AbstractRecently, innovative developments have been made in the rapidly developing field of nanotechnology. However, toxicity due to long-term exposure to various types of metal-based nanoparticles, especially metal and metal oxide nanoparticles, is gradually increasing. The kidney is prone to toxin-induced impairment because it is one of the main organs for eliminating multiple potentially dangerous substances, including nanoparticles (NPs), from mammals. However, the mechanisms underlying cytotoxicity triggered by various NPs have not yet been reported. This review summarizes the various mechanisms involved in metal-based nanoparticle-induced nephrotoxicity, focusing on Reactive Oxygen Species (ROS) production, which leads to oxidative stress and apoptosis. The mechanisms by which metal-based NPs cause serious side effects in the renal system and the specific toxicity of different types of metal NPs are also summarized.
Graphical Abstract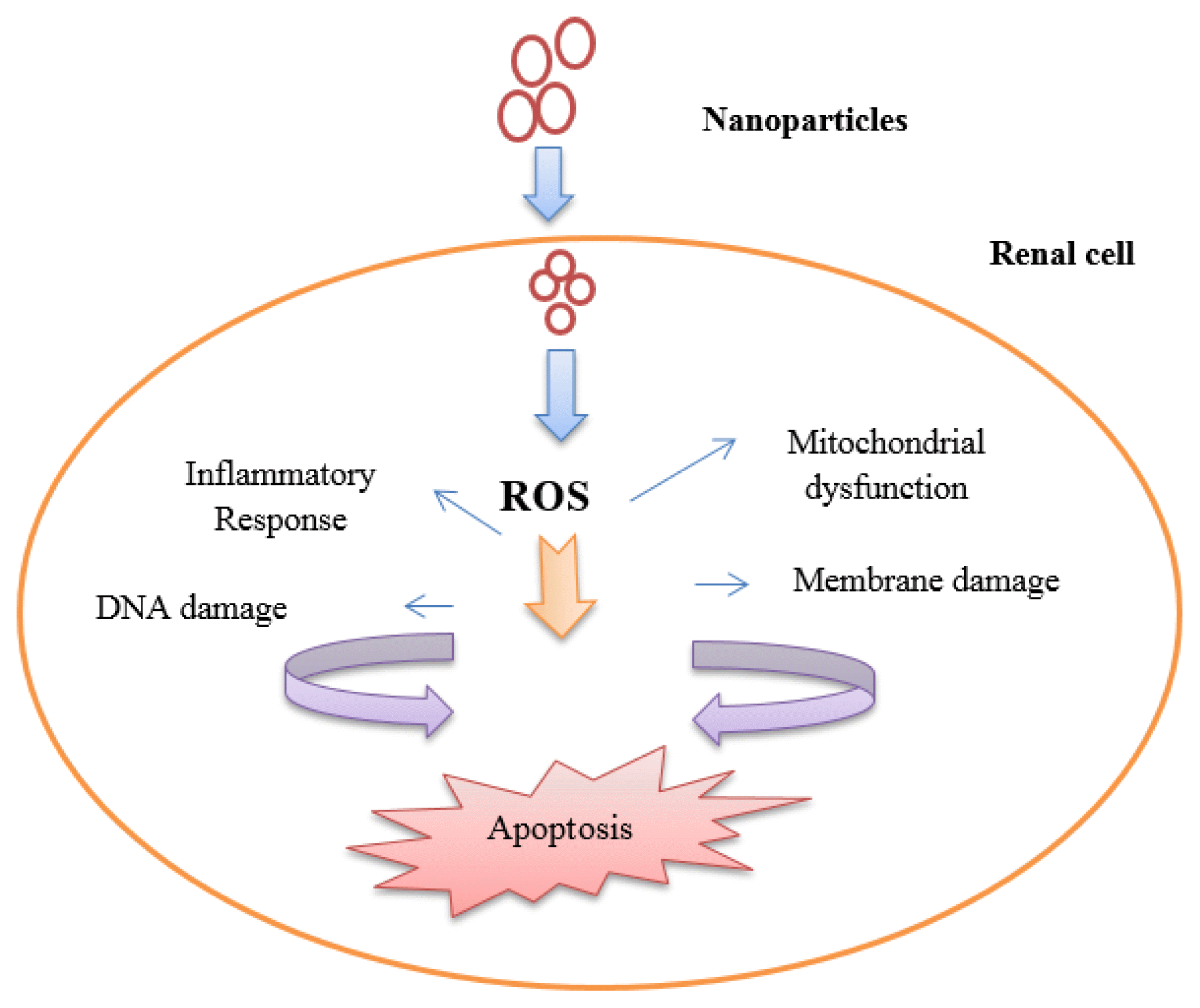
1. IntroductionNanotechnology is an area of research comprising the production and study of nanoparticles (NPs) of 1–100 nm in size [1] which was first presented by Richard Feynman in 1959. Since then, major innovations have been made in this field [2]. They can be zero-dimensional (0D), one-dimensional (1D), or two-dimensional (2D) depending on their shape [3]. Owing to their small size and large surface-to-volume ratio, nanoparticles exhibit characteristic properties and have a wide range of applications. NPs are used in the computer, electronics, biotechnology, and aerospace industries. Nanoparticles have gained considerable attention in the last decade because of their applications in medicine for the diagnosis, prevention, and treatment of various human diseases, especially as drug delivery tools [4].
NPs can be classified into different categories depending on their physicochemical properties. Some of the most well-known classes of NPs include carbon-based nanoparticles, including fullerenes and carbon nanotubes (CNTs). Fullerenes are rounded, hollow cages of carbon atoms. Their high strength, electrical conductivity, electron affinity, and flexibility make them suitable for commercial products. The carbon units in fullerenes are always sp2 hybridized and arranged in pentagonal and hexagonal structures [5]. CNTs are highly elongated tubular structures–2 nm in diameter. They are similar to graphite sheets that are rolled to form a tube. Nanotubes are single-walled, double-walled, and multiwalled because they can roll once, twice, and multiple times, respectively. Carbon precursors, mostly atomic carbon, are deposited to synthesize CNTs in bulk, and have also been fabricated using chemical vapor deposition (CVD) techniques [6].
Metallic nanoparticles (MNPs) are comprised of pure metal precursors. MNPs have numerous applications in many research areas owing to their advanced optical properties [7]. Highly developed and practical techniques for the synthesis of MNPs have enabled their bulk production. They have gained much attention as important ingredients in many consumer product, including creams, shampoos, footwear, plastic containers, and clothing [8].
Ceramic NPs are inorganic nonmetallic solids found in polycrystalline, dense, porous, and hollow forms. The NPs were synthesized by heating and cooling. Ceramic NPs have been used for dye photodegradation, imaging, and catalysis [9].
