AbstractThis work reports the use of highly efficient nanocomposite material fabricated glassy carbon electrode (Ag(NPs)/ChS/GC) in the trace detection of Pb2+ using differential pulse voltammetry (DPV). A facile method yielded the silane-grafted chitosan (ChS) using 3-trimethoxyoctylsilane, and the bioderived Ag(NPs) was in situ decorated on the ChS composite to obtain Ag(NPs)/ChS. X-ray Diffraction (XRD) results showed the presence of Ag(NPs) within the composite material. Spherical-shaped and uniform distribution of Ag(NPs) were observed in SEM and TEM images of the nanocomposite solid. The cyclic voltammetric studies revealed a significant increase in redox peak currents (i.e., 0.1283 mA), which was obtained using the Ag(NP)/ChS/GC compared to the bare glassy carbon electrode. Further, the electrochemical determination of Pb2+ showed good linearity in anodic peak current and Pb2+ concentrations (5.0–80.0 μg/L), and the limit of detection was 1.97 μg/L. With 10-fold increase in several cationic and anionic interfering ions, oxalic acid and glycine only show interference in determination of Pb2+. The reproducibility study showed a %RSD of 2.43%, and the electrode could be efficiently used for prolonged and repetitive detection having reasonably low %RSD of 2.55%. The real water analysis showed almost 100% recovery of Pb2+ in two different real water samples.
Graphical Abstract
1. IntroductionLead (Pb) is a non-essential metal and is highly toxic to human being [1]. Long-term exposure to lead causes kidney damage, cancer, nausea, and lung disease [2]. Because of the extreme toxicity of lead ions (Pb2+), the World Health Organisation (WHO) has reduced the Pb2+ permissible limit to 10.0 μg/L in drinking water [1]. Hence, various removal studies for Pb2+ are performed at low-level in wastewater and stringent monitoring of Pb2+ species in the aquatic environment to safeguard human well-being and marine lifeform [3]. In a line, various analytical methods like spectroscopic technique and chromatographic technique are widely used for low-level determination of Pb2+. However, there are certain drawbacks in these detection methods, such as, high maintenance/operational cost, the requirement of trained personnel for operation, complex sample preparations, time consumption, and lack of on-site detection [4]. On the contrary, electrochemical techniques are practical and viable alternatives because of their easy/robust operation, high sensitivity, cost-effectiveness, and, most importantly, on-site detection [5, 6].
The selectivity and stability of working electrodes are key challenges for possible implications of electrochemical methods in miniaturized sensor development. The use of several advanced materials for electrode modification significantly improves the sensing properties of the working electrode for trace level detection of several analytes. Graphene quantum dots (GQDs) and Nafion (NF) modified glassy carbon (GC) electrode (GC/GQDs-NF) detects simultaneously the Cd2+ and Pb2+ with the limit of detection of 11.30 μg/L for Cd2+ and 8.49 μg/L for Pb2+ [7]. Similarly, the use of multiwalled carbon nanotube (MWCNT) with 2-(5-bromo-2-pyridylazo)-5-diethylaminophenol (5-Br-PADAP) coated GC electrode (5-Br-PADAP/MWCNT/GCE) utilize for Pb2+ detection in the standard alloys and water sample. The complex formation of Pb2+ with 5-Br-PADAP enables the selective determination of Pb2+ in the complex matrix [8]. The nanocomposite material (Fe3O4/PDA/MnO2) functionalised with polydopamine (PDA) polymer showed high sensitivity in the detection of Pb2+. The functionalized material modified electrode shows the detection limit 0.03 μg/L Pb2+ and good linearity achieved within the anodic peak current and concentration of Pb2+ (0.1 to 150.0 μg/L). Moreover, the fabricated electrode gives reproducible results in the repeated detection of Pb2+ [9]. The bismuth film coated electrode detects simultaneously the Cr6+, Pb2+ and Cd2+ utilising anodic stripping voltammetry [10]. Further, the single-walled carbon nanohorns (SWCNHs) modified screen-printed electrode (SPE), (SPE/SWCNHs) is utilised in the simultaneous quantifiation of Cd2+ and Pb2+ in honey and milk samples [11]. Nevertheless, Polymers such as polyaniline, polystyrene, polypyrrole etc. were used for fabricating the working electrode which further enhanced its sensitivity and selectivity toward the target pollutants [12]. Biopolymers show potential for such strategies of making the process cost-effective and non-toxic, and developing advance material for electrodes modification [13].
Chitosan, a natural biopolymer possesses excellent physical properties viz., high film forming capacity, strong adhesion, high permeability, and non-toxicity, make it ideal for a number of applications, including sensor development. Moreover, swelling and hydrophilic nature of chitosan enhances electron transfer processes in the sensor applications [14, 15]. Furthermore, literature review showed that the physical and chemical properties of chitosan is easily altered due to the presence of amino and hydroxyl group in their polymeric chain, which further improves the applicability of chitosan in various applications [16]. Apparently, chemical modification of chitosan using 1,2,7,8-diepoxyoctane [17], glutaraldehyde [18], sodium tripolyphosphate (TPP) [19] and gloxal [20] were previously reported obtaining the crosslinked chitosan matrix. Similarly, grafting of chitosan using inorganic compounds such as 3-isocyanatopropyltriethoxysilane (ICPTES) [21], 3-amino-propyltriethoxysilan [22], 3-mercaptopropyl trimethoxysilane and trimethoxyoctylsilane [16] were used to modify the chitosan, and utilised to remove a number of harmful heavy metal ions from the aqueous medium.
On the other hand, the silver nanoparticles (Ag(NPs)) play an important role in enhancing electron transport properties of advanced materials. They possess high electrical conductivity, and unique chemical and physical properties with biocompatibility which make them interesting for various applications compared to Au or Pt nanoparticles [23, 24]. However, the Ag nanoparticles are seemingly aggregated in the substrate matrix and forming the bulk materials along with the formation of metal oxides. Therefore, preventing nanoparticles aggregation and metal oxide formation is a challenge in material synthesis.
The synthesis of nanoparticles using phytochemicals, known as the greener synthesis route is introduced, which replaces the toxic chemicals used in synthesis and prevents aggregation [25]. Several works are reported using different leaf extracts to synthesize nanoparticles and employed in sensor development. Kumar et al. used Gongura (Hibiscus Sabdariffa) leaf extract for green synthesis of bio-functionalised Ag(NPs) and applied it for sensor development for Hg2+, Pb2+ and Cd2+ while detecting in ppm level in water [26]. Similarly, Holy basil (Ocimum Sanctum) leaf extract synthesizes the Ag(NPs) and the material used to fabricate the carbon paste electrode and employed for sensing of Cd2+ and Pb2+ in the discharge effluent of textile factories [27]. Furthermore, Serunting et al. used Lamtoro Pods (Leucaena leucocephala) extract in the synthesis of Ag(NPs) and employed in Hg2+ detection using a colorimetric study [28]. Babunah (Matricaria recutita) leaf extract was used to synthesize Ag(NPs) and employed for trace detection of Hg2+ under the colorimetric study [29]. The widespread availability of these natural phytochemicals paves the way for alternative, non-toxic potential natural chemicals to be employed for facile material synthesis and in sensor development.
The studies specifically aims to synthesize the novel nanocomposite material (Ag(NPs)/ChS) through greener and indigenous method. Chitosan functionalized with 3-trimethoxyoctylsilane (ChS) improves its settling capacity and provides an extra binding siloxane site and complex formation with Pb2+. Furthermore, silver nanoparticles (Ag(NPs)) were synthesised using natural phytochemicals extracted from Psidium guajava leaf, which prevented the agglomeration of nanoparticle and improving the electron transport at the electrode surface using the nanocomposite solid. Therefore, nanocomposite fabricated electrode employed in the trace detection of Pb2+ in aqueous medium and the stability of the fabricated electrode studied extensively. Additionally, the real implications of electrode in the detection Pb2+ utilizing the natural water samples provides useful input data towards the miniaturized sensor development.
2. Experiment2.2. Methodology2.2.1. Preparation of IThe Mizoram University campus, Aizawl (India) provided the fresh and mature leaf of Psidium guajava. After being cleaned in double-distilled (DD) water and dried for five hours at 50°C in the oven, the leaf was crushed in a mortar to obtain the powder of leaf. 5.0 gm of powder was taken in a round bottom flask with 60.0 mL of deionized water. The solution mixture was agitated for 30 minutes and filtered with a Whatman filter paper (pore size 11μm). The extract was used for screening of various phytochemicals such as alkaloids, glycosides, steroids, flavonoids, saponin, quinones, tannin and terpenoids.
