AbstractSeparation of heavy metals to obtain potable water for domestic and agricultural applications is important considering health effects, bioaccumulation properties and applicability. The separation of Cr and Mn salts by Donnan exclusion are investigated using polysulfone (PSF) based membranes modified by anchoring ZnO nanoparticles. Use of Acid-treated ZnO nanoparticle enhnanced rejection properties for Cr and Mn (97.12 and 98.37%, respectively) for membranes based on 40% PSF, 8% PEG-400 and 0.8% ZnO. The use of polyethylene glycol (PEG)with molecular weight of 200 Da enhanced rejection properties to ~ 99%. This would provide excellent pathway for PSF membrane modification without affecting stability. Separation was dependent uponconcentrations of PSF, PEG, ZnO nanoparticles in dope solution, and bubble point pressure, pore size, number of pores, etc. Analysis of these properties and effect would provide pathway for design of membranes for heavy metal removal and process applications. Hence, they were analyzed using R studio multi-attribute linear regression model. Membrane performance regression analysis provided correlation with 95% fitting accuracy with 0.98 coefficient of regression, suggesting good relationship between predicted and observed data. This shows the applicability of model to save time and cost involved in designing membrane formation parameters and properties with optimized applicability.
Graphical Abstract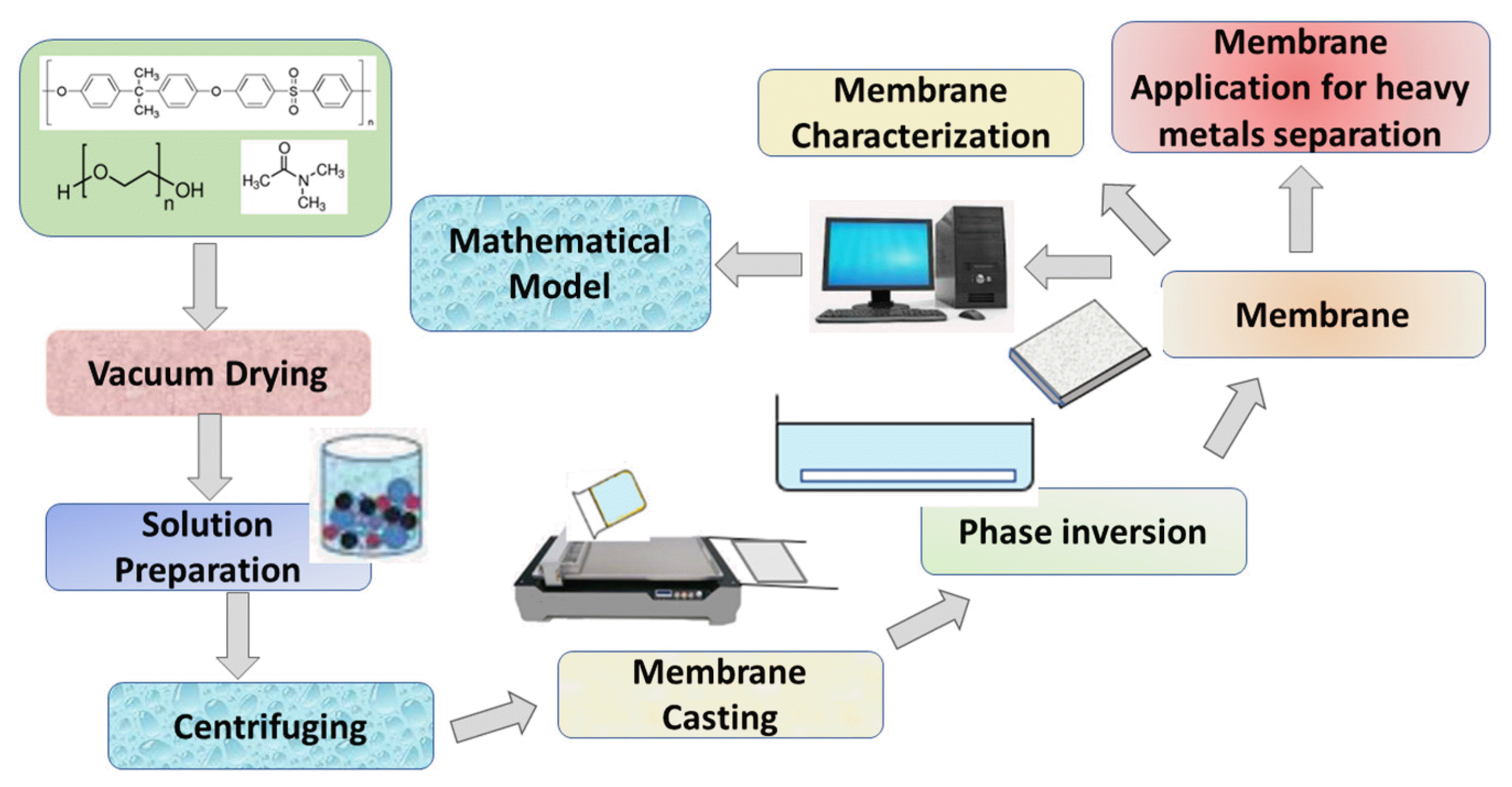
1. IntroductionFreshwater demand is increasing due to population, urbanization, and exponential growth in industrialization, across the globe [1]. This growth resulted in the generation of large amounts of industrial effluent and has become the major source of water pollution. This wastewater be treated for removal of harmful components which would become useful resource [2]. Such treatment of industry effluent for removal and recovery of pollutant and recycle of water would provide a practical solution for the water crisis.
Heavy metal is one of the major pollutants in wastewater due to their toxicity and bioaccumulation properties [2–4]. At the same time, these components have numerous applications in industrial purpose and biological applications like growth support, medicinal application, infection disruption, anticancer activity and soil microbial community modulation, etc. [5–7]. Selection and design of separation methods for this purpose is one of the tedious tasks. The traditional methods have their own challenges of secondary pollution or dumping of toxic waste [5, 8]. The membrane-based separation is reported using reverse osmosis (RO) and nanofiltration (NF) [9,10]. Though efficient separations are reported with RO and NF, they are known to separate other salts [10]. Additionally, there are limitations of high energy consumption, membrane fouling, concentration polarization for these methods [9, 10]. One techno-economically feasible solution could be the modification and formulation of ultrafiltration (UF) membrane for desired permeability and selectivity properties. Forming UF membranes with desired transport properties and surface charge impartation would benefit such applications. Incorporating surface charge would provide desired separation properties for heavy metals by Donnan exclusion [11,12]. Such modification of membranes by coating them with surface active layer has shown enhanced separation properties for Boron and humic acid (HA) [13,14]. The incorporation of charge in membrane surface would provide more stability compared to surface layer coating.
Optimization of membrane properties such as surface charge and smoothness are highly important while defining techno-economic feasibility of membrane processes. It would affect the separation efficiency and transport rate, in turn affect the overall membrane performance and related process design. These parameters are dependent upon different factors like solution composition, casting conditions, base polymer properties, solvent used, concentration of polymer, additive, surface modifier etc. [15–18]. Thus, selection of base polymer and optimization membrane formation parameters combinedly would define the process applicability.
