AbstractThe modification of biochar (BC) with metal/metal oxides is expected to improve its adsorption capacity to pollutants, especially anions dyes. A green chromium ferrite-biochar composites (CF-BC) was synthesized to enhance the adsorption efficiency of Congo red (CR) via a simple co-precipitation method. The samples were characterized by different characterization techniques: XRD, FT-IR, SEM, XPS, etc, which showed that chromium ferrite was successfully loaded on the surface of biochar. The influencing factors of adsorption and recycling properties were discussed, and the adsorption mechanisms such as kinetics, isotherm, thermodynamics were explored. The results show that CF-BC2 achieves a removal rate of 92.29% for CR and maintains a removal rate of 90% even after three cycles. The addition of ferrite not only promoted the adsorption effect, but also increased the magnetic property, making the adsorbents easy to recycle. The equilibrium and kinetic studies suggested that the adsorption process followed Freundlich isotherm and pseudo-second order model, respectively. Furthermore, a study into the adsorption mechanism revealed that CF-BC2 primarily achieves CR adsorption through electrostatic attraction, hydrogen bonding, π-π interactions, and pore filling.
Graphical Abstract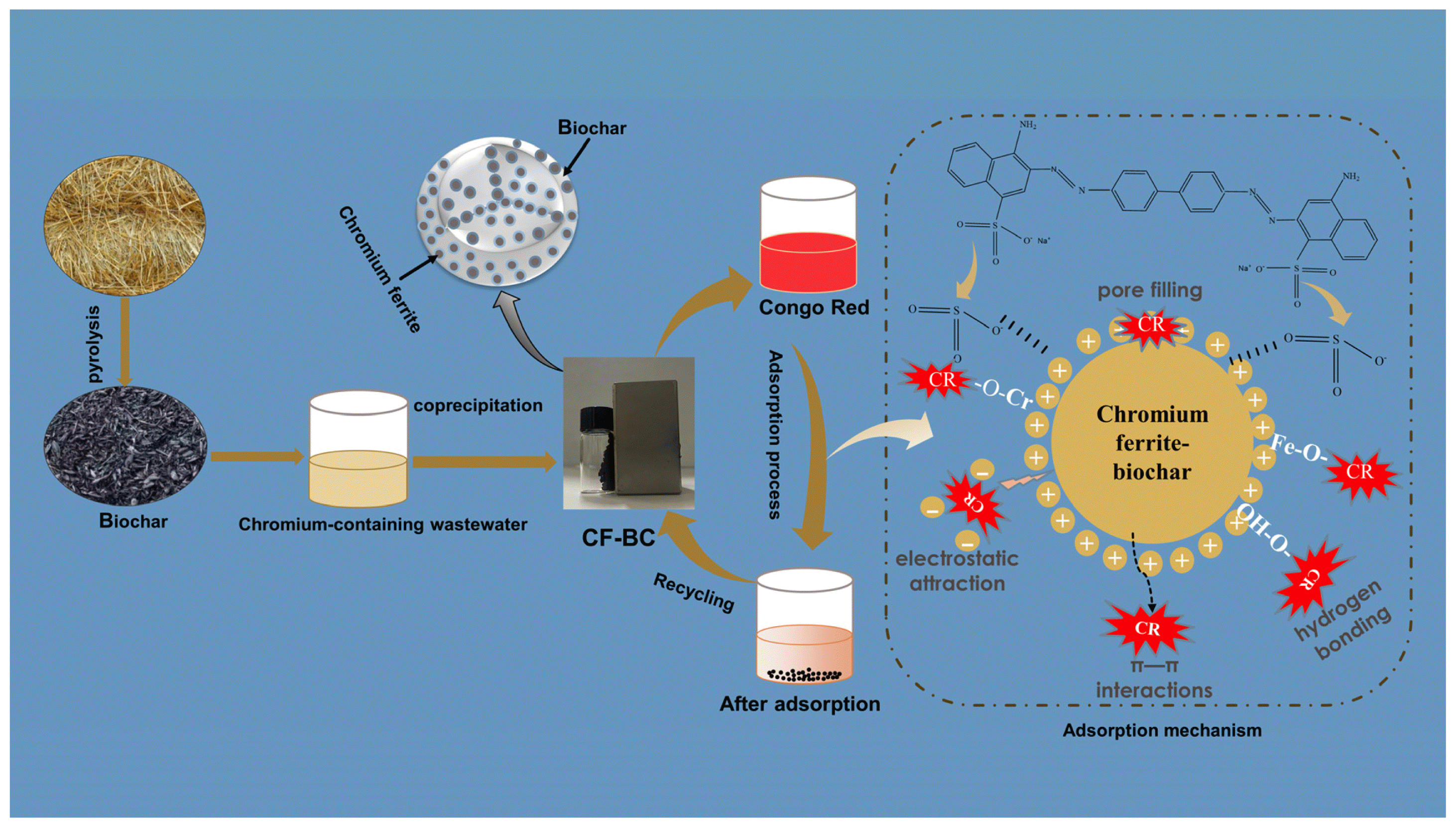
1. IntroductionDye wastewater constitutes one of the most environmentally hazardous types of wastewater. Both the structure and synthesis process of dyes have the potential to induce water pollution. Moreover, industrial-grade dyes possess complex compositions, thereby posing a threat to organisms even at low concentrations [1–4]. Congo Red (CR) is an anionic diazo dye based on the biphenylamine structure. Wastewaters containing CR originate from various industries such as textiles, printing, dyeing, papermaking, rubber, and plastics. Possessing physicochemical, thermal, and optical stability, this dye has been associated with genetic mutations as well as pulmonary and renal infections [5–8]. Due to its structural stability, CR proves resistant to degradation, thus necessitating the imminent development of a novel process for treating CR dye wastewater.
Industrial-grade dyes possess complex compositions, thereby posing a threat to organisms even at low concentrations [5–10]. Currently, various techniques for CR dye removal from wastewater have been extensively investigated, including membrane filtration [11], photocatalysis [12, 13], biodegradation [14–16] and adsorption etc. [11, 17, 18]. Among these, adsorption technology, characterized by its simplicity of operation, low cost, and high removal efficiency, has found wide applications in the treatment of organic dyes in wastewater [19].
Numerous materials have been developed as adsorbents, such as zeolites, metal-doped porous carbon materials, organic clay materials, biopolymer materials, carbon nanotube materials, and graphene, etc. [2, 9, 20–25]. Compared to other adsorbents, biomass-based adsorbents have been demonstrated to be suitable for the removal of various pollutants under different conditions, while also enabling the resourceful utilization of agricultural waste [10, 26]. As an important renewable resource, agricultural solid waste has tremendous potential and value for resource utilization [27]. Its reasonable recovery is not only beneficial to environmental protection, but also can create significant economic benefits. Due to the expansion of planting scale and the excessive use of fertilizers, a large amount of agricultural waste has been generated from plant sources (such as orange peels, wheat straw, corn stalks, rice straw, chestnut shells, etc.), and it difficult to handle them [28]. From the perspective of economy and health, agricultural solid waste can be used to prepare adsorbents to remove organic matter from wastewater, which not only further reduces costs, but also turns waste into treasure and reduces the disposal pressure of agricultural waste.
Crop residues, such as wheat straw, corn stalks, rice straw, etc., are low-cost and readily available biological waste materials, and the pyrolysis for producing biochar (BC) is an effective approach for the resource utilization of these residues. Additionally, BC, due to its well-developed pore structure, large specific surface area, and abundant functional groups, is widely employed for the removal of both organic and inorganic pollutants [29–31]. However, one drawback of BC as a separate adsorbent is its difficulty in recovery and separation after use [31]. Therefore, it is necessary to modify the biochar and develop an environmentally friendly, sustainable, and easily recoverable biochar adsorbent material.
In recent years, magnetic nanoparticles and composite materials with tailored size, composition, magnetic properties, and structure have been employed for wastewater treatment [26, 32–37]. Ferrite, as a magnetic material, is commonly used for the treatment of organic and inorganic compounds, specific dyes, and bacteria in wastewater. Additionally, ferrite and its composites exhibit excellent magnetic properties, low cost, and compatibility, making them effective adsorbents for organic wastewater purification [38–41]. Based on our previous research [42], chromium-containing wastewater can be processed into magnetic chromite by co-precipitation [43], although chromite itself exhibits relatively weak adsorption performance and requires combination with other substances to enhance its adsorption capacity [44]. This deficiency is compensated for by the superior adsorption capability of biochar. Furthermore, compared to other adsorbents, ferrite-biochar composite materials demonstrate excellent dye adsorption performance and possess the high-efficiency magnetic separation characteristics of ferrite materials [33, 45, 46]. In recent years, biochar has been widely used as a low-cost adsorbent for dye removal from water [31, 47], however, the adsorption efficiency of ferrite-biochar composite is limited, and the recycling ability is poor [48]. Therefore, in this study, the ferrite precipitate generated from high-concentration chromium-containing wastewater treatment is combined with the biochar produced by pyrolysis of agricultural crop residues, aiming to prepare a recyclable composite adsorbent with excellent dye adsorption performance.
This study first utilizes the straw residues of wheat, rice, and corn, three major crops, to prepare BC, selecting the most suitable BC carrier through comparative analysis. Subsequently, based on previous research, the product from high-concentration chromium-containing wastewater treatment is loaded on the pyrolyzed BC, and a novel CF-BC composite is prepared using the co-precipitation method. The composite is then applied to the decolorization treatment of organic dye wastewater (CR), followed by an analysis of its purification mechanism. This study not only achieves the resourceful utilization of the ferrite precipitate from chromium-containing wastewater and agricultural waste residues, but also reduces the environmental impact of CR dye wastewater.
