AbstractThe thermolysis of waste tobacco filters (WTFs) was carried out and characterized by thermogravimetric analysis (TGA) and pyrolysis-gas chromatography/mass spectrometry (Py-GC/MS). The WTFs showed four distinctive derivative TG (DTG) peaks. Such distinctive DTG curves were indicative of the thermal desorption of triacetin (< 220°C), thermal desorption of glycerol tricaprylate (221–270°C), decomposition of cellulose acetate (CA, 281–400°C), and char stabilization (> 400°C). The activation energy (Ea) (223–279 kcal kg−1) in the additives desorption region (220–270°C) were higher than those (169 ± 5.1 kcal kg−1) from the WTFs at the main decomposition region of CA (281–400°C). Py-GC/MS suggested that a large amount of acetic acid (AA, 33.0 wt.%) was recovered as the major pyrogenic product stemming from WTF. An increase in AA purity up to 76.2% (except for CO2) was achieved in the 2nd Py step at 400°C after eliminating the non-target chemicals (nicotine, triacetin, glycerol tricaprylate, and 2-(decanoyloxy)propane-1,3-diyl dioctanoate) through the 1st thermal desorption (TD) step to 300°C.
Graphical Abstract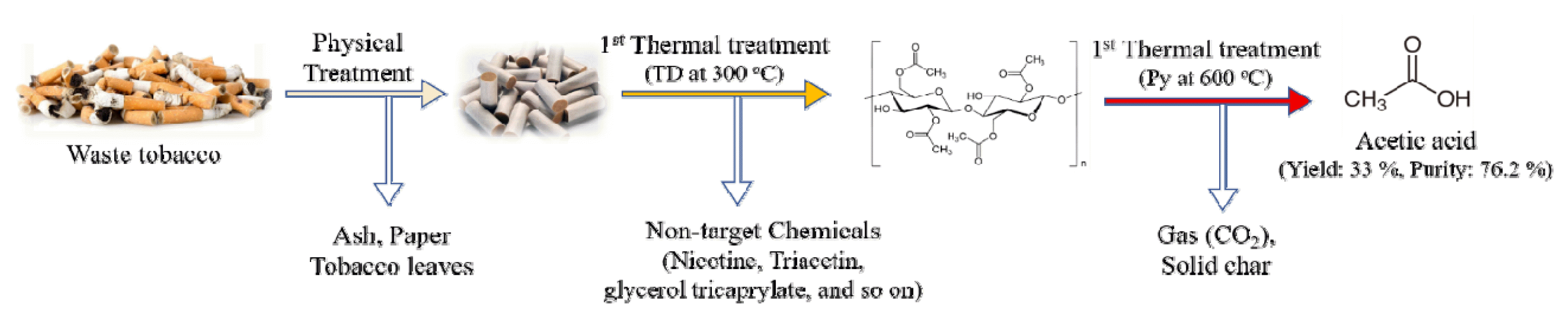
1. IntroductionAn appropriate disposal treatment of tobacco waste is an essential environmental issue because of its hazardous potential and difficult collection [1,2]. Marine pollution by microplastics has been recently identified, and their hazardous risk has highlighted the need for a proper disposal treatment of tobacco waste [3,4]. Indeed, tobacco butts contain various pollutants, including heavy metals and toxic pyrogenic chemicals. On the other hand, the major component of tobacco butts is cellulose acetate (CA) and additives [6,7]. Thus, developing a reliable disposal platform is essential while valorizing them into useful chemicals [5].
Conventional waste treatment methods of waste tobacco butts (landfilling and incineration) can increase the discharge of toxic chemicals [8], and it is impossible to recycle CA and other additives for use as chemical feedstock. Many researchers proposed various tobacco waste recycling technologies to overcome the limited sustainability of conventional waste treatment methods. Kurmus etal. [9] manufactured fired clay bricks by controlling the mixing ratio of tobacco butts and reported a decrease in thermal conductivity, suggesting lower energy costs for heating and cooling. Anna et al. [10] proposed a potential recycling method for tobacco butts by manufacturing raw materials for consumable items made from CA, such as horn-rimmed eyeglass frames, via the extraction of harmful substances from tobacco butts using two-step solvent extraction using water and ethanol and dry at 60°C. Manfrin etal. [11] manufactured tobacco butts-derived activated carbon and reported its Pb2+ adsorption capability by applying various activation techniques. Although various recycling methods have been introduced recently, the residual toxicology in extraction solvents and recycled products can impede environmental sustainability.
