AbstractBioelectroremediation technology (BERT) is a new age greener technology which is currently been heavily studied for treatment of persistent industrial effluents such as polycyclic aromatic hydrocarbons (PAHs), perchloroethylene (PCE), perchlorate, nitrate and petroleum hydrocarbon (PHs) along with simultaneous generation of bioenergy. The focus of this review paper is on these industrial effluents because many of these effluents are toxic in nature and through contaminated water, soil or sediment enter the food chain and start to bioaccumulate thereby entering living organisms and causing severe fatal diseases. While PAHs and nitrates are major contaminant of both water and soil but their removal efficiency through BERT is much more in water sample than from soil samples, studies have shown that perchlorate and nitrate are usually co-contaminants and perchlorate, PCE and PHs are much more prevalent groundwater contaminant with good removal efficiency of these contaminants and simultaneous bioelectricity generation through BERT. Therefore, this review paper focus on role of microbial community structure as a biocatalyst in BERT along with a in-depth review of bioelectroremediation process for industrial effluent treatment involved in removing diverse pollutants integrated with energy generation from fundamentals, challenges, and future prospective dimensions.
Graphical Abstract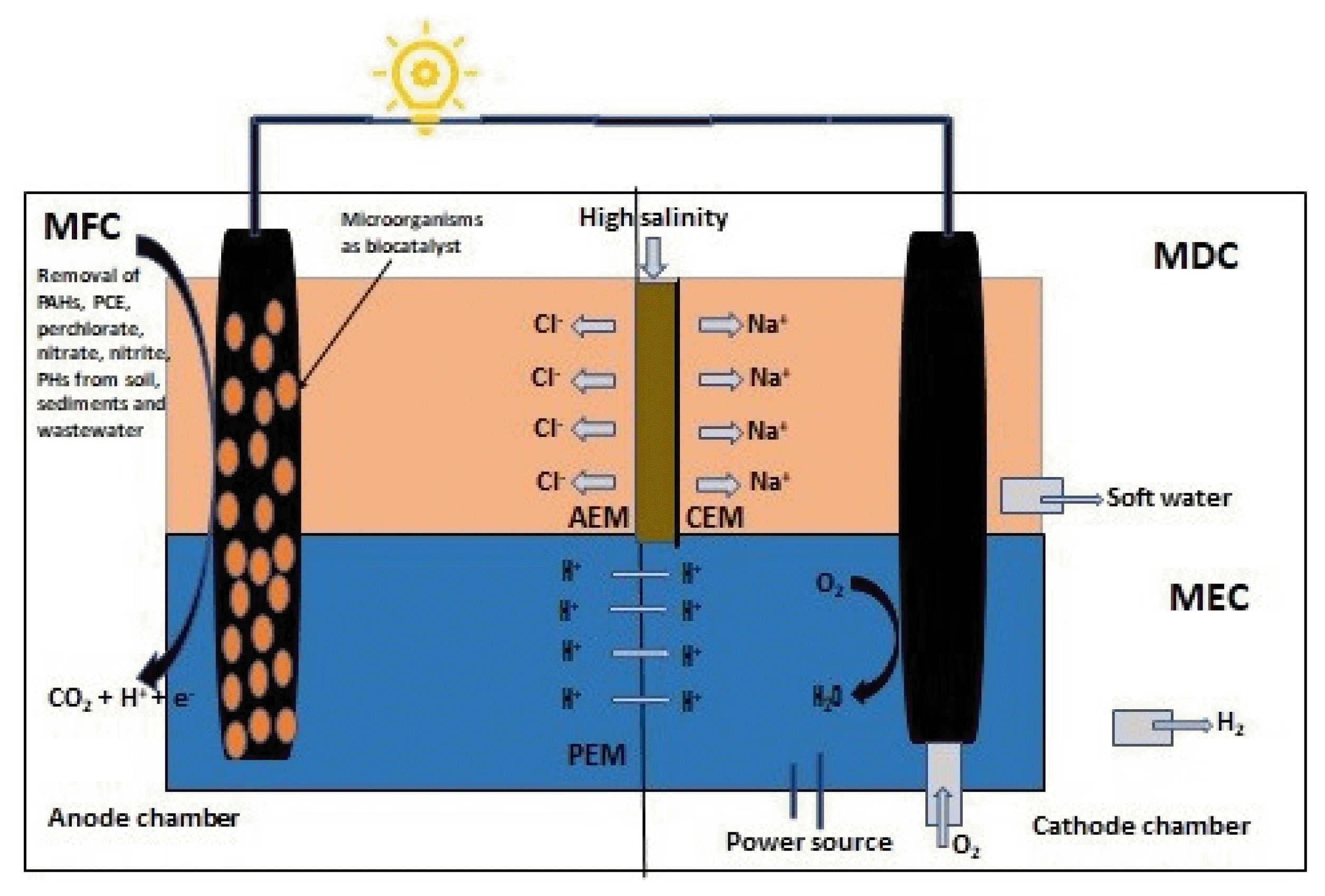
1. IntroductionWith increasing population growth there is a global need to manage the availability of food, water and shelter along with reduction of contamination of air, water and soil. The rise in industries and factories without proper management of effluent treatment has led to large-scale contamination of water and land [1–5]. Effluents released from industries such as heavy metals, petroleum hydrocarbons (PHs), dyes from tanneries and electroplating industry are harmful and toxic to any living organism, as they lead to disruption of cell function and metabolism, posing severe health problems and eventually lead to life threatening diseases as cancer, lung diseases and kidney malfunctioning, due to their potential of bioaccumulation inside living organism [4,6–11]. The release of several industrial effluents are beyond the permissible limits set by World Health Organization (WHO) and Bureau of Indian Standards (BIS) [4,12,13].
Majority of the time conventional physiochemical method such as filtration, coagulation, flocculation, precipitation, adsorption etc. are used to remove pollutants from soil, wastewater as well as groundwater [14–16]. But these techniques are neither absolutely efficient nor are they cost effective, and they also tend to release by-products that are unable to achieve the permissible limits of discharge without requiring additional operating expenses and costly retrofitting to the existing systems [17–21]. Effluent treatment is costly, complicated and therefore, does not receive the required attention before disposal [22–25]. Bioelectroremediation technology (BERT) is much more effective in removing effluents and does not release any toxic by-product also being cost effective. This is a rapidly progressing technology used by the scientific community from all over the globe for its dual ability to treat wastes and recover energy in the form of bioelectricity. With its capability to degrade different organic compounds, BERT provides a sustainable approach to environmental remediation along with resource recovery.
In developing countries due to shortage of technical experts in regards of control and operation of effluent treatment units, usually the input cost overruns the output products particularly in mid-scale factories that are expanding at a rapid pace [26]. BERT treats wastes without producing any secondary pollution along with recovering energy from the waste and have low-cost maintenance [27]. Apart from removing organic compounds, BERT is also capable of removing nitrogen and phosphorus from wastewater and waste streams [28–30]. For complete treatment of wastewater before disposal removal of nutrients is mandatory which would otherwise lead to eutrophication (nitrogen and phosphorus), global warming and ozone depletion (reactive nitrogen) and pose several other risks to human health [31–33]. Instead of nutrient removal, recovery of valuable nutrients is a much more sustainable approach, and current research with BERT technology are focusing on nutrients recovery.
Discharge of industrial effluents into soil and water resources has led to extensive contamination of natural ecosystems such as soil and river sediments [34–36]. And even though the effluents are treated to certain limit before disposal, sludge gets generated as a toxic byproduct where pollutants such as heavy metals, persistent chemicals and recalcitrant organic compounds get concentrated at levels far exceeding the untreated effluent [37–39]. BERT using polluted soil and sediment as substrates has been shown to be effective in-situ treatment of natural ecosystems [40–44] because they do not cause any disturbance to the natural environment and work by activating the microorganisms native to the water, soil or sediment for degradation of the waste [45,46].
In recent times, studies have shown that a number of electrogenic microorganisms have been explored for their ability of bio-catalysing and degrading many of these effluents [47–51]. Putting electrogenic microorganisms to work with electrochemistry makes BERT an excellent choice for treatment and removal of effluents from environment. In BERT, the chosen microbial consortium degrades and consumes the effluents as source of energy and in turn liberates electrons and protons which can be harnessed to produce bioelectricity which is a source of green energy. BERT can be used for both in-situ and ex-situ degradation of contaminants [47,48, 52–54]. Frequently used BERT are microbial fuel cells (MFCs), microbial electrolysis cell (MECs), enzymatic fuel cells (EFCs) and microbial desalination cell (MDCs). While in MFC energy is generated by reducing oxygen at cathode to produce water, in MEC energy is required to be supplied to the system to reduce protons at the cathode to generate hydrogen gas. EFCs utilizes enzymes as biocatalyst in replacement of costly and toxic metal catalyst to treat waste and generate electricity. MDCs removes ions and reduces salinity of water. In MDCs two ion exchange membranes are used such as one membrane is between the desalination and the anodic chamber and other one is in between the desalination and the cathodic chamber.
This review paper attempts to provide a comprehensive examination of bioelectroremediation process for industrial effluent treatment involved in removing diverse pollutants integrated with energy generation from fundamentals, challenges, and future prospective dimensions. It also reviews the role of microbial community (biocatalyst) in industrial effluent degradation. The focus of this paper is on industrial effluents such as polycyclic aromatic hydrocarbons (PAHs), tetrachloroethylene, perchlorate, nitrate and petroleum hydrocarbon (PHs) because these pollutants have higher bioaccumulation capability making them much more penetrable into the food chain and are able to contaminate not only surface water bodies but also groundwater, soil, and sediment which makes these effluents very difficult to bioremediate. Though industrial effluents containing heavy metal also comes under this category but heavy metal degradation is not included in this paper because recycling of metallic industrial effluents has already been thoroughly reviewed [55,56]. Therefore, this paper reviews the degradation efficiency of BERT of these industrial pollutants from either soil, sediment, wastewater or groundwater sources.
2. Bioelectroremediation Technology (BERT)The working principle behind a BER system is oxidation of organic contaminants and harnessing bioenergy from them. It works on the principle of electrochemistry accelerated by microbial catalyst. The substrate which can be anything ranging from soil or wastewater or groundwater pollutants, are fed into anodic chamber separated by a membrane (cation or anion exchange membrane, AEM or CEM/PEM) from cathodic chamber. The substrate gets consumed by the microorganism selected for treating the pollutant in the anodic chamber, in the process releases electrons and protons, the protons cross the membrane and reach cathodic chamber whereas the electrons flow through an outer circuit forming a potential difference as a result bioelectricity is generated (Fig. 1).
Each and every step involved affects the output of BERT, factors such as pH and temperature along with redox potential and ionic strength [57], where optimal pH increases microbial growth thereby increasing current and power production and also minimizing internal resistance in a MFC [58], whereas increased pH has shown to affect current production by decreasing its generation. Temperature also affects microbial growth by affecting microbial proteins activation energies [59]. Power density increases while internal resistance decreases with increase in temperature as it enhances growth of microbes [60]. High redox potential increases microbial growth which thereby facilitates more electron generation which in turn produces more bioelectricity [61].
There are 3 major types of bioelectrochemical systems - microbial fuel cells (MFCs), microbial electrolysis cell (MECs), and microbial desalination cells (MDCs) that are widely used at diverse stages ranging from small scale laboratory studies to pilot scale studies dedicated to remediation of wastewater and groundwater, desalination, generation of hydrogen as a fuel, sequestration of CO2, as well as formation of biosensors and production of value-added by-products. A microbial fuel cells (MFCs), convert biochemical energy (through microbes-assisted bio-oxidation) to electrical energy, while MECs generate hydrogen (by electro-hydrogenesis) or methane or H2O2 depending upon the substrates/reactions involved, by break down of organic compounds when external electrical energy is provided to the system [56]. The basis architecture of a MFC and a MEC are same with an anaerobic anode and an aerobic cathode being separated with a barrier or separator such as PEM or CEM which facilitate proton exchange along with degradation of various types of substrates ranging from simple sugars to complex wastewaters, sediments and soil with the aid of microbial-entities which act as biocatalyst. MDC is majorly used to remove ions and reduce the salinity of water. While in MFC and MEC a single membrane is used in MDC the desalination chamber is kept in between two membranes, one between desalination chamber and anode compartment (CEM) and the other one between desalination chamber and cathode compartment (AEM). The working principle of MDC is oxidation of organic compounds by microbes in the anodic chamber to release electrons which migrate via the bacterial cells to reach anode electrode and finally the cathode electrode via external circuit [56,62]. This generates the required potential difference for the anions and cations present in the desalination chamber to move towards the anode and the cathode, respectively, by crossing their respective ion exchange membranes. Recent studies have greatly improved MDC and their efficiency [63,64]. A schematic diagram of MFC, MEC and MDC are depicted in Fig. 1, 2 and 3 respectively.
While there are several advantages and applications of using BRET system such as they can produce alternate source of energy in form of biohydrogen and biomethane [65–67], they are eco-friendly with low carbon emission [66,68], recovery of valuable compounds from substrates and are much more efficient in conversion of substrate to electricity [68,70–72]. One of the main disadvantage or limitation of using BERT system is it’s upscaling. Power outputs from MFCs commonly have been shown to be high enough to only run small lab scale MFCs but studies are recently been done to overcome design constrains of MFCs by performing life cycle assessments and improving the engineering paraments for upscaling [73]. Other disadvantages and limitations are low growth rate of microorganisms, toxicity caused by the materials used, low power output, lack of durability and strength of electrodes, high cost and low efficiency of membranes, high material and maintenance cost [74]. Recently studies have greatly improved the limitations of BERT system by increasing their efficiency such as studies done to improve anodic electrode material and configuration [75–78] to improve its power generation capacity [79], to study substrate effect on MFCs connected serially in continuous mode [80], agricultural waste as efficient substrate for bioelectricity generation [25,81], improvement of cathodic performance by using catalyst made by binding metal-organic framework and activated carbon [71] and using reduced graphene oxide to an activated-carbon cathode [70]. To improve the polarisation behaviour of BERT system various impedance studies also have been done in different medium pH [82], under influence of external resistance [83,84], biocatalyst and mediators [84–86].
In the following section, removal of industrial effluents coupled with energy production by using BERT and the role of biocatalyst has been discussed.
3. Treatment of Industrial Effluents using Bioelectroremediation Technology3.1. Removal of Polycyclic Aromatic HydrocarbonsStructurally polycyclic aromatic hydrocarbons (PAHs) are made up of atoms of carbon and hydrogen and are generally derived when incomplete combustion of organic matter such as of fuel [87], mining from coal industries [88], or petroleum spills and power plant emissions [89] occurs. Because PAHs are water repelling and fat-soluble compounds [90] it is a challenging task to degrade them [91]. Therefore, once they are discharged into soil and water environment they tend to get adsorbed onto nearby organic particles, making themselves less available for biodegradation in aqueous environment [92] resulting in accumulation of these particles in soils [93] and sediments [94]. And from soil through irrigation and cultivation they enter the food chain. Once they enter living beings, they tend to assemble inside organelles with fatty acid composition instead of organelles having aqueous environment making these compounds more thermodynamically stable and less bioavailable for degradation [95–97]. Apart from food chain these PAH compounds can also enter inside human beings through vapour inhalation or inhalation of suspended air particles or even through dermal contact [98]. Currently researchers are facing a great challenge to limit the amount of PAH bioaccumulation in food chains since these compounds had been accepted as potent carcinogenic, teratogenic and mutagenic contaminants [99].
Bioelectrochemical systems (BESs) works on the principle of bio-catalysis thereby converting the thermodynamically unfavourable reaction of PAH removal into favourable one. Even persistent PAH molecules can be effectively reduced to less toxic compounds when the microorganisms used to treat these PAH molecules are in close vicinity of the electrodes [100].
The first study on removal of PAH using 14C-labeling was conducted by Zhang et al. [101]. In the study, the group exhibited that toluene could be effectively removed in a MFC by using Geobacter metallireducens which oxidizes toluene into carbon dioxide and since the study did not use any other electron acceptor other than anode, it helped to establish the parameter that electrodes also play a part in degradation of PAH from polluted anaerobic sedimentation. Even naphthalene was also removed by degradation in the same study and in a comparison, it was shown that naphthalene got degraded in just 9 days in MFC while there was no removal of naphthalene in both the controls (heat treated abiotic control and open circuit (OC) control). Soil MFCs can easily target 5–6 ring aromatic hydrocarbons present in petroleum sludge while in conventional anaerobic degradation method only 2–3 carbon ring aromatic compounds are targeted [102].
About 90% decrease in phenanthrene concentration within 24 h was achieved when seven inoculum of S. oneidensis (S.O), P. aeruginosa (P.A), a co-culture of S. oneidensis and P. aeruginosa (CO), anaerobic-digested sludge (MC), anaerobic-digested sludge with the co-culture (MCO), anaerobic-digested sludge with S. oneidensis (MCS) and lastly anaerobic-digested sludge with P. aeruginosa, were separately used in bulk solutions [103]. Phenanthrene got adsorption onto the carbon felt electrode, where the anode respiring bacteria utilised these PAHs as carbon sources for their own growth leading to decrement in phenanthrene concentration. In the same experiment it was shown that the above seven inoculate were all highly efficient with a minimum degradation efficiency of 97% when compared with controls in removing phenanthrene [103]. This efficiency value was determined by evaluating the total concentration of phenanthrene existing in aqueous solution after getting absorbed onto the electrode versus the starting concentration of phenanthrene that was used in the experiment. Maximum power density of 1.25 mW/m2 when normalized with the total surface area of the anode (40 cm2) was achieved in this study [103].
