AbstractNatural organic matter (NOM) is a primary component of fouling in low-pressure membrane filtration, either solely, or in concert with colloidal particles. Various preventive measures to interfere with NOM fouling have been developed and extensively tested, such as coagulation, oxidation, ion exchange, carbon adsorption, and mineral oxide adsorption. Therefore, this article aims to conduct a literature review covering the topics of low-pressure membrane processes, NOM characteristics and fouling behaviors, and diverse fouling control strategies. In-depth explanations and discussion are made regarding why some treatment options are able to remove NOM from source water, but do not reduce fouling. This review provides insight for hybridized membrane processes with respect to NOM removal and fouling mitigation in water treatment.
1. IntroductionLow-pressure membrane processes, such as microfiltration (MF) and ultrafiltration (UF), have become increasingly popular as one of the most promising tools for the treatment of drinking water and wastewater. However, inevitable fouling issues, related to a substantial loss of production capacity of the membrane treatment plant, are challenging, requiring more frequent replacement of membranes, leading to higher operating costs. Therefore, various pretreatment technologies have been developed to resolve such demerits.
An important trend in the application of membrane processes for water treatment is to combine diverse pretreatments with MF/UF to increase the removal of natural organic matter (NOM) and so to reduce membrane fouling. The effects of source water pretreatments can be summarized as follows [1]: 1) changing the size distributions of contaminants; 2) altering the affinities of contaminants to membrane surfaces; and 3) suppressing microbial activity. In contrast, pretreatments have not always been positive regarding membrane fouling control. They have been found to be negligible or even negative due to complicated unknown factors, which must have been linked to membrane properties and source water qualities [2].
Pretreatments of source water, such as coagulation with metal salts and adsorption onto powdered activated carbon, were able to remove a portion of NOM, but the effects of such approaches on membrane fouling control were found to be ineffective in some cases. The metal salts added for coagulation can adsorb NOM, but NOM-metal oxide species cannot often be removed well from treated water during clarification. The pH-dependent surface charges and subsequent interactions between flocculated particles may govern settling characteristics. Once settled, however, expensive chemical sludge treatment is required. Thus, alternative approaches have reported that the integration of reusable iron oxide particles or heated iron oxide particles (HIOPs) to UF membranes is possible, while effectively mitigating membrane fouling [3–12]. Previous studies that had investigated alkaline regeneration of NOM-laden iron oxide adsorbents suggested that the regenerant solution could be reused, until the DOC concentration increased to at least 4,000 mg/L [8]. This should reduce the regeneration costs of adsorbents. However, the separation and regeneration steps of used adsorbents in hybridized mineral oxide adsorption plus UF systems have been another obstacle for practical applications.
An attempt to solve separation and regeneration problems was to immobilize iron oxides on the surfaces of granular media, which can be utilized simultaneously for the adsorption of NOM, and the filtration of particulate matter. Various materials have been tested as support/filtration media, which helped enhance their adsorption of NOM onto bare or modified surfaces [13–19]. A key idea of such approaches was to form a hydrothermal coating layer of iron oxides on granular media (e.g., sand and olivine). The advantages of this form of adsorbents over others were: ease of adsorbent preparation, flexibility of system configurations, and ease of collection and regeneration of used adsorbents. However, heat treatment caused a significant loss of sorption sites.
In this work, therefore, we primarily conducted a literature review to assess the characteristics and effects of NOM with regard to the fouling of low-pressure membranes in water treatment. Various strategies to control and minimize NOM fouling in low-pressure membrane filtration were then compared and discussed in depth.
2. Low-Pressure Membrane Processes for Water TreatmentLow-pressure membranes are being widely used for drinking water and wastewater treatment with drastic improvements in membrane properties and costs [1, 20]. In particular, low-pressure membrane processes (e.g., MF and UF) have had a substantial market growth and have become one of the most attractive and reliable technologies for water treatment due to their superior efficacy in producing a high quality of water with compact facilities, relatively low costs, reduced chemical requirements, and great flexibility against fluctuating feedwater qualities.
Compared to MF, UF has been more popular and preferentially adopted for drinking water treatment plants due to enhanced viral removal. However, a major limitation to the use of UF membranes in drinking water treatment is membrane fouling caused by the deposition of particulate and/or dissolved materials on top of or inside the membrane pore, which leads to an increase in hydraulic filtration resistance and consequently requires more operational and maintenance costs [21].
The fouling phenomena of low-pressure membranes can be classified into two based on locations [21–24]: one is surface fouling, and the other is internal pore fouling. Surface fouling is caused by the deposition of solutes (e.g., colloids and NOM) on top of the membrane, while growing over time. This fouling layer can often be controlled by turbulence and hydrodynamic flushing, but not always [25, 26]. The use of hydrophilic or charged membranes helps retard or minimize the adhesion of foulants to the membrane surface [20, 24, 27]. Surface fouling is often considered to be reversible. Internal fouling is caused by pore plugging and/or the adsorption of small substances (e.g., NOM) inside the membrane pores, which may not be restorable, either physically or chemically, or both.
Several physical cleaning approaches, such as hydraulic backwashing, air scrubbing, and surface flushing, provide more sustainable hydrodynamic conditions leading to fouling reduction [25, 28–31]. However, the membrane filtration resistance gradually increases in long-term operations indicating that membrane fouling cannot be completely mitigated by physical cleaning [32]. Such portion of irreversible fouling can be further eliminated by chemical cleaning [33]; but frequent chemical cleaning may shorten the membrane lifetime, while producing a larger amount of spent chemical wastes.
