AbstractElectro-Fenton (E-Fenton) is a versatile treatment method; however, it has not yet been used for the treatment of electroplating wastewater using graphene oxide (GO) coated TiO2 nanotubes (GO/TiO2NTs) electrode. A simple anodization technique has been adopted to synthesize GO/TiO2NTs electrode. The present work aimed to study an E-Fenton treatment to investigate the effect of different parameters on % COD removal and energy consumption using GO/TiO2NTs electrode. The performance of the treatment process was examined in terms of % COD removal and energy consumed at three process parameters: current, time, and ferrous sulfate concentration. The optimum operating conditions were determined using multiple responses optimization based on Box-Behnken design (BBD). At optimum operational parameters, the results revealed that the % COD removal and energy consumed were 96.27% and 15.35 kWh/m3, respectively. The pseudo-first-order reaction kinetics was fitted to the experimental data at optimum conditions. Possible intermediates were identified based on the GC-MS analysis, and a corresponding tentative degradation pathway has been proposed. Furthermore, a recyclability and stability study of the synthesized electrode has also been performed. Moreover, total organic carbon (TOC) study revealed that E-Fenton has the potential to detoxify electroplating effluents. Hence, GO/TiO2NTs electrodes can be highly efficient in degrading hazardous pollutants.
Graphical Abstract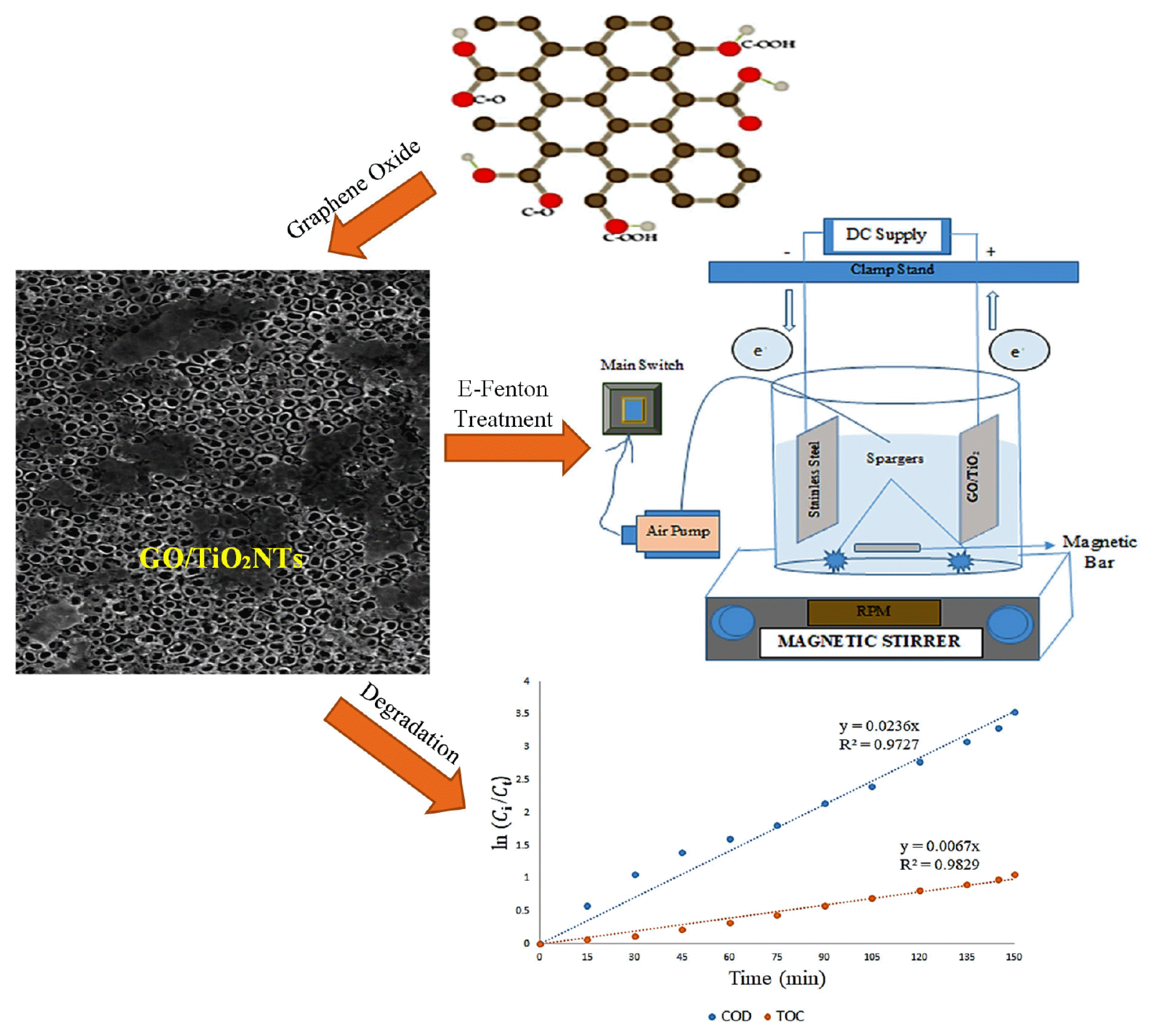
1. IntroductionAs a result of the rapid rise in public awareness of environmental problems and the resulting pressure on governments to introduce legislation that prescribes and limits the emission of pollutants, there has been a noticeable increase in research focusing on the treatment of industrial effluents [1]. The rising concentrations of several contaminants in water have posed a growing concern to humans and ecosystems as a result of the rapid development of urbanization and industry [2, 3]. The world is facing environmental degradation difficulties, particularly water contamination via industrial activities such as mining, electroplating, tannery, textile, pharmaceutical, metallurgical, petrochemicals, and other similar ones [4–7]. Plating is one of the more common industrial processes. Plating is the electroplating procedure used to apply metal to a surface of a substance to prevent corrosion. The industrial electroplating process includes stages that produce many effluents high in heavy metals, COD, cyanides, nitrates, and sulfate complexes. These steps include acid pickling, alkaline cleaning, plating, and rinsing [8]. Furthermore, intensive human activities produce a wide variety of organic compounds from effluents of the electroplating industry, for instance, ethylenediaminetetraacetic acid (EDTA), malic acid (MA), citric acid (CA), sodium lauryl sulfate (SLS) and dihydroxy succinic acid have been attracted great interest in the environmental concerns [9]. In addition, since 1947, the purification of industrial electroplating wastewater has been regarded as a severe challenge due to its chemical composition and effects on the environment in the absence of appropriate and legal laws regulating its discharge into the environment [10]. Due to the frequent exposure to these hazardous contaminants, subtle, acute symptoms have been observed in humans because of their toxicity [11].
Additionally, the effluent from electroplating processes is highly turbid, colored, too acidic (low pH), and capable of causing bio-distortion when absorbed by plants through soil. This harms the ecosystem and indirectly leads to toxins in foods. Therefore, effective initiatives are paramount to reduce the potential risks of these contaminants on humans and the ecosystems. Hence, the contaminants should be removed before being discharged into the natural environment. It is essential to identify an environmentally friendly, cost-effective method of addressing environmental pollution for the long-term development of society and the well-being of individuals. Traditional water treatment techniques are ineffective at removing most contaminants because they are recalcitrant and difficult to degrade [12]. In the recent decade, advanced oxidation processes (AOPs) have become one of the most promising approaches to organic wastewater treatment due to their eco-friendly, superior performance, cost-effectiveness, and lack of secondary contamination [13, 14]. AOPs are processes that generate highly reactive radical species, such as hydroxyl radical (•OH) and sulfate radical (SO4•−), to degrade refractory organic compounds to smaller, less toxic compounds or even mineralize them into the water, CO2, and inorganic salts [15]. E-Fenton is particularly attractive among these advanced electrochemical oxidation processes (EAOPs) because of its simplicity without requiring special equipment, clean, efficient, and highly economical processing of organic pollutants removal. Moreover, electricity is used in the overall process as it is a clean energy source and produces no secondary pollutants [16]. The E-Fenton process is environmentally beneficial for treating water and wastewater because it uses no hazardous chemicals. The electrolyte, pH, current density, Fenton reagents (i.e., Fe2+ and Fe3+), hydrogen peroxide (H2O2) concentration, oxygen flow rate, electrode gap, and temperature are suitable influencing parameters that have an impact on the E-Fenton process’ performance. The solution’s pH significantly affects the E-Fenton process because it is often carried out in an acidic environment. According to prior research, the E-Fenton method is used in an acidic pH range of 2.8 to 3.0 [17–19] to create the hydroxyl radical (•OH) effectively. Numerous alternative catalysts are listed in the literature; however, Fe2+ or Fe3+ have demonstrated good catalytic capabilities even at lower concentrations [20]. In the E-Fenton method, the breakdown of organic pollutants is mediated by the strong •OH radicals produced by catalytic decomposition of electro-generated H2O2 in the treated solution [21]. As H2O2 breaks down into water and oxygen, it is an eco-friendly chemical that does not leave any potentially harmful residues [22].
