1. Introduction
Microalgae are attractive potential raw material for bioenergy because they do not require vast areas for cultivation, short growth period, high growth rate, and high lipid content compared with other plants biooil [1, 2]. Despite several advantages the harvesting of pure microalgae is expensive and required much energy. Especially, small size and low cell density of microalgae are harvested more costly [3]. According to the reports, the harvest of microalgae biomass accounted for 20–30% of total production costs [3, 4].
A number of techniques have been developed to harvest microalgae, such as flotation, filtration, centrifugation, adsorption and flocculation etc. From that, an adsorption and flocculation method is relatively simple and cost effective compared to other harvesting methods [5]. Flocculation has the advantage of high efficiency for microalgae harvesting. However, it requires a significant amount of chemical consumption. For example, typical flocculants include ferric and aluminum salt for flocculation of sample solutions greater than 600 mg L−1 of biomass [3, 6]. Moreover, the use of excess metals should be avoided since they may cause problems if the algae mass is to be used as a source for fuel production or as fish fodder, for instance [2]. Metals in oils extracted from biomass typically need to be removed before further processing [3, 7]. It is therefore necessary to develop a method that is equally efficient and vastly more economical to achieve a commercially adopted means of harvesting this material. The method utilizing methyl-esterified eggshell membrane (MESM) as an adsorbent for the harvesting of microalgae may be an alternative to adsorb algae present in an aqueous phase to effect rapid and gentle enrichment or concentration of algae.
The Korean food industry produces 90,000 tons of eggshell waste per year [3, 7]. The disposal uses for eggshell waste include 6.2% in fertilizer, 10.7% in animal feed ingredients, 3.2% in other applications, and the rest discarded as waste in Korea [3]. However, many landfills are reluctant to accept eggshell waste because the eggshells and the attached membrane attract pests [8, 9]. The eggshell membrane (ESM) is located between the liquid white and the solid inner surface of the eggshell. The contents of C, H, N, O, and S in the ESM are 47.5%, 6.8%, 15.3%, 12.0%, and 3.0%, respectively [10]. Despite its porous structure, ESM is composed of a fibrous protein network. Therefore, ESM has good adsorbent properties, including the ability to remove heavy metal and dye from wastewater. In a previous study, ESM was shown to be positively charged at pH values of 1–6 and negatively charged at pH values of 6–12 [10, 11]. The surface property of the ESM dominates its sorption behaviors toward various ions and species. However, the negatively-charged carboxylic groups play an inhibitory role for adsorption of anion species by ESM [12, 13]. After methyl esterification, the ESM surface charge property is changed according to the esterification of carboxylic groups, which is the main driving force for the improvement of microalgae sorption capacity [10]. MESM has a high cationic charge density and can thus strongly adsorb and destabilize negative particles, such as negatively-charged microalgae cells. MESM from eggshell waste has been recommended for use as an adsorbent because of its low cost, copious availability, non-toxicity, high specific surface area, biodegradable, the high potential of ion exchange for charged pollutants, and safe handling [14, 15]. Many researchers have reported several biological, chemical, and physical processes for heavy metal removal using MESM [8, 10, 16, 17]. However, close examination of MESM for the harvesting of microalgae species has rarely been reported. Therefore, the novelty in this study is the proposal of an effective use of MESM for microalgae harvest in solution. We expect MESM to be used as an eco-friendly bio-sorbent instead of other chemical harvesting materials for microalgae.
2. Materials and Methods
2.1. Microalgae Cultivation
Chlorella vulgaris (C. vulgaris: KMMCC 145) was obtained from KMMCC (Korea Marine Microalgae Culture Center) and was cultivated in Jaworski’s medium (JM) under LED (light-emitting diode) lamps at ambient temperature. The chemical composition of JM is given elsewhere [2, 3]. The microalgae were cultured in a 200 mL conical flask containing 100 mL JM with 10 mL C. vulgaris at 7.2 ± 0.3 of pH. The cultures were maintained in a dark and light cycle of 8 and 16 h, respectively, for 10 days. The temperature was maintained at 23ºC ± 1ºC using LEDs. The LED light source was used because it is efficient and provides the required wavelength light from 430 nm to 670 nm, which is selective for microalgal growth. Moreover, the light intensity was 200–250 μmol m−2s−1. An equivalent CO2 aeration rate of 0.02 vvm was used for cultivation. The initial concentration of the inoculated microalgae was 1.857 g L−1 ± 0.5 g L−1.