2.2.2. Synthesis: Silane grafted chitosan (ChS) and Ag(NPs) decorated ChSA facile sol-gel process was used to prepare silane-grafted chitosan (ChS). The detailed procedure is included elsewhere [30]. The Ag(NPs) were decorated with the ChS (3-trimethoxyoctylsilane grafted chitosan) composite material by in situ process. In brief: 50 mg ChS solid was dispersed in 10 mL of acetic acid (0.1%) under stirring to dissolve the solid. The pH of the solution was brought back to 5.0 using 0.1 M NaOH. Further, 30.0 mM AgNO3 solution was added slowly to the 10.0 mL of ChS slurry. The solution was stirred for 1 hr and then 0.2 mL of Psidium guajava leaf extract was added gently. The solution mixture was agitated at 60°C for 3 hrs. The solution mixture colour was changed from light to deep yellow (Fig. S1. (b)). The formation of Ag nanoparticles was confirmed with UV-visible spectra obtained for these solutions (Fig. S1. (a)). Centrifugation enabled it to separate the solid material, which was then washed thoroughly by deionized water to eliminate the impurities including the Ag+ or NO3− ions. The collected sample was dispensed in 10 mL of deionised water for final casting solution (Ag(NPs)/ChS) and used to fabricate the electrode. The ChS and Ag(NPs)/ChS materials were dried and grinded into powder form for characterisation using XRD and TEM analytical tools where, SEM-EDX characterisation was performed using nanomaterial casted glassy carbon (GC) plate (1 mm thick). Prior to the casting of glassy carbon plate, it was thoroughly cleaned with 0.1 M HNO3 and washed with plenty of deionized water and dried in a hot air oven at 100°C for 2 hrs.
2.2.3. Fabrication of working electrode and electrochemical proceduresFabrication of the glassy carbon electrode was carried out using simple drop-casting method [31]. The surface of the GC electrode was first polished with the 0.05 μm alumina followed by 1.0 μm diamond polishing solutions. The GC electrode was then cleaned with deionized water followed by 2.0 minutes sonication each with ethanol and deionized water. The electrode was dried at 60°C for 30 minutes, cooled at room temperature. The GC electrode surface was drop casted with 0.02 mL of Ag(NPs)/ChS suspension (10 mg Ag(NPs)/ChS + 10 mL of deionized water), which was then dried for 30.0 minutes at 40°C in the oven. The electrode was kept at room temperature for cooling before being submerged for 5.0 minutes in the 0.1% glutaraldehyde solution to facilitate the crosslinking of nanocomposite material [30].
The fabricated electrode and bare GC electrode was electrochemically characterised using cyclic voltammetry in a 2.0 mmol/L standard redox couple [Fe(CN)6]3−/4− prepared in 100.0 mmol/L acetate buffer solution pH 4.5 as supporting electrolyte. Voltammogram was recorded for modified and bare GC electrodes at a potential window of −0.4 to 0.8 V and scan rate of 100 mV/s.
Electrochemical behaviour of Pb2+ 50.0 μg/L at bare GC and Ag(NPs)/ChS modified GC electrode was studied using differential pulse voltammetry (DPV). Parameter such as pH, greatly influence the behaviour of heavy metal ions, hence, the detection of Pb2+ were optimised under varying pH conditions (i.e., 3.5, 4.0, 4.5, 5.0 and 5.5) using Ag(NPs)/ChS/GC. The concentration of Pb2+ was taken at 50.0 μg/L (100.0 mmol/L acetate buffer solution) and keeping other parameters constant viz., deposition potential −0.9 V (vs. Ag/AgCl), and deposition time 180.0 sec. Similarly, the deposition time was studied at different time intervals 60.0, 90.0, 120.0, 150.0, 180.0, 210.0 and 240.0 sec., the other condition such as pH 4.5, deposition potential of −0.9 V remained constant. All the other conditions such as pulse amplitude: 0.05 V; step potential: 0.0001 V; modulation time: 0.05 s; and time interval: 0.5 s remained constant throughout the experiment.
The determination of Pb2+ at varied concentration ranging from 5.0 to 80.0 μg/L under optimised experimental condition was conducted and the corresponding oxidative peak current was recorded. Further, the limit of detection (LOD) and limit of quantification (LOQ) was calculated using the equations: LOD = 3 x SD/m, and LOQ = 10 x SD/m, where m = mean value, SD = standard deviation of the blank sample measurements (n=5) [32].
The interference study was performed for detection of 50.0 μg/L Pb2+ using 10-fold (500.0 μg/L) increase in cationic and anionic species. Cations such as Ni2+, Zn2+, Cr3+, and Cu2+ and anion viz., EDTA (Ethylene Diamine Tetraacetic Acid), OA (Oxalic acid) and GLY (Glycine) were studied for their possible interference in the Pb2+ detection using Ag(NPs)/ChS/GC.
The reproducibility of the fabricated electrode Ag(NPs)/ChS/GC was conducted under optimised experimental conditions utilising 50.0 μg/L Pb2+ solution (100.0 mmol/L acetate buffer). The other parameters were taken according to the previously optimised DPV measurement conditions. The repeatability study was performed in a series of repetitive experiments running the blank sample 10 times and then detecting Pb2+ for 5 times. The recorded oxidative peak current was collected and the RSD% (Relative standard deviation) was calculated using the standard formula RSD = s*100/, where s stands for standard deviation and is the mean of the observed data [33]. Long term stability of the Ag(NPs)/ChS/GC was investigated for varied time intervals i.e., 0, 12, 24, and 48 hours. Each time the experiment was performed for five consecutive detection and mean value was recorded. After completion of each run, the electrode was washed with deionized water and stored in a desiccator at room temperature and utilized for the next cycle of operation. The RSD% value was calculated using the %RSD equation.
Furthermore, recovery rate study was conducted utilising spring water sample and groundwater sample spiked with 5.0, 10.0 and 20.0 μg/L Pb2+ under optimised experimental condition such as pH 4.5 (acetate buffer 100.0 mmol/L), deposition potential −0.9 V and deposition time 180.0 sec.
3. Results and Discussions3.1. Phytochemical Analysis of Leaves Extract (Psidium guajava)The phytochemicals of the leaf extract were identified using standard methods described elsewhere [34]. The test for alkaloids, glycosides, steroids [35], flavonoids [36], saponins [37], quinones [38], tannin and terpenoids [39] were carried out and results are shown in Table S1. The flavonoids have the potential to reduce the Ag+ ions while acting as capping agent for Ag(NPs). It was assumed that numerous forms of −OH groups present with flavonoids reduces Ag+ to Ag0 while capping it, through chelation with the C=O and −OH group and the flavonoid catechol moiety [40].
3.2. Characterisation of Prepared MaterialThe X-ray diffraction pattern of ChS and Ag(NPs)/ChS materials are presented in Fig. 1. The ChS material showed diffraction peaks at 2θ = 9.5 and 20.26, that indicate the semi-crystalline nature, found in a higher degree of deacetylated chitosan [41]. On the other hand, the X-ray diffraction pattern of Ag(NPs)/ChS showed dominant peaks at the 2θ values of 38.35, 44.35, 64.31, and 77.21 which are assigned to the diffraction planes (111), (200), (220), and (311), respectively of Ag(NPs) (JCPDS card no. 04–0783). Further, using the wavelength of Cu K♋ 1.54 Å, the corresponding dhkl values were computed. The d values of the (111), (200), (220), and (311) were found to be 2.35, 2.04, 1.45 and 1.24 Å, respectively. It was reported that the crystalline and cubic shaped Ag(NPs) showed face-centered cubic (FCC) lattice parameter ‘a’ 4.086 Å [42]. Moreover, the additional peaks occurring in the XRD pattern indicated the presence of some impurities, primarily bioorganic compounds/proteins, during the synthesis of nanoparticles [43, 44].
The SEM image of the nanocomposite material showed a dense distribution of the silane grafted composite material (Fig. 2. (a)). The composite material surface is highly uneven and porous. Further, the Ag(NPs) are widely and evenly distributed on the surface of composite material. The small sized Ag(NPs) are predominantly spherical in shape and not aggregated on the solid surface. The in situ decoration of Ag(NPs) enabled to prevent the agglomeration of Ag(NPs) on the surface. Further, the EDX spectra of the solid is shown in Fig. 2. (a) (Inset). EDX spectra showed a predominant silver peak which confirmed the presence of Ag(NPs) within the nanocomposite solid (Ag(NPs)/ChS. Additionally, the presence of Si inferred the grafting of silane with the chitosan network. The TEM image of the Ag(NPs)/ChS showed a distinct distribution of silver nanoparticles. The nanoparticles are smooth and predominantly spherical in shape (Fig. 2. (b)). Moreover, the average particle size was obtained using the J Software and found to be 22.32 nm. Additionally, the d-spacing was obtained for the Ag(NPs) and was found to be 1.45 Å, which is assigned to the (220) plane of the Ag(NPs) (Fig. 2. (c)).