These NPs have been used in drug delivery, biosensors, stimuli-responsive cargo delivery, nanocomposites, and agricultural and environmental applications [10]. 80 % of NPs that enter the body are retained by the kidneys and detoxified in liver and kidney tissues. Kidneys are the major target organs of foreign particles (nanoparticles) therefore, the impacts of NPs on kidney tissues requires serious attention. This review aims to elucidate different mechanisms involved in induction of nephrotoxicity by MNPs. The results of different studies summarized in this review indicate that different mechanisms are responsible for metallic nanoparticle-induced nephrotoxicity. The mechanisms of toxicity of different metallic nanoparticles are discussed in this review. The current review provides information regarding the toxicological effects of NPs and increases our understanding of NP development and applications.
2. Synthesis of NanoparticlesThere are two approaches to the synthesis of nanoparticles. Both are now discussed.
2.1 Top-down ApproachThis approach begins with the reduction of large molecules to nanoparticles after sequential treatments have been conducted on them. The main shortcoming of this approach is the requirement for bulk installation and a large setup. Although large-scale production of nanoparticles is not possible using this approach, it is appropriate for laboratory experiments.
The top-down approach involves multiple processes, which are now discussed.
2.1.1 Mechanical milingMechanical methods are the most commonly used top-down methods for the mass production of nanoparticles because they are cost-effective. This technique involves the use of a ball mill, which is a stainless-steel container designed to contain a large number of small iron, silicon carbide, and tungsten carbide balls, which rotate within it. The powdered substance is placed into this container and reduced to a nanoscale powder by the action of the grinding balls [11]. However, the major shortcoming of this method is that the nanomaterial may be inadvertently contaminated by the grinding environment and milling media. The ball-milling method also causes noise pollution, which is another drawback of this method [12].
2.1.2 NanolithographyNanolithography is a useful and powerful technique for large-scale production of nanoparticles. Nanolithographic techniques include optical, electron beam, nanoimprint, and multiphoton lithography. This technique produces the desired shape or structure material by placing the desired material on a light-sensitive material and then selectively cutting the material. The major drawback of using this technique is the requirement for complex equipment, which increases the cost [13].
2.1.3 Laser ablationDifferent types of nanoparticles can be synthesized by laser ablation. It is a cost-effective and safe method that does not require high temperatures or pressures. In this method, nanoparticles are synthesized by laser irradiation of a metal dipped in a liquid solution by condensing a plasma plume [14].
2.2 Bottom-up SynthesisIn bottom-up methods, nanoparticles are synthesized using chemical or physical forces from atoms that form clusters to form nanostructures. The bottom-up approach resembles the biological systems that use chemical forces to build molecules.
Some of the most common methods in the bottom-up approach are now briefly described.
2.2.1 Sol-gel MethodThis method involves the conversion of a precursor into a sol after a chain reaction involving hydrolysis and polycondensation. The resulting molecules in the sol form are then converted into a solid or gel form during the sol-gel process at room temperature and pressure. The gel is processed at different temperatures for drying purposes and converted into a nanopowder [15]. Advantages of using sol-gel method include formation of thick coating to provide protection against corrosion, simple, economical as well as an efficient method to produce high quality yield. There are some drawbacks of using this method as well. Some of the major drawbacks are contractions that occur during process, long processing time and use of organic solution that might be toxic [16].
2.2.2 SpinningThe synthesis of nanoparticles by spinning method involves the use of a spinning disc reactor. A disk reactor consists of a chamber containing a rotating disk with adjustable physical properties. The process involves the formation of a film of liquid reagent by dropping it onto the center of a rapidly rotating disc (300 to 3000 rpm). The drag forces between the moving liquid film and disc surface make the reagents in the liquid streams that fall to the disc surface highly micromax, which is a requirement for nanoparticle synthesis using this method [17]. Major drawbacks of using this method are use of toxic solvents and low precision in fiber deposition [16].
3. KidneysThe kidneys are two bean-shaped organs that regulate blood volume, excrete waste materials, maintain a general fluid balance, and filter minerals from the blood. The kidneys are located on either side of the spine, in the retroperitoneal area. As the liver is located above the right kidney on the right side of the abdominal cavity, the left kidney is slightly higher than the right kidney. In males and females, the two bean-shaped organs weigh 125, 175 g and 115, 155 g, respectively. The usual dimensions of the kidney are 6 cm in width, 11–14 cm in length, and 4 cm thick. The ribs, muscles, and fat in the back protect the kidneys. The renal fat pad, also known as the perirenal fat, shields the kidneys from damage or external forces. The renal hilum, a medial indentation on the kidneys, enters and leaves the kidneys to supply or drain blood [18].
The 1.3 million nephrons that comprise each kidney are the functional units of kidneys. The outer renal tissue or cortex is found beneath the renal capsule. Compared with other kidney tissues, it has a paler appearance. Here are the proximal and distal convoluted tubules as well as the renal corpuscles. It penetrates the inner renal tissue or medulla and divides it into triangular renal pyramids. The loops of Henle and collecting ducts of each renal tubule are located within renal pyramids [19].
4. Metallic NanaoparticlesNanotechnology has emerged as a field of science and technology that is rapidly expanding worldwide. This has resulted in increased manufacturing and use of nanomaterials in a variety of fields, including automotive, medicinal, cosmetic, and defense industries. In 2016, the market for nanotechnology products and applications was estimated to be worth $39.2 billion, and by 2021, it is anticipated to be worth $90.5 billion [20].
Metal cores formed from metals or metal oxides form metallic nanoparticles (MNPs), which are typically encased in organic or inorganic substances or metal oxide shells. Metal nanoparticles are used in a variety of ways in daily life. The development of new economically viable manufacturing methods for MNPs production has increased the demand for MNPs in a variety of consumer products, including lotions, shampoos, apparel, footwear, and plastic containers [8].
Owing to their various physicochemical characteristics, including mechanical strength, low melting point, large surface area, chemical reactivity, and optical activity, MNPs have been employed in several fields of nanotechnology. MNPs are used in several consumer products, including clothing, cosmetics, food, and sunscreens. Owing to their widespread commercial use, transition metal oxides, including zinc oxide, copper oxide, and titanium dioxide, have drawn much attention [21]. In vivo studies have employed MNPs, particularly AuNPs, as antiangiogenic drugs in tumor therapy. Most semiconductor- and MNPs are employed in radiotherapy, photothermal therapy (PPT), rheumatoid arthritis, leukemia, multiple myeloma, and other medical applications [22].
As NPs are used more frequently, their emanation sources emit more of them, which causes them to be released into large sinks like soil and water. The following three outflow situations are typically taken into consideration for NPs: (i) discharge during the production of raw materials and nano-enhanced products; (ii) discharge during use; and (iii) discharge after the removal of NP-containing products (waste dealing). NPs may be released into the atmosphere directly or indirectly through specialised infrastructure like landfills or wastewater treatment facilities (22WWTPs). Aberrant discharges are likely to occur from landfill leachates, biosolids used as fertiliser, or the gushing of WWTPs [23].