2.2. Adsorbent
Waste eggshell samples were collected from a chicken farm in Gangneung City, Korea. To remove all adhesion or interference materials, such as organics and salts, the sample was rinsed several times with deionized water and then boiled in water. After cleaning, eggshells were dried in an oven at 80°C for 24 h. The dried eggshells were first pretreated with 15% (v/v) HCl overnight to dissolve the outer layer of the shell and leave the ESM. The separated ESM was then cleaned using deionized water, dried at 80°C for 24 h in an oven, and crushed into fine powder using a mortar and pestle for future use. Next, 100 mg of the pretreated ESM powder was immersed into 50 mL methanol containing 2% (v/v) HCl for 10 h at 80°C, during which process the carboxylic groups on the ESM structure were esterified [10]. The resultant MESM was rinsed several times with deionized water, dried at 80°C for 24 h in an oven, and stored in a desiccator for future use. Sorption by MESM should occur mainly through an exchange reaction, and it should be possible to use it as a new biological flocculent of microalgae.
2.3. Methods
The high cationic charge density of MESM allows it to strongly adsorb the negatively-charged regions of microalgae. Sorption by MESMs should occur mainly through an exchange reaction, and it should be possible to use MESM as a new biological harvesting method for microalgae. The experiment was carried out in the form of a batch-test. To determine the effect of microalgae harvesting, MESM doses ranging from 1 to 30 mg L−1 were added in the microalgae contained in 2 L water, and a MESM particle size of 20 to 320 mesh was used. The pH was controlled from 1 to 10 using NaOH and/or H2SO4, and the sample pH values tested ranged from 1 to 10 in single unit increments.
The mineralogy of the MESM and ESM was characterized by an X-ray diffractometer (Rikaku PMG-S2, 30 kV X 10 mA). Surface images of both MESM and ESM were obtained by a scanning electron microscope (SM-300, Topcon, Japan). The zeta potential of the MESM and ESM at different pH values was determined with a Malvern ZetaSizer Nano ZS90 (Malvern Instruments Ltd., UK). One milliliter of suspension was pipetted into a cuvette and inserted into the unit for determination of zeta potential and osmotic adjustment. Zeta potential was measured in triplicate at room temperature, and the average values were determined. MESM was sieved to obtain 0–320 mesh sizes using a mechanical sieve. The particle size and amount of sericite were analyzed using laser diffraction (Laser Diffraction Master Class 3 & 4, Malvern, England) and micro-scales (XP26, Mettler Toledo, Swiss), respectively. The pH was measured using a pH/ORP meter (ISTEK, pH-20N).
To determine the separation efficiency (SE), 10 mL of microalgae cells were placed in a 15 mL falcon tube. MESM and ESM were added at a designated concentration and mixed at 80 rpm/min using a mini orbital shaker (VWR Advanced Orbital Shaker, Model 15000). High-speed mixing tends to break the flocs, causing the adsorbed cells to be redisposed and reintroduced into the medium. After mixing, the algal cells were allowed to settle for 10 min. Supernatant samples were collected over time from the middle of the algae suspension to obtain optical density (OD) measurements. The OD was measured at 680 nm (OD 680) by a spectrophotometer (Beckman Coulter, model DU 730) equipped with a carousel cell holder. The OD 680 values were calibrated against dry cell weight. The separation efficiency (%) was calculated as follows:
where ODt0 is the optical density of samples taken at time zero (t0), and ODt is the optical density at time t.
2.4. Data Analysis
Data presented in tables and figures represent mean values ± 3σ of five replicates. Where error bars are not visible, errors were less than or equal to the symbols. The differences between mean values were calculated using Tukey’s test at the 0.05 level by Origin software (v.7.5, OriginLab, Northampton, MA, USA).