3. NOM Fouling3.1. Characteristics of NOMNOM is a complex mixture of particulate and dissolved organics generated by the microbial decay of plants and vegetables, whose characteristics vary from one water source to another. It has a wide range of distributions in molecular weight (MW) and functional groups (phenolic, hydroxyl, and carboxyl groups), which originate from allochthonous (e.g., terrestrial and vegetative debris) and autochthonous (e.g., algae) inputs. Normally, NOM can be fractionated into three portions [21, 34, 35]: hydrophobic, hydrophilic, and transphilic fractions. As shown in Fig. 1, the hydrophobic fraction represents almost 50% of dissolved organic carbon (DOC). The hydrophilic fraction, which is non-humic, accounts for 25%–40% of DOC. The transphilic fraction occupies approximately 25% of the DOC in the natural water carbon pool. It has been reported that a major fraction of NOM derives from humic substances and corresponds to 60%–70% of total organic carbon (TOC) in soils and 60%–90% of the DOC in most natural waters [36]. Some NOM fractions (e.g., polysaccharide or protein-like substances) are more biodegradable than humic substances [37]. NOM itself is not harmful, but it can react with disinfectants to form carcinogenic disinfection by-products [38].
3.2. NOM Fouling MechanismsNOM is thought to be a primary component of membrane fouling, either acting alone or in concert with metals and particles [33, 39–42]. Although NOM fouling has been substantially studied, its mechanisms are not yet clear. As demonstrated in previous reports [20, 35, 43–45], factors potentially affecting membrane fouling caused by NOM include the following: the nature of NOM, solution characteristics, membrane properties, and system operation conditions. More specifically, the nature of NOM is related to its molecular size, hydrophobicity, charge density, and isoelectric point. The physicochemical properties of membranes include hydrophobicity, charge density, surface roughness, and porosity. The characteristics of the solution phase, such as pH, ionic strength, and specific metals, can affect the NOM-membrane interaction [46]. Finally, the hydrodynamics of membrane systems can control the transport of NOM molecules at the membrane surface as well.
The combined effects of the above factors result in more severe NOM fouling caused by either concentration polarization or pore blocking, which depends on the size of membrane pores and NOM molecules. Pore blocking and cake formation were taken into account to describe humic acid fouling during MF [47, 48]. The results demonstrated that the initial fouling rate was attributed to pore blocking caused by the deposition of large aggregates on the surface of the membrane. After that, a humic deposit layer started to build up on the membrane surface. Similarly, the fouling of a UF membrane by NOM was caused by narrowing of pores at the mouth followed by coverage of the membrane surface by a cake layer [21, 22, 49, 50]. The following fouling mechanisms were proposed: first, membrane pores are narrowed or covered with relatively large particles; then, small particles block the narrowed pores; and finally, a cake layer begins to form on the membrane surface [51, 52]. A previous study reported that cake formation was a dominant fouling mechanism, in NOM fouling of the UF membrane in crossflow mode [39]. Another report suggested that fouling by alginate was caused by pore blockage, and then followed by buildup of a cake layer; while fouling by humic acids occurred due to adsorption [40–42].
NOM fouling mechanisms can thus be summarized into the following three categories (Fig. 2): 1) NOM may attach to the membrane pores and narrow or block the permeation channel; 2) it may block the pores by accumulating a gel or cake layer on the membrane surface; or 3) NOM may bind particles to form a less permeable deposit layer at the membrane surface.
4. NOM Fouling Control Methods
Table 1 summarizes the performances of NOM removal and fouling control achieved by the various control technologies available. The collected information may depend on the types of water sources and operating conditions to be tested, but it implies that treatment options using ozone, chlorine, anion exchange resins, and PAC are not always effective in fouling control, even though they achieve marked NOM removals. Detailed information and discussion are provided in the subsequent sections.
4.1. Chemical CoagulationCoagulation is one of the most common pretreatments for controlling the fouling of UF membranes in water treatment. Several types of coagulants are available in the commercial market [53–56]: inorganic metal salts (e.g., Al or Fe salts) or polymers (e.g., polyaluminum chloride [PACl]) and organic macromolecules (mostly polyelectrolytes). Al or Fe salts added into water react with particles and water molecules, and thus form larger flocs as well as hydroxide precipitates. The combination of charge neutralization and sweep flocculation is essential for effective coagulation, while the coagulation of negatively charged NOM moieties is possibly achieved via their adsorption to Al or Fe precipitates or metal-NOM complexation. PACl coagulants were also compared with Al and Fe salt coagulants in terms of fouling control [55]. The degree of fouling control was found to be a function of the NOM removal by coagulation and the filterability of coagulated particles. Ordinary coagulation was better than in-line coagulation in fouling control during UF. Another important finding was the fact that in-line coagulation substantially mitigated membrane fouling caused by pore narrowing and cake formation, when compared to direct UF [57]. In addition, the types of Fe salt and organic polymer coagulants (e.g., cationic polyamines) were compared and found to have different effects on fouling control [58]. Both coagulated floc size and its mechanical strength against applied shear were critical in UF fouling mitigation. Although the combined coagulation/MF and carbon adsorption/MF processes showed similar performances for TOC removal, coagulation/MF had a significantly lesser flux decline [56].