Thermal conversion technology generates various products by thermally decomposing waste through torrefaction, pyrolysis (Py), and gasification [12]. Although combustion can potentially detoxify harmful substances, its recyclability is lower than other thermal technologies. Recently, Kim et al. [13] suggested the CO2-assisted catalytic Py of tobacco butts over Ni/SiO2 as a technology that can simultaneously achieve the dual goals of detoxification and gas production. On the other hand, the presence of harmful substances, such as nicotine and pyridines, in the Py oil was also reported in a comparative study between Py-gas chromatography/mass spectrometry (GC/MS) and fixed-bed reactors [14,15]. Choi et al. [16] reported a high yield of specific liquid products (caprolactone, acetic acid, triacetin, and limonene) produced by the Py of waste electronic heated tobacco filters. Wang et al. [17] produced the ester-rich bio-oil via microwave-Py with methanol addition. Indeed, CA filter occupies the majority of tobacco waste, making it more desirable to separate waste tobacco filters (WTFs) and apply the Py process for the production of higher-quality oil because the physical separation of TF from tobacco wastes by crushing and sieving is easier than the extraction of a target product from complicated tobacco waste Py oil mixture with a large amount of n- and o-containing products. Nevertheless, most research has been performed to recycle untreated tobacco butts, and the detailed yield of the target chemical of each reaction was also not reported.
This study examined the production of chemical feedstock in WTF, such as acetic acid and other additives physically extracted from tobacco butts, using kinetic and Py-GC/MS analyses. The thermal behavior determined by thermogravimetric analysis (TGA) was interpreted with the activation energy (Ea) derived by a model-free kinetic analysis. Single-shot Py-GC/MS of WTF at 400, 500, and 600°C was performed to determine the optimized conditions for high-quality acetic acid (AA) production. In addition, double-shot Py-GC/MS analysis, thermal desorption (TD) and Py, was applied to produce high-purity AA during the 2nd Py step (400°C) of TF obtained after extracting other additives and impurities via 1st TD step up to 300°C. Obtained results suggest the optimized thermal conversion process, which can produce large amounts and high purity AA by applying the two-step thermal treatment of waste TF after the physical treatment of waste tobacco butts.
2. Experimental Section2.1. WTFWTFs were prepared by collecting the filter parts from waste tobacco butts after smoking and drying them at 60°C for four hours before the experiment. Proximate, ultimate, and heating value measurements were performed according to the procedure reported elsewhere [18].
2.2. Kinetic AnalysisTGA (TGA55, TA instruments, USA) of WTF at multi-heating rates (1, 5, and 10°C/min) was performed by heating 5 mg of WTF from 40°C to 800°C under a nitrogen atmosphere (60 mL/min). TGA of CA and unsmoked TF (UTF) were also performed to understand the kinetic behavior of WTF. The Ozawa method [19], a model-free kinetic analysis, was applied to derive the apparent Ea values without assuming a reaction model using TGA at multi-heating rates.
