1. Introduction
Due to rapid population growth, urbanization and industrialization world is facing problems associated with shortage of fresh water and energy to run people’s daily life. As population rises, the demand of freshwater gets elevated and so is the generation of wastewater and crisis of energy. Wastewater treatment plant requires more energy to treat this higher generation of wastewater. Treatments plants in existence are not 100% effective in removing nutrients at desirable limits for disposal. The ultimate fate of these pollutant nutrients in effluent is either in sea, land or any other water bodies which leads to increase in pollutant load and eutrophication in water bodies. Microalgae is being an acceptable field of interest from last five decades because of their possible applications in biofuels, cosmetics, pharmaceuticals, nutrition and food additives, aquaculture etc. Moreover, it is also potent medium for further removal of polluting nutrients from wastewater treatment plant effluent [1, 2, 3]. The high cost of microalgae cultivation is one of the major obstacles for commercial application of micro-algae and production of biodiesel.
Microalgae have potential of treating sewage by up taking nitrogen and phosphorus to get bloomed [4]. Difficulties in cultivating microalgae in commercial scale could be solved at some extent by using it for further removal of nutrient from sewage but there is still a problem for harvesting it for higher biomass concentration. Microalgae harvesting methods such as chemical coagulation, centrifuge, and other physical means are not economically viable in terms of energy and cost. Chemical coagulation contaminates the microalgae biomass and is not suitable for biodiesel production. Membrane systems such as MF (micro filtration), UF (ultra filtration) and NF (nano filtration) for microalgae harvesting seems economically difficult in terms of energy and membrane cost as well. One potential solution for harvesting issue could be the use of forward osmosis (FO) membrane. FO membrane system which consumes less energy and does not require applied mechanical pressure has potential application including seawater and brackish water desalination and wastewater treatment biomass concentration, and food processing [5, 6, 7, 8].
FO is an osmotically driven membrane process that takes advantage of the osmotic pressure gradient to drive water across the semipermeable membrane from the feed solution (low osmotic pressure) side to the draw solution (high osmotic pressure) side [9]. The concentration difference across facilitates water transport by diffusion from low osmotic pressure impaired feed to the high osmotic pressure draw solution, while rejecting ions and molecules [7, 10]. FO differs from traditional membrane separation technology because unlike MF, UF, NF, and RO (reverse osmosis) a little or no hydraulic pressure is required and fouling propensity is also low [10].
The availability of a suitable draw solution is crucial for advancing FO technology. An appropriate draw solution not only promotes the efficiency of the FO process, but also saves cost of the subsequent steps in recovering and replenishing the draw solute [11]. In addition to have minimal toxicity and low cost, an ideal draw solution needs to fulfill the following requirements. First, it should be able to generate a high osmotic pressure. As the osmotic pressure difference between the draw solution and feed solution across the membrane is the driving force for the FO process, the osmotic pressure of a draw solution must be higher than that of the feed solution to ensure a positive permeate flux [12]. Ideal drawing agent for FO system is problematic and economically ineffective. SWRO concentrate could list its name as a drawing agent for FO system due to its high concentration. The rationale behind selection of seawater as drawing agent is, it consists of various chemical ions at very high concentration compared to sewage effluent which ultimately creates higher osmotic gradient when used as drawing agent for FO system. Seawater has high solubility and is relatively easy to reconcentrate to high concentrations using conventional desalination process without risk of scaling [13]. SWRO concentrate is so highly concentrated with salt ions and elements of seawater which is being problematic for direct disposal to marine system. Engineers and researchers are working for better option for management of SWRO concentrate [14]. One solution for management of SWRO concentrate is to use it as a drawing agent in FO system as it has high concentration and possibly has higher osmotic efficiency.
The objective of this study is to evaluate the potential of forward osmosis membrane process for harvesting microalgae for further biodiesel production. Microalgae performance in removal of nutrient from sewage was studied. Efficiency of seawater and SWRO concentrate as drawing agent was assessed.