3. Results and Discussion
3.1. Characterization of ESM and MESM
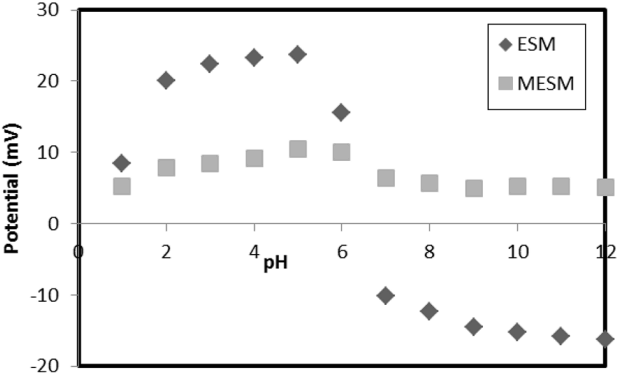
Analyzed ESM and MESM surfaces are noted in Fig. 1. The ESM surface was positively charged at pH values of 1–6 and negatively charged at pH higher than 6. These results suggested the negatively charged carboxyl group activated with increasing of pH. When the negatively charged carboxyl group increased, the surface charge of ESM changed a significantly stronger negative charge. However, MESM was positively charged over the entire pH range. The potential of MESM was demonstrated as 5–10 mV in all pH values, whereas that of ESM varied from 23.7 to -16.2 mV over all pH values and changed significantly at pH 6. The negatively-charged carboxylic groups present on ESM should play an inhibitory role for adsorption of anion species [18]. After methyl esterification, the negative surface charge property of ESM is changed to positive for all pH values for improvement of microalgae sorption capacity. Variations in surface charge affect the interaction between particles and thus affect the adsorption efficiency. Adsorption using MESM is the result of particle collision and charge interaction between the charges of MESM and the microalgae surface in a liquid medium. In general the potential values of ±5 mV represent rapidly adsorption, which values from ±10 to ±30 mV represent instability adsorption, and values more than ±30 mV the particle is in stable and represent almost no adsorption due to the repulsive force between particles [3, 19]. In this study, the MESM potential for all pH ranges was in the range of 0 to 8 mV. The apparent surface charge of the organic/inorganic matter is represented by its zeta potential, which might affect the adsorption efficiency.
Scanning electron microscopic (SEM) images of eggshell membrane samples before and after methyl esterification are shown in Figs. 2(a) and 2(b), respectively. The SEM images of ESM and MESM demonstrate similar morphologies. However after methyl esterification eggshell membrane intertwined like a thin thread and the specific surface area was expanded in order that adsorption of microalgae can better in aqueous solution. The energy dispersive spectroscopic (EDS) evaluation determined that the C, N, and S contents in ESM were 42.6%, 31.5%, and 9.9%, respectively [20]. However, after methyl esterification, the contents of these elements changed to 54.7%, 20.4%, and 7.4%, respectively. Chen et al. [10] reported that after the esterification process the presence of −C = O and -C-O-C bands, which are clear indicators of esterification, was confirmed with two new peaks 1,731 cm−1 and 1,187 cm−1. However, the major absorption bands for the ESM are still clearly observed after esterification, e.g., the absorption at 3,315 cm−1 assigned to the stretching vibration of hydroxyl groups, and the absorption 1,660 cm−1 attributed to the −C = O stretching. The increase in C and decreases in N and S further indicated the esterification of carboxylic groups on the ESM surface. These changes show the negatively-charged ESM surface being changed to a positive surface after methyl esterification.
3.2. Effect of pH on Microalgae Harvest
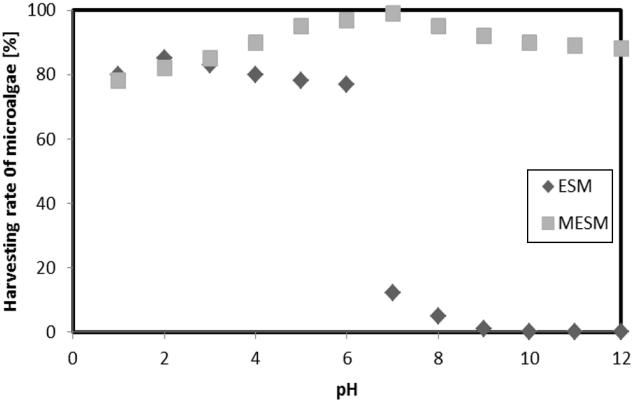
The charge properties of the MESM and ESM surfaces and the microalgae species are closely related to sample pH (Fig. 3). Within the pH range of 1–6, the harvesting efficiency of microalgae was < 77% for ESM; however, the adsorption efficiency by ESM was negligible within the entire pH range studied, i.e., pH 7–12. These trends are associated with zeta potential. By analyzing the ESM surface, we confirmed it was positively charged at pH values 1–6 and negatively charged at pH 7 or greater (Fig. 1). The negatively-charged ESM should play an inhibitory role on the adsorption of negative microalgae. In contrast, the harvesting efficiency of microalgae by MESM reached 78–99% in all pH ranges. After methyl esterification, the negative surface charge of ESM was changed to positive for all pH values, demonstrating improvement of sorption capacity toward negatively-charged microalgae. Therefore, an effective harvesting efficiency by MESM was obtained for all pH values. The maximum harvesting efficiency of 99% by MESM was recorded at pH 7. Therefore the best pH for harvesting of microalgae recommended pH 7.