3.3. Electrochemical Study of Fabricated ElectrodeUtilizing cyclic voltammetry the electrochemical behaviour of fabricated electrode Ag(NPs)/ChS along with the bare GC and ChS were investigated utilizing the 2.0 mmol/L [Fe(CN)6]3−/4− (100.0 mmol/L acetate buffer at pH 4.5) at scan rate of 100.0 mV/s. Voltammograms recorded for these electrodes are displayed in Fig. S2. It is evident that the redox current peaks are quite pronounced and significantly increased using the Ag(NPs)/ChS/GC compared to the bare GC. The reduction and oxidation peaks were observed at an applied potential of 0.14 and 0.30 V (vs Ag/AgCl). The presence of −NH and −OH groups in chitosan and siloxane site (from grafted silane), along with the availability of free energy charge of Ag0 results in catalytic property or enhancement of electron transfer kinetics with enhanced surface selectivity between Ag(NP)/ChS/GC surface and the redox couple [Fe(CN)6]3−/4−. This enhances significantly the peak current (i.e., 0.1283 mA) using the Ag(NP)/ChS/GC fabricated electrode.
Furthermore, compression between voltammograms obtained by different electrode i.e., bare GC, ChS and Ag(NPs)/ChS, show that peak to peak separation was slightly reduced and the calculated ΔE value for Ag(NPs)/ChS is 0.15 V whereas for bare GC and ChS it was 0.17 V. The reduced ΔE value with Ag(NPs)/ChS resulted due to decreasing overpotential which further show that electron transfer is faster on the electrode surface of Ag(NPs)/ChS. Hence, nanocomposite material resulted in increasing electron transfer rate on the electrode surface is due to availability of more binding sites on the ChS material [31]. Further, Ag(NPs) acted as a catalyst and provide faster electron transfer kinetics at the electrode surface.
3.4. Differential Pulse Voltammetric (DPV) Studies of Pb2+ Using Fabricated ElectrodeElectrochemical behaviour of Pb2+ 50.0 μg/L (100.0 mmol/L acetate buffer, pH 4.5) at different electrodes i.e., bare GC and Ag(NPs)/ChS was studied using DPV and the results are returned in Fig. 3. Fig. 3. clearly showed that no oxidative peak current occurred using the bare GC. However, a significantly high oxidative peak current 5.13 μA was observed at potential around −0.35 V using the Ag(NP)/ChS/GC (line (b)). The results showed that the fabricated electrode possessed an enhanced electrochemical response for the Pb2+ in aqueous medium. The high value of peak current is due to the abundance of −NH2 and siloxane sites in the nanocomposite material, which facilitate the preferential chelation of lead ions at the electrode surface and cause of higher oxidative peak current using the fabricated electrode [30]. Additionally, the free energy charge due to Ag0 decoration on the surface of electrode further catalyses the electron transfer rate at the interface of electrode resulting in high oxidative peak current [45]. Previously it was reported that using the silane grafted chitosan (CHTMS) composite caused an increase in oxidative peak current Pb2+ due to available siloxane site within the chitosan matrix [30]. Moreover, using the Ag(NP)/ChS/GC, the oxidative peak current is increased by almost 3 fold compared to the previously reported CHTMS/GC and Ca. 5 fold compared to bare GC. Prakash et al. reported that Ag(NPs)/CS synthesized material employed in simultaneous determination of As3+ and Cu2+, where the presence of Ag(NPs) favoured the current response toward the target pollutants [43]. The present study shows advantages in the efficient and trace detection of Pb2+ using the novel nanocomposite material Ag(NPs)/ChS. Further, the nanocomposite material was derived from the abundant natural biopolymer chitosan, and the use of natural phytochemicals for in situ synthesizing the Ag(NPs).
3.5. Experimental Parameters OptimisationElectrochemical behaviour of Pb2+ at varied pH was obtained and results are returned in Fig. 4. (a). Upon increasing the pH value from 3.5 to 5.5, a rapid increase in oxidative peak current was observed and a maximum peak current was attained at pH 4.5 (Fig. 4. (a)). However, further increase in pH >4.5, rapid decline in peak current was observed which is possibly due to the formation of Pb(OH)x species in solution which hinders the lead species to aggregate at the electrode surface [46]. Similarly, at low pH values, the hydrogen ion actively occupies the active sites at the electrode surface compared to Pb2+, resulting in low peak current values. Similar observations were reported for Pb2+ determination using Co3O4/reduced graphene oxide/chitosan (Co3O4/rGO/CS) modified glassy carbon electrode [47]. These results inferred that pH 4.5 is an optimum pH for further electrochemical studies of Pb2+ in aqueous medium. The other studies indicated that the detection of As3+ is efficient at pH 2.0 using trichloro(octadecyl) silane grafted bentonite [48].
Deposition or the accumulation potential is a crucial parametric study in the stripping voltammetry since it significantly affects the sensitivity of the working electrode [2]. The deposition potential (−0.6 V and −1.1 V) dependence results are shown in Fig. 4. (b). It is evident from the Fig. 4. (b) that the oxidation peak current was maximum at potential −0.9 V. However, potential >−0.9 V caused a decrease in oxidative peak current, which was due to the formation of bubbles and production of hydrogen. Therefore, the optimized accumulation potential for efficient detection of Pb2+ using the Ag(NPs)/ChS/GC was −0.9 V.
Finally, the deposition time at various time intervals ranging from 60.0 to 240.0 sec. was conducted and results are shown in Fig. 4. (c). An increase in the deposition time from 60.0 to 180.0 sec favoured the anodic peak current. Increasing the deposition time favoured the accumulation of Pb2+ at the electrode surface, which increased the oxidative peak current. However, upon further increase in deposition time >180.0 sec, an apparent constant peak current was recorded. This result is due to the saturation of electroactive sites at the electrode surface (Fig. 4. (c)). Hence, the 180.0 sec accumulation time was chosen for enhanced electrochemical response of Pb2+ in aqueous medium.
3.6. Pb2+ DeterminationElectrochemical detection of Pb2+ at varied concentrations of Pb2+ is conducted and the results are returned in Fig. 5. It is evident that increasing the concentration of Pb2+ increases the oxidative peak currents. The oxidative peak current is linearly increased, increasing the Pb2+ concentrations (5.0–80.0 μg/L). Therefore, a linear relationship gives the straight-line equation: y (μA) =0.0368 μg/L + 3.5742 with R2= 0.9811 (Fig. 5. (inset)). The calculated values of LOD and LOQ were 1.97 μg/L and 6.58 μg/L, respectively. The LOD value is considerably less than the WHO guidelines for Pb2+ in drinking water i.e., 10.0 μg/L. Further, the efficacy of detection using the Ag(NP)/ChS/GC is compared with several other materials in the detection of Pb2+ and returned in Table 1. A comparable or even lower detection limit was achieved using the fabricated electrode.
Abundant −NH2 and −OH groups in Ch (Chitosan), along with grafted silane (ChS), increases its settling and adsorption capabilities as well as the availability of siloxane sites, the modified GC with ChS exhibits superior electrochemical response toward Pb2+. Additionally, Ag(NP) increases the electroactive surface area, along with presence of high surface free energy accelerates the transport of electrons [43, 45]. Thus, the combined properties led to an increase in electrostatic attraction and affinity between Ag(NPs)/ChS/GC and Pb2+. The mechanism involved in the DPV studies is followed:
3.7. Interference StudyIn presence of several cationic and anionic species, interfering ion study was conducted, and the results is returned in Fig. 6., which shows that the cationic species do not show any significant effect in determining Pb2+ from aqueous medium. In contrast, anionic species such as GLY and OA interfere in determining Pb2+. GLY which is an amino acid with amino and carboxyl groups in their simple chain, readily oxidised at the working electrode [49], hence, suppressed the Pb2+ oxidation at the working electrode and resulted with reduced oxidative peak current. Additionally, GLY is an excellent antioxidant, hence, preventing Pb2+ oxidation at the working electrode [50].
Similarly, OA shows interference in Pb2+ determination. OA is a strong chelating effect forming chelates with Pb2+, hence, decreases the oxidative peak current. It was reported previously, that Cu2+ detection was interfered in presence of OA using nanoscale hydroxyapatite (nHAP, Ca10(PO4)6(OH)2) modified electrode due to the formation of strong coordinating bond between OA and Cu2+, which significantly lowered the concentration of Cu2+ and further inhibited the ion exchange between Ca of nHAP and Cu2+ [51]. Interesting to observe that a wide range of cations do not interfere with the Pb2+ detection which points to the potential of Ag(NPs)/ChS/GC in the efficient detection of Pb2+ from aqueous medium.