2. Materials and Methods2.1. ChemicalsAll chemicals are analytically pure and can be used without further purification. Deionized water (DI) is used to prepare an aqueous solution. Potassium dichromate (K2Cr2O7, ≥99wt%), ferrous sulfate heptahydrate (FeSO4·7H2O, ≥99wt%), sodium hydroxide (NaOH, ≥97wt%), hydrochloric acid (HCl, ≥98wt%), phosphoric acid ((H3PO4, ≥85wt%), dibenzoyldihydrazine (C13H14N4O, >98.0wt%), acetone (C3H6O, ≥99.5wt%), o-diazophene (C24H14N2Na2O6S2·3H2O, ≥98.0wt% (HPLC)), hydroxylamine hydrochloride (NH2OH·HCl, >99wt%) and sodium acetate (CH3COONa, >99wt%) were all purchased from Sinopsin Chemical Reagents Co., LTD.
2.2. Experimental Methods2.2.1. Preparation of biocharBiochar (BC) was prepared utilizing residues from three distinct agricultural crops. A comprehensive comparative analysis was conducted to select the most favorable option. Initially, corn, rice, and wheat straw were subjected to deionized water rinsing, followed by drying at 60°C. The dried material was subsequently pulverized and sieved to obtain straw particles with a diameter of 0.15 mm. Finally, employing these different straw powders as raw materials, BC was individually prepared through pyrolysis in a tube furnace under a nitrogen atmosphere. The pyrolysis temperatures employed in this study were 500°C, 600°C, and 700°C, each maintained for 2 hours to ensure consistent thermal condition.
2.2.2. Preparation of chromium ferrite-biochar compositeThe co-precipitation method was employed to synthesize chromium ferrite-biochar composite (CF-BC), utilizing BC as the carrier material. Firstly, the simulated wastewater containing Cr (VI) in the concentration of 1000 mg/L was prepared by dissolving K2Cr2O7 (Merck-1.04862) in deionized water. According to the designed molar ratio, the theoretical dosage of Fe2+ should be 5 times that of Cr6+ [42], FeSO4·7H2O was introduced into 50 mL of chromium-containing wastewater and stirred vigorously. After thorough mixing, 5 mol/L NaOH was added to adjust the pH to 10.5. Subsequently, different masses of BC were separately added, and the reaction mixture was stirred at 100°C. Upon completion of the reaction, the mixture was allowed to cool, followed by filtration using a sand core funnel and rinsing with deionized water until the solution reached neutrality. The resulting material was then dried in an electric constant temperature oven at 80°C for 24 hours. After grinding, the obtained powder was identified as the CF-BC composite. The composites prepared with BC amounts of 0.1 g, 0.2 g, and 0.3 g are respectively denoted as CF-BC1, CF-BC2, and CF-BC3.
2.2.3. Adsorption capacity and removal efficiencyTo simulate CR organic dye wastewater in the laboratory, 1 gram of Congo Red (CR) was dispersed in 1000 mL of deionized water, resulting in a concentration of 1000 mg/L of CR dye. Pre-measured adsorbents were then added to a specific volume of the CR solution. The entire experimental process was conducted in a constant-temperature shaking incubator at a rotation speed of 200 revolutions per minute (rpm) for adsorption treatment. Post-treatment, the organic wastewater was analyzed using a UV-visible spectrophotometer at the maximum wavelength of CR (λ = 497 nm) to measure the absorbance of the solution. Adsorption capacity and removal efficiency were calculated based on a standard curve [49].
Calculate the adsorption capacity based on the following Eq. (1)
Here, qe represents the adsorption capacity (mg/g), C0 and Ce denote the initial concentration and equilibrium concentration of CR, respectively (mg/L). “V” stands for the volume of the CR solution (L), and “m” represents the mass of the adsorbent (g).
The percentage of removal efficiency (R) is determined by Eq. (2):
2.3. Materials CharacterizationThe structural and crystalline characteristics of the samples were characterized using an X-ray diffractometer (D/max-2550, Rigaku Corporation, Japan) with the following instrument parameters: Cu Kα radiation (λ = 0.1789 nm), rated tube voltage of 20–40 kV, rated current of 10–450 mA, scanning speed of 8°/min, and scanning range of 2θ from 5 to 80 degrees. The obtained data were analyzed using Jade 6.0 software, and the material structures were compared with the ICDD-JCPDS card database to calculate the grain size. The Fourier transform infrared spectrum of the sample was characterized by an infrared spectrometer (Nicolet iS50, Thermo Fisher Technology Co., LTD.) with a resolution of 6 cm−1, a test range of 4000~400 cm−1, and 64 scanning times. OMNIC infrared analysis software was used to analyze the data. The surface morphology of the samples was analyzed using a scanning electron microscope (Gemini SEM 300, Zeiss, Germany). The acceleration voltage during morphology imaging was set to 3 kV, while it was 15 kV for energy spectrum mapping. The detector was SE2 secondary electronic detector. Automatic microporous physical adsorption analyzer (Autosorb-IO2, Canta Instruments, USA) was used to test the specific surface area and porosity of the sample, and the nitrogen adsorption-desorption isotherm of the sample was determined. Oscillating sample magnetometer (VSM, Model 7407, Lake Shore, USA) was used to measure the hysteresis curve and saturation magnetization of the sample at room temperature. The zero point of charge (pHpzc) of the adsorbents (BC, CF, and CF-BC2) was determined using the salt addition method.
3. Results and Discussion3.1. Characterization of BC and Chromium Ferrite/Biochar Composites3.1.1. Biochar selection and characterizationSpecific surface area is a crucial factor affecting the loading and adsorption efficiency of biochar (BC). The pyrolysis temperature of biochar can influence its adsorption capacity [50]. Therefore, the specific surface area of BC prepared from different agricultural crop residues (wheat, rice, corn [51]) at various pyrolysis temperatures was determined, as shown in Fig. S1(a). Under the preparation conditions of 500°C and 600°C, BC prepared from rice straw has a much larger specific surface area compared to that from wheat straw and maize straw. However, at 700°C, the specific surface area of BC prepared from rice straw decreases instead. Since BC prepared from rice straw at lower temperatures possesses a larger specific surface area, it is more suitable for loading and adsorption. As indicated in Fig. S1(b,c,d), BC prepared from wheat straw at 700°C, corn straw at 700°C, and rice straw at 600°C exhibit Type I adsorption isotherms, indicating a relatively abundant microporous structure. Other BC samples exhibit Type III adsorption isotherms, indicating weak monolayer adsorption and making them less suitable as adsorbents. Fig. S2 shows the adsorption effect of different biochar on CR under certain conditions. The adsorption effect of 700°C wheat straw, 700°C corn straw and 600°C rice straw is significantly better than that of other temperatures in biochar prepared from the same kind of straw. Considering the production cost, the energy consumption of biochar produced with straw as raw material at 600°C is lower. Also considering that China is a major producer of grain, with rice production ranking first, followed by wheat and maize, in order to minimize energy consumption, BC prepared from rice straw at 600°C was used for subsequent experiments.
3.1.2. X-ray diffraction and FT-IR of BC, CF and CF-BC compositesXRD Analysis revealed the crystalline phases of BC, CF and CF-BC composites (Fig. 1(a)). The presence of an amorphous peak around 24° indicates that BC is an amorphous material. The diffraction peak at around 44° corresponds to the graphite crystal structure of BC [52]. The diffraction peaks at 30.3°, 35.2°, 43.2°, 53.9°, 57.4°, and 63.0° correspond to the (220), (311), (400), (422), (511), and (440) hkl planes, respectively. By comparing the XRD data with standard diffraction patterns of spinel, it was confirmed that the characteristic peaks of FeCr2O4 were present.
FT-IR analysis was employed to determine the functional group structures in the composites. As shown in Fig. 1(b), it can be observed that the BC obtained from rice straw pyrolyzed at 600°C exhibits stretching vibration peaks of −OH at 3270 cm−1, a C=C vibration peak at 1589 cm−1, and absorption bands at 1419 and 1017 cm−1, which are mainly attributed to the stretching vibrations of −OH in alcohols or phenols and C-O-C in alcohol ethers or esters. The inherent vibrations of tetrahedral coordination (Fe-O) were observed in the range of 500–600 cm−1, while octahedral coordination (Cr-O) vibrations were detected in the range of 500–400 cm−1[53]. Two peaks corresponding to the metal-oxygen bonds of tetrahedral and octahedral sites appeared at 543 cm−1 (ν1) and 441 cm−1 (ν2), respectively, which closely matched the typical vibrations of spinel ferrite. Additionally, a stretching vibration peak of −OH at 3357 cm−1, stretching vibration of C=C in the composite at 1611 cm−1, and stretching vibration of C-O-C in alcohols, ethers, or esters at 1075 cm−1 were observed, confirming the successful deposition of cubic spinel-type CF on the surface of BC.
3.1.3. Magnetic analysis of CF and CF-BC2 composites
Table S1 and S2 show the elemental analysis of BC and CF-BC composites with different BC doping amounts. The element content of BC is diverse, and CF-BC composite is mainly composed of C, O, Fe and Cr, of which Fe content is the highest. CF brings a large amount of iron to the complex, making it easier to magnetic separation and recovery. In addition, because the amount of CF is fixed, the available active sites are limited. With the increase of BC content, Fe and Cr contents in CF-BC2 and CF-BC3 decreased significantly compared with CF-BC1. In Fig. 1(c), with the increase of BC content, the saturation magnetization of CF-BC1, CF-BC2 and CF-BC3 is 31.33, 25.59 and 23.45 emu/g, respectively. However, the saturation magnetization of CF is 36emu/g, and this decrease is attributed to the dilution effect of non-magnetic BC on magnetic nanoparticles [45]. Magnetic biochar with superparamagnetization can be quickly recovered and reused under the action of applied magnetic field without loss of active site, which provides a basis for the recovery and reuse of adsorbent [26]. The magnetic hysteresis loop of CF showing S-type is consistent with the typical characteristics of spinel ferrite materials. As BC is non-magnetic, the addition of CF makes the composite nanoparticles easy to be magnetized and demagnetized, thus forming soft magnetic materials. Therefore, the overall composite material still has considerable magnetic properties, which provides favorable conditions for subsequent recycling.