3.3. Effect of MESM Dosage on Microalgae Harvesting
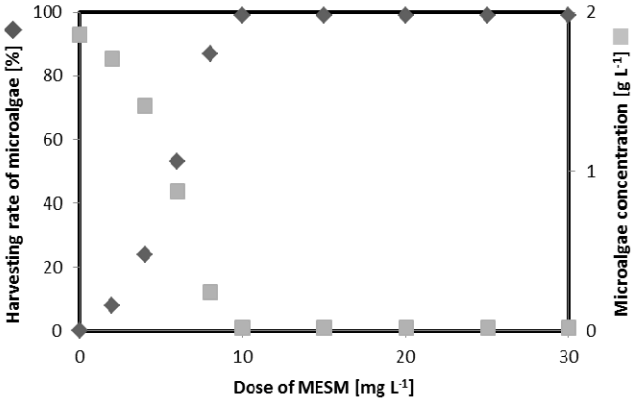
The effect of MESM dosage on microalgae harvesting is depicted in Fig. 4. The harvesting efficiency of microalgae was < 30% with 5 mg L−1 of MESM and > 99% at up to 10 mg L−1 of MESM at pH 7. At up to 10 mg L−1 of MESM, the harvesting efficiency of microalgae was not changed and showed nearly complete harvest. The microalgae concentration also decreased significantly until 10 mg L−1 MESM according to increasing MESM dosage. A large adsorbent surface is very important for microalgae adsorption.
3.4. Effect of Particle Size on Microalgae Harvesting
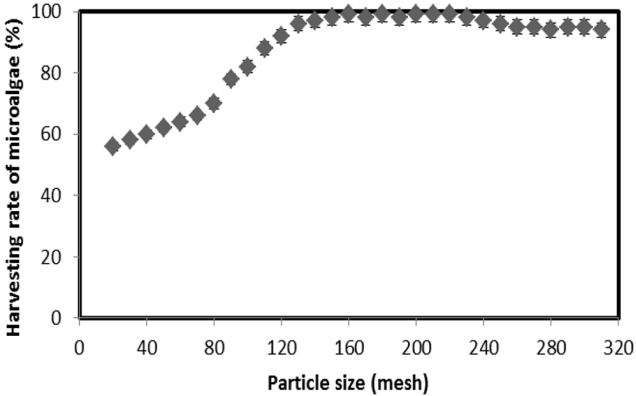
A smaller particle size is better for adsorption of materials, because the total surface of smaller particles is greater than that of larger particles. Accordingly, the adsorption capacity increased with small particles compared with larger particles. The harvesting efficiencies of microalgae by various MESM particle sizes are noted in Fig. 5. The maximum microalgae harvesting efficiency was observed as 56–70% with 20–80 mesh, 90–97% with 120–140 mesh, and greater than 98% with a MESM particle size > 150 mesh. The harvesting efficiency of microalgae depends on the particle size; however, up to 150 mesh, MESM showed similar results. Therefore, the optimum MESM particle size for harvesting is suggested as 150 mesh. Moreover, the MESM surface provides active sites that are capable of adsorbing microalgae from aqueous solutions, and this ability is further enhanced under elevated solution pH conditions [18]. Adsorption using MESM is a consequence of its surface energy being similar to its surface tension. Before equilibrium is reached between the MESM and microalgae, the harvesting efficiency increased with increasing surface area at a constant 10 mg/L MESM. However, if equilibrium is reached between MESM and microalgae, then the adsorption force is weak. After equilibrium, the microalgae sorption by MESM was not changed significantly, thereby further increasing the importance of particle size and dosage. This may be due to the fact that the MESM surface area of the > 150 mesh particle size was not significantly changed compared with that of the < 150 mesh particle size.