2.3. Py-GC/MS AnalysisFurnace-type pyrolyzer (3050TR, Frontier-Lab, Japan)-GC/MS (7890B/5977B, Agilent Technology, USA) was used for the Py of WTF and its product analysis at once. For the experiment, 1.0 ± 0.01 mg of WTF was dropped into a preheated pyrolyzer heater at the target temperature (400, 500, or 600°C) for the single-shot Py of WTF to check the efficient temperature to produce AA. For the double shot Py-GC/MS analysis, the 1st TD mode, from 100°C to 300°C at 20°C/min, and 2nd Py mode at 400°C were applied. The product vapor, emitted from the pyrolyzer, was cryo-focused at the front part of the GC column (UA-5, 30 m × 0.25 mm inner diameter × 0.25 μm film thickness) and separated by a GC oven program. Table S1 lists the detailed GC/MS operation parameters applied in this study (Supplementary Information). The identification of product peaks, obtained from the Py-GC/MS analysis, was performed by comparing the mass spectrum of each peak on the chromatogram with those in mass libraries, NIST 08th and F-Search [20]. The weight yield of AA was quantified by applying an external calibration method, and the purity of AA in the product oil was checked with a peak area% of AA except for CO2 and water peaks on the chromatogram.
3. Results and Discussion3.1. Physicochemical Characterization of WTFThe WTF produced a large amount of volatiles (84.8%), suggesting its high potential as a Py feedstock for oil production (Table 1). In addition, the contents of carbon (51.2%) and oxygen (42.8%) were much higher than hydrogen (4.8%), nitrogen (1.0%), and sulfur (0.4%) because CA and the main additives (triacetin and glycerol tricaprylate) of TF have carbon and oxygen as main elements in their structures [21]. Although the heating value of WTF was not higher than other fossil fuels [22], it was comparable to woody biomass [23], suggesting the possibility of WTF as fuel.
3.2. Kinetic Analysis
Fig. 1 shows the TGA and derivative TG (DTG) curves of CA, UTF, and WTF. CA decomposed rapidly between 280 and 400°C and slowly up to 550°C. Although UTF revealed a similar decomposition pattern to CA, it showed additional weight loss at the low temperature, overlapped with the vaporization temperature of triacetin [24], Zone A at below 220°C. The TG and DTG curves of WTF revealed four weight loss temperature regions assigned to A, B, C, and D. An overlay of the TG and DTG curves with those of CA and UTF showed that Zones A, C, and D could be explained by the vaporization of triacetin, decomposition of CA, and thermal stabilization of char intermediates, respectively.
The newly detected Zone B at between 220 and 270°C on the TGA result of WTF can be explained by the vaporization of other additives in tobacco capsules and hazardous materials transferred from tobacco leaves to TF by smoking. During smoking, many additives in the capsule are vaporized and transferred to TF, and their amounts are increased when smokers break the capsule in the tobacco during smoking. Lim et al. [25] detected many chemicals, such as limonene, benzyl alcohol, menthone, menthol, glycerol tricaprylate, and glycerol tricaprate, in tobacco capsules using GC/MS.
Table 2 lists the initial (Ti), maximum (Tm), and final (Tf) decomposition temperature of each thermal zone on the DTG curves of CA, UTF, and WTF. Although the Tmax on the TGA of UTF (358°C) was not significantly different from CA (357°C), the Tmax of WTF (348°C) was lower than CA (357°C) and UTF (358°C), suggesting the thermal stability change of TF, caused by smoking. This result is comparable to the research for electronically heated tobacco. Choi et al. [16] reported that the Tmax of CA filter in electronically heated tobacco was not largely differentiated by smoking during non-isothermal TGA, even though they contain other additives, such as glycerin, nicotine, and limonene. The difference in the thermal stability of conventional TF and electronically heated TF can be explained by the different heat transferred to the CA filter during smoking. In the case of conventional tobacco, the tobacco temperature can be increased to 850°C [26], but that of electronically heated tobacco can be increased to 400°C [27]. This suggests that the change in the thermal stability of TF is caused by the heat transfer from the smoking flame to the filter.
After TGA, the residual char amount of CA, UTF, and WTF was 11.9%, 10.3%, and 12.6%, respectively. Owing to the larger contents of TF additives, the residual solid amount of UTF can be smaller than CA after TGA because they are vaporized at low temperatures, as shown in Fig. 1(b). On the other hand, a WTF produces a larger amount of solid residue after TGA than UTF. During smoking, harmful organic and inorganic contaminants are trapped in TF, reducing the risk of smoking. In the case of Py, high temperatures can reduce the absolute amount of these toxic contaminants via chemical changes in toxic gas and by fixing the inorganic substances in the form of solid char rather than Py oil [13].