3.8. Reproducibility of the Working ElectrodeThe reproducibility study was performed and shows almost same oxidative peak current in five consecutive detections of Pb2+ under optimised experimental conditions and the RSD was evaluated as 2.43%, revealing that the prepared electrode has good reproducibility in repeated operations. Further, the long-term stability of fabricated electrode was obtained at different time duration from 0 to 48 hours and the observed peak current was evaluated and the %RSD is shown in Table S2. The calculated RSD% is below 5%, which inferred that the stability of Ag(NPs)/ChS/GC electrode is found promising for long-term applications.
3.9. Recovery Rate Study in Natural Water SampleThe applicability of the Ag(NPs)/ChS/GC in natural water samples was greatly studied utilizing the two different natural water samples collected from spring and groundwater sources at the Mizoram University campus, Tanhril, India. The natural water samples were analysed using different analytical methods and results are shown in Table S3. The natural water samples contained high calcium concentrations in groundwater compared to the spring waters, while lower nitrate and sulphate concentrations were found in groundwater samples. High concentrations of calcium and inorganic carbon indicated the hardness of ground water due to the existence of calcium carbonates and bicarbonates. Other reports on hardness of tap water samples were also reported due to the occurrence of high calcium and inorganic carbon concentrations in the water sample [52]. Other water parameters like TOC, conductivity, resistivity, etc., and the presence of minimal concentrations of Cu, and Fe were also recorded. The natural water samples were spiked with 5.0, 10.0, and 20.0 μg/L of Pb2+, and the DPV response was recorded and shown in Fig. 7. (a&b). The linear equation obtained from the calibration curve (Fig. 7. (c)) for spring water: y (μA) =0.0256 μg/L + 3.5068 (R2=0.9292) and y (μA) =0.0213 μg/L + 2.431 (R2=9806) for groundwater samples. The recovery of the spiked concentrations was calculated, and the results are shown in Table S4. Calculated recovery percentage of Pb2+ at natural water samples ranged between 95.52–96.06% and 92.95–102.57% for spring water and groundwater samples, respectively. These results inferred that the determination of Pb2+ in natural water samples is feasible using the novel Ag(NPs)/ChS/GC hence, the material is useful in the miniaturized device development for the detection of Pb2+.
4. ConclusionsA nanocomposite material Ag(NP)/ChS is obtained using the 3-trimethoxyoctylsilane grafted chitosan (ChS) and in situ decorated with Ag(NPs). The Ag0(NP) were obtained using the natural phytochemicals (Psidium guajava leaf extract). Silane grafting into chitosan provided chitosan stability, and siloxane provided different binding sites for heavy metals. Moreover, the Ag(NPs) provided high surface free charge for an improved electrical response. The in situ synthesis and decoration of Ag(NPs) enabled uniform and small-sized distribution of nanoparticles that were not aggregated. The spherically shaped Ag(NP)’s average size was 22.32 nm and the d-spacing was 1.45 Å which referred to the (220) plane. The electrochemical response of Ag(NP)/ChS/GC was significantly increased compared to the bare GC using the standard probe Fe(CN)6]3−/4− (100.0 mmol/L acetate buffer at pH 4.5). The DPV studies showed that the optimum pH, deposition potential and deposition time was 4.5, −0.9 V and 180.0 sec, respectively, in the detection of Pb2+. Optimised conditions enabled trace level detection of Pb2+ under the DPV measurements. Reasonably a good linear line was obtained (y (μA) =0.0368 μg/L + 3.5742) with correlation coefficient, R2=0.981 for a wide concentration range of Pb2+ from 5.0–80.0 μg/L employing the nanocomposite electrode (Ag(NPs)/ChS/GC). Further, the observed LOD was 1.97 μg/L. The presence of 10-fold concentrations of co-ions (cations: Ni2+, Zn2+, Cr3+, and Cu2+ and anionic species: EDTA) does not show any interference in detecting Pb2+ whereas, glycine and oxalic acid show interference in detection of Pb2+. Ag(NPs)/ChS/GC showed excellent reproducibility for Pb2+ detection, having a %RSD of detection as low as 2.43%. Stability of the fabricated electrode was ascertained with repeated operations and their %RSD was found less than 5%, showed reasonably high stability of fabricated electrode. Recovery study using spiked Pb2+ in both the water sample show reasonably good recovery of the analyte (i.e., 95.52–96.06% for spring water and 92.95–102.57% for ground water). Therefore, the studies inferred that the synthesized novel nanocomposite material is promising in developing miniaturized device development for the trace and selective Pb2+ detection in aqueous samples.
AcknowledgmentAuthors acknowledge the DRDO, Govt. of India, New Delhi for providing the financial support in the form of Research Project (vide No.: DFTM/07/3600/NESTC/EWM/M/P-01/01).
NotesAuthor Contributions S (Ph.D.) performed the experiments and drafted the manuscript. L. (Assistant Professor) and J.L. (Ph.D.) finalized the data experimental data obtained and helped in revising the draft manuscript. D.T. (Professor) conceptualized the problem and critically evaluated the manuscript. The review and editing are completed. S.M.L. (Professor) analyzed the data and provided critical comments. J.K.Y. (Professor) provided critical comments and helped in finalizing the manuscript. References1. Wani AL, Ara A, Usmani JA. Lead toxicity: a review. Interdiscip Toxicol. 2015;8(2)55–64.
https://doi.org/10.1515/intox-2015-0009
2. Guo Z, Li D, Luo X-K, et al. Simultaneous determination of trace Cd(II), Pb(II) and Cu(II) by differential pulse anodic stripping voltammetry using a reduced graphene oxide-chitosan/poly-l-lysine nanocomposite modified glassy carbon electrode. J. Colloid. Interface Sci. 2017;490:11–22.
https://doi.org/10.1016/j.jcis.2016.11.006
3. Priya T, Dhanalakshmi N, Thinakaran N. Electrochemical behavior of Pb (II) on a heparin modified chitosan/graphene nanocomposite film coated glassy carbon electrode and its sensitive detection. Int. J. Biol. Macromol. 2017;104:672–680.
https://doi.org/10.1016/j.ijbiomac.2017.06.082
4. Lingerak WA, Wensveen-louter AMV, Slanina J. The Determination of Zinc, Cadmium, Lead and Copper in Precipitation by Computerized Differential Pulse Voltammetry. Int. J. Environ. Anal. Chem. 1985;19(2)85–98.
https://doi.org/10.1080/03067318508077020
5. Seenivasan R, Chang W-J, Gunasekaran S. Highly Sensitive Detection and Removal of Lead Ions in Water Using Cysteine-Functionalized Graphene Oxide/Polypyrrole Nanocomposite Film Electrode. ACS Appl. Mater. Interfaces. 2015;7(29)15935–15943.
https://doi.org/10.1021/acsami.5b03904
6. Zirlianngura , Tiwari D, Ha J-H, Lee S-M. Efficient Use of Porous Hybrid Materials in a Selective Detection of Lead(II) from Aqueous Solutions: An Electrochemical Study. Metals. 2017;7(4)124.
https://doi.org/10.3390/met7040124
7. Pizarro J, Segura R, Tapia D, Navarro F, Fuenzalida F, Jesús Aguirre M. Inexpensive and green electrochemical sensor for the determination of Cd(II) and Pb(II) by square wave anodic stripping voltammetry in bivalve mollusks. Food Chem. 2020;321:126682.
https://doi.org/10.1016/j.foodchem.2020.126682
8. Salmanipour A, Taher MA. An electrochemical sensor for stripping analysis of Pb(II) based on multiwalled carbon nanotube functionalized with 5-Br-PADAP. J. Solid State Electrochem. 2011;15:2695–2702.
https://doi.org/10.1007/s10008-010-1197-3
9. Wang L, Lei T, Ren Z, et al. Fe3O4@PDA@MnO2 core-shell nanocomposites for sensitive electrochemical detection of trace Pb(II) in water. J. Electroanal. Chem. 2020;864:114065.
https://doi.org/10.1016/j.jelechem.2020.114065
10. Li J, Zhang J, Wei H, Wang E. Combining chemical reduction with an electrochemical technique for the simultaneous detection of Cr(VI), Pb(II) and Cd(II). Analyst. 2009;134:273–277.
https://doi.org/10.1039/b804670k
11. Yao Y, Wu H, Ping J. Simultaneous determination of Cd(II) and Pb(II) ions in honey and milk samples using a single-walled carbon nanohorns modified screen-printed electrochemical sensor. Food Chem. 2019;274:8–15.
