BiV1-xTixO4 (x = 5%, 10% and 15%). The products are analyzed using powder X-ray diffraction, Raman spectroscopy, scanning electron microscopy and energy dispersive spectroscopy. The average particle sizes are in the range of 71–82 nm. Using diffuse reflectance spectroscopy, the band gaps are estimated and fall in visible range (2.11–2.41 eV). These nanomaterials are investigated for the photodegradation of malachite green dye solution that can find application in wastewater treatment and their efficiencies are investigated by time-dependent UV-Visible spectroscopy. 15% titanium doped BiVO4 (BVO-15) showed the highest efficiency by completely degrading malachite green in 180 min under visible light irradiation. In such heterogenous processes, separation of the used nanocatalysts remain a challenge. To address this, reusable polymer nanocomposite films are prepared using thermoplastic polyurethane (TPU) to immobilize BVO-15. The polymer nanocomposite film with 0.10 g of catalyst in 1 g of TPU showed maximum efficiency (~ 96%). The powder and film catalysts are investigated for three cycles and found to be effective. Thus, they show remarkable photocatalytic activity, stability and reusability that make them suitable for the wastewater treatment.
1. Introduction
The organic dyes used in textile industries are causing severe threat to the environment as they lead to contamination of water bodies and groundwater. These chemicals are toxic and carcinogenic in nature [1–3]. Especially, the malachite green dye is genotoxic and carcinogenic [4, 5] despite its uses in wide range of industrial applications [6, 7]. Therefore, degradation of toxic dyes such as malachite green in industrial wastewater is a significant step to avoid contamination and hazardous effects in order to protect the waterbodies. Photocatalysis, adsorption and biochemical methods are mainly investigated for the wastewater treatment in recent decades [8–11]. Among these methods, photocatalysis has attracted much interest due to its high efficiency, low-cost and suitability for the practical applications [12–14]. Many semiconducting materials such as TiO2, ZnO, BiVO4, BiOX (X = Cl, Br & I) are studied for their potential applications in the treatment of dangerous organic pollutants or dyes in the recent years [15–19]. Among them, TiO2 is one of the most widely investigated photocatalyst for the treatment of wastewater. Though TiO2 is showing appreciable activity in UV region, it exhibits lower activity in visible region. Other major challenges in such heterogeneous photocatalytic treatments are separation of used nanocatalysts from the reaction medium after the treatment and its reusability. Thus, there is a need to produce a photochemically stable, readily separable, reusable and non-toxic photocatalyst which can exhibit appreciable photocatalytic activity in the visible region for the photodegradation of toxic organic dyes such as malachite green.
BiVO4 is a semiconductor which possesses band gap in the visible region. It is non-toxic, photochemically stable and exhibits appreciable photocatalytic activity [20, 21]. The catalytic activity of BiVO4 can be enhanced by modifying the compound by various methods such as doping as doping modifies the band structure/band gap and decreases the rate of recombination of charges [22, 23]. In this study, an attempt is done to replace some of the vanadium sites (V5+) by titanium ions (Ti4+) to enhance the photocatalytic activity. To overcome the limitations [24, 25] such as separation of the used nanocatalysts, a polymer nanocomposite film is fabricated as recovery of the film from the heterogeneous reaction mixture is simple and more practical compared to the recovery of the dispersed powder nanocatalysts. Further, loss of used catalyst during recovery can also be minimized using films by immobilizing the photocatalyst. Thus, the polymer nanocomposite films can help to achieve better recovery and reuse of the photocatalysts. Therefore, these film catalysts can be used for many cycles. Hence, this work focuses on fabrication of a novel polymer composite film to immobilize titanium doped BiVO4 on thermoplastic polyurethane matrix and further investigates its potential to degrade the harmful malachite green dye solution under visible light irradiation for multiple cycles. Thermoplastic polyurethane was chosen as the matrix material due to its stability in aqueous medium and under visible light irradiation. It also possesses good mechanical properties and flexibility [26, 27].
2. Experimental Section
2.1. Materials Used for the Synthesis
The precursors for the preparation of BiVO4, Bismuth Oxide (Bi2O3, 99.5%) and Vanadium Oxide (V2O5, 99.5%) are purchased from Sigma-Aldrich as analytical grade reagents. TiO2 (99.9%), malachite green (99.8%) and tetrahydrofuran (99.5%) are procured as high purity chemicals from S D Fine-Chem limited. Texin Rx T85A (aromatic thermoplastic polyurethane) is obtained from Bayer material science, USA. All the chemicals are used as purchased without any further purification.
2.2. Synthesis of Undoped and Titanium Doped BiVO4 Compounds by Solid State Reactions
Stoichiometric amount (1:1) of Bi2O3 and V2O5 are taken in an agate mortar and ground for 45 min using acetone as the mixing medium. The mixed powder is calcined at 700°C for 7.5 h to produce the parent compound BiVO4. To prepare the doped compounds, TiO2 is mixed with an appropriate stoichiometric ratio along with Bi2O3 and V2O5. Using the same procedure, titanium doped BiV1-xTixO4 (x = 5%, 10% and 15%) compounds are prepared. The heating (10°C/min) and cooling (3°C/min) rates are identical for all the reactions. The sample codes of undoped BiVO4, 5%, 10% and 15% titanium doped BiVO4 compounds are BVO, BVO-5, BVO-10 and BVO-15, respectively.
2.3. Fabrication of Polymer Nanocomposite Films by Solution-Casting Method
1 g of thermoplastic polyurethane (TPU) is mixed with 15 mL of tetrahydrofuran solvent and using a magnetic stirrer TPU is dissolved completely. A known amount of powder catalyst is taken in a beaker and the polymer solution is added dropwise, further mixed thoroughly. After the complete dispersion of the catalyst, the solution is transferred to a clean and dry glass petri plate. Then, the solution is dried to form a film at room temperature. Likewise, three different polymer nanocomposite films are prepared with differing catalyst loads of 0.05 g, 0.10 g and 0.25 g.