Hence there is a need to obtain the correlation between membrane composition and properties such as transport and selectivity as membrane performance is dependent on these parameters. These parameters and conditions would be used further to optimize membrane formation by developing a conceptual mathematical correlation. It would define the effect of these parameters on membrane performance, so that the membranes with predetermined properties can be developed. Hence data for membrane properties and preparation parameters and their effect on Cr and Mn rejection analysis was utilized for correlating independent (input) variables and dependent (output) variables based on multivariable regression method.
The regression term was first used by Sir Francis Galton in 1877 for defining the process to foresee the effect of one variable on another [1,19]. Whereas further the term multiple regression is evolved which illustrates the method of correlating several variables to predict one variable [1,19]. Multiple regression is a simple, versatile statistical method to predict single variable as function of multiple independent variables [1,19,20]. It gives the relation between known and unknown parameters to evaluate for some unknown parameter [1].
In the case of current work, the PSF was selected as base polymer due to its stability properties [5, 8]. Objectives of current work are optimization of PSF based membrane formation and its transport properties through use of polyethylene glycol as porogen and ZnO as additives. All these would affect the membrane performance by Donnan exclusion through variation in membrane morphology and operational parameters, interaction between solute molecules and membrane surface. These optimization conditions and parameters would be further correlated with membrane performance and metal rejection properties. Such optimized parameters and properties like dope solution concentration, pore size, number of pores, bubble point pressure, etc. would be correlated through data-based modelling. An experimental data-based model is developed and investigated for tolerance and error analysis. It would save the experimental necessity, expenses involved, indirect environment constraints, and time involved.
2. Materials and Methods2.1. MaterialsChemicals essential for the present research study are polysulfone (PSF) of Molecular weight (M.W.) 35000 Dalton (Da) is purchased from Otto Chemie Pvt. Ltd. India. N,N’-Dimethyl acetamide (DMAc) was bought from Loba Chemie Pvt. Ltd. India. Polyethylene glycol (PEG) with M.W. 200 (PEG-200), 400 (PEG-400), 600 (PEG-600) Da were procured from High Purity Lab. Pvt. Ltd. India. PEG of M.W. 1500 and 6000 (Da) were obtained from Loba Chemie Pvt. Ltd. India and Sisco Research Lab Pvt. Ltd. India, respectively. Zinc oxide nano powder of 80–100 nm size was bought from Nanoshel LLC, India. HCl was purchased from Merck Ltd., India. Potassium dichromate (K2Cr2O7), and potassium permanganate (KMnO4) were purchased from Sisco Research Lab. Pvt. Ltd., India. Non-woven polyester backing 3324 was purchased from Ahlstrom Hollytex, Finland.
2.2. Membrane PreparationThe UF membrane used for analysis was prepared using ShivOhm automatic casting system. The polysulfone with concentration ranging from 23 to 43% was dissolved in dimethylacetamide. The polyethylene glycol of different molecular weight and different concentration [8, 21, 22] and ZnO nanoparticles from concentration 0.2 to 1% (nascent or acid treated) were added in dope solution [8, 23]. In particular, the backing was attached to flat glass plate by scotch tape and attached to the caster. The degassed solution was poured on the same and spread using doctor knife attached to the system. A gap of 100 μm was maintained between knife edge and backing. The transition speed of knife was maintained at 100 mm/s using a drive attached. Air dry time was maintained at 10 sec, followed transition into gelation bath maintained at 30°C. Formed membranes were maintained at 4°C, in formalin solution.
The formed membranes were used for property analysis and application. The data was collected in triplicate and average was used so as to avoid issues with manual and experimental errors.
2.3. Membrane Bubble Point, Pore Size, and Number of Pore AnalysisThe membrane samples preserved in formalin solution were dipped into water to avoid effect of formalin on membrane property analysis. Such wet membrane coupon was placed in sample holder and air was pressurized through it. The air was allowed to pass with lowest pressure 0.1 bar for 5 min and then same procedure is repeated with 0.1 bar incremental pressure. The pressure at which maximum pore size of membrane opens, and a continuous air bubble is observed, the corresponding pressure is called bubble point pressure [24]. The pore size of membrane is calculated by using Cantor’s equation expressed as Eq. (1) and pore density by Hagen-Poiseuille’s equation given as Eq. (2).
where rpi is pore radius in m, σ is surface tension (N/m), θ is contact angle (degree), Pi is the applied pressure to open these pores (Pa), Ni is the number of pores per unit area (m2), η is water viscosity (Pa.s), Ji is water flux at ith increment when applied pressure is Pi, l is length of pore which is assumed to be equal to skin layer thickness of membrane, similarly Ji-1 is water flux at corresponding ith decremental pressure Pi-1 [25, 26].
2.4. Rejection and Water Flux AnalysisPure water flux was calculated with the help of dead-end cell. Here the membranes samples were mounted in dead end cell. It is fed with distilled water and pressurized using compressed air. The transported water is collected at regular time interval and is used in calculation of water flux using Eq. (3). The detailed procedure was reported by Dhume et. al. [21].
where J is water flux in lit/m2·h (LMH), V is volume of water collected, A is the cross-sectional area of the membrane (m2) and ΔT is the time (s) to collect water of volume V. Though the LMH is not an SI unit, it is largely used to define membrane properties.
The membrane selectivity is the key factor based on which the membrane will be selected for separation. It is defined with the help of membrane rejection analysis. A synthetic solution of 1000 ppm concentration is fed to the membrane cell. It was maintained with constant stirring to avoid concentration polarization and fouling as reported earlier [8,21]. The permeate solution was collected after disposal of 50 ml solution to avoid effect of water from pores of membranes, as reported earlier [8]. The concentration of metal salts in collected samples were analyzed with the help of UV spectrophotometer. The membrane rejection (%R) can be calculated by Eq. (4)
2.5. Statistical AnalysisMultiple regression method is significant in statistical analysis of data. R-Studio and Statistics online tool has been used in this analysis [27]. Regression analysis provides mathematical relation between dependent and independent variables. If X is an independant variable, k is number of independent variables and Y is dependent variable, then multiple regression model equation is given by Eq. (5)
where β0, β1, … etc. are regression model coefficients and ɛ is random error or supporting element [20]. Y is dependent variable-metal ion rejection efficiency. X1, X2, … etc...are independent variables and β1, β2, … etc. are respective regression model coefficients. Generally, data fitting is validated by R square, adjusted R square, multiple corrélation coefficient R and F-test [16]. R square measures correlation strength on 0 to 100% between dependent variable and linear model. The value of R square ranges from 0 to 1. If R square is zero, it indicates that there is no linear relation between observed and predicted values. If it is one, it means predicted and observed values are identical. While if R square is 0.5, it implies that half of variance is explained by model based upon considered dependent variable The variance measures how each number in the data set is away from average mean. Adjusted R square considers both the number of data points, and independent variables number for predicting data. Adjusted R square is calculated by Eq. (6),
where n is the number of data points, k is number of independent variables.
