AbstractSoil quality assessment is generally performed using a combination of physical, chemical, and biological indicators in a target area, and the indicator measurement data are subjected to sequential processes such as scoring, weighting, and indexing. The selection of indicators is one of the important processes, and their actual application should be carefully considered. The techniques of soil remediation can affect the measurement of traditional biological indicators. In the present study, we aimed to evaluate the applicability of ecotoxicological indicators for soil quality assessment. The paddy and upland soil plots were constructed using contaminated-, remediated-, and reclaimed-soils on field, and the soil quality indicators were measured. We designed a soil quality assessment system including ecotoxicological indicators, and the soil quality index (SQI) was calculated using indicator measurement data of each plot. The SQI with or without ecotoxicological indicators was directly compared with in situ crop productivity. We found that relatively higher SQI can be obtained with ecotoxicological indicators than that without them, and soil quality indexing with ecotoxicological indicators showed high correlations with in situ crop productivity in paddy and upland soil plots.
Graphical Abstract
1. IntroductionSoil quality is defined as the capacity of a soil to function within the ecosystem boundaries [1]. To quantify and manage soil quality, the method of soil quality index is the most commonly employed today, because it has modifiability and flexibility for quantification of soil indicators [2, 3]. The soil indicators are classified by physical, chemical and biological properties, and they are treated through sequential processes such as scoring, weighting and indexing for soil quality assessment [2–6]. Especially, incorporating biological indicators enables a better representation of soil quality in comparison with using physicochemical indicator alone [7, 8]. The organic carbon content, plant cover, mineralizable N content, root quality rating, and soil respiration have been used as biological indicators in previous studies [9–13], and the abundance (individuals/m2) of living organisms has been applied in the target area [9, 11]. These indicators are well-verified parameters for soil quality assessment, but there is a need for in-depth studies to evaluate contaminated or remediated soils.
Many in situ and ex situ soil remediations have been conducted to minimize risks associated with toxic contaminants, and various techniques such as solidification, immobilization, soil washing/flushing, phytoremediation, and amendment applications (e.g., biochar) have been widely used in field remediation [14]. In addition, soil reclamation is often considered as one of the restoration processes for converting the damaged soils before its productive uses such as revegetation [15, 16]. These techniques can change the physicochemical soil properties and disrupt the soil balance for living organisms [14–19], and the application of biological indicators could be difficult in the remediated or reclaimed soil. Previous studies have suggested ecotoxicological bioassay using earthworms and plants as an alternative tool since these assays can be used to assess chemical bioavailability and risk characterization after remediation process [20–23]. In addition, Wang et al. [24] reported that several bioassays can capture the changes in soil physicochemical characteristics.
The ecotoxicological approaches have been used to evaluate the protection level of ecological receptors, and ecotoxicological data is generally applied in terms of the species sensitivity distribution (SSD) concept [25, 26]. Hence, ecotoxicological bioassay has good sensitivity for evaluating chemical sensitivity along with various field soil properties [27–29]. Kim et al. [30] has suggested that the use of authentic ecotoxicological data can be reasonable for interpreting the soil quality since the ecotoxicological indicators provide direct evidence of biological response in the soils. The purpose of this study was to evaluate the applicability of ecotoxicological indicators for soil quality assessment, and the field experiments were conducted to compare with crop productivity in metal contaminated, remediated, and reclaimed soils. The soil quality assessment system using ecotoxicological indicators was designed according to previous studies [30], and the soil quality index (SQI) was calculated with or without ecotoxicological indicators. Several crops (rice, perilla, and soybean) were cultivated, and field productivity under in situ condition was calculated to verify the applicability of these ecotoxicological indicators.
2. Materials and Methods2.1. Descriptions of Soil PlotsThe study sites are located in the western side of South Korea (36°00′48″ N, 126°39′56″ E) (Fig. 1A), and the privately-owned paddy-growing field nearby the metal smelter had been extensively contaminated with heavy metals (arsenic, cadmium, and lead) [31]. Korean Government projects were implemented to remediate metal-contaminated paddy soils, and the acid washing (0.5 M H2SO4 and 0.5 M H3PO4) technique was applied to remove metal contaminants in the privately-owned paddy field soils. In the present study, the metal contaminated and remediated paddy soils were collected, and the in situ test plots were constructed in an area of 5 m (width) × 15 m (length) × 1 (height) m (Fig. 1B). To construct the reclaimed paddy soil plot, a 30-cm layer of clean soil was used to cover the remediated soil of depth 70-cm (total height, 1 m). This layer-covering is the Korean Government-recommended technique to restore privately-owned paddy fields. This reclaimed soil represented the privately-owned agricultural fields since the remediated soils have reconveyed to each site and used for the crop cultivation. The clean soil was obtained from a different area (36°03′17″ N, 126°40′54″ E), which is 4.97 km distant from study area. The soils were fertilized with 4.5 kg of nitrogenous manure, 4.5 kg of phosphorus manure, and 6.1 kg of potassium manure per hectare, and irrigated using stream water.