2.4. Characterization Techniques
All as-prepared compounds are analyzed using room temperature powder X-ray diffraction patterns that are recorded using PANalytical X-ray diffractometer equipped with Cu-Kα radiation (λ = 0.1542 nm) at a voltage of 40 kV and a current of 30 mA. The powder X-ray diffraction patterns are recorded from the 2θ range of 10° to 70° and with a scan rate of 4°/min. Raman spectra of all the as-prepared powder samples are recorded using Horiba, LabRAM HR(UV). Morphology of BiVO4, BiV1-xTixO4 (x = 5%, 10% and 15%) and the polymer nanocomposite films are investigated using scanning electron microscopy (Tescan-Mira 3 LMH) with an accelerating voltage of 25.0 kV. All the as-prepared doped and undoped powder samples are analyzed using energy dispersive X-ray spectroscopy (Bruker Quantax 200) attachment in the SEM. Random spots are chosen from the as-prepared powder samples for EDS spectra and the atomic ratios of the elements are determined. Shimadzu UV-260 spectrophotometer is used to record diffuse reflectance spectra of all the powder samples where barium sulphate is used as the reference material. The spectra are recorded from 200 nm to 1,200 nm with a scan speed of 5 nm/s and the band gaps are estimated by Tauc method using absorbance data. Photodegradation of malachite green dye solution is investigated by time-dependent UV-Visible spectroscopy using Shimadzu UV-260 spectrophotometer.
2.5. Photocatalysis
Photocatalytic degradation of 10 ppm malachite green dye solution is investigated using a home-made reactor which is made of 400 W high pressure mercury lamp (OSRAM MBF-U E-40). The lamp is equipped with a cut-off glass filter that can transmit only wavelengths in visible region (λ > 420 nm) and can filter the UV light. For all the photocatalytic experiments, the dye samples are placed on a magnetic stirrer and kept at a distance of 20 cm from the light source so that same intensity of light will be exposed on all reacting mediums. The dye solution is kept in a water bath to minimize thermal effects due to prolong exposure to the light source. This set-up is completely covered by wooden doors to minimize the scattering light intensity. For each photocatalytic degradation experiment, 250 mL of 10 ppm of malachite green dye solution is taken. 0.25 g of powder catalyst is used as the photocatalyst for each experiment. In similar conditions, instead of powder catalysts the polymer nanocomposite films are used in other experiments for the photocatalytic degradation of malachite green dye solution under visible light irradiation.
3. Results and Discussion
3.1. Structural Analysis
Powder X-ray diffraction patterns are recorded at room temperature for undoped BiVO4 and the titanium doped compounds which are shown in Fig. 1(a) along with the reference patterns from JCPDS data base. PXRD analysis confirms the formation of monoclinic scheelite BiVO4 phase by solid state route without any impurities as the observed reflections match with the JCPDS file no. 14-688 with space group I2/a. All the characteristic reflections of monoclinic BiVO4 phase are observed that are indexed as (020), (110), (011), (−121), (040), (200), (002), (220), (112), (211), (051), (−231), (132), (240), (042), (−202), (202), (161), (013), (251), (170) and (321). The doped samples show similar PXRD patterns as that of parent compound along with few more reflections due to the presence of small amount of tetragonal BiVO4 phase. The reflections at 2θ values 23.4°, 32.4° and 48.1° indicate the formation of tetragonal BiVO4 phase to a small extent which can be matched with the JCPDS file no. 14-0133 with space group I41/amd. These impurity peaks are indicated with * symbols in Fig. 1(a). Thus, attempts to dope the BiVO4 leads to a transition from monoclinic phase to tetragonal phase to a small extent. The changes from monoclinic to tetragonal phases can be caused by doping as there are similar reports available in literature [28]. Thus, it can be understood that attempts to dope titanium ions can induce the formation of small amount of tetragonal BiVO4 phase. According to Fig. 1(b) (where the magnified portion of PXRD from 2θ values 25° to 35° is shown), the PXRD patterns of the doped samples are not showing any appreciable shifts to investigate the substitution of vanadium ions (V5+) by titanium ions (Ti4+). Further, the most intense peak of the undoped BiVO4 shows splitting when it is attempted to dope with Ti4+. This is possibly due to the presence of some amount of unreacted titanium oxide which is used as the source of Ti4+ ions in the preparation of doped samples [29]. This observation indicates that the substitution of vanadium ions by titanium ions did not fully occur as expected according to the stoichiometric ratios used for the preparation of doped samples. However, the possibility of substitution to a small extent cannot be ignored. To investigate this further, Raman studies are carried out.

(a) Powder X-ray diffraction patterns of as-prepared BVO, BVO-5, BVO-10 and BVO-15 compounds along with the reference patterns from JCPDS file no 14-688 (monoclinic BVO phase) and 14-0133 (tetragonal BVO phase) recorded at room temperature where * indicates reflections from the secondary tetragonal BiVO4 phase, (b) Magnified portion of PXRD of as-prepared samples BVO, BVO-5, BVO-10 and BVO-15 from 2θ values 25° to 35°.