3. Results and Discussion3.1. Membrane Transport Properties and Rejection AnalysisThe membranes were formed using PSF, DMAc, PEG and ZnO nanoparticles (nascent or acid treated). PSF is chosen as base polymer because it has excellent chemical inertness across the entire pH range, good compressive strength and has high mechanical, chemical, thermal, and hydraulic stability [31]. Also, it has low water absorption rate, swelling and shrinkage [21]. It is widely available, inexpensive, and easy to process. Major limitation of PSF to be used as membrane is its hydrophobic nature [32]. This can be overcome by proper use and selection of additives and its use in optimum amount. DMAc is chosen as a solvent for PSF as it forms a stable dope solution at a higher concentration of PSF. Also, the use of DMAc as a solvent can give a more porous structure as compared with N,N′-dimethyl formamide (DMF) and N-methyl pyrrolidone (NMP) [18,33]. The polyethylene glycol of different molecular weight and different concentration were added to impart hydrophilicity and smoothness to membrane [22]. It will help to reduce fouling of membrane [8,21,22]. The ZnO nanoparticles from concentration 0.2 to 1.0% were added in dope solution. It helps to improve morphology of membrane and imparts charge to membrane. The use of acid treated ZnO particles shows better performance than nascent [8,23]. Similar enhanced separation of boron and humic acid has been reported [13,14]. The separation principle applied is Donnan exclusion. The different composition membranes used for separation are shown in Table 1.
The experiments were carried out in triplicate with the variation of ± 5% in results, while sequential optimizing the parameters. The results of different membranes for rejection efficiency and membrane morphology are presented in Fig. 1 to 6.
It can be seen from Fig. 1, 2; the pore size, pore density, bubble point pressure, and rejection efficiency depend on composition of dope solution used for formation of membrane. Membrane pore size and number of pores plays an important role to define membrane separation efficiency and transport rate [8,21,25]. To calculate the pore size of membrane, the bubble point pressure is required. The pressure at which maximum size of pores opens is called as bubble point pressure. It was observed that when concentration of polymer increases, its pore size has reduced, resulted in increase in bubble point pressure required [21,24,34]. Similarly, when pore former PEG (0 to 10% concentration) is used the pore density has increased by maintaining rejection properties [31,35]. There is always a trade-off between membrane selectivity and transport rate. For good selectivity lower pore size is preferred whereas it reduces the water flux. The Donnan exclusion separation retains the solute particles in spite of larger membrane pore size [11,12]. For any membrane processes, at low pressure the good water flux is desirable. The Fig. 3 shows the effect of PEG M.W. and Fig. 4 describes the effect of PEG concentration on membrane morphology, permeability, and selectivity.
It can be seen from Fig. 3, decrease in M.W. of PEG resulted in increase in porosity, but reduction in the pore size of membrane. It leads to increase in bubble point pressure, and smaller pores enhanced metal ion rejection properties. Enhanced transport properties with minimum pore size and maximum metal ion rejection were observed at 8% PEG concentration of base polymer. Further a reduction in pore size and enhanced rejection properties with decrease in PEG molecular weight has been observed [21,24,25]. The effect of concentration of PEG-400 and ZnO nanoparticles is shown in Fig. 4. The improvement in rejection efficiency for PEG-400 as compare with higher M.W. PEG was observed.
Fig. 5 and 6 show the effect of acid (HCl) treated ZnO nano particles use in membrane. As seen from Figs. 2 and 6, the use of HCl treated ZnO as an additive to dope solution resulted in large increase in heavy metal rejection efficiency of the membrane formed [11,12] as compared with use of nascent ZnO nanoparticles. The rejection efficiency increased from 90.5% to >99% as seen from the rejection of Mn for membranes with 40% PSF in dope solution (M40 and M36, respectively). The same could be taken to more than 99 % by variation in ZnO acid treatment from 1 hr to 1.5 hr, while the PEG MW changed from 400 to 200 Da. Whereas it was increase from 98.3 to 99% even for Cr [Fig. 6]. This excellent metal rejection efficiency is important in removal of these metals from water. It underlines more than 99% removal of these metals per pass.
This separation and variation in metal rejection properties can be attributed to variation in membrane surface composition. The variation in membrane surface material can be seen varying with composition in dope solution (Fig. S1).
A membrane with a surface charge similar to the salts in solution, for instance, would have a higher rejection rate. The Donnan exclusion principle is recognised for such repulsive interaction [5,21]. As seen from electron dispersive x-ray diffraction (EDS) scanning electron microscopic (SEM) images, is used to study surface qualities, chemical composition, and component distribution. As seen from EDS image (Fig. S1-A) shows presence of C, O, S, and Zn in membrane and there is absence of chlorine. It suggests ZnO nanoparticles are present in their nascent form. Also, the distribution of Zn was observed to be homogeneous in Fig. S1-B. Whereas Fig. S1-C shows the presence of elements C, O, S, Zn, and Cl in membrane matrix, presence of Cl indicates ZnO was treated with HCl. The distribution of Zn is extremely good in Fig. S1-D as compared to Fig. S1-B This suggests the membrane formed from ZnO modified with HCl and has homogeneous distribution, and dispersion of nanoparticles. It supports formation of the highly charge incorporated in membrane with evenly distributed membranes, which resulted producing the maximum rejection properties for Mn and Cr.
This excellent removal efficiency provides a major tool for removal of these metals from industrial effluent and ground water. Such removal would help us design the separation system to obtain the pure water, which can be used in further applications. It has been observed that membranes showed excellent metal rejection efficiency for dope solution containing 8% PEG and 0.8% ZnO nanoparticles concentration. The optimized membranes would be an excellent system for separation of these molecules and purification of water.
3.2. Multi-Attribute Linear Regression Model for ‘Mn’ and ‘Cr’ Rejection AnalysisR studio software was used for multiple regression statistical analysis for correlating metal ion removal efficiency of polysulfone based membranes with its formation and operational parameters. Multiple linear regression expresses the linear equation, outliers and calculates the p-value, R and adjusted Fisher-Pearson coefficient of skewness [36,37]. For example, if the data distribution is skewed, this approach will adjust it to gaussian kind of distribution. A linear regression model which encompasses more than one explanatory or predictor variable is referred to as multiple linear regression model. The Eq. (9) is fitted regression model for Mn ions.
where Y is dependent variable defining metal (Mn) ion rejection efficiency. P1, P2, P3, P4, P5, P6 are independent variables viz., PSF concentration, PEG M.W., ZnO concentration, flux in m3/m2·s, bubble point pressure in bar, and pore size in nm, respectively; while β1, β2, … are respective regression model coefficients.
Overall correlation obtained through multiple linear regression indicated that the metal rejection Y is dependents on PSF concentration, PEG (M.W), PEG concentration, ZnO concentration in membrane formation solution and treatment, flux, bubble point pressure, pore size, no. of pores. The correlation can be defined as Y, (F(6, 37) = 208.02, p < 0.001, R2 = 0.97, R2adj = 0.97).
The individual predictors were calculated further and specified that PSF concentration (t = 5.359, p < 0.001) and PEG (M.W) (t = −4.201, p < 0.001), PEG concentration (t = 3.578, p < 0.001), ZnO concentration (t = 3.336, p = 0.002), ZnO nano treatment (t = 4.557, p < 0.001) and Flux (t = 3.259, p = 0.002) were significant predictors in the model.
In this approach, based on the predictor’s p-value by applying the backward stepwise procedure the preliminary sorting of the predictors is generated. The statistical data correlation analyzed by p–value. It is used to define the statistical confidence level. Since the overall p value is much smaller than 0.01, it indicates there is 99% confidence level for relation between variables [20].
