AbstractAdvanced oxidation process (AORs) is an effective method to remove a wide range of organic contaminants. However, an outstanding catalysis is needed to generate more free radicals. In this work, nano zero-valent iron (nZVI) modified with activated carbon and copper (AC-nZVI/Cu) was synthesized by liquid-phase reduction, and used to catalyze persulfate (PS) to remove norfloxacin (NOR). The effect of various factors, such as diverse adsorbents, pH, temperature, PS dosage, adsorbent dosage, and inorganic anions, on NOR removal efficiency were investigated. The results show that 30 mg/L NOR can be easily removed by 0.5 g/L AC-nZVI/Cu at 313 K through activating 0.5 mM PS, and the removal efficiency is as high as 96.84%. The electron paramagnetic resonance (EPR) analysis proves that numerous sulfate free radicals (·SO4−) appear at the initial stage of the removal process. Furthermore, the quenching experiments manifested that ·SO4− was the predominant radical special in acidic conditions, while adsorption of NOR was predominant under alkaline conditions. Importantly, compared with nZVI, AC-nZVI/Cu has excellent long-term stability because of the introduction of activated carbon and copper. The study laid the groundwork for further development of adsorbent and activator based on nZVI.
Graphical Abstract
1. IntroductionCurrently, emerging antibiotic contaminants are constantly detected in water environments (e.g. surface water, river, and underground water) [1–6]. Norfloxacin (NOR) as a kind of fluoroquinolone antibiotic was employed to treat disease of human beings and animals [7–9]. According to reliable statistics, in 2013 about 17% of 27540 tons of antibiotics came from NOR [10], as well as the discharge of wastewater containing NOR degrades water quality and thus water cannot be directly used for potable water and industrial applications [11–13]. Additionally, the accumulation of NOR constantly occurs in environmental systems [14, 15], toxic and adverse consequences from these medications' residues in food animals include drug-resistant human pathogenic microorganisms, disruption of liver metabolism, destruction and reduction of red and white blood cells, among other negative effects. Unfortunately, the elimination of NOR is insufficient through conventional wastewater treatment technologies (including aerobic, anaerobic, and membrane technologies [16]) due to its poor biodegradability and chemical structure stability [17, 18].
Various advanced oxidation processes (AOPs) have been demonstrated to efficiently remove organic contaminants including antibiotics [19–22]. For instance, Vlissides A.G [23] used an electrochemical oxidation system to treat textile dye wastewater, and after 18 min of electrolysis at 0.89 A/cm2, the removal efficiency of chemical oxygen demand (COD) and biochemical oxygen demand (BOD5) can reach 86.00% and 71.00%. Ji Y et al. [24] took the ozone oxidation process to fix a water system containing pharmaceutical wastewater, and the results show that the removal efficiency of COD is 79.90% and within 60 min. Compare with these above AOPs, a chemical activator as the reagent to activating AOPs system for generating free radicals (·SO4−, ·OH, ·H and Cl·) has advantages of low-cost and high-efficiency. Nano zero-valent iron is not only a highly reactive reducing agent [25–28] but also an effective activator [29, 30], which can activate persulfate (PS) to generate ·SO4− (E0=2.5 V–3.1 V) and thus degrade organic contaminants. For instance, nZVI was employed by Yuan X et al. [31] to activate PS, as well as decompose tetrabromobisphenol A (TBBAA) in soil, the results indicated that degradation efficiency of TTBBA can reach 78.32% within 12 h under the condition of 3 g/kg nZVI, 25 mM PS and pH=5.5 at 25 °C. However, bare nZVI has two fatal faults. On the one hand, it has a heavy accumulation effect because of its intrinsic magnetic interactions and weak van der waals forces[26, 32], on the other hand, oxide and hydroxide are formed on the surface [33–36], which obstruct nZVI react with PS continuously.
To address these issues, transition metals, for instance, palladium (Pd), nickel (Ni), copper (Cu), Zinc (Zn), and silver (Ag), combined with nZVI to form a bimetal system, which not only accelerates the rate of electron transfer [37] and thus cutting down the apparent activation energy [38–41] required for reaction, but also is an outstanding activator for PS. Although Pd and Ni have excellent electron transfer effects, Cu is more feasible in catalytic applications owing to three advantages: 1) cheapness; 2) exceptional accessible; last but not least, enable electrons transfer faster when combined whit other metal as the result of its being in the transition region [42]. Various carriers, such as functional kaolin [43], bentonite [44], attapulgite [45], and activated carbon (AC) [46–48] were applied to disperse nZVI due to their low cost. Among the above supporters, AC is not only an excellent adsorbent with trace oxygen-containing functional groups located on the surface, but can activate PS to produce ·SO4− as well [49, 50].
Although several materials have been utilized to remove NOR, the removal mechanism is still unknown. The foundation of the mechanism must be understood in order to advance NOR elimination research. This study used the liquid phase reduction approach to create a composite known as nano zero-valent iron modified by activated carbon and copper (AC-nZVI/Cu). Investigations into removing NOR from solutions were conducted. The work intends to (i) analyze the activation mechanism of PS by the AC-nZVI/Cu system, (ii) examine the concentration influence of various anions, such as Cl− HCO3− NO3− and H2PO4−, on NOR removal efficiency, (iii) assess the long-term stability of the AC-nZVI/Cu, and (iv) realistically extract the mechanism for the NOR removal process by the AC-nZVI/Cu-PS system.
2. Materials and Methods2.1. MaterialsNorfloxacin (Shanghai Aladdin Biochemical Co., Ltd. Shown in Table S1), ferrous sulfate heptahydrate (FeSO4·7H2O) (Tianjin Baishi Chemical industry Go. Ltd.), copper sulfate pentahydrate (CuSO4·H2O) (Tianjin Yusheng Chemical Co., Ltd.), sodium borohydride (NaBH4) (Sinopharm Chemical Reagent Co., Ltd.), hydrochloric acid (HCl), sodium hydroxide (NaOH), ethanol, tert-butanol (TBA), 1,10-phenanthroline monohydrate, hydroxylamine hydrochloride, and ammonium acetate were all reagents of analytical grade. 5,5-dimethyl-1-pyrrolidine N oxide (DMPO) was used as the trapping agent for ·OH and
.
2.2. Preparation of AdsorbentsThe liquid phase reduction approach was implemented to create AC-nZVI/Cu. As the ratio of nZVI to Cu was 16:1, 0.25 g AC was first added to 250 mL of a water/ethanol solution (2:1, vol/vol) that also contained CuSO4•5H2O (0.296 g) and FeSO4•7H2O (4.96 g). Then, after 30 minutes of sonication, 50 mL of a solution containing 2.20 g of NaBH4 was added to the mixture at a rate of 1–2 drops per second. The reduction reaction (Eq. (1), (2), and 3) then continued for 60 minutes while being stirred mechanically at a rate of 350 rpm, and the entire process was carried out in a nitrogen atmosphere. Blackish nanoparticles were then dried in a vacuum for four hours at 60 °C after being repeatedly cleaned with ethanol and deionized water. Cu, nZVI/Cu, AC-nZVI were manufactured using a similar process to that described above, but without the inclusion of AC FeSO4•7H2O and CuSO4•5H2O. Fig. S1 displayed the synthesis flowchart.
2.3. Characterization of Prepared AdsorbentsThe apparent morphology of adsorbents was investigated by transmission electron microscope (TECHAIG2TF20, American FIE Co., Ltd.). X-ray diffraction (XRD, D/Max-2400 X-ray diffractometer (Rigaku Miniflex, Japan) operated at 35kv and 40mA from 15° to 90° [51]. X-ray photoelectron spectroscopy (XPS, Thermo Fisher Scientific’s ESCALAB 250Xi type) was employed to investigate the valence changes of elements and bonding properties between elements. The concentration of norfloxacin was detected by a UV-visible spectrophotometer (UV-1900) (Shanghai, China Aoyi Instrument Co., Ltd.) at 272 nm. Elemental composition and contents of bimetal were measured by Energy dispersive spectrometer (EDS) and inductively coupled plasma-atomic emission spectrometry (ICP). The electron paramagnetic resonance (EPR) was used to identify radicals.