Although MNP manufacturing and use have advanced significantly, there is a lack of knowledge regarding their toxicity to human health and the environment. Thorough knowledge of the causes of MNPs-induced toxicity is necessary before large-scale manufacturing, despite preliminary findings on their ability to influence biological systems at the cellular and sub-cellular levels being available.
4.1 Effects of MNPs on Human HealthConcerns about the toxicity and safety of MNPs have arisen because of their widespread use. The physicochemical characteristics of NPs, including their small size, large surface area, and adaptable chemical composition and structure, dictate their toxicological impact. MNPs enter the body through the respiratory system and ingestion, and are subsequently distributed to organs, including the brain, liver, and kidneys. They can infiltrate cells and damage the immune system, thereby negatively affecting biological functions [24]. Exposure to MNPs is associated with several adverse effects. For example, patients who used colloidal silver three times per year for two years reported developing diabetes, hyperlipidemia, and hypertension [25]. Mouse brains have been exposed to nanosilver, which causes apoptosis and gene modification [26].
Few studies have investigated the toxicological effects of MNPs on the kidney, compared to pulmonary toxicity, which has been extensively researched. Renal toxicity of MNPs is one of the primary adverse consequences recently investigated. However, the under lying molecular and cellular mechanisms remain poorly understood, despite several studies on the malfunctioning of subcellular organelles, including mitochondria, the nucleus, and cell membranes, induced by metal-based nanoparticles [27]. Because of their high blood volume through flow, capacity to store NPs, the kidneys are particularly vulnerable to these hazardous metals [28].
4.2 Exposure Routes of Metallic Nanoparticles to KidneysMNPs can enter the body through inhalation, ingestion, dermal contact, injection, implantation, and direct penetration into cells [29]. Inhalation and ingestion are the two most common methods of exposure. However, regardless of the exposure method, MNPs can enter the bloodstream through various channels and propagate through the circulatory system to multiple organs [30]. The kidneys, heart, liver, spleen, lungs, brain, and gastrointestinal tract are the main target organs [31]. Hepatobiliary metabolism, urine excretion, and fecal excretion are the three main channels through which MNPs are cleared from mammals [32]. Organs of the reticuloendothelial system (RES), including the liver and spleen, are primarily responsible for eliminating MNPs larger than 6 nm. Glomeruli can effectively filter MNPs smaller than 6 nm, and the urinary system can remove them from the body. The urinary system is the preferred method for excreting MNPs because it is faster and more effective than other channels [33].
The kidney, a vital organ for urine production, is crucial for the removal of bodily metabolites, waste, and toxins, as well as for the retention of water and other beneficial chemicals, including glucose, ions, and nutrients, through reabsorption. The kidneys serve as the final line of defense against MNPs that are eliminated via the bladder [34]. Glomeruli can filter MNPs in the blood through the glomerular filtration barrier. Podocytes, glomerular basement membranes, and glomerular endothelial cells constitute the glomerular filtration barrier [35]. The endothelial layer is the first physical filtration barrier and has fenestrations of 70–90 nm. The functional glycocalyx covers the glomerular endothelial cells. The glomerular basement membrane (GBM) is a non-amorphous meshwork that is extremely well ordered. The glycocalyx covers the podocyte layer, which has pores with diameters of 4–11 nm. MNPs with a particle size of less than 6 nm can be effectively filtered by the glomerulus in healthy states and eliminated in urine [36].
Destruction of the glomerular filtration barrier results in podocyte death in clinical settings. Larger MNPs accumulate in the Bowman space because of leakage and aberrant fenestrae [35]. The strong reabsorption capabilities of renal tubular epithelial cells make them the primary sites where glomerular-filtered MNPs accumulate. Excessive MNP accumulation in glomerular and tubular cells can cause kidney damage and impair normal kidney function, including oxidative stress, inflammation, DNA damage, autophagy, and endoplasmic reticulum stress [37].
5. Mechanisms of Renal Toxicity Induced by MNPsMNPs exhibit numerous mechanisms of toxicity, the most prevalent of which are discussed below. MNPs can interact with most cellular components, including DNA, proteins, and mitochondria. In doing so, they generate reactive oxygen species (ROS) and affect how well the cells perform their various activities. Thus, MNPs interactions can be described to changes in proteins, accumulation of NPs in the Golgi, DNA damage, lysosomal hydrolases, ROS production, mitochondrial dysfunction, apoptosis, cell membrane damage, cytoplasmic impairment, and ATP levels [38].
5.1 Oxidative Stress Induced by MNPsOxidative stress develops in cells and tissues as a result of an imbalance between the production and accumulation of reactive oxygen species (ROS). ROS, also referred to as ‘free radicals’, are metabolic by-products of biological systems produced by mitochondria under healthy and pathological conditions [39]. They are beneficial at low to moderate concentrations. For example, ROS combat pathogens, are essential for cell signaling, and help create different proteins and cellular structures [40]. However, at excessive concentrations, oxidative stress (OS) occurs, as ROS reduce the capacity of living cells and organs to detoxify. Under these high concentrations, proteins, lipids, and nucleic acids are harmed and cellular death and disease is promoted [41].
Two types of oxidative stress biomarkers are (a) formation of ROS-modified molecules; and (b) degradation or loss of enzymes or antioxidants. Traces of these biomarkers can be found in body fluids. It is difficult to accurately measure levels of ROS, however fluorogenic and fluorescent probes have been used to measure cellular levels of ROS [42]. Regular metabolism of oxygen produces ROS as a natural byproduct and this affects cell signalling and homeostasis [43]. Excessive production of ROS due to external inputs including NPs, can have other negative effects, including apoptosis (programmed cell death) and damage to RNA and DNA. Lipid peroxidation, amino acid oxidation in proteins, and enzyme deactivation by the oxidation of cofactors are other undesirable effects of excessive production of ROS caused by NPs [44]. Metallic NPs produce ROS through a mechanism dependent on the chemistry, shape, size, and surface area of the particles. ROS play significant roles in various biological and cellular processes. In addition to causing fluctuations in cellular signaling and thereby affecting cell death, proliferation, and differentiation, ROS formation is a key factor in toxicity caused by the application of NPs [45].
In vitro and in vivo cellular and animal models have been used to examine the underlying mechanisms of kidney toxicity caused by MNPs. In a study of silver-based NPs (AgNP), male Swiss albino mice received 14 days of treatment with 20 nm AgNPs at low exposure dosages (0.1 and 0.2 mg/kg). AgNPs significantly increased the induction of metallothionein and ROS generation in kidney tissue of the mice, at both exposure doses. Under the same experimental conditions, exposure to gold nanoparticles (AuNPs) resulted in lower levels of ROS production and metallothionein induction than for AgNPs. These results demonstrate the significant role of the chemical makeup of NPs and their capacity to release metal ions in oxidative stress [46].