https://dio.org/10.1016/j.foodchem.2018.08.110
12. Promphet N, Rattanarat P, Rangkupan R, Chailapakul O, Rodthongkum N. An electrochemical sensor based on graphene/polyaniline/polystyrene nanoporous fibers modified electrode for simultaneous determination of lead and cadmium. Sens. Actuators B: Chem. 2015;207:526–534.
https://doi.org/10.1016/j.snb.2014.10.126
13. Zouaoui F, Bourouina-Bacha S, Bourouina M, Jaffrezic-Renault N, Zine N, Errachid A. Electrochemical sensors based on molecularly imprinted chitosan: A review. TrAC Trends Anal. Chem. 2020;130:115982.
https://doi.org/10.1016/j.trac.2020.115982
14. Sarikokba S, Tiwari D, Prasad SK, Choi SS, Lee SM. Bio-Composite Materials Precursor to Chitosan in the Development of Electrochemical Sensors: A Critical Overview of Its use with Micro-Pollutants and Heavy Metals Detection. Appl. Chem Eng. 2020;31(3)237–257.
https://doi.org/10.14478/ace.2020.1034
15. Lee SM, Lalchhingpuii , Lalhmunsiama , Tiwari D. Synthesis of functionalized biomaterials and its application in the efficient remediation of aquatic environment contaminated with Cr(VI). Chem. Eng. J. 2016;296:35–44.
https://doi.org/10.1016/j.cej.2016.03.077
16. Lalhmunsiama , Lalchhingpuii , Nautiyal BP, et al. Silane grafted chitosan for the efficient remediation of aquatic environment contaminated with arsenic(V). J. Colloid Interface Sci. 2016;467:203–212.
https://doi.org/10.1016/j.jcis.2016.01.019
17. Vakili M, Deng S, Li T, Wang W, Wang W, Yu G. Novel crosslinked chitosan for enhanced adsorption of hexavalent chromium in acidic solution. Chem. Eng. J. 2018;347:782–790.
https://doi.org/10.1016/j.cej.2018.04.181
18. Song J, Zhou H, Gao R, et al. Selective Determination of Cr(VI) by Glutaraldehyde Cross-Linked Chitosan Polymer Fluorophores. ACS Sens. 2018;3(4)792–798.
https://doi.org/10.1021/acssensors.8b00038
19. Nasution TI, Asrosa R, Nainggolan I, Balyan M, Indah R, Wahyudi A. Sodium tripolyphosphate cross-linked chitosan based sensor for enhacing sensing properties towards acetone. IOP Conf. Ser.: Mater. Sci. Eng. 2018;309:012083.
https://doi.org/10.1088/1757-899X/309/1/012083
20. Gupta KC, Jabrail FH. Glutaraldehyde and glyoxal cross-linked chitosan microspheres for controlled delivery of centchroman. Carbohydr. Res. 2006;341(6)744–756.
https://doi.org/10.1016/j.carres.2006.02.003
21. Silva SS, Ferreira RAS, Fu L, et al. Functional nanostructured chitosan–siloxane hybrids. J. Mater. Chem. 2005;15:3952–3961.
https://doi.org/10.1039/b505875a
22. Lalhmunsiama , Lee S-M, Lalchhingpuii , Tiwari D. Functionalized hybrid material precursor to chitosan in the efficient remediation of aqueous solutions contaminated with As(V). J. Environ. Chem. Eng. 2016;4(2)1537–1544.
https://doi.org/10.1016/j.jece.2016.02.015
23. Lian W, Liu S, Yu J, et al. Electrochemical sensor using neomycin-imprinted film as recognition element based on chitosan-silver nanoparticles/graphene-multiwalled carbon nanotubes composites modified electrode. Biosens. Bioelectron. 2013;44:70–76.
https://doi.org/10.1016/j.bios.2013.01.002
24. Ahmad MB, Lim JJ, Shameli K, Ibrahim NA, Tay MY. Synthesis of Silver Nanoparticles in Chitosan, Gelatin and Chitosan/Gelatin Bionanocomposites by a Chemical Reducing Agent and Their Characterization. Molecules. 2011;16(9)7237–7248.
https://doi.org/10.3390/molecules16097237
25. Bose D, Chatterjee S. Biogenic synthesis of silver nanoparticles using guava (Psidium guajava) leaf extract and its antibacterial activity against Pseudomonas aeruginosa. Appl. Nanosci. 2016;6:895–901.
https://doi.org/10.1007/s13204-015-0496-5
26. Kumar VV, Anbarasan S, Christena LR, SaiSubramanian N, Anthony SP. Bio-functionalized silver nanoparticles for selective colorimetric sensing of toxic metal ions and antimicrobial studies. Spectrochim. Acta-A: Mol. Biomol. Spectrosc. 2014;129:35–42.
https://doi.org/10.1016/j.saa.2014.03.020
27. Amare M, Worku A, Kassa A, Hilluf W. Green synthesized silver nanoparticle modified carbon paste electrode for SWAS voltammetric simultaneous determination of Cd(II) and Pb(II) in Bahir Dar Textile discharged effluent. Heliyon. 2020;6:e04401.
https://doi.org/10.1016/j.heliyon.2020.e04401
28. Serunting MA, Maryana OFT, Syafitri E, Balqis S. Green Synthesis Silver Nanoparticles (AgNPs) Using Lamtoro Pods Extract (Leucaena leucocephala) and Their Potential for Mercury Ion Detection. EVERGREEN J. Nov. Carbon Resource Sci. Green Asian Strategy. 2021;08(01)63–68.
https://doi.org/10.5109/4372261
29. Uddin I, Ahmad K, Khan AA, Kazmi MA. Synthesis of silver nanoparticles using Matricaria recutita (Babunah) plant extract and its study as mercury ions sensor. Sens. Bio-Sens. Res. 2017;16:62–67.
https://doi.org/10.1016/j.sbsr.2017.11.005
30. Sarikokba S, Lalmalsawmi J, Lee SM, Tiwari D. Highly Efficient Functionalized Chitosan in the Development of Electrochemical Sensor for Trace Detection of Pb (II). J. Electrochem. Soc. 2022;169:066513.
https://doi.org/10.1149/1945-7111/ac77c4
31. Sarikokba S, Lalmalsawmi J, Kumar SP, Tiwari D. Development of a novel sensor with high sensitivity for electroanalytical determination of bisphenol A based on chitosan-3-mercaptopropyl trimethoxysilane modified glassy carbon electrode. Microchem J. 2022;181:107748.
https://doi.org/10.1016/j.microc.2022.107748
32. Gao Y, Ierapetritou MG, Muzzio FJ. Determination of the Confidence Interval of the Relative Standard Deviation Using Convolution. J. Pharm. Innov. 2013;8:72–82.
https://doi.org/10.1007/s12247-012-9144-8
33. Shrivastava A, Gupta V. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chron. Young. Sci. 2011;2:21.
https://doi.org/10.4103/2229-5186.79345
34. Kenneth E, Paul T, Istifanus N, et al. Phytochemical analysis and antibacterial activity of Psidium guajava L. leaf extracts. GSC Biol. Pharm. Sci. 2017;01(02)013–019.
https://doi.org/10.30574/gscbps.2017.1.2.0024
35. Chandraker SK, Lal M, Ghosh MK, Tiwari V, Ghoria TK, Shukla R. Green synthesis of copper nanoparticles using leaf extract of Ageratum houstonianum Mill. and study of their photocatalytic and antibacterial activities. Nano Ex. 2020;1:010033.
https://doi.org/10.1088/2632-959X/ab8e99
36. Rattanachaikunsopon P, Phumkhachorn P. Contents and antibacterial activity of flavonoids extracted from leaves of Psidium guajava. J. Med. Plant Res. 2010;4(5)393–396.
https://doi.org/10.5897/jmpr09.485
37. Stephen AO, Akanji AM, Oguntoye SA. Ethanolic leaf extract of Psidium guajava: Phyto-chemical and trypanocidal activity in rats infected with Trypanosoma brucei brucei. J. Med. Plant Res. 2009;3(5)420–423.
https://doi.org/10.5897/jmpr.9000580
38. Vaidyanathan G, Kiruba D. Preliminary phytochemical analysis of leaf powder extracts of Psidium guajava L. Int. J. Pharmacogn Phytochem. Res. 2014;6:332–334.
https://doi.org/10.9734/ajbgmb/2021/v8i430200
39. Biswas B, Rogers K, McLaughlin F, Daniels D, Yadav A. Antimicrobial Activities of Leaf Extracts of Guava (Psidium guajava L.) on Two Gram-Negative and Gram-Positive Bacteria. Int. J. Microbiol. 2013;1–7.