2.4. Removal ExperimentsRemove experiments were carried out in 250 mL erlenmeyer flask, which included five factors (different adsorbents, dosage, initial pH, PS concentration, and different temperatures), corresponding experimental conditions have been attached under the assignation map. In all these situations, the solution was shaken in a vibrator at 230 rpm, and 2 mL liquid was extracted with 0.22 μm leach paper for analysis in a UV-visible spectrophotometer at 272 nm within the periodic time. Residual concentration and capacity of NOR were counted by formulas below Eq. (4) and Eq. (5) [52], additionally, the pseudo-first-order Eq. (6) was employed for dynamic fitting.
Where c0 and ct, (mg/L) is the initial concentration and concentration of NOR at “t” min, “m” is the adsorbent mass (g) and V is the solution volume (mL). kobs is rate constants,
3. Results and Discussion3.1. Activation and Removal System of Different AdsorbentsThe removal and PS-activated system treated by different samples was shown in Fig. 1(a). The removal efficiency of NOR at 120 min by PS and AC was only 16.66% and 26.40% respectively, but it was improved to 32.80% while combining both of them. Two reasons are employed to explain this phenomenon. For one, PS can self-reaction under some temperature points (Eq. (7)) [53]. For two, the oxygen-containing functional group on AC can activate PS (Eq. (8) and Eq. (9)) [54], which is demonstrated by the change of PS concentration in Fig. 1(b). The bare PS system shows a mild decline from 0.500 mM to 0.459 mM, while in the AC-PS system, the concentration of PS decreased from 0.500 mM to 0.415mM indicating that more PS were activated by AC. Additionally, the removal efficiency of nZVI/Cu-PS AC-nZVI-PS and AC-nZVI/Cu-PS increase sequentially (67.63% 90.59%, and 93.98%) within 120 min as a result of activating PS to produce (Eq. (10) and Eq. (11)) by nZVI and Cu [37]. As seen in Fig. 1(b), PS concentration in AC-nZVI/Cu significantly changed from 0.500 mM to 0.101 mM within 120 minutes, proving that this compound has the best activation impact on PS out of all the others. The finest adsorbent for NOR removal and manufacturing, among other samples, is AC-nZVI/Cu, to put it simply.
3.2. Factors Affecting Removal of NOR3.2.1. Effect of adsorbent dosageIn Fig. S2, increasing the dose of AC-nZVI/Cu can improve the reactive sites, leading to an increase in the effectiveness of NOR elimination. Using 0.5 g/L adsorbents, approximately 94.00% of NOR removal efficiency was attained in 120 minutes. However, elevating the concentration of AC-nZVI/Cu to 0.9 g/L (98.90%) rendered the NOR removal efficiency sluggish, which is primarily due to the buildup of adsorbent, causing a limited number of reactive sites. [55, 56]. As a result, a higher dose leads to a quicker rise in removal efficiency. The following experiment was chosen to use 0.5g/L adsorbent because of the financial advantages.
3.2.2. Effect of other conditionsThe effectiveness of NOR removal and the reaction rate constant at various pH levels were displayed in Fig. 2(a), and Fig. 2(b). At a heavy acidic pH (pH=2.0), the greatest NOR removal efficiency (93.98%) and reaction rate constant (0.0425 min−1) were attained. The possible reason is that a heavily acidic condition is beneficial to the formation of Fe2+ as the activator of PS by increasing the corrosion of nZVI included in AC-nZVI/Cu [57], the formation process of ·SO4− on the acidic condition was shown in Eq. (12). However, improving pH to alkaline condition causes the formation of the passivation layer on the AC-nZVI/Cu surface, which obstructing the release of Fe2+. Furthermore, ·SO4− was consumed by reacting with OH− to produce ·OH under alkaline conditions (Eq. (13)), resulting in a dramatic decreasement of removal efficiency. It can be seen that the condition of pH has a significant influence on the NOR removal.
The effect of different PS concentrations (0.100 mM, 0.300 mM, 0.500 mM, 0.700 mM, 0.900 mM) on NOR removal by AC-nZVI/Cu was investigated. As we can see in Fig. 2(c) and Fig. 2(d), the removal efficiency was dramatically enhanced from 68.30% to 96.81% as the PS concentration rose from 0.100 mM to 0.700 mM. A significant enhancement of the rate constant appeared as well (from 0.0361 min−1 to 0.0703 min−1). There was a swift growth trend when the PS concentration increased from 0.100 mM to 0.500 mM, which is attributed to numerous activated sites of adsorbent. However, a lower growth trend of removal efficiency was found because of the saturation of activity sites when PS concentration is changed from 0.500 mM to 0.700 mM. Continuously improvement of PS concentration leads to a decrease to 84.97% of the removal efficiency, because too muchwere consumed by themselves (Eq. (14)) as well as reacting with PS (Eq. (15)) [57]. Thus, the concentration of PS is immobilized at 0.500 mM, corresponding kinetic constant of 0.0683 min−1.
Fig. 2(e) and Fig. 2(f) showed the removal efficiency and rate constant of NOR by AC-nZVI/Cu at different reaction temperatures (293K-313K). Clearly, the removal efficiency (from 86.27% to 96.84%) and rate constant (from 0.0361 min−1 to 0.0672 min−1) was improved as the temperature increased. The reason is that the mobility of NOR from the solution to the adsorbent’s surface and the corrosion of AC-nZVI/Cu was accelerated by the higher temperature [58].
3.2.3. Effect of various inorganic anions on NOR removalNumerous anions such as Cl− NO3− HCO3− and H2PO4− are extensively detected in surface and underground water, which always have a deleterious effect on AOPs performance [59]. So, we were committed to investigating the effect of different concentration anions on NOR removal, and the degradation trend curves and rate constants were shown in Fig. 3. It is noted that Cl− and HCO3− exhibit dual effects [53] (stimulation and inhibition) on NOR removal in Fig. 3(a), Fig. 3(b) and Fig. 3(e), Fig. 3(f). While NO3− and H2PO4− showed a negative influence on the removal of NOR in Fig. 3(c), Fig. 3(d) and Fig. 3(g), Fig. 3(h).
Active chlorine (ECl·=1.36 V) can be produced because of the chain reaction of Cl− with ·SO4− (Eq. (16)) [60]. The existence of a few active chlorine has an advantage in the removal of NOR. But too much Cl− will lead to lots of consumption of ·SO4− and ·OH (Eq. (16), Eq. (17)). More free radicals with higher redox potential were reacted and transformed into free radicals with lower redox potential, which cause lower NOR removal efficiency. This explanation is consistent with experimental results that the removal efficiency can reach 96.51% and the corresponding rate constant is 0.0686 min−1 under the condition of adding 1 mM Cl−. However, with the concentration enhanced to 50 mM, NOR removal efficiency decreased continuously, and the rate constant exhibited resemble trends. Similarly, the above principle can be employed by the effect of HCO3−. Suitable HCO3− can generate carbonate and bicarbonate (ECO3−·=1.59 V EHCO3−·=1.65 V) radicals (Eq: 18, 19, 20), which were reacted with the aromatic anilines and amino acids swiftly. However, with the improvement of HCO3− amount, ·SO4− and ·OH were consumed excessively, as a result, the NOR removal rate was reduced.
Unlike Cl− and HCO3−, NO3− and H2PO4− merely have an inhibitory effect whatever the concentration level. In Fig. 3(c) and Fig. 3(d), the removal efficiency was decreased continuously with the increase of NO3− concentration, corresponding rate constant was cut down from 0.0425 min−1 (no addition) to 0.0074 min−1 (50 mM) due to the passivation layer on the adsorbent surface caused by the reaction of nZVI and NO3− (Eq. (21)) [61]. A similar trend was observed in the NOR removal system containing H2PO4−. The removal efficiency was reduced from 93.89% to 29.60% when H2PO4− concentration increased from 0 mM to 50 mM, and the corresponding rate constant decreased sharply from 0.0425 min−1 to 0.0056 min−1. Although the reactions between H2PO4− and or ·OH produce phosphate radicals (Eq. (22), Eq. (23)), they cannot effectively remove NOR because of the lower redox potential. Meanwhile, the complex was generated because H2PO4− reacted with Fe2+ and Fe3+ [53].