5.2 Damage to Cytoskeleton ComponentsThe cytoskeleton serves as a framework for cell architecture; hence, it is important to carefully analyze how NPs affect the cytoskeletal network. Several types of proteins, including microtubules, actin, intermediate filaments, and other crucial elements of the cytoskeleton, have been identified. Although TiO2 was shown to be non-toxic in two studies [47], another study showed that TiO2 NPs caused the disintegration of actin and tubulin, as well as other changes to cytoskeletal proteins [48]. In this latter study, α and β tubulin and actin were downregulated, however proteomic analysis of co-cultured epithelial cells showed that Ag NPs readjust different types of cytokeratin and gelsolin. The strong dissolution of Ag further supports the potent effects of NPs, rather than Ag ions [49]. ZnO NPs have been shown to cause cytotoxicity in cells by rearranging actin and tubulin networks, which subsequently form wrapped bundles in the periphery of the nucleus. These bundles induce improper chromosome and spindle shapes in the cytoplasm, leading to detrimental effects [50].
5.3 Genotoxicity Induction by MNPsCellular exposure to MNPs can result in DNA damage [51]. Single-and double-strand breaks (SSB and DSBS, respectively) are the primary types of DNA damage [52]. In response to DNA damage, several cellular signaling cascade promote DNA repair, cell cycle arrest, senescence, and cell death. The intricate processes of DNA damage and repair work in concert to determine how affected cells develop [53].
Various MNPs cause damage to DNA in kidneys. ROS is thought to be involved in the genotoxic pathways of MNPs through facilitation of oxidative damage to DNA [54]. AgNPs may generate ROS by mediating mitochondrial malfunction, resulting in DNA damage [55]. AgNPs cause ROS-mediated DNA damage in HK2 cells, leading to arrest of the G2/M cell cycle in human renal epithelial cells. Ataxia telangiectasia-mutated (ATM) and Ataxia- and Rad-related (ATR) kinases are the first to be activated in response to DNA damage caused by AgNPs. In addition, suppression of cell division by cyclin 25 homolog C (CDC25C) and activation of p53 signaling, can activate G2/M checkpoints [56]. CuO and TiO2 NPs may also generate ROS, damage DNA, and ultimately trigger apoptosis in cells [57].
In a study of the mitotic and meiotic effects of Cu and CdS NPs, Cu NPs were found to be more toxic to cells than CdS NPs. Mitotic aberrations can be caused by different phenomena, including: 1) depolymerization of DNA and strict adherence of chromosome bundles; 2) formation of rings, bridges, and micronuclei due to the breaking up of chromosomes; 3) formation of diplochromosome due to the lack of centromeric division; and 4) formation of polyploid cells and laggard due to variability in the spindle apparatus. NPs that induce mitotic aberrations appear to be important because their persistent modifications can result in heritable changes in the genotype [58].
6. Toxic Effects of Metallic NanoparticlesIn animals, the primary role of the kidneys is the elimination of metabolic waste products and control of extracellular fluid volume and electrolyte balance. Kidneys are exposed to high concentrations of xenobiotics in systemic circulation because of their high blood flow and capacity to concentrate solutes and are particularly vulnerable to injury from toxins because of their large blood supply compared to their size. Renal tissue becomes physiologically concentrated in the presence of xenobiotics, thereby impairing its ability to function [59].
6.1 Titanium dioxide NanoparticlesTiO2 NPs are white crystalline powders synthesized worldwide and have wide application in cosmetics, food additives, textiles, paints, and photosensitive materials [60]. Exposure to large amounts of TiO2 NPs due to their overuse, could be hazardous to humans [61].
The impact of injection with Titanium dioxide nanoparticles was examined in Wistar rats. They found that the brains and lymph nodes of the TiO2 NPs exposed rats were unaffected. However, signs of nanoparticle accumulation have been observed in the liver, kidneys, spleen, and lungs [62]. The brains and lymph nodes of the rats exposed to TiO2 NPs were unaffected, however there were signs of nanoparticle accumulation in the liver, kidneys, spleen, and lungs. Comparable findings were observed in another study, where the toxicity caused by TiO2 NPs was linked to the quantity and duration of the given dose. TiO2 NPs were injected at a dose of 20 mg/kg for 20 d to induce vacuolization, congestion, and vasodilation in the liver, eventually resulting in liver dysfunction. High doses of these NPs (1387 mg/kg body weight) caused mortality in the rats within 2 days after injection, whereas low doses (10 mg/kg body weight) induced minor symptoms of toxicity such as an elevated white blood cell count and decreased food consumption.
6.2 Silver NanoparticlesSilver is considered a precious metal in its nanoparticle form, and these nanoparticles are widely used for the treatment of bacterial infections, especially in situations where there is antibiotic resistance [63]. To inhibit microbial growth, Ag NPs must be used at doses below those harmful to human cells. In vitro studies in various cell lines including skin carcinoma, hepatoma, fibroblasts, and fibrosarcoma, have shown that ROS generation and subsequent cytotoxicity depend on the size of AgNPs [64]. Other factors influencing AgNP cytotoxicity and genotoxicity include coating, concentration, exposure length, environmental conditions, particle aggregation, and surface oxidation to generate silver oxides [65].
Silver has a strong affinity for sulfur, hence when Ag NPs interact with cell membrane proteins, signaling pathways for ROS formation can be activated, which in turn can destroy proteins and nucleic acids, ultimately leading to apoptosis and decreased cell proliferation [66]. Silver NPs have been shown to cause various morphological destructions, deformities, and negative effects on reproduction in diverse animal models [67]. For example, rats administered water containing AgNPs for 1–2 weeks showed distribution of AgNPs in the cerebellum, spleen, duodenum, and in cardiac muscles [68]. Additionally, a dose-dependent build-up of Ag NPs has been reported in the liver of rats [69].
Long-term exposure to low concentrations of Ag NPs in the form of salt causes changes in blood cells and fat degradation in the liver and kidneys [70]. A 40 mg/kg dose of intravenous Ag NPs significantly increase liver enzymes and blood serum ROS levels in rats, and TEM images showed particle deposition in the liver and kidney [71].
6.3 Gold NanoparticlesGold NPs are useful in assessing cellular absorption and tissue dispersion of particles and have wide application, as depicted in Fig. 6, including infor pathogen detection, drug delivery, and tumor detection.
Despite their advantages, long-term use of AuNPs lead to a substantial build-up in tissues, inducing cytotoxicity. The likelihood of toxicity in humans is high, given the numerous applications of, and therefore potential exposure to, Au NPs [72]. The cellular toxicity of Au NPs is influenced by their size and shape; some AuNPs have been shown to be hazardous and others non-toxic to humans, depending on their size. For example in a study of AuNPs ranging in size from 0.8 to 15 nm, AuNPs with a diameter of 1.4 nm were shown to be most toxic, causing rapid cell death by necrosis [73], and AuNPs with a diameter of 15 nm appear to be non-toxic [74].