Further it is supported by R2 analysis. R2 value of 0.9712 which shows that the predictors explain 97.1% of the variance of Y variable and excellent data correlation with metal ion rejection. It means data points are scattered less around fitted regression line or there is very small difference between observed and fitted data.
Adjusted R square equals 0.967115 indicates that statistic is well suitable for comparing model with different number of variables. The coefficient of multiple correlation (R) equals 0.9855 which emphasis on a very strong correlation between the observed and predicted data.
It is observed from regression model (Eq. 9), the regression variable ZnO nanoparticle concentration (10.90) and pore size (618864889.9) has higher influence on metal ion rejection as compared to number of pores. This itself supports our theoretical consideration of Donnan exclusion principle based heavy metal separation. A smaller pore size would bring a higher number of heavy metal ions in the sphere of influence. This would result in higher retention. Further the effect would be supported by ZnO concentration. Higher concentration would result in higher charge and interaction-based rejection of heavy metal ions. The number of pores would support transport properties or flux, higher number of pores would enhance transport rate due to availability of larger number of transport passages. This enhancement in pores is provided by leaching of PEG during formation of membrane by phase inversion. Hence the PEG concentration would also affect transport properties significantly not the rejection. Thus, the model defines heavy metal rejection excellently on the basis of theoretical consideration and parameters of metal ion separation by Donnan exclusion principle. The higher R square value and other parameters of tolerance and statistical confidence level shows excellent fit and good quality of regression and model equation, which supports the theoretical considerations [1,19,20].
Similarly for Cr Multiple linear equation is given by Eq. (10).
Here in Eq. (10), Y is dependent variable defining metal (Cr) ion rejection efficiency. Q1, Q2, Q3, Q4, Q5, Q6 are independent variables viz., PSF concentration, PEG M.W., ZnO concentration, bubble point pressure in bar, pore size in nm and number of pores, respectively, which are affecting Cr. Removal efficiency. Whereas β1, β2, … etc. are respective regression model coefficients correlating independent variables with Cr separation efficiency. A variation is observed here compared with Mn based model that the water flux is replaced by pore size and number of pores. These are interdependent parameters, where the water flux is dependent upon combination of number of pores and pore size.
The overall outcomes of the multiple linear regression showed that there was a combined significant noteworthy effect between the PSF concentration, PEG (M.W), PEG concentration, ZnO concentration, ZnO nanoparticles treatment, flux, bubble point pressure, pore size, no. of pores, and Y, (F(6, 37) = 373.9, p <.001, R2 = 0.98, R2adj = 0.98).
In the result, R square is calculated as 0.98377 which shows that the predictors describe 98.4% of the variance of Y variable. The coefficient of multiple correlation (R) square equals 0.9918 which emphasis on a very strong correlation between the observed and predicted data.
The individual predictors were further studied and shown that PSF concentration (t = 7.532, p < 0.001) and PEG (M.W), (t = −3.707, p < 0.001) and PEG concentration (t = 2.622, p = 0.013) and ZnO concentration (t = 6.391, p < 0.001) and ZnO nanoparticles treatment (t = 5.592, p < 0.001) and flux (t = 3.913, p < 0.001) were significant predictors in the model.
3.3. ToleranceThe linear regression assumes there is no, modest, or high multi-collinearity in the data. The multicollinearity is a phenomenon in which one predicted variable is calculated through others with good accuracy. Multicollinearity occurs when these variables are well related to each other. It also determines interrelation between two or more independent variables by calculating the tolerance [19]. The tolerance determines the interrelation between data points. From the data the R square is 0.97 for Mn and 0.98 for Cr, it means tolerance is 0.03 and 0.02 for Mn and Cr, respectively, it indicates there is good multicollinearity in the data.
3.4. Variance Inflation FactorThe VIF shows correlation for predicted values, or it can calculate the intensity of correlation between independent variables. If VIF value is 1, it indicates predictors are not correlated. If VIF is between 1 to 5, it is moderately correlated and if it is larger than 5, it is strongly correlated [20,30]. The use of VIF is to know how much variance of calculated coefficient are affected by multicollinearity [30].
For Mn and Cr, the overall VIF values are 33.33 and 50, respectively. As these VIF values are greater than 10, it suggests a high multicollinearity relevance. The multicollinearity may impact the coefficients or the skill to select the predictors, but not suitable for the dependent variable (Y). The priori power that is the power to test the entire model is strong which gives a value of 0.8294.
3.5. Model Adequacy Testing3.5.1. Quantile-Quantile (Q-Q) plotThe sample data analysis for goodness-of-fit is verified by Q-Q plot [38,39]. It can determine data normality by graphical assessment. The data points are normal if points are closer to straight line, and they are termed as outliers if points are scattered. The residual plots that help to check whether the data matches the normality and homoscedasticity assumptions of linear regression. Residual value is the difference between actual and predicted value. Q-Q plots compares the distribution of data to a normal distribution by drawing the quartiles of data against normal distribution. The residual Q-Q plot from Fig. 7 A1 (for Mn) and A2 (for Cr) indicates the data is homoscedastic and normally distributed. There are rare outliers and almost all data points are close to reference line shows the data normality.
For Mn, the overall regression is right tailed with values of F = 208.0161, p-value = 0. Since p-value < a (0.05), the NULL hypothesis. The independent variables - PEG concentration, ZnO nano particles treatment, no. of pores is not substantial as predictors for Y. Therefore, these variables are omitted from the model. Linear regression presumes normality for residual errors. The p-value equals 0.4916 hence it is supposed the data to be normally distributed. In homoscedasticity the white test p-value equals 0.05998 (F = 3,0161) thus the variance is homogeneous and validates residual normality.
For Cr, the general regression is right tailed with values of F = 373.904, p-value = 0. Since p-value < a (0.05), the NULL hypothesis. The independent variables - PEG concentration, flux, and ZnO nano treatment are not considerable as predictors for Y. Therefore, these variables are omitted from the model.
3.5.2. Residual and predicted versus actual plotIn residual versus actual plot the data points are symmetrically scattered around reference line is shown in Fig. 7-B1 (for Mn) and 7-B2 (for Cr). Whereas in predicted versus actual graph Fig. 7-C1 (for Mn) and 7-C2 (for Cr)., the data is closer to reference line and no outliers. The predicted Vs actual graph shows that all the points are close to the regressed diagonal line, it suggests good fit of data [38]. This study is satisfactory to correlate the input variable influence on different dependent and independent variables. Though, the ZnO nano particles treatment in model suggests some more experimentation is required to find the dependency of rejection on this parameter.
3.5.3. Standard error and deviation in the analysisIn a multiple linear regression model, the error term represents the variability in the data that is not explained by the model. The error term is assumed to be normally distributed with a mean of zero and a constant variance. Error bars can be used to represent the uncertainty in the predicted values of the dependent variable. One way to do this is to calculate the standard error of the estimate, which is a measure of the variability of the predicted values. The standard error can be used to construct confidence intervals around the predicted values, which can be represented graphically using error bars. The residual standard error is 5.81, which measures the average deviation of the observed values of y from the fitted values. The multiple R-squared value is 0.9603, indicating that the model explains a high proportion of the variance in the data [39]. The confidence interval for the predicted values of the dependent variable y is given by the fit, lower, and upper variables of the output. The fit value represents the fitted value, which is the predicted value of the dependent variable based on the regression model. These values represent the fitted value and the lower and upper bounds of the confidence interval, respectively. In Mn case, the fitted value is 59.59136 and the 95% confidence interval for the predicted value of y is [57.81311, 61.36962]. In Cr case the fitted value is 56.09121 and the 95% confidence interval for the predicted value is [54.94518, 57.23723]. This means that we can be 95% confident that the true mean value of y lies within this interval. Error bars can be used to represent this uncertainty in the predicted values graphically. The length of the error bars would correspond to the width of the confidence interval, with longer error bars indicating greater uncertainty in the predictions. The error bar plot with respect to the predicted value y is shown in the supplementary materials (Fig. S2).