The history and location of the upland soils were the same as that of the paddy soils. The upland soils had been contaminated with heavy metals and remediated by soil washing using 0.1 N NaOH and Na2CO3 (alkali reduction or oxidation-reduction). The upland soils were classified as contaminated, remediated, and reclaimed soil plots. The reclaimed upland soil plot was prepared by covering a 30-cm layer of clean soil over the remediated soil of a 70-cm depth (total height, 1 m). The in situ test plots were constructed with same design of paddy plots (Fig. 1C). Each plot was treated with 1.2 kg/m2 of fertilizer (Saengjiwang, Yeongmin Tech., Hwoaseong, South Korea) consisting of 30% peat, 20% vermiculite, 20% biotite, 10% zeolite, 5% treacle, and 15% other substances (e.g., amino acids).
2.2. Soil Sampling and Data CollectionApproximate 2 kg of surface soil samples (0–10 cm) were collected from each plot at the end of crop cultivation, and each soil sample (<2 mm) was air-dried (24°C) for two weeks. Soil physical, chemical, biological, and ecotoxicological indicators were classified according to previous study [30]. Thirteen soil physical, chemical, and biological indicators were chosen, and the five ecotoxicological indicators were selected (Table 1). The aggregate stability (AS), available phosphate (AvP), available silicate (AvS), bulk density (BD), cation exchange capacity (CEC), enzyme activity (EA), electrical conductivity (EC), exchangeable cation (ExC) including Ca, K, and Mg, organic matter (OM), pH, total nitrogen (TN), total phosphorus (TP), and water holding capacity (WHC) have been commonly used in previous studies [3, 11, 13, 32, 33]. The catalase (CAT), urease (UA), and β-glucosidase (βGA) activities were selected as main indicator EAs for soil quality assessment [34, 35].
Earthworm survival (E), nematode reproduction (NR), plant germination and growth (PG), soil algal biomass (SAB), and soil algal photosynthetic capacity (SAP) were measured using standard ecotoxicity bioassay as follows. The earthworm survival (indicator E) was measured according to standard test guidelines [36, 37], and NR bioassay was conducted according to the ISO [38] and Kim et al. [39, 40]. The indicator PG included barley (B, Hordeum vulgare), rice (R, Oryza sativa), sorghum (S, Sorghum biocolor), and wheat (W, Triticum aestivum) [31, 41], and two soil algal species (Chlorococcum infusionum, Ci; Chlamydomonas reinhardtii, Cr) were used to measure the SAB and SAP [42]. To normalize the ecotoxicological indicators, LUFA 2.2, which is widely used as control in soil ecotoxicity studies [43], was selected as reference soils.
2.3. Application of Soil Quality Assessment SystemThe soil quality assessment consisted of indicator classification, measurement, scoring, weighting, and SQI calculation [30]. We used the indicator measurement data of each test plot, and indicator scoring was performed using the following assumptions: “more is better,” “less is better,” and “optimum is the best.” Each type included the standard scoring function (SSF) equations [3, 11, 30], and the SSF equations for “more is better” (Eq. (1)), “less is better” (Eq. (2)), and “optimum is the best” (Eq. (3)) can be described as:
The f(x), x, L, and U indicate the scores (ranged from 0.1 to 1.0), measurement data, and lower and upper threshold values of each indicator, respectively. The lower and upper thresholds were determined according to previous studies (Table 2). According to Kim et al. [30], the changeable thresholds can be applied to the specific indicators including AvP, Bd, EA, ExC (K), and ExC (Ca) (Table 3). These indicators have various threshold values, because the optimal values of AvP, ExC (K), and ExC (Ca) could differ with crop type [32], and the BD indicator has a correlation with soil texture [33, 44]. The EA indicators, including CAT, UA, and βGA, were normalized with the corresponding values in the control soils, such as the international standard soils and non-contaminated field soils [34, 35].
The principal component analysis (PCA) was employed for indicator weighting. Only the principal components (PCs) of eigenvalues ≥ 1 were considered for weighting, and each eigenvector within the available PC was squared and summed [3, 30, 45, 46]. The communality was calculated, and the ratio to total communality was determined as weights [3, 30]. By the end of the process, we used the integrated quality index (IQI) equation approach [2, 3, 6, 13, 47], and the SQI was calculated to quantify the soil class at each plot:
where Wi, Si, and n are the weights of each indicator, indicator scores, and number of indicators, respectively. The SQI can classify soils into five classes: very good (> 0.85), good (0.70–0.85), moderate (0.55–0.69), bad (0.40–0.55), and very bad (< 0.40) [13, 30, 48]. To evaluate whether the ecotoxicological indicators affected the SQI value, the soil quality assessment was performed with or without ecotoxicological indicators.