3.1.4. Micromorphology and pore evidenceIn order to better understand the microstructure of BC, CF and CF-BC composites, SEM tests were performed (Fig. 2 (a–f)). It can be observed that the surface of BC is relatively rough, showing an irregular porous structure, which is typical of agricultural waste-derived carbon materials (Fig. 2 (a)). In addition, the uneven size of pores on the BC surface is consistent with the previously determined large specific surface area. This provides potential for BC as a structural carrier of CF [54]. CF, on the other hand, consists of condensed magnetic nanoparticles. (Fig. 2 (c)) It can be seen that the diameter of CF particles is very small, and the magnetic properties of chromium ferrite make chromium ferrite exist in the form of clusters. For composite materials, SEM images enhance the results of XRD and FT-IR, demonstrating that chromium ferrite particles are successfully attached to the surface of biochar. But as the amount of CF added increases, the number of pores in the complex also decreases, which is more unfavorable to adsorption. Although CF-BC1 has more pores than CF-BC2, too large pores are not conducive to the adsorption of organic matter. However, SEM images of CF-BC2 composites showed that CF nanoparticles were uniformly dispersed in the pores of BC (Fig. 2 (e)), indicating that BC as a carrier could reduce aggregation and enhance the activity of CF particles [55]. This is because the presence of amorphous carbon with a lamellar structure facilitates the efficient separation of nanoparticles. This amorphous carbon inhibits the aggregation of nanoparticles by reducing the binding between particles, thereby increasing the available surface area of nanoparticles and further enhancing their adsorption capacity [56]. In addition, the uniform distribution and coexistence of C, O, Cr and Fe elements were clearly observed on the CF-BC2 surface from the EDS mapping (Fig. 2 (g)). The finding affirmed that ferrite was grown on the surface of BC, in which the low agglomeration phenomenon might make composites possess more active sites and show better adsorption stability.
To further confirm the pore analysis in SEM, the nitrogen adsorption/desorption isotherm and pore size distribution of CF-BC2 composite are shown in Fig. S3(a) and (b), respectively. It can be seen from Fig. S3(a) that the adsorption of CF and CF-BC2 composites follows the Type IV adsorption isotherm classified by IUPAC, which is the adsorption isotherm model for mesoporous materials. However, there is a bulge in the second half of the curve, and there may be an adsorption hysteresis loop in the middle part, corresponding to the system of capillary condensation of the porous adsorbent. From the type of hysteresis loop, it belongs to the H3 type [57], indicating that the adsorbent material is mesoporous material, indicating that the introduction of iron may destroy the micropore wall and increase the proportion of mesoporous biomass in biomaterials [58]. At the same time, the specific surface area of CF-BC2 is 100.93 m2/g, which is smaller than that of BC (141.67m2/g). This may be due to the fact that CF particles are loaded on the surface of BC during the preparation process and block part of the pores, but it is much higher than the surface area of CF 25.55 m2/g. This means that the addition of BC increases the specific surface area of the complex, which is more conducive to adsorption. As shown in Fig. S3(b), the pore size of CF-BC2 is distributed between 2 and 15 nm. The results showed that the pore volume and size increased, and the excellent mesoporous structure was conducive to the diffusion and adsorption of pollutants in biochar [59, 60].
3.1.5. XPS spectral analysis of CF and CF-BC compositesThe XPS wide scanning spectra of CF and the three compounds confirm that Fe, Cr, C and O are mainly present (Fig. 3). High-resolution XPS spectra of C-1s, Cr-2p and Fe-2p are further analyzed (Fig. 3(a)). The C-1S spectrum can be deconvolved into three peaks corresponding to C-C, C-O, and C=O(Fig. 3(b)). The highest intensity peaks at CF-BC are attributed to the graphite structure (C-C), indicating that the carbon components in CF-BC have a highly aromatic structure. The more graphite structure of biochar, the more pore structure, the more favorable for adsorption. After binding with biochar, the peak value of C-O in CF-BC1, CF-BC2 and CF-BC3 changed from 286.08 eV to 286.58 eV, 286.2 eV and 286.5 eV, with relative contents of 19%, 22% and 11%, respectively. By comparing the three compounds, It is observed that the C-O content of CF-BC3 is relatively small, and according to the peak area of C=O, the highest relative content of CF-BC1 is 17%, which is 11% more than the C=O content of CF alone. The C=O content of CF-BC complex gradually decreases, which is because the magnetic nanoparticles are doped in the non-magnetic BC. It is caused by the dispersion of BC to CF nanoparticles. In Fig. 3 (c, d), The Fe 2p peaks of CF-BC1, CF-BC2, and CF-BC3 can be attributed to Fe (III) at 711.73 eV, 711.8 eV, and 712.33 eV, and Fe (II) at 709.61 eV, 709.84eV, and 709.58eV. The Cr2p peaks of CF-BC1 are 575.78 eV and 585.51 eV, CF-BC2 are 575.68 eV and 585.93 eV, and CF-BC3 are 576.34 eV and 586.04 eV, which mainly contribute to the binding energy of Cr (III). The results indicated that Cr (III), Fe (III) and Fe (II) existed in the complex.
3.2. Adsorption of Congo Red on Different Materials
Fig. 4 (a) illustrates the adsorption performance of BC, CF, and CF-BC towards CR. While keeping other conditions constant, 0.4 g of adsorbent was added to 250 mg/L CR solution. Samples were taken at different time intervals to calculate the removal rate of CR and compare the adsorbents. It is evident that the contact time significantly enhances the adsorption capacity of the materials. However, after 24 hours, the change in CR removal efficiency is not substantial. This phenomenon can be explained by the availability of vacant sites on the material surface, which decrease with increasing contact time. When treated with CF alone, the effect is relatively poor, with a removal rate of only 22.75% after 24 hours. BC alone exhibits a removal rate of 62.23%. However, when CF-BC composites are used for treatment, the adsorption effect is significantly enhanced compared to individual materials. With increasing BC content, CF-BC1, CF-BC2, and CF-BC3 exhibit removal rates of 80.52%, 92.29%, and 89.36%, respectively. It can be observed that the effects of CF-BC2 and CF-BC3 are relatively close, indicating that there is no direct relationship between the percentage content of doped BC and the effect. This is consistent with previous research, where a limited number of active sites are required for a certain amount of CF. A higher BC content may lead to sample aggregation, which is unfavorable for adsorption. Moreover, excessive doping of BC can adversely affect the magnetic properties of the product, hindering its recovery. Therefore, further comparative research will focus on CF and CF-BC2.
In order to investigate the impact of adsorbent dosage on CR adsorption stability, while keeping other experimental conditions unchanged, different dosages of adsorbents were added to the CR dye, which were 25 mg, 30 mg, 35 mg, 40 mg, and 45 mg. The concentration of adsorbents varied between 1~1.8 g/L. As shown in Fig. 4(b), the removal rate of CR increased sharply with the increase of adsorbent dosage. When the adsorbent dosage increased from 25 mg to 45 mg, the removal rate increased from 69.1% to 92.4%. When the adsorbent dosage was above 40 mg, the adsorption rate increased slowly and gradually approached equilibrium. This behavior may be due to particle aggregation at higher dosages, leading to a decrease in available adsorption sites for CR absorption. The pH value has a significant impact on the surface charge of adsorbents.
Fig. 4(c) shows the effect of different pH values on the stability of dye adsorption. The pH of the solution was adjusted to between 2 and 10 using HCl and NaOH solutions, while other parameters were kept constant. The results indicate that increasing the pH leads to a decrease in CR removal rate. At lower pH values, CF and CF-BC2 have a positive effect on removing CR from the solution, with the best adsorption removal effect observed at pH 2. This is because under acidic conditions, the surface binding sites of the adsorbent become protonated due to an increase in positive charges, and the electrostatic attraction between the positively charged adsorbent and the anionic dye surface is key to removing CR from the solution. As the pH increases, the adsorbent's ability to remove the dye decreases because the increase in negative charges on the adsorbent surface causes electrostatic repulsion between the adsorbent functional groups and the dye anions, leading to a decrease in dye adsorption[61]. However, chromium ferrite may become unstable under acidic conditions, and lower pH may cause Cr and Fe ions to leach from the composite. Therefore, we analyzed the concentrations of Cr and Fe in the adsorbed solution when pH value was 2, and the results were shown in Table S3. According to Table S3, the concentration of chromium in the reaction solution was 4.81 mg/L, which was below the toxic leaching limit set by the United States Environmental Protection Agency (USEPA), and iron was not detected. These results indicate that heavy metals after adsorption are not easily leach from the complex under acidic conditions and can be effectively fixed in a stable and dense ferrite lattice.
When all other parameters are held constant, the effect of initial concentration (200, 400, 500, 600, 800, 1000 mg/L) on CR removal is studied through adsorption processes. Fig. 4(d) shows that CF-BC2 has a removal rate of over 80% for CR at initial concentrations up to 800 mg/L. However, at high concentrations, due to the fixed number of adsorption sites on the adsorbent, the sites gradually become saturated after reaching a certain adsorption capacity, leading to a decrease in removal rate compared to low concentration solutions.
3.3. Thermodynamics and Kinetics Studies of Adsorption3.3.1. Adsorption thermodynamicsTo examine the relationship between adsorption capacity (qe) and concentration (Ce) at equilibrium, various adsorption isotherm models were extensively employed for data fitting [62]. Among them, the Langmuir and Freundlich equations are the most widely applied, as shown in Eq. (3) and (5) respectively. The Langmuir model assumes that adsorbate molecules undergo single-layer adsorption on a uniform surface, without interaction between the adsorbed molecules. The Freundlich model is suitable for non-ideal adsorption on heterogeneous surface [63]. This heterogeneity arises from the presence of various functional groups on the surface, as well as interactions between adsorbent and adsorbate species [64]. In order to obtain equilibrium data, adsorption experiments were conducted by varying the initial concentration of CR (200, 400, 500, 600, 800, and 1000 mg/L) on the samples.
In Eq. (3), where qe represents the amount of CR adsorbed per unit mass of adsorbent at equilibrium (mg/g), Ce is the equilibrium concentration of CR in the solution (mg/L). Q0 provides the theoretical monolayer adsorption capacity (mg/g), where KL is the Langmuir constant related to the adsorption energy. Q0 and KL can be calculated from the slope and intercept, respectively [65].