Exposure to AuNPs with diameters of 10, 30 and 60 nm were examined in HT-29 and HepG2 cells and Wistar rats [75]. Traces of AuNPs were detected in the liver, intestine, urine, feces, kidneys, and spleen. Transmission electron microscopy study showed that biodistribution and excretion routes are influenced by particle size with the smallest AuNPs causing most damage to the DNA, likely due to their faster circulation and distinct biodistribution [75].
The liver and spleen are specifically involved in detoxification and hence are the first organs targeted by MNPs. For example, studies have shown significant variation in liver enzymes in rats injected with an intraperitoneal dose of AuNPs [153]. Trisodium citrate dihydrate-capped AuNPs cause mild nephrotoxicity and hepatotoxicity [76]. After intravenous administration of AuNPs, mice displayed liver tissue apoptosis and inflammation [77]. Histological examination showed that the testes were not affected, although the kidney and liver portions showed slight modifications [78]. Therefore, it is crucial to assess the toxicological effects of AuNPs and develop early biomarkers of negative impact on health.
6.4 Zinc Oxide NanoparticlesZinc oxide nanoparticles (ZnONPs) have a molecular weight of 81.38 g/mol, and are a white, odourless powder. ZnO NPs have unique electrical, optical, catalytic, and photochemical properties that make them useful in industrial and biomedical applications. ZnO NPs with ultraviolet blocking properties have been used in cosmetics. Due to the antibacterial and antifungal properties of ZnO NPs, they are also used in food packaging, ointments, and in daily care [79]. These multiple, everyday uses are leading to increased human exposure to ZnO NPs. According to the Food and Drug Administration, manufacturers do not need to perform safety assessments of nanoscale substances if the bulk material is found to be safe. The findings of the current study suggest that the physical, chemical, and biological characteristics of NPs are distinct from those of their bulk material. A relatively low concentration of 10 g/ml of ZnO NPs is sufficient to cause breaks in single-stranded DNA, due to their strong reactivity [80].
Direct diffusion and endocytosis allow ZnO NPs to enter cells. When ZnO NPs interact with biological systems, ROS are produced which induce damage through oxidative stress. hHigh surface reactivity results from a larger surface area, exposing more reactive sites on the surface of the ZnO NPs. Protein degeneration, DNA fragmentation, oxidative stress, inflammatory responses, and organelle failure are examples of damage caused by ZnONPs. Antioxidant defenses built within cells allow them to regulate the ROS produced by regular metabolism. In the presence of ZnO NPs, ROS production may exceed the capability of the cellular antioxidant machinery. By producing ROS or blocking antioxidant molecules, ZnO NPs can behave as prooxidant molecules and cause oxidative stress [81]. In one study, 100 nm ZnO NPs (2.5 g/kg bw) administered intraperitoneally led to accumulation in the liver, spleen, lung, kidney, and heart. When these NPs were administered, the concentration of Zn in the liver, spleen, and lungs was higher than when equivalent amounts of 1 μm ZnO particles were administered [82].
ZnO NPs are widely used in biomedical applications, with the types of application increasing with the advancement of nanotechnology. These multiple uses of ZnO NPs pose a danger to ecology and human health. Use and application of ZnO NPs should be supported by extensive data on biocompatibility and safety as uncontrolled use may have unexpected deleterious effects on biological systems. Improved synthetic parameter control will aid in modifying the compatibility and physiochemical characteristics of ZnONP. Understanding the toxicity of ZnO NPsZnONP in greater detail is needed when examining their potential in the future.
7. ConclusionsNanotechnology is an especially interesting and contemporary branch of research owing to its many uses in the food and cosmetics sectors. As a result of these uses nanoparticles are present in everyday life for most people. Although nanoscale materials offer numerous advantages over coarse material, these very small materials can overcome the protective barriers of living cells and tissues, leading to inflammatory problems and other toxic impacts. The scientific discipline of ‘nanotoxicology’ has been developed to clarify the potential negative consequences of NPs and factors influencing cytotoxicity of these materials.
Since metal and metal oxide materials are some of the most widely used NPs and affect the kidneys, which are the major target organs for NPs, this review paper hass focused on examination of the possible mechanisms of renal toxicity induced by metal NPs and review of in vitro and in vivo studies to uncover the potential hazards of these MNPs.
8. Future ProspectsModern NPs are designed with properties which enhance their beneficial effects and minimize any potential toxicity. Toxicity-transmitting species and targets are detected by taking into account the life cycle of the NPs, and various coatings and surface treatments are incorportated to decrease the negative while maintaining the positive properties of these particles. These are just a few of the emerging trends and prospects in metallic nanoparticle toxicity studies. Many aspects relating to these issues remain unresolved and require further studies to overcome the toxicity limitations of metallic NPs and other barriers.
Methods based on the simultaneous use of NPs and antitoxic agents appear to be most promising. In future studies, more attention should be paid to the possible effects of biological fluids and surrounding tissues, biokinetics, mechanisms involved, and other chemical and biological factors. In particular, here is demand for more sophisticated and validated in vitro models that can predict in vivo experimental outcomes.
Finally, emerging recommendations must explain precisely how NPs interact with biological systems, providing an accurate risk assessment. To reliably inform the complicated cellular and molecular events, many scientific disciplines, such as chemistry, physics, medicine, and biology, need to be involved.
AcknowledgmentThis work was supported by the 2021 sabbatical year research grant of the University of Seoul for Yong Jun Choi.
NotesAuthors Contributions A.W. (PhD student) Conceptualized and wrote the original manuscript. S.S. (Professor) Supervised and revised the edited manuscript. S.N. (Associate Professor) Wrote and reviewed the figure designs. F.M. (Professor) Wrote and reviewed the data. F.R. (Professor) Wrote and revised the manuscript. S.T. (Associate Professor) revised and edited the draft. F.J. (Assistant Professor) Wrote and revised the draft. M. H. (Associate Professor) Wrote and revised the final draft. Y.J.C. (Associate Professor) Wrote and revised the final draft. Y.-K.P. (Professor) Supervised and revised the edited manuscript. References1. Iavicoli I, Fontana L, Nordberg G. The effects of nanoparticles on the renal system. Crit. Rev. Toxicol. 2016;46:490–560.
https://doi.org/10.1080/10408444.2016.1181047
2. Khan I, Saeed K, Khan I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019;12:908–931.
https://doi.org/10.1016/j.arabjc.2017.05.011
3. Tiwari JN, Tiwari RN, Kim KS. Zero-dimensional, one-dimensional, two-dimensional and three-dimensional nano structured materials for advanced electrochemical energy devices. Prog. Mater. Sci. 2012;57:724–803.