Fig. 2 presents the Ea changes versus conversion, calculated from TGA results obtained at different heating rates using the Ozawa method. The slopes of the plots of ln β vs. 1/T had high linearities (R2 > 0.99) at all conversions for all samples analyzed in this study (Fig. S1), confirming the reliability of the applied kinetic analysis.
The Ea of CA on non-isothermal thermal decomposition was increased from 151 kJ/mol to 187 kJ/mol with an increase in conversion; the average value was 170 kJ/mol. Although Ea was increased with the conversion, the difference in Ea between the minimum and maximum values was low, 36 kJ/mol, suggesting that CA decomposed via a single-step reaction [28]. Other researchers reported similar results. Conceição et al. [29] indicated that CA decomposed via a single decomposition reaction with the narrow Ea range.
The UTFs and WTFs had higher Ea values at low conversion. The Ea for the Py of UTF at 0.1 conversion was 226 kJ/mol in the triacetin vaporization step and 166 ± 0.9 kJ/mol in the CA decomposition region from 0.2 to 0.8 conversion. In the case of WTF, high Ea values (279–223 kJ/mol) were observed up to 0.2 conversion because of the vaporization of glycerol tricaprylate after the vaporization of triacetin. After these additive vaporization steps, the Ea of the WTF Py step was also 169.0 ± 5.1 kJ/mol from 0.3 to 0.8 conversion. The difference in the pyrolysis of CA during the pyrolysis of TFs was that WTF had a lower Ea value (163 kcal/mol) than that of UTF (171 kcal/mol) at 0.9 conversion, supporting its lower thermal stability compared to UTF. Fig. 2 presents the Ea change on the non-isothermal decomposition of CA, UTF, and WTF.
3.3. Py-GC/MS AnalysisAs obtained from the single-shot Py-GC/MS analysis (Fig. 3), the flash Py of WTF at 400°C produced CO2 (#1), AA (#3), hydroxyethyl acetate (#4), and triacetin (#7) as the main products with the minor amounts of other chemical, acetone (#2), levomenthol (#5), (5-formyl-2-furyl)methyl acetate (#6), nicotine (#8), glycerol tricaprylate (#9), and 2-(decanoyloxy)propane-1,3-diyl dioctanoate (#10). CO2 (#1), acetone (#2), AA (#3), hydroxyethyl acetate (#4), and (5-formyl-2-furyl)methyl acetate (#6) can be generated by the decomposition of CA. Among them, the peak area of AA was the largest because of the effective deacetylation of CA [30]. The production of AA via the pyrolysis of WTF at 400°C is meaningful because it can decrease the AA production cost than petroleum-derived AA [31]. Triacetin (#7), levomenthol (#5), glycerol tricaprylate (#9), and 2-(decanoyloxy)propane-1,3-diyl dioctanoate (#10) were typical additives used as a typical plasticizer [32] and flavoring agents [33]. Based on the peak intensity on the pyrogram of WTF at 400°C, AA and triacetin can be considered as the main chemicals that can be recovered from WTFs using simple Py technology due to their high potential use as the chemical feedstocks for the production of calcium magnesium acetate (CMA) deicer [31] and additives [34]. The yields of AA and triacetin, using an external calibration method, were 33.0 ± 1.0% and 5.0 ± 0.5%, respectively, of the WTF amount fed to the Py (Fig. 4).
The yields of AA and triacetin produced at 500°C were similar to those at 400°C, but they decreased to 27.9 ± 0.2% and 3.1 ± 0.1% at 600°C, respectively, with the additional formation of other pyrolyzates, such as acetone and pentadienes, suggesting secondary cracking at high temperatures. Based on the above results, the optimized temperature for AA production was 400°C, considering the target product yields and input process energy cost. Although a large amount of AA can be generated at 400°C, its purity, supposed by the MS peak area%, was low (55.5%) because triacetin (29.8%) and other additives act as impurities in the WTF pyrolysis oil produced from single-shot Py. The CA Py oil contained a wider variety of chemicals as impurities emitted from tobacco leaves and capsules, such as levomenthol, nicotine, and glycerol triacetate, because they also accumulated in the WTF after smoking and act as impurities in the pyrolysis oil of WTF.