2. Materials and Methods
2.1. Microalgae Cultivation
Species of microalgae used in the experiment was provided by Pusan National University. It was cultivated in BG-11 medium as stock solution. The chlorella sp. ADE4 was inoculated to make the initial microalgae concentration of 0.32 g/L in 2 L Erlenmeyer flask containing secondary sewage effluent. Erlenmeyer flasks were placed on a shaker for mixing at 120 rpm and with illumination at light intensity of 50 μmol/(m2·s). The growth rate of microalgae was calculated using equation provided below.
where, W1 and W2 represent dry weight or cell number at time t1 and t2, respectively.
The secondary effluent was collected from local sewage treatment plant in Jinhae, South Korea. Chemical characteristics of the secondary effluent are shown in table 1. The average concentration of total nitrogen (TN) and total phosphorus (TP) was found to 19.9 and 0.15 mg/L, respectively.
2.2. FO Membrane
Flat sheet membranes specifically developed for FO were used in this study. The membranes were manufactured by HTI (Hydration Technologies Inc., Albany, OR, USA). Salient features and properties of this membrane are repeatedly reported in previous studies [7, 15, 16]. Membrane in this study was made up of Cellulose Triacetate (CTA) with embedded polyester screen mesh for mechanical support. HTI membranes are especially designed to minimize internal concentration polarization, so are thinner (< 50 μm) considering valid assumptions. The filtration area of FO membrane used in this study was of 14 cm × 10 cm.
2.3. Draw and Feed Solute Used
Draw solution used in this experiment were SWRO concentrate and seawater. SWRO concentrate was collected from reverse osmosis desalination plant from Korea Maritime Ocean University. Seawater as a draw solution was collected from Masan, South Korea. Three feed solution were tested to study the dewatering performance of FO system. Three types of feed solutions for the test were super pure water (FW: 18; JT Baker, USA), secondary sewage effluent and solution of microalgae in secondary sewage effluent. Pure water dewatering test was conducted to collect baseline data to compare those of sewage effluent and microalgae mixture in in the seawater effluent.
2.4. Experimental Setup
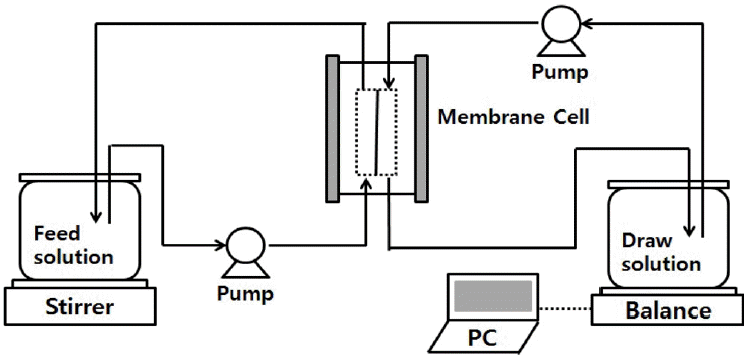
The FO filtration setup is illustrated in Fig. 1. FO experiments were conducted using a bench scale cross flow FO setup. Before conducting each experiment, FO membrane was cleaned. Smooth active layer of the membrane was faced towards feed solution and rough support layer was faced towards draw solution. Feed solution was placed on mechanical stirrer for continuous stirring of the constituents in the solution. Draw solution was placed on precision balance scale for continuous monitoring of change in volume of draw solution. Two peristaltic pumps (L/S 7518-00; Masterflex, USA) were used for pumping draw and feed solution. Flow rate of draw and feed solution over the membrane system were controlled to 0.85 L/min. highly sensitive precision balance (ADAM PGW 4502i) and ADAM DU scale software V4.26 system was used for weighing and continuous monitoring of change in volume of draw solution.