H2O2 is electro-generated in an acidic medium by a two-electron reduction of oxygen on the cathode surface [23, 24] (Eq. (1)):
or in an alkaline solution by the reaction:
Thus, the strong oxidant of •OH radical can be generated in the solution with the addition of Fe2+ as a catalyst (Eq. 2), and this active species can attack and initiate the oxidation of pollutant (RH) as shown in Eq. (3–5). Moreover, the regeneration of Fe2+ by chemical or electrochemical reactions allows the propagation of the process (Eq. (5–8)) [14].
The anode material can influence the association of other products, and the electrode material is a crucial element in the development of electrochemical processes. To obtain the desired electrodes, three requirements must be satisfied: (1) low production costs, (2) excellent stability under anodic polarisation circumstances, and (3) excellent efficiency in pollutant degradation. As a result of the benefits such as minimal cost, chemical stability, non-toxicity, and high oxidation potential, electrochemical treatments utilizing TiO2 have previously been suggested as an effective treatment approach [25]. However, due to its high e/h+ recombination rates, high bandgap, and complex recycling, TiO2’s practical applications in water treatment are greatly restricted [26]. TiO2 has recently been combined with carbon compounds like carbon nanotube, graphene, graphite, and graphene oxide has attracted extensive interest because of their applications in various fields such as wastewater treatment, catalysts, batteries, and electrochemical capacitors and super-capacitors [27].
The catalytic activity of carbon materials is increased because they serve as an electron reservoir and prevent charge recombination. In contrast to the other carbon compounds listed above, GO material is the most significant because of its low cost, suitable electrical conductivity, and simplicity of production [28]. Additionally, the many hydroxyl groups that cover the surface of GO can interact with other chemical groups, making it simple to incorporate GO into other materials. GO has been transformed into a metal-free catalyst or catalytic support by these functional groups, which enables active sites via surface functionalization. As a result, GO has the potential to be a candidate for a variety of wastewater treatment applications. In EAOPs, the performance of the electrolytic system depends on several parameters, among which the electrode material is the most important. So, the development of highly efficient and cost-effective electrode materials is desirable. Anode materials mainly reported in the literature include IrO2, RuO2 [29], PbO2 [30], SnO2, and Boron-Doped Diamond (BDD) [6]. However, these electrodes are costlier than graphite/carbon electrodes. Some research examined the E-Fenton method for removing organic pollutants from wastewater using several efficient coating materials [31, 32]. These coating anodes have proven effective in treating a wide range of contaminants, including industrial/synthetic wastewater, reverse osmosis concentrate, and landfill leachate, etc. The application of graphene oxide (GO) based on titanium oxide (TiO2) electrodes in electroplating wastewater treatment is rarely reported. Thus, there is a need to investigate the use of these graphene oxide-coated anodes for the degradation of electroplating effluents.
Although E-Fenton has demonstrated promising potential in wastewater treatment, it has not yet been used for the treatment of electroplating wastewater using a synthesized electrode. To the best of the author’s knowledge, this is the first study using synthesized GO/TiO2NTs electrodes for the treatment of synthetic electroplating wastewater by the E-Fenton process.
The aim of the present study is to synthesize GO/TiO2NTs electrode to replace the expensive conventional electrodes (BDD, Pt, Ir, Ru). A reported amount of GO is deposited on the TiO2 electrode to investigate its suitability for electroplating wastewater treatment. The novelty of this study demonstrated an E-Fenton treatment of electroplating industry wastewater using the low-cost GO/TiO2 electrode with a unique capacity to remove COD from the wastewater. A laboratory-scale synthesized GO/TiO2NTs electrode was used to assess the higher efficiency of COD removal. Attempts have been made to reduce treatment time while maintaining the durability and stability of electrode.
The present study seeks to investigate the use of synthesized GO-coated TiO2 nanotube electrodes for the treatment of synthetic electroplating effluent by the E-Fenton process. Furthermore, the E-Fenton process was optimized for a number of process variables, including time (t), current (i), and Fenton catalyst i.e., ferrous sulfate concentration (CFe) for % COD degradation (Z1) and energy consumption (Z2). The Box-Behnken design (BBD) under response surface methodology (RSM) was used to optimize process parameters. Maximizing COD degradation and minimizing energy consumption were the applicable optimization constraints. Gas chromatography-mass spectrometry (GC-MS) analyzed the formations of organic intermediates. As a result, this study proposes a tentative degradation pathway for electroplating effluents based on these intermediates. The degradation mechanism of the whole system is also discussed in this study. In addition, we also investigated the degradation kinetics of a COD and TOC removal in an E-Fenton system. Furthermore, the recyclability and stability of the GO/TiO2 electrode was evaluated based on consecutive reaction cycle tests.
2. Materials and Method2.1. MaterialsTitanium (Ti) sheet with a thickness = 1.0 mm (Grade: 2 with purity = 99.5%) and stainless steel (SS) plate (Grade: AISI 304 with purity = 99.99%) were supplied by AUM Scientific suppliers, Jaipur, India. EDTA disodium salt dihydrate (C10H14O8Na2N2.2H2O, purity = 99%, MB Grade, Ultrapure, Thermo Scientific Chemicals), sodium citrate dihydrate (C6H5Na3O7.2H2O, ≥ 99.0%, ACS grade, LabChem™), sodium lauryl sulfate (C12H25NaO4S, 99%, Powder/NF/FCC grade, Fisher Chemical™), sodium oxalate (C2Na2O4, ≥ 99.5%, Pure, Fisher Chemical™) and graphite flakes (purity = ≥ 99%) were provided from M/s Fisher Scientific Pvt. Ltd. India. Sodium sulfate (Na2SO4, purity = ≥ 99%, LR grade, anhydrous, Sigma-Aldrich) was received from M/s Merck Ltd, India. Potassium dichromate (K2Cr2O7, 99.9%, AR grade, Sigma-Aldrich) and mercury sulfate (HgSO4, 99.0%, ACS grade, Sigma-Aldrich) were used as reagents in COD measurements. The Fenton reagents, i.e., ferrous sulfate LR (Heptahydrate) (Fe2SO4.7H2O) with 99% purity, was provided from Molychem, India. Silver sulfate (Ag2SO4, ≥ 99.99%, GR for analysis ACS grade), hydrofluoric acid (HF, 48%, ACS grade), sulphuric acid (H2SO4, 98%, ACS grade), and nitric acid (HNO3, 69%, ACS grade) were obtained from Sigma-Aldrich. As needed, the solution pH was adjusted using AR grade reagents, i.e., hydrochloric acid (HCl) (37%) and sodium hydroxide (NaOH, purity = ≥ 97%) were brought from M/s Merck Ltd, India. Isopropanol (IPA, C3H8O, purity = 99.5%, AR grade, Loba Chemie) and 1,4-benzoquinone (C6H4O2, 99.5%, AR grade, Merck) were acquired from SD Fine Chemicals, India. All the chemicals employed in the experiments were not modified further and used as such. All the solutions were prepared with high-purity water obtained from a Millipore Milli-Q system, with resistivity ≥18.2 MΩ cm−1 at 25°C.
2.2. Synthesis of GO/TiO2NTs ElectrodeTiO2 nanotube electrodes were made on the surface of a Ti sheet using the anodization method, and the sheet was then calcined. The Ti substrate was etched mechanically and chemically before being anodized. The substrate was mechanically cleaned using different-sized sandpapers before being ultrasonically cleaned in double-distilled water for 30 min. Ti sheet was chemically etched using HF: HNO3: H2O (1:4:10, v/v/v) for 10 seconds before being cleaned with acetone and double-distilled water. The Ti electrode was connected to the positive terminal of the D.C. power source, while the stainless steel (SS) electrode was connected to the negative terminal. Both electrodes were held at a distance of 2 cm for the anodization experiment, which was carried out in a glass reactor. An external voltage of 20 V was applied for 20 min using an HF (200 mL, 5%) based electrolyte. The freshly manufactured electrodes were washed with double-distilled water and calcined for 3 hours at 500°C in a muffle furnace.