4. ConclusionsSeparation of heavy metals using PSF based ultrafiltration membranes is based upon membrane formation parameters and properties, viz., PSF concentration, PEG M.W., ZnO concentration and treatment of ZnO nanomaterial by HCl. These parameters affected membrane properties of bubble point pressure in bar, pore size in nm, number of pores, water flux, which affected the removal properties for Mn and Cr. The anchoring of ZnO nanoparticles suitably modified by treatment with strong acids, vary surface charge to PSF based membranes. These charged membranes showed excellent removal properties for Mn and Cr salts as 97.12 and 98.37%, respectively, which can be optimized to 99% for both materials. The combination of PSF as base material for mechanical, chemical and thermal stability and anchoring such charged nanomaterial provide an excellent method for formation of membranes for industrial applications. This formation parameters were correlated with separation properties using multiple linear regression with the help of R studio software. A model was built and was found to possess excellent correlation with experimental as indicated by R square is 0.97 and 0.98 for Mn and Cr, respectively. Further their smaller (0.01) P-value suggests 99% confidence level for relation between variables, while tolerance of 0.02–0.03 implies good multicollinearity in the data. The nature of residual plots implies the data is homoscedastic and normally distributed.
Altogether the optimization approach and statistical analysis can be utilized for further membrane development based upon PSF with desired modification using porogenic PEG and surface modifying ZnO for desired requirement of metal removal. The considered metals are some of the major pollutants in the tannery and metal processing industry. These optimized membranes can be utilized in combination with processes towards real life applications for separation of these metals. Similarly, the optimization approach and membrane properties can be tuned for separation of range of heavy metals owing to variation in their physical and chemical properties. This would provide a techno-economical method for separation of such heavy metals.
AcknowledgementWe thank Bharati Vidyapeeth (Deemed to be) University, College of Engineering, Pune for providing us the useful resources. We also thank for the financial support from DST-Nano-Mission (Sanction No. SR/MN/NT/-1029/2015). We would like to take the opportunity to thank our family members for their understanding, as we have taken away the valuable time of togetherness to accomplish this work.
NotesAuthor contributions P.M.T and S.D. (PhD students) conducted the membrane formation and their analysis. N.J. (Professor, Computer Engineering) helped to model building and data analysis. Y.C. (Guide and Professor) worked towards development of concept, experiment design, data interpretation and correspondence. R.K. (Professor) helped in data interpretation and correlation. S.C. (Professor) helped in experiment design and data collection. V. W. (Professor) helped in analysis and data interpretation. References1. Selvi SR, Baskaran R. Statistical Study Using Multiple Regression Model in Reverse Osmosis. Int. J. Chem. Tech. Res. 2015. 8:211–220.
https://api.semanticscholar.org/CorpusID:73545949
2. De Souza GBM, Pereira MB, Mourao LC, Santos MP, Oliveira JA, Garde IAA, Alonso CG, Jegatheesan VJ, Cardoso FL. Supercritical water technology: an emerging treatment process for contaminated wastewaters and sludge. Rev. Environ. Sci. Biotechnol. 2022;21:75–1041. DOI:https://doi.org/10.1007/s11157-021-09601-0
3. Sonal R, Manish V, Vikas KS. Oxidative degradation of electroplating wastewater by an electro-Fenton process using GO/TiO2NTs electrode. Environ. Eng. Res. 2024;29:220056. DOI:https://doi.org/10.4491/eer.2023.056
4. Sevgi O, Nalan OSK. Development of reusable and long-term storable bacteria attached nanofiber mats for highly efficient removal of Cr(VI) and reactive dye from wastewater. Environ. Eng. Res. 2024;29:230036. DOI:https://doi.org/10.4491/eer.2023.036
5. Mahajan-Tatpate P, Dhume S, Chendake Y. Removal of Heavy Metals from Water: Technological Advances and Today’s Lookout through Membrane Applications. Int. J. Membr. Sci. Technol. 2021;8:1–21.
https://doi.org/10.15379/2410-1869.2021.08.01.01
6. Muhammad N, Temoor A, Usman I, Muhammad S, Muhammad MN, Azizullah JC, White , Dayong L, Fengming S. Bio-Functionalized Manganese Nanoparticles Suppress Fusarium Wilt in Watermelon (Citrullus lanatus L.) by Infection Disruption, Host Defense Response Potentiation, and Soil Microbial Community Modulation. Nano Micro Small. 2023;19:2205687.
https://doi.org/10.1002/smll.202205687
7. Ahmed F, Mohammed Mahdi A, Magdy MY, Gaber MAER. Investigation (IR, UV-visible, fluorescence, X-ray diffraction and thermogravimetric) studies of Mn(II), Fe(III) and Cr(III) complexes of thiosemicarbazone derived from 4-pyridyl thiosemicarbazide and monosodium 5-sulfonatosalicylaldehyde and evaluation of their biological applications. J. Molecular Str. 2023;1271:134139.
https://doi.org/10.1016/j.molstruc.2022.134139
8. Mahajan-Tatpate P, Dhume S, Chendake Y. Recovery of Chromium Using Membrane Containing Charged Material. IOP Conf. Ser. Mater. Sci. Eng. 2021;1146:012022. 10.1088/1757-899x/1146/1/012022
9. Fu F, Wang Q. Removal of Heavy Metal Ions from Wastewaters: A Review. J Environ Manage. 2011;92:407–418. 10.1016/j.jenvman.2010.11.011
10. Linlin Y, Xiaobin Y, Haoze Z, Yuanyuan Z, Yangxue L, Xuezhong H, Jun M, Lu S. Nanocomposite hydrogel engineered hierarchical membranes for efficient oil/water separation and heavy metal removal. J. Membr. Sci. 2023;668:121243.
https://doi.org/10.1016/j.memsci.2022.121243
11. Salih M, Al-Alawy AF. Mathematical Modelling of Zinc Removal from Wastewater by Using Nanofiltration and Reverse Osmosis Membranes. Int. J. Sci. Res. 2018;7:45–52. 10.21275/ART20179156
12. Muthumareeswaran MR, Alhoshan M, Agarwal GP. Ultrafiltration Membrane for Effective Removal of Chromium Ions from Potable Water. Scientific Rep. 2017;7:1–12. 10.1038/srep41423
13. Kok PW, Chai HK, Yean LP, Woon CC, Woei JL. Purifying surface waters contaminated with natural organic matters and bacteria using Ag/PDA-coated PES membranes. Environ. Eng. Res. 2023;28:220097.
https://doi.org/10.4491/eer.2022.097
14. Rajesh KA, Mansour A, Garudachari B, Mansour AR, Jibu PT. Ethylenediamine-graphene oxide impregnated thin film nanocomposite membrane for the enhanced boron separation from seawater. Environ. Eng. Res. 2023;28:220760.