Regarding variation in performances of MFCs in treating PAHs either in soil, sediment or wastewater, efficiency of PHA removal in MFC is based on nature of the sample which can be in solution form or collected from sediment and soil, but studies have shown that the percentage of PAHs removal is usually greater in liquid medium often even reaching 100% [61,103]. Research working with real wastewater from petrochemical industry [104], wastewater containing oil from saline industry [105] or soil contaminated with diesel [106] has shown that removal of PAH varied between 55 to 82%. In the first case while treating real wastewater with a starting concentration of 3 g TPH/L, within 17 days the maximum removal efficiency of PHA compounds inside MFC chamber with aqueous media reached 70%. And when sediment was used in the MFC then there was a 12-fold increment in the removal efficiency when compared to natural removal efficiency of the same compound [107]. In a SMFC phenanthrene and pyrene was removed 95–99% respectively, when the treatment procedure included ferrihydrite [108]. The study also concluded that the removal efficiency of SMFC also depends on the polarity of the contaminant. Alcohols and carboxylic acids which are hydrocarbons with polar nature were more effectively removed than PAHs, cyclo-hexanes and pentanes which are non-polar hydrocarbons [109]. Because soil has very low water content therefore the degradation rate of PAHs from soil sample is very low, with starting concentration of 30 g/L the maximum removal efficiency was 53% [110].
Most studies have shown that maximum power densities in MFCs treating PAHs depends on the complex nature of the substrate, area of the electrode used, maintenance of optimum pH and temperature depending on the type of biocatalyst used and ranges between 2–3 W/m2 [111]. In a study done with low concentration of PAHs containing wastewater it was shown that with a rise in electrode area per volume of reactor, power density up to 1.55 kW/m3 (2.77 W/m2) was achieved [112]. A current density of 299 mA/m2 was achieved in a sediment microbial fuel cell (SMFC) degrading TPHs (total petroleum hydrocarbons/litre), n-alkanes, PAHs in a matrix of saline soil [113]. Study on PAHs, naphthalene, acenaphthene and phenanthrene degradation in a sediment based SMFC, maximum current density of 2 mW/m2 in anaerobic, 5.8 mW/m2 in aerobic condition was achieved [114]. Diesel was degraded in synthetic media of a MFC achieving 90.8 mW/m2 (115 mA/m2 normalized to cathode surface area) of maximum current density [115]. Petroleum wastewater was degraded in a MFC with anoxic cathode achieving maximum current densities of 17.1 mW/m2 (anoxic) and 14.3 mW/m2 (aerobic) conditions [104].
3.2. Removal of TetrachloroethyleneTetrachloroethylene also known as perchloroethylene (PCE) belongs to a class of compound known as chlorinated aliphatic hydrocarbons (CAHs). PCE is hugely used as raw material in refrigerant production and in solvents for dry cleaning [116]. Due to improper handling and disposal practices a significant amount of PCE is leached into groundwater, making this compound a prevalent groundwater pollutant. This compound is also carcinogenic in nature which is why US EPA has listed it among the 126 types of priority pollutants [117]. Although this compound is persistent in nature, but studies have shown that organo-halide-respiring bacteria (OHRB) can lead to anaerobic reductive dechlorination (RD) of this compound. Through RD, PCE is broken down into cis-1,2-dichloroethene (Cis-DCE) and vinyl chloride (VC) which are converted to innocuous ethene (ETH), leading to bioremediation of groundwater and aquifers [116,118,119]. Among all the OHRBs, Dehalococcoides mccartyi is the only microbe that is having the capability to degrade PCE all the way down to ethene because they can produce an enzyme known as reductive dehalogenase (RDases) [120,121]. However, this method has limitations such as low metabolic rate of dechlorinating compounds and also poor presence of electron donors when applied for in situ conversation [122]. One way to enhance this type of CAH dechlorination is to externally add carbon sources or electron donors such as buffers to the polluted site which helps to boost the growth and PCE degrading capability of the microorganisms.
Another newer technology known as electrochemical stimulated microbial dechlorination is now much more on focus because this technology uses electrode as a direct source of electron donor and also generates a favourable environment for redox reaction [123,124]. A schematic diagram of removal of PCE using BERT is shown in Fig. 4. Few studies have been done with biocathode systems where simple substrates such as glucose, sodium acetate, or sodium bicarbonate (NaHCO3) have been tested for PCE dechlorination. Geobacter lovleyi which is a Fe (III)-reducing bacterium had dechlorinated PCE to cis-DCE by using electrode made up of graphite as sole electron donor for RD [118]. In a more recent study electrochemical stimulated microbial dechlorination of PCE to ETH was tested in the anodic chamber of microbial electrochemical system (MES) where electron donor was acetate and electron acceptor was PCE. The analysis of the microbial consortium supported that other electrochemically active microorganisms, such as Geobacteraceae, Desulfuromonas, Desulfitobacteriacea, and Dehalococcoides groups, and the indigenous non-Dehalococcoides community (Spirochaetes, Firmicutes, Bacteroidetes and Protebacteria) were also responsible for electron transfer and PCE reduction in the anodic chamber of the MES suggesting that not only Dehalococcoides species but other electrochemically active microorganisms can also reduce PCE [125].
Bacterial culture from sediment samples when used along with methyl-viologen (MV) as an electron mediator in a MES chamber in association with graphite electrode, a good PCE-dechlorinating activity was seen [126]. Results showed that within 1 day 91% of PCE got reduced to TCE and cis-DCE and within next 7 days, from the total PCE content 80% of PCE got reduced to VC from which 18% got reduced to ETH. The study also compared dechlorination pattern between two MES by adding MV in only one of the MES and concluded that MV is not the primary factor in dechlorination of PCE and that certain bacteria from the sewage sample could have used a self-secretory electron mediator. These results are well coordinating with previous reports that dechlorinating bacteria have the capability to reduce PCE down till ETH via cis DCE when electrode are used as an electron donor [127].
In a study done using constructed biocathode, it was shown that when PCE was fed with different substrates such as glucose, sodium acetate, or NaHCO3 as carbon source, the rate of dechlorination was 4.1–26.5 times higher when compared to individual electrochemical or microbial systems. PCE dechlorination rates followed first order kinetic constant (k) and were 0.110 ± 0.002, 0.056 ± 0.002, and 0.032 ± 0.001 h−1 for glucose, sodium acetate and NaHCO3, respectively. In all the experiments major and minor dechlorination products were cis-DCE and ETH [128].
After initial screening of the sample for PCE dechlorination, in a study done with 10 samples compromising 2 PCE-contaminated groundwater samples, 2 soil samples, 3 sewage sludge samples, and 3 sediment samples (SE) it was found that SE samples showed maximum PCE dechlorinating activity and therefore only SE samples were experimented further. Methyl viologen (MV) was used as an electron donor and PCE acted as electron acceptor. Results showed that 91% of PCE was degraded to trichloroethene (TCE) and cis-DCE (14% and 77%) respectively after only 1 day which got further reduced to VC and ETH (80% and 18%) respectively after 7 days [126].
Most of the studies done till now only focuses on PCE dechlorination efficiency and its by product formation and not much focus is put into energy recovery though several studies have shown columbic efficiency results which takes into consideration number of coulombs recovered as electric current. In a recent study four different experimental conditions for volatile chlorinated hydrocarbons (VCH) dechlorination process were simultaneously studied [128]. First system with an abiotic cathode without any microbial culture as biocatalyst, to study the function of microbes in dechlorination. Second system with open circuit condition without any current passing through the system to study role of electro-stimulation in dechlorination. In the third system inorganic substrate NaHCO3 was supplied in replacement of sodium acetate in the cathodic chamber (solution was replaced minimum 3 times to remove left over sodium acetate) to study if cathode can act as an electron donor. And fourth, a blank control system in no electric current or microbes was studied for volatilization and adsorption of VCH. All experiments were done against saturated calomel electrode (SCE) [128]. Among the four experimental conditions dechlorination rate of PCE and 1, 2-dichloroethane (1, 2-DCA) followed the order: acetate-fed biocathode (k of 0.217 and 0.150 h−1) > NaHCO3-fed biocathode (k of 0.084 and 0.079 h−1) > open circuit (k of 0.010 and 0.008 h−1) > abiotic cathode (k of 0.005 and 0.003 h-1). After 24 hours operation, in closed circuit cathode condition dechlorination activity of PCE and 1,2-DCA were 2.65–5.85 times higher than open circuit condition, verifying the higher dechlorination function in presence of electrical stimulation. After 24 hours in abiotic cathode system dechlorination activity of 10.8 ± 2.8% and 5.5 ± 2.5% for PCE and 1,2-DCA were achieved. This confirmed that sole electrochemical process with no biocatalyst in presence of weak potentials contributed little to VCH dechlorination. In the third condition where inorganic substrate NaHCO3 was used as replacement to sodium acetate, after 24 hours dechlorination rate reached as high as 85%, confirming that cathode can serve as the sole electron donor in dechlorination process and the coulombic efficiency (CE) of the system was greater than 80%. Nevertheless, k values in the experimental condition under NaHCO3-fed biocathode was found out to be 47.5–61.5% lower than the condition where sodium acetate was present. In acetate-fed biocathode, CE for PCE and 1, 2-DCA were 115.5 ± 8.2% and 135.5 ± 3.5% respectively. The reason for more than 100% of CE was the use of acetate as an alternative electron donor in the process of VCE dechlorination by organohalide-respiring bacteria (OHRB) which acted as a biocatalyst [128].
In a study in continuous mode for 570 days, cathode potential was varied −250 mV to −750 mV vs. standard hydrogen electrode. It was shown that in the cathode at −250 mV there was no generation of abiotic hydrogen and dechlorination of TCE to cis-DCE and VC occurred at a rate of 15.6 ± 1.3 μmol e-/L d. Methanogenesis was shown to be totally suppressed at this cathodic potential with dechlorination consuming up to 94.8 ± 0.2% of the electric current (15.1 ± 0.9 μA) generated in the system. At cathodic potential lower than −450 mV much greater rate of dechlorination up to (64 ± 2 μmol e-/L d) occurred but methanogenesis consumed around 60% of the generated current. When cathodic potential was further reduced to −750 mV, the rate of methanogenesis surpassed rate of dechlorination up to (4100 μmol e-/L d). Consequently, the CE for the dechlorination gradually decreased from 23% at −450 mV to less than 1% at −750 mV [129].
3.3. Removal of PerchloratePerchlorate being an inorganic anion and strong oxidizing agent has the capability to contaminate both water and soil in the form of salts of ammonium (NH4+), potassium (K+), magnesium (Mg2+) and sodium (Na+) perchlorate and perchloric acid (HClO4) when in contact in water [130]. Since kinetically perchlorate is stable under normal temperature and pressure condition owing to its tetrahedral structure of oxygen which restricts its central chlorine atom from interacting with reluctant, it can mobilize in water environments due to its strong oxidizing property [131]. Therefore, perchlorate can prevail in soil and water environments and eventually also percolates into groundwater thereby bioaccumulating mainly into vegetables and dairy products [132]. The movement of this compound in the environment poses health risk to human as it inhibits proper thyroid functioning.
By use of stable isotopic mechanisms (assessing triple-oxygen isotope ratios 18O/16O and 17O/16O) naturally occurring perchlorate can be differentiated from anthropogenic perchlorate [133]. In addition, 37Cl/35Cl fractionation method is also used to differentiate the pattern of unique origin of the natural perchlorate from the anthropogenic ones [134]. While the main source of anthropogenic perchlorate contamination comes from industries such as ordnance, chemical and aerospace, the natural source of perchlorate contamination is nitrate deposits for example Chilean nitrate [135]. Nitrogen fertilizers also heavily contribute to perchlorate pollution as they are highly concentrated with perchlorate (3700 mg/kg) [136]. Worldwide perchlorate contamination in drinking water (> 4 μg/L, ESCTP 2000) is a major concern. At higher concentrations, perchlorate restricts proper functioning of the thyroid gland and dietary reference dose (RfD) for perchlorate exposure in food is set at 0.7 μg kg−1 body weight/day while for drinking water the perchlorate exposure level is 24.5 μg L−1 [137].
Perchlorate present in soil is one of the major reasons of perchlorate contamination in groundwater. Anthropogenic activities such as bursting of firecrackers, explosives, high safety flares during military operation and even burning of match sticks lead to perchlorate contamination in soil. Burning of fire cracker during spring festival seasons leads to high concentration of perchlorate in particulate matter which get deposited in soil as well as gets dispersed into remote region via wind [131]. In addition to that in presence of enough precipitation perchlorate gets transported from source to other locations in surface runoff. As perchlorate is negatively charged thereby because of electrostatic repulsion it does not get adsorbed to soil particles however due to capillary forces and surface tension dissolved perchlorate can get into pores of soil and get infiltrated into groundwater. Perchlorate when present in soil quicken the rate of dissolution of minerals leading to heavy or trace metals leaching into surface and groundwater which then enters food chain and starts accumulating in living organisms. Studies have shown that presence of perchloric acids has led to dissolution of many minerals such as ulexite which is a commonly found boron-containing mineral, dissolution of nickel and copper present in laterite [138], dissolution of iron in goethite and hematite [139].
Perchlorate contamination in water (groundwater, surface water or drinking water) are majorly because of soluble nature of perchlorate in water which makes it easier for minimum rainfall to wash off this contaminant from source to other areas. In highly modernized cities such as New York and Oklahoma substantial amount of perchlorate in surface and ground water have been contaminated because of various anthropogenic activities [140,141]. Due to heavy use of propellants and rocket motors in Longhorn army ammunition plant, Texas, USA, 31.43 mg L−1 of perchlorate was detected in a close by wastewater treatment plant [130]. Perchlorate used as cleansing agent in a liquid-crystal display monitor production unit near Nakdong River watershed, South Korea, led to detection of high amount of perchlorate (<1.1–6.2 mg L−1) in tap water [142]. An elaborate study dealing with effect of seasonal changes on concentration of perchlorate in groundwater in Harbin, North China, showed that in summer concentration of perchlorate ranged between <0.008–1.7 mg L−1 while during spring season it increased from 0.1 to 1.72 mg L−1. The factor behind to this increment was attributed to melting of snow in the region which dissolved the surface perchlorate and allowed it to percolate into groundwater [131]. In a time dependent study, the concentration of perchlorate was measured after bursting of fire crackers during festive season in a municipal lake in Ada, Oklahoma, USA. The results showed perchlorate concentration as high as 44 mg L−1 following the fireworks. However due to presence of native microbial population which facilitated natural attenuation of perchlorate, the high concentration of perchlorate rapidly came back to background level within 20–80 days [143].
Water samples were collected from 13 different areas from over 6 states in India (where number of samples n = 66). Samples of saliva were collected from 4 areas from 3 different states in India (n = 74). Concentration of perchlorate were measured using high performance liquid chromatography in association with tandem mass spectrometry (HPLC-MS/MS). Results showed that perchlorate was present in almost 75.6% of water samples above permissible limits of 0.02 μg L−1. Concentration of perchlorate varied between <0.02 to 6.9 μg L−1. Results also showed that mean concentration of perchlorate present in water from direct rain, surface water, bottled can, groundwater, and drinking water were <0.02, 0.05, <0.02, 1.0, and 0.1 μg L−1 respectively. Among all the water samples analysed only 3 samples (all groundwater) showed perchlorate concentration above 1 μg L−1. Saliva samples when analysed showed perchlorate concentration ranging between 0.2–4.7 μg L−1. This study showed that in India amount of perchlorate in water samples are only 1–2 orders of magnitude less than the amounts reported for the United States [144].
Conventional chromatographic biological techniques to remove perchlorate includes continuous flow stirred tank reactor (CSTR) and systems with attached biofilm reactors. But, the drawback of these methods are that they are expensive because of electron donor dosage as well as not so effective [145]. So, perchlorate wastewater is readily dumped into soil from where it contaminates surface and groundwater. In industries, three main techniques that are used to treat perchlorate wastewater are, first high energy organic compounds are removed from the wastewater and then it is send to wastewater treatment plants for further processing. While in the second method dilution treatment is performed on the perchlorate wastewater, in the third method evaporation technique is applied [145].
BES technology is the most recent sustainable technology that has been applied to treat perchlorate wastewater [146–148]. Generally, in nature perchlorate co-occurs with nitrate ions and both can be treated with biocathode in BES. Working principle for bioelectroremediation of perchlorate in BES depends on the electroactive bacteria grown in the cathodic chamber where they act as biocatalyst and as an electron donor these bacteria uses the cathode electrode whereas perchlorate acts as the electron acceptor.
To study the effect of H2SO4 (anodic supplement) and thereafter autotrophic H2 reduction of perchlorate an experiment was conducted in batch mode and the result showed that maximum removal of perchlorate in the cathodic chamber occurred when H2SO4 concentration was ranging between 0.04 to 0.05 mol/L. Phylogenic characterization of hydrogen-autotrophic perchlorate-reducing cultures found WHC2–6, Thermotalea metallivorans, Fulvivirga kasyanovii, Aureibacter tunicatorum, and Thauera sp Q 20 to be the dominant species present on the cathode electrode. The parameters that affected perchlorate reduction were sludge concentration, current density and initial perchlorate concentration. The removal efficiency of perchlorate (95.03, 96.29, 97.56 and 98.99%) positively correlated with the current intensity (of 20, 40, 50, and 60 mA) respectively [149].
