AbstractArsenic pollution is caused by industrial activities of human beings such as the mineral product manufacturing industry and metal industries. Arsenic pollution can cause nervous system and cardiovascular diseases in humans and destroy various water-based ecosystems. So far, different chemical analysis equipment is being utilized to carry out measurements of arsenic. However, conventional chemical analysis techniques are restricted by their needs for large or expensive equipment, which prevents convenient and rapid measurement. Here, we show that convenient measurements of arsenic can be conducted through the utilization of silica that has been modified with methylene blue molecules (MB-Silica). Several studies have reported that the interaction of methylene blue (MB) with arsenic in aqueous solution leads to color variations caused by oxidation-reduction reactions. Taking these alterations in colors into consideration, we have developed a convenient approach for arsenic detection by synthesizing MB-Silica. Furthermore, by varying the MB concentration during synthesis, arsenic ions can be detected with various concentration gradients, validating the feasibility of aqueous arsenic detection. Since the possibility of detecting arsenic using MB-silica, developed in this study, was successfully confirmed, it is anticipated that the MB-silica will be extensively used to detect arsenic in the field.
Graphical Abstract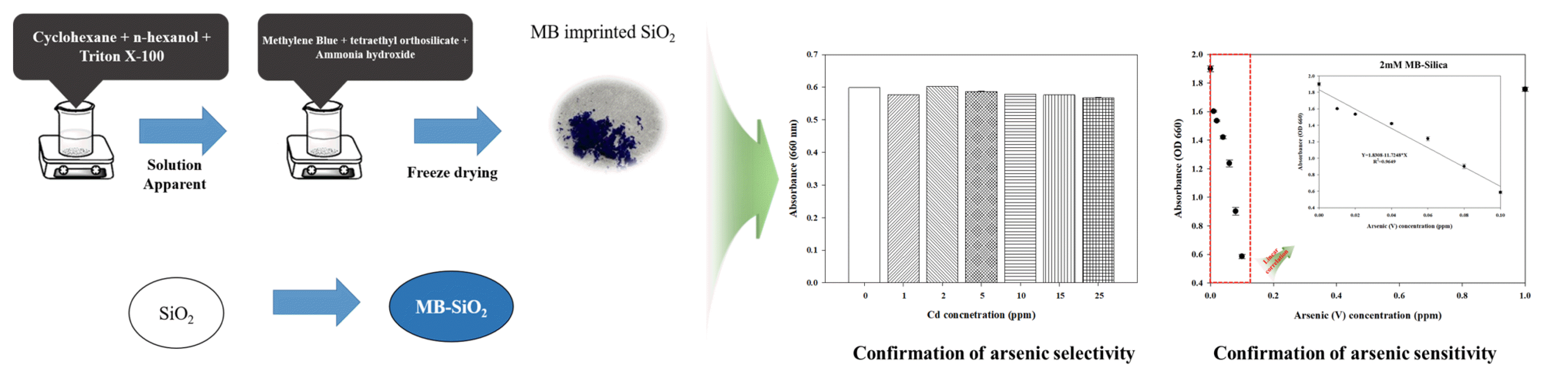
1. IntroductionHeavy metals, such as several groups of metals and metalloids, are widely known to be universal contaminants in various environmental systems including water-based, terrestrial, and marine ecosystems [1]. Furthermore, global industrial exploitation of heavy metals has accelerated an unusual spread of toxic heavy metals into various ecosystems. Upon long-term exposure to these contaminants, the nervous system of humans can undergo acute or chronic damages [2–3]. For instance, long-term exposure to arsenic can cause cardiovascular disease, cancer, and immunological dysfunction in humans [4–6]. Therefore, it is crucial to prevent the contamination of water resources with heavy metals to maintain human health in modern society [7–8]. Therefore, various technologies are being developed to monitor pollutants in order to respond to environmental disasters caused by heavy metals such as arsenic [9–11].
UV-Vis spectroscopy and inductively coupled plasma (ICP) approaches have been extensively employed as established detection methods for determining the heavy metal concentrations in aqueous solutions through physical and chemical reactions. For example, in the Republic of Korea, the ICP-based method is frequently used for monitoring most heavy metals [12]. However, this method is limited to on-site monitoring because it requires expensive and bulky equipment as well as professional personnel for analysis. Another method is based on UV-Vis spectroscopy that measures the absorbance of a sample after its reaction with chemicals. A compact UV-Vis spectroscopy setup has been introduced recently, however the time required for chemical reaction is still too long to be utilized in the field.
To overcome these limitations, many researchers have been developing in-situ analytical methods that can be easily applied in the field, such as biological detection with aptamers, electrochemical detection, optical detection with chemically modified dyes, and chemical-reaction-based detection method [13–20]. In the biological approach, aptamers are utilized to selectively interact with target contaminants, facilitating concentration measurement through signals such as fluorescence and absorption. The electrochemical approach detects contaminant concentrations by monitoring changes in electrical signals when target contaminants are present in solutions or other mediums. Lastly, the optical approach measures contaminant concentrations by utilizing dyes or other chemicals that undergo chemical reactions like oxidation-reduction. In addition to such detection methods, Ion Selective Electrodes (ISEs) have recently been applied for the detection of environmental pollutants [21]. ISEs are ion-selective electrodes equipped with a sensing membrane that selectively responds to the target ions to be detected. When immersed in the sample solution along with reference and counter electrodes, an electromotive force proportional to the concentration of the target ion is generated at the ion-selective membrane. By measuring the potential difference between the reference and indicator electrodes, it becomes possible to determine the ion concentration consistently [22]. ISE, as a non-destructive technique, has the advantage of measuring analytes without their consumption and can provide quick measurements within a short period [23]. However, ISE has been reported to have issues related to selectivity and sensitivity depending on the condition of the electrode [24].
Methylene Blue (MB), a dye widely used in the coloration of cotton fabrics and the manufacturing of industrial products, possesses the characteristic of redox. The redox properties of MB render it be further utilized in various applications such as disease diagnosis [25–26] and arsenic detection [27]. The method for detecting arsenic ion using MB solution offers the advantage of being able to measure arsenic concentration conveniently with a short response time and simplified equipment. In addition, certain studies have yielded results showing that the addition of metal particles as catalysts leads to further reduction in reaction time, and it possesses the advantage of shorter detection time compared to other methods [27]. However, the method for detecting arsenic ion using MB solution has the drawback of increased measurement complexity and limited on-site application, as all reactions need to take place in liquid phase and the reaction reagents must be prepared in advance.
Therefore, in the present study, an arsenic detection method based on MB-Silica has been developed, reducing the need for additional chemicals compared to the MB-Solution based method. The developed MB-silica can be applied for on-site arsenic detection without the input of any additional chemicals. Additionally, a stable reaction was achieved by introducing MB molecules onto silica in the development of MB-Silica, which preserves the color bleaching phenomenon observed in the oxidation-reduction reaction of conventional MB-solution based method. Surface analysis was carried out silica and MB-silica using Fourier transform infrared spectroscopy (FT-IR), X-ray photoelectron spectroscopy (XPS) and Field emission scanning electron microscopy/energy-dispersive X-ray spectroscopy (FE-SEM/EDS) to determine the stable integration of MB onto the silica surface. The detection capability of the developed MB-silica was tested and compared against the conventional MB solution method. To further enhance the detection sensitivity and capability of detecting low concentrations of arsenic in the MB-Silica based arsenic detection method, MB-Silica was synthesized by diversifying the concentration of MB during its preparation and successfully proven its performances over low concentrations of arsenic. The proposed MB-Silica-based arsenic detection method developed herein is expected to be utilized as a field-type detection method to overcome the shortcomings of the existing MB-solution detection method.
2. Materials and Methods2.1. MaterialsMethylene Blue, n-hexanol, Triton X-100, and tetraethyl orthosilicate were purchased from Sigma-Aldrich Korea, Ltd. Cyclohexane (99.5%), acetone (99.5%), and EtOH (99.9%) were bought from DaeJung Chemical & Metals (Siheung-Si, Korea). Ammonia hydroxide was purchased form Supelco, and arsenic standard solution was purchased from Kanto Chemical Ltd. (Tokyo, Japan).
