AbstractThe feasibility of preparing non-sintered lightweight aggregate using textile sludge and coal fly ash as the main raw materials was investigated. Extensive experiments were conducted to analyze the effects of different coal fly ash/sludge ratio, sodium silicate addition and curing time on the performance of non-sintered lightweight aggregates (NSLWAs). The optimal preparation parameters were determined as follows: coal fly ash/cement ratio of 2.2, textile sludge addition of 20%, sodium silicate addition of 10%, and curing time of 28 days. Under these optimal preparation conditions, the cylindrical compressive strength of NSLWAs was relatively high. The test results of Zn leaching toxicity test and S content all met the relevant standards and environmental safety requirements. NSLWA has a dense internal structure with uniformly distributed grains. By analyzing the hydration reaction mechanism during the NSLWAs preparation, it can be obtained that part of Zn2+ was stabilized by conversion into insoluble Ca(Zn(OH)3)2·2H2O, while the remaining part was solidified and stabilized through encapsulation and adsorption of hydration products. Furthermore, it was discovered that pozzolanic admixtures played a crucial role in the hydration and hardening process of NSLWAs, and the presence of C-S-H gel and Mullite contributed to the increase in cylinder compressive strength of NSLWAs.
Graphical Abstract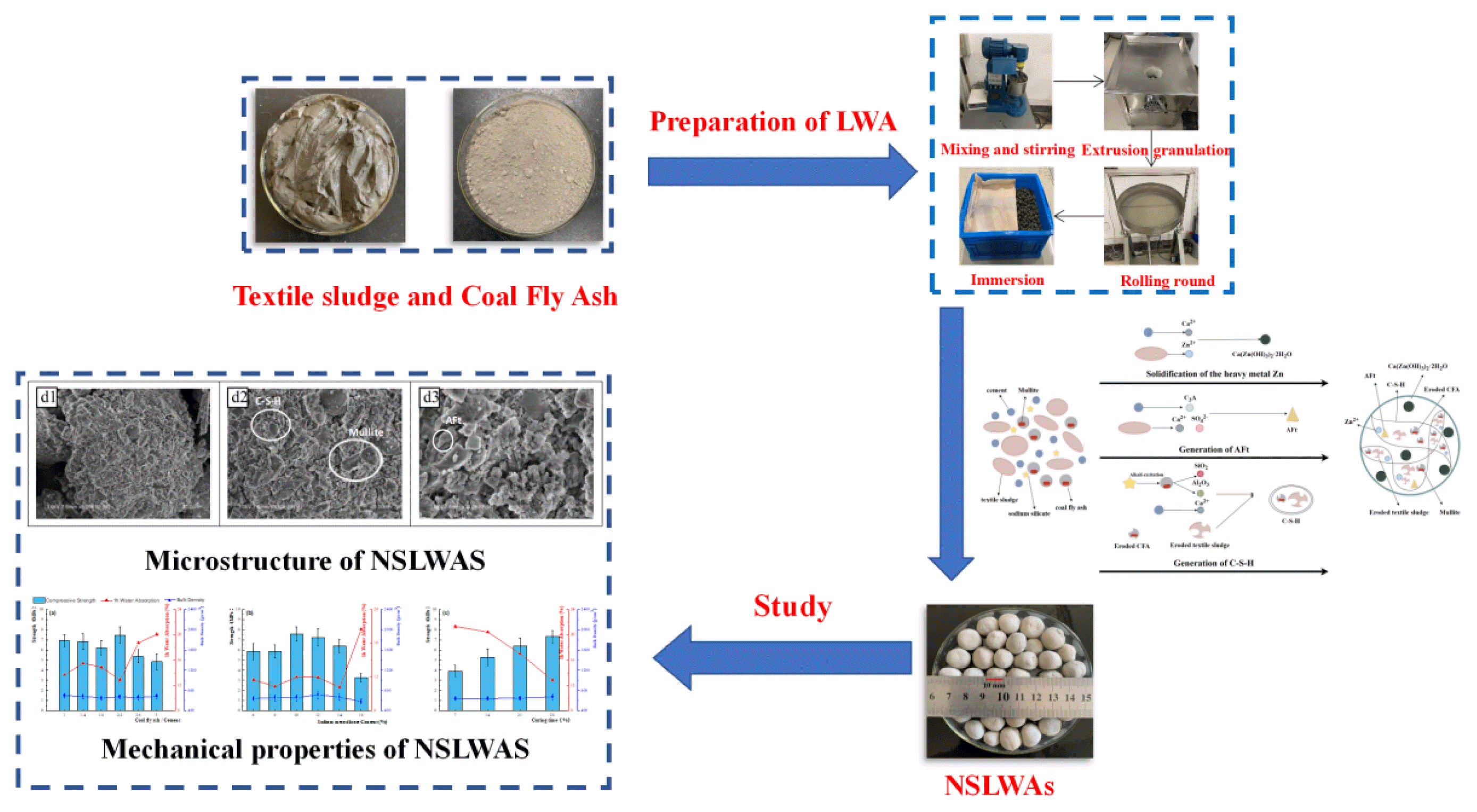
1. IntroductionChina accounts for more than a quarter of global cotton production, giving rise to a large number of cotton textile-related industries. The utilization of cotton to produce viscose staple fibers is its main production process. In the production of viscose staple fibres, a large amount of acidic wastewater containing ZnSO4 is generated during the post-dissolving, filtration and acid bath processes. Meanwhile, corresponding textile sludge can also be produced after neutralization and precipitation treatment. Textile sludge is characterized by its intricate composition, encompassing a variety of carcinogenic, toxic or mutagenic products, including polycyclic aromatic hydrocarbons (PAHs), surfactants, toxic heavy metals such as Cd, Cr, Pb, Zn and Ni detergents, as well as bio-residues and other pollutants [1,2,3], these components pose significant ecological risks and have substantially constrained its further application in the conventional sludge disposal industry [4].
Currently, two main techniques exist for the disposal of textile sludge: incineration and landfill treatment [5]. Incineration is an energy-intensive process that involves substantial energy consumption and entails elevated pre-disposal expenditures, primarily due to the high-water content of the sludge. Additionally, the incineration process is prone to producing other harmful derivatives causing secondary pollution, such as Zn salts contained in the sludge in the incineration process volatilization, resulting in tail gas containing Zn2+ dust [6]. Landfill disposal generates leachate and sludge which have low degradation capacity and can persist in the environment for extended periods [7]. This may lead to considerable pollution and has adverse effects on public health and the ecosystem. Therefore, it is imperative to employ appropriate processes for the recycling and reuse of textile sludge in order to mitigate environmental pollution. One common approach for the resource utilization of textile sludge involves its conversion into functional materials, like building materials [8,9], adsorbents [10,11] and catalysts [12]. This method also addresses the issue of hazardous substances pollution that arises in sludge treatment.