Therefore, purity control of AA in the pyrolysis oil will be a crucial factor in controlling the AA production process, even though it can be achieved by applying various kinds of technology, solvent extraction, and thermal extraction [35] before Py or additional distillation of WTF Py oil.
Fig. 5 revealed the chromatogram obtained by the double-shot Py-GC/MS analysis, selectively applied the 1st TD, from 100°C to 300°C, and 2nd Py zone, at 400°C, from waste TF. As expected, a large amount of triacetin and other flavors could be extracted thermally during the TD step from WTF. CO2 and AA were generated mainly during the Py step of residual TF. This suggested that the process separation for the thermal extraction of additives and the pyrolysis of CA could help recycle the additives and produce higher-purity AA by eliminating impurities. Overall, the yield and purity of the AA by applying two-step thermal treatment, thermal elimination of impurities, and the Py of residual CA after the TD step were 32.4 ± 1.7% and 76.2% (except for water and CO2), highlighting the feasibility of the applied process. The above results suggest the potential use of recycled AA as a chemical feedstock on CMA production because AA production via WTF pyrolysis has an enough potential to reduce the cost of AA, which accounts for the largest cost proportion (75%) of CMA raw materials. However, the feasibility of WTF-derived AA as the chemical feedstock for CMA production has to be further investigated as our future work.
4. ConclusionsThe WTFs recovered from tobacco butts through physical processing contain various toxic compounds, but they also have large amounts of CA and other valuable additives, which can produce chemical feedstock, such as AA and triacetin, via simple Py process. During the TGA, triacetin and glycerol tricarprylate were extracted thermally at temperatures lower than 300°C, and the Ea values at this temperature zone were decreased from 279 to 223 kcal/kg. After the TD region, CA was decomposed via single-step decomposition with a narrow Ea range (169.0 ± 5.1 kcal/kg). The single-shot-GC/MS result of WTFs suggested that the pyrolysis product at 400 and 500°C consisted mainly of AA and triacetin and were not largely different, and the yield of AA decreased. The 2nd Py at 400°C after the 1st TD to 300°C suggested the largest amount (32.4%) and high purity (76.2%) AA can be produced because of the effective impurities elimination via the TD step.
AcknowledgmentsThis research was supported by the National Research Foundation of Korea(NRF) grant funded by the Korea government( MSIT).(No.NRF-2022R1C1C1005976), by the Technology Innovation Program(20016810, Development of high-purity magnesium oxide and magnesium slat manufacturing technology using dolomite), and by the Korea Ministry of Environment as Waste to Energy-Recycling Human Resource Development Project (YL-WE-22-001).
NotesAuthor Contributions B.L (Undergraduate Student) carried out the experiments, data analysis, interpretation and wrote the original draft. H.K (PhD student) and W.B.L (Master’s Student) performed the experiments and data analysis. Y.M.K (Assistant Professor) conceptualized and supervised the project, acquired funding, provided resources for the experiments and contributed to the final revision of the manuscript. References1. World Health Organization. WHO Global Report on Mortality Attributable to Tobacco [Internet]. [cited 16 June 2023]. Available from: https://www.who.int/publications/i/item/9789241564434
2. Shah G, Bhatt U, Soni V. A comprehensive review on triple R eco-management strategies to reduce, reuse and recycle of hazardous cigarette butts. Heliyon. 2023;9:e16642.
https://doi.org/10.1016/j.heliyon.2023.e16642
3. Conradi M, Sánchez-Moyano JE. Toward a sustainable circular economy for cigarette butts, the most common waste worldwide on the coast. Sci. Total Environ. 2022;847:157634.
https://doi.org/10.1016/j.scitotenv.2022.157634
4. Torkashvand J, Farzadkia M, Sobhi HR, Esrafili A. Littered cigarette butt as a well-known hazardous waste: a comprehensive systematic review. J. Hazard. Mater. 2020;383:121242.