A modified Hummers process was used to produce GO from graphite flakes [33]. In addition, GO was electro-deposited onto already-synthesized TiO2 nanotube electrodes to have GO/TiO2NTs electrodes. The anodization process was applied to fabricate a GO TiO2NTs electrode. To produce a GO coated TiO2NTs electrode, the anodization procedure was used. It involved adding 0.5 g of GO to 200 ml of deionized water for 15 min at 15 V, followed by an hour of annealing at 300ºC. Fig. 1 illustrates the schematic representation of the synthesis process that was used.
2.3. Characterization of TiO2NTs and GO/TiO2NTs ElectrodeThe prepared nanotube electrodes were analyzed using the characterization technique. In order to examine the surface morphology, FESEM (Nova Nano FE-SEM 450) was employed. The FESEM was also equipped with an EDS spectroscopy for the elemental analysis of the synthesized nanotube electrode.
2.4. Experimental Setup and Operation2.4.1. Reactor configurationSynthesized GO/TiO2 anodes and parallel-assembled stainless steel (SS) plate cathodes (supplied by AUM Scientific Suppliers, Jaipur, India) were employed in a cubical-shaped batch E-Fenton reactor made of plexiglass with a 0.5 L working capacity (Fig. 1). Each electrode has a surface area of 29.9 cm2 with the following dimensions: 46 mm × 65 mm × 1.0 mm. The distance between electrodes was set at 1 cm. A magnetic stirrer (bought from REMI, India) was employed to mix the synthetic electroplating effluent contained within the E-Fenton reactor. Electricity was supplied to the electrochemical reactor using a DIGITECH DC power supply (Model: 4818A10; 0–25 V, 0–5 A; Roorkee, India).
2.4.2. Experimental procedure and methodologyThe main equipment consisted of a reactor, electrodes, and a power supply. The first step in the research process was to clean the electrode before and after each run. After rinsing with aqua distillate, cleaning was completed by washing the electrode in acetone. The design matrix was followed when conducting the experiments. High precision (± 0.1 mg) weighing balance (purchased from WENSAR, India) to weigh electrolytes and chemicals used for synthetic wastewater preparation. A glass reactor was then filled with 0.5 L of effluent as the next step. The optimum pH for the E-Fenton process is reportedly 3 [34]. Hence, the pH of the wastewater solution was adjusted to 3 before the experiment started. Aqueous HCl solutions were used as necessary to alter the pH of the solution. A conductivity meter was used to determine the conductivity of the initial solution. During the electrocatalytic reaction, the effluent was homogenized using a magnetic stirrer. Wastewater was constantly stirred at a 150-rpm stirrer speed throughout the research. During the experiments, the current was supplied by a precision DC power supply (DIGITECH, Roorkee, India, Model: 4818A10; 0–25 V, 0–5 A). The proper quantity of Na2SO4, which was fixed at 0.1 M for this experiment, was incorporated into the synthetic electroplating solution. Na2SO4 is essential for improving the conductivity of the wastewater sample so that the electrical current can flow through it simultaneously. The anode and cathode are connected to the DC supply by a wire, and electrodes are placed inside the reactor. A 1 cm inter-electrode gap separated the anode-cathode electrodes utilized in this experiment. Power was turned ON, and the time period from this point until the sample was removed was recorded. During the E-Fenton experiment, the current (i) was varied as advised by BBD, and the voltage was periodically recorded to calculate energy consumption. The power supply was turned off after a predetermined time, and a sample was taken from the reactor for further examination. A room temperature of 30°C was used for all runs. After the run was finished, wastewater was filtered using a membrane with a pore size of 0.45 micrometers, and the 100 ml filtrate was then collected for COD analysis. The stages listed above were used to clean the electrode once again. Each experiment was carried out in a lab under extremely exhausting conditions. The wastewater sample was continuously and steadily aerated using air diffusers at the bottom of the reactor. The schematic layout of the experimental design is provided in supplementary data (Fig. S1, supplementary material). Every time an experiment was run, fresh synthetic electroplating wastewater was prepared. The prepared GO/TiO2 electrodes were cleaned and reused for each run.
2.4.3. Wastewater characteristicsSynthetic electroplating wastewater containing 1100 mg/L COD was prepared with sodium citrate, ethylenediaminetetraacetic acid (EDTA), sodium oxalate, sodium lauryl sulfate (SLS), and deionized water [9].
2.5. Analysis and Characterization MethodsThe evaluation of the treatments included the analyses of COD, TOC, pH, and electrical conductivity (EC). The pH solution was monitored and adjusted using a digital pH meter (HANNA model HI2215). The conductivity of the initial solution was taken from a multiparameter instrument (LMMP-30 Labman). The synthetic electroplating wastewater sample in the reactor received a constant flow of air at a rate of 770.00 mL/min using a fish tank pump with an air sparger. The concentration of TOC was determined after the sample was filtered with a 0.45 μm filter paper using an automatic TOC analyzer (Sievers InnovOx Laboratory TOC analyzer). The COD in the wastewater was measured using a COD reactor (HANNA model HI839800), and direct readings were taken using a multiparameter photometer with COD (HANNA model HI83399).
Eq. (9) states that COD0 and CODt are the COD of the initial synthetic and treated wastewater, respectively, whereas Eq. (10) states that TOCt is the TOC at any moment in time and TOC0 is the initial TOC of synthetic wastewater.
Eq.11 calculated the energy consumed (E) during the E-Fenton process.
where, S is the sample volume (L), t is the treatment time (h), i is the current (A), V is the voltage (volts), and CODf is the COD removed (mg/L).
A gas chromatograph–mass spectrometry GC-MS (Agilent 7890B GC & 5977A series GC/MSD) coupled with Flame Ionization Detection (FID) was used to investigate the treated and untreated (at optimum conditions) electroplating wastewater sample by E-Fenton treatment method for disposal study. The GC-MS analysis was performed using an HP-5MS column (30 m × 0.25 mm internal diameter, 0.25 mm thickness) from Agilent Technologies. At splitless injector mode, the injector temperature was 250°C. Inlet line and ion source temperatures were 250°C and 260°C, respectively. The temperature of the GC oven was changed in increments of 10°C between 40°C and 250°C. One microliter of the sample was injected. Helium was used as carrier gas at a flow rate of 15 mL/min. The total time for the GC–MS analysis run was 32 min. All of the aforementioned parameters were measured three times using repeated sampling, and the results were calculated as means. These measurements had relative errors that were less than 5%.
2.6. Experimental Design, Modeling and Data AnalysisExperimental design, modeling, and data analysis were all applied to the BBD under the RSM. The BBD can be used to minimize the number of experimental runs requisite to identify potential inter-parametric interactions and their effects on the E-Fenton degradation of electroplating effluents. RSM helps in experiment design, modeling, assessing the impact of process parameters, and determining the optimal conditions for responses [35]. Using BBD under RSM, it is possible to recognize and quantify the interacting impacts of numerous parameters. The BBD needs a series of experiments according to the following;
where cp is the replicate number for the center point, and k is the factor number. These patterns are produced by merging 2k factorials with incomplete block designs. BBD is a spherical, revolving design consisting of a center point and the midpoints of the cube’s edges, surrounded by a sphere. The BBD is composed of three interconnected 22 factorial designs with points positioned around the design’s center on the surface of a sphere [36]. Numerous chemical and physical processes have benefited from its application, and the number of experimental runs is now determined accordingly [37, 38]. The paramount model is often chosen using the sequential F-test and other adequacy measures. To analyze a system or process that includes a response Z, where Z depends on the input factors: u1, u2,..., uk, the relationship between the response and the input process parameters is given in Eq. (13):
where Z is the response that depends on the input factors (u1, u2,..., uk of k quantitative factors), v is the hypothetical but actual response function, and δr is the residual error that explains the differentiation that the function f can take into account. The percentage of COD removal and energy consumed are referred to as the expected response (Z) in the current study. The statistics and response plots were created using the statistical Design-Expert software, version 13.0.5 (STAT-EASE Inc., Minneapolis, US). A second-order polynomial Eq. (14) was utilized through nonlinear regression to fit the experimental data and find the relevant model terms [39]. The quadratic response model can be defined in terms of all the linear terms, square terms, and linear-by-linear interaction terms as follows:
where k is the number of factors or variables, β0 is the constant, βi is the linear effect of input factor ui, βij is the effect of the linear-by-linear interaction between the input factors ui and uj, βii is the quadratic effect of input factor ui, and δr is the statistical error. An analysis of variance (ANOVA) was used to examine the relationship between the process factors and the responses. In this study, there are two responses for the amount of COD removed (Z1) and the amount of energy consumed (Z2); hence the E-Fenton process parameters were optimized using a multi-response processes approach using a desirability function technique. The desirability function approach is one of the most often utilized methods in the industry for the optimization of multiple response processes [37].
