1. Introduction
The cold-rolling industry generates a considerable amount of oily wastewater, which contains iron pin, lubricating oil, surfactants, and other pollutants [1, 2]. Directly discharging oily wastewater into the environment has negative environmental and ecological impacts. Therefore, numerous chemical and physical technologies, such as coagulation, flotation, gravity, and biodegradation, have been employed to remediate oil-contaminated wastewater [1, 3]. Nevertheless, these conventional methods consume high amounts of energy while exhibiting poor removal efficiency. The low removal rates of these methods are particularly pronounced when used to remove low concentrations of finely dispersed oil droplets from wastewater [3, 4]. Membrane separation technology offers high oil removal efficiency and a facile operational process [5–7].
In general, oil–water separation is significantly affected by the physical and chemical properties of the separation membrane and the conditions of the process. Ohya et al. [8] investigated the effects of average pore size on oil-water emulsion separation with a microfiltration (MF) membrane. The filtration mechanism at the initial stage of the treatment process could be explained by three types of blocking filtration models, which are constructed on the basis of the average pore size of the membrane. The pore size of the membrane should be selected in accordance with oil droplet size to reduce membrane fouling during operation. Hua et al. [9] performed a crossflow MF process using a ceramic (α-Al2O3) membrane with 50 nm pore size to treat oily wastewater (oil concentration ≈ 500 ppm). The TOC removal efficiencies of the process exceeded 92% under all experimental conditions. Lobo et al. [10] studied the effects of crossflow velocity (CFV) and pH on an ultrafiltration (UF) module for emulsion separation with a tubular ceramic membrane under a transmembrane pressure (TMP) range of 0.05–0.4 MPa. They concluded that permeate flux and chemical oxygen demand retention (CODcr) decreased drastically under low pH values.
The application of MF and UF in oily water treatment is mainly limited by the fouling (i.e., the severe decline in flux with operation time through the nonspecific adsorption of oil droplets on membrane surfaces and pores) encountered by these processes [7–10]. The antifouling performance of membranes has been improved through structural modification or process optimization [7–8]. In addition, hydrophilic additives, such as hydrophilic polymers, amphiphilic copolymers, and inorganic nanoparticles, have been introduced to enhance the flux and antifouling properties of membranes. Rajasekhar et al. [11] studied oil-water (1,000 ppm) separation using UF membranes based on novel PVDF and amphiphilictri-block copolymer blends. The modified membrane obtained a water flux that is approximately 2.5 times higher than that of the neat PVDF membrane and maintained 99% oil rejection. Yuliwati and Ismail [12] used titanium dioxide (TiO2) nanoparticles as pore-forming and hydrophilic additives to prepare a PVDF UF membrane. At 1.95 wt% TiO2 concentration, the membrane showed 82.5 L/(m2·h) maximum flux and 98.83% rejection of oily wastewater.
Different separation processes are used for each kind of specific oil/water (O/W) mixture in accordance with the physical nature of the oil, total oil content, and chemical nature of other components. In many case, the combination of two or more separation techniques is required to increase separation efficiencies. The application of destabilization/evaporation and UF-combined processes, UF and activated carbon hybrid processes, and combined UF and bipolar electro-chemical reactor processes in O/W separation has been studied [13–15]. These integrated processes have shown long-term operational stability and are suitable for O/W emulsion treatment.
This work investigated the feasibility of applying a hybrid process that is based on coagulation and a two-stage membrane process with MF + UF in oily wastewater treatment. Wastewater containing micron- or submicron-sized droplets has to be de-emulsified prior to treatment to reduce membrane fouling. Coagulation is a simple pretreatment method for oily wastewater. Oil droplets tend to form large oil globules because of the electrostatic and adsorption effects of coagulants. Thus, large oil globules could be separated easily through the MF + UF process. This work aims to investigate the technical feasibility of coagulation with MF + UF process for the treatment of emulsion wastewater and to provide a superior hybrid membrane process for oily wastewater treatment.