https://doi.org/10.4491/eer.2022.760
15. Abdelrasoul A, Doan H, Lohi A, et al. Morphology Control of Polysulfone Membranes in Filtration Processes: A Critical Review. Chem. Bio. Eng. Rev. 2015;2:22–43. 10.1002/cben.201400030
16. Alvi M, Khalid MW, Ahmad NM, et al. Polymer Concentration and Solvent Variation Correlation with the Morphology and Water Filtration Analysis of Polyether Sulfone Microfiltration Membrane. Adv. Polym. Technol. 2019;2019:1–11. 10.1155/2019/8074626
17. Chakrabarty B, Ghoshal AK, Purkait MK. Effect of Molecular Weight of PEG on Membrane Morphology and Transport Properties. J. Membr. Sci. 2008;309:209–221. 10.1016/j.memsci.2007.10.027
18. Madaeni SS, Rahimpour A. Effect of Type of Solvent and Non-Solvents on Morphology and Performance of Polysulfone and Polyethersulfone Ultrafiltration Membranes for Milk Concentration. Polym. Adv. Technol. 2005;16:717–724. 10.1002/pat.647
19. Sureiman O, Mangera C. F-Test of Overall Significance in Regression Analysis Simplified. J. Pract. Cardiovasc. Sci. 2020;6:116. 10.4103/jpcs.jpcs_18_20
20. Dang HQ. Mathematical Modelling for Predicting Rejection of Trace Organic Contaminants by the Nanofiltration Membrane Nf270. J. Environ. Treat. Tech. 2020. 8:900–907.
https://api.semanticscholar.org/CorpusID:219178729
21. Dhume SS, Mahajan-Tatpate P, Chendake YJ. Optimization of PSF Membrane Transport Properties with the Use of Porogenic Additive. J. Appl. Membr. Sci. Technol. 2020;24:57–73. 10.11113/amst.v24n3.193
22. Javdaneh S, Mehrnia MR, Homayoonfal M. Fabrication of Polysulfone/Zinc Oxide Nanocomposite Membrane: Investigation of Pore Forming Agent on Fouling Behavior. Korean J. Chem. Eng. 2016;33:3184–3193. 10.1007/s11814-016-0178-3
23. Mukherjee R, Mondal M, Sinha A, et al. Application of Nanofiltration Membrane for Treatment of Chloride Rich Steel Plant Effluent. J. Environ. Chem. Eng. 2016;4:1–9. 10.1016/j.jece.2015.10.038
24. Phale JS, Chendake YJ. Polysulfone Based Ultrafiltration Membrane Preparation by Phase Inversion: Parameter Optimization. Int. J. Sci. Res. 2013. 5:2319–7064.
https://www.ijsr.net/archive/v5i6/ART201673.pdf
25. Lohokare HR, Bhole YS, Kharul UK. Effect of Support Material on Ultrafiltration Membrane Performance. J. Appl. Polym. Sci. 2006;99:3389–3395. 10.1002/app.23039
26. Mulder M. Basic principles of membrane technology. Kluwer Academic Publishers; 1996;
https://doi.org/10.1007/978-94-009-1766-8
28. Alin A. Multicollinearity. Wiley Interdiscip. Rev. Comput. Stat. 2010;2:370–374. 10.1002/wics.84.polyether
29. Daoud JI. Multicollinearity and Regression Analysis. J. Phys. Conf. Ser. 2018;949(1)10.1088/1742-6596/949/1/012009
30. Mansfield ER, Helms BP. Detecting Multicollinearity. Am. Stat. 1982;36:158–160. 10.1080/00031305.1982.10482818
31. Pagidi A, Saranya R, Arthanareeswaran G, et al. Enhanced Oil-Water Separation Using Polysulfone Membranes Modified with Polymeric Additives. Desalination. 2014;344:280–288. 10.1016/j.desal.2014.03.033
32. Vatanpour V, Madaeni SS, Moradian R, Zinadini S, Astinchap Bl. Novel Antibifouling Nanofiltration Polyethersulfone Membrane Fabricated from Embedding TiO 2 Coated Multiwalled Carbon Nanotubes. Sep. Purif. Technol. 2012;90:69–82. 10.1016/j.seppur.2012.02.014
33. Arthanareeswaran G, Starov VM. Effect of Solvents on Performance of Polyethersulfone Ultrafiltration Membranes: Investigation of Metal Ion Separations. Desalination. 2011;267:57–63. 10.1016/j.desal.2010.09.006
34. Plisko TV, Bildyukevich AV, Usosky VV, Volkov VV. Influence of the concentration and molecular weight of polyethylene glycol on the structure and permeability of polysulfone hollow fiber membranes. Petroleum Chem. 2016;56:321–329.
https://doi.org/10.1134/S096554411604006X
35. Ma Y, Shi F, Ma J, et al. Effect of PEG Additive on the Morphology and Performance of Polysulfone Ultrafiltration Membranes. Desalination. 2011;272:51–58. 10.1016/j.desal.2010.12.054