2.4. SQI Comparison with In Situ Crop ProductivityCrop productivity data was generated by in situ cultivation under actual field conditions. Rice plants (Oryza sativa) were grown for 108 days on paddy soil plots. Before planting, the rice seeds were sterilized for 1 day using a germicide, washed for 2 days, and germinated at 30–32 °C. They were transplanted into the paddy soil plots, watered, and the weight of 100-grains was measured after 108 days. The detailed description of in situ cultivation on paddy soil plots can be found in the study of Kim et al. [31]. On upland soil plots, perilla (Perilla frutescens) and soybean (Glycine max) were used, and nursery crops were planted with 80 replications for each upland soil plot. After 120 days, the grains of perilla and soybean were threshed, and the weight of total grains (for perilla) and single crop-grains (for soybean) was determined. The in situ crop productivity of each plot was compared with the soil classes (very bad, bad, moderate, good, and very good) estimated by SQI with or without ecotoxicological indicators.
3. Results and Discussion3.1. Paddy Soil PlotsThe indicators in reference and paddy soils (contaminated, remediated, and reclaimed) were measured as shown in Table S1 (Supporting Information). The data were scored using SSF equations (Table 2 and 3), and they were listed as shown in Table S2 (Supporting Information). As the soil textures of reference and paddy soils (contaminated, remediated, and reclaimed) were determined as sand, clay, clay, and silty clay loam respectively, the different SSF thresholds were applied for BD indicator scoring. In addition, we chose “rice” as the target crop, and the SSF thresholds of AvP and ExC (K) were selected as 120 mg/kg and 0.3 cmol+/kg for rice group, respectively. ExC (Ca) was also scored using SSF threshold (6 cmol+/kg) of chilly, Chinese cabbage, apple, Asian pear, rice, sesame, sweat potato group. PCA was conducted using scores of each indicator, and the three available PCs (eigenvalue ≥ 1) were determined (Table S3, Supporting Information), and the communality and weights were calculated as shown in Table S4 (Supporting Information). The highest weights with or without ecotoxicological indicators were determined as PG (S) and pH, respectively.
The SQIs of each paddy soil were exhibited as shown in Table S5 (Supporting Information). The SQI with ecotoxicological indicators were calculated as 0.770, 0.714, 0.716, and 0.589 on reference, contaminated, remediated, and reclaimed soils, respectively, and these values indicated “good,” “good,” “good,” and “moderate” soil classes (Fig. 2A). We run a soil quality assessment system without ecotoxicological indicators (Fig. 2B). The SQI showed 0.678 (moderate), 0.673 (moderate), 0.735 (good), and 0.520 (bad) on reference, contaminated, remediated, and reclaimed soils, respectively. The reference and contaminated soils descended to “moderate” from “good” of soil class, the reclaimed soil decreased to “bad” from “moderate” of soil class, and the remediated soil showed no changes.
3.2. Upland Soil PlotsThe indicator measurement data of upland soils were scored using SSF function as shown in Table S6 (Supporting Information). The soil textures of reference, contaminated, remediated, and reclaimed soils were determined as sand, sandy loam, loamy sand, and sandy loam respectively, and the different SSF thresholds were used in BD indicator scoring. As “bean” was chosen as one of the target crops, the SSF thresholds of AvP, ExC (K), and ExC (Ca) were selected as 250 mg/kg, 0.55 cmol+/kg and 7 cmol+/kg, respectively (Table 3). After the PCA, we obtained the three available PCs (eigenvalue ≥ 1) (Table S7, Supporting Information), and the highest weights with or without ecotoxicological indicators were determined as EA (CAT) (Table S8, Supporting Information).
The SQI were calculated as 0.743, 0.598, 0.634, and 0.408 on reference, contaminated, remediated, and reclaimed soils, respectively, and these values indicated good, moderate, moderate, and very bad soil class (Table S9, Supporting Information). With the soil quality assessment system without ecotoxicological indicators, the SQI was calculated as 0.579 (moderate), 0.528 (bad), 0.572 (moderate), and 0.539 (bad) in reference, contaminated, remediated, and reclaimed soils, respectively. The soil class of the contaminated soil became “bad,” and SQI of the reclaimed soil increased to 0.528 from 0.408 (Fig. 2C and D).
3.3. Comparison of SQI with In Situ Crop ProductivityThe in situ crop productivity of paddy soil plots were intimately reported in our previous study [31]. The weights of 100 rice grains were determined as 2.8 ± 0.3, 2.7 ± 0.2, and 2.9 ± 0.2 g on contaminated, remediated, and reclaimed plots, respectively. The dot data represented the data distribution of SQI versus in situ crop productivity (grains weight) on paddy soil plots (Fig. 3A). The dot data of each paddy soil plot showed dense distribution, because the SQI and in situ crop productivity were similar. In order to evaluate whether the ecotoxicological indicators influenced SQI calculation, the SQI without ecotoxicological indicators was calculated and compared with in situ crop productivity. The dot data of the reclaimed paddy soil plot were SQI sifted to the left side of the x-axis (red arrow in Fig. 3B).