Modified graphite with polyaniline (PANI) was studied as sole electron donor for perchlorate reduction in a membrane-less MEC. With PANI modification enhanced biofilm formation was achieved on the cathode electrode. Hairlike structure (pili) was observed in the biofilm as well as in the electrolyte and this helped in electron movement between the cathode and the electrolyte. Results showed 12% higher perchlorate reduction with PANI than non-modified cathode. The predominant bacteria in the cathodic chamber were reported to be Azospiraoryzae strain DSM 13638. This study concluded that MEC is a new age technology that can be used for reduction of high concentration of perchlorate (60 mg/L) [150].
Presence of high concentration of nitrate ion can slow the reduction of perchlorate ion and it have been shown that at nitrate concentration of around 2.10 mM, reduction of perchlorate becomes almost negligible, and even at concentration as low as 0.07 mM of nitrate ion removal of perchlorate ions is very slow due to the growth of active denitrifies at the cathode electrode which inhibits the efficiency of perchlorate reduction [151]. Cathode current generation was achieved with nitrate as catholyte, and graphite felt cathode as the sole electron donor (maintained at −0.50 V). The generation current can be attributed to bioelectrochemical reduction of perchlorate. Both nitrate and perchlorate were experimented as catholyte at concentration of 0.07 mM and results showed two current peaks where, the 1st peak appeared soon after beginning of the experiment and the second peak appeared after 4 days of operation, but intensity of the 1st peak was much pronounced than the second peak. The 1st and 2nd current peak can be attributed to nitrate and perchlorate reduction respectively which the study further confirmed by degradation data. When the concentration of nitrate was increased to 0.35 mM, 1.40 mM, and 0.70 mM perchlorate removal percentage was 60.32%, 40.05% and 54.73%. The bacteria present on the cathode surface were found to be predominantly Alpha-proteobacteria, Beta-proteobacteria, Gamma-proteobacteria, Bacilli, and Clostridia [151].
In a study done with autotrophic denitrifying biocathode system, in the first experimental condition sole perchlorate concentration was studied by varying its concentration from 0.033 to 0.40 mmol/L in cathodic chamber [147]. In this range of concentration, the perchlorate removal efficiency was 52.87%. But when the concentration of perchlorate exceeded 0.40 mmol/L perchlorate removal efficiency decreased to 10.93%. Reasons behind this decrement has been attributed to adverse effect of perchlorate on the microbes in cathodic chamber due to toxic nature of perchlorate and also low electron transfer rate by autotrophic denitrifying biocathode due to hindrance in its activity by high concentration of perchlorate [147]. Maximum current density generated with 0.40 mmol/L of perchlorate concentration was 5.03 A/m3 and stable current generated was 3.00 A/m3. In the next experimental condition perchlorate removal was studied along with nitrate. Nitrate was maintained at a constant concentration of 0.32 mmol/L while perchlorate concentration was varied from of 0.033 to 1.20 mmol/L where initial influent molar ratio of nitrate and perchlorate (NO3 −/ClO4 −) was altered from 9.6:1 to 1:3.7. Even though nitrate concentration was kept constant still, perchlorate removal efficiency did not improve even when concentration of perchlorate was increased from 0.033 to 0.07 mmol/L (consequently NO3 −/ClO4 − decreasing from 9.6:1 to 4.8:1), but result of denitrification rate achieved was good around 85% indicating that low concentration of perchlorate hasn’t affected nitrate reduction by autotrophic denitrifying bacteria [147]. When molar ratio of NO3 −/ClO4 − was further reduced from 4.8:1 to 1:1, there was gradual increment in perchlorate removal rate (40.97%) while denitrification rate was still good (86.03%). This improved rate in perchlorate reduction can be attributed to enrichment of perchlorate-reducing bacteria (PCRB) as well as adaptation of autotrophic denitrifying bacteria to the higher concentration perchlorate. With further reduction in NO3 −/ClO4 − ratio to 1:3.7 nitrate removal rate was greatly reduced to 42.36% indicating that higher concentration of perchlorate inhibited nitrate reduction rate while perchlorate removal was only 3.94%. From the perspective of energy recovery, it was observed that stable current generation of 3.10 A/m3 occurred at 1:1 NO3 −/ClO4 − molar ratio while maximum current generation of 6.17 A/m3 occurred at 1:1.2 NO3 −/ClO4 − molar ratio [147].
3.4. Removal of NitrateGlobally nitrate (NO3 −) and nitrite (NO2 −) are major surface and ground water contaminant due to extensive use of fertilizers, wastes released from various industries as well as animals and septic tanks [152]. Study has shown that excessive amount of nitrate and nitrite if present in drinking water, can cause severe health conditions such as “blue baby syndrome” in infants, liver toxicity and cancer in adults [153]. According to united states maximum permissible amount of nitrate-nitrogen (NO3 −-N) and nitrite-nitrogen (NO2 −-N) in water is 10 mg/L and 1 mg/L respectively [154]. Though conventional treatment technologies such as reverse osmosis (RO), electrodialysis (ED), heterogeneous catalysis (HC), and physicochemical, chemical and electrochemical methods can be applied for nitrate/nitrite removal, but they are not cost effective and also generate toxic byproducts.
Recent research has studied bioelectroremediation in the form of BES technology (bioelectrochemical denitrification system (BEDS)) for the removal of nitrate from contaminated surface water and groundwater. This technology explores the use of denitrifying bacteria to be used in the cathodic chamber where these microbes colonize the cathode electrode and reduce the nitrate into nitrite (NO2 −), nitric oxide (NO), nitrous oxide (N2O), and dinitrogen (N2), in consecutive order, by utilizing the electrons released from the anodic chamber (Fig. 5). Recent studies on biocathode MFC has shown that MFCs can use nitrate as an alternate electron acceptor instead of oxygen and in turn can generate bioelectricity [155]. Biocathodic nitrate ions reduction can take place either by using denitrification microbes such as in MFC-BEDS [156,157] or by voltage induced electrolysis of water in the anodic chamber (external-powered BEDS) [158]. Magnitude of the current generated by either of the system is a key parameter because it exhibits a linear relationship with the rate of denitrification [159].
In situ removal of nitrate from groundwater was studied in submerged microbial desalination-denitrification cell (SMDDC) [160]. The cell was designed to work as both MDC and MFC. The SMDDC was such design that it will submerge 1.5 cm below synthetic groundwater, which was fed into outer glass reactor. Synthetic wastewater was fed into the anodic chamber of the SMDDC as a fuel and through a looped connection effluent from the anodic connection was fed into the cathodic chamber of SMDDC. As current generation occurred in the anodic chamber by the bacteria NO3 − and Na+ got transferred into the anodic and cathodic chambers of SMDDC from the synthetic groundwater via AEM and CEM; through the looped connection effluent from the anodic chamber was directed to the cathodic chamber where NO3 − was reduced to N2 through autotrophic denitrification. With 12 h of hydraulic retention time (HRT) and 10 Ω of external resistance the system generated 3.4 A/m2 of current density and also removed 90.5% of nitrate from the synthetic groundwater. This system also showed enhanced performance with high ionic strength groundwater (2200 mS/cm) and was capable to reduce the salinity of the groundwater as well [160].
Nitrite and nitrate ions along with separate nitrite and nitrate ions were studied in 3 different bioelectrochemical denitrification systems (BEDS) in biotic and abiotic mode. The biotic cathode using NO3 −-N as electron acceptor showed 88% removal efficiency while NO2 −-N treatment showed only 85%. The result of this study showed that at the end of 55 days of operation the initial sludge culture changed to Firmicutes and Proteobacteria in BEDS operation with NO3 −-N and NO2 −-N respectively where BEDS simultaneous nitrite and nitrate reduction was enriched with only Proteobacteria [161].
In a separate study BEDS was used to determine removal of nitrate using different catalyst in cathodic chamber [162]. Results showed that 91% of nitrate ions was removed by biocathode (carbon cathode enriched by biofilm) while nearly same 90% of nitrate removal rate was shown by Pt-coated electrode at 1.0 V. Maximum current density of 0.0083 A/m2 was achieved by biocathode which was 22-fold greater than what was achieved with an abiotic plain carbon cathode. Cell voltage is one of the key factors on which generation of intermediate products from reduction of nitrate depends. A major portion of nitrate was converted to NH3 + at around 0.5 V whereas at much increased voltage of around 0.7 to 1 V majorly NO2 − was formed with little generation of NH3 +. Highest nitrate removal rate of 0.204 mg NO3 −-N/cm2d was shown by biocathode, while plain carbon cathode showed only 0.176 mg NO3 −-N/cm2d. Highest current was also generated by biocathode (3.1 mA) when compared to Pt-coated cathode and plain carbon which generated (2.8 mA and 0.6 mA respectively) at 0.7 V [162].
Three different types of cathodes (abiotic cathode, biocathode and biohydrogen facilitated biocathode) was utilized for nitrate removal efficiency in a BES. Results showed that while biohydrogen facilitated biocathode had 95% efficiency in nitrate removal, biocathode and abiotic cathode had only 59% and 13% efficiency. Activity of the enzyme nitrate reductase was 9.3-fold higher in biohydrogen facilitated biocathode system (reaching 0.701 g-N/L h) than the biocathode system. Energy recovery in terms of power density from the biohydrogen facilitated group was 76.96 mW/m3 which was more than the other 2 systems [163].
A BES reactor with a novel membrane was built to fit two dual chamber MFCs one of which was an aerobic chamber, to simultaneously conduct nitrification and denitrification. In comparison of this coalescent system with control system there was a lower membrane fouling achieved and nitrate ion removal rate was found to be 84.30%. When energy generation is considered 8.50 A/m3 was the highest current density recorded while the highest power density was 1800 mW/m3 [164].
Sole nitrate system was studied for effect of increasing concentration of nitrate (0.08–0.64 mmol/L) on its removal efficiency in the cathodic chamber of the system. Results showed that when the inlet nitrate concentration was lower than 0.32 mmol/L, efficiency of nitrate removal was good around 87.05% but as the inlet nitrate concentration exceeded 0.32 mmol/L the nitrate removal efficiency started declining. At nitrate concentration of 0.65 mmol/L, maximum and stable current density achieved were 3.17 and 1.64 A/m3, respectively [147].
Comparison between the performance of the two MFC setups where in the 1st setup eggshell a bio waste was introduced in the cathode chamber with groundwater as catholyte and in the 2nd setup only groundwater with no eggshell was studied. In both the setups secondary sewage water was used as the anolyte. Nitrate removal and energy recovery in terms of bioelectricity generation was monitored for both the system for 3 weeks. Results showed improved reduction kinetics at the cathode chamber of the 1st setup due to use of eggshell which had bio-absorbed the nitrate and also showed improved microbial growth. After operation of 3 weeks a maximum power density of 228 ± 1.3 mW/m2 and 97% nitrate removal was achieved [165].
3.5. Removal of Petroleum HydrocarbonPetroleum products are regularly used in vast quantities as a source of energy majorly in industrial and transportation sector leading to these compounds being released into the environment either via water or by directly being released into soil and sediment [166,167]. Major types of petroleum products can be categorized into four different groups-alkanes, aromatics, nitrogen-sulphuroxygen compounds (NSO) and asphaltenes [168]. PHs through contaminated soil and water enter the food chain and due to their certain properties, such as lipophilicity and electrochemical stability tends to bioaccumulate in organic tissues. Petroleum hydrocarbons are heavily toxic and mutagenic in nature thereby increasing the need to remediate this type of pollutants [169].
Conventional methodologies to treat petroleum hydrocarbons for examples biodegradation, membrane processes, electrocoagulation, adsorption and advanced oxidation processes (AOPs) [170–174] have disadvantages such as biodegradation is a substantially slow moving process having long durations of reaction time lasting for days or even months. This type of treatment often needs larger treatment plants which demands higher energy consumption and also encounters with problem of membrane fouling. Similarly, use of activated carbon for adsorption of petroleum hydrocarbons using phase change technique is both complicated and costly due to frequent interval regeneration of the saturated activated carbon with petroleum hydrocarbons as well as other pollutants. Other methodologies such as AOPs are high energy demanding and complex to operate requiring skilled technicians which is not always available. Electrocoagulation generates large amount of sludge, and upon that high operational cost and energy demand has made this a non-approachable process during scale-up to industrial level [175].
Bioelectroremediation has been successfully studied for bioremediation of petroleum hydrocarbon majorly from polluted soil [176,177]. In recent years various configuration of BES has been studied to achieve effluent with minimum quality of hydrocarbon along with being cost effective [178]. The most commonly used BES for this purpose is H type two chambered MFC. Laboratory scale MFC has been well studied regarding petroleum hydrocarbon polluted soil but scaling up these reactors and their implementation in real case scenarios is a real challenge [179]. To increase the sustainability of PH treating MFCs, be it from soil, wastewater, sediments or petroleum containing anaerobic sludge, air cathode reactors with single chamber have been vividly studied [180]. In this type of reactors, anode compartment is filled with petrogenic hydrocarbon and the cathode is always exposed to air by suspending it over the aqueous part [45,108].
Multi-anode system has been studied for their better ability to remove petroleum hydrocarbons. Here three anodes were parallelly connected, and an air cathode made of activated carbon was fixed at the bottom of the chamber. The result showed that pH removal efficiency of multi-anode MFCs are higher than a MFC with single anode system because greater the surface area of the electrode higher is the movement of electrons and protons for generation of bioelectricity [150]. In a study with MFCs where anode electrode was made up of rolled carbon cloth. Three electron collectors were used while pH contaminated sediment and ocean water was used as a substrate in anodic chamber. Results of the study showed reliable performance as the system generated high power density [181]. Three different MFCs with various configuration were studied each with either single, horizontal, or vertical arrangement. The results revealed that the MFC with vertical electrode has lower internal resistance leading to generation of more power and exhibiting greater performance [182]. A single cell MFC showed that carbon electrodes are much more effective with 83.4% diesel removal rate along with production of 90.81 mW/m2 of power [115]. Carbon felt electrodes were used for PHs removal and achieved 90% removal rate for both in situ and ex situ MFC. The result of this study with ex situ MFC validated that this system works well even in anoxic conditions and under harsh weather [183].
A plexiglass SMFC with five different electrodes graphite felt (GF), carbon cloth (CC), activated carbon fiber felt (ACFF), graphite paper, and aluminium sheet was studied for petroleum hydrocarbon removal efficiency. The results revealed that GF anode had the most efficient petroleum hydrocarbon removal rate of 59.14% from soil polluted with petroleum spill, owing to its large surface area, low internal resistance and rapid transfer of mass from substrate to electrode surface [184]. To lower the internal resistance which decreases electron and proton movement bioreactors made up of stainless steel was studied. The anode electrode was made up of 80 stainless-steel meshes of 1 mm thickness while cathode electrode was made up of stainless-steel cylinder of 4 cm height and 9.6 cm diameter. This system showed above 90% degradation rate of phenanthrene and pyrene [108].
In terms of energy recovery, effect of packaging material such as graphite granules (GG) and granular activated carbon (GAC) on power generation when treating petroleum refinery wastewater as a substrate have been monitored. Results showed excellent performance of MFC in terms of energy recovery with GAC generating up to 330 mW/cm3 of power and GG generating around 262 mW/cm3 when compared with control (with no packaging material) which generated around 241 mW/cm3 of power [185]. Degradation of benzene and naphthalene in MFC operated at room temperature (30°C) and compared it to MFC operated at thermophilic temperature (40°C). Power density generation was more (292 mW/m2 at 40°C) in thermophilic condition in comparison to room temperature (30o C, 156 mW/m2) demonstrating that due to reduction in activation energy at thermophilic conditions it enhances power generation by increasing degradation rate of petroleum hydrocarbons [186].
Effect of varying organic loading rate (OLR) on degradation of petroleum sludge in a single chambered BES showed that when OLR was increased from 3 to 30 g/L corresponding to 1.11 to 11.1 g TPH/L, though there was a decrease in power density generation from 20.62 to 0.12 mW/m2 but, higher substrate degradation was achieved at high OLR [100].
Similarly, the concentration of glucose (0.1 to 0.5% W/V) as a co-substrate was studied on a soil MFC to enhance the degradation of petroleum hydrocarbons in soil. MFC which operated with a high glucose concentration showed better performance (450 mV) when compared to the MFC which operated with low glucose concentration (400 mV) and control system which had no glucose additions (220 mV) [113].
4. Metal Catalyst for Enhancement of BioelectroremediationAn effective catalyst should have crucial properties making them practically applicable, first of which is having high catalytic activity. Second, they should have durability and longevity. Third, they should possess high electrical conductivity compensating for any internal resistances. Fourth, they should apparently be inexpensive making it easy for their large-scale synthesis. Catalyst function is to lower the activation energy and to form a low energy intermediate thereby enhancing the kinetics of cathodic oxygen reduction reaction (ORR). Since cathodic reduction of O2 has a direct effect on power production, cathodic catalyst plays a major role in it [187].