3.3. Thermodynamic Research3.3.1. Thermodynamic fittingInvestigating the relationship between adsorbent and adsorbate is crucial for the removal process. Hence, three thermodynamic formulas named Langmuir, Freundlich, and Liu were applied to fit the experimental results. These equations are as follows:
where ce and qe is concentration and capacity at adsorption equilibrium, qm represents the maximum adsorption capacity. The constants of these models are KL, KF, and Kg respectively. nF and nL are the exponents of the Freundlich and Liu models.
Three different temperature (293 K, 303 K, 313 K) adsorption equilibrium isotherms were fitted in Fig. 4, and the results were shown in Table S2. The correlation coefficient (R2) of the Liu model is the largest one by comparison, which is definite and predicts that adsorption-activated sites cannot possess the same energy [62]. Additionally, the adsorption capacity increased gradually with an advancement of the temperature from 293 K to 313 K, indicating that the removal process of NOR by AC-nZVI/Cu-PS system is an endothermic reaction.
3.3.2. Calculation of various thermodynamic function valuesThermodynamic parameters like Gibbs free energy (ΔG), entropy (ΔH), and enthalpy (ΔS) are very important, which help us to further understand the removal process of pollution. Therefore, three formulas were applied to count these values.
where R (8.314 J/mol/K) and K is the ideal gas constant and temperature (Kelvin), Ke can be counted by Eq. (27), in which KL is equal to Kg of the Liu model and γ represents the activity coefficient of the adsorbate. Reference [63] for a detailed calculation process. Various thermodynamic function values are listed in Table S3. Negative Gibbs free energy demonstrates that the removal process of NOR by AC-nZVI/Cu-PS system is a spontaneous reaction. The values of Gibbs free energy gradually decreased from −27.88 to −31.08 kJ/mol with an increment of temperature, which suggests that raising the temperature increases the impetus for the removal process. The positive enthalpy means that the reaction process is endothermic. The positive entropy implies that the interface chaos between solid adsorbent and liquid pollutants is proliferated [55].
A comparison of the removal ability of AC-nZVI/Cu with other nanomaterials reported in the literature is listed in Table S4. Apparently, AC-nZVI/Cu can achieve high removal at a high initial concentration of NOR.
3.4. The Characterization of Materials3.4.1. TEM and EDX mapping of AC-nZVI/Cu compositesThe material’s performance is extremely related to its morphology. Hence, TEM surface morphology, EDX elemental maps, and EDS diagram of AC-nZVI/Cu were examined in Fig. 5. It is a known fact that bare nZVI has an intensive aggregation effect as a result of strong magnetism (Fig. 5(a)) [60]. However, the dispersion of nZVI was stupendously enhanced by activated carbon and metal Cu, which was illustrated in Fig. 5(b) and (c) [42]. Additionally, EDX elemental maps demonstrated that the composite materials contain four elements of C, O, Fe and Cu, and the chief component of C is AC.
3.4.2. XRD and ICPThe structure of AC-nZVI/Cu before and after reacting with NOR was detected by X-ray diffraction (XRD). As shown in Fig. S3, the broad diffraction peak appeared at 22.58°, corresponding to the (002) crystal plane, indicating the presence of AC [64]. The peak at 2θ=44.85°(110) and 2θ=65.15°(200) [63] demonstrated that nZVI included AC-nZVI/Cu was synthesized successfully. However, the peaks appeared at 2θ=26.87°, 36.29°, 46.73° and 60.44° after the reaction demonstrated the formation of iron oxide and consumption of nZVI. Consequently, nZVI plays an indispensable role in the removal process of NOR.
The inductively coupled plasma emission spectrometer (ICP) was employed to detect the ratio of nZVI: Cu. As shown in Table S5, it is easy to see that the ratio of nZVI/Cu is about 16:1, which is very close to the original design ratio.
3.4.3. XPS spectrum of adsorbentTo understand the surface characteristic of the composite, XPS analysis of fresh and used AC-nZVI/Cu was shown in Fig. S4. The spectrum for C1s in Fig. S4(a) and Fig. S4(b) included various peaks at 284.68 eV, 286.67 eV, and 289.19 eV, attributing to C-C, C-OH, and CO-OH, respectively. The proportion of these functional groups was listed in Table 1. The ratio of groups has changed after the reaction, which may be explained by Eq. (8) and Eq. (9). During the reaction process, AC could be an electron transferal mediator involved in the activation of PS. The character peak at 706.56 eV (Fig. S4(c)) was typically ascribed to Fe0, however, it has disappeared (Fig. S4(d)) after reacting with NOR, which demonstrates that Fe0 was converted to iron oxide. This consequence was consistent with XRD results.
3.5. Identification of Dominant Radical Species in ACnZVI/Cu-PS SystemThe above experiments have indicated that PS is activated to generate ·SO4−. To verify this fact, EPR was employed to capture ·SO4− and ·OH and the result was presented in Fig. S5. There are not only ·SO4− but also ·OH in the early stage of the reaction. Meanwhile, the intensity of ·OH is stronger than ·SO4−, suggesting that the amount of ·OH was higher than ·SO4−. Two reasons can be applied to explain this phenomenon. In the first strategy, nZVI and Fe2+ can activate PS to generate ·SO4−, but they also activate oxygen dissolved in water to generate ·OH (Eq. (31), Eq. (32)). In the second strategy, it would oxidize water to produce hydroxyl as result of has a high redox potential (Eq. (33)).
To further find out the contribution of free radical species to the degradation of NOR in the AC-nZVI/Cu-PS system, quenching experiments were performed. Ethanol (EtOH) and tert-butanol (TBA) are well-known to be effective probe agents for ·SO4− (EtOH for ·SO4− 1.6×107–7.7×107 M−1s−1) and ·OH (TBA for ·OH: 3.8×108–7.6×108 M−1s−1) [65]. Experimental data was shown in Table 2. The removal efficiency decreased from 93.98% and 85.75% to 31.31% and 35.48% after adding EtOH under acidic conditions. While the removal efficiency only drops to 70.97% and 70.16% by adding TBA at the same condition. It is fully proven that ·SO4− is the predominant free radical in the AC-nZVI/Cu-PS system under acidic conditions. Meanwhile, the lower removal efficiency of 49.1% further dropped to 29.30% and 30.54% under alkaline conditions while adding TBA and EtOH, suggesting that the removal of NOR mainly depends on the adsorption of passivation layer appears on the surface of AC-nZVI/Cu at alkaline conditions [39].
3.6. Removal Mechanism of NORDepending on the method reported in the reference [66], we detected the concentration of Fe2+ and Fe3+ by UV-spectrophotometry. In Fig. 6(a), the concentration of Fe2+ increases with the reaction going on, and the highest point of 27.09 mg/L was reached at 20 min. In Fig. 6(c), PS concentration [67] was decreased continuously and the downward trend was the fastest in 5 min until stabilized at 20 min, which demonstrated that nZVI and PS were consumed fastest at the beginning of 20 min. As a result, the generation of free radicals is fastest, and NOR was mainly removed within 20 min, as shown in Fig. 6(b).
