1. Introduction
Tailing is a waste generated from mineral processing [1]. The mineral processing is carried out by amalgamation techniques that mix mineral ore with mercury to form amalgam with water as a medium [2]. Traditional gold mining in Kulon Progo uses a simple technique using mercury in the amalgamation process. According to Larasati et al. [3], tailings from gold processing overflowed from existing shelters and flowed out to the surrounding environment. In some mine sites, tailings form a heap without further processing. According to Ogola et al. [4], there are several heavy metals contained in the tailings from the amalgamation process, such as mercury, lead, arsenic, and cadmium. Besides a mercury concentration test, this study also measured heavy metal concentrations such as As, Pb, Cd, Cr and Ni but the result was still below the specified environmental standard, so it can be neglected. Mercury is the most dominant heavy metal in tailings from the traditional gold mining process [5]. According to Government Regulation no. 101 Year 2014 on the Management of Hazardous and Toxic Wastes [6], tailings from mineral processes fall into category 2. The resulting tailings shall be kept in accordance with the Decision of the Head of the Environmental Impact Management Agency No. 1 of 1995 on Technical Procedures and Technical Requirements for the Storage and Collection of Hazardous and Dangerous Wastes. Based on the results of the tailings sampling test in mining, the total mercury concentrations ranged from 164 mg/kg – 314 mg/kg which compare to applied regulation standard, the maximum total concentration of mercury permitted in the tailings is 75 mg/kg. Mercury concentration in the tailings has exceeded the specified quality standard, means that tailing processing techniques is required to reduce the pollution level to predetermined quality standards.
Remediation technology using physical/chemical processes consists of in situ and ex situ [7]. The in situ remediation are chemical oxidation, electrokinetic separation, fracturing, soil flushing, soil vapor extraction, solidification/stabilization (S/S). Whereas ex situ remediation are chemical reduction/oxidation, dehalogenation, separation, soil washing, S/S [8].
The remediation technologies that can be applied either in situ or ex situ using physical chemical process are S/S and chemical oxidation. In this research is using S/S because it can reduce mercury waste in the tailings. Andrés et al. [9], claimed that S/S technology works by limiting the contamination of hazardous material compounds.
The advantage of S/S technology for hazardous waste treatment are cheap, environmentally friendly, and easy to apply [10]. In S/S technology, the waste is converted into solid compounds which directing on reducing the rate of contaminants [11]. The S/S technique requires an additional binder to encapsulate the physical and chemical contaminants to become more stable formation than before [12]. Some additive materials that can be used in S/S process are Portland cement, fly ash, lime, clay, zeolite [13].
The most common binder used in S/S technology is Portland cement due to its ease to obtain and it can be applied to various types of waste [14]. The S/S technique combine with Portland cement is very effective to bind inorganic compounds [15]. In the other hand, disadvantages of S/S process can not be used for waste containing hydrocarbons [16].
The cost effectiveness of remediation techniques can be determined by type of waste. Moisture content in the sludge drives up costs compared to solid; contaminant concentration and type determine the amount of reagents added to the waste to attain the required treatment standards. Size of the mobile S/S system (choosing the correct size mobile S/S system to adequately handle the throughput of waste volume). Cost analysis (the estimated costs by common unit of measure to apply S/S technology at sites of varying size and complexity [17].
This study will tested the ability of S/S technology to stabilize mercury contained in the tailings by using a mixture of Portland cement. The characteristics of tailings should be known as considerations in performing S/S. In the S/S techniques, variations of Portland cement composition and tailings will affect product quality. The optimum composition of Portland cement and tailings should be considered for obtaining the desired S/S product, with the lowest amount of additional portland cement.
2. Materials and Methods
2.1. Materials
Ordinary Portland Cement (OPC) supplied from PT Semen Indonesia was used throughout this research. Tailings were taken at the Kulon Progo gold mine site at 5 specific locations. Sampling was done by using a core sampler which refers to US EPA [18]. The main sampler method used in this research is sub sampling and composite sampling (multiple core samples) with variation of depth are 30 cm, 60 cm and 90 cm for each location. Each sample was inserted into Poly Ethylene Terephthalate (PET) plastic and labeled, then inserted into an ice box with temperature of 4°C and brought to the laboratory for analysis of total mercury concentrations and tailing characteristics. The results of the five samples sites are composited into one sample based on the depth. The tailings sample location can be seen in Fig. 1 [19].
2.2. Test of Mercury and Tailings Characteristics
All samples were analyzed soil physical characterization based on particle size distribution analysis refers to ASTM D422. Whereas the test for chemical characteristics i.e. water content test is done by gravimetric method and pH is measured by pH-meter. Particle size analysis methods and chemical characteristics of soil samples were performed at the Soil Mechanics Laboratory and Stone in Civil Engineering FTSP ITS. Mercury level test was conducted at LPPT UGM Yogyakarta by using Mercury Analyzer Type VM-3000. All samples were analyzed twice.