In addition, to elucidate the fundamental characteristics of the Langmuir isotherm, a dimensionless constant termed as the separation factor, denoted as RL, is introduced. It is defined as shown in Eq. (4):
where C0 stands for the initial concentration of CR (mg/L), and RL represents the dimensionless constant. When the value of RL falls between 0 and 1, it indicates favorable adsorption.
In Eq. (5), KF represents the constant related to the adsorption capacity, Ce represents the concentration of the dye in the solution at equilibrium (mg/L), qe represents the equilibrium adsorption capacity of the adsorbent (mg/g), and n represents the parameter related to the adsorption intensity. When n is greater than 1, it indicates good adsorption performance [66].
The Langmuir and Freundlich isotherm models for CF are shown in Fig. 5 (a, b), and the specific parameters for the fitted equations are provided in Table S4. As shown in Fig. 5(a, b), the adsorption capacity of CF for CR increases with the initial concentration, indicating that higher concentrations intensify the collision between adsorbent and pollutant. Furthermore, with the rise in temperature, the adsorption capacity of CF for CR steadily increases, suggesting that temperature favors the progress of the reaction, indicating an endothermic process. From Table S4, it is evident that the Langmuir correlation coefficient for CF is higher than that for the Freundlich isotherm. This indicates that the Langmuir model provides a better fit to the experimental data compared to the Freundlich model, suggesting that CF undergoes monolayer adsorption of CR molecules. The adsorption sites on the surface of the adsorbent are evenly distributed, and the energy is equivalent. Additionally, the value of 0 < RL < 1 indicates that the adsorption process is favorable.
The Langmuir and Freundlich Isotherm models for CF-BC2 are shown in Fig. 5(c, d), and the specific fitting parameters are listed in Table S5. From Fig. 5(c, d), it is observed that the adsorption capacity of CF-BC2 increases with the initial concentration of CR. This is attributed to the fact that higher concentrations can capture more dye molecules. Furthermore, as the temperature rises, the adsorption capacity of CF-BC2 for CR continues to increase, indicating that the process is more favorable for adsorption at higher temperatures, suggesting an endothermic reaction.
According to Table S5, the Freundlich model provides a better fit for the adsorption isotherm of CF-BC2. This suggests that the adsorption of CR onto CF-BC2 follows the coverage principle, occurring on heterogeneous surfaces and non-equivalent sites, which is related to the developed pores within CF-BC2 due to the load on BC. Moreover, the value of n being greater than 1 indicates that the conditions are favorable for the adsorption reaction.
3.3.2. Adsorption KineticsObserving adsorption kinetics is crucial in adsorption studies, as it can predict adsorption rates and potential mechanisms [62]. In this study, pseudo-first-order and pseudo-second-order kinetic models [67] were employed to analyze the adsorption process of CF and CF-BC2 for the dye. The conformity between experimental data and model-predicted values was evaluated using the correlation coefficient (R2). Higher R2 values indicate that the model successfully describes the dynamic adsorption process of different materials.
Here, in Eq. (6), qe and qt represent the adsorption capacities at equilibrium and time t (mg/g), respectively. k1 is the first-order adsorption rate constant (min−1). Plotting log(qe-qt) against t yields a straight line. The values of k1 and qe are obtained from the slope and intercept of this line.
In Eq. (7), qe and qt represent the adsorption capacities at equilibrium and time t, (mg/g), respectively. k2 stands for the rate constant (g/(mg ·min)). The values of qe and k2 are determined by the slope and intercept of the curve, respectively.
The fitting results of the pseudo-first-order and pseudo-second-order kinetic models for different adsorbents are shown in Fig. 5(e, f). The specific fitting parameters can be found in Table 1.
The degree of fit of the fitted curves in Fig. 5(e, f) indicates that both materials are better described by the pseudo-second-order kinetic model. Table 1 shows that the correlation coefficients of the pseudo-second-order kinetic equations for CF and CF-BC2 composites are 0.9814 and 0.9990, respectively, which are higher than those of the pseudo-first-order kinetic equations. The adsorption capacities calculated by the pseudo-second-order kinetic model are more in line with the experimental results. This suggests that the adsorption of CR by CF and CF-BC2 follows the pseudo-second-order kinetic model. According to the assumption of the pseudo-second-order kinetic model, it can be inferred that chemical adsorption is the main process in this adsorption. The value of k2 indicates that the chemical adsorption rate of CR on the surface of CF is slower than that of CF-BC2 composite, which is attributed to the increase in active sites due to the addition of BC, thereby facilitating the adsorption process [68].
3.4. Analysis of Adsorption MechanismThe results of the sorption test and sorption model fitting can be extruded, and the sorption process is multi-layer sorption on heterogeneous surface. Chemical adsorption is the main method. From BET and SEM analysis, it is evident that BC possesses a large specific surface area and well-developed microporous structure, providing numerous active sites for CF loading. The significantly larger specific surface area and pore volume of the CF-BC2 composite accelerate the adsorption of CR by the adsorbent. This suggests that pore filling is one of the mechanisms by which CF-BC2 adsorbs CR. The ferrite particles loaded on biochar may be the determinant factor for the removal of CR by CF-BC2. In order to further explore the mechanism of CF-BC2 removal of CR, the materials before and after CR absorption were characterized with FT-IR (Fig. 6), and the zero-point charge was tested to explore the electrostatic interaction.
In Fig. 6(a), the FT-IR spectrum of CF-BC2 composite after adsorption of CR shows the asymmetric (1173 and 1225 cm−1) and symmetric stretching vibrations (1046 and 1112 cm−1) of the sulfonic acid groups on CR molecules, and the C-N bond on CR molecules also appears at 1356 cm−1. These results indicate that CR is successfully adsorbed onto the surface of the CF-BC2 composite. The CF-BC2 composite surface has two abundant −OH groups (Cr-OH and Fe-OH), which are the primary binding sites for various cationic and anionic species. They interact with CR during the adsorption process and may form Cr-O-CR and Fe-O-CR. It is well known that due to the reaction of water molecules with the surface of metal oxides, the outermost layer of metal oxide colloids forms −OH (S-OH) groups [69]. It is speculated that at acidic pH values, hydrogen bonding may exist between the −OH groups of CF-BC2 composite and the sulfonic acid or amine sites of the dye molecules. However, after CR adsorption, the −OH peak at 3357 cm−1 increases, which may be due to the presence of the N-H peak (3420 cm−1) in CR. A comparison before and after CR adsorption by CF-BC2 composite reveals an enhanced C=C functional group vibration peak at 1615 cm−1, suggesting structural disruption possibly due to the opening of the benzene ring during the reaction. Furthermore, in the C=C aromatic ring structure of the CF-BC2 composite, there is a π electron orbital that can also form π-π interactions with the C=C bonds of CR. Therefore, hydrogen bonding and π-π interactions have a significant impact on the removal of CR.
Finally, electrostatic attraction also plays a crucial role in adsorption. The zero charge point (pHpzc) is the pH at which the surface charge of the adsorbent is neutral, the positive and negative charges are balanced. Below this pH, due to functional group protonation, the adsorbent acquires a net positive charge, leading to strong electrostatic attraction between anionic dyes and the adsorbent. Beyond this pH, the adsorbent surface becomes negatively charged [70]. Therefore, adsorbing anionic pollutants (dyes) is effective at pH < pHpzc, when the adsorbent surface is positively charged, facilitating electrostatic attraction. Since CR is an acidic dye, when the pH is lower than the adsorbent's zero charge point, the adsorbent surface carries a positive charge. As CR is an anionic dye, electrostatic attraction occurs between the positively charged adsorbent and the anionic dye, leading to anionic adsorption. As the pH of the CR solution increases, the adsorption capacity sharply decreases. Typically, at higher pH levels, hydroxide anions (OH−) can compete with the anionic sites of the CR dye and adsorb onto the positive charges of the composite. As shown in Fig. 6(b), the pHpzc values for BC, CF, and CF-BC2 composite are 9.78, 3.16, and 5.14, respectively. This validates that at lower pH values, both CF and CF-BC2 play a positive role in removing CR. Compared to CF, CF-BC2 has a relatively higher zero charge point, indicating that CF-BC2 exhibits better tolerance towards the acidity of the CR solution compared to CF. This is one of the reasons why the CF-BC2 composite shows superior adsorption performance.
In summary, The primary adsorption mechanisms are illustrated in Fig. 7: (1) The results of the sorption test and sorption model fitting can be extruded, and the sorption process is multi-layer sorption on heterogeneous surface; (2) The CF-BC composite surface has two abundant −OH groups (Cr-OH and Fe-OH), they interact with CR during the adsorption process and may form Cr-O-CR and Fe-O-CR.; (3) Hydrogen bonding and π-π interactions have a significant impact on the removal of CR; 4) There is electrostatic attraction between CR and CF-BC.
3.5. Study on Renewable Recyclability and Economic AdaptabilityAn important indicator to measure the performance of an adsorbent is its regeneration ability. Therefore, regeneration cycling experiments are conducted to test the stability of the adsorbent and determine its efficiency in repeated binding with the target pollutant. In this study, the adsorbent is collected from the solution using an external magnetic field, and the collected adsorbent is immersed in a 1.0 mol/L NaOH solution. The mixture is then stirred at room temperature at 200 r/min for 24 hours. This process is repeated for three cycles using the regenerated adsorbent. The removal efficiency of CR is measured after each cycle, and the results are shown in Fig. S4. It can be observed that in the cyclic experiments with 200 mg/L of CR wastewater, the regeneration and reuse performance of CF is lower compared to CF-BC2 composite material. The three cycles of regeneration for CF resulted in removal rates of 76%, 69%, and 54% for CR. In contrast, CF-BC2 showed higher stability, with 95% of the initial adsorption efficiency in the first cycle, 92% in the second cycle, and 90% in the third cycle. This suggests that CF-BC2 possesses a certain degree of stability. This could be attributed to the presence of BC, which provides CF with a supportive matrix, and enhances its buffering capacity towards the NaOH solution. In addition, As can be seen from Table S6, CF-BC2 has good cycle stability compared to other adsorbents.