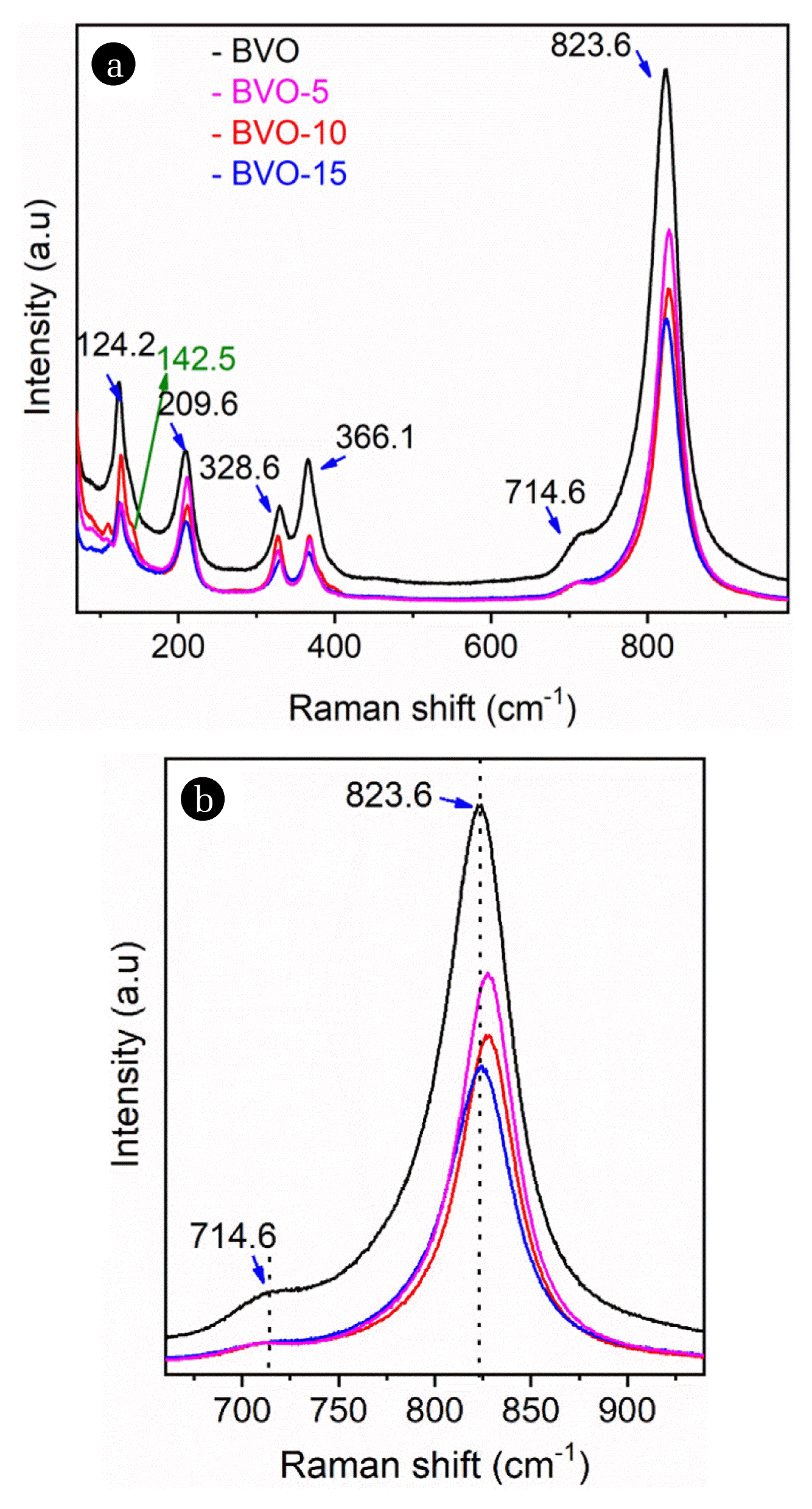
Raman spectra of as-prepared BVO, BVO-5, BVO-10 and BVO-15 powder samples are shown in Fig. 2(a) and all the characteristic bands of monoclinic BiVO4 are observed [30, 31]. The band at 823.6 cm−1 corresponding to the symmetric V-O stretching mode. Asymmetric V–O stretching mode is observed at 714.6 cm−1. The bands of symmetric and asymmetric bending modes of vanadate units (
3.2. Band Gap Calculation
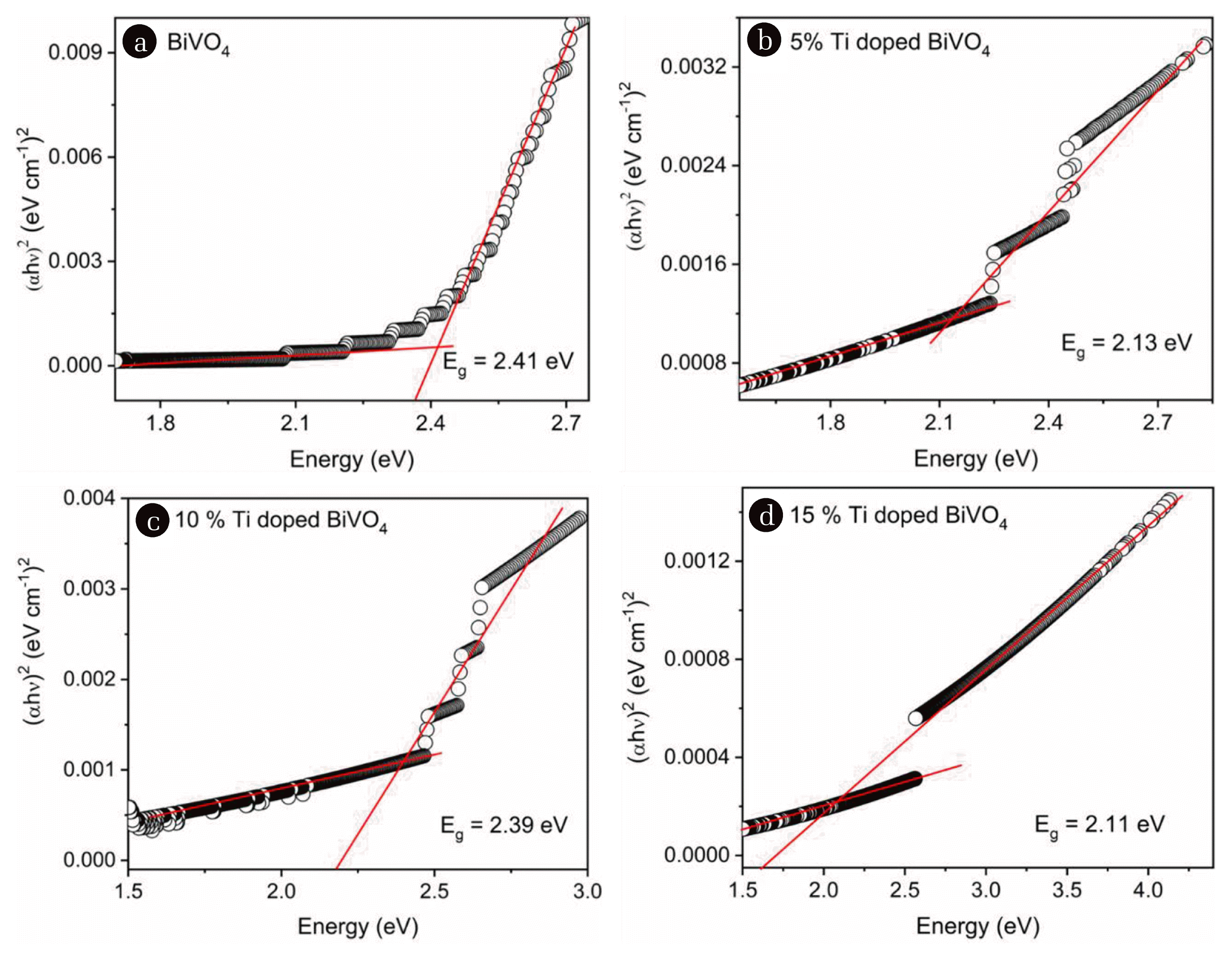
Fig. 3 shows the estimated band gaps of as-prepared BVO, BVO-5, BVO-10 and BVO-15 powder samples. Absorbance data from the diffuse reflectance spectra are utilized to calculate the band gaps using the respective Tauc plots. BVO shows a band gap of 2.41 eV which is comparable to the band gap values reported in the literature for monoclinic BiVO4 phase [35, 36]. BVO-5, BVO-10 and BVO-15 samples showed band gap values of 2.13 eV, 2.39 eV and 2.11 eV respectively. The band gap values of doped samples are smaller compared to the undoped BVO due to the formation of a dopant energy level near the valance band which is explained in Fig. S1. This is due to the incorporation of some of the titanium ions in the sites of vanadium ions. Similarly, decrease in band gap was reported when vanadium sites in BiVO4 were doped by Ni2+ ions and Cu2+ ions [37, 38]. Decrease in band gap leads to the utilization of longer wavelengths and enhanced photo-induced change carrier density. Formation of dopant energy level helps to decrease the recombination rate of