2.2. Synthesis of Methylene Blue (MB) Imprinted SilicaTo synthesize MB-Silica, 75 mL of cyclohexane, 18 mL of n-hexanol, and 18 mL of Triton X-100 were mixed until the mixture became transparent. Thereafter, 4 mL of 20 mM MB solution (in DI water) and 1 mL of tetraethyl orthosilicate solution were slowly injected into the mixture by using a syringe pump (NE-1000-ES, New Era Pump Systems, Inc.). After 20 min of reaction, 3 mL of ammonia hydroxide solution was added into the mixture, followed by stirring for 24 h to complete the chemical reaction. Upon completion of the stirring process, the compounds of silica and MB-silica were separated using an ultrasonic cleaner (SD-200H, Korea) and acetone. The separated components were washed with EtOH and deionized (DI) water at least three times. The silica and MB-silica obtained from this process were dried using a freeze dryer (FDU-2110, EYELA) for 24 h. To further improve the sensitivity and accuracy of arsenic detection, MB-silica was synthesized using different concentrations of MB solution in accordance with target arsenic concentrations. The MB concentrations used for MB-silica synthesis were 2 mM (for arsenic detection of 0–0.1 ppm), 5 mM (for detection of 0.1–1 ppm), and 20 mM (for detection of 1–100 ppm).
2.3. Surface Characterization of MB SilicaTo characterize the surface properties of the developed MB-silica, we utilized FT-IR, XPS, and FE-SEM/EDS. The FT-IR (Cary 630 FT-IR, Agilent Technology, USA) measurement was conducted in the wavelength range of 650–4000 cm−1, and the attenuated total reflection (ATR) method was used to examine the change in functional group during the synthesis of MB-silica.
To investigate the surface modification of silica through the introduction of methylene blue, XPS signal analysis of C1S, O1S and Si2P was performed using an ULVAC PHI XPS instrument equipped with Al source (24.1 W, 15 kV, Al = Ka =1486.6 eV) in order to observe the changes in surface functionalities. The pressure in the analysis chamber was maintained at 7.49 × 10−7 Pa during the XPS measurement. All binding energies were referred to the C1s peak to identify the peak of the main component of the sorbents.
FE-SEM was used to examine the surfaces of silica and MB-silica. The synthesized compounds were coated with platinum for 60 s (4-nm-thick coating) by means of sputtering in vacuum (Auto Fine Coater, JFC-1600, JEOL, Tokyo, Japan), and then analyzed using the FE-SEM (Quanta 250 FEG, FEI, USA). Additionally, the Pt-modified synthesized compounds were subjected to EDS analysis to confirm the distribution of elements on their surfaces.
2.4. Characterization of the Developed MB Silica Method for Arsenic DetectionThe MB-silica synthesized using 20mM MB was suspended in distilled water (DW) at a concentration of 0.005g/mL. Subsequently, the solution was dispensed into a 96-well plate. After the completion of uniform dispensing, the sample to be used for measurement was dried in an oven at 60 °C for one day to evaporate the distilled water. To proceed with absorbance-based arsenic detection, 200 μl of arsenic standard solution of a specific concentration (0, 1, 5, 10, 25, 40, 60, and 100 ppm) was added to the 96-well plate at room temperature. Then, the arsenic reaction was carried out using a shaker operated at 100 rpm for 30 min. After the reaction, absorbance was measured at the wavelength of 660 nm by using a microplate reader (Hidex Multi-Technology Microplate Reader, Finland). The performance of the MB-silica method was compared to the MB solution method. The detection of arsenic using the MB solution was carried out according to the previously reported method [28].
To address the challenge of distinguishing measurements below 1 ppm arsenic concentration in 20mM MB-Silica, the following experiments were conducted. The preparation of MB-Silica involved the utilization of 2 mM and 5 mM MB to extend the range of bleaching during arsenic measurement. The manufacturing process of 2mM and 5mM MB-Silica was carried out following the previously mentioned procedure. The 2mM MB-Silica aimed to measure arsenic concentrations in the range of 0–0.1 ppm, while the 5mM MB-Silica targeted the measurement of arsenic concentrations in the range of 0.1–1 ppm, and both experiments were conducted following the same procedure as mentioned earlier, with three repetitions for each experiment.
2.5. Comparative Analysis of Arsenic Detection Using ICP-OES and MB SilicaThe arsenic detection performance of the proposed MB-silica method was compared with inductively coupled plasma-optical emission spectroscopy (ICP-OES, Agilent, Santa Clara, CA, USA), a conventionally widely used arsenic detection method. For the ICP-OES analysis, a calibration curve was prepared using an arsenic standard solution. To compare the accuracy between MB-Silica and the widely used arsenic detection method, ICP-OES analysis, solutions containing arsenic concentration of 0.03, 0.05, 0.3 and 0.7 ppm were prepared and subjected to comparative analysis. All comparative analysis were conducted with three repetitions. To validate the arsenic-detection capacity of the MB-Silica-based method, the analysis was performed using MB-Silica, which is detectable at the concentration employed in ICP-OES detection and repeated over three times at 660 nm using a Microplate reader (Hidex Multi-Technology Microplate Reader, Finland).
3. Results and Discussion3.1. Surface Characterization of MB SilicaTo determine the functional groups of the synthesized compounds, FT-IR analyses were performed using MB, silica, and MB-silica, which results are presented in Fig. 1. In the FT-IR spectrum of the MB (Fig. 1A), peaks were observed in the wavelength bands of 2816, 1635, 1600, 1390, 1347, and 879 cm−1. The peak at 2816 cm−1 corresponds to the -CH-aromatic bond in MB. The peaks at 1635, 1600, 1390, and 1347 represent the -N+, C=N, C-H, and C-S bonds, respectively [29–32]. In the FT-IR spectrum of silica (Fig. 1B), peaks were observed at 1064, 947, and 800 cm−1. The peak at 1064 cm−1 was attributed to Si-O-Si conjugation [33]. The absorption band at 947 cm−1 was ascribed to Si-OH [34]. The peak at 800 cm−1 was assigned to Si-CH3 stretching [35]. In the FT-IR spectrum of the MB-silica, as illustrated in Fig. 1C, the main peaks representing both MB and silica were all observed. For example, the main peaks of the silica (1064, 947, 800 cm−1) and the MB (1635, 1600, 1390, 1347, and 879 cm−1) were all found in the MB-silica. Comparing the silica spectrum, the MB-silica displays the characteristic peaks of the MB, clearly indicating that the MB was successfully synthesized into the silica. From the FT-IR analysis of the synthesized MB-silicas, it was confirmed that MB was chemically conjugated with the silica surface, and MB functional groups were introduced on the surface. Lee et al. [36] reported the FT-IR spectrum of MB solution, where MB contains many C=N bonds and S functional groups that do not exist in silica. This report was consistent with the FT-IR spectrum of MB obtained herein. From these FT-IR spectra, it can be inferred that MB was conjugated with the silica surface because C=N (1600 cm−1) and C=S (1347 cm−1) bonds were formed.
To further confirm the change in functional groups in detail, XPS was utilized to analyze the C1s, O1s, and Si2p signals of the silica and MB-silica. Table 1 summarizes the types of chemical bonds and the degrees of bonding of each element on the basis of Si2p, C1s, and O1s in the XPS results. The two peaks observed at the binding energies of 101.9 and 102.4 eV in the Si2p XPS spectrum of silica (Fig. 2A) were assigned to the Si-O and Si-O2 bonds, respectively [37]. However, the peak at 102.8 eV (Fig. 2B) was newly assigned to the Si-O3 bond on the synthesized MB-silica [38–39]. Furthermore, the newly introduced peak at 101.1 eV in the Si2P measurement of MB-Silica confirms the presence of methylene blue introduced to the surface of silica [40]. No other peaks were found in the C1s XPS spectrum of the silica (Fig. 2C) while the MB-Silica exhibits several peaks at 282.6, 284.2, and 285.9 eV owing to the chemical reaction of MB with silica (Fig. 2D). The peaks at the binding energies of 282.6 and 284.2 eV were newly assigned to the C=C and C-C bonds, respectively [41–42]. Moreover, the peak at the binding energy of 285.9 eV was newly assigned to the C-S and C-N bonds on MB-Silica [43]. Lastly, in the O1s XPS spectra of silica and MB-silica (Fig. 2E, F), the peak at 531.7 eV (silica, Fig. 2E) shifted to 532.1 eV (MB-silica, Fig. 2F), and it was assigned to the Si-O bond [43]. The XPS C1s and O1s spectral analysis results confirmed that MB and silica were chemically combined, indicating that the properties of MB were introduced to silica.