The water flux (Jw) was determined from the volume increase in the draw solution, detected and recorded by ADAM DU software. Increase in draw solution was also observed on the draw solution flask. The water flux was directed from the feed waterside to the draw solution side. The water flux is given by following equation [17].
where, A is active working area of membrane (m2), ΔV is change in volume (L) of draw solution, ΔT is time (hrs) and Jw is flux in LMH.
3. Results and Discussion
3.1. TN and TP Uptake by Microalgae
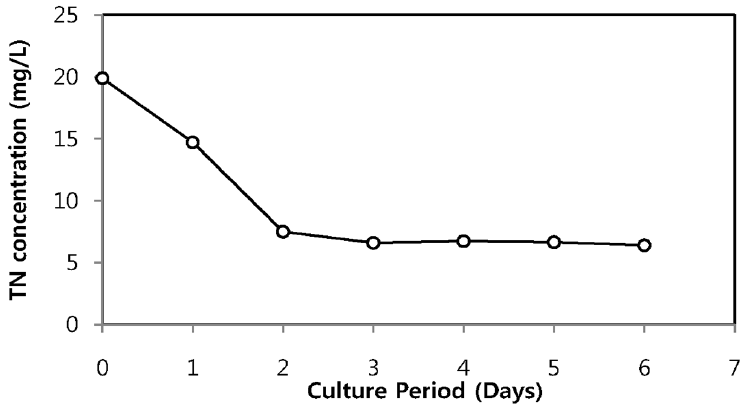
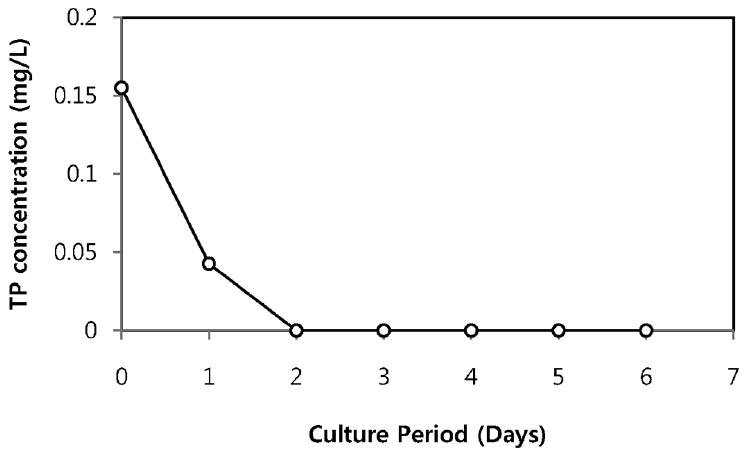
The TN and TP removal from secondary sewage effluent using microalgae is illustrated in Fig. 2 and Fig. 3, respectively. Chlorella sp. ADE4 culture provided moderate removal of TN. Initial TN concentration of sewage effluent was 19.9 mg/L and final TN concentration was 6.4 mg/L. TN removal efficiency of Chlorella sp. ADE4 was found to be 68% for 6 days operation. 62% of TN was removed after 2 days. This implies that Chlorella sp. ADE4 is not effective enough to remove TN with longer mean cell residence time (MCRT). More than 99% of TP removal was achieved. Initial TP concentration of secondary sewage effluent was 0.15 mg/L. The depletion of the P source was believed to be associated with the N assimilation observed from N concentration in sewage effluent rapidly removed in the first 2 days. Previous study suggested that N and P are considered as biochemically dependent [18].