Equilibrium modeling was studied for adsorption of microalgae onto MESM. The results obtained using the Langmuir and Freundlich isotherm models are represented in Table 1. The Langmuir isotherm provides a better fit than the Freundlich isotherm. The maximum adsorption capacity of MESM was 59.6 mg g−1 according to the Langmuir isotherm.
Microalgae are taken up by MESM through adsorption. The microalgae are quickly adsorbed over the MESM surface; this process is called physical adsorption. In the present investigation, by decreasing the particle size, the percentage of harvested microalgae slowed gradually, and an apparent equilibrium was established. Upon reaching equilibrium, the microalgae sorption by MESM was not changed significantly, despite further increases in the dosage and operation time. MESM is an excellent biosorbent due to pore structure and protein acid mucopolysaccharide contents. ESM has been used by many researchers for the adsorption and immobilization of several metal and inorganic ions to prevent their hazardous impacts on humanity and the environment as a whole [17, 21, 22].
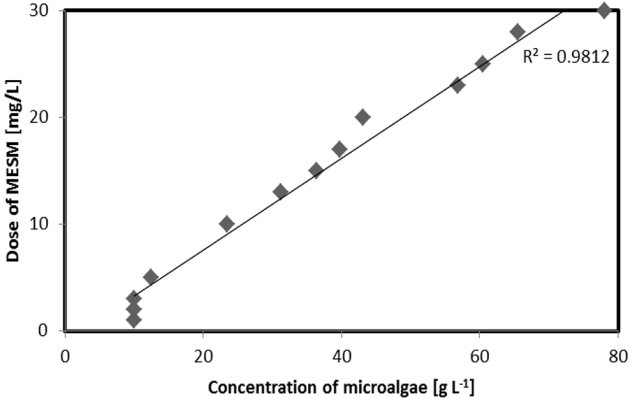
3.5. Microalgae Concentration and Dose of MESM
The relationship of microalgae concentration and MESM is represented in Fig. 6. The relation coefficient (R2) of microalgae concentration and MESM dosage was determined as 0.9812. Increasing microalgae concentration increased the required amount of MESM. In the experiment, the 2.3–2.5 mg L−1 of MESM adsorbed approximately 1 g L−1 microalgae. MESM is a very effective bio adsorption material for microalgae harvesting. The adsorption capacity of MESM is superior due to higher contents of carbonate groups. The effects of process parameters such as pH, microalgae concentration, amount of adsorbent, and adsorbent pore size on process equilibrium showed that the uptake of microalgae by MESM was increased by increasing the microalgae concentration and adsorbent dosage and decreased by increasing the adsorbent size.
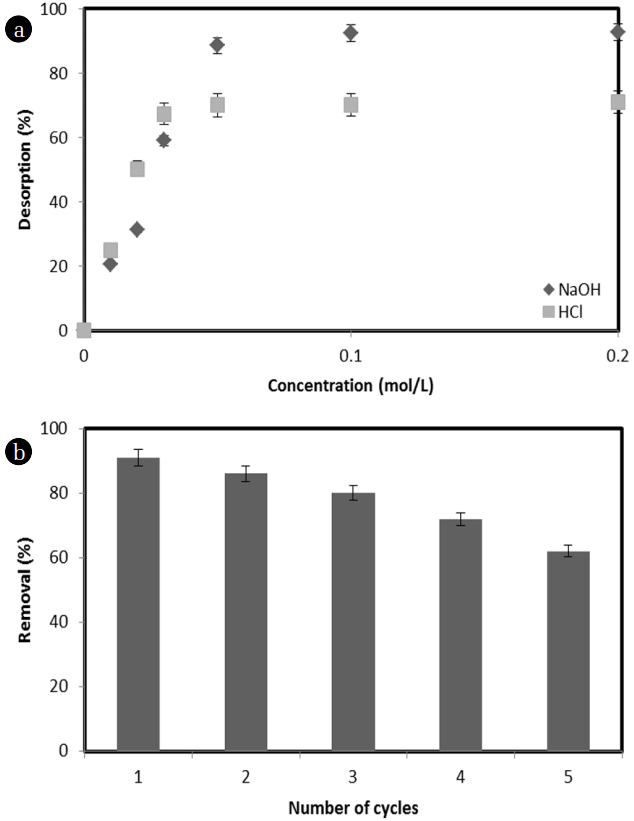
3.6. Desorption and Reusability
It is necessary to find a suitable eluent which allows the reusability of the adsorbents. In this study, NaOH is found to be a suitable eluent compared with HCl and the percentage of microalge desorbed using various concentration of NaOH or HCl is given in Fig. 7(a). It is observed that 0.05 mol/L NaOH could desorb 90.75% of microalgae within 2 h contact. The percentage of desorption with HCl was obtained lower than using NaOH and it desorb 69.95% of microalgae by 0.05 mol/L HCl. Although ~ 92.75% by 0.2 mol/L NaOH and ~ 70.92% by 0.2 mol/L HCl for microalgae, further increase in NaOH and HCl concentration above 0.05 mol/L did not significant increase in desorption of microalgae. Therefore, 0.05 mol NaOH solution was recommend for regeneration of MESM. The desorption with NaOH was higher than that of HCl. This suggests that an increase in the OH− ion in the aqueous solution with a higher concentration of NaOH increased the repulsion between the OH− ion and the microalgae. Thus, desorption of MESM was increased with increasing of NaOH concentration. Furthermore, reusability of MESM with 0.05 mol NaOH for adsorption of microalgae was studied and results were shown in Fig. 7(b). The adsorption of microalgae was decreased as the number of adsorption-desorption cycles increased; however, Ca 62% of microalgae was adsorbed even after the material passed through five replicates of adsorption-desorption cycles. Therefore, this study indicates that the MESM is a suitable and excellent material for multiple uses in the adsorption of microalgae simultaneously from aqueous solution.