Finally, the surfaces of the silica and MB-silica were analyzed through FE-SEM/EDS to investigate and compare the distribution of elements (Fig. S1). As shown in Table S1, the proportion of S atoms on the MB-silica surface increased while the proportion of O atoms decreased, compared to those of silica surface. The change on the MB-silica surface resulted from FE-SEM/EDS observed that MB molecules are bonded to the O in silica, resulting in decrease in the proportion of O, while the proportion of S increases with the introduction of MB molecules.
In sum, the FT-IR, XPS, and FE-SEM/EDS results confirmed that MB and Silica were chemically combined, where the chemical properties of MB were successfully introduced on the surface of MB-Silica [43]. From the above characterization results, we hypothesized that the developed MB-silica can be used as a tool for detecting and characterization the arsenic concentration.
3.2. Comparative Analysis of Arsenic Detection in Aqueous Solution by Using MB Solution and MB SilicaMB-solution arsenic detection method has been reported extensively in the literature [16], [24], [44]. Kundu et al. [16] reported a limit of detection (LOD) of 1.3 ppm when using MB solution to detect arsenic. Based on this previous study, we synthesized 20 mM MB-silica. When conducting the arsenic measurement using the MB solutions with a microplate reader, the detection results were like those reported in the literature, but the arsenic concentrations lower than 25 ppm were hardly distinguishable (Fig. 3A). Furthermore, the rapid decrease in absorbance values at arsenic concentrations ranging from 0 to 20 ppm poses challenges for precise measurement, making it difficult to achieve accurate measurement even at concentration below 20 ppm. This issue leads to a significant margin of error in measurements, resulting in decreased accuracy of the measured concentrations. Moreover, in the MB solution measurement, various other chemical solutions, such as sodium dodecyl sulfate and sodium borohydride must be prepared. These chemicals need to be mixed with the MB solution right before the measurement, which requires additional sample preparation steps and limits its on-site applicability. Additionally, significant impact on the accurate measurement of arsenic concentrations can arise when encountering contamination of chemicals or changes in concentration during the preparation process when they need to be mixed with MB solution, as well as issues regarding the chemical reactivity of the prepared chemicals, which can hinder precise arsenic concentration measurement.
To address these issues in MB solution, a MB-silica method was conceived and proposed in this study with the development of MB-Silica, achieved by introducing MB molecules onto the surface of silica, eliminate the need for additional preparation and usage of chemical solutions. The proposed MB-silica method can simplify the measurement process and be applied to on-site arsenic detection since this method requires less preparation and its measurement is easier than the MB solution. Based on the measurement results (Fig. 3B), it was observed that unlike the conventional MB solution method, the R2 value exhibits a high linear relationship, measuring at 0.9836. By establishing a stronger linear relationship, it was possible to overcome the limitation of the original MB-Solution, where the bleaching of the oxidation-reduction reaction ceased beyond a concentration of 10 ppm, ultimately enabling more precise measurement of a wide range of arsenic concentrations. Furthermore, it was observed that the accuracy within the detection range of the measurement was significantly improved, as the errors between the measured values were minimal during repeated measurements.
Taking these results into consideration, the newly developed MB-Silica arsenic detection method can be proposed to improve accuracy and detection resolution compared to the conventional MB solution. Furthermore, it was observed that, like the MB solution method, bleaching of absorbance occurs through arsenic and oxidation-reduction reactions, ultimately confirming the ability to detect arsenic by introducing MB molecules onto the silica surface without compromising their chemical properties.
The reason why the MB-Silica exhibits higher accuracy compared to the methylene blue-based detection can be attributed to the following factors. First, methylene blue molecules vital for detecting arsenic are dispersed within the methylene blue solution, whereas on MB-Silica, methylene blue is densely concentrated on the silica surface. As a result, the sensitivity of the reaction between essential methylene blue and arsenic ions for arsenic detection was increased. Additionally, it can be anticipated that silica with methylene blue imprinted on the surface as a catalyst would enhance the chemical reaction, thereby improving reactivity. According to previous literature [45], silica has been reported as an oxidizing agent, and several reports [46–47] have highlighted that the large surface area and stability of silica can enhance chemical reactions. Furthermore, several previous studies have reported that specific chemical molecules were introduced onto the surface for use as catalysts in chemical reactions such as oxidation-reduction reactions [48–49]. Consequently, by imprinting methylene blue molecules onto the silica surface, the reactivity with arsenic is amplified, and it is expected to boost the stability oxidation-reduction reactions owing to silica’s physical and chemical properties, ultimately resulting in enhanced accuracy in arsenic detection.
3.3. Improving Arsenic Detection Limit through Concentration Based Development of MB SilicaWhen the arsenic ions in aqueous solutions were analyzed by the previously reported MB-solution method (16, 24), it was difficult to distinguish the arsenic concentrations of 1 ppm or lower (Fig. S2). In this low arsenic concentration range, bleaching according to the concentration gradient was not observed, and thus the trend of absorbance measurement could not be confirmed. These results highlighted the difficulty associated with the MB-solution measurement of arsenic at concentrations of 1 ppm or lower. Similarly, the 20 mM MB-Silica exhibited increased accuracy and detection resolution in arsenic detection; however, it faces the challenge of difficult measurement below 1 ppm arsenic concentration. To overcome these issues, experiments were conducted to enhance the detection accuracy and resolution of the detection method for low arsenic concentrations by expanding the range of bleaching caused by oxidation-reduction reactions. In order to expand the range of bleaching, the concentration of MB used in the synthesis of MB-Silica was reduced from 20 mM to 2 mM and 5 mM. After the synthesis of MB-Silica, experiments were conducted with the aim of measuring arsenic concentration below 1ppm, which was a technological hurdle to differentiate in the previous 20mM MB-Silica. The experiment was conducted by following the aforementioned procedure, involving the reaction of the arsenic solution and measurement of wavelength changes at 660 nm.
Various concentrations of arsenic ions (0–10 ppm) were measured using the both 2mM and 5mM MB-Silica, and the detection results are summarized in Fig. 4 and Supplementary Fig. S3. When using the 2mM MB-silica, a linear relationship was derived at concentrations of 0–0.1 ppm, and for the 5mM MB-silica, a linear relationship was observed at concentrations of 0–1 ppm. The measurement of arsenic solution with a concentration range of 0–0.1 ppm using 2mM MB-Silica (Fig.4A) showed a more linear relationship compared to 5mM MB-Silica, resulting in improved detection accuracy and resolution. On the other hand, when measuring arsenic solution with a concentration range of 0.1–1 ppm, the use of 5mM MB-Silica resulted in a more linear relationship compared to 2mM MB-Silica, leading to improved detection accuracy and resolution in the measurement (Fig.4B).
In summary, based on the combined results, it was confirmed that the arsenic solution with concentrations of 1 ppm or lower, which could not be measured using 20 mM MB-Silica, were successfully measured using 2mM and 5mM MB-Silica. In the measurement of arsenic concentrations less than 1 ppm, 2mM MB-Silica exhibited a linear relationship within the range of 0–0.1 ppm, while 5mM MB-Silica exhibited a linear relationship within the range of 0.1–1 ppm, both allowing for clear differentiation of the tested arsenic concentrations. Therefore, it has been confirmed that MB-Silica can be measured differently based on the arsenic concentration levels, with a linear relationship observed at concentrations ranging from 0 to 0.1 ppm for 2 mM MB-Silica, 0 to 1 ppm for 5 mM MB-Silica, and 0 to 100 ppm for 20 mM MB-Silica. The MB-Silica method is expected to improve the accuracy and sensitivity of the arsenic detection by applying different MB-silica depending on the arsenic concentrations to be measured.