https://doi.org/10.1155/2013/746165
40. Makarov VV, Love AJ, Sinitsyna OV, et al. “Green” Nanotechnologies: Synthesis of Metal Nanoparticles Using Plants. Acta Naturae. 2014;6(1)35–44.
https://doi.org/10.32607/20758251-2014-6-1-35-44
41. Bangyekan C, Aht-Ong D, Srikulkit K. Preparation and properties evaluation of chitosan-coated cassava starch films. Carbohydr. Polym. 2006;63(1)61–71.
https://doi.org/10.1016/j.carbpol.2005.07.032
42. Raghunandan D, Mahesh BD, Basavaraja S, Balaji SD, Manjunath SY, Venkataraman A. Venkataraman A. Microwave-assisted rapid extracellular synthesis of stable bio-functionalized silver nanoparticles from guava (Psidium guajava) leaf extract. J. Nanopart. Res. 2011;13:2021–2028.
https://doi.org/10.1007/s11051-010-9956-8
43. Prakash S, Chakrabarty T, Singh AK, Shahi VK. Silver nanoparticles built-in chitosan modified glassy carbon electrode for anodic stripping analysis of As(III) and its removal from water. Electrochim. Acta. 2012;72:157–164.
https://doi.org/10.1016/j.electacta.2012.04.025
44. Kalaivani R, Maruthupandy M, Muneeswaran T, et al. Synthesis of chitosan mediated silver nanoparticles (Ag NPs) for potential antimicrobial applications. Front. Lab. Med. 2018;2(1)30–35.
https://doi.org/10.1016/j.flm.2018.04.002
45. Zahran M, Khalifa Z, Zahran A-H, Azzem MA. Recent advances in silver nanoparticle-based electrochemical sensors for determining organic pollutants in water: a review. Mater Adv. 2021;2:7350–7365.
https://doi.org/10.1039/d1ma00769f
46. Eshlaghi MA, Kowsari E, Ehsani A, Akbari-Adergani B, Hekmati M. Functionalized graphene oxide GO-[imi-(CH2)2-NH2] as a high efficient material for electrochemical sensing of lead: Synthesis surface and electrochemical characterization. J Electroanal. Chem. 2020;858:113784.