https://doi.org/10.1016/j.pmatsci.2011.08.003
4. Waris A, Sharif S, Naz S, et al. Hepatotoxicity induced by metallic nanoparticles at the cellular level: A review. Environ. Eng. Res. 2023;28:220.
https://doi.org/10.4491/eer.2022.625
5. Astefanei A, Núñez O, Galceran M. Characterisation and determination of fullerenes: A critical review. Anal. Chim. Acta. 2015;882:1–2.
https://doi.org/10.1016/j.aca.2015.03.025
6. Elliott A, Shibuta Y, Amara H, Bichara C, Neyts E. Atomistic modelling of CVD synthesis of carbon nanotubes and graphene. Nanoscale. 2013;5:6662–6676.
https://doi.org/10.1039/C3NR01925J
7. Saad AM. Chemical extraction and quantification of extracellular polymeric substances in unspiked-metaldehyde and spiked-metaldehyde of rubber aerobic granular sludge and molasses aerobic granular sludge. Environ. Eng. Res. 2023;28:230–239.
https://doi.org/10.1039/C1CS15237H
8. Diegoli S, Manciulea L, Begum S, et al. Interaction between manufactured gold nanoparticles and naturally occurring organic macro-molecules. Sci. Total Environ. 2008;402:51–61.
https://doi.org/10.1016/j.scitotenv.2008.04.023
9. Sigmund W, Yuh J, Park H, et al. Processing and Structure Relationships in Electrospinning of Ceramic Fiber Systems. J. Am. Ceram. Soc. 2006;89:395–407.
https://doi.org/10.1111/j.1551-2916.2005.00807.x
10. Mansha M, Khan I, Ullah N, Qurashi A. Synthesis, characterization and visible-light-driven photoelectrochemical hydrogen evolution reaction of carbazole-containing conjugated polymers. Int. J. Hydrogen. Energy. 2017;42:10952–10961.
https://doi.org/10.1016/j.ijhydene.2017.02.053
11. Zhang DL. Processing of advanced materials using high-energy mechanical milling. Prog. Mater. Sci. 2004;49:537–560.
https://doi.org/10.1016/S0079-6425(03)00034-3
12. Tavakoli A, Sohrabi M, Kargari A. A review of methods for synthesis of nanostructured metals with emphasis on iron compounds. Chem. Pap. 2007;61:151–170.
https://doi.org/10.2478/s11696-007-0014-7
13. Hulteen C, Treichel DA, Smith MT, et al. Nanosphere Lithography: Size-Tunable Silver Nanoparticle and Surface Cluster Arrays. J. Phys. Chem. B. 1999;103:3854–3863.
https://doi.org/10.1021/jp9904771
14. Makarov GN. Laser applications in nanotechnology: nanofabrication using laser ablation and laser nanolithography. Sov. phys. Usp. 2013;56:643–647.
https://doi.org/3367/UFNe.0183.201307a.0673
15. Nur AM, Sultana M, Mondal A. A review on the development of elemental and codoped TiO2 photocatalysts for enhanced dye degradation under UV–vis irradiation. J. Water Proc. Engineering. 2022;47:102728.
https://doi.org/10.1016/j.jwpe.2022.102728
16. Bokov D, Turki A, Chupradit S, et al. Nanomaterial by Sol-Gel Method: Synthesis and Application. Adv. Mater. Sci. Eng. 2021;202:5102014.
https://doi.org/10.1155/2021/5102014
17. Mohammadi S, Harvey A, Boodhoo KVK. Synthesis of TiO2 nanoparticles in a spinning disc reactor. J. Chem. Eng. 2014;258:171–184.
https://doi.org/10.1016/j.cej.2014.07.042
18. Barrie J, Murray MD. The Kidney: Structure and Function in Health and Disease. Arch. Intern. Med. 2020;88:418418.
https://doi.org/10.1001/archinte.1951.03810090149040
19. Kariyanna SS, Light RP, Agarwal R. A longitudinal study of kidney structure and function in adults. Nephrol. Dial. Transplant. 2009;25:1120–1126.
https://doi.org/10.1093/ndt/gfp654
20. Sengul AB, Asmatulu E. Toxicity of metal and metal oxide nanoparticles: a review. Environ. Chem. Lett. 2020;18:1659–1683.
https://doi.org/10.1007/s10311-020-01033-6
21. Gupta VK, Nayak A, Agarwal S. Bioadsorbents for remediation of heavy metals: Current status and their future prospects. Environ. Eng. Res. 2015;20:1–18.
https://doi.org/10.1155/2013/942916
22. Khanna P, Ong C, Bay BH, Baeg GH. Nanotoxicity: An Interplay of Oxidative Stress, Inflammation and Cell Death. Nanomaterials (Basel). 2015;5:1163–1180.
https://doi.org/10.3390/nano5031163
23. Gottschalk F, Sun T, Nowack B. Environmental concentrations of engineered nanomaterials: review of modeling and analytical studies. Environ. Pollut. 2013;181:287–300.
https://doi.org/10.1016/j.envpol.2013.06.003
24. Kumar H, Venkatesh N, Bhowmik H, Kuila A. Metallic nanoparticle: a review. Biomed. J. Sci. Technol. Res. 2018;4:3765–3775.
https://doi.org/10.26717/BJSTR.2018.04.001011
25. Chang AS, Khosravi V, Egbert B. A case of argyria after colloidal silver ingestion. J. Cutan. Pathol. 2006;33:809–811.
https://doi.org/10.1111/j.1600-0560.2006.00557.x
26. Rahman M, Wang J, Patterson T, et al. Expression of genes related to oxidative stress in the mouse brain after exposure to silver-25 nanoparticles. Toxicol. Lett. 2009;187:15–21.
https://doi.org/10.1016/j.toxlet.2009.01.020
27. Luo YH, Wu SB, Wei YH, et al. Cadmium-based quantum dot induced autophagy formation for cell survival via oxidative stress. Chem. Res. Toxicol. 2013;26:662–673.
https://doi.org/10.1021/tx300455k
28. Detjob A, Boonnorat J, Phattarapattamawong S. Synergistic decolorization and detoxication of reactive dye Navy Blue 250 (NB250) and dye wastewater by the UV/Chlorine process. Environ. Eng. Res. 2023;28:220–221.
https://doi.org/10.1161/hc0402.104118
29. Gonzalez C, Rosas-Hernandez H, Ramirez-Lee MA, Salazar-García S, Ali SF. Role of silver nanoparticles (AgNPs) on the cardiovascular system. Arch. Toxicol. 2016;90:493–511.
https://doi.org/10.1007/s00204-014-1447-8
30. Docter D, Westmeier D, Markiewicz M, Stolte S, Knauer S, Stauber R. The nanoparticle biomolecule corona: lessons learned–challenge accepted? Chem. Soc. Rev. 2015;44:6094–6121.