3.2. Growth of Microalgae
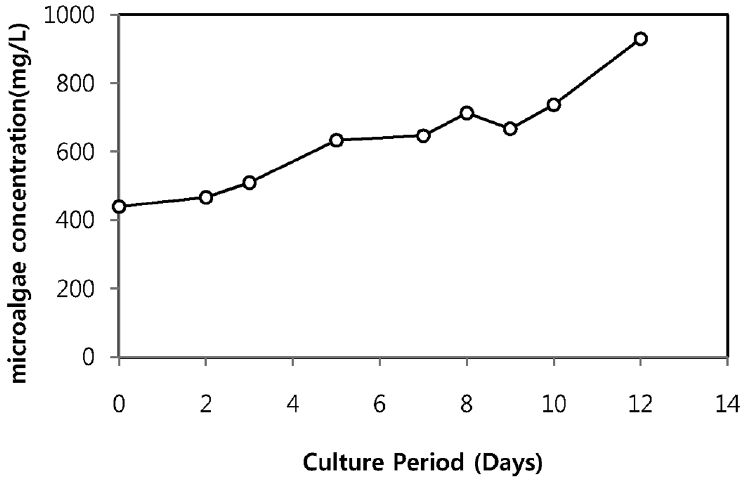
As illustrated in Fig. 4, the microalgae concentration increased along with cell culture duration without showing lag phase. After 12 days of cultivation, maximum microalgae concentration reached to 930 mg/L and kept on rising. The result revealed that, other microorganisms present in sewage effluent did not affect or compete the growth of Chlorella sp. ADE4 and indicates that Chlorella sp. ADE4 was tolerant in sewage effluent.
3.3. FO Membrane for Microalgae Harvesting
3.3.1. Seawater as draw solution
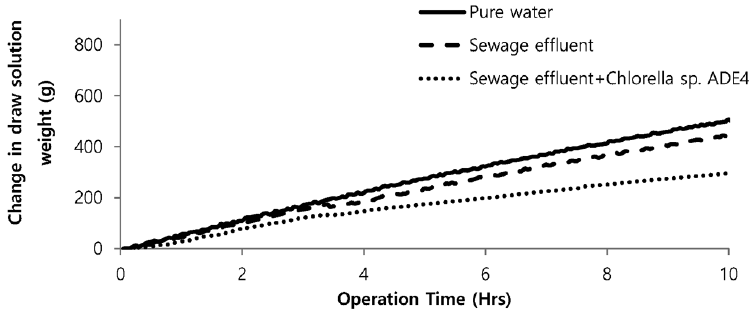
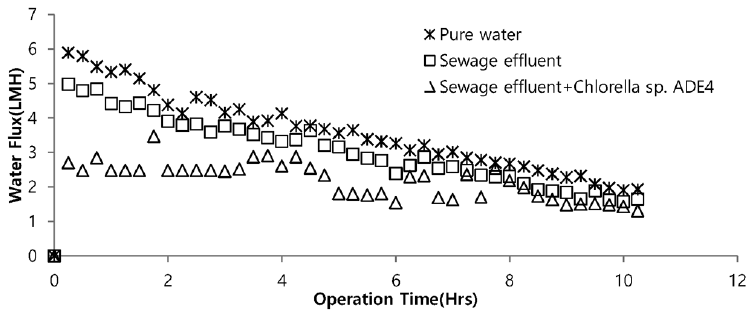
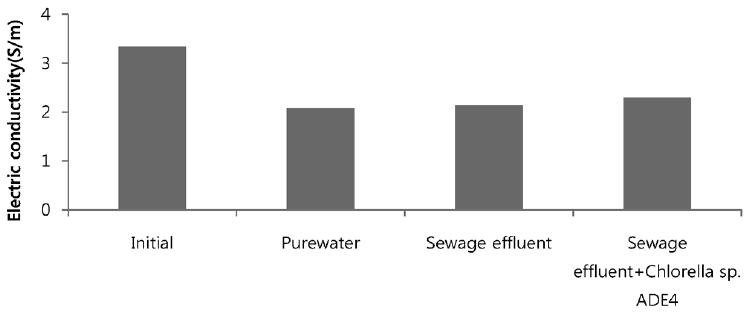
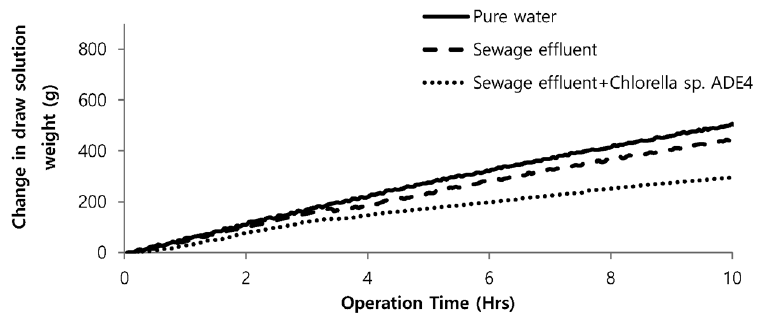
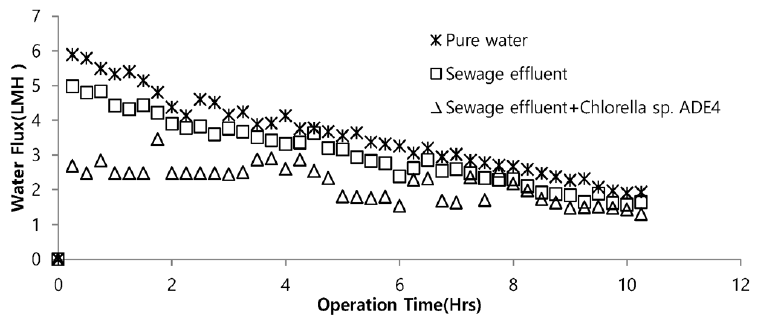
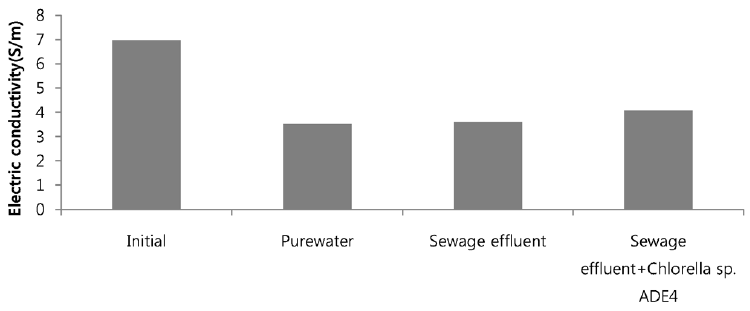
Sea water is considered good as a drawing agent due to its ability of being soluble with water easily and has high concentration of compound [19]. Fig. 5 depicts the dewatering performance of FO system with seawater as a draw solution. Result illustrates the high dewatering rate is achieved for pure water. Dewatering performance is directly proportional to the change in weight of draw solution. As pure water have low ions and almost no contaminants present, the water flux is relatively high compared to other solutions from weight increase of the draw solution. The filtration test of pure water is performed to evaluate dewatering rate of the FO membrane. Second test was carried out to investigate the performance of seawater as drawing agent in FO membrane system to dewater sewage effluent. The dewatering of