The desirability function converts each response in the optimization of multi-response processes into a corresponding desirability value ranging from 0 to 1. All of the desirability is combined to generate a composite desirability function in order to reduce a multi-response system to a single response. The experiments were carried out by utilizing the BBD with three-level under RSM operational parameters for time, t (30–150 min), current, i (0.4–1.6 A), and ferrous sulfate concentration, CFe (0.2–1.0 mM), on E-Fenton responses such as percent COD degradation (Z1), and energy consumption (Z2) are provided in supplementary data (Table S1, supplementary information). The experiments were designed using the statistical Design-Expert software version 13.0.5 (STAT-EASE Inc., Minneapolis, US). To find the regression equations with the best fit, second-order polynomials were utilized to describe the experimental data. The second-order model must be considered if accurate predictions are to be made for the entire research area. The variance of the expected response was the same at all points due to the rotational capability of the second-order response surface design [40]. The experimental data could not be modelled using the cubic model since it was observed to be aliased.
3. Results and Discussion3.1. CharacterizationFESEM was used to investigate the morphology of synthetic GO coated TiO2 electrodes made through anodization, and the results for the SEM pictures are shown in Fig. 2. As seen in Fig. 2a, the nanotubes in pure TiO2 seemed to be spherical and to have an aggregated porous structure. These nanotubes had diameters ranging from ~ 30 to 50 nm and wall thicknesses of ~ 3 to 7 nm. The results agree with the previously published findings wherein the nanotubes of TiO2 and GO/TiO2 electrodes have been reported to be almost similar [41, 42]. After determining that the TiO2NTs on top of the Ti sheet had an ordered structure, their top-view orientation was used as a substrate to deposit GO nanoparticles. As demonstrated in Fig. 2b, the SEM image of GO coated TiO2 reveals a nonporous, compact, linked structure with irregularly sized particles that completely cover the exposed TiO2. The outcomes show that GO is present over the TiO2NTs electrode in scattered form. EDS analysis was used to perform an elemental analysis of synthesized electrodes, and the findings are displayed in Fig. 2b. The deposited material consisted mainly of non-uniform GO nanoparticles with an average crystal size of 15–25 nm. The loading of GO on TiO2NTs was discovered to be 13.56 wt % with the addition of 0.15 g/L GO as a substrate. Results endorsed the presence of O, Ti, and C elements as illustrated in Fig. 2b, with atom percentages of 29.53%, 56.91%, and 13.56%, respectively. Therefore, the SEM + EDS analysis confirms that GO/TiO2 nanotube electrodes were successfully synthesized.
3.2. Regression Model DevelopmentThe optimum conditions were identified using Design-Expert software version 13.0.5 (STAT-EASE Inc., Minneapolis, US). A three-level, full-factorial BBD design was used along with three independent variables: time (t) (min), current i (Ampere), and concentration of ferrous sulfate (CFe) (mM). The experimental and predicted responses of the 17 runs of experiments on the % of COD removal and energy consumption are shown in Table 1. The two responses Z1 and Z2 were correlated by time (t), current (i), and ferrous sulfate concentration (CFe). In terms of important process parameters, the quadratic model equation for Z1 and Z2 is provided below. Final equation in terms of coded factors are shown as;
The model was effectively matched to the observed quadratic equation based on Eq. (15–16), and only a 2% nonconformity between experimental and predicted values were found. Additionally, it was discovered from Eq. (15–16) that the % COD degradation was positively influenced linearly by all three parameters, namely time (t), current (i) and ferrous sulfate concentration (CFe). At the same time, CFe exerted a negative linear effect, whereas time (t) and current (i) exerted a positive linear effect, on energy consumption. A negative coefficient value of the coefficient was found for the mutual impact of CFe and (i) on energy consumption, whereas a positive value was produced for t and i; t and CFe; i and CFe on % COD removal.
3.2.1. Statistical and ANOVA analysisThe model is validated by an ANOVA, which offers useful details about the significance of the variables, their impact on the response, and their impact on the variables’ effect. The statistical analysis results performed using ANOVA for the responses Z1 and Z2 for the E-Fenton treatment of electroplating wastewater are given in supplementary data (Table S2, supplementary information). The ANOVA assessed the variation with the F-test to see whether the model was viable. Because the model F-values obtained for the responses Z1 and Z2 were 32.75 and 273.73, respectively, it was decided that the model validated by the ANOVA was significant. These strong F-values indicate the appropriateness of the variation around its mean values. A t-test was used to determine the significance of each coefficient in Eq. (15–16). With a 95% confidence level, Prob > F values less than 0.05 show that the regression model is competent and that the model terms are significant [43, 44]. Low coefficient-of-variation (C.V.%) values (1.14 and 4.09) for the % COD degradation and energy consumption indicate a high degree of accuracy. The sequential F-test and other adequacy measures were used to suggest a quadratic model. For the responses Z1 and Z2, adequate precision was found at 21.0112 and 61.3909, respectively.
The two tests were conducted to determine whether the model was adequate for the efficiency of % COD degradation and energy consumption are given in supplementary data (Table S3, supplementary information). These tests included the sequential model sum of squares and model summary statistics. All of the regressions had p-values that were less than 0.01. This means that at least one of the terms in the regression equation had a significant correlation with the response variable. The RSM implied that the interaction between two factors (2FI) and the linear model was insignificant. According to the model summary statistic, the quadratic model had the highest regression coefficients (R2 = 0.9768 and 0.9972) for the % COD degradation and energy consumption, respectively (Table S3).
The signal-to-noise ratio is expressed by adequate precision, and a sufficient precision ratio greater than 4 shows that the model effectively navigates the design space. The ANOVA of the regression model was highly significant for these two quadratic equations, with a p-value < 0.0001. High values of R2, adjusted R2, and predicted R2 regression coefficients were found in the model summary statistics for responses Z1 and Z2 are provided in supplementary data (Table S4, supplementary information), supporting a satisfactory agreement between the observed and predicted values. The adjusted R2 value of 0.9470 and predicted R2 value of 0.8949 showed a difference of less than 0.2. The lack of fit was confirmed by a lack of fit F-value of 0.3449 for % COD degradation, which indicated that it is insignificant. R2 also provided the total variation in the response variable, and the proposed model was found to be highly adequate, as indicated by high R2 values (0.9768 and 0.9972, respectively, for % COD degradation and energy consumption). Furthermore, ANOVA indicated the significant terms for Z1: t, i, CFe, t*i, t*CFe, i2 and Z2: t, i, t*i, t2.
3.2.2. Residual analysisThe impacts of the process variables on % COD degradation and energy consumption under optimal conditions were visualized using the model Eq. (15–16). Diagnostic plots (Fig. S2, supplementary material) provided as supplementary data), which are used to ensure the feasibility of the assumptions and practical experimental values. These diagnostics plots are also employed to evaluate the chosen model’s applicability and define the nature of the models’ residuals [45]. The normal plot of residues (Fig. S2a and S2d) demonstrates that the standard deviation between actual and predicted degradation efficacy is within a reasonable range, indicating no abnormal experimental results. The residuals vs. predicted % COD degradation efficiency and energy consumption plots obtained by quadratic model fitting (Fig. S2b and S2e) demonstrate that the actual experimental results are randomly distributed within the ±4.819 range of externally studentized residuals, indicating that the fitted quadratic model is appropriate. The predicted vs. actual plot (Fig. S2c and S2f) of % COD degradation efficiency and energy consumption infering that they are in good synergy. The experimental values and predicted model points were found to agree, with most of the predicted model points lying extremely close to the diagonal line. All the plots for energy consumption were found to be slightly similar to the plots of % COD degradation efficiency.