2. Methods
2.1. Materials and Experiment
Oily wastewater was collected from Heli Metal Rolling Company (Anyang, Henan, China). The precise formulation of the oily wastewater is proprietary. The wastewater contains a mixture of mineral oils and several additives, such as emulsifiers, stabilizers, biocides, and corrosion inhibitors. The wastewater had an oil concentration of approximately 550 mg/L, COD of 6,500 mg/L, a pH value of 7.2, and turbidity of 1,100 NTU. The size of the oil droplets was measured through laser-light scattering (LS-POP-9, Zhuhai OMEC Instruments Co., Ltd., China) and ranged from 0.02 μm to 4 μm with an average size of 0.23 μm.
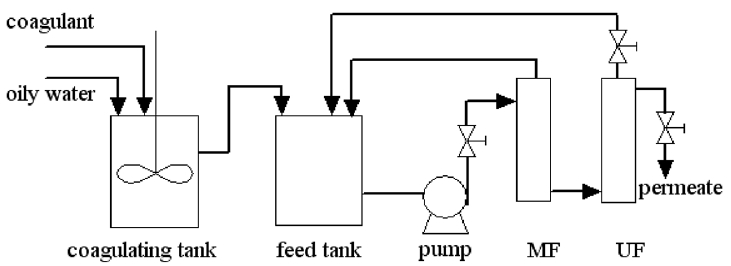
A schematic of the experimental setup is shown in Fig. 1. Raw wastewater collected from the plant was coagulated for 40 min. A certain amount of coagulant was added to the wastewater at 35°C. The mixture was stirred at 100 r/min for 2 min and precipitated through gravity for 40 min. After coagulation, the supernatant was removed, and the remaining aqueous phase was sent to MF and UF modules. The material of the MF was ceramic membrane with the pore size of 0.2 μm and that of the UF was PVDF (cutoff = 500 kDa). Experiments were performed under crossflow filtration conditions, and temperature was maintained at 35°C.
After each UF was performed, the membrane module was cleaned through the following steps: (1) Rinsing with NaOH aqueous solution for 10 min. (2) Rinsing with water for 5 min. (3) Rinsing with HNO3 aqueous solution for 10 min. (4) Rinsing with water for 5 min.
Oil concentration in water was determined through UV–vis spectroscopy at 270 nm [11]. CODcr was determined through potassium dichromate oxidation method. Water turbidity was determined with the turbidity meter WQ770-B. The flux J was defined as Eq. (2).
where V is the volume of permeated water, A is the membrane area, and Δt is the permeation time.
3. Results and Discussion
3.1. Pretreatment by Coagulation
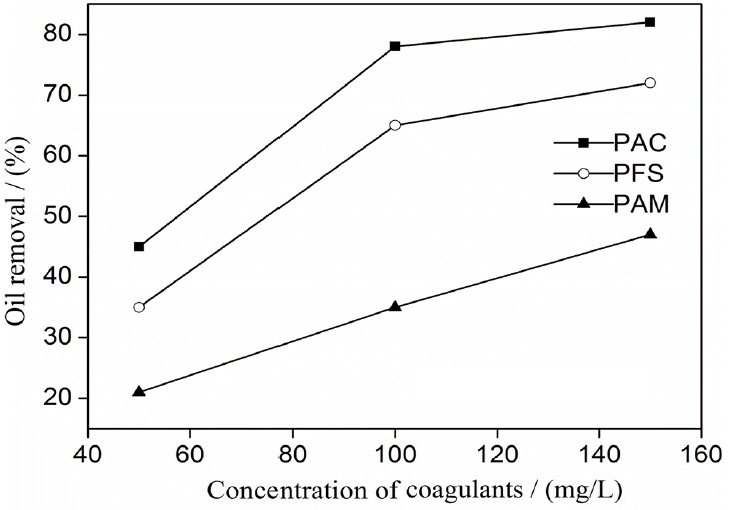
Polyaluminium chloride (PAC), polyferric sulfate (PFS), and poly-acrylamide (PAM) were used as coagulants to destabilize oily wastewater. The effects of coagulant type on oil removal efficiency are shown in Fig. 2. PAC and PFS were more effective than PAM for destabilization. Treatment with 150 mg/L of PAC decreased the oil content of the wastewater by 83%. Coagulation significantly decreased absolute zeta potential values, and neutrality was approximated. A free oil film formed at the emulsion surface after coalescence. Similar results have been reported in the literature [16, 17]. The addition of excess coagulants is uneconomical because it does not improve coagulation effects. Thus, PAC was used as the coagulant at the concentration of 130 mg/L for the other tests.