sewage is relatively lower than that of pure water. Last test was conducted to evaluate dewatering of the microalgae in sewage effluent using seawater as draw solution. Initially, the dewatering of all three samples was increasing rapidly for 3.5 hrs of operation of system. Sewage dewatering showed similar result as pure water, but slight difference was noted. Sewage dewatering is bit lower than pure water due to presence of pollutants micro particles which reduced the dewatering rate with settling contaminants on FO membrane surface. However, bio fouling is not only the reason for decline in dewatering rate. Dewatering rate of depends on water flux over membrane surface. Water flux decreases as draw solution concentration decreases, which ultimately leads to minimization of osmotic efficiency of system [20]. Fig. 6 illustrates the change in flux within the operation time. Average dewatering rate of pure water, sewage effluent and microalgae grown in sewage effluent was found to be 3.5, 3.12 and 2.08 L/(m2·hr), respectively. Average dewatering rate of 2.9 L/(m2·hr) was found in this study using seawater as drawing agent. Similar result of dewatering rate 2 L/(m2·hr) was obtained by Buckwalter et al. [21] with ocean experiments using X-flow pack [21]. Dewatering of different samples made a significant alter in conductivity of draw solute. Initially, seawater conductivity was found to be 3.34 S/m after dewatering test conductivity of seawater was found to be 2.08, 2.14. 2.3 S/m for pure water, sewage effluent and microalgae grown in sewage effluent respectively. Variation in conductivity is illustrated in Fig. 7.