Coagulation was always effective in removing NOM, but occasionally it exacerbated fouling, presumably because either metal-NOM complexes or metal hydroxide colloids, formed during coagulation, act as secondary foulants [58, 59]. The addition of coagulants enhanced the flux of an UF membrane, but not for an MF membrane [59, 60]. It was found that small molecular weight, non-ionic, hydrophilic NOM that were poorly removed by coagulation were still responsible for fouling after the coagulation process [61]. This might also be associated with other findings that the removal of NOM was highly affected by the pH condition of coagulation [62, 63].
4.2. Chemical OxidationIn conventional water treatment, it has been demonstrated that chlorine and permanganate added to feedwater were able to suppress the growth of microorganisms and to maintain oxidative conditions in the water. Preoxidation using ozone has been found to improve the removal efficiency of organics or particles in secondary effluent, but not that significantly the organics (e.g., NOM) in river water [64, 65]. It is possible that ozone degrades or modifies organic matter that can foul the membrane; but fouling mitigation appeared to be dependent on the types of feedwater. Another critical issue of ozone injection is that the residual ozone can damage the polymeric membrane used. Thus, either ozone-resistant ceramic membranes should be employed [66, 67]; or the residual ozone should be destroyed in advance.
The presence of metal species in the feedwater may give an adverse effect on the preoxidative fouling control. It was found that prechlorination caused the precipitation of Fe and Mn species on the surface of MF and UF membranes, which led to severe fouling [68, 69]. The addition of chlorine into permeate was also problematic leading to manganese oxide fouling inside the pore during backwashing, when the feedwater contained Mn2+ ions. A large dose of chlorine formed a denser cake layer on top of the membrane, because the chlorine added played a role in reducing the size of colloidal particles present in feedwater, and hence caused more serious membrane fouling [5, 69].
TiO2 mediated photocatalysis enabled significant removal of NOM (including humic acids in natural and synthetic waters) and secondary effluent organic matter, and thus organics fouling during MF/UF was significantly reduced [6, 26]. However, the interaction of colloids with TiO2 photocatalysts caused an unexpected decline in membrane permeability for a specific type of source water containing small particles with a size of <40 μm [7]. The photocatalytic degradation of seawater organic matter was not achievable due to the hydroxyl radical scavenging effect by chloride ions [70].
4.3. Anion Exchange Resin ProcessAnion exchange processes seem to be an effective solution for the removal of NOM that is normally negatively charged [71–77]. The application of magnetic ion-exchange resins (MIEX) to membrane filtration has been studied. The magnetic resin with quaternary ammonia functional groups is effective in removing anionic substances in natural waters. Pretreatments using MIEX resins can increase NOM removal, but the fouling control of an UF membrane was questionable [78, 79]. It was found that MIEX resins selectively adsorbed the low- and medium-MW fractions of the NOM, but they had relatively little effect on the sorption of high-MW molecules, which are more responsible for membrane fouling [80, 81]. Combined pretreatment methods, such as MIEX + alum and MIEX + PAC + alum, removed the majority DOC of all MW ranges and successfully prevented short-term MF fouling; whereas, these treatments did not remove the colloidal components [82]. The addition of coagulants after ion exchange resin treatment seems to be illogical, but may actually help reduce membrane fouling [81].
4.4. Activated Carbon AdsorptionPowdered activated carbon (PAC) was one of the most popular adsorbents used in combination with MF or UF. The efficacy of PAC in removing organic contaminants depends on the type and dose of PAC, the properties of organics, and water chemistries. Granular activated carbon also effectively reduced irreversible fouling of some UF membranes in surface water treatment [83, 84]. However, it was found that PAC pretreatments for UF membrane systems cannot reduce membrane fouling [85–87]. It was demonstrated that PAC preferentially adsorbed low-MW, non-fouling molecules; and thus PAC-treated humic acid solution still fouled UF membranes as the untreated solution did. Other findings supported the fact that PAC adsorbed non-fouling molecules preferentially over foulants [23, 88]. In fact, PAC was ineffective for removing highly hydrophilic extracellular organic matter [89], even though it can remove hydrophobic synthetic organic chemicals well. Carbons are still relatively costly and create sludge that should be disposed of.
4.5. Mineral Oxide AdsorptionSeveral mineral oxide particles have been developed and tested as adsorbents for NOM removal in place of carbon based adsorbents [11, 90–92]. HIOPs and heated aluminum oxide particles (HAOPs) have advantages over Fe and Al salt coagulants with regard to NOM removal and fouling control [8–11, 93, 94]. In those studies, HIOPs/HAOPs inhibited the growth of a NOM gel layer on the membrane surface serving as a protective, secondary dynamic layer on top of the membrane. In addition to HIOPs and HAOPs, freshly precipitated ferrihydrite particles also effectively removed NOM from natural water; and they also substantially reduced membrane fouling, through either their addition to feed or pre-deposition on the membrane surface [3,4].
Some studies focused on the removal of anion contaminants (e.g., arsenate, phosphate, and NOM) using a granular iron oxide adsorbent (e.g., granular ferric hydroxide [GFH]) in a fixed-bed filter [95, 96]. Adsorption onto GFH with subsequent in situ regeneration was studied as a new process for NOM removal from groundwater [97]. However, little information is available in the literature regarding NOM adsorption by GFH and membrane fouling control. More recently, iron oxide coated polymer beads were employed prior to UF, and thus achieved a substantial fouling reduction with moderate NOM removal [92].