Lightweight Aggregate (LWA), also known as ceramsite, is widely used in the construction industry and environmental protection industry due to its advantages of low density and high strength, chemical stability, and good thermal insulation properties [13]. Conventional sintering technology is currently the most widely used method of preparing LWAs. The sintering process involves combustion of non-renewable resources, including shale clay, to create sintered products under high temperatures and pressures [14]. In the sintering process, the formation of pollutants is primarily due to the volatilization of organic matter and the decomposition of salts, resulting in the release of waste gases. The particulate matter and exhaust gases generated during the sintering process mainly include SO2, NOx, H2S, HCl, HF, and other acid gases, dioxins, which have a great impact on the environment. Furthermore, it is worth noting that manufacturing 1 cubic meter of sintered ceramsite requires energy input ranging from 240 to 480 RMB/m3, whereas non-sintering ceramsite production costs between 55 to 132 RMB/m3 [15]. These statistics demonstrate that sintering technology consumes an excessive amount of energy and causes environmental pollution, which conflicts with the energy-saving emission reduction strategy and impedes the development of LWAs production. Non-sintering technology, in comparison to the sintering process, obviates the long firing duration and instead relies on natural or autoclave curing, resulting in significantly lower energy consumption. The curing process generates bubbles and coagulation to expand the volume of the ceramics to create non-sintering ceramsite. However, the strength and water absorption of conventional non-sintered ceramsites is usually lower than sintered ceramsites [16]. Additionally, these materials require raw materials with volcanic ash activity, resulting in relatively high production costs. Researchers have been exploring alternative materials to replace traditional resources like cement and gypsum the production of LWAs. Currently, the most extensively studied substitutes are sewage sludge [17,18,19], sewage ash [20], and incinerator fly ash [21,22]. Incorporating solid waste into the production process of non-sintering lightweight aggregates delivers environmental benefits, reducing the impact on the environment due to cement manufacture and depletion of non-renewable resources. Moreover, this approach greatly diminishes energy consumption, such as electricity and fuel, and reduces the emission of dust particles, CO2, SO2, and NOx during the sintering process. These environmental advantages make it an attractive and sustainable option for the production of LWAs. Sludge ceramsite has been extensively researched by both domestic and international scholars. However, the abundance of organic matter and inadequate silica-aluminum ratio in sludge hinder the hydration reaction and affect the curing efficacy. Consequently, researchers have synergistically mixed sludge with other substances to prepare ceramsite. Among them, coal fly ash (CFA) is a typical industrial by-product, which is a spherical particle in a glassy state produced by high-temperature combustion in the process of coal-fired thermal power generation. Such intrinsic inert structure makes CFA hard to be reutilized as valuable resources. The annual emission of CFA in China is increasing annually. Considering the huge quantity of CFA and the low utilization rate, it is imperative to enhance the recycling of CFA and develop it into higher value-added products [23]. CFA contains a large number of metal oxides such as Al2O3 and its Ca/Si is large, which can provide a compositional complement for the preparation of sludge ceramsite, Therefore, introducing CFA into the production of sludge ceramistes is a rational approach to optimize their properties [24,25].
Currently, there is limited literature pertaining to the production of lightweight aggregates (LWA) utilizing textile sludge. Based on our previous work [26], it was found that the use of textile sludge as the main raw material for the preparation of no-sintering ceramsite can make full use of the water content of textile sludge, greatly reducing the energy demand of the drying and dewatering and the sintering process of LWA in the traditional sludge treatment process and reduce the secondary pollution. However, it's essential to be vigilant about the elevated sulfur (S) and zinc (Zn) content in textile sludge. Considering the characteristics of textile sludge rich in Si and Al salts and the chemical composition of fly ash with a large Ca/Si ratio, this study will further improve the performance of textile sludge-based no-sintering ceramsites and the stabilization and curing of Zn and S elements in textile sludge. In this study, textile sludge/CFA based NSLWA with low environmental risk was prepared by mixing appropriate amount of industrial solid waste CFA and textile sludge. The influencing mechanism of CFA/cement ratio, sodium silicate addition and curing time on the physical properties of NSLWAs was elucidated and the optimal corresponding parameters of preparing textile sludge/CFA based NSLWA were also determined. Specific characterizations including XRD and SEM analysis techniques were performed to assist the analysis of the variation in phase composition and micro-structure inside NSLWA samples. The solidification and stabilization reaction mechanism relating to the excess S and Zn in textile sludge was also investigated. To the best of our knowledge, this is the first time to simultaneously utilize CFA and Zn containing industrial waste sludge for preparing non-sintered aggregates. The experimental and mechanism study in this work can be of great value to both environmental and construction domain.
2. Materials and Methods2.1. Raw MaterialsThe textile sludge used in the study was obtained from the wastewater treatment process of a cotton textile company. CFA was obtained from a coal-fired power plant and can be classified as Class F according to ASTM C618-22 classification standard based on its chemical properties and composition.
Table S1 shows the oxide composition of the main raw materials. The textile sludge contains a high proportion of CaO and SO2, a relatively low proportion of SiO2 and Al2O3, and a certain amount of ZnO. CFA contains a high proportion of SiO2 and Al2O3, and a small amount of CaO, Fe2O3 and MgO, but it does not contain the corresponding oxides of S and Zn elements.
The XRD characterization of the textile sludge material was shown in Fig. S1. The most dominant crystalline phases in the sludge were CaSO4·2H2O, ZnS and CaCO3. ZnS and SO42− appear in the mineral phase as a result of the injection of CS2 and ZnSO4 during the production of viscose staple fibers. The CaSO4·2H2O diffraction peak has high peak intensity and narrow peak shape, in which Ca2+ originates from the limestone (mainly CaCO3) used in the neutralizing precipitation. The textile sludge contains a certain amount of organic components, which causes many miscellaneous weak peaks in the XRD pattern.
2.2. Preparation of NSLWAsAs has been reported in our previous work [26], due to the high S content in the textile sludge, the S content in the NSLWAs produced by single use of textile sludge exceeded the limit value specified in GB/T17431.2-2010 “Light Aggregates and Test Methods”(sulfide and sulfate content ≥1.5%). Through further experimental analysis, the maximum mass proportion of textile sludge in the raw material was determined to be 20% in order to control the S content of the NSLWAs. Therefore, the composition of each raw and auxiliary material used in this experiment is shown in Table S2. The CFA/cement ratio, the addition of sodium silicate, and the curing time were used as the main variables to investigate the effects of each variable on the main physical properties of the textile sludge/CFA-based NSLWA, including the barrel compression strength, bulk density, and 1 h water absorption.
The preparation process of NSLWAs is shown in Fig. 1: dry materials such as CFA, cement, sodium silicate and binder were mixed and stirred evenly, and then mixed into quantitative textile sludge and stirred evenly to form sticky billet. Then the viscous billet was extruded by spiral extruder and continuously cut into cylindrical granules, the extrusion diameter was 15 mm, the cutting length was 15 mm. Cylindrical particles were rounded to nearly spherical NSLWAs by a disk granular. The initial blanks of NSLWAs were dried at room temperature for 12~48 h and then immersed in water for 60~80 h. Finally, the NSLWAs were produced by curing the water-immersed NSLWAs blanks in a curing box covered with water for 28 days.
2.3. Performance Testing and CharacterizationThe bulk density, compressive strength, 1 h water absorption rate and S contents were determined according to GB/T17431.2-2010, Lightweight aggregates and its test methods - Part 2: Test methods for Lightweight aggregates (in China).
Measuring the mass (m) and minimum perimeter (d) of NSLWA, the barrel compressive strength (S) is calculated as Eq. (1).