3.3.2. SWRO concentrates as draw solution
As mentioned previously, drawing agent should be of higher solubility and higher concentration. SWRO is 1.4 times more concentrated than seawater and is a good option for use as a draw solution. Fig. 8 depicts the dewatering rate of different samples, using SWRO concentrate as draw solution. The nature of dewatering performance of FO system with SWRO concentrate as draw solution is identical to seawater as draw solution. Dewatering rate of all three samples was inflated by almost 2 folds in case of using SWRO concentrate as drawing agent. Dewatering rate of pure water and sewage effluent is somehow similar and increased in continuous manner. For the first three hours of operation, sewage water dewatering overlaps the dewatering rate of pure water. This might possibly be due to higher concentration of RO concentrate because of which osmotic pressure was increased and water across the membrane was dragged to draw solution side in higher rate. Dewatering rate of microalgae is also increased by two times. As stated earlier, increment in dewatering rate depends on increasing water flux. SWRO concentrate as drawing agent is more than two times concentrated which creates higher osmotic pressure and drags feed solution with higher water flux. Typical osmotic pressure of seawater and SWRO concentrate is mentioned to be 23 and 47 bar respectively [22]. Fig. 9 illustrates the flux over time of operation. Using SWRO concentrate as drawing agent in FO dewatering test, average dewatering rate of pure water, sewage and microalgae grown in sewage is 5.5, 5.2 and 3.6 L/(m2·hr) respectively. The flux result shows that the permeability of water is increase by almost 1.5 times using SWRO concentrate compared with sea-water as a drawing agent. This increase in flux is due to higher osmotic gradient between feed solution and draw solution side. Dewatering of different samples made a significant alter in conductivity of draw solute. Initially SWRO concentrate conductivity was found to be 6.98 S/m. After dewatering test, conductivity of SWRO concentrate was found to be 3.52, 3.6 and 4.07 S/m for pure water, sewage and microalgae grown in sewage respectively. Variation in conductivity is illustrated in Fig. 10. Conductivity of SWRO concentrated decreased by 49%, 48% and 41% for pure water, sewage effluent and microalgae with sewage respectively. These drop in conductivity of draw solution result in drop of the driving force to some extent [23].
3.4. Microalgae Separation Efficiency
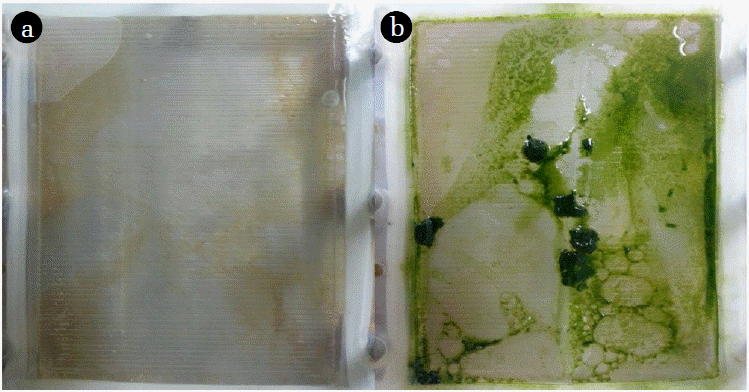
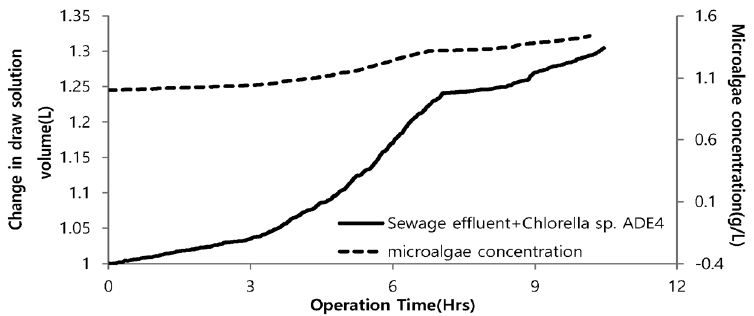
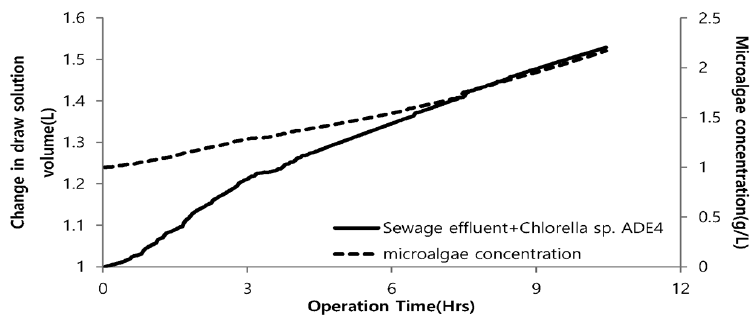