The weight of total perilla-grains was determined as 1,111 and 1,742 g at the contaminated and remediated upland soil plots, respectively, and the weight of single soybean-grains was determined as 9.4 ± 6.1 and 33.9 ± 18.8 g at the contaminated and remediated upland soil plots. As the crops at the reclaimed upland soil plot were all withered since planting, the in situ data could not be obtained (Fig. S1). This can be attributed to the high soil EC level at the reclaimed upland soil plot (Table S1, Supplementary material). The SQIs of each upland plot were compared with in situ crop productivity (grains weight), and the dot data showed good linear shape (Fig. 4A and B). However, in the SQI without ecotoxicological indicators, the SQI of the contaminated and reclaimed upland soils was SQI sifted to the left (“bad” area) and right (“moderate” area) of the x-axis, respectively (red arrows in Fig. 4C and D). The SQIs of each upland soils showed a good correlation with in situ crop productivity when the ecotoxicological indicators are used. These results imply that ecotoxicological indicators play an important role in representing productivity function of the upland soil plots since they directly response to low functionality of soils for their survival. Although EC of the reclaimed upland soil showed higher level compared to other soils (Supporting Table S1), but SQI without the ecotoxicological indicators was similar levels with other upland soils (Fig. 2). However, the ecotoxicological indicators, especially P (plants) and E (earthworm), directly response to the amended soil (Supporting Table S1), and SQI showed good correlations with in situ crop productivity (Fig. 4).
3.4. Application of Ecotoxicological Indicator for Soil Quality AssessmentThe remediation techniques can disrupt physicochemical properties of soil and can be fatal to soil organisms because it changes the balance of the soil ecosystem [17–19]. These disruptions can affect the measurement of traditional biological indicators such as microbial C/N, plant cover, root quality rating, and earthworm/nematode abundances [9–13]. The biological monitoring methods such as QBS, EMI, TRIAD, which evaluate the biodiversity by actual field survey, were also suggested in previous studies [49–51], and these approaches enable to correctly decide the degree of soil organism’s vulnerability and biological activity. However, the biological monitoring methods could not evaluate severely contaminated or disrupted soils where life of soil organisms are not allowed [17–19]. Currently, for in situ assessment using laboratorial animals, the previous studies employed earthworm and soil algal species into field situation, and they found some possibility as rapid tool for soil quality evaluation on the contaminated or remediated sites [52–54]. The ecotoxicological approaches also investigated using field soil samples on laboratorial condition, and the bioactivities of earthworm, nematode, and soil algae were evaluated through the modified standard bioassay methods [31, 36, 40]. Kim et al. [30] suggested the soil quality assessment system using ecotoxicological indicators on contaminated and remediated sites, and they reported that the use of ecotoxicological indicator can serve the soil quality assessment even in contaminated or physically and chemically treated soil. We also speculated that the ecotoxicological indicators can show more sensitive response to the damaged soils compared with the other indicators, because the ecotoxicological indicators can provide direct evidence of biological response.
4. ConclusionBefore in situ crop cultivation, we expected that the remediated plots would have low crop productivity, and the contaminated- and reclaimed-plots would exhibit similar trends on both paddy and upland soil plots. In terms of the actual field data, there were no differences between paddy plots, and the reclaimed soil plot was uncultivable, and therefore, in situ crop productivity could not be calculated for this plot. Despite this unexpected field variation, the SQI enabled the estimation of relevant soil class, and it showed high correlation with in situ crop productivity. We conclude that the use of ecotoxicological indicators can help soil quality assessment, and future studies should verify this approach.
AcknowledgementThis work was supported by the Korea Environment Industry & Technology Institute (KEITI) funded by Korea Ministry of Environment (MOE) (No.2022002450002). This paper was supported by Konkuk University Researcher Fund in 2022.
NotesAuthor Contributions S.W.K. (Postdoc), Y.-J.A. (Professor), and S.-W.J. (Professor) generated the concepts and experimental design. S.W.K., S.-H.N. (Research Professor), J.I.K. (Research Professor), Y.C. (Postdoc), and D.K. (Postdoc) conducted all the experiments. S.W.K., Y.-J.A., and S.-W.J. wrote and revised the manuscript. References1. Larson WE, Pierce FJ. The dynamics of soil quality as a measure of sustainable management. Defining Soil Quality for a Sustainable Environment. Soil Science Society of America; 1994. p. 37–52.
2. Andrews SS, Mitchell JP, Mancinelli R, Karlen DL, Hartz TK, Horwath WR, Pettygrove GS, Scow KM, Munk DS. On-farm assessment of soil quality in California’s central valley. Agron. J. 2002;94:12–23. https://doi.org/10.2134/agronj2002.1200
3. Qi Y, Darilek JL, Huang B, Zhao Y, Sun W, Gu Z. Evaluating soil quality indices in an agricultural region of Jiangsu Province, China. Geoderma. 2009;149:325–334. https://doi.org/10.1016/j.geoderma.2008.12.015
4. Karlen DL, Scott DEA. Framework for evaluating physical and chemical indicators of soil quality. Defining Soil Quality for a Sustainable Environment. ASA and SSSA. 1994;53–72.