Measuring the diameter (X) and fracture load (Pc) of NSLWA, the barrel compressive strength (S) is calculated as Eq. (2).
Measuring the mass (m) and the mass after 1 h water absorption (m1) of NSLWA, the 1 h water absorption (W) is calculated Eq. (3).
In order to study the curing effect of Zn, according to the current standards HJ/T299-2007, solid waste-extraction procedure for leaching toxicity-sulfuric acid & nitric acid method and HJ 781-2016, solid waste determination of 22 metal elements-Inductively coupled plasma optical emission spectrometry, the leaching concentration of Zn2+ in NSLWAs samples was measured and judged whether it exceeded the limit.
To further investigate the preparation mechanism of the NSLWAs, the main components of the raw materials were analyzed by XRF-1800 (Japan) XRF analyzer. The composition of the main phases was detected by D-max 2500 VL/PC X-ray diffractometer (Japan), the microstructure morphology of the samples was detected by JSM-5610LV scanning electron microscope (Japan).
3. Result and Discussion3.1. Performance Testing and Analysis for NSLWAsThis experiment determined that the textile sludge addition amount was 20% and CFA was added as a supplementary raw material. The CFA/cement ratio, sodium silicate addition, and curing time were determined as the main variables to investigate the effect patterns of each variable on the compressive strength, bulk density, and 1 h water absorption of textile sludge/coal fly ash based NSLWAs.
Fig. 2(a) investigated the performance test results of NSLWAs (coal fly ash content 5%, curing time 28 d, additive content 1%) related to CFA/cement ratios. As can be seen from the figure, the CFA/cement ratio had a great influence on the physical properties of NSLWAs. When the coal fly ash/cement ratio was 2.2, the barrel compressive strength of NSLWAs reached the maximum value (7.481 MPa) and the 1 h water absorption rate was the lowest (12.9%). The bulk density was basically stable in the range of 655 kg/m3~ 697 kg/m3. In addition, when it was less than or equal to 2.2, the comprehensive physical properties of NSLWAs conformed to the requirements of GBT17431.1-2010, Lightweight aggregates, and its test methods. When the CFA/cement ratio was greater than 2.2, the cylinder compression strength of NSLWAs decreased and the 1 h water absorption rate increased rapidly. When the CFA/cement ratio increased to 3, due to the low cylinder compression strength and the large water absorption rate, the physical properties of NSLWAs cannot reach the performance index required by the national standard.
Fig. 2(b) shows the effects of different sodium silicate (Na2SiO3· 9H2O) additions on the physical properties of NSLWA samples. The physical performance change trend shown in the figure was that when the sodium silicate content was less than or equal to 10%, the addition of sodium silicate had a positive effect on the physical properties of NSLWAs, and the cylinder compressive strength of NSLWAs increased from 5.83 MPa to 7.481 MPa as the addition of sodium silicate increased from 6% to 10%, and the 1 h water absorption rate fluctuated steadily while the bulk density increased gradually. It was because the alkaline environment brought by the sodium silicate promoted the hydration reaction inside the NSLWAs and generated more strength-causing substances [27]. However, when the sodium silicate addition was greater than 14%, the cylinder compressive strength of NSLWA showed a continuous downward trend and decreased significantly after the addition amount reached 16%.
Fig. 2(c) shows the effects of different curing times on the physical properties of NSLWA sample. As shown in the figure, with the extension of the curing time, the cylinder compressive strength of NSLWAs increased from 3.93 MPa to 7.481 MPa, and the 1 h water absorption gradually decreased from 21.33% to 12.9%. Meanwhile, the bulk density showed a slow increasing trend, and increased slowly to 673 kg/m3 when the curing time was 28 days.
Based on the above experimental analysis results, the optimal raw material composition ratio and curing process for preparing NSLWAs were determined as follows: coal fly ash/cement ratio of 2.2, textile sludge addition of 20%, sodium silicate addition of 10%, binder addition of 1%, and curing time of 28 days.
3.2. Crystal Phase Analysis
Fig. 3a shows the XRD patterns of NSLWA samples with different coal fly ash/cement ratios. According to Fig. 3a, the main phases in the NSLWAs were CaCO3, Mullite, C-S-H (CaO·SiO2·nH2O) gel, SiO2 and Ca(Zn(OH)3)2. The diffraction peaks of the C-S-H gels weakened progressively with the increasing CFA/cement ratio. This was attributed to the generation of Ca(OH)2 (CH) resulting from the hydration of cement, creating an alkaline reaction environment, in turn allowing the dissociation of Si-O and Al-O vitreous from CFA and the secondary hydration reaction with CH to produce C-S-H gel [28], which bonds internal organizational structure and densifies the internal structure of NSLWA [29]. When the CFA/cement ratio exceeded 2.2, the amount of cement in the reaction system decreased, causing a reduction in the CH content of the hydration products [30,31]. As a result, the system could not provide an adequately alkaline environment for the CFA hydration reaction, leading to a decrease in the secondary hydration products, C-S-H. Macroscopically, it is manifested that the NSLWA strength first enhances and then decreases with the increase of CFA content. Meanwhile, the diffraction peak of Mullite became stronger, because mullite, acting as the main skeleton bearing force, was an important component of CFA, accounting for about 40%–50%. Therefore, the increase of CFA corresponded to the increase of mullite content.
In order to explore the role of sodium silicate addition in the formation of NSLWAs, the XRD patterns of NSLWAs samples with different sodium silicate addition levels were analyzed (Fig. 3b). When the sodium silicate content was increased from 6% to 10%, the C-S-H gel diffraction peak increased continuously, the peak shapes became narrower, and double peaks appeared. When the addition amount of sodium silicate exceeded 10%, the C-S-H gel diffraction peak decreased until the addition amount exceeded 14%, and the shape of its peak changed from double peaks to single peaks. This is due to the hydrolysis reaction of the appropriate amount of water glass under the action of an aqueous medium to form NaOH and Si(OH)4− silicate gel, in which Si(OH)4− reacts with CH to form C-S-H gel-like products [32]. In addition, sodium silicate acted as an alkali exciter, causing CFA to dissolve and release a large number of ions under strong alkali conditions, forming C-S-H gels with Ca2+ in the cement, which was conducive to improving the strength of NSLWA [33]. When there was an excess of sodium silicate, the silicate hydrolysis products increased and were highly reactive, and the curing rate was too fast, resulting in an incomplete hydration reaction in the NSLWA, leading to a reduction in C-S-H, which is consistent with the findings of Hu et al. [34] and Fang et al. [35].
Fig. 3c shows the XRD patterns of NSLWAs with different curing times. The sharp and high diffraction peaks of Ca(Zn(OH)3)2·2H2O and SiO2 with prominent advantages were significant under curing time of 7 d, accompanied by weaker and wider diffraction peaks of CaSO4 and C-S-H gel. This is because the excitation of sodium silicate stimulated a large amount of reactive SiO2 in the CFA, which facilitated the subsequent hydration reaction. Additionally, the textile sludge itself contained a certain amount of organic matter, CaSO4 and Zn2+, and the delayed coagulation effect of heavy metals, organic matter, and sulfate slowed down the hydration reaction within the NSLWA [36]. In addition, in the highly alkaline environment produced by cement hydration, Zn2+ combined with sodium silicate hydrolysis product OH− or Si(OH)4− to form hydrated calcium hydroxyl zincate (Ca(Zn(OH)3)2), which wrapped around the surface of cement particles and inhibited the hydration product, C-S-H gel. So macroscopically, it showed low strength and high 1 h water absorption of NSLWAs.
