AbstractIn this paper, Cobalt-doped α-MnO2 (i.e., Co-α-MnO2) were synthesized through hydrothermal method. Phenol was employed as targeted pollutants to investigate the catalytic ozonation performance of Co-α-MnO2. Results showed that Co-α-MnO2 significantly improved the phenol removal increased to 97.47 % after 40 min, which was 16.46 %, 38.92 % higher than that of α-MnO2 catalytic ozonation and single ozonation without catalyst. Additionally, the physicochemical properties of α-MnO2 and Co-α-MnO2 were analyzed using technologies such as XRD, TEM, BET and XPS. Compared to α-MnO2, Co-α-MnO2 has larger specific surface area (79.496 m2/g) and pore volume (0.0396 cm3/g), higher Mn3+ relative content (41.16 %) and adsorbed oxygen content (18.99 %). Also, the oxygen vacancy content, lattice defect content and surface hydroxyl content of Co-α-MnO2 are higher than that of α-MnO2, which could result in higher catalytic oxidation performance of Co-α-MnO2. The influence of masking agent showed that surface hydroxyl group, •OH and •O2− were involved in the catalytic ozonation of phenol. This study could help recognize the role of surface hydroxyl groups and active free radicals and demonstrate the contribution of reactive oxygen species on phenol removal in Co-α-MnO2 systems.
Graphical Abstract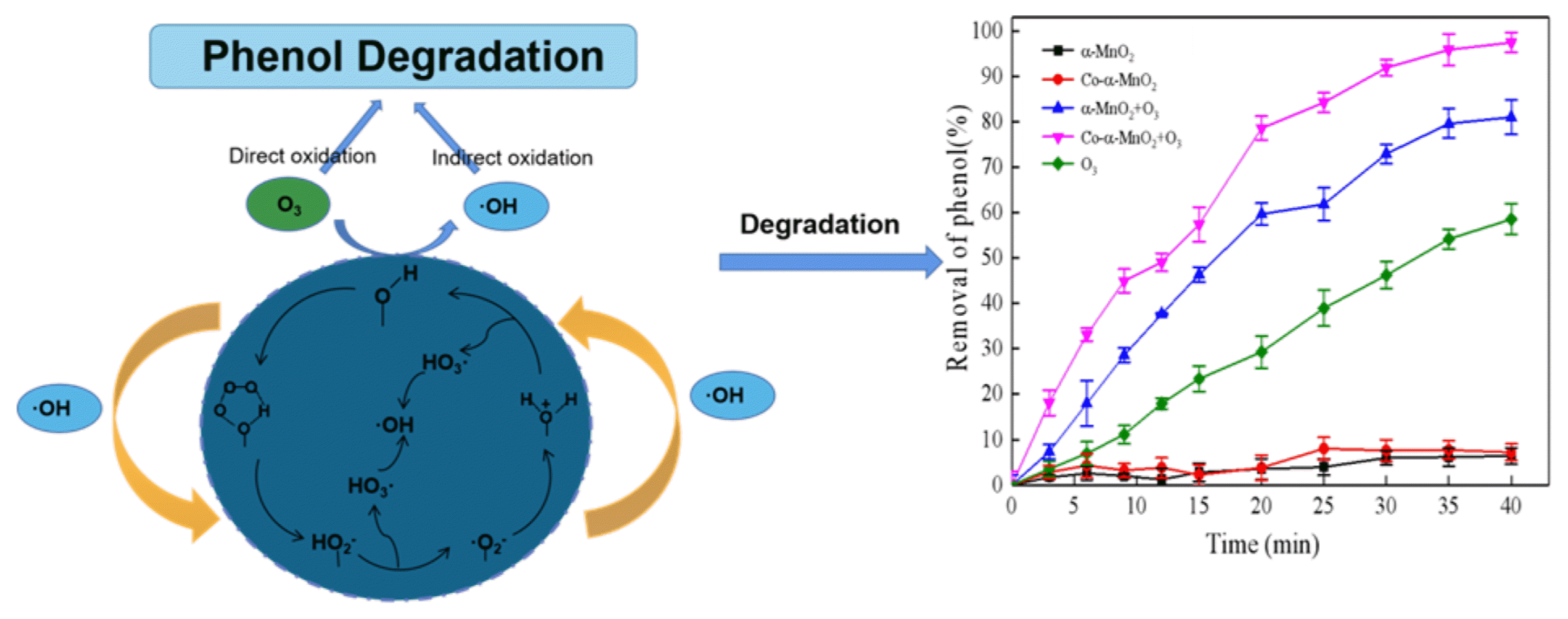
1. IntroductionAdvanced oxidation process (AOP) using free radical reaction is considered to be an effective and promising method for degradation and mineralization of organic compounds in wastewater treatment. Many studies have been carried out on the degradation of organic compounds by AOPs technologies, such as photocatalysis[1], Fenton oxidation technology[2]. etc. Now, ozonation has been widely undertaken in industrial wastewater treatment because ozone poses the outstanding oxidation potential on a large number of organic pollutants [3, 4]. However, single ozonation commonly presented a low removal rate on organic compounds degradation [5]. To overcome the limitation of this process, ozonation coupled with catalyst has been attracted much attention. Heterogeneous catalytic ozonation can enhance the oxidation of ozone by selecting appropriate solid catalysts. This method can promote ozone decomposition, which will generate more reactive oxygen species (ROS) and further enhance the degradation of organic compounds [6, 7].