3.4. Verification of Arsenic Detection Reactivity and interference factors of MB-silicaTo assess the potential applicability of MB-Silica in environmental conditions, experiments were conducted to evaluate its arsenic detection reactivity and interference factors. Initially, arsenic is present in environmental conditions (both arsenic (III) and arsenic (V) ions), to verify their reactivity in arsenic detection. Given that the arsenic standard solution used in aforementioned experiments consists solely of arsenic (III), we prepared an arsenic (V) solution to confirm the reactivity of MB-Silica toward arsenic (V) ions. The preparation of the arsenic (V) solution was conducted following the established method outlined in previous research [50].
Again, the prepared 2 Mm MB-Silica was used for assessing the potential for detecting various ranges of arsenic (V) ions. The measurement results (Fig. 5A) revealed that, when investigating the reactivity of As (V) using 2 mM MB-Silica, a linear relationship was affirmed within the 0 to 0.1 ppm range, similar to As (III). Furthermore, within this range, a linear relationship equation was established as Y=1.8308−11.7248*X, with an R2 value of 0.9649, indicating a higher linearity compared to As (III) measurements. Following the same procedure as previously detailed, we conducted an evaluation of the reactivity of As (V) within the extended range of 0 to 1 ppm with the use of 5 mM MB-Silica, and in addition, we also affirmed the reactivity of arsenic at a further higher concentration, specifically 100 ppm. The analysis results (Fig. 5B) revealed that when measuring As (V) using 5 mM MB-Silica, a linear relationship within the concentration range of 0 to 1 ppm, similar to that of As (III), was observed. Moreover, within the linear range, we verified the linear relationship equation (Y=2.5815−0.9198*X) and obtained an R2 value of 0.9178. signifying a superior linearity compared to As (III) measurements. As the last step, measurements were carried out using 20 mM MB-Silica within further extended range of 0 to 100 ppm (Fig. 5C). Notably, unlike those in As (III), we were able to establish linear relationship within the range of 0 to 40 ppm, with a linear equation of Y=2.6141−0.0209*X and R2 value of 0.9278. This confirms that 20 mM MB-Silica is more efficient for As (III) detection but also capable of detecting As (V) as well. Upon comprehensive consideration of the results, it can be affirmed that MB-Silica enables the concurrent measurement of arsenic ions in their coexisting forms, As (III) and As (V), within aqueous solution.
Through this approach, we affirmed the reactivity of arsenic detection and, in addition, tested the responsiveness of MB-Silica towards various metal ions that might pose interference when quantifying arsenic levels in environmental sample and similar conditions. To verify the reactivity towards interfering factors, mercury (Hg) and cadmium (Cd) were selected from various metal ions, which can cause significant harm to aquatic ecosystems and the environment. For the purpose of assessing the interference caused by metal ions, we employed 20 mM MB-Silica to validate its reactivity, and our preliminary analysis (Fig. 6A) of its response to Hg indicated the absence of a linear relationship and reactivity when exposed to Hg concentrations ranging from 0 to 25 ppm. Moreover, we verified the reactivity of Cd by employing 20 mM MB-Silica, and resembling Hg, we noticed that Cd did not exhibit a linear correlation or reactivity.
While there are differences in reactivity depending on the oxidation-reduction reaction extent of MB-Silica, it has been confirmed that reactivity exists for both As (III) and As (V) in aqueous solution. Furthermore, it is likely that the difference in reactivity would be originated from the distinct chemical properties inherent to As (III) and As (V) [51]. In the case of As (III), it possesses the characteristic of being more readily reducible in its oxidized state, whereas As (V) has the feature of having a high oxidation rate, making it more challenging to reduce. Hence, at lower concentration (0 to 0.1 ppm, 0 to 1 ppm) of both As (III) and As (V), linear relationships could be established within the same range, indicating reactively for both ion forms, but at higher concentration range (0 to 100 ppm), differences in reactivity arise, resulting in slightly altered linear relationship, possibly due to chemical reaction disparities.
Furthermore, although the potential for interference by all metal ions has not been verified, it has been confirmed that reactivity and linear relationship do not exist when reacting with representative environmental pollutant such as Hg and Cd, indicating that they do not act as interfering factors. Thus, reactivity concerning the arsenic ion forms and metal ions that can potentially acts as interfering factors has been verified, confirming the feasibility of employing MB-Silica for arsenic detection. However, for practical application in environmental samples, it is necessary to verify the potential interference not only from metal ions but also from various organic contaminants, solid materials [52–53]. Therefore, in future research, additional verification of reactivity towards various interfering factors and optimization for detection performance improvement will be conducted to facilitate the detection of total arsenic from environmental sample using MB-Silica.
3.5. Comparative Analysis of Arsenic Concentration in MB Silica Using Conventional Arsenic Detection Method (ICP-OES)The accuracy of the measurement values was verified by comparing the measured arsenic concentration obtained using MB-Silica with those obtained through ICP-OES method, which is widely used for the measurement of heavy metals and other water contaminants, using prepared samples. As an application strategy of MB-silica for arsenic detection, different MB concentrations were set, and the arsenic detection concentration ranges were derived for each synthesized material and applied. In the arsenic concentration range of 0–0.1 ppm, for the arsenic concentration of 0.03 ppm, the ICP-OES method yielded an arsenic concentration 0.0301 ± 0.0018. For the same concentration, when the measurement was performed using MB-silica prepared with 2 mM MB, the measured arsenic concentration was 0.0300 ± 0.0017. At the arsenic concentration of 0.05 ppm, the results of the ICP-OES- and MB-silica-based methods were 0.0529 ± 0.0006 and 0.0519 ± 0.002, respectively (Fig. 7A). At arsenic concentrations of 0–1 ppm, measurement was performed using MB-silica synthesized using 5mM MB, and the result was compared to those obtained using the ICP-OES-based method. When the arsenic concentration of the measured solution was 0.3 ppm, the ICP-OES- and MB-silica-based method yielded concentration values of 0.3748 ± 0.0013 and 0.3252 ± 0.027 ppm, respectively. In addition, for the 0.7 ppm arsenic solution, the ICP-OES- and MB-silica-based method yielded concentration values of 0.7365 ± 0.0023 and 0.7025 ± 0.0223, respectively (Fig. 7B). These results confirmed that the concentration values obtained using the proposed MB-silica-based method were closer to the standard solution than those obtained using the ICP-OES-based method. Thus, the MB-silica-based method can be considered more accurate than the ICP-OES-based method.
As described above, when arsenic was detected using the MB-silica, the detection capability similar to the conventional method, ICP-OES was achieved. In addition, a higher arsenic detection sensitivity was achieved using the proposed method compared to the existing MB-solution method. In conclusion, the possibility of arsenic detection was successfully verified through the use of the solidified MB-silica arsenic detector. Moreover, the possibility of detecting arsenic in a more precise range was confirmed by varying the MB concentration used to synthesize MB-silica.
4. ConclusionsIn this study, we developed the MB-silica capable of detecting the well-known pollutant arsenic in aqueous solutions. Utilizing the principle of arsenic detection through oxidation-reduction reaction, we developed MB-Silica by immobilizing MB molecules on the surface of Silica. When detecting arsenic using MB-Silica instead of the MB solution method, an increase in detection accuracy and resolution was observed. The synthesized MB-Silica can detect arsenic more easily and accurately compared to the existing MB solution method. For detecting arsenic at various concentrations, MB-silica samples were developed using various MB concentrations, and specific arsenic detection ranges were set for each MB-silica sample. When measuring arsenic at concentrations of 0–0.1 ppm and 0–1 ppm, MB-silica synthesized using of 2 mM and 5 mM MB solutions, respectively, provided higher detection sensitivity compared to the conventional ICP-OES-based detection method. The proposed MB-silica-based detection method is expected to be applied for facile arsenic detection in the field.