https://doi.org/10.1016/j.jelechem.2019.113784
47. Zuo Y, Xu J, Jiang F, et al. Voltammetric sensing of Pb(II) using a glassy carbon electrode modified with composites consisting of Co3O4 nanoparticles, reduced graphene oxide and chitosan. J. Electroanal. Chem. 2017;801:146–152.
https://doi.org/10.1016/j.jelechem.2017.07.046
48. Lalmalsawmi J, Zirlianngura Z, Tiwari D, Lee SM. Low cost, highly sensitive and selective electrochemical detection of arsenic (III) using silane grafted based nanocomposite. Environ Eng. Res. 2020;25:579–587.
https://doi.org/10.4491/eer.2019.245
49. Olejnik A, Karczewski J, Dołęga A, Siuzdak K, Grochowska K. Novel approach to interference analysis of glucose sensing materials coated with Nafion. Bioelectrochem. 2020;135:107575.
https://doi.org/10.1016/j.bioelechem.2020.107575
50. Alcaraz-Contreras Y, Garza-Ocañas L, Carcaño-Díaz K, Ramírez-Gómez XS. Effect of Glycine on Lead Mobilization, Lead-Induced Oxidative Stress, and Hepatic Toxicity in Rats. J. Toxicol. 2011;2011:e430539.
https://doi.org/10.1155/2011/430539
51. Wei W, Han X, Shao Y, et al. Comparing the effects of humic acid and oxalic acid on Pb(II) immobilization by a green synthesized nanocrystalline hydroxyapatite. Chemosphere. 2021;285:131411.
https://doi.org/10.1016/j.chemosphere.2021.131411
52. Tiwari D, Zirlianngura , Lee SM. Fabrication of efficient and selective total arsenic sensor using the hybrid materials modified carbon paste electrodes. J. Electroanal. Chem. 2017;784:109–114.
https://doi.org/10.1016/j.jelechem.2016.11.051
Fig. 2(a) SEM image of Ag(NPs)/ChS, (Inset) XRD pattern of Ag(NPs)/ChS, (b) TEM image of spherical Ag(NPs) and (c) TEM image of Ag(NPs) showing d-spacing. 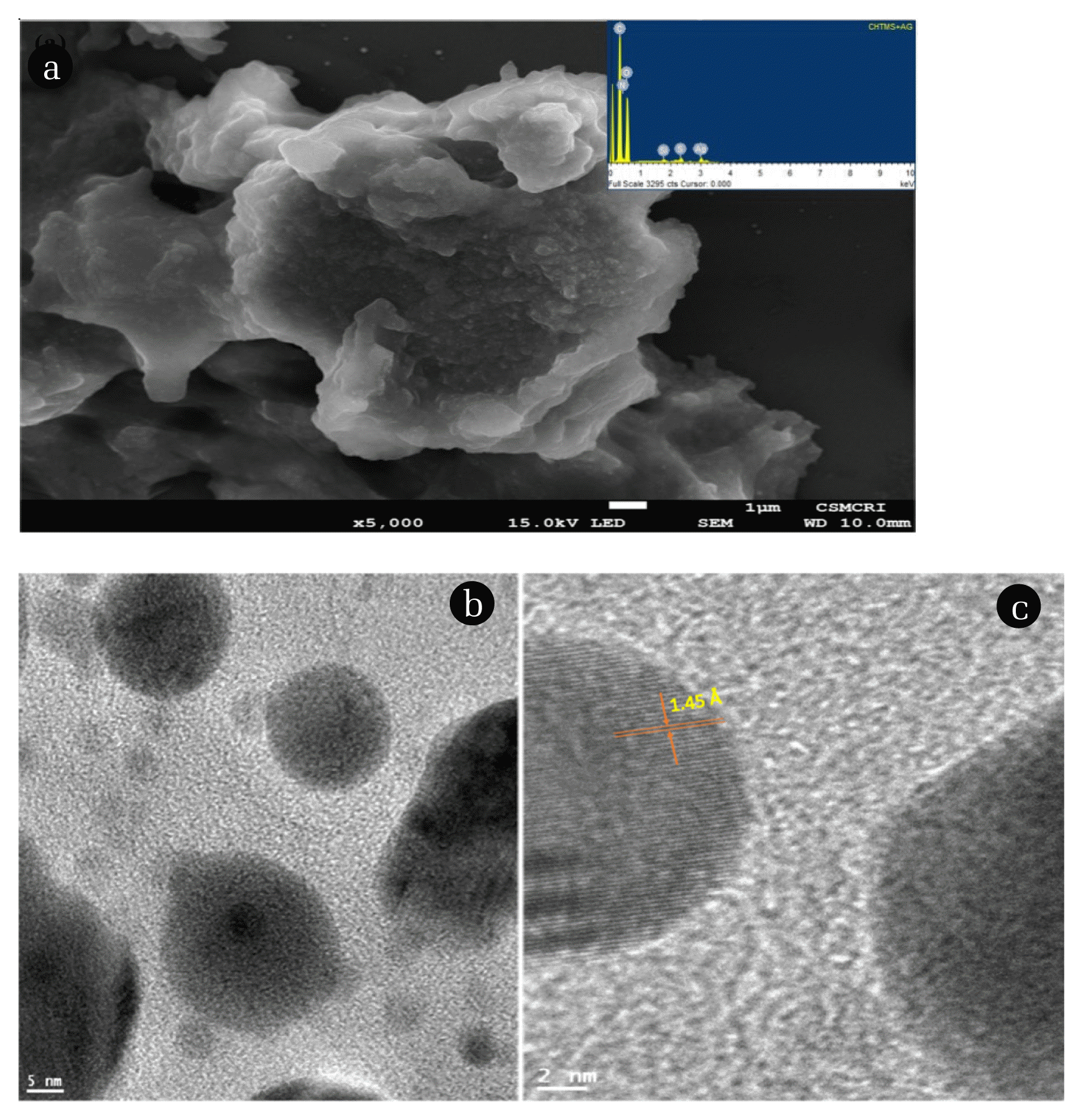