Graphite along with other carbon electrodes have low ORR, thus there is a need to use catalyst that increase cathodic ORR efficiency [188,189]. Noble metal such as platinum (Pt) is the most frequently used catalyst but its high price, low earth abundance and issues of catalyst poisoning limits its practical use [190]. Therefore, current research focuses on development of non-Pt catalysts as alternative. Approaches that have been considered are- 1) reconstruction of Pt nanostructure by tuning its shape and size. 2) building multi-metallic Pt-based nanostructure such as PtM or PtM1M2. 3) development of catalyst by using earth abundant elements.
To promote less dependency on Pt based catalyst, electrodes built with well-defined three-dimensional (3D) structures and explicit porosity are being thoroughly studied. Research done with foam-based 3D architecture nano Pd-Cu and activated carbon-Ni had shown a power output of 1240 mWm−2 with an effective ORR catalytic activity [191,192]. In a study on nanoparticles made up of CoNi alloy enclosed within a bamboo structured carbon nanotube (CNT) with numerous graphene (GR) layers making up its inner cavity [193]. This modified CNT nanotube had an ORR catalytic activity of 3.63 comparable to Pt/C ORR catalytic activity of 3.9. And it generated power density of 2.0 ± 0.1 W/m2 which is approximately close to power density of 2.6 ± 0.2 W/m2 generated by platinum carbon nanotube (Pt/C).
Owing to easy synthesis, low cost, high ORR catalytic efficiency and less harmful effect on the environment metal oxide (MO) based catalyst such as manganese oxides (MnOx) have been thoroughly examined as cathode catalysts in MFC [187]. In recent years approaches have been taken to synthesis catalyst made up of metal complexes with macro cyclic ligand bounded to them. Example of these type of catalyst are phthalocyanines and tetramethoxy phenylporphyrins. Even though phthalocyanines works well in neutral, alkaline and acidic conditions but their stability decreases below pH 3 reason being macro cyclic ring gets demetallized below this pH. To remove this limitation macro cycles are added via pyrolysis to activated carbon (AC) increasing their stability and catalytic efficiency in acidic environment [194].
Metal carbides (MCs) is another class of catalyst that are recently been studied due to their property of resistance towards corrosion along with being mechanically durable and having high catalytic activity [195]. MCs efficiency of catalytic activity depends on their chemical composition of MxCy therefore, to increase their ORR catalytic activity appropriate composition of MxCy needs to be selected [195].
The mentioned abiotic catalyst has several limitations. ORR being an irreversible electrochemical event which contribute to notable amount of energy loss. In most of the catalyst high standard reduction potential (1.23 V vs. SHE) becomes the prime limiting factor of ORR. Apart from that, most of the noble metals are unstable at high potential making them less applicable than non-noble metal-based ORR catalysts [196]. In MO based catalyst poor electronic conductivity is a major limiting factor which increases the internal resistance of the system. To overcome this limitation, supports that are electrically conductive for example AC, graphitized carbon, CNTs, graphene (GR) are usually added. MnO2/CNT and NiO/CNT are two such catalyst that were evaluated for their ORR activity in MFC [197]. Other than these, limitations for abiotic catalyst include high cost, complex fabricated operating protocol, and are non-environmentally friendly.
5. Study on Microbial Community Structure (Biocatalyst) for BioelectroremediationMicrobial community analysis has become a crucial step in increasing the efficiency of BERT because microbes growing on the electrodes act as biocatalyst and enhances ORR which in turn increases the efficiency of wastewater treatment and generation of bioelectricity. In the anode formation of biofilm by the microbial community by attaching themselves on the electrode is one of the crucial determining factors in generation of bioelectricity [198–200], while in the cathode ORR plays a crucial role in improving the catalytic efficiency thereby affecting cathodic potential and power output [201]. Therefore, studying the types of microorganism growing on the electrode and their morphology determines the efficiency of the biocatalyst being used. In this regard two commonly used exoelectrogens, G. sulfurreducens PCA and S. oneidensis MR-1, have been studied in detail to elucidate their biofilm formation and electron transfer mechanism. Both strains have distuiguished electrochemical physiologies. G. sulfurreducens uses conductive protein pilus, nanowire, as the major appendage for electron transfer onto the anode surface whereas S. oneidensis uses flavin as electron shuttle. Due to these properties, G. sulfurreducens grow on the anode electrode, but S. oneidensis favors growing in suspended medium [84,202].
Bacterial community analysis in recent studies uses DNA extraction kit and follows standard protocol to extract DNA from the biofilm and get the purified DNA ligated into a suitable plasmid vector. Transformants are then screened using appropriate screening technique and positive clones are sequenced against Gen Bank nucleotide database. Two microbes are considered to belong to the same species if there is more than 97% identity in between their sequences. Rarefaction curves are drawn to check if enough number of clones from each library has been sequenced. Then sample coverage value, diversity index and evenness are calculated [203]. In the same study, four 16S RNA gene libraries were prepared to study the effect of material used in biocathode synthesis on the formation and attachment of biofilm on the electrode. Results showed that the microbial community on the biofilm belonged to four different phylum Alphaproteobacteria, Betaproteobacteria, Bacteroidetes and Acidobacteria [203].
Microbes on the developed biofilm of biocathode were identified using methodologies such as modified Hartree-Lowry protein analysis for determination of volatile suspended solids (VSS) whereas to study the morphology as well as distribution of the microbes, fluorescence microscopy and scanning electron microscopy was used [204]. 16S rRNA gene sequencing was performed and blasted against reference sequences in the NCBI BLAST database to obtain a phylogenetic classification. The result of this classification showed three type of archaea phyla and six types of bacterial phylum. The dominating strain of the archaea phyla was found to be Methanobacterium palustre strain DSM3108 and Methanobacterium aarhusense strain H2-LR whereas the dominating strain of bacterial phyla was Methylocystis sp. SC2 strain SC2, Acidovorax caeni strain R-24608, Desulfovibrio putealis strain B7-43, Petrimonas sulfuriphila strain BN3, and Ottowia thiooxydans. Both fluorescence microscopy and SEM revealed that the microbes were rod shaped 1–3 μm long cells where the length of the cells varied from <1 μm and 10 μm. and single cells or present in colonies. SEM exclusively revealed both the areas of the electrode 1) where the rod cells have densely formed the biofilm 2) area where only few cells have attached themselves. It also revealed the capability of the microorganism to form filaments, and even their size [204].
In a dual chambered MFC degrading phenanthrene, pseudomonas was found out to be dominant in both planktonic and biofilm area. Phenanthrene was more easily absorbed and assimilated by surfactant producing pseudomonas [205]. A study showed Pseudomonas and Rhodococcus work synergistically to degrade PAHs partly because of their capability to bio emulsify [206]. In native PAH polluted areas, hydrogenotrophic or acetotrophic microorganisms such as proteobacteria and firmicutes co-exist and synergistically work towards PAH degradation [207,208]. The dominance of Rhizobium (Alphaproteobacteria) and Thauera (Betaproteobacteria) which is also an endosymbiotic nitrogen-fixer elicits PAHs degradation as these roots associated microbes increases the expression of genes and in turn mRNAs which produces hydrogen degrading enzymes for example alkane monooxygenase and naphthalene dioxygenase. They also secrete organic acids, siderophore, and phytochelatin to enhance PAH degradation [207,209,210].
Use of BERT technology to degrade PAH using bacterial population are well studied but use of archaea in PAH degrading MFC are rarely reported. A soil MFC was supplemented with biochar made from chicken manure (CB), wheat straw (SB) and wood sawdust (WB) along with soil at 2% mass ratio and studied for removal of PAHs [211]. In the same study high throughput amplicon sequencing of archaea was also studied and the results generated 50,794–92,928 valid tags with 277–288 base pair (bp) of average length. In comparison to control, number and species richness were less in WB biochar while they were notably higher in CB biochar. Unique OTUs were found out to be much higher in SB biochar (174) and CB biochar (514) in comparison to control (120) whereas shared OTUs among control and CB biochar, control and SB biochar, and control and WB biochar were 859, 820, and 750 respectively. In the control predominant phylum were Euryarchaeota (85–92%) and Thaumarchaeota (1–2.4%). Addition of CB, SB and WB biochar significantly reduced growth of phylum Thaumarchaeota by 32, 54, and 61% respectively. While addition of SB biochar facilitated the growth of class Halobacteria, addition of CB and WB biochar were detrimental for class Methanomicrobia (Euryarchaeota). Dominant genera in control were Methanosarcina (6–12%) and Methanoculleus (5–7%), Halogranum (2–4%) and Halovivax (2–3%). After addition of CB and WB biochar the growth of Methanosarcina was seen to get reduced by 40–45%. Abundance of other genera were also affected by biochar addition. One species Methanolobus chelungpuianus was found out to be in abundance in control soil than in any other biochar treated soil making it a good candidate for being used as a biomarker for the control soil. Methanoculleus and Halobacteria of class Methanomicrobia and Halobacteria respectively could have contributed to degradation of PAH compounds after biochar treatment [211]. Because other study has shown Methanoculleus to play active role in PAH degradation through methanogenic metabolism [212], while genera of methanogenic archaea has been shown to be dominant in anthracene and phenanthrene degradation [213].
Network analysis and microbiome community structure study disclosed that all the three different (glucose, sodium acetate (NaAc), or NaHCO3) fed carbon substrate systems were dominated by dechlorinators and cathode-respiring bacteria, but their richness was notably distinct in each of the system. Lactococcus and Anaeroarcus; Geobacter and Pseudomonas; and Bacillus was dominant in the glucose fed system, NaAc fed system, and NaHCO3 fed system respectively. Some of the cathode-respiring dechlorinators were among the shared network between all the three different systems such as Desulfovibrio and Pseudomonas. Results of difference in microbiome community structure was reaffirmed by the abundance of putative functional genes which showed that heterotrophic systems such as that fed with either glucose or NaAc were more favourable to express dehalogenase (pceA and tceA) and electroactive omcX genes than the autotrophic system fed with NaHCO3 [128].
Metagenomic methods such as 16S rRNA gene amplicon sequencing and whole shotgun technique was applied at the biocathode sample containing TCE-contaminated and TCE/Cr(VI)-contaminated mineral medium which majorly revealed bacterial community interaction in the biofilm [214]. At the biocathode TCE reductive dechlorination (RD), hydrogenotrophic methanogenesis, and Cr (VI) reduction occurred. When the bioreactor was operated with TCE (111 ± 2 μeq/Ld) and TCE/Cr (VI) (146 ± 2 μeq/Ld), results of RD rates showed that degradation of TCE was not adversely affected by presence of Cr (VI) and Dehalococcoides mccartyi was found out to be the dominant marker of the RD process in the biocathode as it was able to degrade both TCE-contaminated and TCE/Cr (VI)-contaminated mineral medium. Results of metagenomic analysis revealed that D. mccartyi was the sole bacteria with dechlorinating properties due to its uptake of H2 as the sole electron supply mechanism demonstrating that electroactivity is not a characteristic of this bacteria. Methanobrevibacter arboriphilus and Methanobacterium formicicum was also shown to populate the biocathode as consumers of H2 and producers of CH4 and these microbes also released cofactors that helped in cobalamin biosynthesis in D. mccartyi. Metagenomic analysis also revealed that in M. formicicum there are genes present which are associated with Cr(VI) reduction through extracellular and intracellular mechanisms [214].
A MFC system for perchlorate degradation utilizing a denitrifying biocathode was studied to identify putative biocathode-utilizing perchlorate-reducing bacteria (PCRB). Perchlorate degradation was monitored by increasing the concentration of perchlorate in the biocathode of perchlorate-reducing MFC while lowering the concentration of nitrate in denitrifying MFC. Microbial community structure analysis revealed that perchlorate reducing biocathode MFC was mainly dominated by putative denitrifying Betaproteobacteria while denitrifying MFC was dominated by putative iron-oxidizing genera. Though both the MFCs had different cathodic roles, the anodic communities from both the system were found to be similar [146]. In a study with autotrophic denitrifying biocathode MFC treating perchlorate with simultaneously generation of bioelectricity 16S rRNA amplicon sequencing was studied for four experimental conditions, in system with initial seed sludge (S1), in biocathode fed with sole nitrate (S2), sole perchlorate (S3) and their mixture (S4). While Proteobacteria and Chloroflexi was primarily dominant in S1 but it was found to be present all the biocathodes also (55.22%, 49.96% and 51.65% in S2, S3 and S4, respectively). Other dominant genera in the biocathodes were Acidobacteria, Firmicutes and Thermi [147]. Study done on an externally powered biocathode BEDS to elucidate bacterial community structure; high-throughput pyrosequencing was done to get 16S rRNA gene sequences. Results showed that initial inoculum was dominated by Proteobacteria, Bacteroidetes, and Acidobacteria but after 45 days of operation Bacteroidetes and Firmicutes were dominant in the biocathode. Phylogenetic analysis showed the dominance of genus Clostridia belonging to phylum Firmicutes which are denitrifying bacteria found ubiquitously in autotrophic and heterotrophic denitrification processes as well as in BEDSs [162]. Metagenomic sequence analysis which targeted V4-V5 hyper variable segments of the 16S rRNA gene showed dominance of denitrifying bacteria such as Pseudomonas (17%), Azoarcus (15%), Thauera (10%) which utilized nitrate as terminal electron acceptor (TEA) in denitrification process. Other bacterial species in the community were Wolinella (1%), Myroids (1%), Petrimonas (8%) which also contributed in denitrification [165]. Deinococcus sp. and Paenibacillus sp. were studied in combination in a MFC to elucidate their synergistic role in bioelectricity production and PHs removal. Results showed indeed these two species acted synergistically where Deinococcus sp. were able to degrade PHs to fatty acids and esters while these simple compounds were further acted upon by Paenibacillus sp. [215].
6. Scaling of Bioelectrochemical Systems for BioelectroremediationAmidst this huge demand of energy consumption in today’s world there is an urgent need to scale up BERT from laboratory to industrial level. Factors that hinder scalability of BERT is that many of these technologies bank upon external power sources and also few times electrochemical reaction ORR rate is poor which increases the overall operation cost whereas decreases the recovery rate of valuable resources. To increase the scalability of this technique, optimizing the operational conditions such as reactor design, material selection for electrode and characterisation of wastewater can prove beneficial [216,217]. A major contributor (50–60%) towards the fabrication cost of reactor is the choice of membrane hence recent researches has been focused on increasing the cost effectiveness of the membranes along with conducting experiments with low-cost membrane materials [218,219].
Another factor that has led to the failure of commercialization of this technique and its scale-up is the selection of the microbial consortium without considering its electron transfer pathway because the electron transfer pathway is the most important contributor towards driving this process forward. Commercialization of MECs, MDCs, and MESs require their modular units to be either connected in series or parallel to manage the huge inflow of wastewater. Apart from that, maintaining uniformity and homogeneity across all operating parameters is the most challenging task that involves fluid dynamics [220].
Onsite work done with microbial electrochemical technologies (METs) has shown that the main challenges to apply these systems in real life scenarios majorly includes characterization of substrate used, occurrence of antimicrobial agents in-situ, various aesthetics related to recovery of resource from waste materials [221]. Other factors such as manhandling of the instrument, operational constrains, maintenance cost are few important areas which requires to be addressed to make this process more sustainable as well as suitable for up scaling from laboratory to commercialisation stage [222].
7. Challenges and Future ProspectiveThough there has been various significant research in this area but still BERT is regarded as low energy generating system. This low energy output has been accredited to thermodynamic restrains and losses at different stages such as activation and ohmic losses, less coulombic efficiency, as well as various factors involved with different structural, technological, electrochemical and microbial processes that attributes to high cost of the system. Theoretically any MFCs produces a maximum of 1160 mV when the carbon source is acetate, and the terminal electron acceptor is oxygen but in practical application the voltage obtained from the system is always less than the theoretical value due to the above-mentioned losses. Major challenges occur in optimising different operational parameters which can lead to higher efficiency of waste treatment and resource recovery, leading to better energy generation. For up-scaling of the system to industrial level, factor such as establishment and operational cost, longevity of the materials used against deterioration as well as biofouling should be considered. More pilot scale studies are required to understand and document the real potential of these systems. Future prospective of BERT is very promising as these technologies apart from treating wastewater along with resource recovery and generating bioelectricity, are also being incorporated into benthic biosensors in oceanic floors, to make robots such as gastrobot, and also for generating electricity from urine for providing power to urinals. In future this technology will become one of the sustainable sources of energy for many small devices. To be a sustainable source of energy for larger units, stacked modules or multi-anode/cathode systems will work better in continuous mode which may be able to solve the problem of toxic by-product generation and decreasing bioelectricity production over time.
8. ConclusionBERT can effectively be used for bioremediation of industrial effluents from soil, water or sediment. PAHs removal using BERT usually varies between 55 to 82% (soil or sediment samples), to even 100% removal (wastewater sample) [61,103]. PCE is a major ground water pollutant and 91% of PCE was degraded to trichloroethene (TCE) and cis-DCE (14% and 77%) respectively after only 1 day from sediment samples contaminanted with PCE [126]. Perchlorate in nature co-occurs with nitrate ions and BERT can effectively remove both from contaminated samples though presence of high concentration of nitrate ion has shown to slow down the reduction of perchlorate ion. A high concentration of perchlorate (60 mg/L) was removed in a MEC study with Azospiraoryzae strain DSM 13638 [150]. When nitrate removal was studied along with perchlorate with NO3 −/ClO4 − molar ratio of 1:3.7, nitrate removal rate was greatly reduced to 42.36% indicating that higher concentration of perchlorate inhibited nitrate reduction rate while perchlorate removal was only 3.94% [147]. Multi-anode system has shown better ability to remove PHs than single anode system. Few single cell MFCs have achieved 83.4% diesel removal rate and 59.14% removal of petroleum spill from polluted soil [115]. From bioenergy (in form of bioelectricity) generation point of view, good power generation has been shown in lab scale studies but yet factors such as architectural design of reactor, microbial kinetics and the synergic/antagonist behaviour of the microbial consortium challenges up-scaling of BERT in industrial effluent treatment. Use of microorganisms as biocatalyst is much more efficient and effective in treating industrial effluents in terms of energy recovery as it overcomes many of the disadvantages of metal catalysts. Increased number of pilot scale studies are required to estimate the real potential of the system when applied to in situ contaminated sites.