AcknowledgmentThis research was supported by the Korea Basic Science Institute (KBSI) (Project No. C070300). This work was supported by the Korea Environment Industry & Technology Institute (KEITI) through a project to develop eco-friendly new materials and processing technology derived from wildlife and Aquatic Ecosystem Conservation Research Program, funded by the Korea Ministry of Environment (MOE) (2021003280004 and 2022003040001).
NotesAuthor Contributions Y.H.P.: Conceptualization, Investigation, Formal analysis, Writing–Original draft, Writing–Review & Editing, Visualization. H.S.K.: Writing –Original draft, Writing – Review & Editing, Supervision, Project administration. J.S.C.: Conceptualization, Investigation, Formal analysis. J.P.: Formal analysis, Investigation. J.S.C.: Conceptualization, Investigation, Formal analysis. Y.-E.C.: Writing – Original draft, Writing – Review & Editing, Supervision, Project administration, Funding acquisition. Reference1. Duffus JH. “Heavy metals” a meaningless term? (IUPAC Technical Report). Pure Appl. Chem. 2002;74(5)793–807.
https://doi.org/10.1351/pac200274050793
2. Godt J, Scheidig F, Grosse-Siestrup C, et al. The toxicity of cadmium and resulting hazards for human health. J. Occup. Med. Toxicol. 2006;1(1)1–6.
https://doi.org/10.1186/1745-6673-1-22
3. Järup L, Åkesson A. Current status of cadmium as an environmental health problem. Toxicol. Appl. Pharmacol. 2009;238:201–208.
https://doi.org/10.1016/j.taap.2009.04.020
4. Chen Y, Wu F, Liu M, et al. A prospective study of arsenic exposure, arsenic methylation capacity, and risk of cardiovascular disease in Bangladesh. Environ. Health Perspect. 2013;121:832–838.
https://doi.org/10.1289/ehp.1205797
5. Smith AH, Hopenhayn-Rich C, Bates MN, et al. Cancer risks from arsenic in drinking water. Environ. Health Perspect. 1992;97:259–267.
https://doi.org/10.1289/ehp.9297259
6. Yu S, Liao WT, Lee CH, Chai CY, Yu CL, Yu HS. Immunological dysfunction in chronic arsenic exposure: From subclinical condition to skin cancer. J. Dermatol. 2018;45:1271–1277.
https://doi.org/10.1111/1346-8138.14620
7. Soleimani H, Abbasnia A, Yousefi M, Mohammadi AA, Khorasgani FC. Data on assessment of groundwater quality for drinking and irrigation in rural area Sarpol-e Zahab city, Kermanshah province, Iran. Data Brief. 2018;17:148–156.
https://doi.org/10.1016/j.dib.2017.12.061
8. Zhang Y, Chu C, Li T, Xu S, Liu L, Ju M. A water quality management strategy for regionally protected water through health risk assessment and spatial distribution of heavy metal pollution in 3 marine reserves. Sci. Total Environ. 2017;599:721–731.
https://doi.org/10.1016/j.scitotenv.2017.04.232
9. Bundschuh J, Armienta MA, Simfors NM, Alam MA, López DL, Quezada VD. Arsenic in Latin America: New findings on source, mobilization and mobility in human environments in 20 countries based on decadal research 2010–2020. Crit. Rev. Environ. Sci. Technol. 2021;51(16)1727–1865.
https://doi.org/10.1080/10643389.2020.1770527
10. Dasgupta N, Ranjan S, Ramalingam C. Applications of nanotechnology in agriculture and water quality management. Environ. Chem. Lett. 2017;15:591–605.
https://doi.org/10.1007/s10311-017-0648-9
11. Lee MS, Lee JH, An YJ, et al. Development of water quality criteria for arsenic to protect aquatic life based on species sensitivity distribution. Ecotox. Environ. Safe. 2020;189:109933.
https://doi.org/10.1016/j.ecoenv.2019.109933
12. Chang SW, Baek SH, Yang HC, et al. Heavy metal analysis of ortho MTA and ProRoot MTA. J. Endod. 2011;37(12)1673–1676.
https://doi.org/10.1016/j.joen.2011.08.020
13. Forzani ES, Foley K, Westerhoff P, Tao N. Detection of arsenic in groundwater using a surface plasmon resonance sensor. Sens. Actuator B-Chem. 2007;123(1)82–88.
https://doi.org/10.1016/j.snb.2006.07.033
14. Gu H, Yang Y, Chen F, et al. Electrochemical detection of arsenic contamination based on hybridization chain reaction and RecJf exonuclease-mediated amplification. Chem. Eng. J. 2018;353:305–310.
https://doi.org/10.1016/j.cej.2018.07.137
15. Kumar JB, Retna RC. Gold nanoelectrode ensembles for the simultaneous electrochemical detection of ultratrace arsenic, mercury, and copper. Anal. Chem. 2008;80(13)4836–4844.
https://doi.org/10.1021/ac071064w
16. Kundu S, Ghosh SK, Mandal M, Pal T, Pal A. Spectrophotometric determination of arsenic via arsine generation and in-situ colour bleaching of methylene blue (MB) in micellar medium. Talanta. 2002;58(5)935–942.
https://doi.org/10.1016/S0039-9140(02)00434-4
17. Pan J, Li Q, Zhou D, Chen J. Ultrasensitive aptamer biosensor for arsenic (III) detection based on label-free triple-helix molecular switch and fluorescence sensing platform. Talanta. 2018;189:370–376.
https://doi.org/10.1016/j.talanta.2018.07.024
18. Shrivas K, Shankar R, Dewamgam K. Gold nanoparticles as a localized surface plasmon resonance based chemical sensor for on-site colorimetric detection of arsenic in water samples. Sens. Actuator B-Chem. 2015;220:1376–1383.
https://doi.org/10.1016/j.snb.2015.07.058
19. Song L, Mao K, Zhou X, Hu J. A novel biosensor based on Au@Ag core–shell nanoparticles for SERS detection of arsenic (III). Talanta. 2016;146:285–290.
https://doi.org/10.1016/j.talanta.2015.08.052
20. Uda MNA, Hashim U, Gopinath SCB, et al. Label-free aptamer based biosensor for heavy metal detection. In : AIP Conference Proceedings; AIP Publishing. 2020.
https://doi.org/10.1063/5.0022834
21. Rousseau CR, Bühlmann P. Calibration-free potentiometric sensing with solid-contact ion-selective electrodes. Trac-Trends Anal. Chem. 2021;140:116277.
https://doi.org/10.1016/j.trac.2021.116277
22. Shao Y, Ying Y, Ping J. Recent advances in solid-contact ion-selective electrodes: Functional materials, transduction mechanisms, and development trends. Chem. Soc. Rev. 2020;49(13)4405–4465.
https://doi.org/10.1039/C9CS00587K
23. Gan AW, Seah GEKK, Kwek LH, Goh SS. Ion transfer voltammetry of amino acids with an all-solid-state ion-selective electrode for non-destructive phenylalanine sensing. Electroanalysis. 2023;e202200501.
https://doi.org/10.1002/elan.202200501
24. Richa A, Fizir M, Touil S. Advanced monitoring of hydroponic solutions using ion-selective electrodes and the internet of things: a review. Environ. Chem. Lett. 2021;19(4)3445–3463.
https://doi.org/10.1007/s10311-021-01233-8
25. Andresen M, Dougnac A, Diaz O, et al. Use of methylene blue in patients with refractory septic shock: impact on hemodynamics and gas exchange. J. Crit. Care. 1998;13(4)164–168.
https://doi.org/10.1016/S0883-9441(98)90001-6
26. Van ML, Handgraaf HJM, Diana M, et al. A practical guide for the use of indocyanine green and methylene blue in fluorescence-guided abdominal surgery. J. Surg. Oncol. 2018;118(2)283–300.
https://doi.org/10.1002/jso.25105
27. Kundu S, Ghosh SK, Mandal M, Pal T. Silver and gold nano-cluster catalyzed reduction of methylene blue by arsine in micellar medium. Bull. Mat. Sci. 2002;25:577–579.
https://doi.org/10.1007/BF02710555
28. Ghosh SK, Kundu S, Mandal M, Pal T. Silver and gold nano-cluster catalyzed reduction of methylene blue by arsine in a micellar medium. Langmuir. 2002;18(23)8756–8760.