2.3. Manufacturing of Test Material
The test object is made by using a mold specimen’s cuboid with 5 cm size per side. Variations in the composition of the specimen between Portland cement and tailings are 100:0; 90:10; 80:20; 30:70; 60:40; 50:50; 40:60; 30:70; 20:80 and 10:90. The procedure of stirring, preparing the specimen, compacting and treating the specimens shall refer to SNI 2493: 2011. The determination of the water requirement for the manufacture of the specimen is performed by a normal consistency test using Vicat tool. Cement, tailings and water are mixed until homogen and printed using a specimen mold.
2.4. Mortar Treatment (Curing)
The mortar treatment done by kept the mortar moist to prevent crack in the test objects. Mortar was placed in the room temperature for 28 d. In this study, moisture curing was performed by placing the test objects around a bucket of water and sealed using a large tub. The treatment aimed to minimize the leaching of heavy metals contained in Portland cement: tailing mortar.
2.5. Compressive Strength Test
A compressive strength test was performed by using ASTM C109-93 test method. Compressive strength test was performed on a mortar that had been through the curing process for 28 d. Compressive strengths of specimens were measure using Toorse Universal Testing Machine. Compressive strength test is performed to determine the maximum load that can be received by the specimen. The quality standard of compressive strength is 10 ton/m2 based on the Decree of Head of the Indonesia Environmental Impact Management Agency (Kepka Bapedal) No. 3 of 1995 [20].
2.6. TCLP Test
This is a decomposition method using an extraction method containing glacial acetic acid with a low pH. The rotation-agitation process in this test was performed with a rotary agitator at 30 ± 2 rpm speed for 18 ± 2 h. The sample solution was precipitated and the filtrate is taken. The filtrate is stored in a brown glass bottle and tested for mercury concentration. Mercury level tests were held at the Laboratory.
2.7. Data Analysis and Conclusions
The analysis includes the discussion of the relationship between water requirements, compressive strength values and TCLP test results on the composition between Portland cement and tailings. The optimum composition is determined by looking at the quality standards of each test with comparison of lowest portland cement usage.
3. Results and Discussion
3.1. Characteristics of Soil Samples
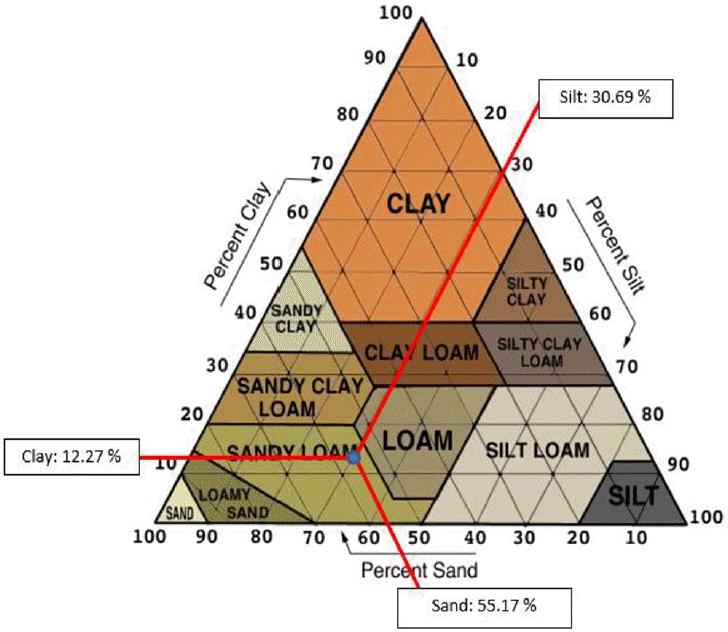
Materials used in this study are Portland cement type I and tailings. Sampling activities conducted on October 2015. The soil samples of the mine site were physically dominated by sand, silt and clay. Physical characterization of tailings can be seen in Table 1. Based on soil texture triangle of the total content of each type of grain, tailings including sandy loam category (Fig. 2). The test result of chemical characterization of soil samples such as pH and total mercury concentration (Table 2). Compressive strength test was performed on each variation of the composition between Portland cement and the tailings formed into mold (duplicate) on each specimen. The value of compressive strength can be seen in Table 3.
In Table 3, it can be seen that all specimens met the minimum required of compressive strengths with the compressive strength value of 53 ton/m2 to 3,895 ton/m2. The highest compressive strength value was on the 80:20 composition, and the lowest is in the 10:90 composition.
The existence of Portland cement would add compressive strength value to the specimen. According to Pollard et al. [22], the hydration reaction of cement will produce calcium silicate hydrate (CSH) which plays an important role in the binding of the specimen’s strength. Overall, the value of compressive strength decreased and contrast to the increasing number of tailings. According to Ganjidoust et al. [14], the greater the surface area will reduce the capability of the cement to achieve the required compressive strength. Large amounts of soil will cover the surface of an aggregate and prevent the adhesion process from cement paste.