5. Karlen DL, Ditzler CA, Andrews SS. Soil quality: Why and how? Geoderma. 2003;114:145–156. https://doi.org/10.1016/S0016-7061(03)00039-9
6. Li P, Zhang T, Wang X, Yu D. Development of biological soil quality indicator system for subtrophical China. Soil Till. Res. 2013;126:112–118. https://doi.org/10.1016/j.still.2012.07.011
7. Dawson JJC, Godsiffe EJ, Thompson IP, Ralebitso-Senior TK, Killham KS, Paton GI. Application of biological indicators to assess recovery of hydrocarbon impacted soils. Soil Biol. Biochem. 2007;39:164–177. https://doi.org/10.1016/j.soilbio.2006.06.020
8. Dexter AR, Pagliai M, Jones R. Soil structure: the key to soil function. Advan. Geoecol. 2002;35:57–69.
9. Andrews SS, Carroll CR. Designing a soil quality assessment tool for sustainable agroecosystem management. Ecol. Appl. 2001;11:1573–1585. https://doi.org/10.1890/1051-0761(2001)011[1573:DASQAT]2.0.CO;2
10. Bone J, Head M, Barraclough D, Archer M, Scheib C, Flight D, Voulvoulis N. Soil quality assessment under emerging regulatory requirements. Environ. Int. 2010;36:609–622. https://doi.org/10.1016/j.envint.2010.04.010
11. Glover JD, Reganold JP, Andrews PK. Systematic method for rating soil quality of conventional, organic, and integrated apple orchards in Washington state. Agri. Eco. Environ. 2000;80:29–45. https://doi.org/10.1016/S0167-8809(00)00131-6
12. Govaerts B, Sayre KD, Deckers J. A minimum data set for soil quality assessment of wheat and maize cropping in the highlands of Mexico. Soil Till. Res. 2006;87:163–174. https://doi.org/10.1016/j.still.2005.03.005
13. Volchko Y, Norrman J, Rosén L, Norberg T. SF-box—A tool for evaluating the effects on soil functions in remediation projects. Integr. Environ. Assess. Manag. 2014;10:566–575. https://doi.org/10.1002/ieam.1552
14. Palansooriya KN, Shaheen SM, Chen SS, Tsang DC, Hashimoto Y, Hou D, Bolan NS, Rinklebe J, Ok YS. Soil amendments for immobilization of potentially toxic elements in contaminated soils: A critical review. Environ. Int. 2020;134:105046. https://doi.org/10.1016/j.envint.2019.105046
15. Monterroso C, Balseiro-Romero M, Garbisu C, Kidd PS, Qafoku NP, Baveye PC. Searching for solutions to soil pollution: Underlying soil-contaminant interactions and development of innovative land remediation and reclamation techniques. Front. Environ. Sci. 2022;9:706. https://doi.org/10.3389/fenvs.2021.830337
16. Sheoran V, Sheoran AS, Poonia P. Soil reclamation of abandoned mine land by revegetation: a review. Int. J soil Sed. Water. 2010;3:13.
17. Reed BE, Carriere PC, Moore R. Flushing of a Pb(II) contaminated soil using HCl, EDTA, and CaCl2. J. Environ. Eng. 1996;122:48–50. https://doi.org/10.1061/(ASCE)0733-9372(1996)122:1(48)
18. Abumaizar RJ, Smith EH. Heavy metal contaminants removal by soil washing. J. Hazard. Mat. 1999;70:71–86. https://doi.org/10.1016/S0304-3894(99)00149-1
19. van Benschoten JE, Reed BE, Matsumoto MR, McGarvey PJ. Metal removal by soil washing for an iron oxide coated sandy soil. Water Environ. Res. 1994;66:168–174. https://doi.org/10.2175/WER.66.2.11
20. Larsson M, Hagberg J, Rotander A, van Bavel B, Engwall M. Chemical and bioanalytical characterization of PAHs in risk assessment of remediated PAH-contaminated soils. Environ. Sci. Pollut. Res. 2013;20:8511–8520. https://doi.org/10.1007/s11356-013-1787-6
21. Maenpaa KA, Kukkonen JVK, Lydy MJ. Remediation of heavy metal-contaminated soils using phosphorus: Evaluation of bioavailability using an earthworm bioassay. Arch. Environ. Con. Tox. 2002;43:389–398. https://doi.org/10.1007/s00244-002-1248-6
22. Chang LW, Meier JR, Smith MK. Application of plant and earthworm bioassays to evaluate remediation of a lead-contaminated soil. Arch. Environ. Con. Tox. 1997;32:166–171. https://doi.org/10.1007/s002449900170
23. van Gestel CAM, van der Waarde JJ, Derksen JGM, van der Hoek EE, Veul MFXW, Bouwens S, Rusch B, Kronenburg R, Stokman GNM. The use of acute and chronic bioassays to determine the ecological risk and bioremediation efficiency of oil-polluted soils. Environ. Toxicol. Chem. 2001;20:1438–1149. https://doi.org/10.1002/etc.5620200705
24. Wang Q-Y, Zhou D-M, Cang L, Sun T-R. Application of bioassays to evaluate a cooper contaminated soil before and after a pilot-scale electrokinetic remediation. Environ. Pollut. 2009;157:410–416. https://doi.org/10.1016/j.envpol.2008.09.036
25. European Chemicals Bureau (ECB). Technical Guidance Document on Risk Assessment. EUR 20418 En/2. European Commission, Joint Research Centre; 2003.