3.3. Interaction of Process ParametersTo assess the overall effect of the E-Fenton process parameters, namely t, i, and CFe on Z1 and Z2, ANOVA-based p-values were utilized. (P < 0.05) demonstrates the significance of the model terms, indicating that they significantly impacted the responses’ variability [46]. RSM-generated 3-D response surface graphs were examined in order to investigate the individual and collaborative effects of these E-Fenton parameters on the responses. Fig. 3a–3C and Fig. 4a–4c demonstrate the effects of time, current, and CFe on the response Z1 (% COD degradation) and Z2 (energy consumption) during the process.
Fig. 3a shows that there was a significant increase in the Z1 when the E-Fenton system’s current (i) increased with increasing time (t) [47]. With an increase in i from 0.4 A to 1.3 A, a gradual increase in Z1 can be seen with increasing time (t). Now for current (i) in-between 1.3 A to 1.6 A and time (t) 135–150 min, Z1 practically remains constant with 95% degradation of COD. However, at a steady state (t = 145 min) for i = 1.3 A, maximum Z1 was observed (Fig. 3a). It took longer with a lower current to reach the desired higher value. Additionally, after i = 1.3 A current, there is almost no or minimal change in COD degradation. Fig. 3c depicts the impact of increasing CFe with increasing current (i) on the % COD degradation (Z1). Z1 value varies as a result of the steadily increasing current value, just as time (t) affects Z1 value (Fig. 3a). At 0.4 A current, there is a slight increment in the Z1, but when it increases to the i = 1 A, Z1 is increased to 85%–95%, and around i = 1.3 A current it gives almost 97% COD degradation. A further small increment in the current (i) only gives small changes to the Z1 at a higher concentration of CFe. Increasing current (i) from 1.3 A to 1.6 A sharply increases the Z1 at 0.9 Mm. Z1 increases with an increase in CFe at all current values, but after CFe = 0.8 mM to 1.0 mM only marginal changes are seen or relatively remain constant.
On the other hand, Z1 noticed only slight variations when CFe changed for any current (i). A similar phenomenon was shown in Fig. 3b; Z1 increases as the time value increases. Z1 is only minimally influenced over the interval between t = 30–130 min. Any CFe value showed this trend to be accurate. Additionally, increasing time (t) value always results in an increase in Z1 for higher CFe values (CFe > 0.8mM). At low CFe concentrations, Z1 does not change significantly before t = 130 min [48]. As a result, smaller CFe required more time to reach desired higher values. And at CFe = 0.9 mM, there is an almost immediate change in Z1 after t = 140 min.
Fig. 4a–4c depict the effects of the parameters i, t, and CFe on the Z2. Fig. 4a shows the interaction of current (i) and time (t) on Z2. It can be observed that Z2 is increasing for 1.0 A < i < 1.6 A; however, for all i < 1.0 A, it is decreasing. As shown in Fig. 4a, Z2 increases with current (i) for all values of time (t), but the shift in slope is significantly greater for time intervals longer than 90 min as compared to t < 90 min. Similarly, any value of current (i) Z2 increases with time (t), although the change in slope is much bigger for current i > 1 A than for current i < 1 A. That the Z2 is a function of both the applied current (i) and time (t) is true. The maximum energy requirement is shown in Fig. 4a at t = 150 min and i = 1.6 A. Energy consumption is negligible at lower currents (i) at t = 150 min, while at higher currents (i), energy consumption is faster at t = 45 min than at lower currents (i).
The impact of CFe (mM) v/s time (t) and current (i) on energy consumption (Z2) is depicted in Fig. 4b and 4c. It is evident that the Z2 value increases linearly with time (t) and current (i). On the other hand, for every time (t), Z2 remains unchanged as CFe changes. The same phenomenon was seen in Fig. 4c. It is evident from Fig. 4e and 4f that for any value of current and time, Z2 does not change with a change in CFe. On the other hand, it is also evident that the value of Z2 increases linearly as current is provided to the system; however, up to i = 1 A slightly more energy is consumed, about (5–10 kWh/m3).
3.4. Optimization AnalysisThe experimental conditions were optimized using Design-Expert software version 13.0.5 (STAT-EASE Inc., Minneapolis, MN, USA). For synchronized optimization, the time (t), current (i), and ferrous sulfate concentration (CFe) of the E-Fenton process were chosen as process parameters that remained within the acceptable range, and responses (Z1) were fixed as maximum, but responses (Z2) fixed as a minimum. The operational parameters were optimised using a multi-response optimization technique with a desirability function [49] Eq. (17).
One-sided desirability di is used in the study given by:
where, Zi-min and Zi-max represent the lowest and highest allowable values of response i, respectively, while r is weight and a positive constant. An equal weight was assigned to each response, which was set to r = 1 [50].
Eq. (18) determines the desired response Z1, with appropriate Z1-min and Z1-max values of 81.15 and 97.45 percent, respectively.
Similar to equation (18), desirability for responses Z2 was calculated by Eq. (19).
The overall desirability (D) was calculated by the following Eq. (20).
where, n is the total number of responses, and d1 and d2 are the individual desirability.
The optimal parameter values for simultaneous optimization were determined to be t = 145 min, i = 1.3 A, and CFe = 0.9 mM, with an overall desirability value of 0.85. The Z1 and Z2 proposed by BBD were found to be 97.80% and 14.78 kWh/m3, respectively, under optimal conditions. Actual experiments were carried out in duplicate to confirm the validity of the optimization analysis. At the optimum conditions, the average responses Z1 and Z2 were found to be 96.27 % and 15.35 kWh/m3, respectively, which were nearer to the expected values, as shown in Table 2. The model for promoting the E-Fenton degradation of organic molecules from electroplating wastewater is accurate, as shown by the strong agreement between the predicted and experimental values. It also proves that modeling and optimization using RSM under BBD were carried out satisfactorily.
3.5. Degradation KineticsTo determine the order of the reaction, experimental data was recorded under optimal conditions. Pseudo-first-order, first-order, and second-order models were used to analyze the kinetics of degradation. The COD and TOC degradation from synthetic electroplating wastewater by the E-Fenton process is extremely well followed by the pseudo-first-order model Eq. (21) at any time.
On integrating between known limits and rearranging, the above model becomes
where, Ci (mg/L) and Ct (mg/L) are COD and TOC degradation at t = 0 and at any degradation time t (min), k1 is the reaction rate constant.
The experimental data are fitted to pseudo-first-order reaction kinetics (Eq. (22)) is shown in Fig. 5. A pseudo-first-order kinetic study was performed at optimized conditions obtained from BBD through RSM (i.e. time = 145 min, current = 1.3 A and CFe = 0.90 mM) for the COD and TOC concentration change according to the above rate equation. Consequently, degradation kinetics of COD and TOC were investigated within the time range of 150 min. The experimental data were collected under the optimum conditions available for the batch process, and Fig. 5 shows the pseudo-first-order reaction kinetics of the fitted model for those data. For the E-Fenton process to degrade organic compounds, a pseudo-first-order kinetic model was accurately fitted with a high R2 (~ 0.99) value.
3.6. GC-MS Analysis for Intermediates IdentificationFor the initial and final samples, GC-MS analysis was performed to determine the disposability of the wastewater after treatment produced by the E-Fenton process under optimum circumstances. E-Fenton was used for the treatment of 1100 mg/L synthetic electroplating wastewater solution under optimal circumstances for 145 min to assess the transformation byproducts of organic components. At certain times during the reaction, samples for the GC-MS analysis were removed. The process of mediated oxidation for the E-Fenton treatment may be shown to involve •OH radicals [50]. The GC-MS study are provided in supplementary data (Table S5, supplementary information) found numerous organics that contribute to the COD of the electroplating effluent, including stabilizers, brighteners, coupling agents, polishing and coating agents, cleaning fluid, etc. Before treatment. GC–MS analysis revealed the presence of oxalic acid and 1-Dodecanol (bleaching or cleaning agents) in wastewater. The transformation products or intermediate chemicals found after the treatment of electroplating wastewater samples are given in supplementary data (Table S6, supplementary information). Furthermore, as a result of degradation during the E-Fenton treatment method, these bleaching or cleaning agents were not found in the treated electroplating effluent (Table S6). Except for these bleaching or cleaning agents, other compounds such as Methylpent-4-enylamine, 1-Methyldodecylamine, 1,2-Benzenedicarboxylic acid, butyl 2-methylpropyl ester, and Sarcosine ethyl ester hydrochloride were also eliminated during the E-Fenton process (Table S5 and S6).