charges. Thus, incorporating titanium ions in BiVO4 can enhance the photocatalytic efficiency. Since, all the band gap values are in visible region, the prepared compounds can show potential catalytic activity under visible light irradiation. However, the band gaps values are not decreasing linearly as a function of dopant concentration (x). This could be due to the ineffective doping of titanium ions in vanadium sites according to the powder X-ray diffraction and Raman analysis (Section 3.1).
3.3. Morphology Analysis
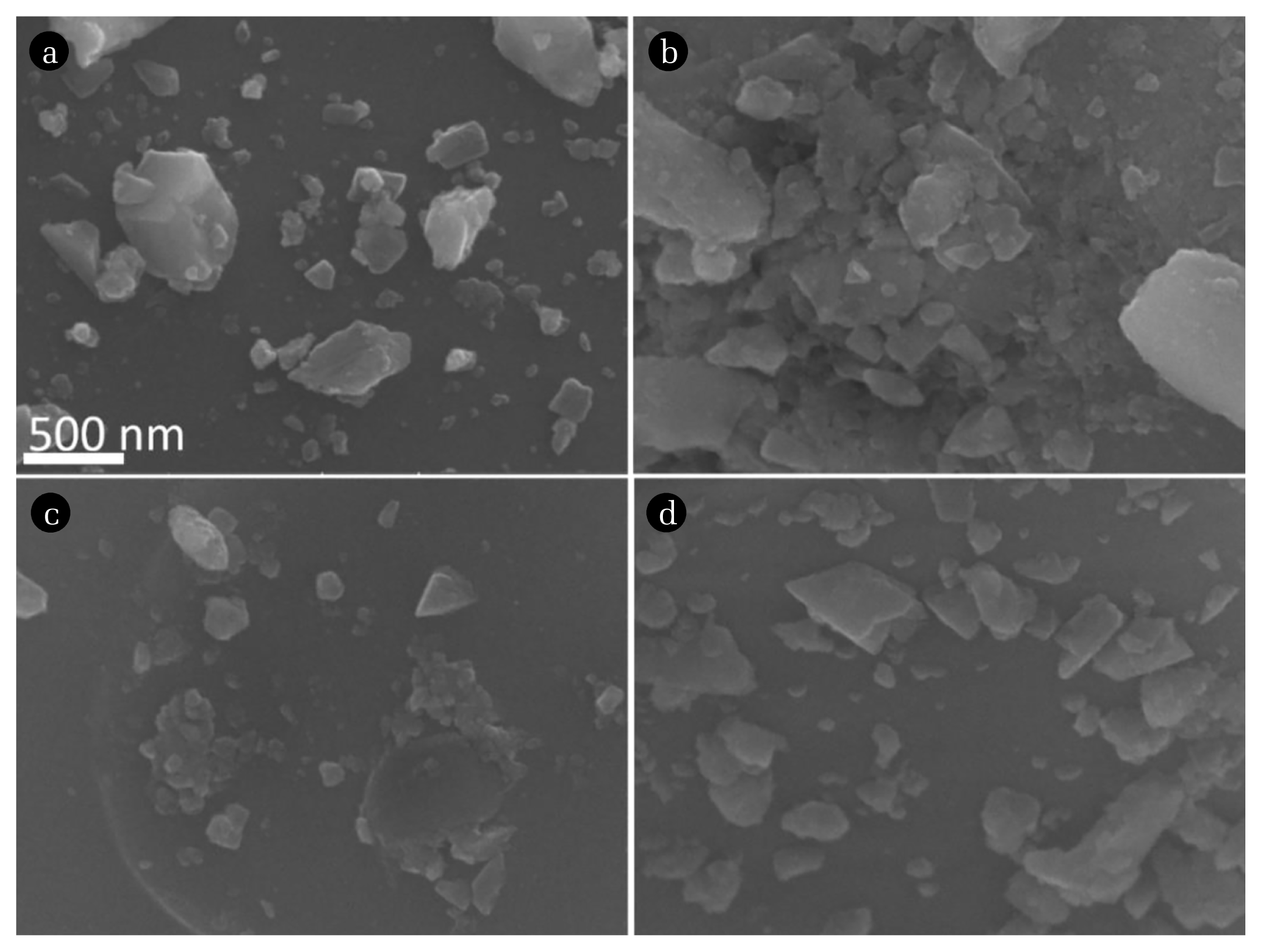
Morphology of all the as-prepared powder samples are investigated using scanning electron microscopy. The SEM images are given in Fig. 4. All the images are shown in same magnification and scale for better clarity. Most of the particles are irregular in shape but some of them are spherical or elongated. Smaller and larger particles are identified in the SEM images along with aggregation of particles. The average particle sizes are calculated for all the samples by taking approximately 100 particles from each sample. The average particle sizes are 74 nm, 71 nm, 82 nm and 77 nm for BVO, BVO-5, BVO-10 and BVO-15, respectively. EDS spectra are recorded by choosing some of the random spots on the as-prepared powder samples and the presence of all elements is confirmed in the doped (Bi, V, Ti and O) and undoped (Bi, V and O) samples. Any impurities from container material (alumina) are not observed. The EDS spectra of all the as-prepared samples are given in Fig. S2.