26. National Environment Protection Council (NEPC). Guideline on methodology to derive ecological investigation levels in contaminated soils. Natl. Environ. Protect. Meas. 1999;Schedule B5b
27. Elliot ET, Anderson RV, Coleman DC, Cole CV. Habitable pore space and microbial trophic interactions. Oikos. 1980;35:327–335. https://doi.org/10.2307/3544648
28. Grossman RB, Harms DS, Kingsbury DS, Shaw RK, Jenkins AB. Assessment of soil organic carbon using the U.S Soil Survey. Assessment methods for soil carbon. Boca Raton: 2001. p. 87–104.
29. Jänsch S, Amorim MJB, Römbke J. Identification of the ecological requirements of important terrestrial ecotoxicological test species. Environ. Rev. 2005;13:51–83. https://doi.org/10.1139/a05-007
30. Kim SW, Jeong S-W, An Y-J. Application of a soil quality assessment system using ecotoxicological indicators to evaluate contaminated and remediated soils. Environ. Geochem. Health. 2020;42:1681–1690. https://doi.org/10.1007/s10653-019-00321-7
31. Kim SW, Chae Y, Moon J, Kim D, Cui R, An G, Jeong S-W, An Y-J. In situ evaluation of crop productivity and bioaccumulation of heavy metals in paddy soils after remediation of metal contaminated soils. J. Agr. Food Chem. 2017;65:1239–1246. https://doi.org/10.1021/acs.jafc.6b04339
32. Rural Development Administration (RDA). Standards for fertilizer prescription of crops. National Academy of Agricultural Science; 2010.
33. United States Department of Agriculture (USDA). Guidelines for soil quality assessment in Conservation planning. 2001;
34. An Y-J, Kim M. Effect of antimony on the microbial growth and the activities of soil enzymes. Chemosphere. 2009;74:654–659. https://doi.org/10.1016/j.chemosphere.2008.10.023
35. Chae Y, Cui R, Kim SW, An G, Jeong S-W, An Y-J. Exoenzyme activity in contaminated soils before and after soil washing: ß-glucosidase activity as a biological indicator of soil quality. Ecotox. Environ. Safety. 2017;135:368–374. https://doi.org/10.1016/j.ecoenv.2016.10.007
36. Kwak JI, Nam S-H, Kim SW, Bajagain R, Jeong S-W, An Y-J. Changes in soil properties after remediation influence the performance and survival of soil algae and earthworm. Ecotox. Environ. Safety. 2019;174:189–196. https://doi.org/10.1016/j.ecoenv.2019.02.079
37. Organization for Economic Cooperation and Development (OECD). Earthworm, Acute toxicity tests. OECD guideline for testing of chemicals 207. OECD; 1984.
38. International Organization for Standardization (ISO). Water Quality Determination of the Toxic Effect of Sediment and Soil Samples on Growth, Fertility and Reproduction of Caenorhabditis elegans (Nematoda). ISO 10872; 2010.
39. Kim SW, Moon J, An Y-J. A highly efficient nonchemical method for isolating live nematodes (Caenorhabditis elegans) from soil during toxicity assays. Environ. Toxicol. Chem. 2014;34:208–213. https://doi.org/10.1002/etc.2788
40. Kim SW, Moon J, An Y-J. Development of a nematode offspring counting assay for rapid and simple soil toxicity assessment. Environ. Pollut. 2018;236:91–99. https://doi.org/10.1016/j.envpol.2018.01.037
41. An Y-J, Kampbell DH, McGill ME. Toxicity of methyl tert-butyl ether to plants (Avena sativa, Zea mays, Triticum asestivum, and Lactuca sativa). Environ. Toxicol. Chem. 2002;21:1679–1682. https://doi.org/10.1002/etc.5620210820
42. Nam SH, An Y-J. A rapid screening method to assess soil algal toxicity: Non-destructive sampling of algal cells using culture medium extraction. Appl. Soil Ecol. 2017;120:143–152. https://doi.org/10.1016/j.apsoil.2017.08.008
43. Løkke H, van Gestel CAM. Handbook of soil invertebrate toxicity. John Wiley & Sons; 1998.
44. Andrews SS, Kareln DL, Cambardella CA. The soil management assessment framework: A quantitative soil quality evaluation method. Soil Sci. Soc. Am. J. 2004;68:1945–1962. https://doi.org/10.2136/sssaj2004.1945
45. Shukla MK, Lal R, Ebinger M. Determining soil quality indicators by factor analysis. Soil Till. Res. 2006;87:194–204. https://doi.org/10.1016/j.still.2005.03.011
46. Sun B, Zhou S, Zhao Q. Evaluation of spatial and temporal changes of soil quality based on geostatistical analysis in the hill region of subtropical China. Geoderma. 2003;115:58–99. https://doi.org/10.1016/S0016-7061(03)00078-8
47. Doran JW, Parkin BT. Defining and assessing soil quality. Defining Soil Quality for a sustainable Environment. Soil Science Society of America; 1994. p. 3–21.