In the present research, the esterification of carboxylic groups on ESM with methanol was investigated to harvest microalgae. The high cationic charge density of MESM allows it to strongly adsorb negative particles. The mechanism of microalgae binding by activated MESM may depend on the pH, microalgae concentration, dose, and MESM particle size. MESM is an abundant, low-cost material and has valuable applications in chemistry and chemical technology. It has attractive adsorption properties; additionally, it can be regenerated by a number of applications. The main component of MESM is protein, which contains amines and carboxylic groups, as well as fiber and a small amount of polysaccharide [23, 24]. Therefore, the harvested microalgae using MESM is applicable for use in biodiesel, animal feed, food, and medicine. In the business world cost is very important. Organic flocculants such as cationic polyacrylamide, cationic starch, and chitosan are biodegradable and offer low toxicity [25] but have high prices i.e., $10 to 100 USD per kilogram for chitosan [5, 26] and $5 to 30 USD per kilogram for cationic starch [6]. In contrast, MESM is inexpensive ($0.1 to 0.5 USD) and represents a reuse of waste. This harvesting method helps reduce the production cost of algae for biodiesel.
4. Conclusions
This study investigated the harvesting of microalgae using MESM. The negatively-charged ESM can play an inhibitory role for the adsorption of negatively-charged microalgae. In contrast, the harvesting efficiency of microalgae by MESM reached 78–99% for all pH values tested. After methyl esterification, the negative surface charge property of ESM was changed to positive, thereby improving sorption capacity toward the negatively-charged surface of microalgae. Therefore, the harvesting efficiency obtained by MESM was highly effective for all pH values. The maximum harvesting efficiency of 99% by MESM was recorded at pH 7. A harvesting efficiency < 30% was obtained within 5 mg L−1 of MESM, and that > 99% was noted at up to 10 mg L−1 of MESM at pH 7. Microalgae concentration decreased within increasing MESM dosage, as the MESM surface area also increased. The maximum microalgae harvesting efficiency was observed as 56% - 70% for 20–80 mesh, 90% - 97% for 120–140 mesh, and greater than 98% for > 150 mesh MESM particle size. After the equilibrium period, the microalgae sorption by MESM was not changed significantly, despite further increases in particle size and dosage. The maximum adsorption capacity of MESM was 59.6 mg g−1 according to the Langmuir isotherm. In the experiment, 2.3–2.5 mg L−1 MESM absorbed approximately 1 g L−1 microalgae. Thus, MESM is a highly effective bio-adsorbent material for microalgae harvesting.