The adsorption of NOM on iron oxide surfaces is explained by the following mechanisms: surface complexation-ligand exchange, hydrophobic interaction, entropic effect, hydrogen bonding, and cation bridging. Among these proposed mechanisms, surface complexation-ligand exchange reactions (i.e., surface coordination) are most widely accepted. Fig. 3 illustrates the ligand exchange reactions between several different functional groups (e.g., carboxyl, benzoic, and phenolic groups) of NOM and iron oxide surfaces under charge neutral conditions [91, 98–100]. The solution pH level plays a crucial role in the surface complexation-ligand exchange reactions, because the hydroxide groups on the iron oxide surface act as diprotic acids creating three different surface charges: ≡FeOH2+, ≡FeOH, and ≡FeO−. In addition, the charges of functional groups in the NOM are pH-dependent. Thus, anionic functional groups of NOM (e.g., carboxyl and phenolic groups) undergo site-specific NOM adsorption onto oxide surfaces [101, 102].
The hydrophobic fraction of NOM with high molecular weights was better sorbed on iron oxide surfaces than those with low molecular hydrophilic fractions [91, 98–100]. Acidic fractions of NOM, which is rich in aromatic carbon moieties, were found to preferentially adsorb onto iron oxide surfaces especially under the low pH condition that is lower than the point of zero charges (i.e., pH < pHzpc) [98, 102]. These findings show that hydrophobic interactions enhance sorptive NOM removal along with surface complexation.
5. ConclusionsLow-pressure membrane filtration processes have been increasingly popular for water treatment; but the impairment of membrane permeability over time is the major bottleneck that should be resolved. Heterogeneously dispersed NOM molecules, composed of humic substances, carbohydrates, and amino acids, are key constituents to membrane fouling either alone or in combination with metals and particles. Pore narrowing and blocking, followed by surface coverage and cake formation, seem to be a plausible mechanism of NOM fouling during low-pressure membrane filtration.
Coagulation pretreatments improved membrane fluxes, but the types (e.g., Fe and Al salts, inorganic and organic polymers), dosages, and dosing methods (e.g., ordinary or in-line) of coagulants had different impacts on fouling control. Preoxidation using ozone helped reduce fouling, but residual ozone should be quenched, unless ozone-resistant membranes are employed. Metal precipitation by oxidation (e.g., Mn2+) can bring a negative influence on water permeability. Photocatalytic oxidation contributed to fouling reduction, but its efficacy depended on the content of ionic species (e.g., Cl−) that can scavenge hydroxyl radicals. Anion exchange resins (e.g., MIEX) were able to remove low to medium molecular weight NOM, but their effect was marginal. Although activated carbons always removed some fraction of NOM, they occasionally exacerbated fouling due to the formation of denser NOM laden carbon layers on the membrane. In contrast, mineral oxide adsorbents (such as iron oxide and aluminum oxide) always helped reduce fouling. Unlike activated carbon adsorbents, mineral oxide particles appeared to capture relatively large sized NOM components, which might otherwise tightly block membrane pores.
This review thus sheds insight on what pretreatment options would be most appropriate for NOM fouling control. A hybridization of mineral oxide adsorption with low-pressure membrane filtration enables the prevention or minimization of unwanted NOM fouling most efficiently. Further investigations on the optimal design and operation of such process combinations are needed considering source water qualities, membrane properties, adsorbent regeneration, and system operation flexibilities.
AcknowledgmentsThis research was supported by a project (No. 2013001390002) from the Korean Environmental Industry & Technology Institute funded by the Korean Ministry of Environment.
References1. Huang H, Schwab K, Jacangelo JG. Pretreatment for low pressure membranes in water treatment: a review. Environ. Sci. Technol. 2009;43:3011–3019.
2. Yuan W, Zydney AL. Effects of solution environment on humic acid fouling during microfiltration. Desalination. 1999;122:63–76.
3. Choo KH, Kang SK. Removal of residual organic matter from secondary effluent by iron oxides adsorption. Desalination. 2003;154:139–146.
4. Lee KW, Choo KH, Choi SJ, Yamamoto K. Development of an integrated iron oxide adsorption/membrane separation system for water treatment. Water Sci. Technol. Water Supply. 2002;2:293–300.
5. Ha TW, Choo KH, Choi SJ. Effect of chlorine on adsorption/ultrafiltration treatment for removing natural organic matter in drinking water. J. Colloid Interface Sci. 2004;274:587–593.
6. Choo KH, Tao R, Kim MJ. Use of a photocatalytic membrane reactor for the removal of natural organic matter in water: effect of photoinduced desorption and ferrihydrite adsorption. J. Membr. Sci. 2008;322:368–374.
7. Yao P, Choo KH, Kim MH. A hybridized photocatalysis-microfiltration system with iron oxide-coated membranes for the removal of natural organic matter in water treatment: effects of iron oxide layers and colloids. Water Res. 2009;43:4238–4248.
8. Chang YJ, Choo KH, Benjamin MM, Reiber S. Combined adsorption-UF process increases TOC removal. J. Am. Water Works Assoc. 1998;90:90–102.
9. Zhang M, Li C, Benjamin MM, Chang Y. Fouling and natural organic matter removal in adsorbent/membrane systems for drinking water treatment. Environ. Sci. Technol. 2003;37:1663–1669.
10. Kim J, Cai Z, Benjamin MM. Effects of adsorbents on membrane fouling by natural organic matter. J. Membr. Sci. 2008;310:356–364.
11. Cai Z, Kim J, Benjamin MM. NOM removal by adsorption and membrane filtration using heated aluminum oxide particles. Environ. Sci. Technol. 2008;42:619–623.