48. Volchko Y, Norrman J, Rosén L, Norberg T. A minimum data set for evaluating the ecological soil functions in remediation projects. J. Soil. Sediment. 2014;14:1850–1860. https://doi.org/10.1007/s11368-014-0939-8
49. Filip Z. International approach to assessing soil quality by ecologically-related biological parameters. Agri. Eco. Environ. 2002;88:169–174. https://doi.org/10.1016/S0167-8809(01)00254-7
50. International Organization for Standardization (ISO). Soil quality – Procedure for site-specific ecological risk assessment of soil contamination (Soil quality TRIAD approach. ISO 19204; 2017.
51. Menta C, Conti FD, Pinto S, Bodini A. Soil Biological Quality index (QBS-ar): 15 years of application at global scale. Ecol. Indic. 2018;85:773–780. https://doi.org/10.1016/j.ecolind.2017.11.030
52. Kim SW, Kim D, Moon J, Chae Y, Kwak JI, Park Y, Jeong S-W, An Y-J. Earthworm dispersal assay for rapidly evaluating soil quality. Environ. Toxicol. Chem. 2017;36:2766–2772. https://doi.org/10.1002/etc.3832
53. Nam S-H, Moom J, Kim SW, Kim H, Jeong S-W, An Y-J. Rapid in situ assessment for predicting soil quality using an algae-soaked disc seeding assay. Environ. Monit. Assess. 2017;189:637. https://doi.org/10.1007/s10661-017-6358-8
54. Kwak JI, Nam S-H, An Y-J. Soil algae pipe assay: ex situ method for the evaluation of soil quality based on soil algae and its application to the pot test. Chemosphere. 2019;224:634–636. https://doi.org/10.1016/j.chemosphere.2019.02.198
Fig. 1(A) Location of target paddy and upland soil plots, and the construction design of the (B) paddy and (C) upland soil plots. 