AcknowledgmentThis work was supported by Science and Engineering Research Board (SERB) Grant no SRG/2021/001460.
NotesReferences1. Chabukdhara M, Gupta SK, Kotecha Y, Nema AK. Calidad del agua subterránea en el distrito de Ghaziabad, Uttar Pradesh, India: multivariante y evaluación de riesgos para la salud. Chemosphere. 2017;179:167–78.
2. Chandra R, Kumar V. Biodegradation of Chemical Pollutants of Tannery Wastewater. Environ. Waste Manag. CRC Press; 2016;385–412.
https://doi.org/10.1201/b19243-17
3. Chandra R. Advances in biodegradation and bioremediation of industrial waste. Adv. Biodegrad. Bioremediation Ind Waste. 2015;1–426.
https://doi.org/10.1201/b18218
4. Sakoda M, Tokida T, Sakai Y, Senoo K, Nishizawa T. Mitigation of Paddy Field Soil Methane Emissions by Betaproteobacterium Azoarcus Inoculation of Rice Seeds. Microbes Environ. 2022;37:ME22052.
https://doi.org/10.1264/jsme2.ME22052
5. Dong K, Hochman G, Kong X, Sun R, Wang Z. Spatial econometric analysis of China’s PM10 pollution and its influential factors: Evidence from the provincial level. Ecol. Indic. 2019;96:317–28.
https://doi.org/10.1016/j.ecolind.2018.09.014
6. Chandra R, Kumar V, Tripathi S, Sharma P. Heavy metal phytoextraction potential of native weeds and grasses from endocrine-disrupting chemicals rich complex distillery sludge and their histological observations during in-situ phytoremediation. Ecol. Eng. 2018;111:143–56.
https://doi.org/10.1016/j.ecoleng.2017.12.007
7. Kumar V, Chandra R. Bioremediation of Melanoidins Containing Distillery Waste for Environmental. Chapter 20. Bioremediation of Melanoidins Containing Distillery Waste for Environmental Safety. 2020;
https://doi.org/10.1007/978-981-13-3426-9
8. Naha S, Joshi C, Chandrashekhar B, Sreekrishnan TR, Goswami P, Sevda S. Bioelectrosynthesis of Organic and Inorganic Chemicals in Bioelectrochemical System. J. Hazardous, Toxic, Radioact. Waste. 2020;24:03120001.
https://doi.org/10.1061/(asce)hz.2153-5515.0000491
9. Ahsan A, Alamgir M, El-Sergany MM, Shams S, Rowshon MK, Daud NNN. Assessment of Municipal Solid Waste Management System in a Developing Country. Chinese J. Eng. 2014;2014:1–11.
https://doi.org/10.1155/2014/561935
10. Sharma NK, Sharma S. Municipal solid waste management in developing countries: Future challenges and possible opportunities. J. Green Eng. 2020;10:8788–97.
https://doi.org/10.5772/16438
11. Yadav IC, Devi NL, Syed JH, Cheng Z, Li J, Zhang G, et al. Current status of persistent organic pesticides residues in air, water, and soil, and their possible effect on neighboring countries: A comprehensive review of India. Sci. Total Environ. 2015;511:123–37.
https://doi.org/10.1016/j.scitotenv.2014.12.041
12. Prasad S, Singh A, Korres NE, Rathore D, Sevda S, Pant D. Sustainable utilization of crop residues for energy generation: A life cycle assessment (LCA) perspective. Bioresour. Technol. 2020:303.
https://doi.org/10.1016/j.biortech.2020.122964
13. Varanasi JL, Prasad S, Singh H, Das D. Improvement of bioelectricity generation and microalgal productivity with concomitant wastewater treatment in flat-plate microbial carbon capture cell. Fuel. 2020;263:116696.
https://doi.org/10.1016/j.fuel.2019.116696
14. Kumar SS, Kumar V, Kumar R, Malyan SK, Pugazhendhi A. Microbial fuel cells as a sustainable platform technology for bioenergy, biosensing, environmental monitoring, and other low power device applications. Fuel. 2019;255:115682.
https://doi.org/10.1016/j.fuel.2019.115682
15. Singh DP, Singh HB, Prabha R. Plant-Microbe Interactions in Agro-Ecological Perspectives. Plant-Microbe Interact. Agro-Ecological Perspect. 2017;2:1–763.
https://doi.org/10.1007/978-981-10-6593-4
16. Daghio M, Aulenta F, Vaiopoulou E, Franzetti A, Arends JBA, Sherry A, et al. Electrobioremediation of oil spills. Water Res. 2017;114:351–70.
https://doi.org/10.1016/j.watres.2017.02.030
17. Yong X, Gu D, Wu Y, Yan Z, Zhou J. Bio-Electron-Fenton (BEF) process driven by microbial fuel cells for triphenyltin chloride (TPTC) degradation. J. Hazard Mater. 2017;324:178–83.
https://doi.org/10.1016/j.jhazmat.2016.10.047
18. Rajasulochana P, Preethy V. Comparison on efficiency of various techniques in treatment of waste and sewage water - A comprehensive review. Resour. Technol. 2016;2:175–84.
https://doi.org/10.1016/j.reffit.2016.09.004
19. Kumar V, Sharma DC. Distillery Effluent: Pollution Profile, Eco-friendly Treatment Strategies Challenges and Future Prospects, Chapter 17. Microbial Metablosim of Xenobiotic Compounds. 2019;337–357.
https://doi.org/10.1007/978-981-13-7462-3
20. Kumar SS, Malyan SK, Kumar A, Bishnoi NR. Optimization of fenton’s oxidation by box-behnken design of response surface methodology for landfill leachate. J. Mater. Environ. Sci. 2016;7:4456–66.
21. Kumar SS, Yadav C, Malyan SK, Manju , Bajwa K, Bishnoi NR. Dose optimization for aluminium and iron based coagulants viz., aluminium sulphate, ferric chloride and ferrous sulphate for COD removal from landfill leachate at its natural pH. Ann. Agri. Bio. Res. 2016;21:120–3.
22. Abdel-Shafy HI, Mansour MSM. Solid waste issue: Sources, composition, disposal, recycling, and valorization. Egypt J. Pet. 2018;27(4)1275–1290.
https://doi.org/10.1016/j.ejpe.2018.07.003
23. Sarker NK, Sarkar S. A comparative study on cost analysis, efficiency, and process mechanism of effluent treatment plants in Bangladesh. Environ. Qual. Manag. 2018;27:127–33.
https://doi.org/10.1002/tqem.21533
24. Abedin MA, Jahiruddin M. Asian Journal of Medical and Biological Research Waste generation and management in Bangladesh: An overview. Asian J. Med. Biol. Res. 2015;1:114–20.
25. Quraishi M, Wani K, Pandit S, Gupta PK, Rai AK, Lahiri D, et al. Valorisation of CO2 into Value-Added Products via Microbial Electrosynthesis (MES) and Electro-Fermentation Technology. Fermentation. 2021;7:291.
https://doi.org/10.3390/fermentation7040291
26. Khan MS, Ahmed S, Evans AEV, Chadwick M. Methodology for Performance Analysis of Textile Effluent Treatment Plants in Bangladesh. Chem. Eng. Res. Bull. 2009;13:61–6.
https://doi.org/10.3329/cerb.v13i2.3939
27. Huang L, Cheng S, Chen G. Bioelectrochemical systems for efficient recalcitrant wastes treatment. J. Chem. Technol. Biotechnol. 2011;86:481–91.
https://doi.org/10.1002/jctb.2551
28. Ding W, Cheng S, Yu L, Huang H. Chemosphere Effective swine wastewater treatment by combining microbial fuel cells with fl occulation. Chemosphere. 2017;182:567–73.
https://doi.org/10.1016/j.chemosphere.2017.05.006
29. Zhang Y, Noori JS, Angelidaki I. Simultaneous organic carbon, nutrients removal and energy production in a photomicrobial fuel cell (PFC). Energy Environ. Sci. 2011;4:4340–4346.
https://doi.org/10.1039/c1ee02089g
30. Kang H, Kim E, Jung SP. Influence of flowrates to a reverse electro-dialysis (RED) stack on performance and electrochemistry of a microbial reverse electrodialysis cell (MRC). Int. J. Hydrogen Energy. 2017;42:27685–92.
https://doi.org/10.1016/j.ijhydene.2017.06.187
31. Pinder RW, Bettez ND, Bonan GB, Greaver TL, Wieder WR, Schlesinger WH, et al. Impacts of human alteration of the nitrogen cycle in the US on radiative forcing. Biogeochemistry. 2013;114:25–40.
https://doi.org/10.1007/s10533-012-9787-z
32. Smil V. Nitrogen and food production: Proteins for human diets. Ambio. 2002;31:126–31.
https://doi.org/10.1579/0044-7447-31.2.126
33. Litke DW. Review of Phosphorus Control Measures in the United States and Their Effects on Water Quality. Water-Resources Investig. Rep. 99–4007. 1999;1–38.
34. Thevenon F, Graham ND, Chiaradia M, Arpagaus P, Wildi W, Poté J. Local to regional scale industrial heavy metal pollution recorded in sediments of large freshwater lakes in central Europe (lakes Geneva and Lucerne) over the last centuries. Sci. Total Environ. 2011;412–413:239–47.
https://doi.org/10.1016/j.scitotenv.2011.09.025
35. Amin MM, Golbini Mofrad MM, Pourzamani H, Sebaradar SM, Ebrahim K. Treatment of industrial wastewater contaminated with recalcitrant metal working fluids by the photo-Fenton process as post-treatment for DAF. J. Ind. Eng. Chem. 2017;45:412–20.
https://doi.org/10.1016/j.jiec.2016.10.010
36. Badmus KO, Tijani JO, Massima E, Petrik L. Treatment of persistent organic pollutants in wastewater using hydrodynamic cavitation in synergy with advanced oxidation process. Environ. Sci. Pollut. Res. 2018;25:7299–314.
https://doi.org/10.1007/s11356-017-1171-z
37. Katsoyiannis A, Samara C. Persistent organic pollutants (POPs) in the conventional activated sludge treatment process: Fate and mass balance. Environ. Res. 2005;97:245–57.
https://doi.org/10.1016/j.envres.2004.09.001
38. Mohapatra DP, Brar SK, Tyagi RD, Picard P, Surampalli RY. Analysis and advanced oxidation treatment of a persistent pharmaceutical compound in wastewater and wastewater sludge-carbamazepine. Sci. Total Environ. 2014;470–471:58–75.
https://doi.org/10.1016/j.scitotenv.2013.09.034
39. Johnson AC, Jürgens MD, Lawlor AJ, Cisowska I, Williams RJ. Particulate and colloidal silver in sewage effluent and sludge discharged from British wastewater treatment plants. Chemosphere. 2014;112:49–55.
https://doi.org/10.1016/j.chemosphere.2014.03.039
40. Abbas SZ, Rafatullah M, Khan MA, Siddiqui MR. Bioremediation and electricity generation by using open and closed sediment microbial fuel cells. Front. Microbiol. 2019;10:1–12.
https://doi.org/10.3389/fmicb.2018.03348
41. Li X, Wang X, Weng L, Zhou Q, Li Y. Microbial Fuel Cells for Organic-Contaminated Soil Remedial Applications: A Review. Energy Technol. 2017;5:1156–64.
https://doi.org/10.1002/ente.201600674
42. Wang C, Deng H, Zhao F. The Remediation of Chromium (VI)-Contaminated Soils Using Microbial Fuel Cells. Soil Sediment. Contam. 2016;25:1–12.
https://doi.org/10.1080/15320383.2016.1085833
43. Li H, He W, Qu Y, Li C, Tian Y, Feng Y. Pilot-scale benthic microbial electrochemical system (BMES) for the bioremediation of polluted river sediment. J. Power Sources. 2017;356:430–7.
https://doi.org/10.1016/j.jpowsour.2017.03.066
44. Son S, Jung SP. Trends and Prospects of Sediment Microbial Fuel Cells as Sustainable Aquatic Ecosystem Remediation Technology. J Korean Soc. Environ. Eng. 2022;44:468–92.
https://doi.org/10.4491/ksee.2022.44.11.468
45. Nastro RA, Gambino E, Toscanesi M, Arienzo M, Trifuoggi M. Microbial Fuel Cells (MFCs) Remediation Activity of Marine Sediments Sampled at a Dismissed Industrial Site: What Opportunities? J. Clean Prod. 2019;235:1559–1566.
https://doi.org/10.1016/j.jclepro.2019.07.019
46. Logan BE. Exoelectrogenic bacteria that power microbial fuel cells. Nat. Rev. Microbiol. 2009;7:375–81.
https://doi.org/10.1038/nrmicro2113
47. Chandra R, Kumar V. Detection of Androgenic-Mutagenic Compounds and Potential Autochthonous Bacterial Communities during In Situ Bioremediation of Post-methanated Distillery Sludge. Front. Microbiol. 2017;8:1–18.
https://doi.org/10.3389/fmicb.2017.00887
48. Chandra R, Kumar V. Detection of Bacillus and Stenotrophomonas species growing in an organic acid and endocrine-disrupting chemical-rich environment of distillery spent wash and its phytotoxicity. Environ. Monit. Assess. 2017;
https://doi.org/10.1007/s10661-016-5746-9
49. Chandra R, Kumar V, Tripathi S. Evaluation of molasses-melanoidin decolourisation by potential bacterial consortium discharged in distillery effluent. 3. Biotech. 2018;8:1–16.
https://doi.org/10.1007/s13205-018-1205-3
50. Kumar V, Singh J, Saini A, Kumar P. Phytoremediation of copper, iron and mercury from aqueous solution by water lettuce (Pistia stratiotes L.). Environ. Sustain. 2019;2:55–65.
https://doi.org/10.1007/s42398-019-00050-8
51. Singh JS, Kumar A, Rai AN, Singh DP. Cyanobacteria: A precious bio-resource in agriculture, ecosystem, and environmental sustainability. Front. Microbiol. 2016;7:1–19.
https://doi.org/10.3389/fmicb.2016.00529
52. Azubuike CC, Chikere CB, Okpokwasili GC. Bioremediation techniques-classification based on site of application: principles, advantages, limitations and prospects. World J. Microbiol. Biotechnol. 2016;32:
https://doi.org/10.1007/s11274-016-2137-x
53. Kumar V, Singh J, Chopra AK. Assessment of plant growth attributes, bioaccumulation, enrichment, and translocation of heavy metals in water lettuce (Pistia stratiotes L.) grown in sugar mill effluent. Int. J. Phytoremediation. 2018;20:507–21.
https://doi.org/10.1080/15226514.2017.1393391
54. Raj K, Sardar UR, Bhargavi E, Devi I, Bhunia B, Tiwari ON. Advances in exopolysaccharides based bioremediation of heavy metals in soil and water: A critical review. Carbohydr. Polym. 2018;199:353–64.
https://doi.org/10.1016/j.carbpol.2018.07.037
55. Hemdan B, Garlapati VK, Sharma S, Bhadra S, Maddirala S, Varsha KM, et al. Bioelectrochemical systems-based metal recovery: Resource, conservation and recycling of metallic industrial effluents. Environ. Res. 2021:112346.
https://doi.org/10.1016/j.envres.2021.112346
56. Bhadra S, Sevda S. Use of bio-electrochemical system for industry effluents and resource recovery. Novel Approaches Towards Wastewater Treatment and Resource Recovery Technologies. 2022;109–30.
https://doi.org/10.1016/B978-0-323-90627-2.00018-6
57. Liu Z-D, Li H-R. Effects of bio- and abio-factors on electricity production in a mediatorless microbial fuel cell. Biochem. Eng. J. 2007;36:209–14.
https://doi.org/10.1016/j.bej.2007.02.021
58. Puig S, Serra M, Coma M, Cabré M, Balaguer MD, Colprim J. Effect of pH on nutrient dynamics and electricity production using microbial fuel cells. Bioresource Technology. 2010;101:9594–9.
https://doi.org/10.1016/j.biortech.2010.07.082
59. Oliveira VB, Simões M, Melo LF, Pinto AMFR. Overview on the developments of microbial fuel cells. Biochem. Eng. J. 2013;73:53–64.
https://doi.org/10.1016/j.bej.2013.01.012
60. Larrosa-Guerrero a, Scott K, Head IM, Mateo F, Ginesta a, Godinez C. Effect of temperature on the performance of microbial fuel cells. Fuel. 2010;89:3985–94.
https://doi.org/10.1016/j.fuel.2010.06.025
61. Adelaja O, Keshavarz T, Kyazze G. The effect of salinity, redox mediators and temperature on anaerobic biodegradation of petroleum hydrocarbons in microbial fuel cells. J. Hazard Mater. 2015;283:211–7.