https://doi.org/10.1016/j.jhazmat.2019.121242
5. Marinello S, Lolli F, Gamberini R, Rimini B. A second life for cigarette butts? A review of recycling solutions. J. Hazard Mater. 2020;384:121245.
https://doi.org/10.1016/j.jhazmat.2019.121245
6. Michael M, Meyyazhagan A, Velayudhannair K, et al. The Content of Heavy Metals in Cigarettes and the Impact of Their Leachates on the Aquatic Ecosystem. Sustainability. 2022;14:4752.
https://doi.org/10.3390/su14084752
7. Hossain MM, Abdulla F, Rahman A, Huq MN. Cigarette waste: A burden to the health, environment and economy. Ecotoxicol Environ. Saf. 2022;239:113661.
https://doi.org/10.1016/j.ecoenv.2022.113661
8. Novotny TE, Slaughter E. Tobacco product waste: an environmental approach to reduce tobacco consumption. Curr. Environ Health Rep. 2014;1:208–216.
https://doi.org/10.1007/s40572-014-0016-x
9. Kurmus H, Mohajerani A. Recycling of cigarette butts in fired clay bricks: A new laboratory investigation. Mater. 2020;13:790.
https://doi.org/10.3390/ma13030790
10. De Fenzo A, Giordano M, Sansone L. A clean process for obtaining high-quality cellulose acetate from cigarette butts. Mater. 2020;13:4710.
https://doi.org/10.3390/ma13214710
11. Manfrin J, Gonçalves AC, Schwantes D, Conradi E, Zimmermann J, Ziemer GL. Development of biochar and activated carbon from cigarettes wastes and their applications in Pb2+ adsorption. J. Environ. Chem. Eng. 2021;9:104980.
https://doi.org/10.1016/j.jece.2020.104980
12. Kim S, Lee S, Lee HS, Yang W, Lee J. Catalytic pyrolysis of Abandoned Fishing Nets using Activated Carbon Derived from Wasted Clamshell with in-situ and ex-situ Catalyst Configuration. Environ. Eng. Res. 2023;28:220162.
https://doi.org/10.4491/eer.2022.162
13. Kim Y, Cho SH, Lee S, Jung S, Chen WH, Kwon EE. Environmental benefits from the use of CO2 in the thermal disposal of cigarette butts. Environ. Res. 2023;220:115217.
https://doi.org/10.1016/j.envres.2023.115217
14. Muzyka R, Chrubasik M, Dudziak M, Ouadi M, Sajdak M. Pyrolysis of tobacco waste: a comparative study between Py-GC/MS and fixed-bed reactors. J. Anal. Appl. Pyrolysis. 2022;167:105702.
https://doi.org/10.1016/j.jaap.2022.105702
15. Akalin MK, Karagöz S. Pyrolysis of tobacco residue: Part 1. Thermal. Bioresources. 2011;6:1520–1531.
https://doi.org/10.15376/biores.6.2.1520-1531
16. Choi Y, Jeong S, Park YK, et al. Chemical Feedstock Recovery via the Pyrolysis of Electronically Heated Tobacco Wastes. Sustainability. 2021;13:12856.
https://doi.org/10.3390/su132212856
17. Wang W, Wang M, Huang J, et al. High efficiency pyrolysis of used cigarette filters for ester-rich bio-oil through microwave-assisted heating. J. Clean. Prod. 2020;257:120596.
https://doi.org/10.1016/j.jclepro.2020.120596
18. Park C, Jang ES, Kim YM. The temperature effect on the production of liquid and solid fuel via wood pellet torrefaction. Korean J. Chem. Eng. 2023;40:1373–1379.
https://doi.org/10.1007/s11814-022-1305-y
19. Govardhan P, Anantharaman AP, Patil SS, Dasari HP, Dasari H, Shourya A. Effect of Ag loading on praseodymium doped ceria catalyst for soot oxidation activity. Korean J. Chem. Eng. 2022;39:328–342.