https://doi.org/10.1039/C5CS00217F
31. Lanone S, Boczkowski J. Biomedical applications and potential health risks of nanomaterials: molecular mechanisms. Curr. Mol. Med. 2006;66:651–663.
https://doi.org/10.2174/156652406778195026
32. Chen F, Goel S, Hernandez R, et al. Dynamic positron emission tomography imaging of renal clearable gold nanoparticles. Small. 2016;12:2775–2782.
https://doi.org/10.1002/smll.201600194
33. Longmire M, Choyke PL, Kobayashi H. Clearance properties of nano-sized particles and molecules as imaging agents: considerations and caveats. Nanomedicine. 2008;15:217–219.
https://doi.org/10.2217/17435889.3.5.703
34. George B, You D, Joy MS, Aleksunes LM. Xenobiotic transporters and kidney injury. Adv. Drug Deliv. Rev. 2017;116:73–91.
https://doi.org/10.1016/j.addr.2017.01.005
35. Kamaly N, He JC, Ausiello DA, Farokhzad OC. Nanomedicines for renal disease: current status and future applications. Nat. Rev. Nephrol. 2016;12:738–753.
https://doi/org/10.1038/nrneph.2016.156
36. Wang J, Liu G. Imaging nano–bio interactions in the kidney: toward a better understanding of nanoparticle clearance. Angew. Chem. Int. Ed. 2018;57:3008–3010.
https://doi.org/10.1002/anie.201711705
37. Milić M, Leitinger G, Pavičić I, et al. Cellular uptake and toxicity effects of silver nanoparticles in mammalian kidney cells. J. Appl. Toxicol. 2015;35:581–592.
https://doi.org/10.1002/jat.3081
38. Attarilar S, Yang J, Ebrahimi M, et al. The Toxicity Phenomenon and the Related Occurrence in Metal and Metal Oxide Nanoparticles: A Brief Review From the Biomedical Perspective. Front. Bioeng. Biotechnol. 2020;8:217–224.
https://doi.org/10.3389/fbioe.2020.00822
39. Pizzino G, Irrera N, Cucinotta M, et al. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017;201:841–876.
https://doi.org/10.1155/2017/8416763
40. Dröge W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002;82:47–95.
https://doi.org/10.1152/physrev.00018.2001
41. Katerji M, Filippova M, Duerksen-Hughes P. Approaches and methods to measure oxidative stress in clinical samples: Research applications in the cancer field. Oxid. Med. Cell. Longev. 2019;21:437–453.
https://doi.org/10.1155/2019/1279250
42. Tsukahara H. Biomarkers for oxidative stress: clinical application in pediatric medicine. Curr. Med. Chem. 2007;14:339–351.
https://doi.org/10.2174/092986707779941177
43. Devasagayam T, Tilak J, Boloor K, Sane KS, Ghaskadbi SS, Lele R. Free radicals and antioxidants in human health: current status and future prospects. Japi. 2004;52:4–18.
https://doi.org/10.1016/j.ajme.2017.09.001
44. Wan R, Mo Y, Feng L, Chien S, Tollerud DJ, Zhang Q. DNA damage caused by metal nanoparticles: involvement of oxidative stress and activation of ATM. Chem. Res. Toxicol. 2012;25:1402–1411.
https://doi.org/10.1021/tx200513t
45. Abdal A, Hossain MK, Lee SB, et al. The role of reactive oxygen species (ROS) in the biological activities of metallic nanoparticles. Int. J. Mol. Sci. 2017;18:120.
https://doi.org/10.3390/ijms18010120
46. Shrivastava R, Kushwaha P, Bhutia YC, Flora S. Oxidative stress following exposure to silver and gold nanoparticles in mice. Toxicol. Ind. Health. 2016;32:1391–1404.
https://doi.org/10.1177/0748233714562623
47. Nyyssönen T, Isakov M, Peura P, Kuokkala V. Iterative determination of the orientation relationship between austenite and martensite from a large amount of grain pair misorientations. Metall. Mater. Trans. A: Phys. 2016;47:2587–2590.
https://doi.org/10.1007/s11661-016-3462-2
48. Vuong Q, Goegan P, Mohottalage S, et al. Proteomic changes in human lung epithelial cells (A549) in response to carbon black and titanium dioxide exposures. J. Proteomics. 2016;149:53–63.
https://doi.org/10.1016/j.jprot.2016.03.046
49. Georgantzopoulou A, Serchi T, Cambier S, et al. Effects of silver nanoparticles and ions on a co-culture model for the gastrointestinal epithelium. Part. Fibre Toxicol. 2015;13:1–17.
https://doi.org/10.1186/s12989-016-0117-9
50. García-Hevia L, Valiente R, Martín-Rodríguez R, et al. Nano-ZnO leads to tubulin macrotube assembly and actin bundling, triggering cytoskeletal catastrophe and cell necrosis. Nanoscale. 2016;8:10963–10973.
https://doi.org/10.1039/C6NR00391E
51. Li Z, Pearlman H, Hsieh P. DNA mismatch repair and the DNA damage response. DNA Repair. 2016;38:94–101.
https://doi.org.10.1016/j.dnarep.2015.11.019
52. Jackson P, Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461:1071–1078.
https://doi.org/10.1038/nature08467
53. Yan M, Tang C, Ma Z, Huang S, Dong Z. DNA damage response in nephrotoxic and ischemic kidney injury. Toxicol. Appl. Pharmacol. 2016;313:104–108.
https://doi.org/10.1016/j.taap.2016.10.022
54. Johnston HJ, Hutchison G, Christensen FM, Peters S, Hankin S, Stone V. A review of the in vivo and in vitro toxicity of silver and gold particulates: particle attributes and biological mechanisms responsible for the observed toxicity. Crit. Rev. Toxicol. 2010;40:328–346.
https://doi.org/10.3109/10408440903453074
55. Ahamed M, Karns M, Goodson M, et al. DNA damage response to different surface chemistry of silver nanoparticles in mammalian cells. Toxicol. Appl. Pharmacol. 2008;233:404–410.
https://doi.org/10.1016/j.taap.2008.09.015
56. Kang SJ, Lee YJ, Lee E-K, Kwak M-K. Silver nanoparticlesmediated G2/M cycle arrest of renal epithelial cells is associated with NRF2-GSH signaling. Toxicol. Lett. 2012;211:334–341.
https://doi.org/10.1016/j.toxlet.2012.04.01
57. Meena R, Rani M, Pal R, Rajamani P. Nano-TiO2-induced apoptosis by oxidative stress-mediated DNA damage and activation of p53 in human embryonic kidney cells. Appl. Biochem. Biotechnol. 2012;167:791–808.
https://doi.org/10.1007/s12010-012-9699-3
58. Kumbhakar DV, Datta AK, Mandal A, et al. Effectivity of copper and cadmium sulphide nanoparticles in mitotic and meiotic cells of Nigella sativa L.(black cumin)–can nanoparticles act as mutagenic agents? J. Exp. Nanosci. 2016;11:823–839.