https://doi.org/10.1016/j.jhazmat.2014.08.066
62. Tran VHH, Kim E, Jung SP. Anode biofilm maturation time, stable cell performance time, and time-course electrochemistry in a single-chamber microbial fuel cell with a brush-anode. J. Ind. Eng. Chem. 2022;106:269–78.
https://doi.org/10.1016/j.jiec.2021.11.001
63. Odunlami OA, Vershima DA, Tagbo CV, Ogunlade S, Nkongho S. Microbial desalination cell technique - A review. South African J. Chem. Eng. 2023;46:312–29.
https://doi.org/10.1016/j.sajce.2023.07.011
64. Savla N, Pandit S, Khanna N, Mathuriya AS, Jung SP. Microbially Powered Electrochemical Systems Coupled with Membrane-based Technology for Sustainable Desalination and Efficient Wastewater Treatment. J. Korean Soc. Environ. Eng. 2020;42:360–80.
https://doi.org/10.4491/ksee.2020.42.7.360
65. Son S, Koo B, Chai H, Tran HVH, Pandit S, Jung SP. Comparison of hydrogen production and system performance in a microbial electrolysis cell containing cathodes made of non-platinum catalysts and binders. J. Water Process Eng. 2021;40:101844.
https://doi.org/10.1016/j.jwpe.2020.101844
66. Koo B, Jung SP. Trends and perspectives of microbial electrolysis cell technology for ultimate green hydrogen production. J. Korean Soc. Environ. Eng. 2022;44:383–96.
https://doi.org/10.4491/ksee.2022.44.10.383
67. Pawar AA, Karthic A, Lee S, Pandit S, Jung SP. Microbial electrolysis cells for electromethanogenesis: Materials, configurations and operations. Environ. Eng. Res. 2022:27.
https://doi.org/10.4491/eer.2020.484
68. Son S, Kim Y, Kim MW, Jung SP. Recent Trends and Prospects of Microbial Fuel Cell Technology for Energy Positive Wastewater Treatment Plants Treating Organic Waste Resources. J. Korean Soc. Environ. Eng. 2021;43:623–53.
https://doi.org/10.4491/ksee.2021.43.10.623
69. Chai H, Choi Y, Kim M, Kim Y, Jung SP. Trends of microbial electrochemical technologies for nitrogen removal in wastewater treatment. J. Korean Soc. Water Wastewater. 2020;34:345–56.
https://doi.org/10.11001/jksww.2020.34.5.345
70. Koo B, Lee SM, Oh SE, Kim EJ, Hwang Y, Seo D, et al. Addition of reduced graphene oxide to an activated-carbon cathode increases electrical power generation of a microbial fuel cell by enhancing cathodic performance. Electrochim. Acta. 2019;297:613–22.
https://doi.org/10.1016/j.electacta.2018.12.024
71. Koo B, Jung SP. Improvement of air cathode performance in microbial fuel cells by using catalysts made by binding metal-organic framework and activated carbon through ultrasonication and solution precipitation. Chem. Eng. J. 2021;424:130388.
https://doi.org/10.1016/j.cej.2021.130388
72. Jung SP, Son S, Koo B. Reproducible polarization test methods and fair evaluation of polarization data by using interconversion factors in a single chamber cubic microbial fuel cell with a brush anode. J. Clean. Prod. 2023;390:136157.
https://doi.org/10.1016/j.jclepro.2023.136157
73. Pandit S, Savla N, Jung SP. Recent advancements in scaling up microbial fuel cells. In Integrated Microbial Fuel Cells for Wastewater Treatment. 2020:349–368.
https://doi.org/10.1016/B978-0-12-817493-7.00016-3
74. Khoo KS, Chia WY, Show P, Wayne CK. Nanomaterials Utilization in Biomass for Biofuel and Bioenergy Production. Energies. 2020;13(4)892.
https://doi.org/10.3390/en13040892
75. Jung S, Ahn YH, Oh SE, Lee J, Cho KT, Kim Y, et al. Impedance and thermodynamic analysis of bioanode, abiotic anode, and riboflavin-amended anode in microbial fuel cells. Bull. Korean Chem. Soc. 2012;33:3349–54.
https://doi.org/10.5012/bkcs.2012.33.10.3349
76. Kang H, Jeong J, Gupta PL, Jung SP. Effects of brush-anode configurations on performance and electrochemistry of microbial fuel cells. Int. J. Hydrogen Energy. 2017;42:27693–700.
https://doi.org/10.1016/j.ijhydene.2017.06.181
77. Nam T, Son S, Koo B, Hoa Tran HV, Kim JR, Choi Y, et al. Comparative evaluation of performance and electrochemistry of microbial fuel cells with different anode structures and materials. Int. J. Hydrogen Energy. 2017;42:27677–84.
https://doi.org/10.1016/j.ijhydene.2017.07.180
78. Nam T, Kang H, Pandit S, Kim SH, Yoon S, Bae S, et al. Effects of vertical and horizontal configurations of different numbers of brush anodes on performance and electrochemistry of microbial fuel cells. J. Clean. Prod. 2020;277:124125.
https://doi.org/10.1016/j.jclepro.2020
79. Jung SP, Kim E, Koo B. Effects of wire-type and mesh-type anode current collectors on performance and electrochemistry of microbial fuel cells. Chemosphere. 2018;209:542–50.
https://doi.org/10.1016/j.chemosphere.2018.06.070
80. Gurung A, Kim J, Jung S, Jeon BH, Yang JE, Oh SE. Effects of substrate concentrations on performance of serially connected microbial fuel cells (MFCs) operated in a continuous mode. Biotechnol. Lett. 2012;34:1833–9.
https://doi.org/10.1007/s10529-012-0979-3
81. Pandit S, Savla N, Sonawane JM, Sani AM, Gupta PK, Mathuriya AS, et al. Agricultural waste and wastewater as feedstock for bioelectricity generation using microbial fuel cells: Recent advances. Fermentation. 2021:7.
https://doi.org/10.3390/fermentation7030169
82. Jung S, Mench MM, Regan JM. Impedance Characteristics and Polarization Behavior of a Microbial Fuel Cell in Response to Short-Term Changes in Medium pH. Environ. Sci. Technol. 2011;45(20)9069–9074.
https://doi.org/10.1021/es201737g
83. Jung S, Regan JM. Influence of External Resistance on Electrogenesis, Methanogenesis, and Anode Prokaryotic Communities in Microbial Fuel Cells. Appl. Environ. Microbiol. 2011;77:564–71.
https://doi.org/10.1128/AEM.01392-10
84. Jung S. Impedance Analysis of Geobacter sulfurreducens PCA, Shewanella oneidensis MR-1, and their Coculture in Bioeletrochemical Systems. Int. J. Electrochem. Sci. 2012;7:11091–100.
https://doi.org/10.1016/S1452-3981(23)16929-X
85. Tran VHH, Kim E, Jung SP. Anode biofilm maturation time, stable cell performance time, and time-course electrochemistry in a single-chamber microbial fuel cell with a brush-anode. J. Ind. Eng. Chem. 2022;106:269–278.
https://doi.org/10.1016/j.jiec.2021.11.001
86. Jung S, Mench MM, Regan JM. Impedance characteristics and polarization behavior of a microbial fuel cell in response to short-term changes in medium pH. Environ. Sci. Technol. 2011;45:9069–9074.
https://doi.org/10.1021/es201737g
87. Lan J, Sun Y, Xiao S, Yuan D. Polycyclic aromatic hydrocarbon contamination in a highly vulnerable underground river system in Chongqing, Southwest China. J. Geochemical. Explor. 2016;168:65–71.
https://doi.org/10.1016/j.gexplo.2016.05.013
88. Yakovleva EV, Gabov DN, Beznosikov VA, Kondratenok BM. Accumulation of Polycyclic Aromatic Hydrocarbons in Soils and Plants of the Tundra Zone under the Impact of Coal-Mining Industry. Eurasian J. Soil. Sci. 2016;49:1319–28.
https://doi.org/10.1134/S1064229316090143
89. Lübeck JS, Poulsen KG, Knudsen B, Soleimani M, Furbo S, Tomasi G, et al. Baseline source apportionment of polycyclic aromatic hydrocarbons (PAHs) in sediments from Khuzestan province, Iran. Mar. Pollut. Bull. 2016;110(1)584–590.
https://doi.org/10.1016/j.marpolbul.2016.05.057
90. Jones KC, de Voogt P. Persistent organic pollutants (POPs): state of the science. Environ. Pollut. 1999;100:209–21.
https://doi.org/10.1016/S0269-7491(99)00098-6
91. Barrie LA, Gregor D, Hargrave B, Lake R, Muir D, Shearer R, et al. Arctic contaminants: sources, occurrence and pathways. Sci. Total Environ. 1992;122:1–74.
https://doi.org/10.1016/0048-9697(92)90245-N
92. Axelman J, Palm A, Cousins I, Grunder K, Broman D, Brorstro E. Evaluation of sequentially-coupled POP fluxes estimated from simultaneous measurements in multiple compartments of an air - water - sediment system. Environ. Pollut. 2004;128:85–97.
https://doi.org/10.1016/j.envpol.2003.08.023
93. Yang Y, Woodward LA, Li QX, Wang J. Concentrations, Source and Risk Assessment of Polycyclic Aromatic Hydrocarbons in Soils from Midway Atoll, North Pacific Ocean. PLOS. ONE. 2014;9(1)e86441.
https://doi.org/10.1371/journal.pone.0086441
94. Feng J, Zhai M, Sun J, Liu Q. Distribution and sources of polycyclic aromatic hydrocarbons (PAHs) in sediment from the upper reach of Huaihe River, East China. Environ. Sci. Pollut. Res. 2012;1097–106.
https://doi.org/10.1007/s11356-011-0620-3
95. Huang L, Wang Q, Quan X, Liu Y, Chen GPT. Bioanodes/biocathodes formed at optimal potentials enhance subsequent pentachlorophenol degradation and power generation from microbial fuel cells. Bioelectrochemistry. 2013;94:13–22.
https://doi.org/10.1016/j.bioelechem.2013.05.001
96. Bolognesi S, Bañeras L, Perona-Vico E, Capodaglio AG, Balaguer MD, Puig S. Carbon dioxide to bio-oil in a bioelectrochemical system-assisted microalgae biorefinery process. Sustain. Energy Fuels. 2022;6:150–61.
https://doi.org/10.1039/d1se01701b
97. She Y, Wu J, Zhang Y, Peng Y, Mo L, Luo X, et al. Bioaccumulation of polybrominated diphenyl ethers and several alternative halogenated flame retardants in a small herbivorous food chain. Environ. Pollut. 2013;174:164–170.
https://doi.org/10.1016/j.envpol.2012.11.024
98. Domingo JL. Human exposure to polybrominated diphenyl ethers through the diet. J. Chromatogr. A. 2004;1054:321–6.
https://doi.org/10.1016/j.chroma.2004.03.042
99. Kim K, Ara S, Kabir E, Brown RJC. A review of airborne polycyclic aromatic hydrocarbons (PAHs) and their human health effects. Environ. Int. 2013;60:71–80.
https://doi.org/10.1016/j.envint.2013.07.019
100. Chandrasekhar K, Mohan SV. Bioresource Technology Bio-electrochemical remediation of real field petroleum sludge as an electron donor with simultaneous power generation facilitates biotransformation of PAH: Effect of substrate concentration. Bioresour. Technol. 2012;110:517–25.
https://doi.org/10.1016/j.biortech.2012.01.128
101. Zhang T, Gannon SM, Nevin KP, Franks AE, Lovley DR. Stimulating the anaerobic degradation of aromatic hydrocarbons in contaminated sediments by providing an electrode as the electron acceptor. Environ. Micrbiol. 2010;12:1011–20.
https://doi.org/10.1111/j.1462-2920.2009.02145.x
102. Mohan SV, Chandrasekhar K. Bioresource Technology Self-induced bio-potential and graphite electron accepting conditions enhances petroleum sludge degradation in bioelectrochemical system with simultaneous power generation. Bioresour. Technol. 2011;102:9532–41.
https://doi.org/10.1016/j.biortech.2011.07.038
103. Adelaja O, Keshavarz T, Kyazze G. Enhanced biodegradation of phenanthrene using different inoculum types in a microbial fuel cell. Eng. Life. Sci. 2014;14:218–28.
https://doi.org/10.1002/elsc.201300089
104. Yeruva DK, Jukuri S, Velvizhi G, Kumar AN, Swamy YV, Mohan SV. Integrating sequencing batch reactor with bio-electrochemical treatment for augmenting remediation efficiency of complex petrochemical wastewater. Bioresour. Technol. 2015;118:32–42.
https://doi.org/10.1016/j.biortech.2015.02.014
105. Sabina K, Fayidh Ma, Archana G, Sivarajan M, Babuskin S, Babu PAS, et al. Microbial desalination cell for enhanced biodegradation of waste engine oil using a novel bacterial strain Bacillus subtilis moh3. Environ. Technol. 2014;35:2194–203.
https://doi.org/10.1080/09593330.2014.896951
106. Morris JM, Jin S, Crimi B, Pruden A. Microbial fuel cell in enhancing anaerobic biodegradation of diesel. Chem. Eng. J. 2009;146:161–7.
https://doi.org/10.1016/j.cej.2008.05.028
107. Morris JM, Jin S. Enhanced biodegradation of hydrocarbon-contaminated sediments using microbial fuel cells. J. Hazard. Mater. 2012;213–214:474–7.
https://doi.org/10.1016/j.jhazmat.2012.02.029
108. Yan Z, Song N, Cai H, Tay J, Jiang H. Enhanced degradation of phenanthrene and pyrene in freshwater sediments by combined employment of sediment microbial fuel cell and amorphous ferric hydroxide. J. Hazard. Mater. 2012;199–200:217–25.
https://doi.org/10.1016/j.jhazmat.2011.10.087
109. Xia C, Xu M, Liu J, Guo J, Yang Y. Sediment microbial fuel cell prefers to degrade organic chemicals with higher polarity. Bioresour. Technol. 2015;190:420–423.
https://doi.org/10.1016/j.biortech.2015.04.072
110. Wang X, Cai Z, Zhou Q, Zhang Z, Chen C. Bioelectrochemical stimulation of petroleum hydrocarbon degradation in saline soil using U-tube microbial fuel cells. Biotechnol. Bioeng. 2012;109:426–33.
https://doi.org/10.1002/bit.23351
111. Logan BE, Rabaey K. Conversion of Wastes into Bioelectricity and Chemicals by Using Microbial Electrochemical Technologies. Science. 2012;337(6095)686–690.
https://doi.org/10.1126/science.1217412
112. Lowy DA, Tender LM. Harvesting energy from the marine sediment-water interface. J. Power. Sources. 2008;185:70–5.
https://doi.org/10.1016/j.jpowsour.2008.06.079
113. Li X, Wang X, Zhang Y, Zhao Q, Yu B, Li Y, et al. Salinity and Conductivity Amendment of Soil Enhanced the Bioelectrochemical Degradation of Petroleum Hydrocarbons. Sci. Rep. 2016;6:1–11.
https://doi.org/10.1038/srep32861
114. Sherafatmand M, Ng HY. Using sediment microbial fuel cells (SMFCs) for bioremediation of polycyclic aromatic hydrocarbons (PAHs). Bioresour. Technol. 2015;195:122–130.
https://doi.org/10.1016/j.biortech.2015.06.002
115. Venkidusamy K, Megharaj M, Marzorati M, Lockington R, Naidu R. Enhanced removal of petroleum hydrocarbons using a bioelectrochemical remediation system with pre-cultured anodes. Sci. Total Environ. 2016;539:61–69.
https://doi.org/10.1016/j.scitotenv.2015.08.098
116. Huang B, Lei C, Wei C, Zeng G. Chlorinated volatile organic compounds (Cl-VOCs) in environment — sources, potential human health impacts, and current remediation technologies. Environ. Int. 2014;71:118–38.
https://doi.org/10.1016/j.envint.2014.06.013
117. Sheu YT, Lien PJ, Chen KF, Ou JH, Kao CM. Application of NZVI-contained emulsified substrate to bioremediate PCE-contaminated groundwater - A pilot-scale study. Chem. Eng. J. 2016;304:714–27.
https://doi.org/10.1016/j.cej.2016.06.126
118. Strycharz SM, Woodard TL, Johnson JP, Nevin KP, Sanford RA, Lo FE, et al. Graphite Electrode as a Sole Electron Donor for Reductive Dechlorination of Tetrachlorethene by Geobacter lovleyi. Appl. Environ. Microbiol. 2008;74:5943–5947.
https://doi.org/10.1128/AEM.00961-08
119. Löffler FE, Yan J, Ritalahti KM, Adrian L, Edwards EA, Konstantinidis KT, et al. Dehalococcoides mccartyi gen. nov., sp. nov., obligately organohalide-respiring anaerobic bacteria relevant to halogen cycling and bioremediation, belong to a novel bacterial class, Dehalococcoidia classis nov., order Dehalococcoidales ord. nov. and famil. Int. J. Syst. Evol. Microbiol. 2013;63:625–635.