https://doi.org/10.1021/la0201974
29. Gieroba B, Krysa M, Wojtowicz K, et al. The FT-IR and Raman spectroscopies as tools for biofilm characterization created by cariogenic streptococci. Int. J. Mol. Sci. 2020;21(11)3811.
https://doi.org/10.3390/ijms21113811
30. Firouzjaei MD, Afkhami FA, Esfahani MR, Turner CH, Nejati S. Experimental and molecular dynamics study on dye removal from water by a graphene oxide-copper-metal organic framework nanocomposite. J. Water Process. Eng. 2020;34:101180.
https://doi.org/10.1016/j.jwpe.2020.101180
31. Mezzetta A, Pomelli CS, D’Andrea F, Guazzelli L. 1-Octyl-3-(3-(1-methylpyrrolidiniumyl) propyl) imidazolium Bis (trifluoromethane) sulfonimide. Molbank. 2019;2019(4)M1089.
https://doi.org/10.3390/M1089
32. Tahmasbi B, Ghorbani-Choghamarani A. Magnetic MCM-41 nanoparticles as a support for the immobilization of a palladium organometallic catalyst and its application in C–C coupling reactions. New J. Chem. 2019;43(36)14485–14501.
https://doi.org/10.1039/C9NJ02727K
33. Portaccio M, Ventura BD, Manolova N, et al. FT-IR microscopy characterization of sol–gel layers prior and after glucose oxidase immobilization for biosensing applications. J. Sol-Gel Sci. Technol. 2011;57:204–211.
https://doi.org/10.1007/s10971-010-2343-1
34. Hirashita N, Kobayakawa M, Arimatsu A, Yokoyama F, Ajioka T. Thermal Desorption Studies of Phosphorus-Doped Spin-on-Glass Films. J. Electrochem. Soc. 1992;139(3)794.
https://doi.org/10.1149/1.2069304
35. Kopani M, Mikula M, Kosnac D, Gregus J, Pincik E. Morphology and FT IR spectra of porous silicon. J. Electr. Eng. 2017;68(7)53–57.
https://doi.org/10.1515/jee-2017-0056
36. Lee SH, Lee KS, Sorcar S, Razzaq A, Grimes CA, In SI. Wastewater treatment and electricity generation from a sunlight-powered single chamber microbial fuel cell. J. Photochem. Photobiol. A-Chem. 2018;358:432–440.
https://doi.org/10.1016/j.jphotochem.2017.10.030
37. Cao Z, Duan A, Zhao Z, et al. A simple two-step method to synthesize the well-ordered mesoporous composite Ti-FDU-12 and its application in the hydrodesulfurization of DBT and 4, 6-DMDBT. J. Mater. Chem. A. 2014;2(46)19738–19749.
https://doi.org/10.1039/C4TA03691C
38. Gao Y, Liang X, Bao W, Wu C, Li S. Failure analysis of a field brittle fracture composite insulator: characterisation by X-ray photoelectron spectroscopy analysis. High Volt. 2019;4(2)89–96.
https://doi.org/10.1049/hve.2018.5084
39. Meškinis Š, Vasiliauskas A, Andrulevičius M, Peckus D, Tamulevičius S, Viskontas K. Diamond like carbon films containing Si: structure and nonlinear optical properties. Materials. 2020;13(4)1003.
https://doi.org/10.3390/ma13041003
36. Ameen S, Akhtar MS, Kim YS, Shin HS. Nanocomposites of poly (1-naphthylamine)/SiO2 and poly (1-naphthylamine)/TiO2: Comparative photocatalytic activity evaluation towards methylene blue dye. Appl. Catal. B-Environ. 2011;103(1–2)136–142.
https://doi.org/10.1016/j.apcatb.2011.01.019
40. Tantawy HR, Kengne BAF, Mcllroy DN, et al. X-ray photoelectron spectroscopy analysis for the chemical impact of solvent addition rate on electromagnetic shielding effectiveness of HCl-doped polyaniline nanopowders. J. Appl. Phys. 2015;118(17)175501.
https://doi.org/10.1063/1.4934851
41. Zhang Q, Bao N, Wang X, et al. Advanced fabrication of chemically bonded graphene/TiO2 continuous fibers with enhanced broadband photocatalytic properties and involved mechanisms exploration. Sci Rep. 2016;6(1)1–15.
https://doi.org/10.1038/
42. Liu Z, Wang Y. Characterization of triazinedithiolsilane polymeric nanofilm fabricated by galvanostatic technique on copper surface. Int. J. Electrochem. Sci. 2016;11:1434–1455.
https://doi.org/10.1016/S1452-3981(23)15932-3
43. Wang W, Yang L, Sun H, Yang Z, Du Q, Li C. Improved photodynamic efficiency for methylene blue from silica-methylene blue@tannic acid-Fe (III) ions complexes in aqueous solutions. Adv. Powder Technol. 2018;29(2)341–348.
https://doi.org/10.1016/j.apt.2017.11.021
44. Kundu S, Ghosh SK, Nah S, et al. Ion-associate of arsenic (V)-salicylic acid chelate with methylene blue in toluene: application for arsenic quantification. Indian J. Chem. Sect A-Inorg. Bio-Inorg. Phys. Theor. Anal. Chem. 2005;44A:2030–2033.
45. Wong C, McCabe RW. Effects of oxidation/reduction treatments on the morphology of silica-supported rhodium catalysts. J. Catal. 1987;107(2)535–547. https://doi.org/10.1016/0021-9517(87)90317–4.
46. Yu X, Williams CT. Recent advances in the applications of mesoporous silica in heterogeneous catalysis. Catal. Sci. Technol. 2022;12(19)5765–5794. https://doi.org/10.1039/D2CY00001F
47. Han SJ, Lee SW, Kim HW, Kim SK, Kim YT. Nonoxidative direct conversion of methane on silica-based iron catalysts: effect of catalytic surface. ACS Catal. 2019;9(9)7984–7997.
https://doi.org/10.1021/acscatal.9b01643
48. Mohammadi M, Ghorbani-Choghamarani A. Complexation of guanidino containing l-arginine with nickel on silica-modified Hercynite MNPs: a novel catalyst for the Hantzsch synthesis of polyhydroquinolines and 2, 3-Dihydroquinazolin-4 (1H)-ones. Res. Chem. Intermed. 2022;48(6)2641–2663.
https://doi.org/10.1007/s11164-022-04706-9
49. Taheri-Ledari R, Qazi FS, Saeidirad M, Maleki A. A diselenobis-functionalized magnetic catalyst based on iron oxide/silica nanoparticles suggested for amidation reactions. Sci Rep. 2022;12(1)14865.
https://doi.org/10.1038/s41598-022-19030-w
50. Foroutan R, Mohammadi R, Adeleye AS, et al. Efficient arsenic (V) removal from contaminated water using natural clay and clay composite adsorbents. Environ. Sci. Pollut. Res. 2019;26:29748–29762.
https://doi.org/10.1007/s11356-019-06070-5
51. Páez-Espino D, Tamames J, Lorenzo Vd, Cánovas D. Microbial responses to environmental arsenic. Biometals. 2009;22:117–130.
https://doi.org/10.1007/s10534-008-9195-y
52. Jiao L, Zhong N, Zhao X, et al. Recent advances in fiber-optic evanescent wave sensors for monitoring organic and inorganic pollutants in water. Trac-Trends Anal. Chem. 2020;127:115892.
https://doi.org/10.1016/j.trac.2020.115892
53. Salman MS, Znad H, Hasan MN, Hasan MM. Optimization of innovative composite sensor for Pb (II) detection and capturing from water samples. Microchem J. 2021;160:105765.