In the middle stage of curing (14–21 d), the SiO2 and CaSO4 diffraction peaks decreased, while the C-S-H gel diffraction peak increased. This is because as the hydration reaction proceeds, CaSO4 gradually reacts with tricalcium silicate (C3A) in the cement to produce a small amount of alumina (AFt) by the hydration reaction [37,38]. Under the alkaline excitation of sodium silicate, the activity of SiO2 and Al2O3 in fly ash gradually increased, and the secondary hydration products C-S-H increased.
The decrease in intensity of the diffraction peak corresponding to Ca(Zn(OH)3)2 was due to the fact that a part of Ca(Zn(OH)3)2 dissolved into Ca2+ and Zn(OH)3− distributed in the sample with the extension of the curing time, while the other part crystallized and precipitated to form Ca(Zn(OH)3)2·2H2O [39]. The formation of Ca(Zn(OH)3)2·2H2O reduced the encapsulation of cement particles and accelerated the hydration reaction of cement, which was macroscopically manifested by the increasing strength of NSLWAs. The analysis shows that the main chemical reactions in this stage could be expressed as Eq. (4–9).
In the late maintenance (28 d), the Ca(Zn(OH)3)2 and mullite diffraction peak remained basically unchanged, while the C-S-H gel diffraction peak continued to increase and the peak shape became narrower. This indicates a further deepening of the hydration reaction in the samples and an increase in C-S-H gels. Due to the higher calcium-to-silicon ratio of the C-S-H gels, their silica-oxygen tetrahedral chains became shorter. Meanwhile, Ca(Zn(OH)3)2 was most likely to dissolve gradually with the prolongation of curing time, and thus it was easier to enter into the chain-layer structural space in the C-S-H and undergo the ionic substitution reaction with Ca2+, thus forming stable compounds for the stable curing of heavy metals [40,41]. Macroscopically, the intensity of NSLWAs continued to gain and tend to stabilize.
Analyzing the hydration reaction process of NSLWAs, it can be determined that a small amount of Zn2+, the main heavy metal contained in textile sludge, was converted from the soluble ion to the insoluble states Ca(Zn(OH)3)2·2H2O, and the other part was encapsulated and adsorbed by the hydration products in the ionic form. So that Zn2+ transformed from an unstable state to a stable state.
According to the results, the reuse of textile sludge/coal fly ash can be realized rationally by using appropriate raw material ratio and reasonable non-sintered curing technology, and the technology was conducive to the solidification and stabilization of heavy metal elements contained in such textile sludge.
3.3. Morphological Structures Analyses
Fig. 4 shows the SEM images of the NSLWA samples with different coal fly ash/cement ratios. Combined with the XRD analysis results, the flocculent and cylindrical materials inside the samples were C-S-H gel and mullite, respectively. When the CFA/cement ratio was 2.2, the secondary hydration product C-S-H gel cemented the textile sludge particles and eroded coal fly ash inside the NSLWA into a whole, with a dense internal structure and small voids. Meanwhile, mullite, as the main skeleton load-bearing force [42], formed an intertwined network with the C-S-H gel, which together increased the cylindrical compressive strength and bulk density of the NSLWAs. As the CFA/cement ratio increased above 2.2, the hydration products CH and C-S-H decreased, the interparticle bonding within the sample decreased, and the pores became larger. In addition, the excess CFA not involved in the hydration reaction had a loose structure, and mullite did not contribute to increase the strength under the loose structure, resulting in a decrease in the strength of the samples, which is consistent with the performance test results in Section 3.1.
Fig. 5 shows the SEM images of NSLWAs with different sodium silicate additions. When the sodium silicate addition was less than 10%, the internal structure of the sample was loose and there was a glass bead structure of CFA. When sodium silicate was added at a rate of 10%–14%, small-pored dense agglomerates were formed inside the sample. In contrast, when sodium silicate was added at a rate higher than 14%, the particles inside the sample exhibited large gaps and the structure was loose. Fig. 3b analysis results reveal that the agglomerate was the hydration product C-S-H gel. The excessive hydrolysis of sodium silicate occurs after a fast hydration reaction, and its hydrolysis products enwrap unreacted CFA particles and other slurries, which hinder the reaction's hydration [43]. This results in inadequate internal cementation of the sample and significantly decreased macroscopic sample strength.
Fig. 6 shows the SEM images of NSLWA samples at different curing times. At the initial curing period (7 d), the NSLWAs had a loose internal structure with large pores and a large number of granular substances, which was presumed to be Ca(Zn(OH)3)2· 2H2O generated by Zn2+ and Ca2+ in cement, combined with the analysis results in Fig. 3c. In the middle curing period (14–21 d), the granular material inside the sample decreased and the phases were agglomerated into blocks, while the pores increased. It was because alkaline substances dissolved out and continuously corroded the textile sludge interior, resulting in the formation of a large number of irregular lamellar structural phases. Simultaneously, rod-like particles and flocculent tissues began to appear on the surface of the phase and continued to increase. Combined with the analysis results in Fig. 3c, it was judged that the flocculent tissues were C-S-H gel, and the rod-like particles were mullites, which both could improve the particle strength of NSLWAs. A small number of acicular substances appeared inside NSLWAs, which was presumed that such substances were a small amount of AFt generated by CaSO4 participating in the hydration reaction, and the main chemical reactions were as Eq. (10–12).
In the later curing period (28 d), closed pores were formed on the surface of NSLWAs, and the material phases were tightly bound to each other, and the internal structure was dense. The increase of NSLWAs strength was due to the fact that the process of inter-material hydration reaction was continuously completed with the extension of the curing time, and the generated hydration hardening products gradually increased, which reduced the internal pore space. Simultaneously, due to the continuous generation of C-S-H gel constructed the dense structure inside NSLWAs, mullite contributed strength to NSLWAs a lot under this structure. The high-density components also prevented the water from entering into NSLWAs, making the 1 h water absorption rate of NSLWAs gradually decrease. The above analysis was consistent with the physical performance test results.
According to the changes of the crystalline phase composition and internal structure of NSLWA at different curing times, it can be found that the pozzolanic properties admixture played a major role in the hydration and hardening process of NSLWA. Among them, the active components (e.g. SiO2, Al2O3) excited by sodium silicate in the mixture would be hydrated with Ca(OH)2 generated in the raw cement curing process to generate C-S-H type gel hydration products. The reaction process equation is shown as Eq. (13–15) [29].
The NSLWAs curing reaction process is shown in Fig. 7. In the above hydration reaction process, the key phases affecting the hydration reaction process and the properties of NSLWA finished products were the generation of C-S-H gel (CaO·SiO2·nH2O, CaO·Al2O3·SiO2·nH2O, CaO·Al2O3·nH2O). With the increase of the curing time, C-S-H gel was generated and aggregated continuously, so that the physical properties of NSLWA products were constantly optimized.