Most organics that cause COD are broken down during the E-Fenton process. Comparative analysis of Table S5 and S6 further reveals the production of specific transformation/degradation compounds during the E-Fenton treatment process, including butyl phthalate, Benzenemethanol, -(1-aminoethyl), and 1-Propanamine, N,2-dimethyl. The probable mechanism of the E-Fenton treatment method for electroplating wastewater is provided in supplementary data (Fig. S3, supplementary material). According to GC-MS analysis, bleaching or cleaning substances have been reduced but not entirely removed. Before being released into direct waterways, electroplating wastewater needs to undergo additional treatment.
3.7. Mechanisms of Electroplating Effluents Removal in E-Fenton SystemThe oxidation/degradation mechanisms were predicted based on GC–MS analysis. Fig. 6 depicts a possible catalytic mechanism involved in the degradation of electroplating effluents during the E-Fenton treatment process. This proposed mechanism is in line with the proposed pathway for the degradation of electroplating effluents, as shown in Fig. S3, supplementary material). (i) It was observed that the electrolysis of H2O produces gaseous and O2 and H2 at the anode and cathode surfaces, respectively. (ii) The O2 molecule diffuses to the synthesized electrode surface, where it receives one electron transfer and forms •O2−. As a reactive species, the latter has a high oxidizing capacity for organic compounds. (iii) Both H2 and O2 molecules diffuse to the GO/TiO2 electrode surface and are chemisorbed along with atomic hydrogen and activated O2 formations. After that, H2O2 is produced in-situ by combining atomic hydrogen and O2 [51], and Fe+2 is added externally [52]. (iv) The Fe (II) species, either as a solid or as a Fe2+ ion, can catalyse the decomposition of H2O2, producing •OH, which can oxidize almost all of the organic compounds in the system. A good recycling of Fe3+ to Fe2+ can be accomplished in favor of atomic hydrogen and cathodic reduction. (v) In the degradation of electroplating effluents, direct anodic oxidation with the production of •OH on the electrode surface is also involved. It should be noted that the Fenton-like oxidation of electroplating effluents can also be initiated by electro-generated H2O2, which produces •OH, despite the fact that this process was found to play a minor role in COD degradation. As a result, the synergistic effect of electrochemistry and the GO/TiO2NTs electrode in the E-Fenton process constantly donate a series of reactive species such as H2O2, O2, and •OH in-situ, effectively removing COD from electroplating wastewater.
In order to better understand the oxidation mechanisms for electroplating effluents generated by the E-Fenton process, the contributions of various reactive oxidizing species were determined using radical scavenging experiments.
3.8. Radical OxidationMany studies suggested that anodic oxidation in the E-Fenton system was responsible for micro-pollutant degradation [11, 20]. •OH and HO2 •/•O2− radicals are frequently found in the E-Fenton system (Eqs. (3) and (23)–(30)) [53]. •OH is abundantly produced in the Fenton’s reaction (Eqs. (3) and (23)). A Fenton-like reaction produces HO2• with an oxidation potential of 1.65 V in the E-Fenton system (Eq. (24)). HO2• has the ability to reduce Fe3+ to Fe2+ (Eq. (29). Besides that, O2 can be reduced at the cathode to produce •O2− with an oxidation potential of 0.89 V (Eq. (26)). With a pH of 3, HO2• is the dominant species due to its pKa of 4.8 (Eq. (30), which eliminates the contribution of •O2−. As a result, it was concluded that the contribution of all radicals was collectively essential for COD degradation [54].
In the EAOPs, contaminants can be directly oxidized to form oxidizing pollutant cation, and water molecules on the electrode surface can also be decomposed to form hydroxyl radicals (•OH) adsorbed on the electrode surface. The generated electrons will react with oxygen molecules near the anode to produce superoxide radicals (•O2−), allowing electrons to flow to the stainless-steel (SS) cathode under electrochemical conditions and thus increase efficiency. As a result, hydroxyl radicals, superoxide radicals, and oxidizing pollutant cations can directly oxidize pollutants until mineralization. A free radical quenching study was carried out to determine the effect of active radicals on the degradation of electroplating effluents. Different types of scavengers were introduced into the reaction system to further evaluate the role of active species such as hydroxyl radical (•OH), superoxide ions (•O2−), and holes (h+). Scavengers for superoxide radical (•O2−), hole (h+), and hydroxyl radical (•OH) were 1,4-benzoquinone, Ethylenediaminetetraacetic acid, and iso-propanol (IPA), respectively. The average catalytic activity of GO/TiO2NTs electrodes before and after the addition of 1,4-benzoquinone, ethylenediaminetetraacetic acid, and iso-propanol (IPA) has been provided in supplementary data (Fig. S4, supplementary information). Catalytic activity in terms of average COD degradation was reduced from 96.27% to 41.5% in the presence of scavengers within 145 min of the E-Fenton reaction at optimized operational conditions. Furthermore, E-Fenton degradation of electroplating effluents was ≥ 96% within 145 min in the absence of scavengers. Moreover, a free radical quenching study confirmed that the generation of •OH during E-Fenton treatment was greater than that of •O2− radicals (Fig. S4b), which ultimately aids in mineralization by converting the intermediate into simpler molecules such as CO2 and H2O. According to the results, the degradation and mineralization of electroplating wastewater can occur via oxidation pathways by reacting with oxidizing •OH radicals. When IPA was used as an •OH scavenger in the reaction system, the COD degradation efficacy (59.7%) was significantly reduced. Furthermore, when 1,4-benzoquinone and EDTA were added to the system, the degradation of electroplating effluents was moderately reduced. According to the observations, each selected reactive species played a significant role in regulating the E-Fenton process, and the involvement of •OH was primarily responsible for the degradation of electroplating effluents [55].
3.9. Reusability and Stability StudyFor practical applications, the reusability and stability of the synthesized nanotube electrodes for wastewater treatment are of vital concern [56]. In order to evaluate the reusability of the electrodes, the thirty cycles of experiments were performed using the synthesized GO/TiO2NTs electrode under the optimized electrocatalytic conditions (i.e. time = 145 min, current = 1.3 A and CFe = 0.90 mM). After each cycle, the used electrodes were cleaned and air-dried before being used in the next experiment with a fresh aqueous solution of electroplating effluents. The performance of the synthesized GO/TiO2 electrode remained consistent over seven runs.
Moreover, the E-Fenton process degradation using synthesized electrodes decreased marginally from 96.21% to 95.02% after the ten repeated cycles. It was slightly reduced to 86.12% by the end of the 30th cycle, as shown in Fig. S5 (supplementary material) provided as supplementary data, highlighting the reusability potential of GO coated TiO2NTs electrode. The decrease in active sites due to the deposition of contaminants on the electrode surface may be responsible for the loss of 10.09% in effluent degradation after repeated use. The synthetic GO coated TiO2NTs electrode also showed remarkable stability after repeated use in the E-Fenton degradation process, as no corrosion effects were noticeable even after the thirty consecutive cycles.
4. ConclusionsThis study aims to demonstrate the effective degradation of COD by modifying TiO2 nanotube electrodes with graphene oxide (GO) coated material. This study investigates the feasibility of the Electro-Fenton (E-Fenton) technology for the treatment of electroplating wastewater. A Box-Behnken design (BBD) was used in batch E-Fenton experiments to optimize the effect of influencing process parameters such as electrolysis time, current, and ferrous sulfate concentration (CFe) on degradation efficiency and energy consumption. 96.27% COD degradation and 15.35 (kWh/m3) energy consumption were observed after t = 145 min at i = 1.3 A current with CFe of 0.90 mM. The pseudo-first-order reaction kinetics was fitted to the experimental data at optimum conditions with the kinetic rate constants for the removal of COD = 0.0238 min−1 and TOC = 0.0067 min−1 were observed. According to the Gas chromatography-mass spectrometry (GC-MS) analysis, the electroplating wastewater compounds are not fully mineralized but are minimized. The role of reactive species in the degradation process was investigated using radical scavengers, and hydroxyl radical (•OH) was found to be the most effective. It was concluded that E-Fenton technology could be used as a polishing step for electroplating industry wastewater to improve the water quality for potential reuse.