3.2. Effects of Pretreatment Process on UF Flux
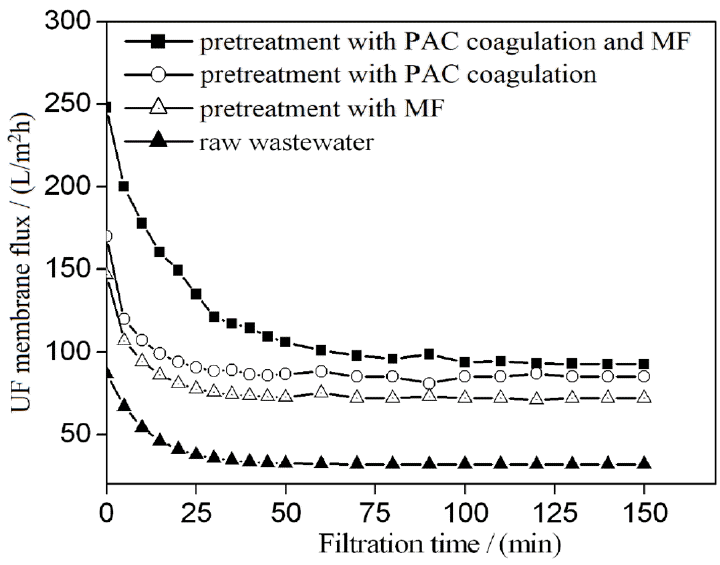
Pretreatment reduces membrane fouling and has an important role in the efficiency of membrane separation [13]. Wastewater was coagulated with 130 mg/L of PAC for 40 min and then pretreated through MF. UF flux values obtained through coagulation integrated with MF, MF only, and coagulation only as a pretreatment are shown in Fig. 3. A shown in Fig. 3, the maximum UF flux was obtained with coagulation integrated with MF as a pretreatment at any filtration time-point. For example, after 50 min of filtration, UF flux reached approximately 95 L/(m2·h), 85 L/(m2·h), and 72 L/(m2·h) with coagulation integrated with MF, coagulation alone, or MF alone as a pretreatment, respectively. The UF flux obtained through coagulation integrated with MF as a pretreatment was 2.5 times higher than that obtained through coagulation without pretreatment. The surfaces of oil droplets are covered by an electric double layer with a negative charge. The electrostatic interactions of PAC disrupted the stability of oil droplets and other suspended particles. Therefore, aggregation was enhanced by the absence of the net charge of oil droplets and suspended matter. In addition, oil droplets were easily separated through sedimentation and MF. These results revealed that the proposed coagulation process with PAC and MF is an effective pretreatment for the UF feed.
3.3. Effects of TMP on UF Flux
UF flux as a function of filtration time under different TMPs is shown in Fig. 4. UF flux rapidly initially declined and then gradually declined with every increment in TMP. Concentration polarization and gel layer formation occurred as soon as filtration began, causing UF flux to decrease rapidly. Subsequently, a compact and ordered deposit progressively formed from the outlet to the inlet of the membrane device. This effect caused the flux to gradually decline. Compared with that under the TMP of 0.2 MPa, flux declined sharply at the beginning of filtration under the TMP range of 0.3 MPa to 0.6 MPa. Moreover, flux rate decreased slowly after 30 min. These findings indicated that under high TMP, some of the oil droplets adsorbed on the membrane surface or were embedded in the membrane pore. These effects caused the permeate flux to decrease quickly at high TMP. Flux declined slightly after 60 min because the fouling layer resistance on the membrane stabilized. At long operation times, the flux appeared to approach a nearly constant value. Membrane cleaning failed to restore the flux to its original value when the pressure exceeded 0.6 MPa. The operation pressure was set at 0.4 MPa for other UF experiments.