12. Kim J, Cai Z, Benjamin MM. NOM fouling mechanisms in a hybrid adsorption/membrane system. J. Membr. Sci. 2010;349:35–43.
13. Chi FH, Amy GL. Kinetic study on the sorption of dissolved natural organic matter onto different aquifer materials: the effects of hydrophobicity and functional groups. J. Colloid Interface Sci. 2004;274:380–391.
14. Kitis M, Kaplan SS, Karakaya E, Yigit NO, Civelekoglu G. Adsorption of natural organic matter from waters by iron coated pumice. Chemosphere. 2007;66:130–138.
15. Kitis M, Kaplan SS. Advanced oxidation of natural organic matter using hydrogen peroxide and iron-coated pumice particles. Chemosphere. 2007;68:1846–1853.
16. Ding C, Yang X, Liu W, Chang Y, Shang C. Removal of natural organic matter using surfactant-modified iron oxide-coated sand. J. Hazard. Mater. 2010;174:567–572.
17. Foppen JW, Liem Y, Schijven J. Effect of humic acid on the attachment of Escherichia coli in columns of goethite-coated sand. Water Res. 2008;42:211–219.
18. Benjamin MM, Sletten RS, Bailey RP, Bennett T. Sorption and filtration of metals using iron-oxide-coated sand. Water Res. 1996;30:2609–2620.
19. Lai CH, Chen CY. Removal of metal ions and humic acid from water by iron-coated filter media. Chemosphere. 2001;44:1177–1184.
20. Lee EK, Chen V, Fane AG. Natural organic matter (NOM) fouling in low pressure membrane filtration: effect of membranes and operation modes. Desalination. 2008;218:257–270.
21. Lee N, Amy G, Croue JP, Buisson H. Identification and understanding of fouling in low-pressure membrane (MF/UF) filtration by natural organic matter (NOM). Water Res. 2004;38:4511–4523.
22. Lee N, Amy G, Croue JP. Low-pressure membrane (MF/UF) fouling associated with allochthonous versus autochthonous natural organic matter. Water Res. 2006;40:2357–2368.
23. Lee N, Amy G, Croue JP, Buisson H. Morphological analyses of natural organic matter (NOM) fouling of low-pressure membranes (MF/UF). J. Membr. Sci. 2005;261:7–16.
24. Lee SJ, Choo KH, Lee CH. Conjunctive use of ultrafiltration with powdered activated carbon adsorption for removal of synthetic and natural organic matter. J. Ind. Eng. Chem. 2000;6:357–364.
25. Huang H, Lee N, Young T, Gary A, Lozier JC, Jacangelo JG. Natural organic matter fouling of low-pressure, hollow-fiber membranes: effects of NOM source and hydrodynamic conditions. Water Res. 2007;41:3823–3832.
26. Lee SA, Choo KH, Lee CH, et al. Use of ultrafiltration membranes for the separation of TiO2 photocatalysts in drinking water treatment. Ind. Eng. Chem. Res. 2001;40:1712–1719.
27. Qu F, Liang H, Zhou J, et al. Ultrafiltration membrane fouling caused by extracellular organic matter (EOM) from Micro-cystis aeruginosa: effects of membrane pore size and surface hydrophobicity. J. Membr. Sci. 2014;449:58–66.
28. Katsoufidou K, Yiantsios SG, Karabelas AJ. A study of ultrafiltration membrane fouling by humic acids and flux recovery by backwashing: experiments and modeling. J. Membr. Sci. 2005;266:40–50.
29. Katsoufidou K, Yiantsios SG, Karabelas AJ. An experimental study of UF membrane fouling by humic acid and sodium alginate solutions: the effect of backwashing on flux recovery. Desalination. 2008;220:214–227.
30. Wray HE, Andrews RC, Berube PR. Surface shear stress and retention of emerging contaminants during ultrafiltration for drinking water treatment. Sep. Purif. Technol. 2014;122:183–191.
31. Wray HE, Andrews RC, Berube PR. Surface shear stress and membrane fouling when considering natural water matrices. Desalination. 2013;330:22–27.
32. Akhondi E, Wicaksana F, Fane AG. Evaluation of fouling deposition, fouling reversibility and energy consumption of submerged hollow fiber membrane systems with periodic backwash. J. Membr. Sci. 2014;452:319–331.
33. Kimura K, Hane Y, Watanabe Y, Amy G, Ohkuma N. Irreversible membrane fouling during ultrafiltration of surface water. Water Res. 2004;38:3431–3441.
34. Fan L, Harris JL, Roddick FA, Booker NA. Influence of the characteristics of natural organic matter on the fouling of microfiltration membranes. Water Res. 2001;35:4455–4463.
35. Zularisam AW, Ismail AF, Salim MR, Sakinah M, Ozaki H. The effects of natural organic matter (NOM) fractions on fouling characteristics and flux recovery of ultrafiltration membranes. Desalination. 2007;212:191–208.
36. Senesi N. Nature of interactions between organic chemicals and dissolved humic substances and the influence of environmental factors. Beck AJ, Jones KC, Hayes MH, editorsOrganic substances in soil and water: natural constituents and their influence on contaminant behavior. Cambridge: Royal Society of Chemistry; 1993. p. 73–101.
37. Huang G, Meng F, Zheng X, et al. Biodegradation behavior of natural organic matter (NOM) in a biological aerated filter (BAF) as a pretreatment for ultrafiltration (UF) of river water. Appl. Microbiol. Biotechnol. 2011;90:1795–1803.