https://doi.org/10.1007/s11814-021-0933-y
20. Rathore NS, Paul AS, Panwar NL. Experimental investigation on the production of bio-oil from maize straw at a pilot scale. Environ. Eng. Res. 2022;27:200592.
https://doi.org/10.4491/eer.2020.592
21. Sharaf SS, El-Shafei AM, Refaie R, Gibriel AA, Abdel-Sattar R. Antibacterial and wound healing properties of cellulose acetate electrospun nanofibers loaded with bioactive glass nanoparticles; in-vivo study. Cellulose. 2022;29:4565–4577.
https://doi.org/10.1007/s10570-022-04570-1
22. Nunes LJR, Matias JCO, Loureiro LMEF, et al. Evaluation of the Potential of Agricultural Waste Recovery: Energy Densification as a Factor for Residual Biomass Logistics Optimization. Appl. Sci. 2021;11:20.
https://doi.org/10.3390/app11010020
23. Castello D, Rolli B, Kruse A, Fiori L. Supercritical Water Gasification of Biomass in a Ceramic Reactor: Long-Time Batch Experiments. Energies. 2017;10:1734.
https://doi.org/10.3390/en10111734
24. Johar M, Zarkasi KZ, Zaini NAM, Rusli A. Enhancement of mechanical, rheological and antifungal properties of polylactic acid/ethylene–vinyl-acetate blend by triacetin plasticizer. J Polym. Res. 2023;30:259.
https://doi-org-ssl.sa.skku.edu/10.1007/s10965-023-03630-9
25. Lim HH, Choi KY, Shin HS. Flavor components in tobacco capsules identified through non-targeted quantitative analysis. J. Mass Spectrom. 2022;57:e4811.
https://doi.org/10.1002/jms.4811
26. Farsalinos KE, Yannovits N, Sarri T, Voudris V, Poulas K. Nicotine delivery to the aerosol of a heat-not-burn tobacco product: comparison with a tobacco cigarette and E-cigarettes. Nicotine Tob. Res. 2018;20:1004–1009.
https://doi.org/10.1093/ntr/ntx138
27. Bitzer ZT, Goel R, Trushin N, Muscat J, Richie JP. Free radial production and characterization of heat-not-burn cigarettes in comparison to conventional and electronic cigarettes. Chem. Res. Toxicol. 2020;33:1882–1887.
https://doi.org/10.1021/acs.chemrestox.0c00088
28. Han TU, Kim YM, Watanabe A, Teramae N, Park YK, Kim S. Pyrolysis kinetic analysis of poly(methyl methacrylate) using evolved gas analysis-mass spectrometry. Korean J. Chem. Eng. 2017;34:1214–1221.
https://doi.org/10.1007/s11814-016-0354-5
29. Lucena MdCC, de Alencar AEV, Mazzeto SE, Soares SdA. The effect of additives on the thermal degradation of cellulose acetate. Polym. Degrad. Stab. 2003;80:149–155.
https://doi.org/10.1016/S0141-3910(02)00396-8
30. Giachet MT, Schilling M, McCormick K, et al. Assessment of the composition and condition of animation cels made from cellulose acetate. Polym. Degrad. Stab. 2014;107:223–230.
https://doi.org/10.1016/j.polymdegradstab.2014.03.009
31. Oh SJ, Choi GG, Kim JS. Preparation of Calcium Magnesium Acetate Deicer Using Raw AceticAcid-Rich Bio-oil Obtained from Continuous Two-Stage Pyrolysis of Corncob. ACS Sustainable Chem. Eng. 2018;6:4362–4369.
https://doi.org/10.1021/acssuschemeng.8b00013
32. Kemper B, Herm C. Transparent Figures: Researching and Preserving Objects of Cellulose Acetate. Polymers. 2023;15:2838.
https://doi.org/10.3390/polym15132838
33. Jang DY, Kim HS, Pack EC, Koo YJ, Lim KM, Choi DW. Development of a Method for Simultaneous Analysis of Allergenic Flavoring Agents in Cigarettes and Quantitative Risk Assessment for Consumer Safety. Toxics. 2021;9:87.