36. Kronthaler F, Zöllner S. Data Analysis with R Studio. Springer Spektrum; Berlin, Heidelberg: 2021.
https://doi.org/10.1007/978-3-662-62518-7
37. Osborne JW, Waters E. Four Assumptions of Multiple Regression That Researchers Should Always Test. Pract. Assessment, Res. Eval. 2003;8:2002–2003. DOI:https://doi.org/10.7275/r222-hv23
Fig. 2Effect of membrane composition on their properties of water flux in (lit/m2.h, LMH) and Cr, Mn rejection. 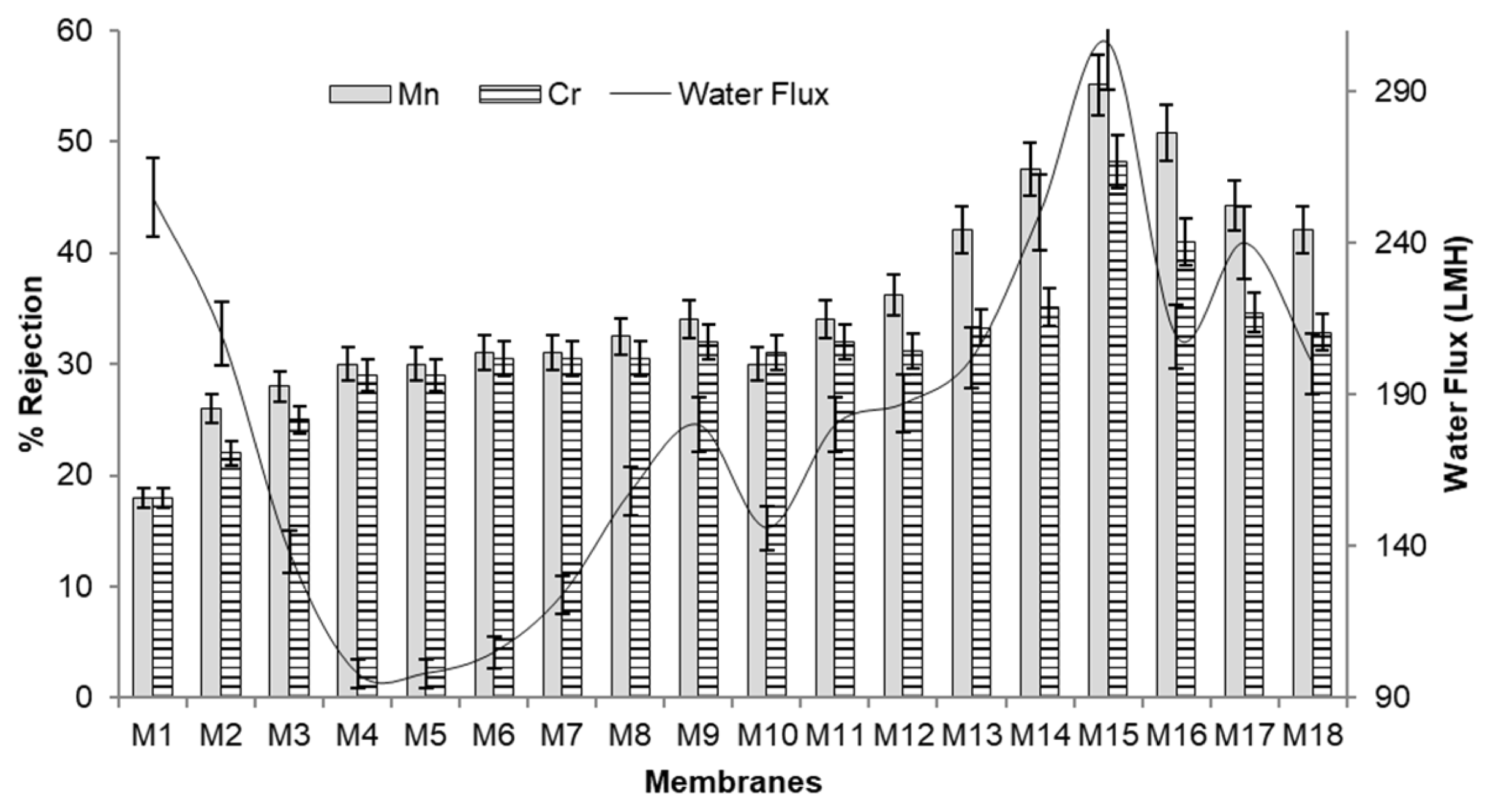
Fig. 3Effect of PEG Molecular weight (M.W.) on bubble point, number of pores, water flux, and Cr and Mn rejection properties. 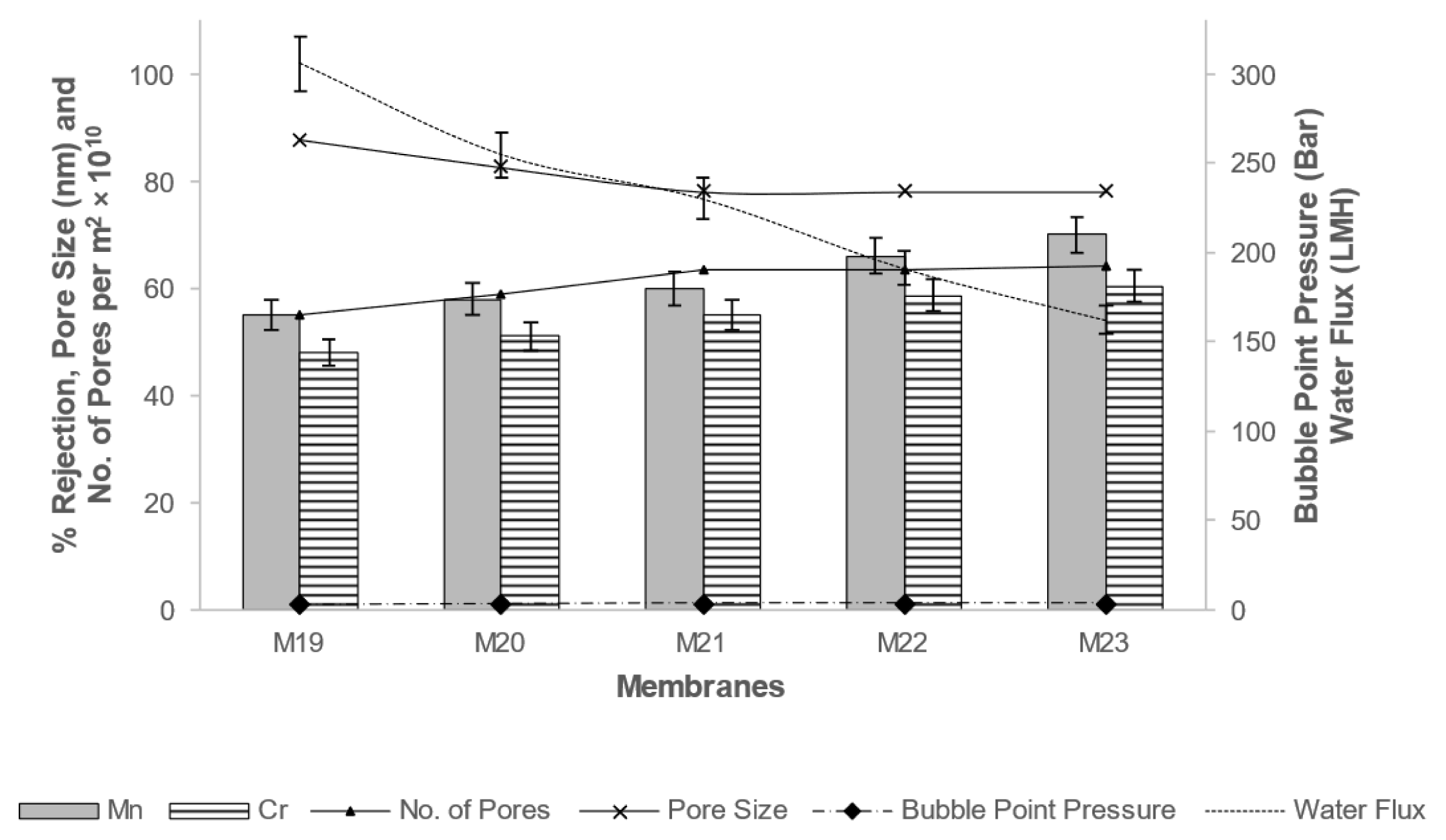