The treatment of chromium-containing wastewater is often costly, as chromium, while an essential industrial raw material, poses as a harmful contaminant in wastewater. Fortunately, the coprecipitation method offers a promising solution, converting the chromium and iron elements in the wastewater into magnetic nanomaterials. This conversion not only mitigates the environmental pollution caused by the wastewater but also enables the recycling of chromium, thus promoting the circular utilization of resources. Moreover, this method is not only cost-effective but also simple to operate and energy-efficient, marking a significant breakthrough in industrial wastewater treatment. Furthermore, this magnetic material can be combined with biochar and other materials to purify organic wastewater. Biochar, renowned for its excellent adsorption properties, can effectively adsorb organic pollutants from wastewater. The addition of magnetic material facilitates the separation of this composite from the wastewater, thereby enhancing the efficiency of wastewater treatment. Crucially, the application of this composite material also enhances the reusability of adsorbents. Traditionally, adsorbents used in wastewater treatment are often disposable, requiring disposal or treatment after use. However, this iron-containing composite can be recovered and regenerated through a simple magnetic separation process, enabling the reuse of adsorbents and further reducing treatment costs.
In summary, the application of this material in wastewater purification exhibits remarkable advantages. It not only reduces treatment costs and improves treatment efficiency but also facilitates the circular utilization of resources and the reuse of adsorbents. Therefore, this method holds immense promise and significant value in industrial applications.
4. ConclusionsThe magnetic adsorbent CF-BC was successfully prepared and applied to the adsorption of Congo red dye. The results of adsorption experiments indicate that compared with CF and BC as separate materials, the removal rate of CR by the composite material (CF-BC2) reached 92.29%, and the removal rate of the composite material remained above 90% even after three cycles of treatment, indicating that CF-BC2 has good reusability. The influence of factors such as temperature, pH and concentration of reactants on the adsorption reaction were investigated. The thermodynamic and kinetic adsorption analysis showed that the increase of temperature was conducive to the adsorption of CR, and the adsorption of CR by CF-BC2 was consistent with the Freundlich isothermal model. The sorption process is multi-layer sorption on heterogeneous surface and chemical adsorption is the main method. The adsorption process conforms to the quasi-second-order kinetic model. The mechanism of CF-BC2 adsorption of CR is mainly carried out through pore filling, hydrogen bonding, π-π interaction and electrostatic attraction, etc. In this study, biochar from agricultural waste is successfully combined with chromium ferrite obtained from chromium-containing wastewater, and an efficient adsorbent for the degradation of Congo red in organic dye wastewater is developed. It is of great practical significance to develop a new type of waste based magnetic mesoporous adsorbent to treat dye wastewater.
AcknowledgementsThis work was supported by National Nature Science Foundation of China Nos. (51274138, 50974086 and 50704023). We thank Shanghai University for the support of this work. The sponsor was not involved in the study design; the collection, analysis or interpretation of the data; the preparation of the manuscript or the decision where to submit the manuscript for publication.
NotesConflict-of-Interest Statement The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Author Contributions Y.M. (M.S. student) performed all experiments and analyses along with data collection and discussion of the results and wrote the manuscript. D.C. (Professor) contributed to the conceptualization of the work, gave the ideas and goal, revised and edited the manuscript. Y.L. (Professor) gave the ideas. W.S. (M.S.) performed some experiments and analyses along with data collection. Y.L. (M.E. student) and H.Y. (M.S. student) worked on chemical analysis and data plotting. G.Q. (Professor) revised and edited the manuscript. References1. Santos Klienchen Dalari BL, Lisboa Giroletti C, et al. Application of a phosphonium-based ionic liquid for reactive textile dye removal: Extraction study and toxicological evaluation. J. Environ. Manage. 2022;304:114322.
https://doi.org/10.1016/j.jenvman.2021.114322
2. Wang H, Li Z, Yahyaoui S, et al. Effective adsorption of dyes on an activated carbon prepared from carboxymethyl cellulose: Experiments, characterization and advanced modelling. Chem. Eng. J. 2021;417:128116.
https://doi.org/10.1016/j.cej.2020.128116
3. Yan X, Cheng S, Ma C, Li J, Wang G, Yang C. D-spacing controllable GO membrane intercalated by sodium tetraborate pentahydrate for dye contamination wastewater treatment. J. Hazard. Mater. 2022;422:126939.
https://doi.org/10.1016/j.jhazmat.2021.126939
4. Zhao J, Liu H, Xue P, et al. Construction of a multi-layer protection of CS polymer brush grafted DA@CNTs coating on PVDF membrane for effective removal of dye effluent. J. Hazard. Mater. 2023;460:132435.
https://doi.org/10.1016/j.jhazmat.2023.132435
5. Solayman HM, Hossen MA, Aziz AA, et al. Performance evaluation of dye wastewater treatment technologies: A review. J. Environ. Chem. Eng. 2023;11:109610.
https://doi.org/10.1016/j.jece.2023.109610
6. Jin W, Nan J, Chen M, Wu F. Superior performance of novel chitosan-based flocculants in decolorization of anionic dyes: Responses of flocculation performance to flocculant molecular structures and hydrophobicity and flocculation mechanism. J. Hazard. Mater. 2023;452:131273.
https://doi.org/10.1016/j.jhazmat.2023.131273
7. Girish YR, Udayabhanu , Byrappa NM, et al. Rapid and facile synthesis of Z-scheme ZnO/g-C3N4 heterostructure as efficient visible light-driven photocatalysts for dye degradation and hydrogen evolution reaction. J. Hazard. Mater. Adv. 2023;9:100230.
https://doi.org/10.1016/j.hazadv.2023.100230
8. Yagub MT, Sen TK, Afroze S, Ang HM. Dye and its removal from aqueous solution by adsorption: A review. Adv. Colloid Interface Sci. 2014;209:172–184.
https://doi.org/10.1016/j.cis.2014.04.002
9. Uddin MJ, Ampiaw RE, Lee W. Adsorptive removal of dyes from wastewater using a metal-organic framework: A review. Chemosphere. 2021;284:131314.
https://doi.org/10.1016/j.chemosphere.2021.131314
10. Sridhar A, Ponnuchamy M, Kapoor A, Prabhakar S. Valorization of food waste as adsorbents for toxic dye removal from contaminated waters: A review. J. Hazard. Mater. 2022;424:127432.
https://doi.org/10.1016/j.jhazmat.2021.127432
11. Kloster GA, Mosiewicki MA, Marcovich NE. Chitosan/iron oxide nanocomposite films: Effect of the composition and preparation methods on the adsorption of Congo red. Carbohydr. Polym. 2019;221:186–194.
https://doi.org/10.1016/j.carbpol.2019.05.089
12. You J, Liu C, Feng X, Lu B, Xia L, Zhuang X. In situ synthesis of ZnS nanoparticles onto cellulose/chitosan sponge for adsorption–photocatalytic removal of Congo red. Carbohydr. Polym. 2022;288:119332.
https://doi.org/10.1016/j.carbpol.2022.119332
13. Ma C, Wang F, Zhang C, et al. Photocatalytic decomposition of Congo red under visible light irradiation using MgZnCr-TiO2 layered double hydroxide. Chemosphere. 2017;168:80–90.
https://doi.org/10.1016/j.chemosphere.2016.10.063
14. Babu SS, Mohandass C, Vijayaraj AS, Dhale MA. Detoxification and color removal of Congo red by a novel Dietzia sp. (DTS26) – A microcosm approach. Ecotoxicol Environ Saf. 2015;114:52–60.
https://doi.org/10.1016/j.ecoenv.2015.01.002
15. Sharma B, Tiwari S, Kumar R, Kumar M, Tewari L. Eco-friendly detoxification of hazardous Congo red dye using novel fungal strain Trametes flavida WTFP2: Deduced enzymatic bio-mineralization process through combinatorial in-silico and in-vitro studies. J. Hazard. Mater. 2023;455:131503.
https://doi.org/10.1016/j.jhazmat.2023.131503
16. Kishor R, Purchase D, Saratale GD, et al. Environment friendly degradation and detoxification of Congo red dye and textile industry wastewater by a newly isolated Bacillus cohnni (RKS9). Environ. Technol. Innov. 2021;22:101425.
https://doi.org/10.1016/j.eti.2021.101425
17. Madan S, Shaw R, Tiwari S, Tiwari SK. Adsorption dynamics of Congo red dye removal using ZnO functionalized high silica zeolitic particles. Appl Surf Sci. 2019;487:907–917.
https://doi.org/10.1016/j.apsusc.2019.04.273
18. Dawood S, Sen TK. Removal of anionic dye Congo red from aqueous solution by raw pine and acid-treated pine cone powder as adsorbent: Equilibrium, thermodynamic, kinetics, mechanism and process design. Water. Res. 2012;46:1933–1946.
https://doi.org/10.1016/j.watres.2012.01.009
19. Pavithra KG, PSK , VJP . Removal of colorants from wastewater: A review on sources and treatment strategies. J. Ind. Eng. Chem. 2019;75:1–19.
https://doi.org/10.1016/j.jiec.2019.02.011
20. Zhou Y, Lu J, Zhou Y, et al. Recent advances for dyes removal using novel adsorbents: A review. Environ. Pollut. 2019;252:352–365.
https://doi.org/10.1016/j.envpol.2019.05.072
21. Xiao W, Jiang X, Liu X, et al. Adsorption of organic dyes from wastewater by metal-doped porous carbon materials. J. Clean. Prod. 2021;284:124773.