3.4. Environmental Impact Assessment3.4.1. Leaching toxicity analysisDespite the uperior mechanical performance of LWAs and resource utilisation of solid wastes such as textile sludge and CFA, the environmental impacts [44,45], including leaching toxicity of soluble metal salts and S content, as has been reported previously [46,47,48], are also critical for the future real-world application. As can be seen from Table S3, the leaching test results of Zn2+ from the NSLWAs did not reach the leaching limit of Zn2+ stipulated in GB 5085.3-2007 “Identification standards for hazardous wastes-Identification for extraction toxicity”. The results indicated that, using the optimal raw material ratio and NSLWAs preparation method determined in this experiment, the obtained NSLWAs were effective for curing Zn2+ residues in the original textile sludge. Combining the results of XRD and SEM characterization analysis, it was concluded that in the process of this study, Zn2+ residue in textile sludge were encapsulated, adsorbed or produced insoluble state of Ca(Zn(OH)3)2·2H2O by the hydration products, which reached solidification and stabilization, resulting in the difficulty of dissolved leaching of Zn2+ from NSLWAs.
3.4.2. S content analysisWhile curing the heavy metal ions, the sulphur content of NSLWA should also meet the specification requirements for good durability and environmental safety in construction materials. However, the S content of textile sludge is usually too high. Therefore, we need to pay attention to the S content of NSLWA. As shown in Table S4, the S content of the NSLWAs with 20% textile sludge addition complied with the limit values specified in GB/T17431.2-2010 “Lightweight aggregates and its test methods - Part 2: Test methods for lightweight” (sulfide and sulfate content ≥ 1.5%).
4. ConclusionBased on the experimental analysis, the optimum raw material ratio and curing process parameters for the preparation of NSLWA with textile sludge and coal fly ash as the main raw materials were finally determined as follows: textile sludge addition of 20%, coal fly ash/cement ratio of 2.2, sodium silicate addition of 10%, binder addition of 1%, and curing time of 28 days, corresponding to physical properties, compression strength of 7.481 MPa, bulk density of 673 kg/m3, and 1 h water absorption rate of 12.9%, which met the performance indexes of common lightweight aggregate specified in GB/T17431.2-2010 “Light Aggregates and Test Methods”. Under the optimal preparation conditions, the internal pores of NSLWAs were closed, the grains were evenly distributed, the structure was dense, and the internal organization was stable. Coal fly ash was stimulated by sodium silicate to release active SiO2 and Al2O3, which hydrated with CH to generate C-S-H. Meanwhile, mullite played a skeleton role in the dense structure, which was macroscopically manifested in the high strength and low water absorption of NSLWA samples. In addition, a small amount of Zn2+ was converted from the soluble state ionic form to the insoluble state Ca(Zn(OH)3)2·2H2O, while a large amount of Zn2+ was wrapped and adsorbed by the hydration products, realizing the stabilization of heavy metal unstable factors. The Zn leaching toxicity test and S content test were conducted, and the results all met the requirements of relevant standards and environmental safety requirements.
AcknowledgementsThis work was supported by the National Key R&D Program of China [2022YFC3901405], the Financial Budget Project of the Ministry of Ecology and Environment (706301)
NotesAuthor Contributions Y.Q.X. (Assistant Researcher): Conceptualization, Methodology, Formal analysis, Investigation, Writing original draft, Visualization. X.Y.W. (Assistant Researcher): Validation, Investigation. H.H.Z. (Ph.D.): Conceptualization, Project administration. Q.Y.Z. (Postgraduate student): Methodology, Formal analysis, Visualization. Y.F.X. (Ph.D.): Conceptualization, Methodology, Visualization, Formal analysis, Writing - review & editing. References1. Guo SX. Research on Sludge Treatment and Recycling Approaches and New Technologies. China Resour. Compr. Uti. 2019;37(12)68–70.
https://doi.org/10.3969/j.issn.1008-9500.2019.12.020
2. Liang X, Ning X, Chen G, et al. Concentrations and speciation of heavy metals in sludge from nine textile dyeing plants. Ecotoxicol. Environ. Saf. 2013;98:128–134.
https://doi.org/10.1016/j.ecoenv.2013.09.012
3. Rosa EVC, Giuradelli TM, Corrêa AXR, et al. Ecotoxicological evaluation of the short term effects of fresh and stabilized textile sludges before application in forest soil restoration. Environ. Pollut. 2007;146(2)463–469.
https://doi.org/10.1016/j.envpol.2006.07.005
4. Jiang J, He X, Xiong X, Zhang X, Ren H. Research progress on toxicity characteristics and control technology of textile printing and dyeing wastewater. Ind. Water Treat. 2021;41(06)77–87.
https://doi.org/10.11894/iwt.2021-0180
5. Karthik T, Rathinamoorthy R. Recycling and reuse of textile effluent sludge. Environ. Implic. Recycl. Recycled Prod. 2015;213–258.
https://doi.org/10.1007/978-981-287-643-0_9
6. Oladejo J, Shi K, Luo X, Yang G, Wu T. A review of sludge-to-energy recovery methods. Energies. 2018;12(1)60.
https://doi.org/10.3390/en12010060
7. Wiszniowski J, Surmacz-Gorska J, Robert D, Weber J. The effect of landfill leachate composition on organics and nitrogen removal in an activated sludge system with bentonite additive. J. Environ. Manage. 2007;85(1)59–68.
https://doi.org/10.1016/j.jenvman.2006.08.001
8. Chen C, Wu H. Lightweight bricks manufactured from ground soil, textile sludge, and coal ash. Environ. Technol. 2018;39(11)1359–1367.
https://doi.org/10.1080/09593330.2017.1329353
9. Goyal S, Siddique R, Sharma D, Jain G. Reutilization of textile sludge stabilized with low grade-MgO as a replacement of cement in mortars. Constr. Build. Mater. 2022;338:127643.
https://doi.org/10.1016/j.conbuildmat.2022.127643
10. Tang SH, Zaini MAA. Development of activated carbon pellets using a facile low-cost binder for effective malachite green dye removal. J. Cleaner Prod. 2020;253:119970.
https://doi.org/10.1016/j.jclepro.2020.119970
11. Sohaimi KSA, Ngadi N, Mat H, Inuwa IM, Wong S. Synthesis, characterization and application of textile sludge biochars for oil removal. J. Environ. Chem. Eng. 2017;5(2)1415–1422.
https://doi.org/10.1016/j.jece.2017.02.002
12. Zhang Y, Chen L, Yan B, Zhang F, Shi Y, Guo X. Regeneration of textile sludge into Cu8S5 decorated N, S self-doped interconnected porous carbon as an advanced bifunctional electrocatalyst for overall water splitting. Chem. Eng. J. 2023;451:138497.
https://doi.org/10.1016/j.cej.2022.138497
13. Algin HM, Turgut P. Cotton and limestone powder wastes as brick material. Constr. Build. Mater. 2008;22(6)1074–1080.
https://doi.org/10.1016/j.conbuildmat.2007.03.006
14. Industrial minerals & rocks: commodities, markets, and uses. Choice Rev. Online. 2006;44(03)
https://doi.org/10.5860/choice.44-1280
15. Zhang R, Chen G, Gu C, Hu Y. Development and Performance Test of Fly Ash Non-fired Ceramsite. Fly Ash Compr. Util. 2022;36(03)99–104.