Based on the above statement, the activation and removal mechanism of using AC-nZVI/Cu was extracted. The consumption of nZVI containing two strategies, firstly, participates in the activation of PS directly (Eq. (9)), and secondly, hydrogen peroxide was produced by a reaction combined nZVI with dissolved oxygen in water (Eq. (31), Eq. (32)). Besides, the generation of Fe2+ with the nZVI consumption goes on, which activated PS continuously (Eq. (34)) and form the Fenton system with H2O2 to degrade NOR. The results of the NOR removal rate are fastest in the early 20 min (Fig. 6(b)), which indicates that the generation of numerous free radicals at the beginning of the reaction. Moreover, Fe3+ concentration reached the highest point at 10min as a result of largely Fe0 and Fe2+ consumed. However, it appeared to decrease trend from 10 min to 30 min, which may be because of the reaction of nZVI and Fe3+ (Eq. (35)). AC as a carrier removes NOR by direct adsorption, another strategy is oxygen-containing functional groups on its surface can activate PS (Fig. S4(a) and S4(b)) (Eq. (8), Eq. (9)), and thus generate ·SO4− to remove NOR. Bare Cu cannot remove NOR, which can be proved in Fig. 1(a). However, it can not only activate PS to produce ·SO4− (Eq. (11)), and catalyze the hydrogen in the system to produce hydrogen radical (Eq. (36), Eq. (37)), which can remove NOR through reduction reactions (Eq. (38)), but Cu, as a transition metal, can form a galvanic cell with nZVI (called nZVI/Cu bimetal) to accelerate the electron transfer rate.
3.7. The Long-term Stability of AC-nZVI/CuZero-valent iron is easy to be oxidized, causing a dense oxide layer on the surface and reducing the reactivity. The long-term stability of AC-nZVI/Cu was tested by being exposed to the air without any protection. As shown in Fig. S6, compared with nZVI, nZVI/Cu, and AC-nZVI, AC-nZVI/Cu showed outstanding long-term stability, which remain at 83.03% after 60 days, however, that of nZVI, nZVI/Cu, and AC-nZVI was merely 28.71%, 31.87%, and 76.46%. The removal efficiency of AC-nZVI/Cu can reach 60.35% by continuously exposing it to air for 100 days, which was highest among nZVI (22.24%), nZVI/Cu (28.43%), and AC-nZVI (55.17%). Obviously, the long-term performance of nZVI was increased after modification by activated carbon and Cu, which can be explained by the changes of specific surface area and dispersibility of composites [68, 69]. Therefore, both activated carbon and Cu play a vital role in improving the long-term performance of nZVI. Furthermore, the amount of metal leaching in the solution was particularly concerning to further understand the stability of AC-nZVI/Cu. Hence, ultraviolet spectrophotometry was employed to measure the concentration of iron and copper ions after reacting 120 min (T=303 K; pH=2.0±0.2; [NOR]=30 mg/L; [adsorbent]=0.5 g/L) by recording the absorbance at 510 nm and 440 nm. The results indicated that the total iron content was only 0.33 mg/L, much below 2 mg/L which is the legal limit imposed by the directives of the European Union [60]. No copper ions were detected in the solution, suggesting AC-nZVI/Cu has excellent stability.
4. ConclusionIn this paper, AC-nZVI/Cu prepared via the liquid phase reduction method was employed to activate PS to remove NOR in the solution. The performance of batch experiments illustrated that high removal efficiency (93.98%) was achieved by AC-nZVI/Cu with the condition of pH=2±0.2, T=313 K, PS concentration=0.5 mM, and [adsorbent]=0.5 g/L. Additionally, and showed an inhibition effect on NOR removal regardless of their concentration, but and Cl− play a dual role (promotion and inhibition) in NOR removal, Furthermore, the removal process of NOR is an endothermic, entropy-increasing, and spontaneous reaction, being consistent with Liu adsorption isotherm. Meanwhile, the EPR analysis and quenching experiments demonstrate that ·SO4− dominants removal reaction under acidic conditions, which mainly occurs on an early stage of reaction, and XRD and XPS illustrate that nZVI is basically a contributor to the system of NOR removal and PS activation. Importantly, AC-nZVI/Cu exhibits excellent long-term stability after being exposed to the air without any protection for 100 days owing to the common modification of activated carbon and Cu.
Consequently, these findings indicated that the proposed AC-nZVI/Cu-PS system is capable of treating wastewater from hospital and pharmaceutical factory containing a high concentration of NOR. It is hoped that this research can provide an effective strategy to overcome the mechanism interpretation for NOR removal and build a forceful oxidative system for the depuration of antibiotic wastewater.
AcknowledgementsThis work was supported by the National Natural Science Foundations of China (52163025, 51503092, and 51763015), and the National Natural Science Foundation of Gansu Province (21JR7RA229).
References1. Than NQ, Sabbah A, Chen LC, Chen KN, Cao TM, Pham VV. High-efficient photocatalytic degradation of commercial drugs for pharmaceutical wastewater treatment prospects: A case study of Ag/g-C3N4/ZnO nanocomposite materials. Chemosphere. 2021;282:130971.
https://doi.org/10.1016/j.chemosphere.2021.130971
2. Liu H, Gao Y, Wang J, Pan J, Gao BY, Yue Q. Catalytic ozonation performance and mechanism of Mn-CeOx@γ-Al2O3/O3 in the treatment of sulfate-containing hypersaline antibiotic wastewater. Sci. Total Environ. 2022;807(1)150867.
https://doi.org/10.1016/j.scitotenv.2021.150867
3. Guo R-F, Liang P, Li X-Y, Liu Z-H. Fabrication of a dual Z-scheme GACN/NiO/Ni3(BO3)2 composite with excellent photocatalytic activity for methylene blue and tetracycline removal. Sep. Purif. Technol. 2021;264:118414.
https://doi.org/10.1016/j.seppur.2021.118414
4. Rasheed T, Ahmad N, Ali Z, et al. Nano and micro architectured cues as smart materials to mitigate recalcitrant pharmaceutical pollutants from wastewater. Chemosphere. 2021;274:129785.
https://doi.org/10.1016/j.chemosphere.2021.129785
5. Rasheed T, Ahmad N, Nawaz S, Sher F. Photocatalytic and adsorptive remediation of hazardous environmental pollutants by hybrid nanocomposites. Case Studies Chem. Environ. Eng. 2020;2:100037.
https://doi.org/10.1016/j.cscee.2020.100037
6. Rasheed T, Anwar MT, Ahmad N, et al. Valorisation and emerging perspective of biomass based waste-to-energy technologies and their socio-environmental impact: A review. J. Environ. Manag. 2021;287:112257.
https://doi.org/10.1016/j.jenvman.2021.112257
7. Zhou G, Wang PF, Li H, et al. Bimetallic-atom-hybridization-driven catalytic reaction kinetics and solar utilization to accelerate norfloxacin degradation. Appl. Catal. B Environ. 2021;298:120525.
https://doi.org/
10.1016/j.apcatb.2021.120525
8. Wang Z, Yu XD, Pan B, Xing BS. Norfloxacin sorption and its thermodynamics on surface-modified carbon nanotubes. Environ. Sci. Technol. 2010;44(3)978–84.
https://doi.org/10.1021/es902775u
9. Messaoudi NE, Khomri ME, Ablouh E-H, et al. Biosynthesis of SiO2 nanoparticles using extract of Nerium oleander leaves for the removal of tetracycline antibiotic. Chemosphere. 2022;287:132453.
https://doi.org/10.1016/j.chemosphere.2021.132453
10. Zhang QQ, Ying GG, Pan CG, Liu YS, Zhao JL. Comprehensive evaluation of antibiotics emission and fate in the river basins of China: source analysis, multimedia modeling, and linkage to bacterial resistance. Environ. Sci. Technol. 2015;49(11)6772–6782.
https://doi.org/10.1021/acs.est.5b00729
11. Panagopoulos A, Giannika V. Comparative techno-economic and environmental analysis of minimal liquid discharge (MLD) and zero liquid discharge (ZLD) desalination systems for seawater brine treatment and valorization. Sustain. Energy Technol. Assess. 2022;53:102477.
https://doi.org/10.1016/j.seta.2022.102477
12. Panagopoulos A. Brine management (saline water & wastewater effluents): Sustainable utilization and resource recovery strategy through Minimal and Zero Liquid Discharge (MLD&ZLD) desalination systems. Chem. Eng. Process. Process Intensif. 2022;176:108944.
https://doi.org/10.1016/j.cep.2022.108944
13. Panagopoulos A. Techno-economic assessment and feasibility study of a zero liquid discharge (ZLD) desalination hybrid system in the Eastern Mediterranean. Chem. Eng. Process. Process Intensif. 2022;178:109029.