3.4. Photocatalytic Dye Degradation by Doped and Undoped BiVO4 Powders
The photocatalytic efficiency of the as-prepared compounds (BVO, BVO-5, BVO-10 and BVO-15) are investigated using 10 ppm of malachite green dye solution. 250 mL of malachite green dye solution is taken for all the experiments along with 0.25 g of photocatalyst. The optimum catalyst load is taken as 0.25 g/250 mL as any increase in the amount of the photocatalyst does not improve the photodegradation according to our previous investigations [39]. Prior to all the photocatalytic experiments, the mixture of dye solution and powder catalyst is stirred for 30 min under dark condition to attain adsorption-desorption equilibrium. Then, the dye solution along with powder catalyst is exposed to the visible light source and 10 mL of dye solution is withdrawn from the reaction mixture at regular time intervals (0 min, 30 min, 60 min, 90 min, 120 min, 150 min and 180 min) to analyze the decrease in concentration of the dye solution due to photocatalytic degradation using time-dependent UV-Visible spectroscopy. Fig. 5(a) shows the decrease in concentration of malachite green dye solution as a function of time under visible light irradiation where ‘C’ represents the concentration of dye solution at time ‘t’ and ‘C0’ represents the initial concentration of the dye solution. BVO shows ~89% decrease in concentration of malachite green in 3 h under visible light irradiation. BVO-5, BVO-10 and BVO-15, respectively exhibit ~90%, ~94% and ~100% decrease in concentration of malachite green in 3 h under visible light irradiation. Thus, BVO-15 shows the maximum photocatalytic efficiency by completely degrading the dye solution in 3 h under visible light irradiation. Here, the effect of photolysis and adsorption of dye molecules at the surface of the catalyst should also be analyzed to understand whether the decrease in concentration of the dye solution is observed due to the photocatalytic activity of the prepared catalysts or by photolysis or adsorption of dye molecules by the catalyst. To address this, following two experiments are performed and the results are given in Fig. 5(b). The dye solution without any catalyst is exposed to the visible light source for 3 h and this leads to ~20% decrease in concentration due to photolysis. The dye solution with catalyst is kept for 3 h under dark condition (without light) which leads to ~8% decrease in concentration of the dye solution possibly due to the adsorption of dye molecules at the surface of the photocatalyst. Thus, the effect of photolysis and adsorption of dye by catalyst are not significantly decreasing the concentration of malachite green dye solution. But when the combination of photocatalyst and visible light is used that leads to complete degradation of the dye solution in 3 h. This observation proves that the decrease in dye concentration occurs due to the effective photodegradation of malachite green by the photocatalyst. In the first 30 min, drastic decrease in the concentration of malachite green dye solution is observed due to the enhanced excitation process that produces large number of electrons and holes at the surface of the semiconductor photocatalyst. These charge carriers react further and generate the reactive intermediate species (which are explained in the proposed mechanism: Eq. (1) to Eq. (4)) that can degrade the malachite green dye solution effectively. Later, due to the consumption of the catalyst the degradation rate is reduced. Similar observations are available in the literature [40–41]. Thus, the prepared materials show potential photocatalytic activity in the degradation of malachite green dye solution under visible light irradiation. The results of the present study are compared with the reports from recent literature [22, 39, 42–46] in Table S1.

(a) Photocatalytic degradation of malachite green dye solution by the photocatalysts (BVO, BVO-5, BVO-10 & BVO-15) under visible light irradiation, (b) Effect of photolysis in the absence of catalyst and adsorption of dye molecules by the catalyst under dark condition (without light).
Photodegradation of malachite green dye solution by the photocatalysts, possibly follows the proposed mechanism which is schematically explained in Fig. S1. Irradiation of the photocatalyst in the aqueous dye medium leads to the separation of holes and electrons. They further react as explained in the following reactions to generate the reactive species. These active species further interact with the dye molecules and degradation of malachite green is carried out [47–49].