Fig. 4Effect of concentration of PEG400 and ZnO nanoparticles on membrane water flux, bubble point, number of pores, and Cr and Mn rejection properties. 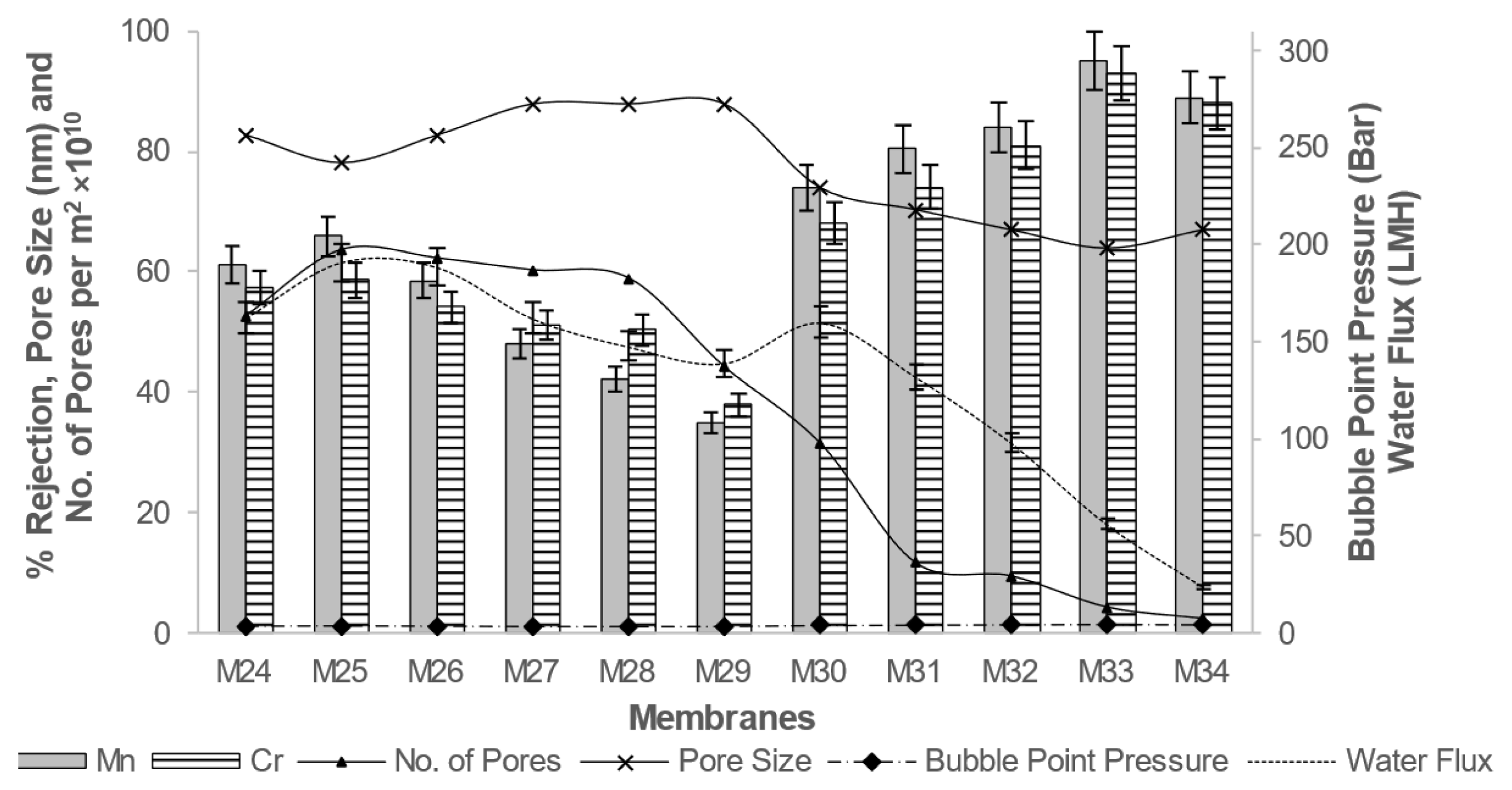
Fig. 5Effect of acid treated ZnO nanoparticles concentration on pore size, number of pores and bubble point properties of PSF based membranes with PEG-200. 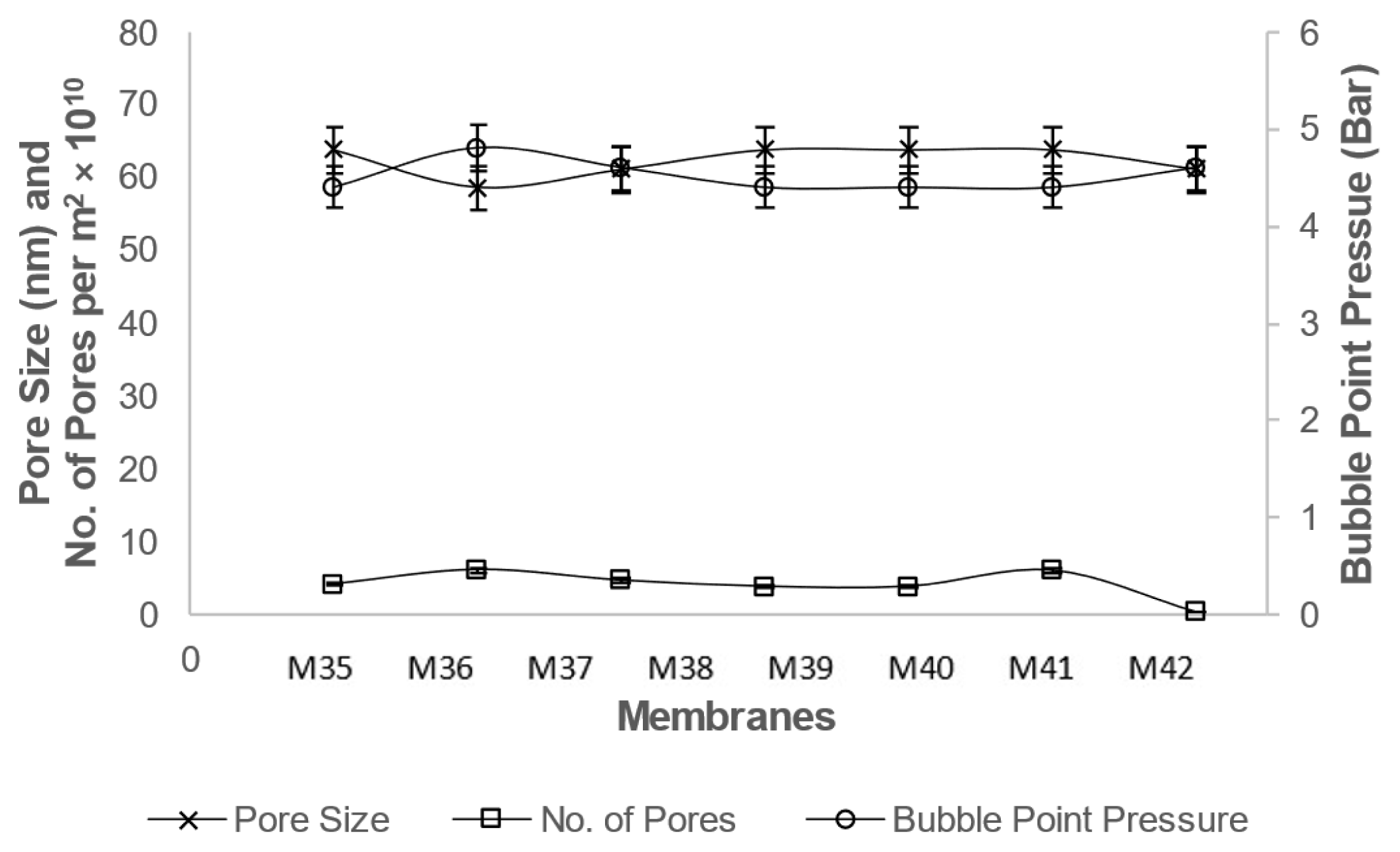
Fig. 6Effect of acid treated ZnO nanoparticles concentration on water flux and rejection on PSF based membranes containing PEG-200. 
Table 1Membranes compositions and their nomenclature |
|
||||||||||||||||||||||||||||||||||||