https://doi.org/10.19860/j.cnki.issn1005-8249.2022.03.016
16. Pang C, Zhou Y, Li P, Zhang C. Review on Research Status and Development of Non-Sintered Lightweight Aggregate. Bull. Chin. Ceram. Soc. 2022;41(06)1849–1860.
https://doi.org/10.16552/j.cnki.issn1001-1625.20220410.009
17. Białowiec A, Janczukowicz W, Gusiatin ZM, Thornton A, Rodziewicz J, Zielińskaet M. Recycling potential of air pollution control residue from sewage sludge thermal treatment as artificial lightweight aggregates. Waste Manage. Res. 2014;32(3)221–227.
https://doi.org/10.1177/0734242x14523483
18. Kockal NU, Ozturan T. Characteristics of lightweight fly ash aggregates produced with different binders and heat treatments. Cem. Concr. Compos. 2011;33(1)61–67.
https://doi.org/10.1016/j.cemconcomp.2010.09.007
19. Tay JH, Show KY, Hong SY. Concrete aggregates made from sludge-marine clay mixes. J. Mater. Civ. Eng. 2002;14(5)392–398.
https://doi.org/10.1061/(asce)0899-1561(2002)14:5(392)
20. Hu SH, Hu SC, Fu YP. Resource recycling through artificial lightweight aggregates from sewage sludge and derived ash using boric acid flux to lower co-melting temperature. J. Air Waste Manage. Assoc. 2012;62(2)262–269.
https://doi.org/10.1080/10473289.2011.646051
21. Quina MJ, Garcia R, Simões AS, Quinta-Ferreira RM. Life cycle assessment of lightweight aggregates produced with ashes from municipal solid waste incineration. J. Mater. Cycles Waste Manage. 2020;22:1922–1931.
https://doi.org/10.1007/s10163-020-01079-2
22. Chiou IJ, Wang KS, Chen CH, Lin YT. Lightweight aggregate made from sewage sludge and incinerated ash. Waste Manage. 2006;26(12)1453–1461.
https://doi.org/10.1016/j.wasman.2005.11.024
23. Cui J, Wang L, He Y, Li Y, Wang R, Han J. Hazards of Fly Ash and Its Utilization Status. China Resour. Compr. Uti. 2022;(003)040.
https://doi.org/10.3969/j.issn.1008-9500.2022.03.034
24. Ma C, Bao S, Zhang Y, Luo Y, Gui Y, Ren Y. Preparation of non-sintered sewage sludge based ceramsite by alkali-thermal activation and hydration mechanism. Ceram. Int. 2022;48(21)31606–31613.
https://doi.org/10.1016/j.ceramint.2022.07.082
25. Jia G, Wang Y, Yang F, Ma Z. Preparation of CFB fly ash/sewage sludge ceramsite and the morphological transformation and release properties of sulfur. Constr. Build. Mater. 2023;373:130864.
https://doi.org/10.1016/j.conbuildmat.2023.130864
26. Xu Y, Ma B, Zhang H, Yan X, Shen X, Liu R. Effect of textile sludge on preparation and properties of non-fired ceramide. J. Funct. Mater. 20152(08)8179–8187.
https://doi.org/10.3969/j.issn.1001-9731.2021.08.026
27. Wang LS, Wang YX, Sun W, Wang ChW, Meng XS, editorsPreparation of lightweight and high-strength ceramsite from highly doped coal fly ash. Trans. Nonferrous Met. Soc. China. 1–26. [2023-07-09]. http://kns.cnki.net/kcms/detail/43.1239.tg.20230222.1302.018.html
28. Mi G. Studies on the hydration performance of complex cementitious materials [dissertation]. Tsinghua University; 2016.
29. John E, Matschei T, Stephan D. Nucleation seeding with calcium silicate hydrate–A review. Cem. Concr. Res. 2018;113:74–85.
https://doi.org/10.1016/j.cemconres.2018.07.003
30. Pakbaz MS, Alipour R. Influence of cement addition on the geotechnical properties of an Iranian clay. Appl. Clay Sci. 2012;67:1–4.
https://doi.org/10.1016/j.clay.2012.07.006
31. Fu K, Lu M, Zhu T, Zhang Q. Alkali Activates Fly Ash to Produce Cementitious Materials and It’s Growth Mechanism. Multipurp. Util. Miner. Resour. 2007;(6)4.
https://doi.org/10.3969/j.issn.1000-6532.2007.06.012
32. Li F. Investigation on the production of soluble silicate with ricehusk ash and its activity to the potential hydraulicity of fly ash [dissertation]. Xi'an University of Architecture and Technology; 2016.
33. Luo J, Li J, Lu Z. Effects of Fly Ash and Circulating Fluidized Bed Combustion on Alkaline Activator Sinter-free Lightweight Aggregates (SLWA). China Concr. Cem. Prod. 2017;(2)7.
https://doi.org/10.19761/j.1000-4637.2017.02.020
34. Hu S, Gong X, Li Q, Fan Z. Preparation and Characterization of Non-Sintered Ceramsites From Alkali-Activated Foundry Dust. Int. J. Metalcast. 2023;1–11.
https://doi.org/10.1007/s40962-023-01050-5
35. Fang S, Lam ESS, Li B, Wu B. Effect of alkali contents, moduli and curing time on engineering properties of alkali activated slag. Constr. Build. Mater. 2020;249:118799.
https://doi.org/10.1016/j.conbuildmat.2020.118799
36. Patel H, Pandey S. Evaluation of physical stability and leachability of Portland Pozzolona Cement (PPC) solidified chemical sludge generated from textile wastewater treatment plants. J. Hazard. Mater. 2012;207:56–64.
https://doi.org/10.1016/j.jhazmat.2011.05.028
37. Jansen D, Goetz-Neunhoeffer F, Lothenbach B, Neubauer J. The early hydration of Ordinary Portland Cement (OPC): An approach comparing measured heat flow with calculated heat flow from QXRD. Cem. Concr. Res. 2012;42(1)134–138.
https://doi.org/10.1016/j.cemconres.2011.09.001
38. Xu J. Preparation properties of ionic rare earth tailings-based ceramsite [dissertation]. Jiangxi University of Science and Technology; 2015.
39. Wu B, Xue XH. Analysis of Mechanical Properties of Cement Solidified Zinc Contaminated Soils. Sci. Technol. Eng. 2016;(16)277–282.
https://doi.org/10.3969/j.issn.1671-1815.2016.19.048
40. Huang T, Zhang Y, Chen X. Effect of Gypsum Contents on Cement-Based Self-Leveling Mortar in Ternary Binder system. Bull. Chin. Ceram. Soc. 2019;38(6)1738–1742.
https://doi.org/10.16552/j.cnki.issn1001-1625.2019.06.016
41. Li W. Development heavy metal ions solidification mechanism study of uhpc including municiple solid wasteincineration fly ash [dissertation]. Qingdao University of Technology; 2022.