References1. Panizza M, Cerisola G. Electro-Fenton degradation of synthetic dyes. Water Res. 2009;43:339–344.
https://doi.org/10.1016/j.watres.2008.10.028
2. Ahmed BS, Hamasalih LO, Aziz KHH, Salih YM, Mustafa FS, Omer KM. Efficient Oxidative Desulfurization of High-Sulfur Diesel via Peroxide Oxidation Using Citric, Pimelic, and α-Ketoglutaric Acids. Separations. 2023;10:1–12.
https://doi.org/10.3390/separations10030206
3. Xing L, Wei J, Zhang Y, et al. Boosting active sites of protogenetic sludge-based biochar by boron doping for electro-Fenton degradation towards emerging organic contaminants. Sep. Purif. Technol. 2022;294:121160.
https://doi.org/10.1016/j.seppur.2022.121160
4. Radhakrishnan K, Sethuraman L, Panjanathan R, Natarajan A, Solaiappan V, Thilagaraj WR. Biosorption of heavy metals from actual electroplating wastewater using encapsulated Moringa oleifera beads in fixed bed column. Desalin. Water Treat. 2016;57:3572–3587.
https://doi.org/10.1080/19443994.2014.985725
5. Bankole MT, Abdulkareem AS, Mohammed IA, et al. Selected Heavy Metals Removal From Electroplating Wastewater by Purified and Polyhydroxylbutyrate Functionalized Carbon Nanotubes Adsorbents. Sci. Rep. 2019;9:1–19.
https://doi.org/10.1038/s41598-018-37899-4
6. Tao NC, LeLuu T. Different behaviours of biologically textile wastewater treatment using persulfate catalyzed electrochemical oxidation process on Ti/BDD and Ti/SnO2-Nb2O5 anodes. Environ. Eng. Res. 2023. 28:1–13.
https://doi.org/10.4491/eer.2022.555
7. Chakraborty S, Mukherjee A, Das S, Maddela NR, Iram S, Das P. Study on isotherm, kinetics, and thermodynamics of adsorption of crystal violet dye by calcium oxide modified fly ash. Environ. Eng. Res. 2021;26:1–9.
https://doi.org/10.4491/eer.2019.372
8. Martin-Lara MA, Blazquez G, Trujillo MC, Perez A, Calero M. New treatment of real electroplating wastewater containing heavy metal ions by adsorption onto olive stone. J. Clean. Prod. 2014;81:120–129.
https://doi.org/10.1016/j.jclepro.2014.06.036
9. Kong X, Zhou Y, Xu T, et al. A novel technique of COD removal from electroplating wastewater by Fenton—alternating current electrocoagulation. Environ. Sci. Pollut. Res. 2020;27:15198– 15210.
https://doi.org/10.1007/s11356-020-07804-6
10. De Beni E, Giurlani W, Fabbri L, et al. Graphene-based nanomaterials in the electroplating industry: A suitable choice for heavy metal removal from wastewater. Chemosphere. 2022;292:133448.
https://doi.org/10.1016/j.chemosphere.2021.133448
11. Abbas ZI, Abbas AS. Oxidative degradation of phenolic wastewater by electro-fenton process using MnO2-graphite electrode. J. Environ. Chem. Eng. 2019;7:103108.
https://doi.org/10.1016/j.jece.2019.103108
12. Rajoria S, Vashishtha M, Sangal VK. Treatment of electroplating industry wastewater: a review on the various techniques. Environ. Sci. Pollut. Res. 2022;29:72196–72246.
https://doi.org/10.1007/s11356-022-18643-y
13. Asaithambi P, Yesuf MB, Govindarajan R, Hariharan NM, Thangavelu P, Alemayehu E. A Review of Hybrid Process Development Based on Electrochemical and Advanced Oxidation Processes for the Treatment of Industrial Wastewater. Int. J. Chem. Eng. 2022;2022:1–15.
https://doi.org/10.1155/2022/1105376
14. Zhou M, Yu Q, Lei L, Barton G. Electro-Fenton method for the removal of methyl red in an efficient electrochemical system. Sep. Purif. Technol. 2007;57:380–387.
https://doi.org/10.1016/j.seppur.2007.04.021
15. Mustafa FS, Aziz KHH. Heterogeneous catalytic activation of persulfate for the removal of rhodamine B and diclofenac pollutants from water using iron-impregnated biochar derived from the waste of black seed pomace. Process Saf. Environ. Prot. 2023;170:436–448.
https://doi.org/10.1016/j.psep.2022.12.030
16. Nidheesh PV, Gandhimathi R. Trends in electro-Fenton process for water and wastewater treatment: An overview. Desalination. 2012;299:1–15.
https://doi.org/10.1016/j.desal.2012.05.011
17. Huang YH, Su HT, Lin LW. Removal of citrate and hypophosphite binary components using Fenton, photo-Fenton and electro-Fenton processes. J. Environ. Sci. 2009;21:35–40.
https://doi.org/10.1016/s1001-0742(09)60008-5
18. Huang B, Qi C, Yang Z, et al. Pd/Fe3O4 nanocatalysts for highly effective and simultaneous removal of humic acids and Cr(VI) by electro-Fenton with H2O2 in situ electro-generated on the catalyst surface. J. Catal. 2017;352:337–350.
https://doi.org/10.1016/j.jcat.2017.06.004
19. Sun Y, Pignatello JJ. Photochemical Reactions Involved in the Total Mineralization of 2,4-D by Fe3+/H2O2/UV. Environ. Sci. Technol. 1993;27:304–310.
https://doi.org/10.1021/es00039a010
20. Kaur P, Sangal VK, Kushwaha JP. Parametric study of electro-Fenton treatment for real textile wastewater, disposal study and its cost analysis. Int. J. Environ. Sci. Technol. 2019;16:801–810.
https://doi.org/10.1007/s13762-018-1696-9
21. Xu T, Zheng X, Zhou Y, et al. Study on the treatment of Cu2+ organic compound wastewater by electro-Fenton coupled pulsed AC coagulation. Chemosphere. 2021;280:130679.
https://doi.org/10.1016/j.chemosphere.2021.130679
22. Rajoria S, Vashishtha M, Sangal VK. Review on the treatment of electroplating industry wastewater by electrochemical methods. Mater. Today. Proc. 2021;47:1472–1479.
https://doi.org/10.1016/j.matpr.2021.04.165
23. Lv J, Wang W, Zhao Q, Wang K. Bioelectrochemical performance of microbial fuel cell powered electro-Fenton system (MFC ⓅEFs) with composite PANI-Mn/CF anode. Environ. Eng. Res. 2022;28:220204.
https://doi.org/10.4491/eer.2022.204
24. Öztürk H, Barışçı S, Turkay O. Paracetamol degradation and kinetics by advanced oxidation processes (AOPs): Electro-peroxone, ozonation, goethite catalyzed electro-fenton and electro-oxidation. Environ. Eng. Res. 2021;26:180332.
https://doi.org/10.4491/eer.2018.332
25. Baqer AR, Beddai AA, Farhan MM, Badday BA, Mejbel MK. Efficient coating of titanium composite electrodes with various metal oxides for electrochemical removal of ammonia. Results Eng. 2021;9:100199.
https://doi.org/10.1016/j.rineng.2020.100199
26. Rajput H, Sangal VK, Dhir A. Synthesis of highly stable and efficient Ag loaded GO/TiO2 nanotube electrodes for the photo-electrocatalytic degradation of pentachlorophenol. J. Electroanal. Chem. 2018;814:118–126.
https://doi.org/10.1016/j.jelechem.2018.02.053
27. Veeresh S, Ganesha H, Nagaraju YS, et al. Graphene oxide/cobalt oxide nanocomposite for high-performance electrode for super-capacitor application. J. Energy Storage. 2022;52:104715.
https://doi.org/10.1016/j.est.2022.104715
28. Bigdeloo M, Kowsari E, Ehsani A, Chinnappan A, Ramakrishna S. Functionalized graphene oxide/activated carbon from canola waste as sustainable nanomaterials to improve pseudocapacitance performance of the electroactive conductive polymer. J. Energy Storage. 2022;50:104279.
https://doi.org/10.1016/j.est.2022.104279
29. Deng M, Wu K, Yang T, et al. Construction of Novel Electro-Fenton Systems by Magnetically Decorating Zero-Valent Iron onto RuO2-IrO2/Ti Electrode for Highly Efficient Pharmaceutical Wastewater Treatment. Water. 2022;14:1–19.