https://doi.org/10.1099/ijs.0.034926-0
120. Pérez-de-mora A, Lacourt A, Mcmaster ML, Liang X. Chlorinated Electron Acceptor Abundance Drives Selection of Dehalococcoides mccartyi (D. mccartyi) Strains in Dechlorinating Enrichment Cultures and Groundwater Environments. Front. Microbiol. 2018;9:1–14.
https://doi.org/10.3389/fmicb.2018.00812
121. Hermon L, Hellal J, Denonfoux J, Vuilleumier S, Imfeld G, Urien C, et al. Functional Genes and Bacterial Communities During Organohalide Respiration of Chloroethenes in Microcosms of Multi-Contaminated Groundwater. Front. Microbiol. 2019;10:1–16.
https://doi.org/10.3389/fmicb.2019.00089
122. Leys D, Adrian L, Smidt H. Organohalide respiration: microbes breathing chlorinated molecules. Philos. Trans. R. Soc. B. Biol. Sci. 2013;368:20120316.
https://doi.org/10.1098/rstb.2012.0316
123. Wang H, Luo H, Fallgren PH, Jin S, Ren ZJ. Bioelectrochemical system platform for sustainable environmental remediation and energy generation. Biotechnol. Adv. 2015;33:317–34.
https://doi.org/10.1016/j.biotechadv.2015.04.003
124. Huang L, Regan JM, Quan X. Electron transfer mechanisms, new applications, and performance of biocathode microbial fuel cells. Bioresour. Technol. 2011;102:316–23.
https://doi.org/10.1016/j.biortech.2010.06.096
125. Patil SS, Adetutu EM, Mitchell JG, Ball AS. Sustainable remediation: electrochemically assisted microbial dechlorination of tetrachloroethene-contaminated groundwater. Microb. Biotechnol. 2014;7(1)54–63.
https://doi.org/10.1111/1751-7915.12089
126. Yu J, Park Y, Nguyen VK, Lee T. PCE dechlorination by non Dehalococcoides in a microbial electrochemical system. J. Ind. Microbiol. Biotechnol. 2016;43:1095–1103.
https://doi.org/10.1007/s10295-016-1791-1
127. Aulenta F, Canosa A, Reale P, Rossetti S, Panero S, Majone M. Microbial reductive dechlorination of trichloroethene to ethene with electrodes serving as electron donors without the external addition of redox mediators. Biotechnol. Bioeng. 2009;103:85–91.
https://doi.org/10.1002/bit.22234
128. Chen F, Li Z, Yang J, Liang B, Lin X, Nan J, et al. Short communication E ff ects of di ff erent carbon substrates on performance, microbiome community structure and function for bioelectrochemical-stimulated dechlorination of tetrachloroethylene. Chem. Eng. J. 2018;352:730–6.
https://doi.org/10.1016/j.cej.2018.07.082
129. Aulenta F, Tocca L, Verdini R, Reale P, Majone M. Dechlorination of Trichloroethene in a Continuous-Flow Bioelectrochemical Reactor: Effect of Cathode Potential on Rate, Selectivity, and Electron Transfer Mechanisms. Environ. Sci. Technol. 2011;45(19)8444–8451.
130. Smith PN, Theodorakis CW, Anderson TA, Kendall RJ. Preliminary Assessment of Perchlorate in Ecological Receptors at the Longhorn Army Ammunition Plant (LHAAP), Karnack, Texas. Ecotoxicology. 2001;10:305–313.
131. Ye L, You H, Yao J, Kang X, Tang L. Chemosphere Seasonal variation and factors influencing perchlorate in water, snow, soil and corns in Northeastern China. Chemosphere. 2013;90:2493–8.
https://doi.org/10.1016/j.chemosphere.2012.10.058
132. Greer MA, Goodman G, Pleus RC, Greer SE. Health Effects Assessment for Environmental Perchlorate Contamination: The Dose Response for Inhibition of Thyroidal Radioiodine Uptake in Humans. Environ. Health Perspect. 2002;110:927–37.
133. Bao H, Gu B. Natural perchlorate has a unique oxygen isotope signature. Environ. Sci. Technol. 2004;38:5073–7.
https://doi.org/10.1021/es049516z
134. Plummer LN, Böhlke JK, Doughten MW. Perchlorate in pleistocene and holocene groundwater in North-Central New Mexico. Environ. Sci. Technol. 2006;40:1757–63.
https://doi.org/10.1021/es051739h
135. Urbansky ET, Brown SK, Magnuson ML, Kelty CA. Perchlorate levels in samples of sodium nitrate fertilizer derived from Chilean caliche. Environ. Pollut. 2001;112:299–302.
136. Anoop Raj JR, Muruganandam L. Biodegradation of perchlorate from real and synthetic effluent by Proteobacterium ARJR SMBS in a stirred tank bioreactor system. Environ. Technol. 2013;34:841–52.
https://doi.org/10.1080/09593330.2012.720715
137. Kumarathilaka P, Oze C, Indraratne SP, Vithanage M. Chemosphere Perchlorate as an emerging contaminant in soil, water and food. Chemosphere. 2016;
https://doi.org/10.1016/j.chemosphere.2016.01.109
138. Senanayake G, Childs J, Akerstrom BD, Pugaev D. Hydrometallurgy Reductive acid leaching of laterite and metal oxides — A review with new data for Fe (Ni, Co) OOH and a limonitic ore. Hydrometallurgy. 2011;110:13–32.
https://doi.org/10.1016/j.hydromet.2011.07.011
139. Majima H, Awakura Y, Mishima T. The Leaching of Hematite in Acid Solutions. Metall. Trans. B. 1985;16:23–30.
https://doi.org/10.1007/BF02657484
140. Wan Y, Wu Q, Abualnaja KO, Asimakopoulos AG, Covaci A, Gevao B, et al. Occurrence of perchlorate in indoor dust from the United States and eleven other countries: Implications for human exposure. Environ. Int. 2015;75:166–71.
https://doi.org/10.1016/j.envint.2014.11.005
141. Wu Q, Oldi JF, Kannan K. Fate of perchlorate in a man-made reflecting pond following a fireworks display in Albany, New York, USA. Environ. Toxicol. Chem. 2011;30:2449–55.
https://doi.org/10.1002/etc.648
142. Her N, Yoon Y. Occurrence of Perchlorate in Drinking Water and Seawater in South Korea Occurrence of Perchlorate in Drinking Water and Seawater in South Korea. Arch. Environ. Con. Tox. 2010;61:166–172.
https://doi.org/10.1007/s00244-010-9616-0
143. Wilkin RT, Fine DD, Burnett NG. Perchlorate Behavior in a Municipal Lake Following Fireworks Displays. Environ. Sci. Technol. 2007;41:3966–71.
https://doi.org/10.1021/es0700698
144. Kannan K, Praamsma ML, Oldi JF, Kunisue T, Sinha RK. Occurrence of perchlorate in drinking water, groundwater, surface water and human saliva from India. Chemosphere. 2009;76:22–26.
https://doi.org/10.1016/j.chemosphere.2009.02.054
145. Bardiya N, Bae J. Dissimilatory perchlorate reduction: A review. Microbiol. Res. 2011;166:237–254.
https://doi.org/10.1016/j.micres.2010.11.005
146. Butler C, Clauwaert P, Green S, Verstraete W, Nerenberg R. Bioelectrochemical perchlorate reduction in a microbial fuel cell. ACS. Natl. Meet. B. Abstr. 2010;4685–4691.
147. Jiang C, Yang Q, Wang D, Zhong Y, Chen F, Li X, et al. Simultaneous perchlorate and nitrate removal coupled with electricity generation in autotrophic denitrifying biocathode microbial fuel cell. Chem. Eng. J. 2017;308:783–790.
https://doi.org/10.1016/j.cej.2016.09.121
148. Yang Q, Zhang F, Zhan J, Gao C, Liu M. Perchlorate removal in microbial electrochemical systems with iron/carbon electrodes. Front. Chem. 2019;7:1–7.
https://doi.org/10.3389/fchem.2019.00019
149. Wang Z, Gao M, Zhang Y, She Z, Ren Y, Wang Z. Perchlorate reduction by hydrogen autotrophic bacteria in a bioelectrochemical reactor. J. Environ. Manage. 2014;142:10–6.
https://doi.org/10.1016/j.jenvman.2014.04.003
150. Li J, Gao M, Zhang G, Wang X, Wang S, Song C, et al. Perchlorate reduction in microbial electrolysis cell with polyaniline modified cathode. Bioresour. Technol. 2015;177:74–9.
https://doi.org/10.1016/j.biortech.2014.11.065
151. Xie D, Yu H, Li C, Ren Y, Wei C, Feng C. Electrochimica Acta Competitive microbial reduction of perchlorate and nitrate with a cathode directly serving as the electron donor. Electrochim. Acta. 2014;133:217–23.
https://doi.org/10.1016/j.electacta.2014.04.016
152. Szpyrkowicz L, Daniele S, Radaelli M, Specchia S. Removal of NO3 − from water by electrochemical reduction in different reactor configurations. Appl. Catal. B. Environ. 2006;66:40–50.
https://doi.org/10.1016/j.apcatb.2006.02.020
153. Tannenbaum SR, Moran D, Rand W, Cuello C, Correa P. Gastric cancer in Colombia. IV. Nitrite and other ions in gastric contents of residents from a high-risk region. J. Natl. Cancer. Inst. 1979;62:9–12.
154. Park JY, Yoo YJ. Biological nitrate removal in industrial wastewater treatment: which electron donor we can choose. Appl. Microbiol. Biotechnol. 2009;82:415–29.
https://doi.org/10.1007/s00253-008-1799-1
155. Rodrigo MA, Cañizares P, Lobato J. Bioresource Technology Effect of the electron-acceptors on the performance of a MFC. Bioresour. Technol. 2010;101:7014–8701.
https://doi.org/10.1016/j.biortech.2010.04.013
156. Gregory KB, Bond DR, Lovley DR. Graphite electrodes as electron donors for anaerobic respiration. Environ. Microbiol. 2004;6:596–604.
https://doi.org/10.1111/j.1462-2920.2004.00593.x
157. Zhang F, He Z. Integrated organic and nitrogen removal with electricity generation in a tubular dual-cathode microbial fuel cell. Process Biochem. 2012;47:2146–2151.
https://doi.org/10.1016/j.procbio.2012.08.002
158. Park H, kun Kim D, Choi Y, Pak D. Nitrate reduction using an electrode as direct electron donor in a biofilm-electrode reactor. Process Biochem. 2005;40:3383–3388.
https://doi.org/10.1016/j.procbio.2005.03.017
159. Sakakibara Y, Kuroda M. Electric prompting and control of denitrification. Biotechnol. Bioeng. 1993;42:535–7.
https://doi.org/10.1002/bit.260420418
160. Zhang Y, Angelidaki I. A new method for in situ nitrate removal from groundwater using submerged microbial desalination-denitrification cell (SMDDC). Water Res. 2013;47:1827–36.
https://doi.org/10.1016/j.watres.2013.01.005
161. Kondaveeti S, Lee S, Park H, Min B. Bacterial communities in a bioelectrochemical denitrification system: The effects of supplemental electron acceptors. Water Res. 2013;51:25–36.
https://doi.org/10.1016/j.watres.2013.12.023
162. Lee SH, Kondaveeti S, Min B, Park HD. Enrichment of Clostridia during the operation of an external-powered bio-electrochemical denitrification system. Process Biochem. 2013;48:306–11.
https://doi.org/10.1016/j.procbio.2012.11.020
163. Liu H, Yan Q, Shen W. Biohydrogen facilitated denitrification at biocathode in bioelectrochemical system (BES). Bioresour. Technol. 2014;171:187–192.
https://doi.org/10.1016/j.biortech.2014.08.056
164. Li H, Zuo W, Tian Y, Zhang J, Di S, Li L, et al. Simultaneous nitrification and denitrification in a novel membrane bioelectrochemical reactor with low membrane fouling tendency. Environ. Sci. Pollut. Res. 2017;24:5106–117.
https://doi.org/10.1007/s11356-016-6084-8
165. Kugarajah V, Solomon J, Rajendran K, Dharmalingam S. Enhancement of nitrate removal and electricity generation in microbial fuel cell using eggshell supported biocathode. Process Biochem. 2022;113:1–10.
https://doi.org/10.1016/j.procbio.2021.12.013
166. Chen M, Xu P, Zeng G, Yang C, Huang D, Zhang J. Bioremediation of soils contaminated with polycyclic aromatic hydrocarbons, petroleum, pesticides, chlorophenols and heavy metals by composting: Applications, microbes and future research needs. Biotechnol. Adv. 2015;33:745–55.
https://doi.org/10.1016/j.biotechadv.2015.05.003
167. Ngueleu SK, Rezanezhad F, Al-Raoush RI, Van Cappellen P. Sorption of benzene and naphthalene on (semi)-arid coastal soil as a function of salinity and temperature. J. Contam. Hydrol. 2018;219:61–71.
https://doi.org/10.1016/j.jconhyd.2018.11.001
168. Varjani SJ. Microbial degradation of petroleum hydrocarbons. Bioresour. Technol. 2017;223:277–286.
https://doi.org/10.1016/j.biortech.2016.10.037
169. Errington I, King CK, Wilkins D, Spedding T, Hose GC. Ecosystem effects and the management of petroleum-contaminated soils on subantarctic islands. Chemosphere. 2018;194:200–210.
https://doi.org/10.1016/j.chemosphere.2017.11.157
170. Moussavi G, Khosravi R, Farzadkia M. Removal of petroleum hydrocarbons from contaminated groundwater using an electrocoagulation process: Batch and continuous experiments. Desalination. 2011;278:288–94.
https://doi.org/10.1016/j.desal.2011.05.039
171. Brandão PC, Souza TC, Ferreira CA, Hori CE, Romanielo LL. Removal of petroleum hydrocarbons from aqueous solution using sugarcane bagasse as adsorbent. J. Hazard Mater. 2010;175:1106–12.
https://doi.org/10.1016/j.jhazmat.2009.10.060
172. Esmaeili A, Saremnia B. Journal of Petroleum Science and Engineering Comparison study of adsorption and nano fi ltration methods for removal of total petroleum hydrocarbons from oil-field wastewater. J. Pet. Sci. Eng. 2018;171:403–13.
https://doi.org/10.1016/j.petrol.2018.07.058
173. Varjani SJ, Upasani VN. Bioresource Technology Biodegradation of petroleum hydrocarbons by oleophilic strain of Pseudomonas aeruginosa NCIM 5514. Bioresour. Technol. 2016;222:195–201.
https://doi.org/10.1016/j.biortech.2016.10.006
174. Bajagain R, Jeong S. Chemosphere Degradation of petroleum hydrocarbons in soil via advanced oxidation process using peroxymonosulfate activated by nanoscale zero-valent iron. Chemosphere. 2020:128627.
https://doi.org/10.1016/j.chemosphere.2020.128627
175. Mohammadi L, Rahdar A, Bazrafshan E, Dahmardeh H, Susan MABH, Kyzas GZ. Petroleum Hydrocarbon Removal from Wastewaters: A Review. Processes. 2020;8:447.
https://doi.org/10.3390/pr8040447
176. Mohanakrishna G, Butti SK, Kannaiah Goud R, Venkata Mohan S. Spatiometabolic stratification of anoxic biofilm in prototype bioelectrogenic system. Bioelectrochemistry. 2017;115:11–8.
https://doi.org/10.1016/j.bioelechem.2017.01.002
177. Mohanakrishna G, Abu-Reesh IM, Kondaveeti S, Al-Raoush RI, He Z. Enhanced treatment of petroleum refinery wastewater by short-term applied voltage in single chamber microbial fuel cell. Bioresour. Technol. 2018;253:16–21.
https://doi.org/10.1016/j.biortech.2018.01.005
178. Pasupuleti SB, Srikanth S, Venkata Mohan S, Pant D. Continuous mode operation of microbial fuel cell (MFC) stack with dual gas diffusion cathode design for the treatment of dark fermentation effluent. Int. J. Hydrogen Energy. 2015;40:12424–35.
https://doi.org/10.1016/j.ijhydene.2015.07.049
179. Wang H, Cui Y, Lu L, Jin S, Zuo Y, Ge Z, et al. Moisture retention extended enhanced bioelectrochemical remediation of unsaturated soil. Sci. Total Environ. 2020;724:138169.
https://doi.org/10.1016/j.scitotenv.2020.138169
180. Zhou Y, Zou Q, Fan M, Xu Y, Chen Y. Highly efficient anaerobic co-degradation of complex persistent polycyclic aromatic hydrocarbons by a bioelectrochemical system. J. Hazard Mater. 2020;381:120945.
https://doi.org/10.1016/j.jhazmat.2019.120945
181. Liu B, Williams I, Li Y, Wang L, Bagtzoglou A, Mccutcheon J, et al. Towards high power output of scaled-up benthic microbial fuel cells (BMFCs) using multiple electron collectors. Biosens. Bioelectron. 2016;79:435–441.
https://doi.org/10.1016/j.bios.2015.12.077
182. Prakash O, Pushkar P, Mungray AK, Mungray A, Kailasa SKSC. Effect of geometrical position of a multi-anode system in power output and nutritional variation in benthic microbial fuel cells. J. Environ. Chem. Eng. 2018;6(1)1558–1568.
https://doi.org/10.1016/j.jece.2018.01.058
183. Adelaja O, Keshavarz T, Kyazze G. Treatment of phenanthrene and benzene using microbial fuel cells operated continuously for possible in situ and ex situ applications. Int. Biodeterior. Biodegrad. 2017;116:91–103.
https://doi.org/10.1016/j.ibiod.2016.10.021
184. Yu B, Feng L, He Y, Yang L, Xun Y. Effects of anode materials on the performance and anode microbial community of soil microbial fuel cell. J. Hazard Mater. 2021;401:123394.
https://doi.org/10.1016/j.jhazmat.2020.123394
185. Guo X, Zhan Y, Chen C, Cai B, Wang Y, Guo S. In fluence of packing material characteristics on the performance of microbial fuel cells using petroleum refinery wastewater as fuel. Renew. Energy. 2016;87:437–444.
https://doi.org/10.1016/j.renene.2015.10.041
186. Al-Shehri ANZ. Employment of microbial fuel cell technology to biodegrade naphthalene and benzidine for bioelectricity generation. Int. J. Curr. Microbiol. Appl . 2015;4(1)134–9.