https://doi.org/10.1080/17458080.2016.1149236
59. Escarcega Gonzalez C, Rodriguez-Vazquez M, Jaramillo-Juarez F, Cerda-Gonzalez E, Ruvalcaba H. The renal effects of a single and low dose of titanium dioxide nanoparticles in adult male rats. Toxicol. Lett. 2014;229:23–28.
https://doi.org/10.1016/j.toxlet.2014.06.644
60. Ghosh M, Chakraborty A, Mukherjee A. Cytotoxic, genotoxic and the hemolytic effect of titanium dioxide (TiO2) nanoparticles on human erythrocyte and lymphocyte cells in vitro. J. Appl. Toxicol. 2013;33:33–37.
https://doi.org/1097-110.10.1002/jat.2863
61. Sha B, Gao W, Wang S, Xu F, Lu TJ. Cytotoxicity of titanium dioxide nanoparticles differs in four liver cells from human and rat. Compos. B. Eng. 2011;42:2136–2144.
https://doi.org/10.1016/j.compositesb.2011.05.009
62. Fabian E, Landsiedel R, Ma-Hock L, Wiench K, Wohlleben W, Ravenzwaay B. Tissue distribution and toxicity of intravenously administered titanium dioxide nanoparticles in rats. Arch. Toxicol. 2008;82:151–157.
https://doi.org/10.1007/s00204-007-0253-y
63. Sintubin L, Verstraete W, Boon N. Biologically produced nanosilver: current state and future perspectives. Biotechnol. Bioeng. 2012;109:2422–2436.
https://doi.org/10.1002/bit.24570
64. Samberg ME, Oldenburg SJ, Monteiro-Riviere NA. Evaluation of silver nanoparticle toxicity in skin in vivo and keratinocytes in vitro. Environ. Health. Perspect. 2010;118:407–413.
https://doi.org/10.1289/ehp.0901398
65. Akter M, Sikder MT, Rahman MM, et al. A systematic review on silver nanoparticles-induced cytotoxicity: Physicochemical properties and perspectives. J. Adv. Res. 2018;9:1–16.
https://doi.org/10.1016/j.jare.2017.10.008
66. Haase A, Rott S, Mantion A, et al. Effects of silver nanoparticles on primary mixed neural cell cultures: uptake, oxidative stress and acute calcium responses. Toxicol. Sci. 2012;126:457–468.
https://doi.org/10.1093/toxsci/kfs003
67. Zhang XF, Gurunathan S, Kim JH, et al. Effects of silver nanoparticles on neonatal testis development in mice. Int. J. Nanomedicine. 2015;10:6243–6256.
https://doi.org/10.2147/ijn.s90733
68. Pelkonen KH, Heinonen-Tanski H, Hänninen OO. Accumulation of silver from drinking water into cerebellum and musculus soleus in mice. Toxicology. 2003;186:151–157.
https://doi.org/10.1016/s0300-483x(02)00743-6
69. Kim YS, Kim JS, Cho HS. Twenty-eight-day oral toxicity, genotoxicity, and gender-related tissue distribution of silver nanoparticles in Sprague-Dawley rats. Inhal. Toxicol. 2008;20:575–583.
http://doi.org/10.1080/08958370701874663
70. Wijnhoven SWP, Peijnenburg WJGM, Herberts CA. Nano-silver – a review of available data and knowledge gaps in human and environmental risk assessment. Nanotoxicology. 2009;3(2)109–138.
http://doi.org/10.1080/17435390902725914
71. Tiwari DK, Jin T, Behari J. Dose-dependent in-vivo toxicity assessment of silver nanoparticle in Wistar rats. Toxicol. Mech. Methods. 2011;21:13–24.
https://doi.org/10.3109/15376516.2010.529184
72. Coradeghini R, Gioria S, García CP, et al. Size-dependent toxicity and cell interaction mechanisms of gold nanoparticles on mouse fibroblasts. Toxicol. Let. 2013;217:205–216.
https://doi.org/10.1016/j.toxlet.2012.11.022
73. Pan Y, Neuss S, Leifert A, et al. Size-dependent cytotoxicity of gold nanoparticles. Small. 2007;3:1941–1949.
https://doi.org/10.1002/smll.200700378
74. Chen YS, Hung YC, Liau I, Huang GS. Assessment of the In Vivo Toxicity of Gold Nanoparticles. Nanoscale. Res. Lett. 2009;4:858–864.
https://doi.org/10.1007/s11671-009-9334-6
75. Schmid G, Kreyling WG, Simon U. Toxic effects and biodistribution of ultrasmall gold nanoparticles. Arch. Toxicol. 2017;91:3011–3037.
https://doi.org/10.1007/s00204-017-2016-8
76. Das S, Debnath N, Mitra S, Datta A, Goswami A. Comparative analysis of stability and toxicity profile of three differently capped gold nanoparticles for biomedical usage. Biometals. 2012;25:1009–1022.
https://doi.org/10.1007/s10534-012-9567-1
77. Cho EC, Xie J, Wurm PA, Xia Y. Understanding the role of surface charges in cellular adsorption versus internalization by selectively removing gold nanoparticles on the cell surface with a I2/KI etchant. Nano. Lett. 2009;9:1080–1084.
https://doi.org/10.1021/nl803487r
78. Yahyaei B, Nouri M, Bakherad S, Hassani M, Pourali P. Effects of biologically produced gold nanoparticles: toxicity assessment in different rat organs after intraperitoneal injection. AMB. Express. 2019;9:1–12.
https://doi.org/10.1186/s13568-019-0762-0
79. Taratula O, Galoppini E, Wang D, et al. Binding studies of molecular linkers to ZnO and MgZnO nanotip films. J. Phys. Chem. B. 2006;110:6506–6515.
https://doi.org/10.1021/jp0570317
80. Chang Y-N, Zhang M, Xia L, Zhang J, Xing G. The toxic effects and mechanisms of CuO and ZnO nanoparticles. Materials. 2012;5:2850–2871.
https://doi.org/10.3390/ma5122850
81. Soenen SJ, Rivera-Gil P, Montenegro J-M, Parak WJ, De Smedt SC, Braeckmans K. Cellular toxicity of inorganic nanoparticles: common aspects and guidelines for improved nanotoxicity evaluation. Nano. Today. 2011;6:446–465.
https://doi.org/10.1016/j.nantod.2011.08.001
82. Li C-H, Shen C-C, Cheng Y-W, et al. Organ biodistribution, clearance, and genotoxicity of orally administered zinc oxide nanoparticles in mice. Nanotoxicology. 2012;6:746–756.
https://doi.org/10.3109/17435390.2011.620717
|
|
||||||||||||||||||||||||||||||||||