https://doi.org/10.1016/j.microc.2020.105765
Fig. 1FT-IR results for identifying functional groups according to surface characteristics of (A) Methylene Blue (MB), (B) silica, and (C) MB-Silica 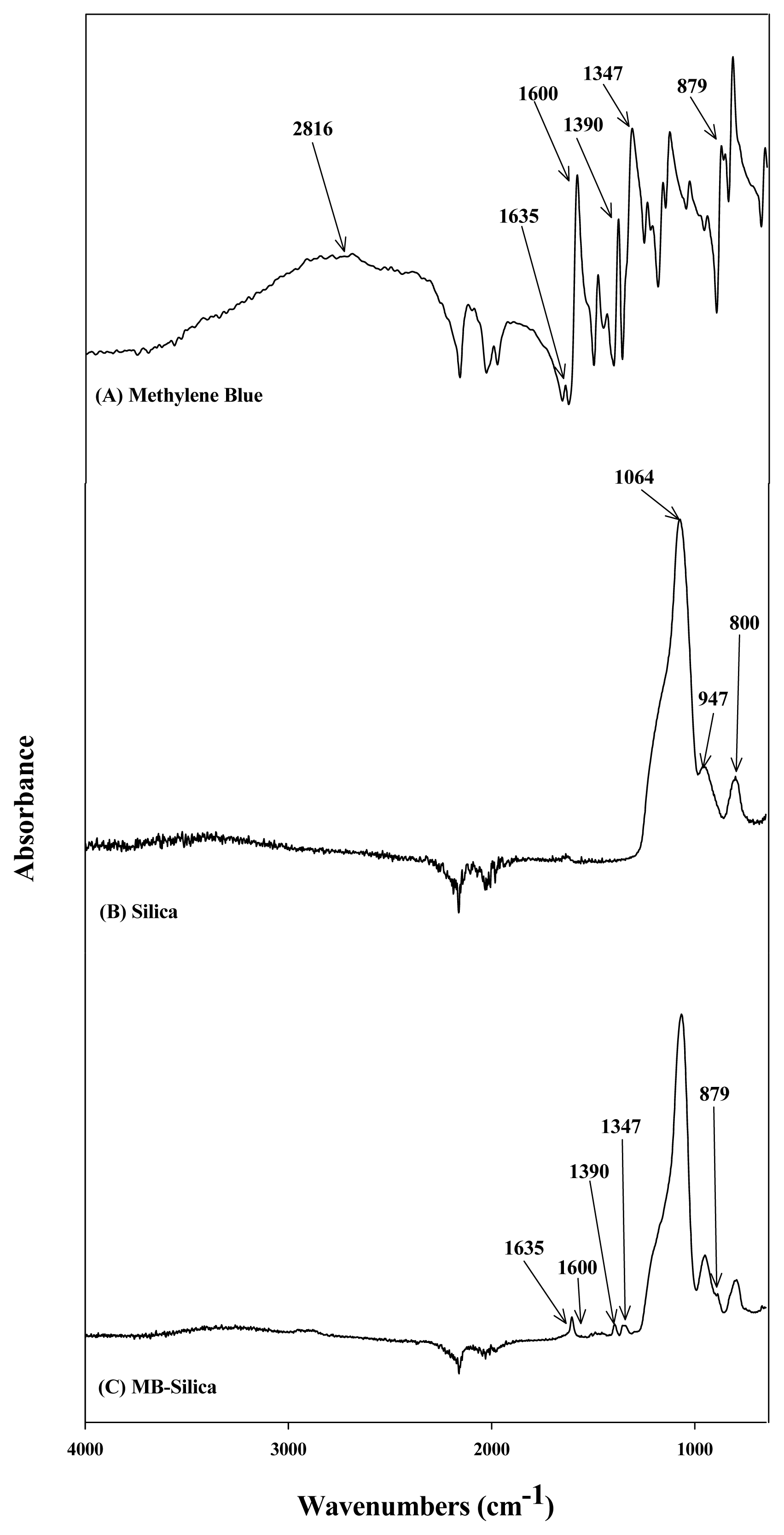
Fig. 2Si2p, C1s, and O1s XPS spectra of the chemical compounds: (A) Si2p spectrum of silica, (B) Si2p spectrum of MB-silica, (C) C1s spectrum of silica, (D) C1s spectrum of MB-silica, (E) O1s spectrum of silica, and (F) O1S spectrum of MB-silica. 
Fig. 3Comparative analysis of arsenic detection in aqueous solutions by using (A) MB solution and (B) MB-silica. 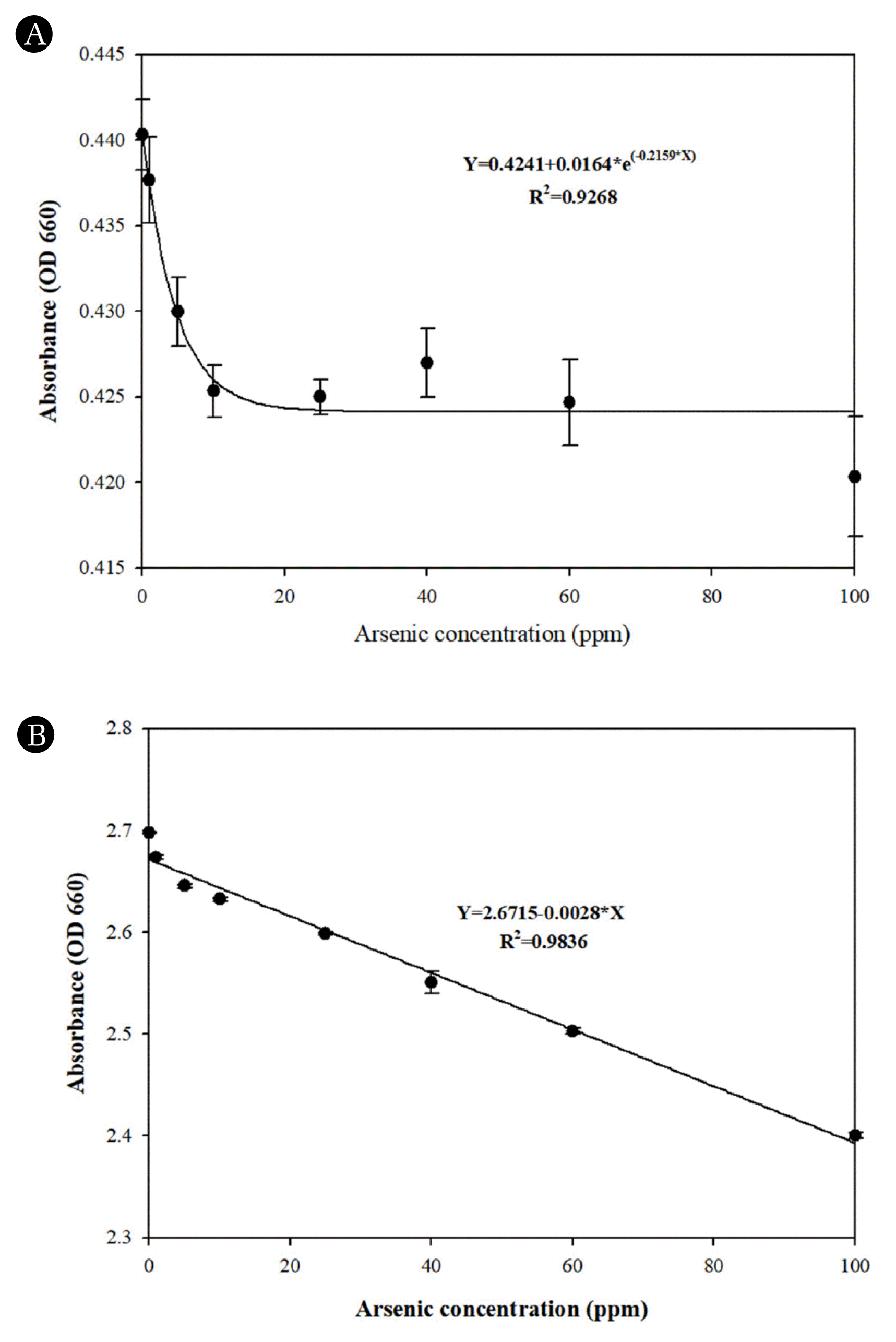
Fig. 4Absorbance profiling of MB-silica (at 660nm) vs. concentration of arsenic standard solution (in ppm) conditions: [MB] = 2 mM and 5 mM for (A) and (B), respectively 