38. Kim HC, Yu MJ. Characterization of natural organic matter in conventional water treatment processes for selection of treatment processes focused on DBPs control. Water Res. 2005;39:4779–4789.
39. Taniguchi M, Kilduff JE, Belfort G. Modes of natural organic matter fouling during ultrafiltration. Environ. Sci. Technol. 2003;37:1676–1683.
40. Jermann D, Pronk W, Meylan S, Boller M. Interplay of different NOM fouling mechanisms during ultrafiltration for drinking water production. Water Res. 2007;41:1713–1722.
41. Jermann D, Pronk W, Kagi R, Halbeisen M, Boller M. Influence of interactions between NOM and particles on UF fouling mechanisms. Water Res. 2008;42:3870–3878.
42. Jermann D, Pronk W, Boller M. Mutual influences between natural organic matter and inorganic particles and their combined effect on ultrafiltration membrane fouling. Environ. Sci. Technol. 2008;42:9129–9136.
43. Howe KJ, Clark MM. Fouling of microfiltration and ultrafiltration membranes by natural waters. Environ. Sci. Technol. 2002;36:3571–3576.
44. Yuan W, Zydney AL. Humic acid fouling during ultrafiltration. Environ. Sci. Technol. 2000;34:5043–5050.
45. Yu CH, Wu CH, Lin CH, Hsiao CH, Lin CF. Hydrophobicity and molecular weight of humic substances on ultrafiltration fouling and resistance. Sep. Purif. Technol. 2008;64:206–212.
46. Zularisam AW, Ahmad A, Sakinah M, Ismail AF, Matsuura T. Role of natural organic matter (NOM), colloidal particles, and solution chemistry on ultrafiltration performance. Sep. Purif. Technol. 2011;78:189–200.
48. Yuan W, Kocic A, Zydney AL. Analysis of humic acid fouling during microfiltration using a pore blockage-cake filtration model. J. Membr. Sci. 2002;198:51–62.
49. Costa AR, de Pinho MN, Elimelech M. Mechanisms of colloidal natural organic matter fouling in ultrafiltration. J. Membr. Sci. 2006;281:716–725.
50. Kim J, Shi W, Yuan Y, Benjamin MM. A serial filtration investigation of membrane fouling by natural organic matter. J. Membr. Sci. 2007;294:115–126.
51. Yamamura H, Chae S, Kimura K, Watanabe Y. Transition in fouling mechanism in microfiltration of a surface water. Water Res. 2007;41:3812–3822.
52. Yamamura H, Kimura K, Watanabe Y. Mechanism involved in the evolution of physically irreversible fouling in microfiltration and ultrafiltration membranes used for drinking water treatment. Environ. Sci. Technol. 2007;41:6789–6794.
53. Haberkamp J, Ruhl AS, Ernst M, Jekel M. Impact of coagulation and adsorption on DOC fractions of secondary effluent and resulting fouling behaviour in ultrafiltration. Water Res. 2007;41:3794–3802.
54. Guigui C, Rouch JC, Durand-Bourlier L, Bonnelye V, Aptel P. Impact of coagulation conditions on the in-line coagulation/UF process for drinking water production. Desalination. 2002;147:95–100.
55. Park P, Lee C, Choi SJ, Choo KH, Kim SH, Yoon CH. Effect of the removal of DOMs on the performance of a coagulation-UF membrane system for drinking water production. Desalination. 2002;145:237–245.
56. Tran T, Gray S, Naughton R, Bolto B. Polysilicato-iron for improved NOM removal and membrane performance. J. Mem-br. Sci. 2006;280:560–571.
57. Wang J, Wang XC. Ultrafiltration with in-line coagulation for the removal of natural humic acid and membrane fouling mechanism. J. Environ. Sci. (China). 2006;18:880–884.
58. Barbot E, Moustier S, Bottero JY, Moulin P. Coagulation and ultrafiltration: understanding of the key parameters of the hybrid process. J. Membr. Sci. 2008;325:520–527.
59. Lee BB, Choo KH, Chang D, Choi SJ. Optimizing the coagulant dose to control membrane fouling in combined coagulation/ultrafiltration systems for textile wastewater reclamation. Chem. Eng. J. 2009;155:101–107.
60. Maartens A, Swart P, Jacobs EP. Feed-water pretreatment: methods to reduce membrane fouling by natural organic matter. J. Membr. Sci. 1999;163:51–62.
61. Carroll T, King S, Gray SR, Bolto BA, Booker NA. The fouling of microfiltration membranes by NOM after coagulation treatment. Water Res. 2000;34:2861–2868.
62. Kabsch-Korbutowicz M. Application of ultrafiltration integrated with coagulation for improved NOM removal. Desalination. 2005;174:13–22.
63. Kimura K, Maeda T, Yamamura H, Watanabe Y. Irreversible membrane fouling in microfiltration membranes filtering coagulated surface water. J. Membr. Sci. 2008;320:356–362.
64. Song Y, Dong B, Gao N, Xia S. Huangpu River water treatment by microfiltration with ozone pretreatment. Desalination. 2010;250:71–75.
65. Wang X, Wang L, Liu Y, Duan W. Ozonation pretreatment for ultrafiltration of the secondary effluent. J. Membr. Sci. 2007;287:187–191.
66. Karnik BS, Davies SH, Chen KC, Jaglowski DR, Baumann MJ, Masten SJ. Effects of ozonation on the permeate flux of nano-crystalline ceramic membranes. Water Res. 2005;39:728–34.