22. Tan KB, Vakili M, Horri BA, Poh PE, Abdullah AZ, Salamatinia B. Adsorption of dyes by nanomaterials: Recent developments and adsorption mechanisms. Sep. Purif. Technol. 2015;150:229–242.
https://doi.org/10.1016/j.seppur.2015.07.009
23. Ngulube T, Gumbo JR, Masindi V, Maity A. An update on synthetic dyes adsorption onto clay based minerals: A state-of-art review. J. Environ. Manage. 2017;191:35–57.
https://doi.org/10.1016/j.jenvman.2016.12.031
24. Kausar A, Iqbal M, Javed A, et al. Dyes adsorption using clay and modified clay: A review. J. Mol. Liq. 2018;256:395–407.
https://doi.org/10.1016/j.molliq.2018.02.034
25. Kubra KT, Salman MS, Hasan MN. Enhanced toxic dye removal from wastewater using biodegradable polymeric natural adsorbent. J. Mol. Liq. 2021;328:115468.
https://doi.org/10.1016/j.molliq.2021.115468
26. Hasanzadeh M, Simchi A, Shahriyari H. Far. Nanoporous composites of activated carbon-metal organic frameworks for organic dye adsorption: Synthesis, adsorption mechanism and kinetics studies. J. Ind. Eng. Chem. 2020;81:405–414.
https://doi.org/10.1016/j.jiec.2019.09.031
27. Wang X, Jiang C, Hou B, et al. Carbon composite lignin-based adsorbents for the adsorption of dyes. Chemosphere. 2018;206:587–596.
https://doi.org/10.1016/j.chemosphere.2018.04.183
28. Guo F, Li X, Jiang X, Zhao X, Guo C, Rao Z. Characteristics and toxic dye adsorption of magnetic activated carbon prepared from biomass waste by modified one-step synthesis. Colloids Surf Physicochem Eng Asp. 2018;555:43–54.
https://doi.org/10.1016/j.colsurfa.2018.06.061
29. Akubo K, Nahil MA, Williams PT. Pyrolysis-catalytic steam reforming of agricultural biomass wastes and biomass components for production of hydrogen/syngas. J. Energy Inst. 2019;92:1987–1996.
https://doi.org/10.1016/j.joei.2018.10.013
30. Ahmed MJ, Hameed BH. Adsorption behavior of salicylic acid on biochar as derived from the thermal pyrolysis of barley straws. J. Clean. Prod. 2018;195:1162–1169.
https://doi.org/10.1016/j.jclepro.2018.05.257
31. Ambika S, Kumar M, Pisharody L, et al. Modified biochar as a green adsorbent for removal of hexavalent chromium from various environmental matrices: Mechanisms, methods, and prospects. Chem. Eng. J. 2022;439:135716.
https://doi.org/10.1016/j.cej.2022.135716
32. Berslin D, Reshmi A, Sivaprakash B, Rajamohan N, Kumar PS. Remediation of emerging metal pollutants using environment friendly biochar-Review on applications and mechanism. Chemosphere. 2022;290:133384.
https://doi.org/10.1016/j.chemosphere.2021.133384
33. Campion L, Bekchanova M, Malina R, Kuppens T. The costs and benefits of biochar production and use: A systematic review. J. Clean. Prod. 2023;408:137138.
https://doi.org/10.1016/j.jclepro.2023.137138
34. You X, Zhou R, Zhu Y, Bu D, Cheng D. Adsorption of dyes methyl violet and malachite green from aqueous solution on multi-step modified rice husk powder in single and binary systems: Characterization, adsorption behavior and physical interpretations. J. Hazard. Mater. 2022;430:128445.
https://doi.org/10.1016/j.jhazmat.2022.128445
35. Ahmed MJK, Ahmaruzzaman M. A facile synthesis of Fe3O4–charcoal composite for the sorption of a hazardous dye from aquatic environment. J. Environ. Manage. 2015;163:163–173.
http://dx.doi.org/10.1016/j.jenvman.2015.08.011
36. Zhang J, Hu X, Yan X, Feng R, Zhou M, Xue J. Enhanced adsorption of Rhodamine B by magnetic nitrogen-doped porous carbon prepared from bimetallic ZIFs. Colloids Surf. Physicochem. Eng. Asp. 2019;575:10–17.
https://doi.org/10.1016/j.colsurfa.2019.04.091
37. Rasoulzadeh H, Sheikhmohammadi A, Asgari E, et al. The adsorption behaviour of triclosan onto magnetic bio polymer beads impregnated with diatomite. Int J Environ An Ch. 2021;4130–4142.
https://doi.org/10.1080/03067319.2021.1922684
38. Safari M, Rezaee R, Soltani RDC, Asgari E. Dual immobilization of magnetite nanoparticles and biosilica within alginate matrix for the adsorption of Cd (II) from aquatic phase. Sci. Rep. 2022;12:11473.
https://doi.org/10.1038/s41598-022-15844-w
39. Sheikhmohammadi A, Asgari E, Yeganeh J. Application of the Fe3O4@activated carbon magnetic nanoparticles for the adsorption of metronidazole from wastewater: optimization, kinetics, thermodynamics and equilibrium studies. Desalination. Water. Treat. 2021;222:354–365.
https://doi.org/10.5004/dwt.2021.27101
40. Debnath S, Das R. Strong adsorption of CV dye by Ni ferrite nanoparticles for waste water purification: Fits well the pseudo second order kinetic and Freundlich isotherm model. Ceram. Int. 2023;49:16199–16215.
https://doi.org/10.1016/j.ceramint.2023.01.218
41. Reddy DHK, Yun Y-S. Spinel ferrite magnetic adsorbents: Alternative future materials for water purification? Coord. Chem. Rev. 2016;315:90–111.
http://dx.doi.org/10.1016/j.ccr.2016.01.012
42. Wang L, Li J, Wang Y, Zhao L, Jiang Q. Adsorption capability for Congo red on nanocrystalline MFe2O4 (M=Mn, Fe, Co, Ni) spinel ferrites. Chem Eng J. 2012;181–182:72–79.
https://doi.org/10.1016/j.cej.2011.10.088
43. Sun Y, Ma C, Wu D, et al. Coating CoFe2O4 shell on Fe particles to increase the utilization efficiencies of Fe and peroxymonosulfate for low-cost Fenton-like reactions. Water. Res. 2023;244:120542.
https://doi.org/10.1016/j.watres.2023.120542
44. Chen D, Sheng W, Wang D, et al. Effective purification of high concentration chromium-containing wastewater and preparation of chromium ferrite. Environ. Eng. Res. 2023;28(6)220706.
https://doi.org/10.4491/eer.2022.706
45. Petcharoen K, Sirivat A. Synthesis and characterization of magnetite nanoparticles via the chemical co-precipitation method. Mater. Sci. Eng. B. 2012;177:421–427.
https://doi.org/10.1016/j.mseb.2012.01.003
46. Bibi F, Iqbal S, Kalsoom A, et al. Magnetically separable Nd and Mn co-doped SrFe12O19 hexa-ferrites nanostructures for the evaluation of structural, magnetic and photo-catalytic studies under solar irradiation for the crystal violet dye removal from the industrial wastes. Ceram. Int. 2023;49:15990–16001.
https://doi.org/10.1016/j.ceramint.2023.01.196
47. Chen D, Li Y, Zhang J, Zhou J, Guo Y, Liu H. Magnetic Fe3O4/ZnCr-layered double hydroxide composite with enhanced adsorption and photocatalytic activity. Chem Eng J. 2012;185–186:120–126.
https://doi.org/10.1016/j.cej.2012.01.059
48. Jiang C, Wang X, Qin D, et al. Construction of magnetic lignin-based adsorbent and its adsorption properties for dyes. J. Hazard. Mater. 2019;369:50–61.
https://doi.org/10.1016/j.jhazmat.2019.02.021
49. Oraon A, Ram M, Kumar Gupta A, Dutta S, Kumar Saxena V, Kumar Gaurav G. An efficient waste garlic skins biochar nanocomposite: An advanced cleaner approach for secondary waste utilization. J. Mol. Liq. 2022;364:119997.
https://doi.org/10.1016/j.molliq.2022.119997
50. Iqbal J, Shah NS, Sayed M, et al. Nano-zerovalent manganese/biochar composite for the adsorptive and oxidative removal of Congo-red dye from aqueous solutions. J. Hazard. Mater. 2021;403:123854.
https://doi.org/10.1016/j.jhazmat.2020.123854
51. Mubarak M, Jeon H, Islam MS, et al. One-pot synthesis of layered double hydroxide hollow nanospheres with ultrafast removal efficiency for heavy metal ions and organic contaminants. Chemosphere. 2018;201:676–686.
https://doi.org/10.1016/j.chemosphere.2018.03.046
52. Zhang Z, Sun L, Pei Z, et al. New insight into the adsorption of sulfadiazine on graphite-like biochars prepared at different pyrolytic temperatures. J. Clean. Prod. 2023;413:137468.
https://doi.org/10.1016/j.jclepro.2023.137468
53. Zhang Y, Liang S, He R, et al. Enhanced adsorption and degradation of antibiotics by doping corncob biochar/PMS with heteroatoms at different preparation temperatures: Mechanism, pathway, and relative contribution of reactive oxygen species. J. Water. Process. Eng. 2022;46:102626.
https://doi.org/10.1016/j.jwpe.2022.102626
54. Liu Y, Chen Y, Li Y, et al. Fabrication, application, and mechanism of metal and heteroatom co-doped biochar composites (MHBCs) for the removal of contaminants in water: A review. J. Hazard. Mater. 2022;431:128584.
https://doi.org/10.1016/j.jhazmat.2022.128584
55. Anwar A, Zulfiqar S, Yousuf MA, et al. Impact of rare earth Dy+3 cations on the various parameters of nanocrystalline nickel spinel ferrite. J. Mater. Res. Technol. 2020;9:5313–5325.
https://doi.org/10.1016/j.jmrt.2020.03.057
56. Song G, Qin F, Yu J, et al. Tailoring biochar for persulfate-based environmental catalysis: Impact of biomass feedstocks. J. Hazard. Mater. 2022;424:127663.
https://doi.org/10.1016/j.jhazmat.2021.127663
57. Welter N, Leichtweis J, Silvestri S, Sánchez PIZ, Mejía ACC, Carissimi E. Preparation of a new green composite based on chitin biochar and ZnFe2O4 for photo-Fenton degradation of Rhodamine B. J. Alloys. Compd. 2022;901:163758.
https://doi.org/10.1016/j.jallcom.2022.163758
58. Kaur M, Kaur N, Jeet K, Kaur P. MgFe2O4 nanoparticles loaded on activated charcoal for effective removal of Cr (VI) – A novel approach. Ceram. Int. 2015;41:13739–13750.