Fig. 12 illustrates the change in volume of drawing agent and dewatering rate of microalgae using seawater as a drawing agent. Plot depicts the sharp increase in volume of drawing agent. Initially, with increase in volume of drawing agent very slow increase in concentration of microalgae was noticed. After 3hr of operation there was slight increase in concentration. This might be due to lower osmotic gradient and clogging of microalgae species in FO chamber. Final concentration of microalgae in feed solution after dewatering was found to be 1.45 g/L. Microalgae dewatering rate from sewage effluent was low due to fouling (Fig. 11) of membrane surface by microalgae. Accumulation of microalgae species on membrane surface diminished the permeability. This decline in permeability is associated with decline of water flux due to predominance of algal cell cake layer on the membrane. Along with bio fouling by accumulation of microalgae on membrane surface feed drawing capacity of draw solute decreased due to dilution. Fig. 13 illustrates the change in volume of drawing agent and dewatering rate of microalgae species using SWRO concentrate as draw solution. With increase in volume of drawing agent concentration of microalgae increased. The steep slope in increase of microalgae shows the higher osmotic gradient across the membrane. This might possibly be due to higher concentration of ions in SWRO concentrate. Final concentration of microalgae in feed solute was almost double compared to final concentration of microalgae in feed solute using seawater as drawing agent. The concentration of microalgae after dewatering was 2.17 g/L. Although the dewatering rate of microalgae is slower than that of other dewatering techniques like chemical coagulation, high speed centrifugation and other physical means. FO dewatering method can have small footprint and economically viable in terms of energy and O&M [24].
4. Conclusions
The result in this study depicted that chlorella sp.ADE4 is a moderate performer in removal of TN (68%) and best in TP removal (99%). Chlorella sp. ADE4 adapts quickly in sewage effluent and increased its biomass by uptake of nutrients efficiently. From the lab scale experiment conducted for dewatering tests, it could be asserted that FO membrane could be a potent technology for dewatering and harvesting of microalgae. Average dewatering rate of 2.9 L/(m2·hr) and 4.8 L/(m2·hr) was obtained using seawater and SWRO concentrate as drawing agents, respectively. From the experimental study, FO membrane dewatering could be an alternative of other conventional methods when SWRO concentrate was used as a drawing agent and it helps in safe disposal of the brine. This study might offer new insight into development of low energy wastewater treatment. However, further researches are required to study the efficacy of SWRO concentrate and enhance dewatering performance.