3.5. Study on Polymer Nanocomposite Films
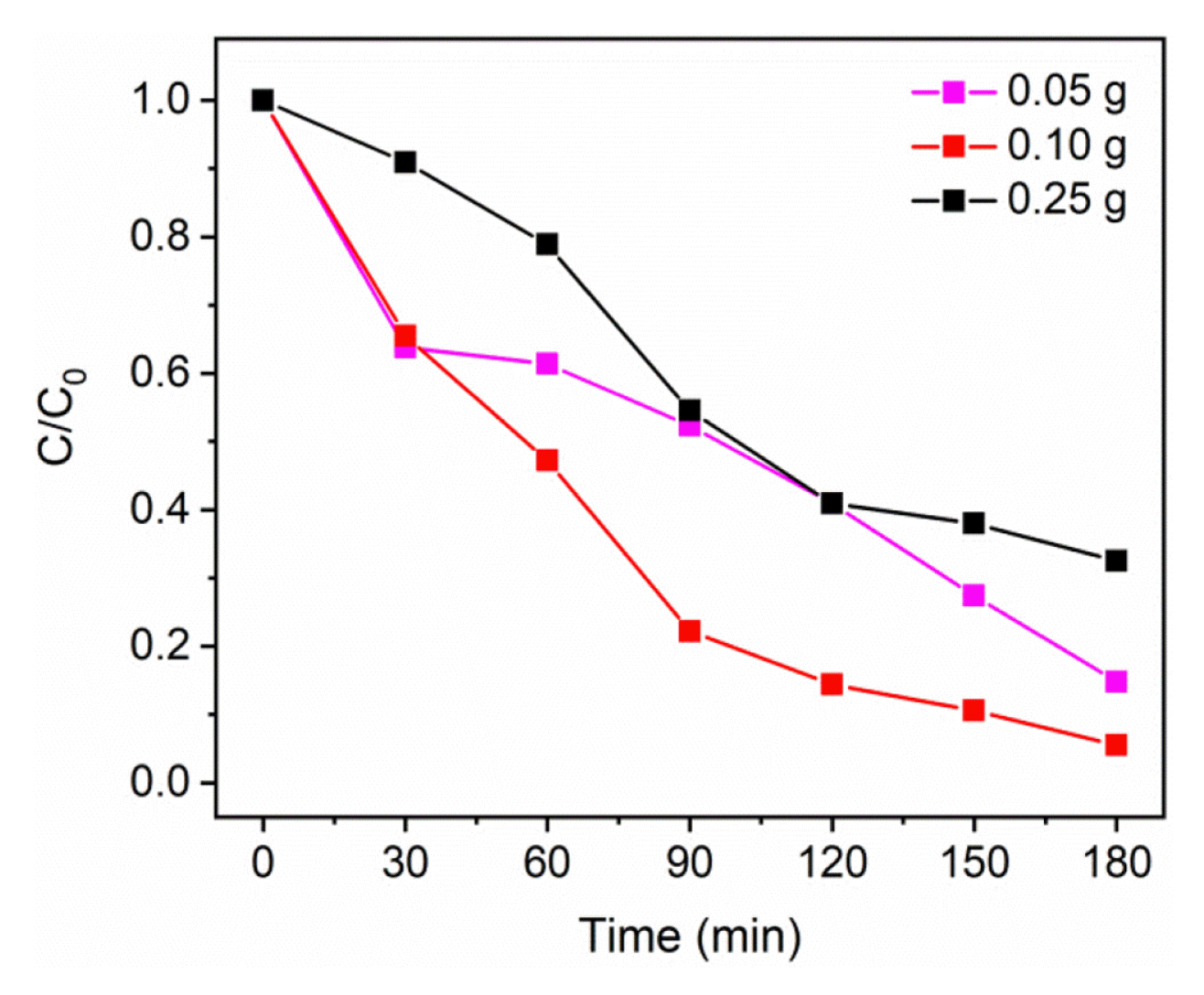
The major challenge in heterogeneous photocatalytic systems is the recovery of the used catalysts. The nanomaterials which are used as photocatalysts can float as dispersions in the reacting medium and cannot be separated effectively after the photocatalytic treatment. To address this issue, polymer nanocomposite films are prepared using thermoplastic polyurethane to immobilize the most active photocatalyst (BVO-15). Thermoplastic polyurethane has excellent mechanical stability, appreciable flexibility and abrasion resistance. It can be easily recycled and processed. Apart from that, the thermoplastic polyurethane is stable in aqueous mediums and under visible light irradiation [26, 27]. Due to such desirable physical properties thermoplastic polyurethane is chosen for the fabrication of the polymer nanocomposite films. Different catalyst loads such as 0.05 g, 0.10 g and 0.25 g of BVO-15 are taken to prepare the films in order to find the optimum amount of catalyst required for the film preparation which can show higher activity under visible light irradiation. The scanning electron microscopy images of all the as-prepared films are given in Fig. 6. The particles are dispersed in the films but not all the particles are present in the surface of the polymer film. Some particles could be buried in the polymer film which cannot effectively take part in the photodegradation process. Thus, when the polymer nanocomposite film is used the efficiency is expected to be lesser compared to the powder catalysts. It is evident from the SEM images that there is particle agglomeration to some extent in all the films. Especially, 0.25 g of BVO-15 loaded film shows more agglomeration of particles than the other two films which indicates a possibility of showing lower catalytic activity compared to other films. The photocatalytic activity of all the three films is investigated using malachite green dye solution of 10 ppm concentration. In place of powder catalysts, the polymer nanocomposite films are used for the photocatalytic experiments and the results are shown in Fig. 7. The polymer nanocomposite film with 0.05 g catalyst shows ~85% decrease in the concentration of malachite green dye solution under visible light irradiation in 3 h. The films with 0.10 g and 0.25 g of BVO-15 show ~96% and ~67% decrease in concentration of malachite green dye solution respectively under visible light irradiation in 3 h. Thus, the polymer nanocomposite film made of thermoplastic polyurethane with 0.10 g of BVO-15 exhibits maximum photocatalytic efficiency in the degradation of malachite green dye solution. The efficiency (~96%) of the polymer nanocomposite film with 0.10 g of BVO-15 is almost closer to the powder catalyst (~100%). Hence, the use of polymer nanocomposite film over powder catalyst is preferred due to its potential photocatalytic activity and ease of recovery.
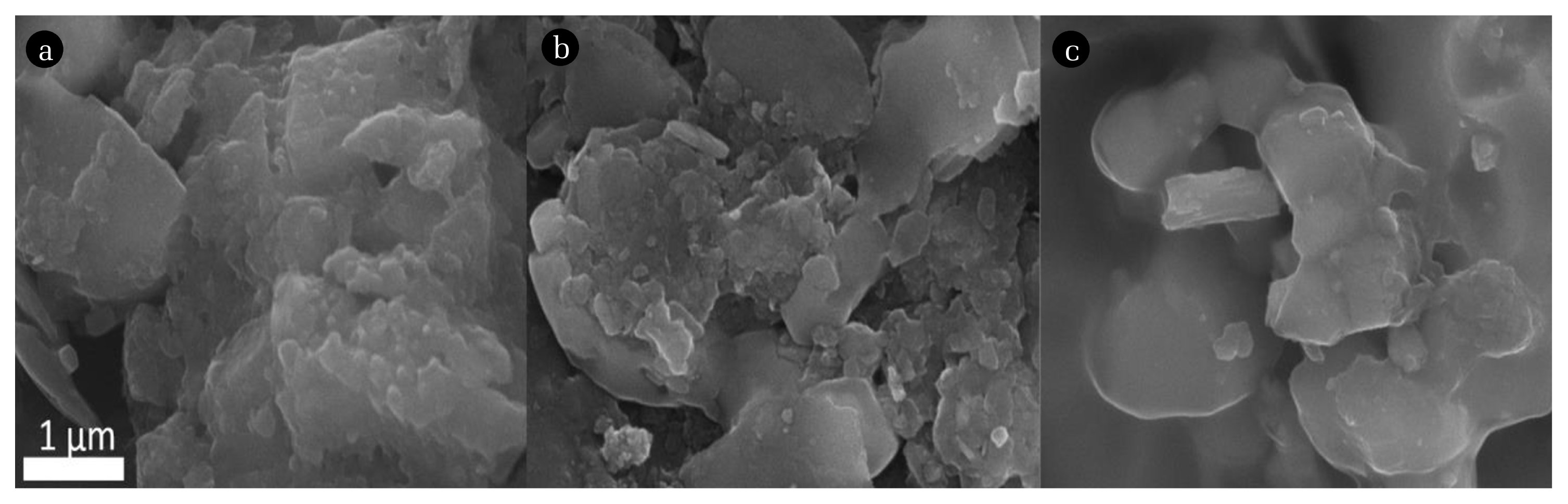
Scanning electron microscopy images of polymer nanocomposite films. (a) 0.05 g of BVO-15 loaded film, (b) 0.10 g of BVO-15 loaded film and (c) 0.25 g of BVO-15 loaded film.