42. Hao J, Ma H, Feng X, et al. Microstructure and fracture mechanism of low density ceramic proppants. Mater. Lett. 2018;213:92–94.
https://doi.org/10.1016/j.matlet.2017.11.021
43. Ali B. Effect of aqueous sodium silicate on properties of recycled aggregate mortar. SN Appl. Sci. 2019;1(10)1296.
https://doi.org/10.1007/s42452-019-1342-2
44. Petrillo A, Colangelo F, Farina I, Travaglioni M, Salzano C, Cioffi R. Multi-criteria analysis for Life Cycle Assessment and Life Cycle Costing of lightweight artificial aggregates from industrial waste by double-step cold bonding palletization. J. Cleaner Prod. 2022;351:131395.
https://doi.org/10.1016/j.jclepro.2022.131395
45. Colangelo F, Petrillo A, Farina I. Comparative environmental evaluation of recycled aggregates from construction and demolition wastes in Italy. Sci. Total Environ. 2021;798:149250.
https://doi.org/10.1016/j.scitotenv.2021.149250
46. Ferraro A, Ducman V, Colangelo F, Korat L, Spasiano D, Farina I. Production and characterization of lightweight aggregates from municipal solid waste incineration fly-ash through single- and double-step pelletization process. J. Cleaner Prod. 2023;383:135275.
https://doi.org/10.1016/j.jclepro.2022.135275
47. Patil U, Raut SP, Ralegaonkar RV, Madurwaret MV. Sustainable building materials using textile effluent treatment plant sludge: a review. Green Mater. 2021;10(4)154–168.