https://doi.org/10.1016/j.cep.2022.109029
14. Ma Y, Li M, Wu M, Li Z, Liu X. Occurrences and regional distributions of 20 antibiotics in water bodies during groundwater recharge. Sci. Total Environ. 2015;518–519:498–506.
https://doi.org/10.1016/j.scitotenv.2015.02.100
15. Gao P, Mao D, Luo Y, Wang Y, Xu B, Xu L. Occurrence of sulfonamide and tetracycline-resistant bacteria and resistance genes in aquaculture environment. Water Res. 2012;46(7)2355–2364.
https://doi.org/10.1016/j.watres.2012.02.004
16. Pan S, Li J, Noonan O, Fang X, Wan G, Yu C, Wang L. Dual-functional ultrafiltration membrane for simultaneous removal of multiple pollutants with high-performance. Environ. Sci. Technol. 2017;51(9)5098.
https://doi.org/10.1021/acs.est.6b05295
17. Zhang Y, Fan N, He JS, et al. Mechanistic insight into different adsorption of norfloxacin on microplastics in simulated natural water and real surface water. Environ. Pollut. 2021;284:117537.
https://doi.org/10.1016/j.envpol.2021.117537
18. Chen X, Wang J. Degradation of norfloxacin in aqueous solution by ionizing irradiation: kinetics, pathway and biological toxicity. Chem. Eng. J. 2020;395:125095.
https://doi.org/10.1016/j.cej.2020.125095
19. Zhang G, Li W, Chen S, Zhou W, Chen J. Problems of conventional disinfection and new sterilization methods for antibiotic resistance control. Chemosphere. 2020;254:126831.
https://doi.org/10.1016/j.chemosphere.2020.126831
20. IM-K , PK , DF-K . The role of operating parameters and oxidative damage mechanisms of advanced chemical oxidation processes in the combat against antibiotic-resistant bacteria and resistance genes present in urban wastewater. Water Res. 2018;129:208–230.
https://doi.org/10.1016/j.watres.2017.10.007
21. Sharma A, Ahmad J, Flora SJS. Application of advanced oxidation processes and toxicity assessment of transformation products. Environ. Res. 2018;167:223–233.
https://doi.org/10.1016/j.envres.2018.07.010
22. Wang JL, Zhuan R. Degradation of antibiotics by advanced oxidation processes: An overview. Sci. Total Environ. 2020;701:135023.
https://doi.org/10.1016/j.scitotenv.2019.135023
23. Vlyssides AG, Loizidou M, Karlis PK, Zorpas AA, Papaioannou BD. Electrochemical oxidation of a textile dye wastewater using a Pt/Ti electrode. J. Hazard. Mater. 1999;70(1–2)41–52.
https://doi.org/10.1016/S0304-3894(99)00130-2
24. Ji Y, Pan C, Yuan D, Lai B. Advanced Treatment of the Antibiotic Production Wastewater by Ozone/Zero-Valent Iron Process. Soil Air Water. 2018;170066:
https://doi.org/10.1002/clen.201700666
25. Lefevre E, Bossa N, Wiesner RM, Gunsch CK. A review of the environmental implications of in situ remediation by nanoscale zero valent iron (nZVI): behavior, transport and impacts on microbial communities. Sci. Total Environ. 2016;565:889–901.
https://doi.org/10.1016/j.scitotenv.2016.02.003
26. Song M, Hu X, Gu T, Zhang WX, Deng Z. Nanocelluloses affixed nanoscale zero-valent Iron (nZVI) for nickel removal: synthesis, characterization and mechanisms. J. Environ. Chem. Eng. 2022;10(3)107466.
https://doi.org/10.1016/j.jece.2022.107466
27. Zhang S, Kong Z, Wang H. Enhanced nitrate removal by biochar supported nano zero-valent iron (nZVI) at biocathode in bio-electrochemical system (BES). Chem. Eng. J. 2022;433(2)133–535.
https://doi.org/10.1016/j.cej.2021.133535
28. Zhang P, Song D, Xu XJ, Hao Y, Sun H. Sulfidated zero valent iron as a persulfate activator for oxidizing organophosphorus pesticides (OPPs) in aqueous solution and aged contaminated soil columns. Chemosphere. 2021;281:130–760.
https://doi.org/10.1016/j.chemosphere.2021.130760
29. Kim C, Ahn JY, Kim TY, Shin WS, Hwang I. Activation of persulfate by nanosized zero-valent iron (NZVI): mechanisms and transformation products of NZVI. Environ. Sci. Technol. 2018;52(6)3625–3633.
https://doi.org/10.102/acs.est.7b05847
30. Reinsch BC, Forsberg B, Penn RL, Kim CS, Lowry GV. Chemical transformations during aging of zerovalent iron nanoparticles in the presence of common groundwater dissolved constituents. Environ. Sci. Technol. 2010;44(9)3455.
https://doi.org/10.1021/es902924h
31. Yuan X, Li T, He Y, Xue N. Degradation of TBBPA by nZVI activated persulfate in soil systems. Chemosphere. 2021;284(38)131166.
https://doi.org/10.1016/j.chemosphere.2021.131166
32. Mangayayam M, Dideriksen K, Ceccato M, Tobler DJ. The structure of sulfidized zero-valent iron by one-pot synthesis: impact on contaminant selectivity and long-term performance. Environ. Sci. Technol. 2019;53(8)4389–4396.
https://doi.org/10.1021/acs.est.8b06480
33. Liu J, Liu A, Guo J, Zhou T, Zhang W-X. Enhanced aggregation and sedimentation of nanoscale zero-valent iron (nZVI) with polyacrylamide modification. Chemosphere. 2020;263:127875.
https://doi.org/10.1016/j.chemosphere.2020.127875
34. Xu H, Tian W, Zhang Y, Tang J, Zhao Z, Chen Y. Reduced graphene oxide/attapulgite-supported nanoscale zero-valent iron removal of acid red 18 from aqueous solution. Water Air Soil Pollut. 2018;229(12)388.
https://doi.org/10.1007/s11270-018-4033-5
35. Xu H, Zhang Y, Cheng Y, Tian W, Zhao Z, Tang J. Polyaniline/attapulgite-supported nanoscale zero-valent iron for the rival removal of azo dyes in aqueous solution. Adsorp. Sci. Technol. 2019;1–19.
https://doi.org/10.1177/0263617418822917
36. Xu H, Zhang Y, Tang J, Tian W, Zhao Z, Cheng Y. Removal of cationic dyes from aqueous solutions using adsorbents based on GO/APTG nanocomposites. Desali. Water Treat. 2020;174:414–427.
https://doi:10.5004/dwt2020.24888
37. Shan A, Farooq U, Lyu S. Efficient removal of trichloroethylene in surfac-tant amended solution by nano Fe0-Nickel bimetallic composite activated sodium persulfate process. Chem. Eng. J. 2020;386:123995.
https://doi.org/10.1016/j.cej.2019.123995
38. Mario R-H, Marshal WD. Reduction of hexavalent chromium mediated by micro-and nano-sized mixed metallic particles. J. Hazard. Mater. 2009;169(1–3)1081–1087.
https://doi.org/10.1016/j.jhazmat.2009.04.062
39. Fang Z, Li L, Ren W, Deng W, Tao L. Effect of pH, temperature, humic acid and coexisting anions on reduction of Cr (VI) in the soil leachate by nZVI/Ni bimetal material. Environ. Pollut. 2017;227:444–450.
https://doi.org/10.1016/j.envpol.2017.04.074
40. Gao Y, Wang FF, Yan Wu, Naidu R, Chen ZL. Comparison of degradation mechanisms of microcystin-LR using nanoscale zero-valent iron (nZVI) and bimetallic Fe/Ni and Fe/Pd nanoparticles. Chem. Eng. J. 2016;285:459–466.
https://doi.org/10.1016/j.cej.2015.09.078
41. Wang Y, Zhao H, Zhao G. Iron-copper bimetallic nanoparticles embedded within ordered mesoporous carbon as effective and stable heterogeneous fenton catalyst for the degradation of organic contaminants. Appl. Catal. B Environ. 2015;164:396–406.