3.6. Reusability of Powder Catalyst and Polymer Nanocomposite Film
All the powder catalysts (BVO, BVO-5, BVO-10 and BVO-15) are found to be stable after the photocatalytic treatment which is confirmed by PXRD and given in Fig. S3. Reusability of the powder catalyst is investigated for up to three cycles for the degradation of malachite green dye solution under visible light irradiation (for 3 h) for the BVO-15 catalyst and shown in Fig. 8. It is showing ~100% degradation of malachite green dye solution in the first cycle. Then, the powder catalyst is filtered carefully, further washed several times with water and ethanol. Then, the catalyst was dried overnight at room temperature and used for the second cycle where it showed ~94% degradation towards the degradation of malachite green. Further, it is used for the third cycle and found to exhibit ~92% degradation of malachite green. The plots for all the three cycles are given in Fig. 8 which indicates that the compound is not losing its catalytic activity significantly and so it is suitable for the repeated usage. The stability of the catalyst after 3 cycles is analyzed by PXRD. There are no significant changes in the PXRD patterns of fresh catalyst and the used catalyst after 3 cycles (Fig. S4) which confirms that the powder catalyst is stable after 3 cycles. Similarly, the polymer nanocomposite film prepared with 0.10 g of BVO-15 is investigated for the reusability up to 3 cycles which is also shown in Fig. 8. In the first cycle ~96% degradation is observed. The film is manually separated from the reaction mixture after the photocatalytic experiment. It is washed with plenty of water then soaked in a solution of thiourea (0.2 g of thiourea in 100 mL of 0.2 M HCl) for one day to desorb the small amount of dye molecules which got absorbed from its surface [50]. Later, the film is soaked in distilled water for 3–4 h and further dried at room temperature overnight so that it can be used for the next cycle. In the second and third cycles respectively ~65% and ~53% degradation efficiencies are observed. Here, the efficiency decreases considerably which is probably due to the loss of active catalyst surface area. The images of as-prepared polymer nanocomposite film before and after three cycles of photocatalytic treatment are given in Fig. S5. Absorbance as a function of time for all the three cycles for both powder catalysts and polymer nanocomposite films are given in Fig. S6 and Fig. S7, respectively. The film is stable and showing appreciable photocatalytic properties for the degradation of malachite green dye solution under visible light irradiation. Detailed desorption studies as well as identification of reasons behind the decrease in photocatalytic efficiency of the polymer nanocomposite film after multiple cycles will be explored in the future studies.
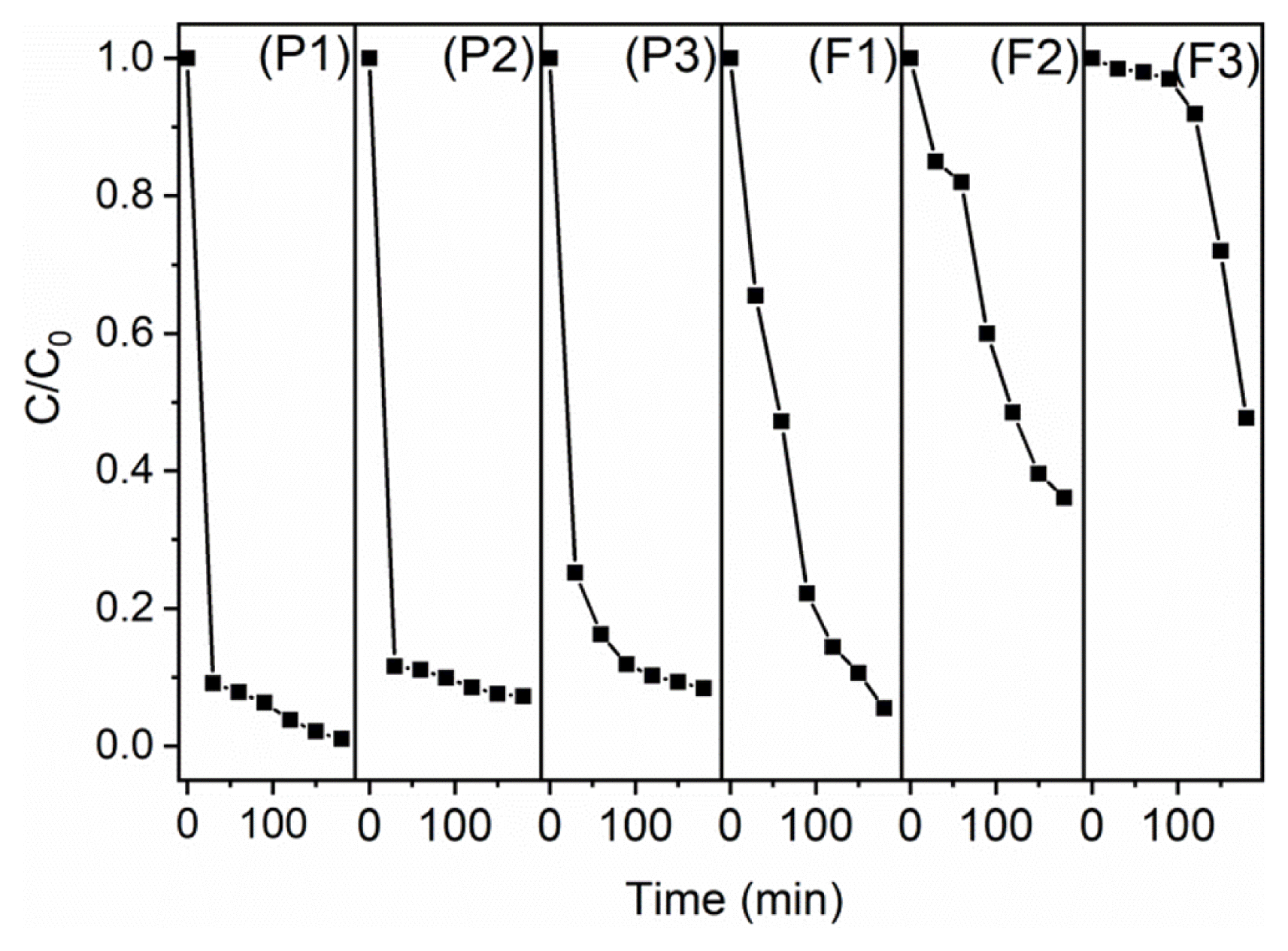
Photocatalytic efficiency of the powder catalyst (BVO-15) and polymer nanocomposite film with 0.10 g of BVO-15 to degrade malachite green dye solution for three cycles, (P1) Powder catalyst-1st cycle, (P2) Powder catalyst-2nd cycle, (P3) Powder catalyst-3rd cycle, (F1) Film-1st cycle, (F2) Film-2nd cycle and (F3) Film-3rd cycle.
The mechanism of action for the powder catalyst and the polymer nanocomposite film can be compared as follows. The dye molecules are attracted by the hydrophilic polymer composite film and they are interacting with the particles of the photocatalyst which are dispersed in the film. Thus, when the polymer nanocomposite films are used for the photodegradation of malachite green, degradation is carried out by the photocatalytic nanoparticles. TPU is photochemically inert under visible light irradiation due to its insulating nature and chemically stable [51]. Thus, the pure polymer cannot generate any reactive species that are shown in Eq. (1) to Eq. (4) which are required for the photodegradation of malachite green dye solution. Hence, it cannot show any potential photocatalytic activity towards the degradation of malachite green dye solution. The role of the polymer film in the degradation process is to provide a stable medium to disperse/immobilize the powder catalyst and attract the dye molecules which are further exposed to the