https://doi.org/10.3390/toxics9040087
34. Zare A, Nabi M, Bodisco T, Hossain F, et al. The effect of triacetin as a fuel additive to waste cooking biodiesel on engine performance and exhaust emissions. Fuel. 2016;182:640–649.
https://doi.org/10.1016/j.fuel.2016.06.039
35. Torkashvand J, Farzadkia M. A systematic review on cigarette butt management as a hazardous waste and prevalent litter: control and recycling. Environ. Sci. Pollut. Res. 2019;26:11618–11630.
https://doi.org/10.1007/s11356-019-04250-x
Fig. 3Chromatograms obtained from the Py-GC/MS analysis of WTF at 400°C (1: CO2, 2: Acetone, 3: Acetic acid, 4: Hydroxyethyl acetate, 5: Levomenthol, 6: (5-Formyl-2-furyl)methyl acetate, 7: Triacetin, 8: Nicotine, 9: Glycerol tricaprylate, 10: 2-(Decanoyloxy)propane-1,3-diyl dioctanoate). 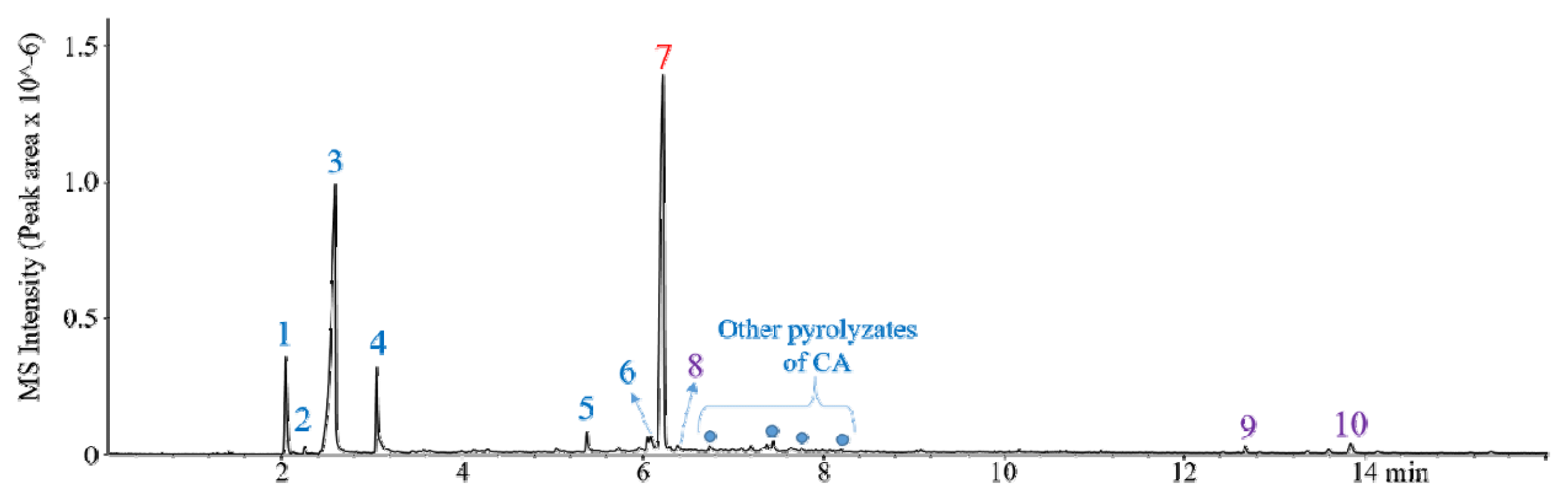
Fig. 4Yields of AA and triacetin obtained from the single-shot Py-GC/MS analysis of WTF at 400, 500, and 600°C (n:3); (a) AA, (b) Triacetin 
Table 1Physicochemical properties of WTF
Table 2Main thermal decomposition characteristics of CA and TFs obtained from TG analysis |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||