https://doi.org/10.1016/j.ceramint.2015.08.040
59. Yoon K, Cho DW, Tsang YF, Tsang DCW, Kwon EE, Song H. Synthesis of functionalised biochar using red mud, lignin, and carbon dioxide as raw materials. Chem. Eng. J. 2019;361:1597–1604.
https://doi.org/10.1016/j.cej.2018.11.012
60. Qiao Q, Zhou H, Guo F, et al. Facile and scalable synthesis of mesoporous composite materials from coal gasification fine slag for enhanced adsorption of malachite green. J. Clean. Prod. 2022;379:134739.
https://doi.org/10.1016/j.jclepro.2022.134739
61. Xiao F, Pignatello JJ. Interactions of triazine herbicides with biochar: Steric and electronic effects. Water. Res. 2015;80:179–188.
https://doi.org/10.1016/j.watres.2015.04.040
62. Wang Z, Alinezhad A, Nason S, Xiao F, Pignatello JJ. Enhancement of per- and polyfluoroalkyl substances removal from water by pyrogenic carbons: Tailoring carbon surface chemistry and pore properties. Water. Res. 2023;229:119467.
https://doi.org/10.1016/j.watres.2022.119467
63. Perveen S, Nadeem R, Nosheen F, Asjad MI, Awrejcewicz J, Anwar T. Biochar-Mediated Zirconium Ferrite Nanocomposites for Tartrazine Dye Removal from Textile Wastewater. Nanomaterials. 2022;12(16)2828.
https://doi.org/10.3390/nano12162828
64. Choi WS, Lee H-J. Nanostructured Materials for Water Purification: Adsorption of Heavy Metal Ions and Organic Dyes. Polymers. 2022;14:2183.
https://doi.org/10.3390/polym14112183
65. Andersson KI, Eriksson M, Norgren M. Removal of Lignin from Wastewater Generated by Mechanical Pulping Using Activated Charcoal and Fly Ash: Adsorption Isotherms and Thermodynamics. Ind. Eng. Chem. Res. 2011;50:7722–7732.
https://doi.org/10.1021/ie200378s
66. Zhu H, Guo A, Wang S, Long Y, Fan G, Yu X. Efficient tetracycline degradation via peroxymonosulfate activation by magnetic Co/N co-doped biochar: Emphasizing the important role of biochar graphitization. Chem. Eng. J. 2022;450:138428.
https://doi.org/10.1016/j.cej.2022.138428