dispersed powder catalysts. This was confirmed by taking a film of pure polymer (without catalyst load) with the dye solution for three hours and no significant dye degradation is observed. Thus, it is evident that the dye molecules are not degraded by the pure polymer film. Thus, the mechanism of photodegradation is slightly different when the films are used. This difference leads to some changes in degradation rates of polymer nanocomposite films and powder catalysts. Photodegradation is more powerful in the first 30 min for the powder catalysts due to the effective photoinduced charge separation at the surface of the semiconducting photocatalyst and later it is slow due to the consumption of the catalysts. But when the films are used there is a steady decrease in the concentration of malachite green dye solution up to 180 min possibly due to the slower photoinduced charge separation. Powder BVO-15 shows more activity in successive cycles due to its high surface area and the polymer nanocomposite film with BVO-15 shows comparably less activity in successive cycles. This is possibly due to the excess loss of active surface sites of the catalyst as not all the particles are in the surface of the polymer film which reduces the exposure of the dye molecules to the dispersed catalyst particles in the film and also the sites are blocked by the degradation products that can bind with the film due to its hydrophilic nature.
Some of the major engineering problems in processing such as enhancement of photocatalytic efficiency, recovery of used catalyst and reusability is addressed in this study as enhancement of photocatalytic efficiency is achieved by doping and effective recovery/reuse of catalyst is achieved by the use of polymer nanocomposite film. This method offers a possibility to design fixed catalyst reactors of batch type in the near future by suitably modifying the required parameters [52–54] to meet the industrial requirements in wastewater treatment. Thus, this study explores the application of photochemically stable, potential and reusable photocatalysts for the effective photodegradation of malachite green dye solution under visible light irradiation which can find application in the treatment of toxic industrial wastewater.
4. Conclusions
Photocatalytic BiVO4 is prepared successfully by solid state reactions. PXRD confirms the formation of monoclinic BiVO4 phase without any impurities. Attempts are taken to dope the vanadium sites by Ti4+ ions. The Raman and PXRD analysis indicate that doping is not as effective as expected. Band gap calculations are carried out using DRS which showed all the as-prepared compounds exhibit band gaps in visible region that makes them suitable for the photocatalytic applications. Their particle sizes are in the order of nanometers (71–82 nm). Hence, the powder catalysts are investigated for the photocatalytic degradation of 10 ppm of malachite green dye solution. BVO-15 showed the highest efficiency by degrading the dye solution completely in three hours under visible light irradiation. Further, it is stable up to three cycles and does not lose its catalytic activity significantly. Then, a polymer nanocomposite film is prepared by using the most active catalyst (BVO-15) made of thermoplastic polyurethane. The film also showed ~96% efficiency in the degradation of malachite green dye solution under visible light irradiation in three hours. Further, it is used for second and third cycles where the efficiency considerably decreases possibly due to the loss of active catalyst surface area. Overall, this study investigates the possibility to produce an effective, stable and reusable catalytic film for the photodegradation of malachite green dye solution under visible light irradiation which can serve as a potential candidate in wastewater treatment.
Supplementary Information
Acknowledgments
The authors thank the support of M S Ramaiah University of Applied Sciences, Bangalore, India to carry out this work.
Notes
Author Contributions
R.M. (M. Sc.) carried out all the experiments. A.G.J (Asst. Prof.) designed and characterized the polymer composite films. N.M.P (Asst. Prof.) supervised the project, characterized the materials, analyzed the data and wrote the manuscript.