Fig. 2Soil quality index (SQI) calculation in the target (A,B) paddy (reference, contaminated, remediated, and reclaimed) and (C,D) upland (reference, contaminated, remediated, and reclaimed) soil plots (A,C) with or (B,D) without ecotoxicological indicators. All the indicators in each plot are expressed in one bar graph. 
Fig. 3Dot plot of soil quality index (SQI) versus in situ crop productivity (grain weight of rice) of paddy soil plots (contaminated, remediated, and reclaimed). The SQI was calculated (A) with or (B) without ecotoxicological indicators. The x- and y-axes indicate the SQI and in situ crop productivity, respectively, and the x-axis represents the five soil classes (very good, good, moderate, bad, and very bad). 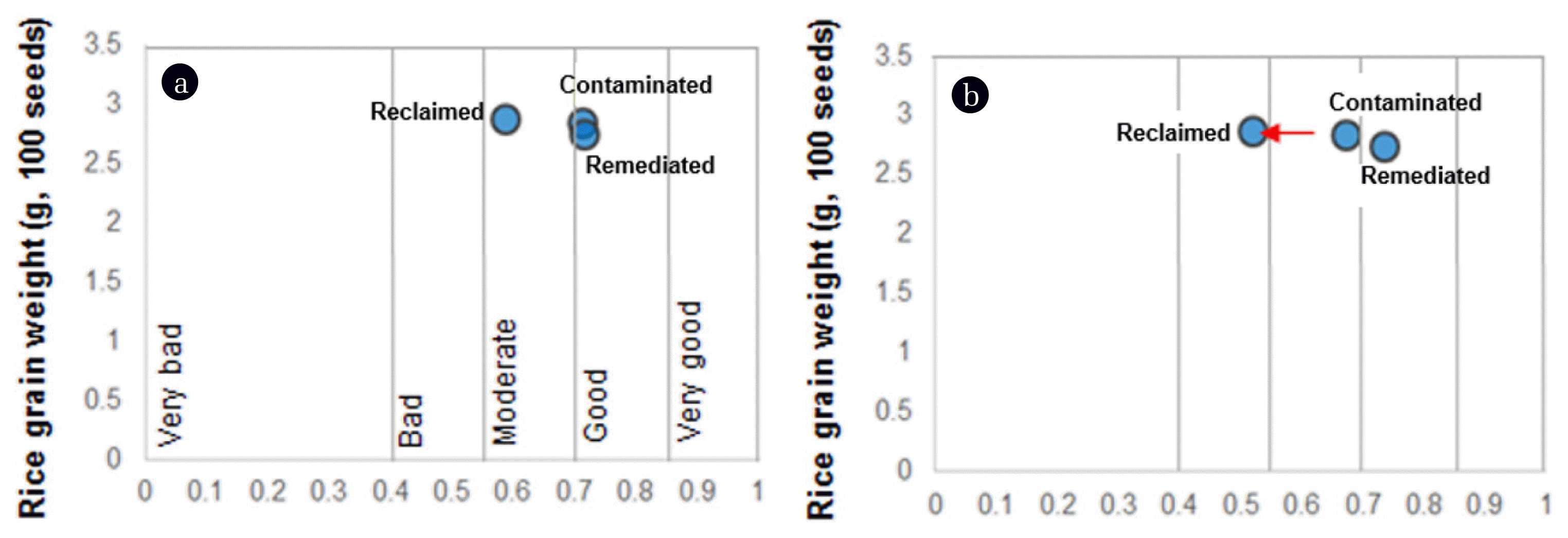
Fig. 4Dot plot of soil quality index (SQI) versus in situ crop productivity (grain weight of perilla and soybean) in the upland soil plots (contaminated, remediated, and reclaimed). The SQI was calculated (A) with or (B) without ecotoxicological indicators. The x- and y-axes indicate the SQI and in situ crop productivity, respectively, and the x-axis represents the five soil classes (very good, good, moderate, bad, and very bad). 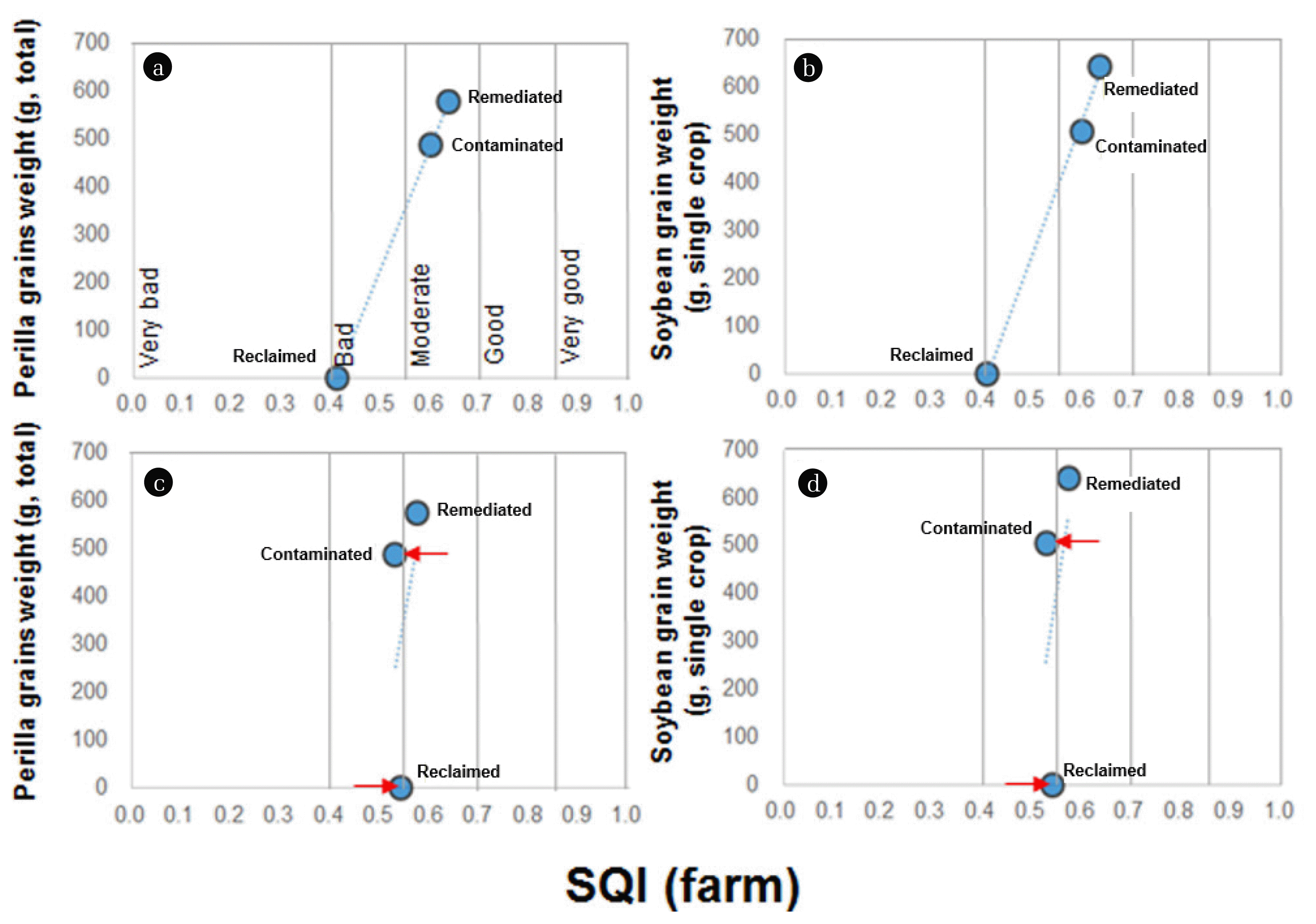
Table 1Classification of physical, chemical, biological, and ecotoxicological indicators [30]. Table 2Standard scoring function (SSF) and lower/upper thresholds of each indicator [30].
Table 3Standard scoring function (SSF) and changeable thresholds of available phosphate (AvP), bulk density (BD), enzyme activity (EA), and the exchangeable cations (ExC) [30].
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||