67. Oh BS, Jang HY, Hwang TM, Kang JW. Role of ozone for reducing fouling due to pharmaceuticals in MF (microfiltration) process. J. Membr. Sci. 2007;289:178–186.
68. Chae SR, Yamamura H, Ikeda K, Watanabe Y. Comparison of fouling characteristics of two different poly-vinylidene fluoride microfiltration membranes in a pilot-scale drinking water treatment system using pre-coagulation/sedimentation, sand filtration, and chlorination. Water Res. 2008;42:2029–2042.
69. Choo KH, Lee H, Choi SJ. Iron and manganese removal and membrane fouling during UF in conjunction with pre-chlorination for drinking water treatment. J. Membr. Sci. 2005;267:18–26.
70. Kim MJ, Choo KH, Park HS. Photocatalytic degradation of seawater organic matter using a submerged membrane reactor. J. Photochem. Photobiol. A Chem. 2010;216:215–220.
71. Bolto B, Dixon D, Eldridge R, King S. Removal of THM precursors by coagulation or ion exchange. Water Res. 2002;36:5066–5073.
72. Bolto B, Dixon D, Eldridge R, King S, Linge K. Removal of natural organic matter by ion exchange. Water Res. 2002;36:5057–5065.
73. Bolto B, Dixon D, Eldridge R. Ion exchange for the removal of natural organic matter. React. Funct. Polym. 2004;60:171–182.
74. Fearing DA, Banks J, Guyetand S, et al. Combination of ferric and MIEX for the treatment of a humic rich water. Water Res. 2004;38:2551–2558.
75. Cornelissen ER, Moreau N, Siegers WG, et al. Selection of anionic exchange resins for removal of natural organic matter (NOM) fractions. Water Res. 2008;42:413–423.
76. Cornelissen ER, Beerendonk EF, Nederlof MN, van der Hoek JP, Wessels LP. Fluidized ion exchange (FIX) to control NOM fouling in ultrafiltration. Desalination. 2009;236:334–341.
77. Han J, Kong C, Heo J, Yoon Y, Lee H, Her N. Removal of perchlorate using reverse osmosis and nanofiltration membranes. Environ. Eng. Res. 2012;17:185–190.
78. Kabsch-Korbutowicz M, Majewska-Nowak K, Winnicki T. Water treatment using MIEX®DOC/ultrafiltration process. Desalination. 2008;221:338–344.
79. Huang H, Cho HH, Schwab KJ, Jacangelo JG. Effects of magnetic ion exchange pretreatment on low pressure membrane filtration of natural surface water. Water Res. 2012;46:5483–5490.
80. Humbert H, Gallard H, Suty H, Croue JP. Performance of selected anion exchange resins for the treatment of a high DOC content surface water. Water Res. 2005;39:1699–1708.
81. Humbert H, Gallard H, Jacquemet V, Croue JP. Combination of coagulation and ion exchange for the reduction of UF fouling properties of a high DOC content surface water. Water Res. 2007;41:3803–3811.
82. Fabris R, Lee EK, Chow CWK, Chen V, Drikas M. Pre-treatments to reduce fouling of low pressure micro-filtration (MF) membranes. J. Membr. Sci. 2007;289:231–240.
83. Tsujimoto W, Kimura H, Izu T, Irie T. Membrane filtration and pre-treatment by GAC. Desalination. 1998;119:323–326.
84. Kim KY, Kim HS, Kim J, Nam JW, Kim JM, Son S. A hybrid microfiltration-granular activated carbon system for water purification and wastewater reclamation/reuse. Desalination. 2009;243:132–144.
85. Lin CF, Huang YJ, Hao OJ. Ultrafiltration processes for removing humic substances: effect of molecular weight fractions and PAC treatment. Water Res. 1999;33:1252–1264.
86. Li K, Qu F, Liang H, et al. Performance of mesoporous adsorbent resin and powdered activated carbon in mitigating ultrafiltration membrane fouling caused by algal extracellular organic matter. Desalination. 2014;336:129–137.
87. Uyak V, Akdagli M, Cakmakci M, Koyuncu I. Natural organic matter removal and fouling in a low pressure hybrid membrane systems. Sci. World J. 2014;2014:11.
88. Kang SK, Choo KH. Why does a mineral oxide adsorbent control fouling better than powdered activated carbon in hybrid ultrafiltration water treatment? J. Membr. Sci. 2010;355:69–77.
89. Campinas M, Rosa MJ. Assessing PAC contribution to the NOM fouling control in PAC/UF systems. Water Res. 2010;44:1636–1644.
90. Ding C, Shang C. Mechanisms controlling adsorption of natural organic matter on surfactant-modified iron oxide-coated sand. Water Res. 2010;44:3651–3658.
91. Gu B, Schmitt J, Chen Z, Liang L, McCarthy JF. Adsorption and desorption of natural organic matter on iron oxide: mechanisms and models. Environ. Sci. Technol. 1994;28:38–46.
92. Cui X, Choo KH. Granular iron oxide adsorbents to control natural organic matter and membrane fouling in ultrafiltration water treatment. Water Res. 2013;47:4227–4237.
93. Kim J, Deng Q, Benjamin MM. Simultaneous removal of phosphorus and foulants in a hybrid coagulation/membrane filtration system. Water Res. 2008;42:2017–2024.
94. Shi W, Benjamin MM. Membrane interactions with NOM and an adsorbent in a vibratory shear enhanced filtration process (VSEP) system. J. Membr. Sci. 2008;312:23–33.