Fig. 5Verification of the reactivity of As (V) using MB-Silica: (A) 2 mM MB-Silica, (B) 5 mM MB-Silica, (C) 20 mM MB-Silica 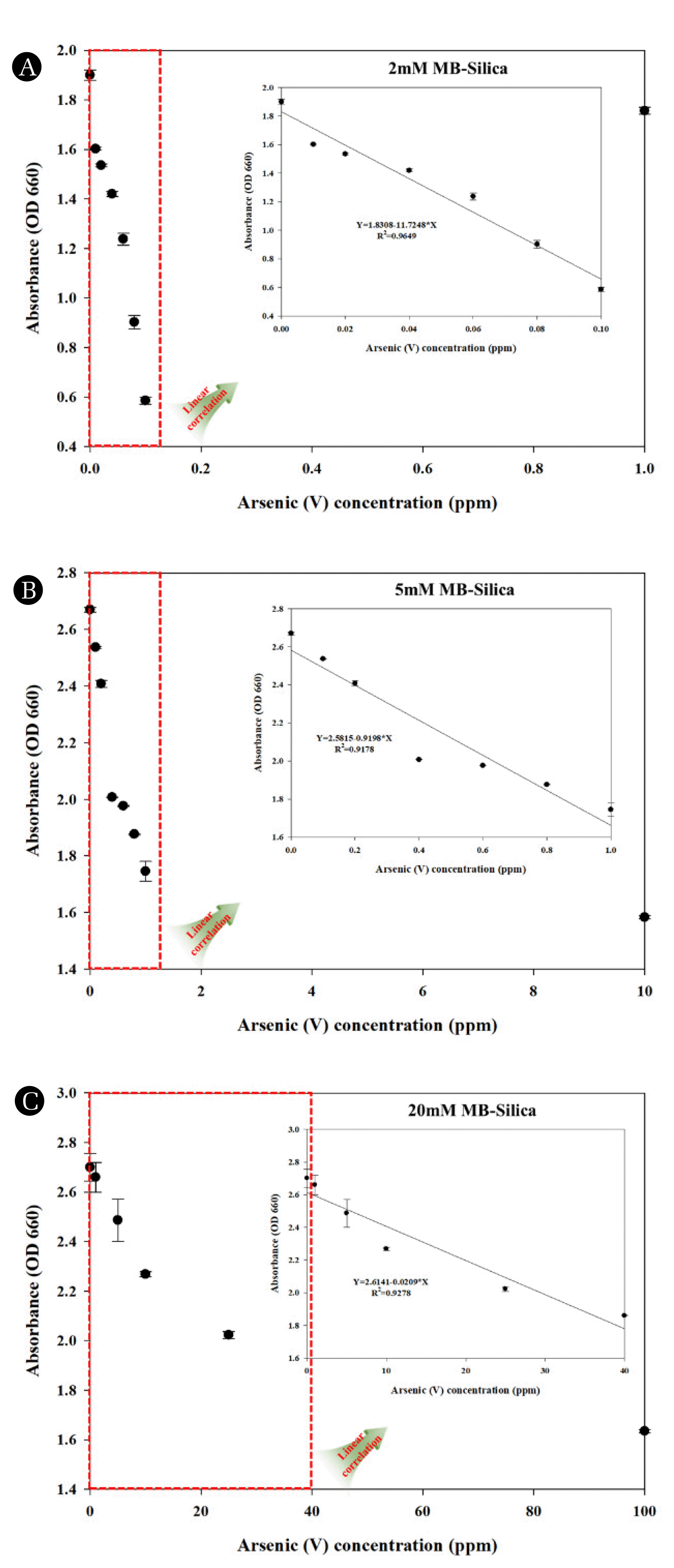
Fig. 6Verification of MB-Silica reactivity of metal ions as an interference factor in arsenic detection: (A) mercury (Hg), (B) cadmium (Cd). 
Fig. 7Comparative analysis of arsenic detection using ICP-OES and MB-silica: (A) 0 0.1 ppm arsenic detection using MB-silica. (B) 0 1 ppm arsenic detection using MB-silica. 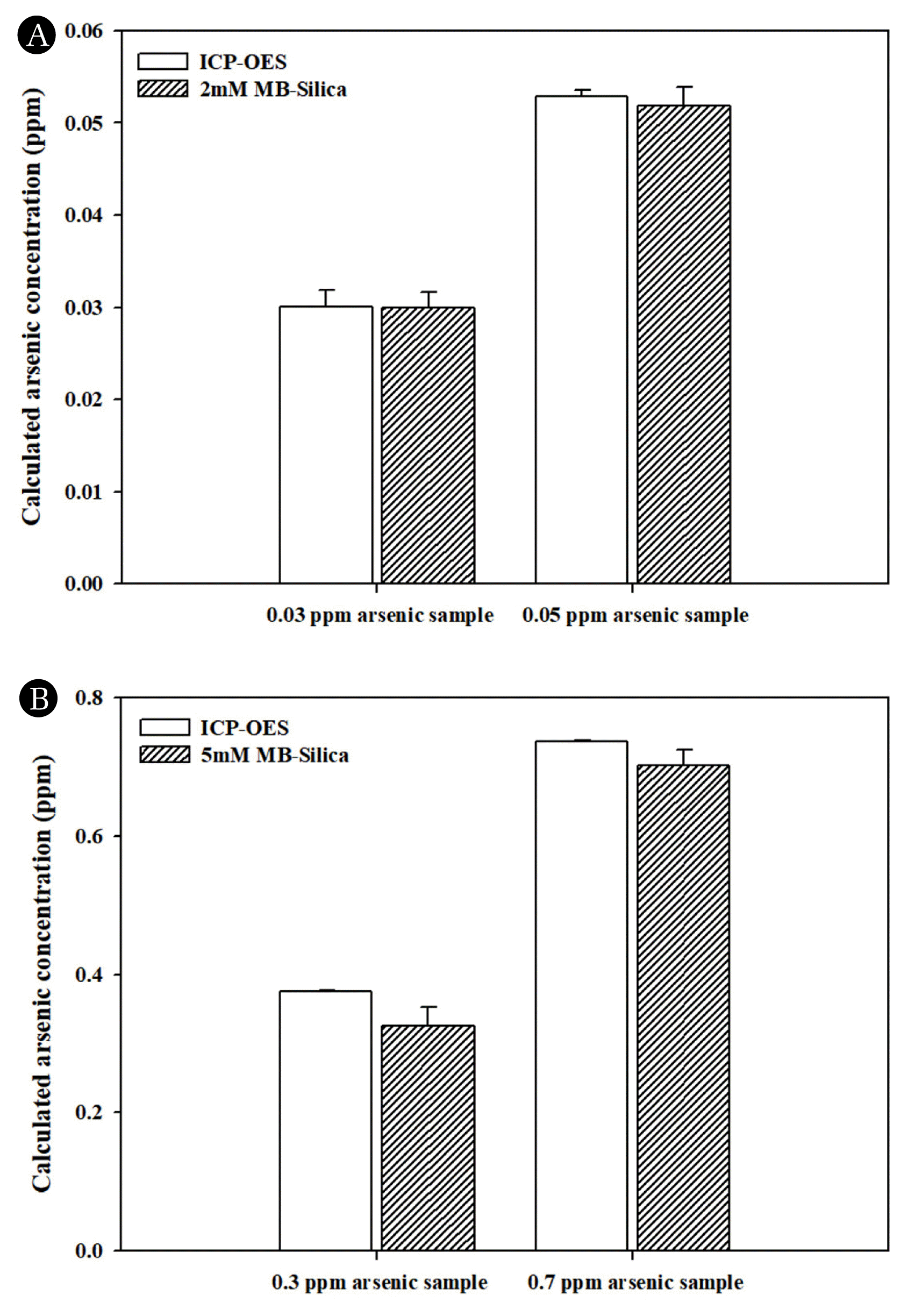
Table 1Si2p, C1s and O1s XPS spectral bands based on the binding energies of silica and MB-silica. |
|
|||||||||||||||||||||||||||||||||||