https://doi.org/10.1016/j.apcatb.2014.09.047
42. Chen L, Yuan T, Ni R, Yue Q, Gao B. Multivariate optimization of ciprofloxacin removal by polyvinylpyrrolidone stabilized NZVI/Cu bimetallic particles. Chem. Eng. J. 2019;365:183–192.
https://doi.org/10.1016/j.cej.2019.02.051
43. Lin J, Sun M, Liu X, Chen Z. Functional kaolin supported nanoscale zero-valent iron as a fenton-like catalyst for the degradation of Direct Black G. Chemosphere. 2017;184:664–672.
https://doi.org/10.1016/j.chemosphere.2017.06.038
44. Shi L, Zhang X, Chen Z. Removal of chromium (VI) from wastewater using bentonite-supported nanoscale zero-valent iron. Water Res. 2011;45(2)886–892.
https://doi.org/10.1016/j.watres.2010.09.025
45. Zhang W, Qian L, Han L, et al. Synergistic roles of Fe (II) on simultaneous removal of hexavalent chromium and trichloroethylene by attapulgite-supported nanoscale zero-valent iron/persulfate system. Chem. Eng. J. 2022;430:132841.
https://doi.org/10.1016/j.cej.2021.132841
46. Mortazavian S, An H, Chun D, Moon J. Activated carbon impregnated by zero-valent iron nanoparticles (AC/nZVI) optimized for simultaneous adsorption and reduction of aqueous hexavalent chromium: Material characterizations and kinetic studies. Chem. Eng. J. 2018;353:781–795.
https://doi.org/10.1016/j.cej.2018.07.170
47. Bagheri AR, Armesh N, Sher F, Bilel M. Covalent organic frameworks as robust materials for mitigation of environmental pollutants. Chemosphere. 2021;270:129523.
https://doi.org/10.1016/j.chemosphere.2020.129523
48. Ali N, Bilal M, Yang Y, et al. Adsorptive remediation of environmental pollutants using magnetic hybrid materials as platform adsorbents. Chemosphere. 2021;284:131279.
https://doi.org/10.1016/j.chemosphere.2021.131279
49. Wang JL, Wang SZ. Preparation, modification and environmental application of biochar: A review. J. Clean. Prod. 2019;277:1002–1022.
https://doi.org/10.1016/j.jclepro.2019.04.282
50. Peng Z, Liu X, Zhang W, Zeng Z, et al. Advances in the application, toxicity and degradation of carbon nanomaterials in environment: A review. Environ. Int. 2019;134:105298.
https://doi.org/10.1016/j.envint.2019.105298
51. Xu CY, Song Q, Merdanoglu N, Liu H, Klemm E. Identifying Monomeric Fe Species for Efficient Direct Methane Oxidation to C1 Oxygenates with H2O2 over Fe/MOR Catalysts. Methane. 2022;1(2)107–124.
https://doi.org/10.3390/methane1020010
52. Teixeira R, Lima E, Benetti A, et al. Preparation of hybrids of wood sawdust with 3-aminopropyl-triethoxysilane. Application as an adsorbent to remove Reactive Blue 4 dye from wastewater effluents. J. Taiwan Inst. Chem. Eng. 2021;125:141–152.
https://doi.org/10.1016/j.jtice.2021.06.007
53. Li H, Yang L, He L, Ma Y, Zhang Z. Kinetics and mechanisms of chloramphenicol degradation in aqueous solutions using heat-assisted nZVI activation of persulfate. J. Mol. Liq. 2020;313:113511.
https://doi.org/10.1016/j.molliq.2020.113511
54. Shan A, Idrees A, Zaman WQ, et al. Synthesis of nZVI-Ni@BC composite as a stable catalyst to activate persulfate: Trichloroethylene degradation and insight mechanism. J. Environ. Chem. Eng. 2021;9(1)104808.
https://doi.org/10.1016/j.jece.2020.104808
55. Chen Y, Lin Z, Hao R, Xu H, Huang C. Rapid adsorption and reductive degradation of Naphthol Green B from aqueous solution by Polypyrrole/Attapulgite composites supported nanoscale zero-valent iron. J. Hazard. Mater. 371:2019;8–17.
https://doi.org/10.1016/j.jhazmat.2019.02.096
56. Rashtbari Y, Sher F, Afshin S, et al. Green synthesis of zero-valent iron nanoparticles and loading effect on activated carbon for furfural adsorption. Chemosphere. 2022;287:132114.
https://doi.org/10.1016/j.chemosphere.2021.132114
57. Xue W, Wang L, Li J, Qiu J, Hui Z. Degradation of acid orange 7 by persulfate activated with zero valent iron in the presence of ultrasonic irradiation. Sep. Purif. Technol. 2014;122:41–46.
https://doi.org/10.1016/j.seppur.2013.10.037
58. Xia Z, Liu H, Wang S, Meng Z, Ren N. Preparation and dechlorination of a poly(vinylidene difluoride)-grafted acrylic acid film immobilized with Pd/Fe bimetallic nanoparticles for monochloroacetic acid. Chem. Eng. J. 2012;200–202:214–223.
https://doi.org/10.1016/j.cej.2012.06.056
59. Wang Y, Tian D, Chu W, Li M, Lu X. Nanoscaled magnetic CuFe2O4 as an activator of peroxymonosulfate for the degradation of antibiotics norfloxacin. Sep. Purif. Technol. 2019;212:536–544.
https://doi.org/10.1016/j.seppur.2018.11.051
60. Liu J, Du Y, Sun W, Chang Q, Peng C. A granular adsorbent-supported Fe/Ni nanoparticles activating persulfate system for simultaneous adsorption and degradation of ciprofloxacin. Chin. J. Chem. Eng. 2020;28(4)1077–1084.
https://doi.org/10.1016/j.cjche.2019.12.019
61. Nie M, Yan C, Li M, Wang X, Bi W, Dong W. Degradation of chloramphenicol by persulfate activated by Fe2+ and zero-valent iron. Chem. Eng. J. 2015;279:507–515.4.
https://doi.org/10.1016/j.cej.2015.05.055
62. Lima ÉC, Adebayo MA, Machado FM. Kinetic and equilibrium models of adsorption, Carbon nanomaterials as adsorbents for environmental and biological applications. Carbon Nanostruct. 2015;33–69.
https://doi.org/10.1007/978-3-31918875-1-3
63. Chen Y, Zhang J, Xu H. Exploration of the degradation mechanism of ciprofloxacin in water by nano zero-valent iron combined with activated carbon and nickel. J. Mol. Liq. 2022;345:118212.
https://doi.org/10.1016/j.molliq.2021.118212
64. Cardoso RM, Montes RHO, Lima AP, et al. Multi-walled carbon nanotubes: Size-dependent electrochemistry of phenolic compounds. Electrochim. Acta. 2015;176:36–43.
https://doi.org/10.1016/j.electacta.2015.06.117
65. Yang S, Yang X, Shao X, Niu R, Wang L. Activated carbon catalyzed persulfate oxidation of Azo dye acid orange 7 at ambient temperature. J. Hazard. Mater. 2011;186659–666.
https://doi.org/10.1016/j.jhazmat.2010.11.057
66. Eljamal O, Mokete R, Matsunaga N, Sugihara Y. Chemical pathways of Nanoscale Zero-Valent Iron (NZVI) during its transformation in aqueous solutions. J. Environ. Chem. Eng. 2018;6(5)6207–6220.
https://doi.org/10.1016/j.jece.2018.09.012
67. Liang C, Huang CF, Mohanty N, Mohanty N, Kurakalva RM. A rapid spectrophotometric determination of persulfate anion in ISCO. Chemosphere. 2008;73(9)1540–1543.
https://doi.org/10.1016/j.chemosphere.2008.08.043
68. Cho Y, Choi S. Degradation of PCE, TCE and 1,1,1-TCA by nanosized Fe/Pd bimetallic particles under various experimental conditions. Chemosphere. 2010;81:940–945.