https://doi.org/10.1680/jgrma.21.00027
48. Ferraro A, Farina I, Race M, Colangelo F, Cioffi R, Fabbricino M. Pre-treatments of MSWI fly-ashes: A comprehensive review to determine optimal conditions for their reuse and/or environmentally sustainable disposal. Rev. Environ. Sci. Bio/Technol. 2019;18:453–471.
https://doi.org/10.1007/s11157-019-09504-1
Fig. 2Different process parameters (a. coal fly ash/cement ratios, b. sodium silicate content, c. curing time) in relation to physical properties of NSLWAs. 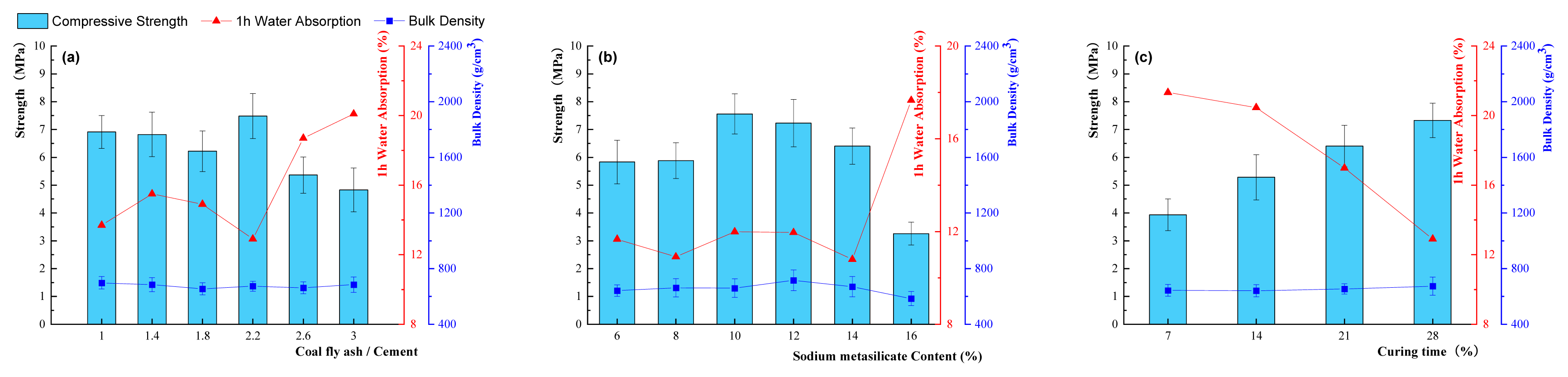
Fig. 3Influences of (a) coal fly ash/cement ratios, (b) sodium silicate content, (c) curing time on X-ray diffraction (XRD) patterns of non-sintered lightweight aggregate. 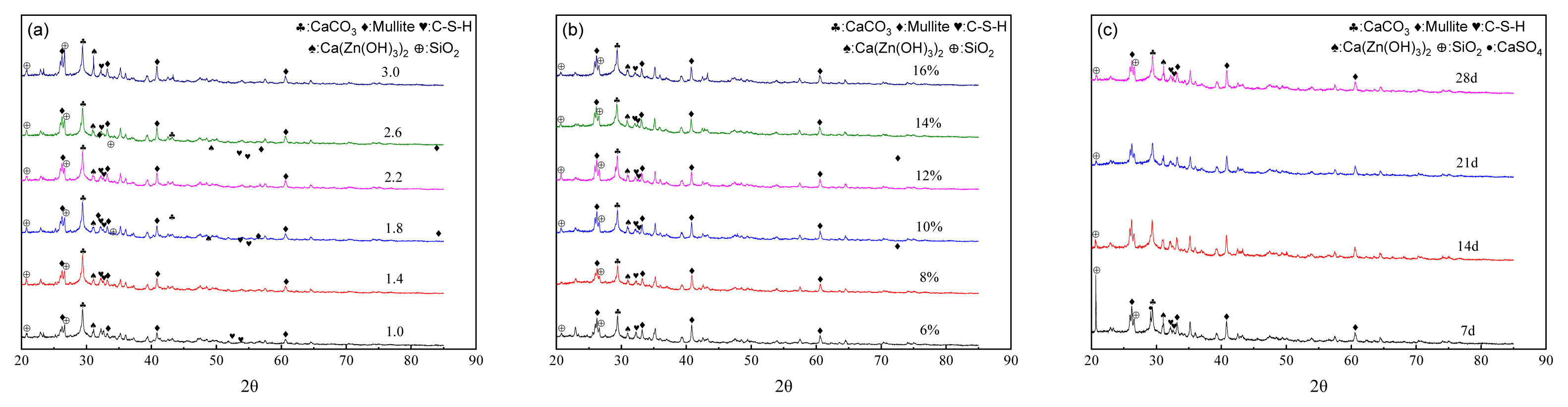
Fig. 4Different coal fly ash/cement ratios on the scanning electron microscope (SEM) of non-sintered lightweight aggregate (a: 1.0; b: 2.2; c: 3.0 1: ×5000; 2: ×10000; 3: ×30000). (Textile sludge addition 20%, sodium silicate addition 5%, binder addition 1%, curing time 2d) 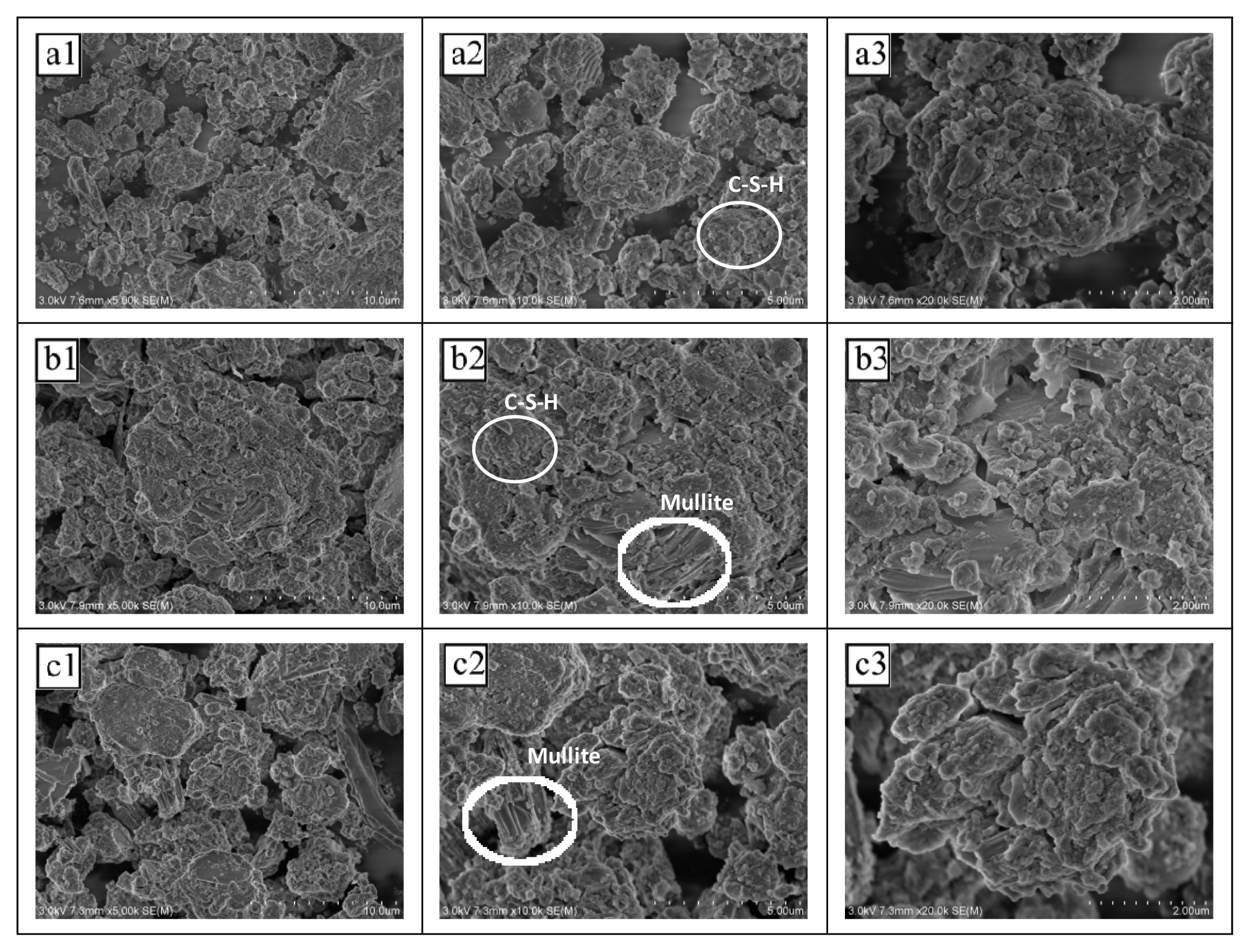
Fig. 5Different sodium silicate content on the scanning electron microscope (SEM) of non-sintered lightweight aggregate (a:6%, b:10%, c:16% 1: ×5000; 2: ×10000; 3: ×30000). (Textile sludge content 20%, fly ash/cement ratio 2.2, binder content 1%, curing time 28 d) 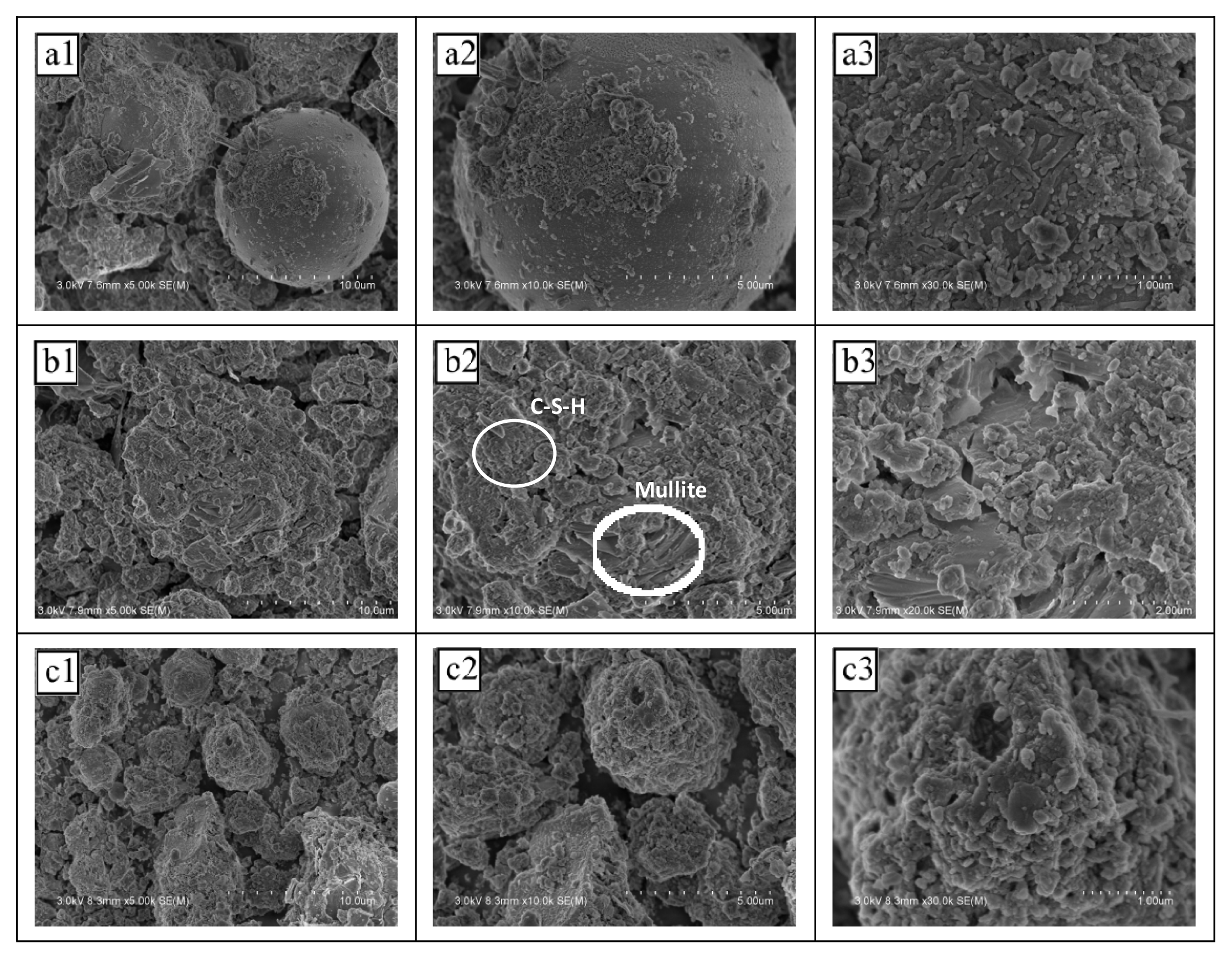
|
|
|||||||||||||||||||||||||||||||||||||