Decreasing compressive strength also can be caused by the unstable water level required for the hardening process during curing, thus the cement hydration process was disrupted [23]. Disruption of the cement hydration process will result in reduced concrete strength [24].
The result was obtained because of the influence of cement, also because of the existence of clay on the tailings [25]. Although the more tailings would reduce the value of compressive strength, the composition of adequate clay-type soil actually might increase the value of compressive strength. According to He et al. [26], refers to ASTM C 618 that the content of SiO2 + Al2O3 + Fe2O3 in natural pozzolan should reach 70 wt%, space clay generally complies this chemical composition. The structure of crystal, chemical, and particle properties of each clay type differ and influence the activity of pozzolan in achieving the resulting mortar strength. Clay permeability also affects the value of compressive strength because the density is better if the permeability is low. So the potential of the cavity contained in the specimen will be decreased [27].
At 100:0 composition in both samples, the value of compressive strength decreased although 100% cement composites. This could be caused by the large amount of Ca(OH)2 content contained in the specimen. According to Sari [28], during the hydration process on cement, a Ca(OH)2 particle will arise which causes the cement strength to decrease and cause the expansion of the concrete surface. The addition of pozzolan containing silica and alumina into cement will react with Ca(OH)2 to form calcium silicate hydrate and calcium aluminate hydrate. [29]. Calcium silicate hydrate and calcium aluminate hydrate plays the main roles in hardening and cement resistance. In this study, the soil contained in clay-type tailings might have silica and aluminate content. According to Faisal [30], reactions that occur between Ca(OH)2 and SiO2 can be written as follows:
Where: x, y, and z = equivalence value
3.2. TCLP Test
Prior to TCLP testing, sample preparation should be performed according to US EPA Method 1311. TCLP preparation included pH checking and determining the extraction fluid used. There were two extraction liquids, namely extraction fluid 1 and extraction fluid 2. Extraction fluid 1 was used if the pH sample < 5, and the extraction fluid 2 was used if the pH sample is > 5 after addition of 3.5 mL of HCl 1 N. The extraction fluid 1 comprised a mixture between a 1 N NaOH solution, glacial acetic acid, and aquades with a mixed pH of 4.93 ± 0.05. The extraction fluid 2 comprised a mixture of glacial acetic acid solution and aquades with a pH of 2.88 ± 0.05. Mixed samples with the extraction solution agitated with a rotary agitator for 18 ± 2 h at a speed of 30 ± 2 rpm. The filter was filtered using Whatman filter paper type GF/F 47 mm with a pore size of 0.7 μm.
Mercury TCLP testing was performed using Mercury Analyzer Type VM-3000. The result of mercury TCLP in both sample variations can be seen in Table 4.
The mercury TCLP test resulted in all samples met the TCLP-B quality standard in government Regulations (PP) No. 101 Year 2014 that is equal to 0.05 mg/L. The TCLP value met the quality standard with a much lower value. The results obtained all samples had a value of < 0.0005 mg/L.
From the data obtained, it can be concluded that mercury had a good precipitation process so the mercury mobility became lower. Precipitation occurs due to the reaction between ionic mercury (Hg2+) with alkaline and carbonic compounds present in cement [30]. The reaction can be written as follows:
The crystalline structure formed from the hydration process will bind heavy metals from the waste after it becomes a precipitate of hydroxide and carbonate salt [31]. In this study, the precipitates and carbonates formed were mercury (II) oxide, mercury (II) hydroxide, and mercury (II) carbonate. The formation of hydroxide precipitates and carbonate salts on mercury was evidenced by the value of TCLP obtained in this study. The result of mercury TCLP had a much lower than the quality standard, so it could be concluded that mercury was well bonded in the form of hydroxide precipitates and carbonate salts.
4. Conclusions
In summary, the soil texture triangle of the total content of each type of grain, tailings are included in the category of sandy loam. Initial mercury tailings exceeded the quality standard of Government Regulations (PP) No. 101 of 2014 of 75 mg/kg, in which the active tailings contained mercury with a total concentration betwen 164.19 until 383.21 mg/kg, with pH betwen 7.48 until 8.56. The S/S method using Portland cement on a mercury contaminated tailings was capable of meeting the required minimum compressive strength values and meeting the required TCLP value. All composition variations of tailings had higher compressive strength value than the quality standard with a minimum compressive strength value is 257 ton/m2 in composition 10% cement Portland and 90% tailings. Whereas, the TCLP value was much lower than the quality standard value < 0.0005 mg / L.
Based on TCLP results stabilized materials meet the established quality standards. The product of S/S in Indonesia even meet the environmental quality standard of Head of BAPEDAL No. 04 Year 1995 which collected at the place of hazardous and toxic waste. Material for practical purposes from S/S result needs further study and new regulations to be used.