https://doi.org/10.1016/j.chemosphere.2010.07.054
69. He F, Zhao D. Hydrodechlorination of trichloroethene using stabilized Fe-Pd nanoparticles: reaction mechanism and effects of stabilizers, catalysts and reaction conditions. Appl. Catal. B Environ. 2008;84:533–540.
https://doi.org/10.1016/j.apcatb.2008.05.008
Fig. 1Effect of different activation systems on the degradation of NOR (a); PS concentration in different activation systems (b). pH=2±0.2, [NOR]=30 mg/L, [adsorbent]0=0.5 g/L, [PS]=0.5 mM, T=303 K 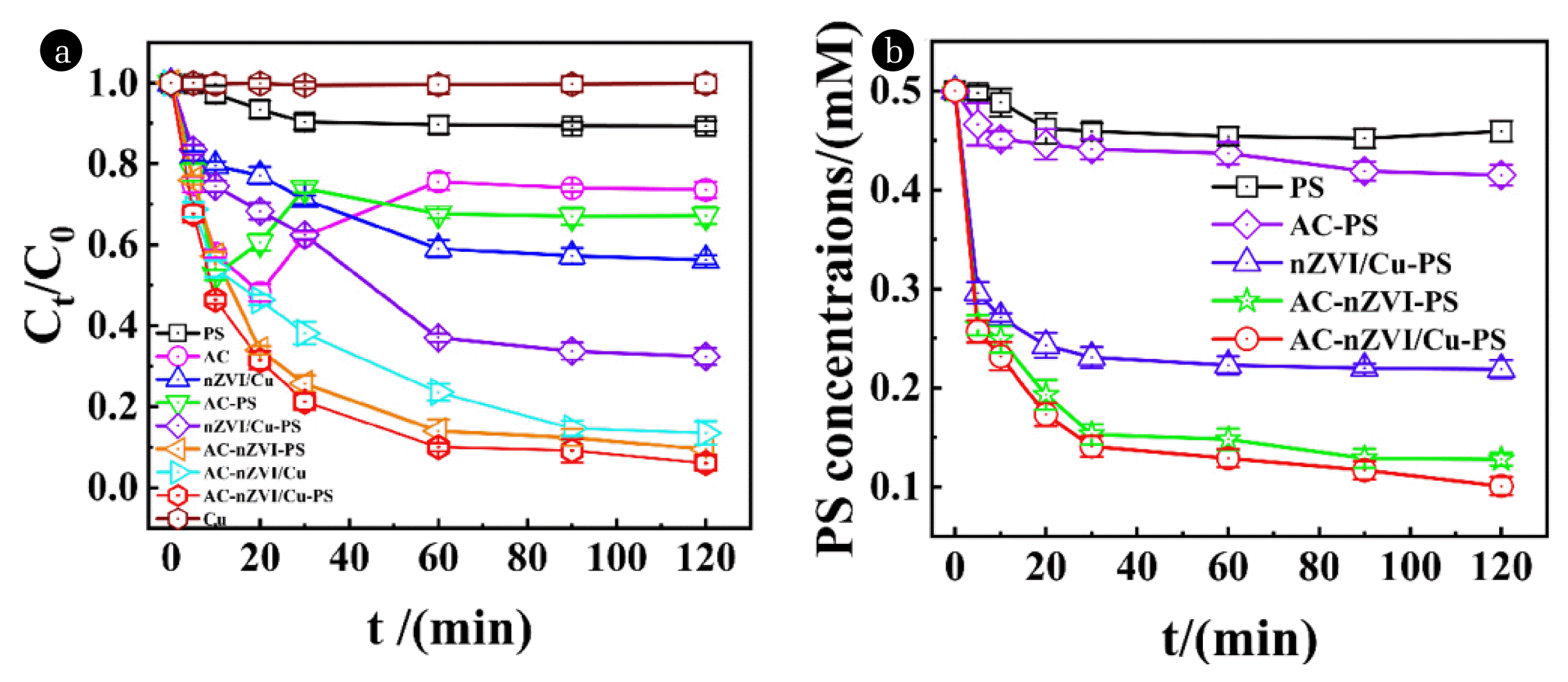
Fig. 2Effect of different pH on the removal of NOR (a) and the kinetic fitting constant of different pH (b) [NOR]=30 mg/L, T=303 K, [PS]=0.5 mM, [adsorbent]=0.5 g/L; Effect of different PS concentration on the removal of NOR (c) and kinetic fitting constant of different PS concentration (d) pH=2±0.2, [NOR]=30 mg/L, T=303 K, [adsorbent]=0.5 g/L; Effect of