https://doi.org/10.3390/w14071044
30. Wang J, Xu M, Liang X, et al. Development of a novel 2D Ni-MOF derived NiO@C nanosheet arrays modified Ti/TiO2NTs/PbO2 electrode for efficient electrochemical degradation of salicylic acid wastewater. Sep. Purif. Technol. 2021;263:118368.
https://doi.org/10.1016/j.seppur.2021.118368
31. Kaur P, Park Y, Sillanpaa M, Imteaz MA. Synthesis of a novel SnO2/graphene-like carbon/TiO2 electrodes for the degradation of recalcitrant emergent pharmaceutical pollutants in a photo-electrocatalytic system. J. Clean. Prod. 2021;313:127915.
https://doi.org/10.1016/j.jclepro.2021.127915
32. Kaur P, Park Y, Imteaz MA, Sillanpaa M. Synthesis of non-active electrode( TiO2/GO/Ag) for the photo-electro-Fenton oxidation of micropollutants in wastewater. Int. J. Environ. Sci. Technol. 2022;20:639–652.
https://doi.org/Https://doi.org/10.1007/s13762-022-04065-3
33. Paulchamy B, Arthi G, Lignesh BD. A Simple Approach to Stepwise Synthesis of Graphene Oxide Nanomaterial. J. Nanomed. Nanotechnol. 2015;6:1–4.
https://doi.org/10.4172/2157-7439.1000253
34. Qu C, Liang DW. Novel electrochemical advanced oxidation processes with H2O2 generation cathode for water treatment: A review. J. Environ. Chem. Eng. 2022;10:107896.
https://doi.org/10.1016/j.jece.2022.107896
35. Singla J, Thakur I, Sangal V, Verma A. Dimensionally stable anode (Doped-MMO) mediated electro-oxidation and multi-response optimization study for remediation of urea wastewater. Chemosphere. 2021;285:131498.
https://doi.org/10.1016/j.chemosphere.2021.131498
36. Singh P, Dhir A, Sangal VK. Optimization of photocatalytic process parameters for the degradation of acrylonitrile using Box Behnken Design. Desalin. Water Treat. 2015;55:1501–1508.
https://doi.org/10.1080/19443994.2014.929032
37. Sangal VK, Kumar V, Mishra IM. Optimization of a divided wall column for the separation of C4–C6 normal paraffin mixture using Box-Behnken Design. Chem. Ind. Chem. Eng. Q. 2013;19:107–119.
https://doi.org/10.2298/ciceq121019047s
38. Tarangini K, Kumar A, Satpathy GR, Sangal VK. Statistical optimization of process parameters for Cr (VI) biosorption onto mixed cultures of Pseudomonas aeruginosa and Bacillus subtilis. Clean. 2009;37:319–327.
https://doi.org/10.1002/clen.200900033
39. Nachiyar GKV, Surendra TV, Kalaiselvi V, et al. Box – Behnken response surface methodology design for amaranth dye degradation using gold nanoparticles. Optik. 2022;267:169633.
https://doi.org/10.1016/j.ijleo.2022.169633
40. Sangal VK, Kumar V, Mishra IM. Optimization of structural and operational variables for the energy efficiency of a divided wall distillation column. Comput. Chem. Eng. 2012;40:33–40.
https://doi.org/10.1016/j.compchemeng.2012.01.015
41. Rajput H, Changotra R, Kumar Sangal V, Dhir A. Photoelectrocatalytic treatment of recalcitrant compounds and bleach stage pulp and paper mill effluent using Au-TiO2 nanotube electrode. Chem. Eng. J. 2021;408:127287.
https://doi.org/10.1016/j.cej.2020.127287
42. Rajput H, Dhir A, Sangal V, kumar GO. Mediated TiO2 Nanotube Electrode for the Photoelectrocatalytic Degradation of Pentachlorophenol. J. Electrochem. Soc. 2018;165:H16–H26.
https://doi.org/10.1149/2.0871802jes
43. Guvenc SY, Daniser Y, Can-Güven E, Varank G, Demir A. Pre-coagulated landfill leachate treatment by Electro-oxidation using MMO/Ti, Pt/Ti, and graphite anodes. Environ. Eng. Res. 2022;28:210419.
https://doi.org/10.4491/eer.2021.419
44. Hosseini SS, Nazif A, Alaei Shahmirzadi MA, Ortiz I. Fabrication, tuning and optimization of poly (acrilonitryle) nanofiltration membranes for effective nickel and chromium removal from electroplating wastewater. Sep. Purif. Technol. 2017;187:46–59.
https://doi.org/10.1016/j.seppur.2017.06.018
45. Pathak A, Khandegar V, Kumar A. Statistical Investigation in Conjunction with a Box–Behnken Design for the Removal of Dyes Using Electrocoagulation. J. Hazard., Toxic, Radioact. Waste. 2022;26:04022001.
https://doi.org/10.1061/(asce)hz.2153-5515.0000679
46. Singh A, Mishra BK. Removal of chlorhexidine digluconate from aqueous solution by heterogenous photocatalysis using sunlight-driven Ni-doped TiO2 material. Environ. Eng. Res. 2022;28:210546.
https://doi.org/10.4491/eer.2021.546
47. Guo F, Lou Y, Yan Q, et al. Insight into the Fe–Ni/biochar composite supported three-dimensional electro-Fenton removal of electronic industry wastewater. J. Environ. Manage. 2023;325:116466.
https://doi.org/10.1016/j.jenvman.2022.116466
48. Pajootan E, Arami M, Rahimdokht M. Discoloration of wastewater in a continuous electro-Fenton process using modified graphite electrode with multi-walled carbon nanotubes/surfactant. Sep. Purif. Technol. 2014;130:34–44.
https://doi.org/10.1016/j.seppur.2014.04.025
49. Kumar M, Bishnoi NR. Statistical approach in optimum conditions simulation and kinetic modeling for the confirmed electroplating industrial effluents treatment. Bioresour. Technol. Reports. 2020;10:100416.
https://doi.org/10.1016/j.biteb.2020.100416
50. Kaur P, Kushwaha JP, Sangal VK. Transformation products and degradation pathway of textile industry wastewater pollutants in Electro-Fenton process. Chemosphere. 2018;207:690– 698.
https://doi.org/10.1016/j.chemosphere.2018.05.114
51. Nguyen TT, Nam SN. Sunlight-driven photocatalysis of dissolved organic matter: Tracking by excitation emission matrix- parallel factor analysis and optimization using response surface methodology. Environ. Eng. Res. 2021;26:200201.
https://doi.org/10.4491/eer.2020.201
52. Gümüş D. Electrochemical treatment of a real textile wastewater using cheap electrodes and improvement in COD removal. Res. Sq. 2022;0–14.
https://doi.org/10.21203/rs.3.rs-2401313/v1
53. Brillas E. Fenton, photo-Fenton, electro-Fenton, and their combined treatments for the removal of insecticides from waters and soils. A review. Sep. Purif. Technol. 2022;284:120290.
https://doi.org/10.1016/j.seppur.2021.120290
54. Wang A, Jiang Y, Yan Y, et al. Mechanistic and quantitative profiling of electro-Fenton process for wastewater treatment. Water Res. 2023;235:119838.
https://doi.org/10.1016/j.watres.2023.119838
55. Hien NT, Nguyen LH, Van HT, et al. Heterogeneous catalyst ozonation of Direct Black 22 from aqueous solution in the presence of metal slags originating from industrial solid wastes. Sep. Purif. Technol. 2020;233:115961.
https://doi.org/10.1016/j.seppur.2019.115961
56. Rahman KO, Aziz KHH. Utilizing scrap printed circuit boards to fabricate efficient Fenton-like catalysts for the removal of pharmaceutical diclofenac and ibuprofen from water. J. Environ. Chem. Eng. 2022;10:109015.
https://doi.org/10.1016/j.jece.2022.109015
Fig. 33-D response surface graph for the E-Fenton of electroplating wastewater (a–c) for % COD degradation v/s time (t), current (i), and CFe (mM). 
Fig. 43-D response surface graph for the E-Fenton of electroplating wastewater (a–c) for energy consumption v/s time (t), current (i), and CFe (mM). 
Table 1Design of experiment matrix for the E-Fenton of electroplating industry wastewater |
|
|||||||||||||||||||||||||||||||||||||||