3.4. Effects of CFV on UF Flux
CFV highly influences membrane fouling [8, 18]. The permeate flux of UF at different CFVs is shown in Fig. 5. The permeate flux increased with increasing CFV. The flux increased significantly as CFV increased from 1 m/s to 4 m/s. As expected, high CFV decreased concentration polarization and fouling layer thickness, thus decreasing filtration resistance. Considering the effect of velocity on membrane flux and energy consumption, the membrane surface velocity was set at 3 m/s.
Table 1 presents the quality of UF permeates. The oil content of the effluent was less than 10 mg/L, and oil removal efficiency exceeded 98.5% after UF treatment. TMP had a negligible effect on wastewater oil concentration. Oil droplet size may be the main factor that affects UF permeates. The sizes of UF pores are smaller than those of oil droplets. Therefore, turbidity and oil concentration negligibly changed with increasing TMP. These results also showed that oil and colloidal particles were effectively removed from wastewater. Water turbidity decreased to 99%, and CODcr decreased by 95.2%.
3.5. Membrane Cleaning
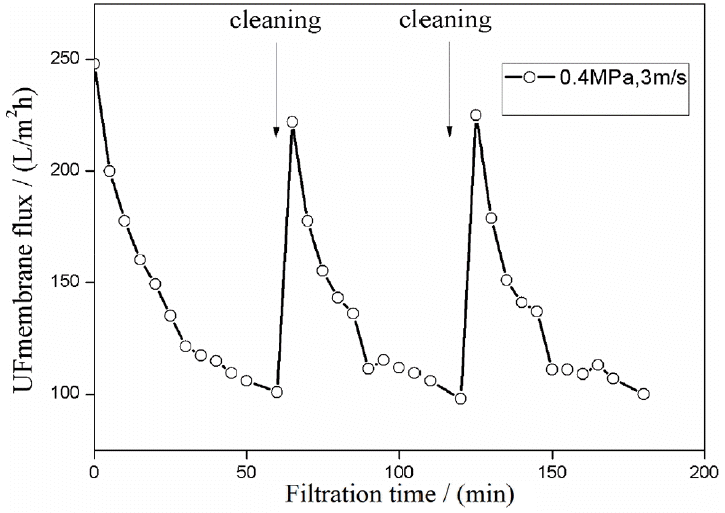
The effects of membrane cleaning are shown in Fig. 6. The decrease in flux to 60% after 60 min of filtration time with the UF membrane could be mainly attributed to the absorption of oil droplets on mfiltration time with the UF membrane could be mainly attributed to the absorption of oil droplets on membrane pores and surfaces. Adsorbed oil blocked the membrane pores and increased membrane resistance during the operation. Hence, membrane cleaning is important to improve the efficiency of the pretreatment. Inorganic detergents were used to clean the fouling layer from membranes because organic detergent would increase COD and could change the interfacial tension between the membrane and wastewater. These effects would cause further membrane fouling. UF flux recovered to 90% after 10 min of membrane cleaning with NaOH and HNO3 aqueous solutions. Inorganic cleaners also had good removal efficiency for insoluble inorganic, organic, and colloidal pollutants. This finding indicated that the UF membrane could be cleaned with NaOH and HNO3 aqueous solutions.
4. Conclusions
Experimental studies on the treatment of oily wastewater through a hybrid-integrated membrane process based on coagulation and MF + UF were conducted. The optimal operation conditions are as follows: Coagulant concentration of 130 mg/L PAC, TMP of 0.4 MPa, and CFV of 3 m/s. The oil removal rate and CODcr reduction rate of the process were 98.5% and 95.2%, respectively, under the optimized conditions. Furthermore, the quality of UF permeates met the local standards for sewage treatment (<10 mg/L of oil content and <500 mg/L of COD). Cleaning the fouling membrane with NaOH and HNO3 aqueous solutions led to the UF flux recovery of 90%.