temperature on the degradation of NOR (e), and kinetic fitting constant of different temperature (f) [NOR]=30 mg/L, [adsorbent]=0.5 g/L, pH=2±0.2, [PS]=0.5 mM. 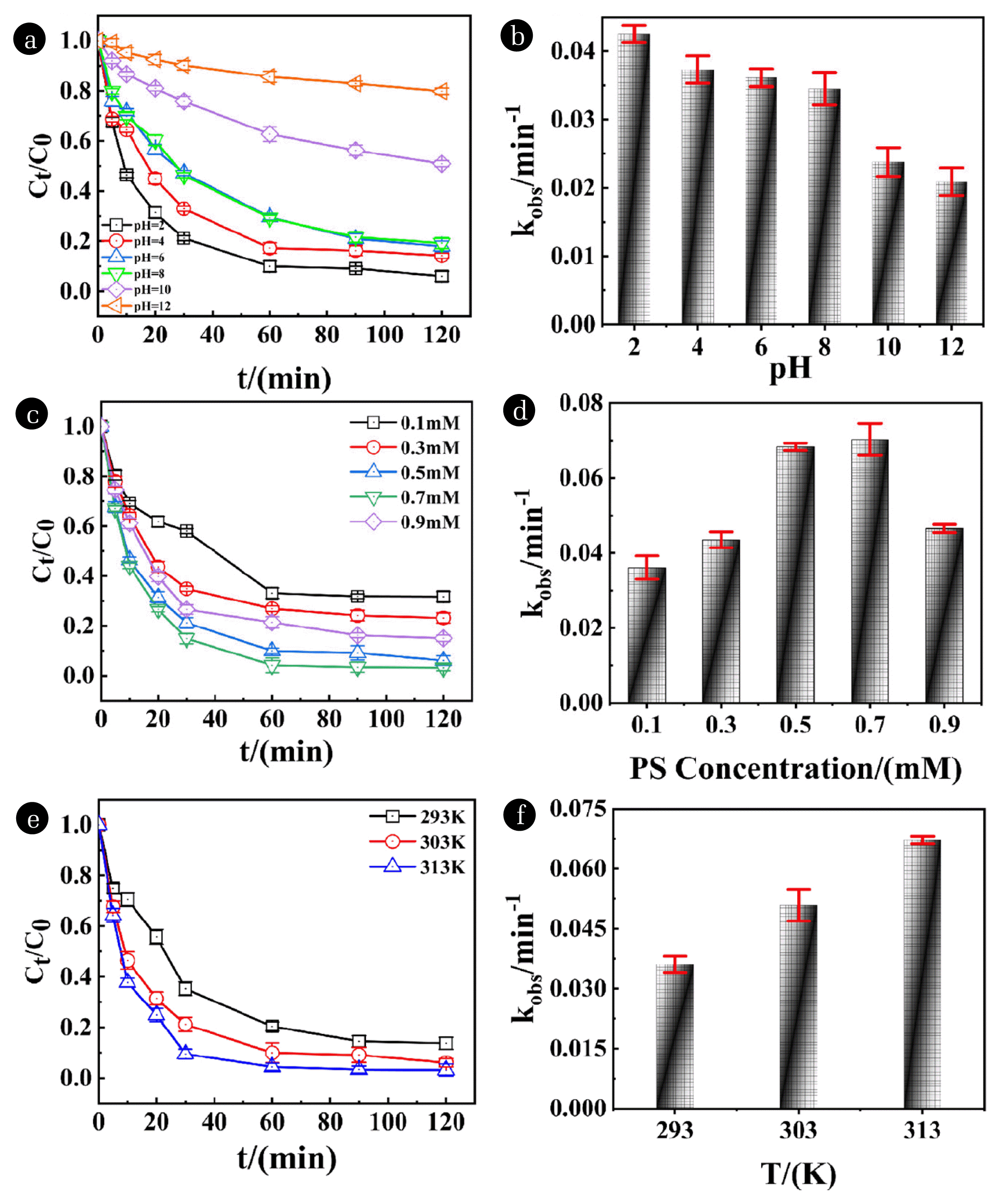
Fig. 3Effect of various inorganic anions on NOR removal and rate constants values in AC-nZVI/Cu-PS system: Cl- (a, b), NO3- (c, d), HCO3- (e, f), H2PO4- (g, h). [NOR]=30 mg/L, [adsorbent]=0.5 g/L, pH=2±0.2, [PS]=0.5 mM. 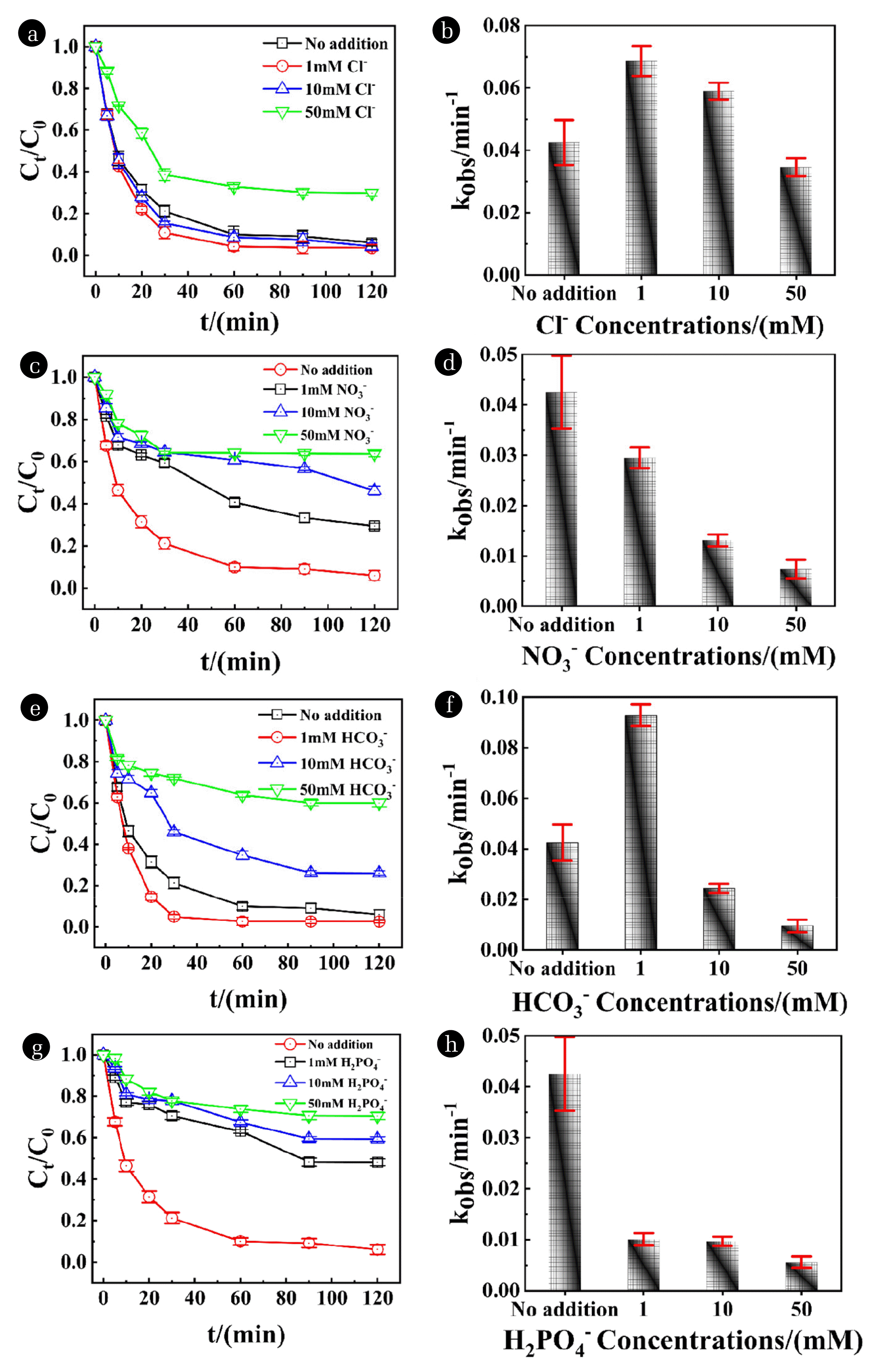
Fig. 5TEM image of nZVI (a); AC-nZVI (b); AC-nZVI/Cu (c); EDS diagrams of AC-nZVI/Cu (d); and EDX elemental maps of AC-nZVI/Cu. 
Fig. 6The trend of Fe3+ and Fe2+ concentration in AC-nZVI/Cu-PS system (a); Concentration of NOR in AC-nZVI/Cu-PS system and AC-nZVI-PS (b); Concentration of PS in AC-nZVI/Cu-PS and AC-nZVI-PS system. [NOR]0=30 mg/L, pH=2.0±0.2, [adsorbent]=0.5 g/L 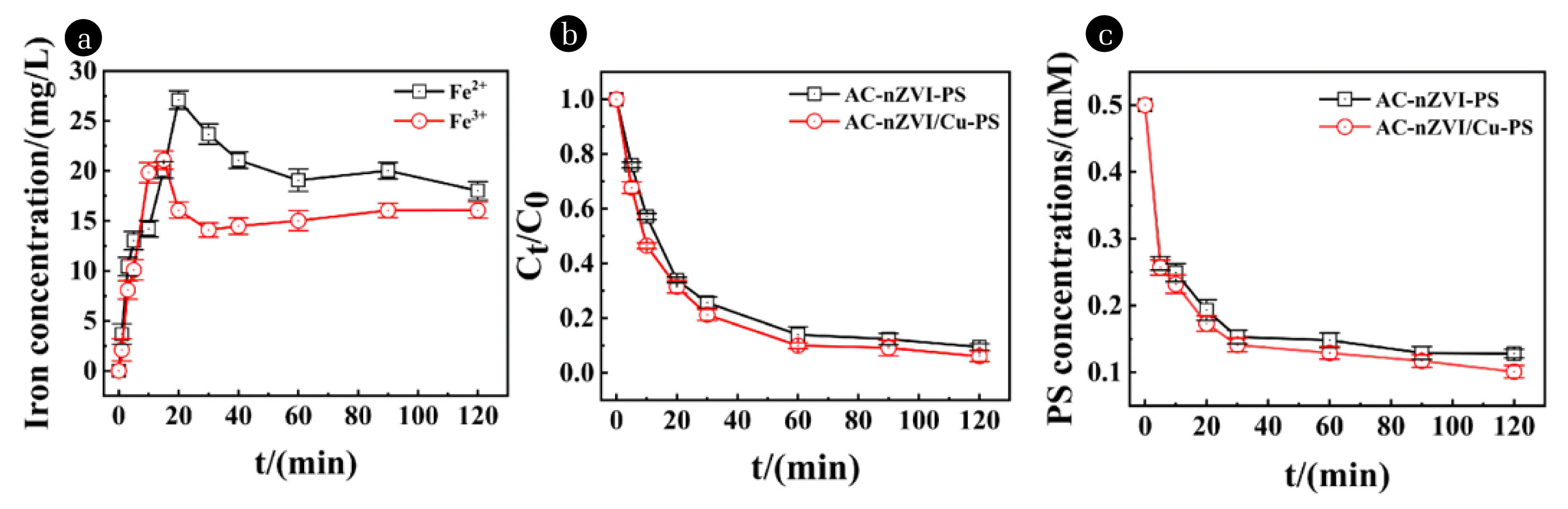
|
|
||||||||||||||||||||||||||||||||||||