Fig. 350.0 μg/L Pb2+ under DPV study using bare GC, and Ag(NP)/ChS/GC under the optimised parameters [Deposition time: 180.0 sec; Deposition potential: −0.9 V (vs. Ag/AgCl); and pH: 4.5 (acetate buffer 100.0 mmol/L); Pulse amplitude: 0.05 V; Step potential: 0.0001 V; Modulation time: 0.05 sec; and time interval: 0.5 sec]. 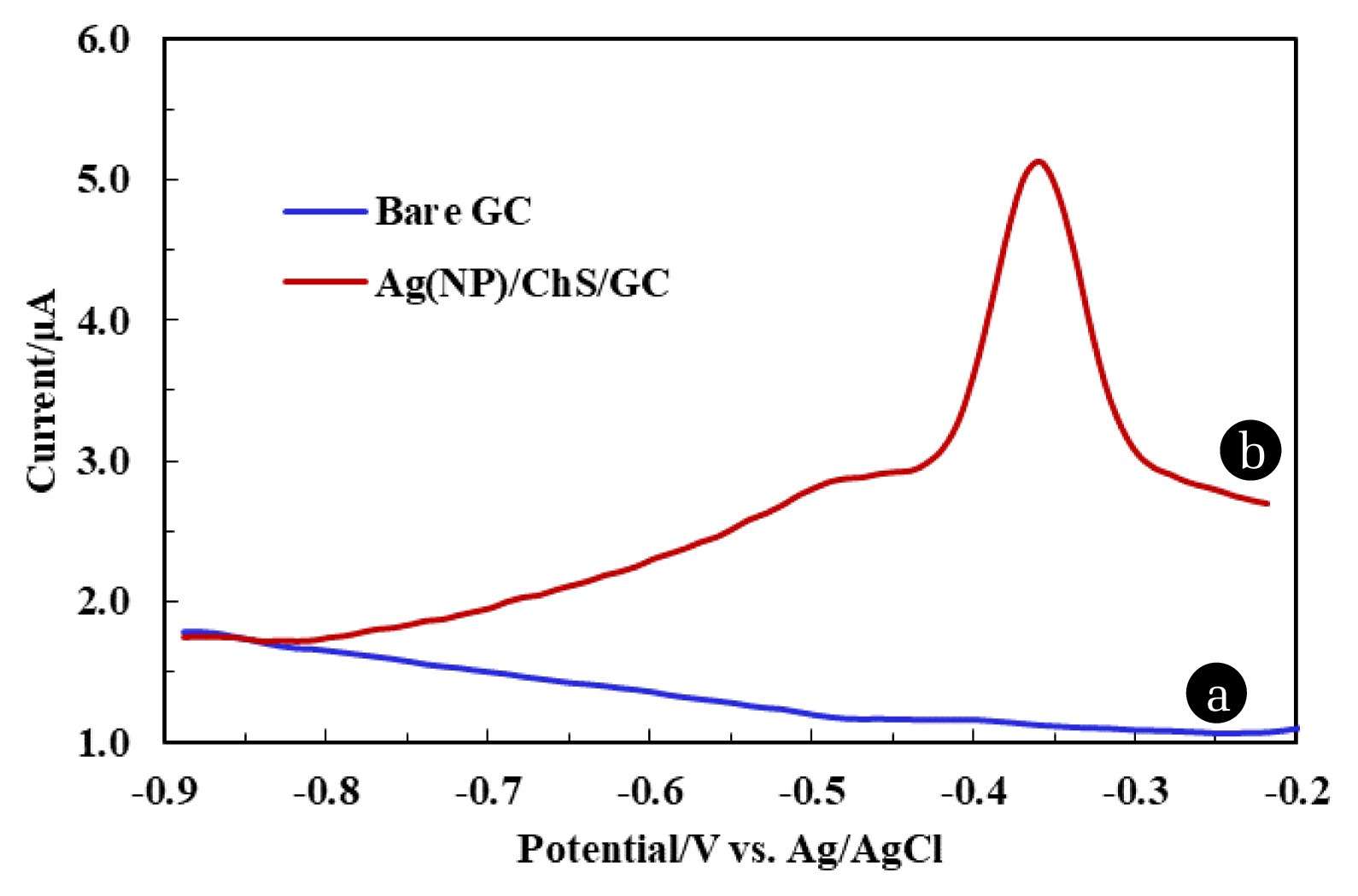
Fig. 4Parametric studies for optimization of 50.0 μg/L Pb2+ (100.0 mmol/L acetate buffer) detection in the DPV measurements using Ag(NPs)/ChS/GC (a) pH dependence studies; (b) Deposition potential studies; and (c) Deposition time studies. 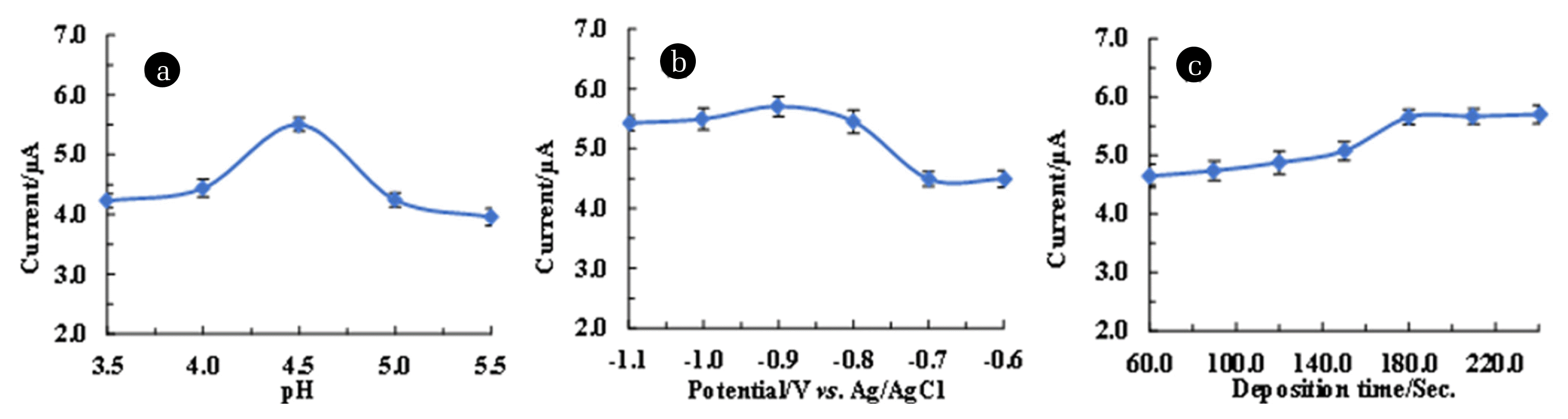
Fig. 5Determination of Pb2+ using Ag(NPs)/ChS/GC under the DPV measurements and the calibration line (Inset). The experiment was carried out under optimised experimental conditions. 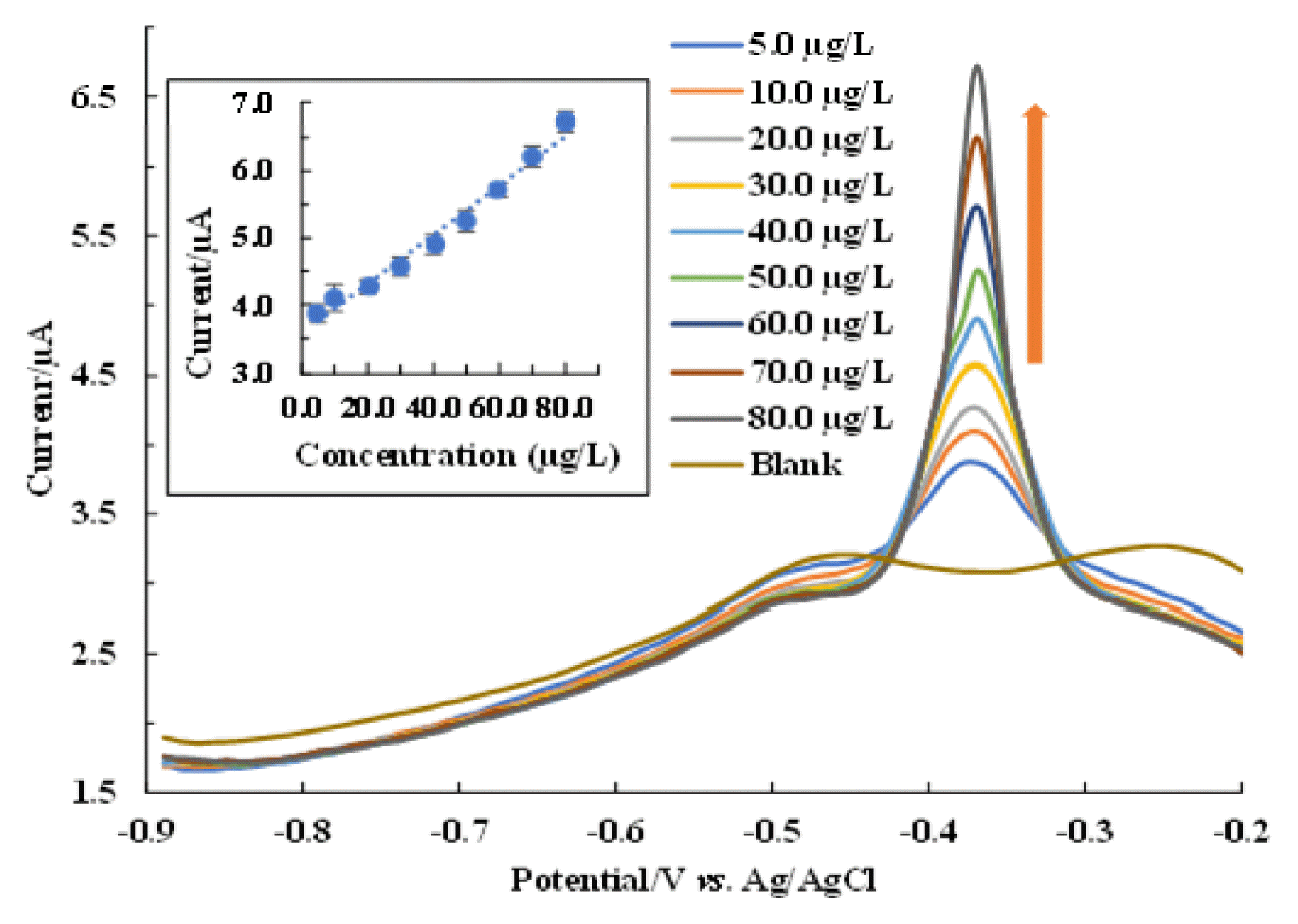
Fig. 6Studies of interfering ions in the detection of Pb2+ (50.0 μg/L) using Ag(NPs)/ChS/GC [Concentration of interfering ions: 500.0 μg/L]. 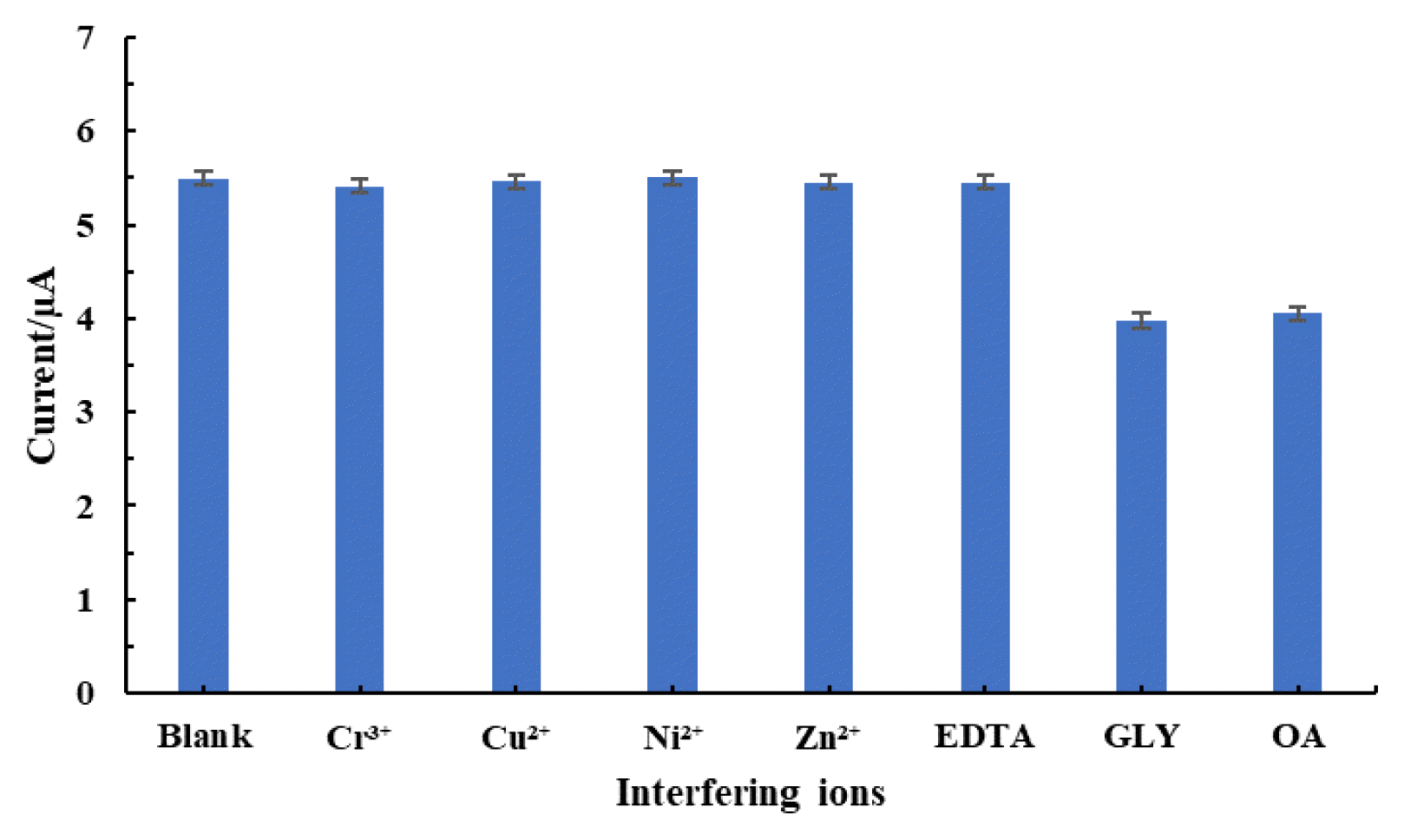
Fig. 7DPV response and the recovery of Pb2+ in spiked (a) Spring water; (b) Ground water) samples under optimised experimental conditions; and (c) Calibration lines for the Pb2+ using natural water samples. 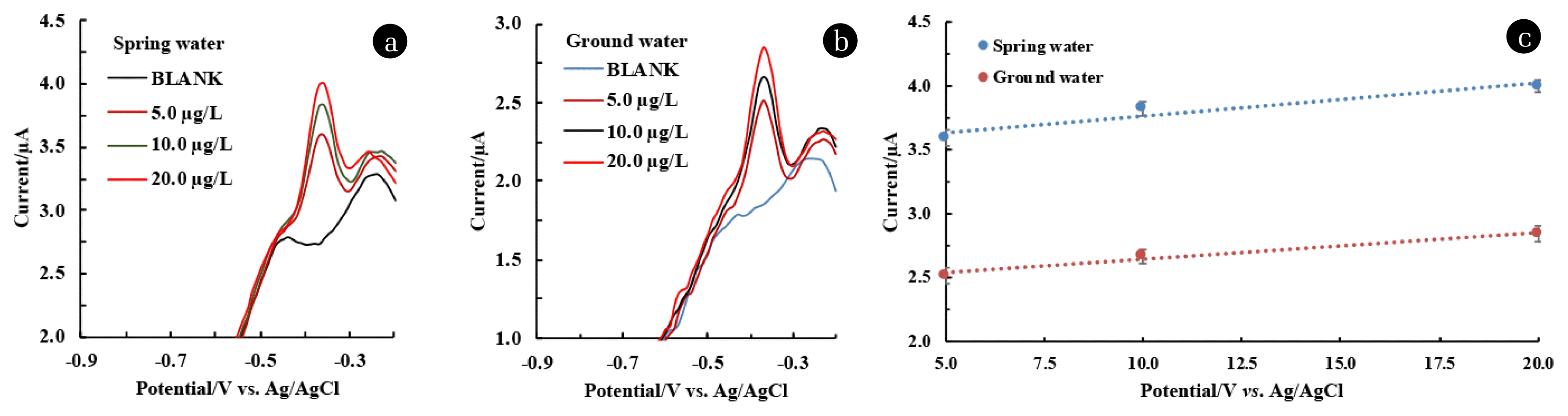
Table 1Comparison of fabricated electrode using the Ag(NPs)/ChS/GC nanocomposite material with previously reported Pb2+ detection works.
SWASV - Square wave anodic stripping voltammetry, GC/GQDs-NF-Glassy Carbon Electrode/Graphene Quantum Dots-Nafion, 5-Br-PADAP/MWCNT-2-(5-bromo-2-pyridylazo)-5-diethylaminophenol/Multiwalled carbon nanotube, PDA-polymer of typical dopamine, BEFs-Bismuth Film Electrodes, SPE/SWCNHs-Screen Printed Electrode/single-walled carbon nanohorns. |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||