67. Deng L, Shi Z, Wang L, Zhou S. Fabrication of a novel NiFe2O4/Zn-Al layered double hydroxide intercalated with EDTA composite and its adsorption behavior for Cr(VI) from aqueous solution. J. Phys. Chem. Solids. 2017;104:79–90.
http://dx.doi.org/10.1016/j.jpcs.2016.12.030
68. Zhou P, Li X, Zhou J, Peng Z, Shen L, Li W. Insights of the adsorption mechanism of methylene blue on biochar from phytoextraction residues of Citrus aurantium L.: Adsorption model and DFT calculations. J. Environ. Chem. Eng. 2023;11:110496.
https://doi.org/10.1016/j.jece.2023.110496
69. Rahimi R, Kerdari H, Rabbani M, Shafiee M. Synthesis, characterization and adsorbing properties of hollow Zn-Fe2O4 nanospheres on removal of Congo red from aqueous solution. Desalination. 2011;280:412–418.
https://doi.org/10.1016/j.desal.2011.04.073
70. Kefeni KK, Mamba BB. Photocatalytic application of spinel ferrite nanoparticles and nanocomposites in wastewater treatment: Review. Sustain. Mater. Technol. 2020;23:e00140.
https://doi.org/10.1016/j.susmat.2019.e00140
Fig. 1(a)XRD; (b) FT-IR of BC, CF and CF-BC composite; (c) Saturation magnetization of CF and CF-BC composite. 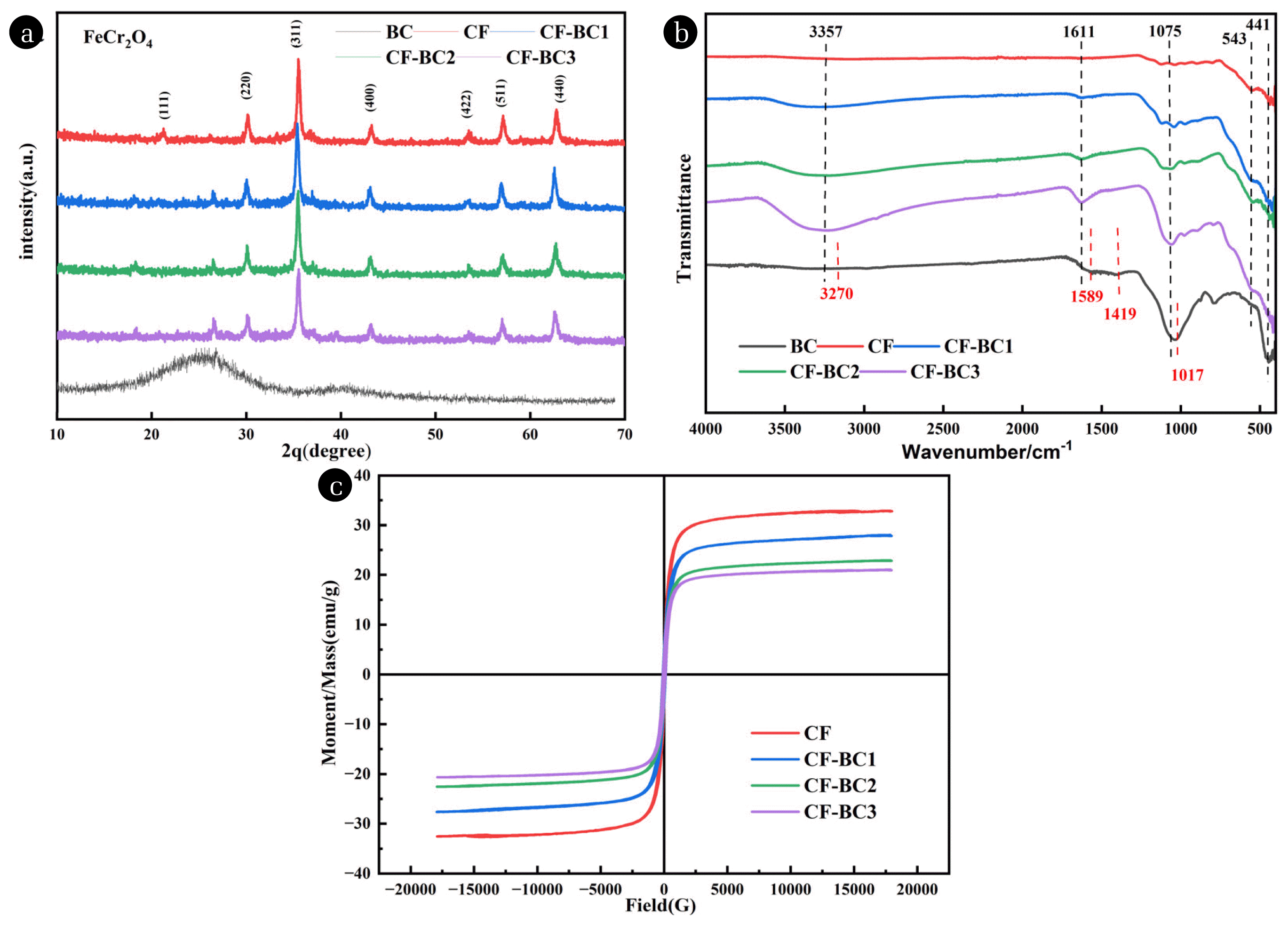
Fig. 2(a–f) SEM diagram of BC CF and CF-BC composites with different magnification; (g) mapping images for the CF-BC2 composite. 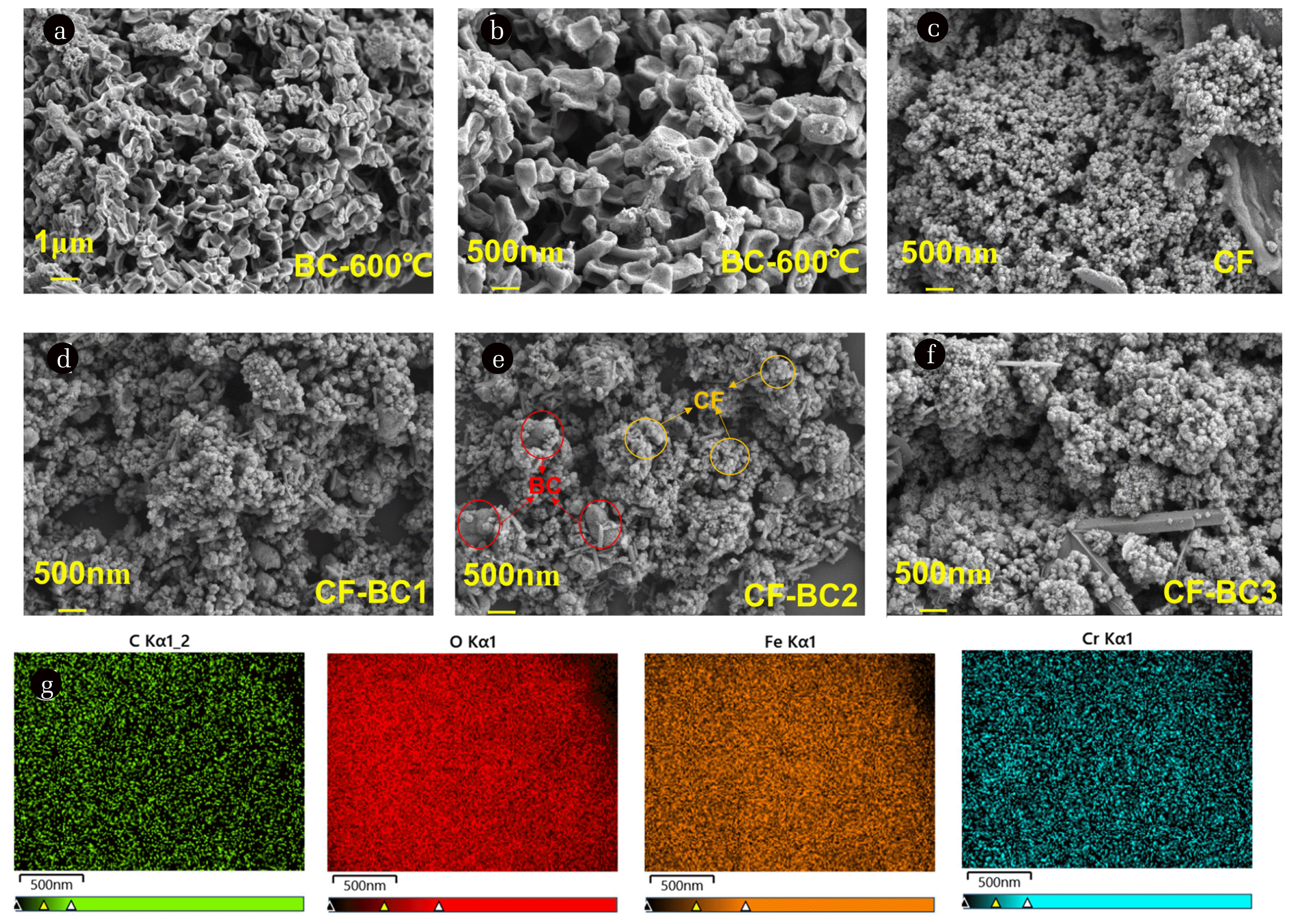
Fig. 4(a) Removal rate of CR by different materials; (b) Effect of adsorbent dose on CR adsorption by CF and CF-BC2; (c) Effect of pH on the adsorption of CR by CF and CF-BC2; (d) Effect of initial concentration of solution on CR adsorption for CF and CF-BC2. 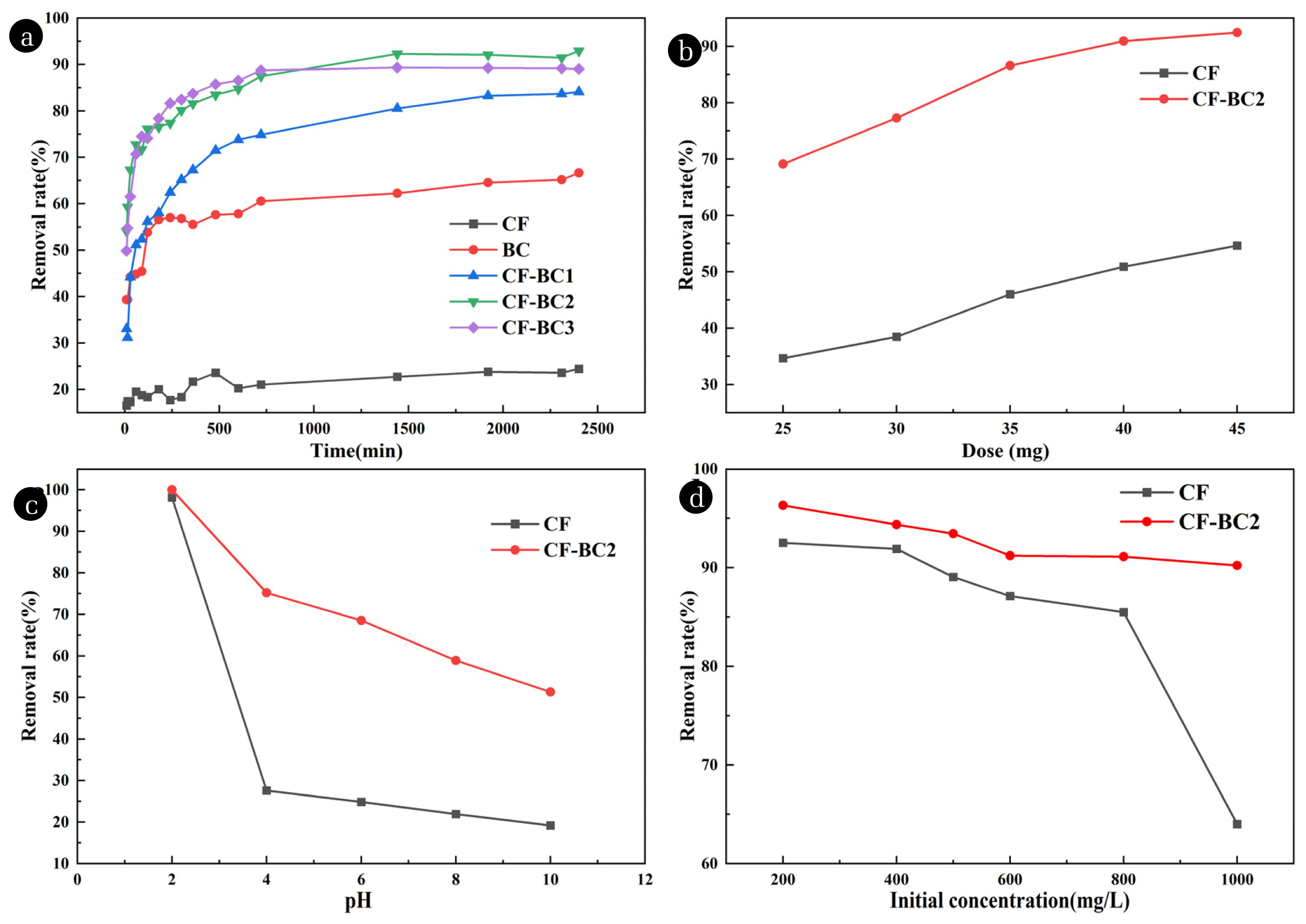
Fig. 5Adsorption isotherm for CR on CF: (a) Langmuir (b) Freundlich; Adsorption isotherm for CR on CF-BC2: (c) Langmuir (d) Freundlich; Kinetic models for the adsorption of CR by CF and CF-BC2: (e) Pseudo-first-order kinetic model (f) pseudo-second-order kinetic model. 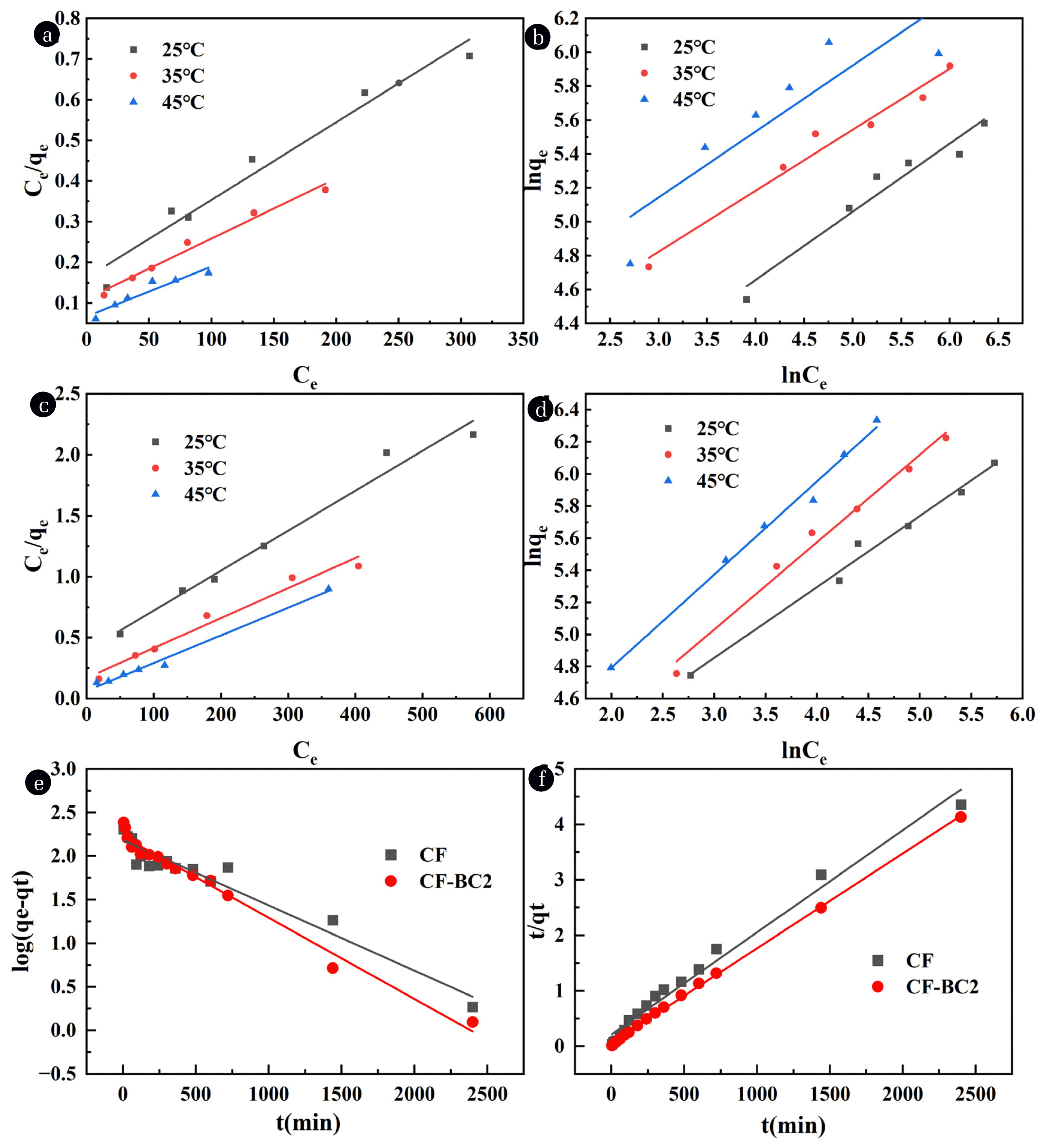
|
|
||||||||||||||||||||||||||||||||||||