95. Sperlich A, Werner A, Genz A, Amy G, Worch E, Jekel M. Breakthrough behavior of granular ferric hydroxide (GFH) fixed-bed adsorption filters: modeling and experimental approaches. Water Res. 2005;39:1190–1198.
96. Sperlich A, Schimmelpfennig S, Baumgarten B, et al. Predicting anion breakthrough in granular ferric hydroxide (GFH) adsorption filters. Water Res. 2008;42:2073–2082.
97. Genz A, Baumgarten B, Goernitz M, Jekel M. NOM removal by adsorption onto granular ferric hydroxide: equilibrium, kinetics, filter and regeneration studies. Water Res. 2008;42:238–248.
98. Gu B, Schmitt J, Chen Z, Liang L, McCarthy JF. Adsorption and desorption of different organic matter fractions on iron oxide. Geochim. Cosmochim. Acta. 1995;59:219–229.
99. Gu B, Mehlhorn TL, Liang L, McCarthy JF. Competitive adsorption, displacement, and transport of organic matter on iron oxide: I. Competitive adsorption. Geochim. Cosmochim. Acta. 1996;60:1943–1950.
100. Gu B, Mehlhorn TL, Liang L, McCarthy JF. Competitive adsorption, displacement, and transport of organic matter on iron oxide: II. Displacement and transport. Geochim. Cosmochim. Acta. 1996;60:2977–2992.
Fig. 1A distribution of natural organic matter fractions present in surface water based on dissolved organic carbon (reproduced with permission from [35]). 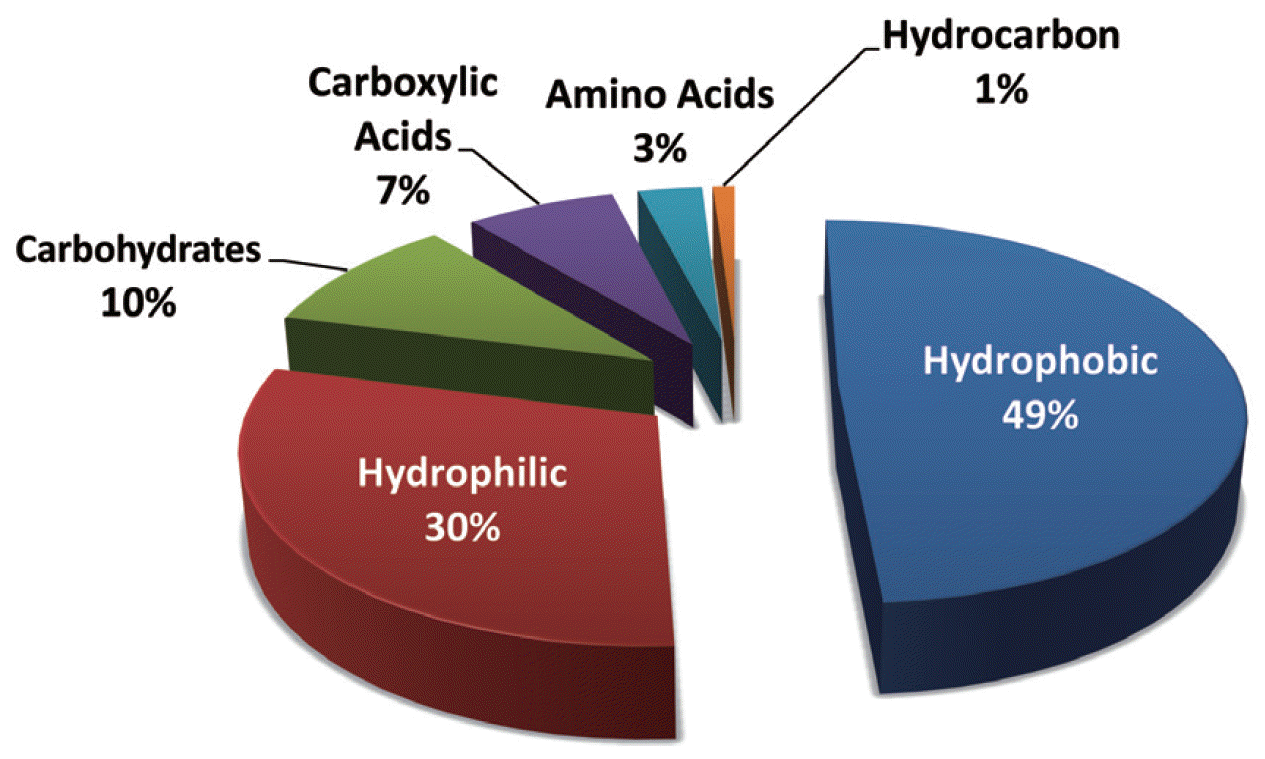
Fig. 2Schematic illustrations of natural organic matter (NOM) fouling in low-pressure membrane filtration: (a) NOM attaching to the membrane pores, (b) NOM accumulating on the membrane surface, and (c) NOM bridging large particles at the membrane surface. 
Fig. 3Possible surface complexation-ligand exchange reactions between iron oxide and natural organic matter with (a) carboxyl, (b) benzoic, and (c) phenolic groups. All functional groups are assumed to have neutral forms in this depiction, but they vary depending on solution pH levels. 
Table 1Summary of the extent of NOM removal and fouling control in low-pressure membrane treatment systems, when various technological options were hybridized
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||