187. Khilari S, Pandit S, Das D, Pradhan D. Manganese cobaltite/polypyrrole nanocomposite-based air-cathode for sustainable power generation in the single-chambered microbial. Biosens. Bioelectron. 2014;54:534–540.
https://doi.org/10.1016/j.bios.2013.11.044
188. Kannan MV, Gnana kumar G. Current status, key challenges and its solutions in the design and development of graphene based ORR catalysts for the microbial fuel cell applications. Biosens. Bioelectron. 2016;77:1208–1220.
https://doi.org/10.1016/j.bios.2015.10.018
189. Xia W, Mahmood A, Liang Z, Zou R, Guo S. Earth-Abundant Nanomaterials for Oxygen Reduction. Angew. Chemie. Int. Ed. 2016;55:2650–76.
https://doi.org/10.1002/anie.201504830
190. Wang Z, Yan Z, Wang M, Zhao J. Glycerol Stabilized NaBH4 Reduction for Preparation Carbon Supported Pt-Ni Alloy Nanoparticles Used as Oxygen-Reduction Electrocatalysts for Microbial Fuel Cells. Int. J. Electrochem. Sci. 2015;10:1953–1965.
https://doi.org/10.1016/S1452-3981(23)04820-4
191. Cheng S, Wu J. Air-cathode preparation with activated carbon as catalyst, PTFE as binder and nickel foam as current collector for microbial fuel cells. Bioelectrochemistry. 2013;92:22–26.
https://doi.org/10.1016/j.bioelechem.2013.03.001
192. Xiong L, Huang Y, Liu X, Sheng G, Li W, Yu H. Electrochimica Acta Three-dimensional bimetallic Pd - Cu nanodendrites with superior electrochemical performance for oxygen reduction reaction. Electrochim. Acta. 2013;89:24–8.
https://doi.org/10.1016/j.electacta.2012.10.162
193. Hou Y, Yuan H, Wen Z, Cui S, Guo X, He Z, et al. Nitrogen-doped graphene/CoNi alloy encased within bamboo-like carbon nanotube hybrids as cathode catalysts in microbial fuel cells. J. Power. Sources. 2016;307:561–568.
https://doi.org/10.1016/j.jpowsour.2016.01.018
194. Zhao F, Bogdanoff P, Herrmann I. Application of pyrolysed iron (II) phthalocyanine and CoTMPP based oxygen reduction catalysts as cathode materials in microbial fuel cells. Electrochem. Commun. 2005;7:1405–1410.
https://doi.org/10.1016/j.elecom.2005.09.032
195. Ham DJ, Lee JS. Transition Metal Carbides and Nitrides as Electrode Materials for Low Temperature Fuel Cells. Energies. 2009;873–899.
https://doi.org/10.3390/en20400873
196. Singh KP, Song MY, Yu J. Iodine-treated heteroatom-doped carbon: conductivity driven electrocatalytic activity. J. Mater. Chem. A. Mater. Energy. Sustain. 2014;2:18115–18124.
https://doi.org/10.1039/C4TA03706E
197. Huang J, Zhu N, Yang T, Zhang T, Wu P. Nickel oxide and carbon nanotube composite (NiO/CNT) as a novel cathode non-precious metal catalyst in microbial fuel cells. Biosens. Bioelectron. 2015;72:332–9.
https://doi.org/10.1016/j.bios.2015.05.035
198. Rabaey K, Rozendal RA. Microbial electrosynthesis - revisiting the electrical route for microbial production. Nat. Rev. Microbiol. 2010;8:706–16.
https://doi.org/10.1038/nrmicro2422
199. Bond DR, Lovley DR. Electricity Production by Geobacter sulfurreducens Attached to Electrodes. Appl. Environ. Microbiol. 2003;69:1548–55.
https://doi.org/10.1128/AEM.69.3.1548-1555.2003
200. Marsili E, Rollefson JB, Baron DB, Hozalski RM, Bond DR. Microbial biofilm voltammetry: Direct electrochemical characterization of catalytic electrode-attached biofilms. Appl. Environ. Microbiol. 2008;74:7329–37.
https://doi.org/10.1128/AEM.00177-08
201. Osman MH, Shah AA, Walsh FC. Biosensors and Bioelectronics Recent progress and continuing challenges in bio-fuel cells. Part II: Microbial. Biosens. Bioelectron. 2010;26:953–63.
https://doi.org/10.1016/j.bios.2010.08.057
202. Marsili E, Baron DB, Shikhare ID, Coursolle D, Gralnick JA, Bond DR. Shewanella secretes flavins that mediate extracellular electron transfer. Proc. Natl. Acad. Sci. 2008;105:3968–73.
https://doi.org/10.1073/pnas.0710525105
203. Sun Y, Wei J, Liang P, Huang X. Microbial community analysis in biocathode microbial fuel cells packed with different materials. AMB. Express. 2012;2:1–8.
https://doi.org/10.1186/2191-0855-2-21
204. Van Eerten-Jansen MCAA, Veldhoen AB, Plugge CM, Stams AJM, Buisman CJN, Ter Heijne A. Microbial community analysis of a methane-producing biocathode in a bioelectrochemical system. Archaea. 2013;2013;
https://doi.org/10.1155/2013/481784
205. Rabodonirina S, Rasolomampianina R, Krier F, Drider D, Merhaby D, Net S. Degradation of fluorene and phenanthrene in PAHs-contaminated soil using Pseudomonas and Bacillus strains isolated from oil spill sites. J. Environ. Manage. 2019;232:1–7.
https://doi.org/10.1016/j.jenvman.2018.11.005
206. Ferrero MA. Improved PAHs removal performance by a de fi ned bacterial consortium of indigenous Pseudomonas and actinobacteria from Patagonia, Argentina. Int. Biodeterior. Biodegrad. 2015;101:23–31.
https://doi.org/10.1016/j.ibiod.2015.03.014
207. Sharma M, Nandy A, Taylor N, Venkatesan SV, Kollath VO, Karan K, et al. Bioelectrochemical remediation of phenanthrene in a microbial fuel cell using an anaerobic consortium enriched from a hydrocarbon-contaminated site. J. Hazard Mater. 2019;12:1845;
https://doi.org/10.1016/j.jhazmat.2019.121845
208. Toth CRA, Gieg LM. Time Course-Dependent Methanogenic Crude Oil Biodegradation: Dynamics of Fumarate Addition Metabolites, Biodegradative Genes, and Microbial Community Composition. Front. Microbiol. 2018;8:1–16.
https://doi.org/10.3389/fmicb.2017.02610
209. Bisht S, Pandey P, Bhargava B, Sharma S, Kumar V, Sharma KD. Bioremediation of polyaromatic hydrocarbons (PAHs) using rhizosphere technology. Brazilian J. Microbiol. 2015;46:7–21.
https://doi.org/10.1590/S1517-838246120131354
210. Thomas F, Cébron A. Short-Term Rhizosphere Effect on Available Carbon Sources, Phenanthrene Degradation, and Active Microbiome in an Aged-Contaminated Industrial Soil. Front. Microbiol. 2016;7:1–15.
https://doi.org/10.3389/fmicb.2016.00092
211. Li X, Li Y, Zhang X, Zhao X, Sun Y, Weng L, et al. Science of the Total Environment Long-term effect of biochar amendment on the biodegradation of petroleum hydrocarbons in soil microbial fuel cells. Sci. Total Environ. 2019;651:796–806.
https://doi.org/10.1016/j.scitotenv.2018.09.098
212. Berdugo-Clavijo C, Dong X, Soh J, Sensen CW, Gieg LM. Methanogenic biodegradation of two-ringed polycyclic aromatic hydrocarbons. FEMS Microbiol. Ecol. 2012;81:124–133.
https://doi.org/10.1111/j.1574-6941.2012.01328.x
213. Ye Q, Liang C, Chen X, Fang T, Wang Y, Wang H. ScienceDirect Molecular characterization of methanogenic microbial communities for degrading various types of polycyclic aromatic hydrocarbon. J. Environ. Sci. 2019;86:
https://doi.org/10.1016/j.jes.2019.04.027
214. Matturro B, Zeppilli M, Lai A, Majone M, Rossetti S. Metagenomic Analysis Reveals Microbial Interactions at the Biocathode of a Bioelectrochemical System Capable of Simultaneous Trichloroethylene and Cr(VI) Reduction. Front. Microbiol. 2021:12.
https://doi.org/10.3389/fmicb.2021.747670
215. Guo X, Zhan Y, Chen C, Zhao L, Guo S. The in fl uence of microbial synergistic and antagonistic effects on the performance of re fi nery wastewater microbial fuel cells. J. Power Sources. 2014;251:229–36.
https://doi.org/10.1016/j.jpowsour.2013.11.066
216. Seelam JS, Rundel CT, Boghani HC, Mohanakrishna G. Scaling Up of MFCs: Challenges and Case Studies. Microb. Fuel Cell, Cham: Springer International Publishing. 2018;459–81.
https://doi.org/10.1007/978-3-319-66793-5_24
217. Nath D, Chakraborty I, Ghangrekar MM. Integrating microbial electrochemical technologies for methane-to-bioelectricity and water-splitting to impart self-sustainability to wastewater treatment plants. Bioresour. Technol. Reports. 2021;13:100644.
https://doi.org/10.1016/j.biteb.2021.100644
218. Tejedor-sanz S, DeGregoris TB, Salas JJ, Pastor L, Esteve-núñez A. Integrating a Microbial Electrochemical System in a classical wastewater treatment configuration for removing nitrogen from low COD effluents Environmental Science into a classical wastewater treatment. Environ. Sci. Water Res. Technol. 2016;2:884–893.
https://doi.org/10.1039/C6EW00100A
219. Park S, Chae K, Lee M. A sulfonated poly (arylene ether sulfone)/polyimide nano fi ber composite proton exchange membrane for microbial electrolysis cell application under the coexistence of diverse competitive cations and protons. J. Memb. Sci. 2017;540:165–173.
https://doi.org/10.1016/j.memsci.2017.06.048
220. Noorman HJ, Heijnen JJ. Biochemical engineering’s grand adventure. Chem. Eng. Sci. 2017;170:677–693.
https://doi.org/10.1016/j.ces.2016.12.065
221. Kishore S, Velvizhi G, Sulonen MLK, Haavisto JM, Oguz E, Yusuf A, et al. Microbial electrochemical technologies with the perspective of harnessing bioenergy: Maneuvering towards upscaling. Renew. Sustain. Energy Rev. 2016;53:462–76.
https://doi.org/10.1016/j.rser.2015.08.058
222. Leicester D, Amezaga J, Heidrich E. Is bioelectrochemical energy production from wastewater a reality ? Identifying and standardising the progress made in scaling up microbial electrolysis cells. Renew. Sustain. Energy Rev. 2020;133:110279.
https://doi.org/10.1016/j.rser.2020.110279
223. Lu L, Yazdi H, Jin S, Zuo Y, Fallgren PH, Ren ZJ. Enhanced bioremediation of hydrocarbon-contaminated soil using pilot-scale bioelectrochemical systems. J. Hazard. Mater. 2014;274:8–15.
https://doi.org/10.1016/j.jhazmat.2014.03.060
224. Li X, Wang X, Zhao Q, Wan L, Li Y, Zhou Q. Biosensors and Bioelectronics Carbon fi ber enhanced bioelectricity generation in soil microbial fuel cells. Biosens. Bioelectron. 2016;85:135–141.
https://doi.org/10.1016/j.bios.2016.05.001
225. Liang Y, Zhai H, Liu B, Ji M, Li J. Carbon nanomaterial-modified graphite felt as an anode enhanced the power production and polycyclic aromatic hydrocarbon removal in sediment microbial fuel cells. Sci. Total Environ. 2020;713:136483.
https://doi.org/10.1016/j.scitotenv.2019.136483
226. Zhao L, Deng J, Hou H, Li J, Yang Y. Investigation of PAH & Oil degradation along with Electricity generation in soil using an enhanced plant-microbial fuel cell. J. Clean. Prod. 2019;221:678–683.
https://doi.org/10.1016/j.jclepro.2019.02.212
227. Mohanakrishna G, Al-raoush RI, Abu-reesh IM, Aljaml K. Science of the Total Environment Removal of petroleum hydrocarbons and sulfates from produced water using different bioelectrochemical reactor con fi gurations. Sci. Total Environ. 2019;665:820–827.
https://doi.org/10.1016/j.scitotenv.2019.02.181
Fig. 4Schematic diagram of removal of tetrachloroethene (PCE) from contaminated groundwater using BERT. 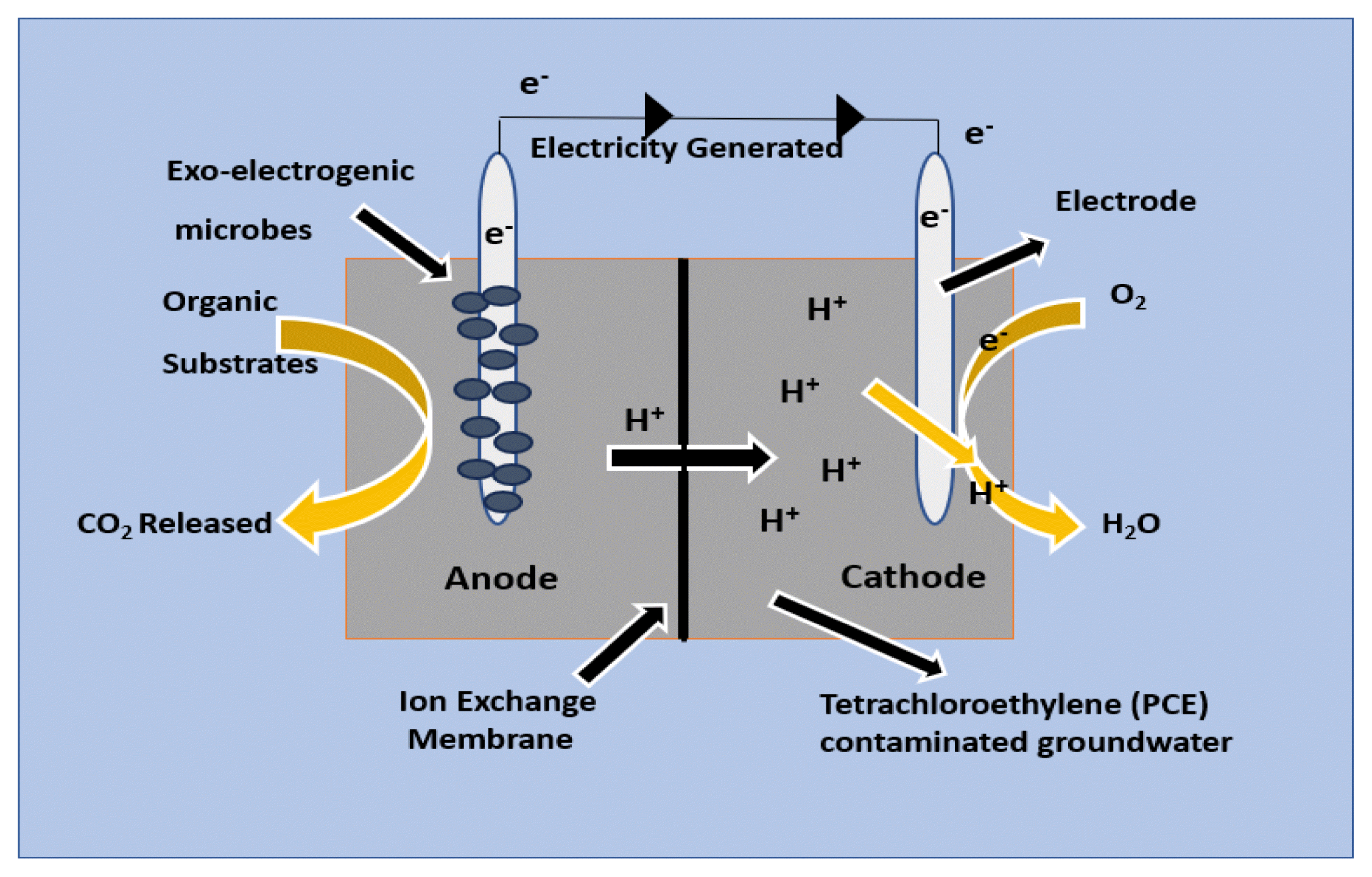
Table 1Shows data from studies on BERT using MFCs and MECs that has been used to treat various industrial effluents and the maximum power/current generated from them.
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||