Metal oxides, such as TiO2 [8–11], MnO2 [12], Fe oxides [13] and some polymetallic oxides (CoFe2O4, et al.) [14] have been widely used as the catalysts in heterogeneous catalytic ozonation. Among these metal oxides, MnO2 showed the excellent catalytic performance on benzene series degradation due to its strong redox coples of Mn2+/Mn3+ and Mn3+/Mn4+ on the surface of catalysts, diverse and crystallographic structure [7,15]. MnO2 has great structural flexibility and crystallographic polymorphs (e.g., α-, β-, γ-, and δ-MnO2) because the basic structural MnO6 units can be linked in different manners forming tunnels [16]. α-MnO2 has been extensively studied due to its structural characteristics and excellent activity for ozone decomposition and catalytic ozonation [17, 18]. Moreover, recent studies have showed that metal doping can improve the catalytic performance by modifying α-MnO2 structure. Wang et al. synthesized α-MnO2 nanowires doped with Zr4+ and observed that Zr4+ ions originally occupied the positions belonging to elemental manganese in the crystal structure and resulted in a mutual action between Zr4+ ions and Mn3+ ions, thus improving the catalytic performance of α-MnO2 [19]. Uematsu et al. reported that the morphology of α-MnO2 was changed by doping with Mo6+, leading to an increase in its specific surface area and the number of catalytically active surface positions, which in turn improved the catalytic performance of the catalyst [20].
As a kind of transition metal, Co has a strong Co2+/Co3+ redox cycle which can also promote electron transfer and thus improve catalytic oxidation efficiency [21]. Lv et al. reported that Co doping on Fe3O4 increased the catalytic activity and stability of Fe3O4 [22]. Li et al. observed that an interface synergistic effect between the doped metal Co and cerium oxide on catalyst Co-Ce-MCM-48 improved the interface electronic behavior and promoted the production of ROS [23]. Faleh et al. developed a new heterogeneous cobalt (Co) catalyst supported on activated carbon (Co/AC) and found the doping of Co improved the surface adsorption capacity of activated carbon which enhanced the degradation efficiency of oxalic acid in catalytic ozonation process [24]. Additionally, some studies have showed that the synergistic role can be well presented when the radius of doped metal ions is close to that of metal ions in the catalyst [25]. Therefore, due to the close ionic radius between Co2+ and Mn2+, the catalyst with Co doping on the α-MnO2 might have a better catalytic activity than that of the sole α-MnO2 catalyst. However, it is not clear how influence cobalt doping on its structure and physical and chemical properties.
In this article, α-MnO2 catalyst doped with Co2+ was prepared by hydrothermal method, and then investigate the catalytic ozonation activities on phenol removal. BET, XRD, XPS and FTIR were used to analyze the phase, morphology and structural properties of the synthesized catalysts. The catalytic ozonation mechanism of Co-doped α-MnO2 catalyst on phenol removal was explored in depth by the masking experiment of free radicals combining with catalyst structure characteristics.
2. Experimental2.1. Materials and ReagentsPotassium permanganate (KMnO4, ≥99 %), Manganese sulphate (MnSO4·H2O, ≥98 %), Cobalt Sulfate (CoSO4·7H2O, ≥98 %) were purchased from Sinopharm Chemical Reagent Co., Ltd. Deionized water was obtained from a Millipore Q water purification system. All reagents and chemicals were of analytical grade.
2.2. Synthesis of CatalystsCo-doped α-MnO2 were synthesized using a modified hydrothermal method, which was showed in Fig. S1. Co-doped α-MnO2 were synthesized via a one-step hydrothermal method according to a previous report [26]. 36 mmol of MnSO4·H2O and certain molar of CoSO4·7H2O were dissolved in 50 mL of deionized water under stirring, and then the above mixed solution was added into 50 mL Potassium homologate solution (KMnO4 was 20 mmol) dropwise, followed by stirring magnetically for about 30 min until the solution became homogeneous. After that, it was transferred into a 200 mL Teflon-lined stainless-steel autoclave. The autoclave was kept at 160 °C for 16 h in an oven and then cooled to room temperature, and then, the product was collected by filtration and fully rinsed several times with deionized water to remove K+, followed by drying at 105 °C for 8 h. In order to determine the optimal doping ratio, we prepared catalysts with different initial molar ratio of Co and Mn (0.1, 0.2, 0.3, 0.4 and 0.5 respectively). The sample with Co/Mn = 0.2 had the highest catalytic ozonation of phenol (Fig. S2). Therefore, catalysts with Co/Mn = 0.2 named Co-α-MnO2 was the materials synthesized. The synthetic procedures for α-MnO2 was similar to that for Co-doped α-MnO2 with the exception of adding CoSO4·7H2O to the initial solution.
2.3. Catalysts CharacterizationThe catalyst was purged 5 h at 120 °C under nitrogen atmosphere protection and then determined the BET surface area and pore-size distribution on a Micromeritics ASAP2020 analyser (Micromeritics TriStar 3000, U.S.) when the sample was cooled. Powder X-ray diffraction (XRD) analysis was carried out on a Bruker D8 ADVANCE Phaser (Rigaku D/MAX-2200PC, Japan) using Cu Ka radiation (k = 0.15418 nm) with a LYNXEYE detector at 40 kV and 30 mA. X-ray photoelectron spectroscopy (XPS) was performed with an ESCALAB250 system (ESCALAB 250, U.S.) equipped with an Al Kα excitation source and operated at 15 kW and 1486.6 eV. Fourier transformed infrared (FTIR) analyses (Nicolet iS10, U.S.) were carried out using a NicoletiS10 FT-IR plus spectrophotometer in a wavelength range of 4000–400 cm−1.
2.4. Catalytic Ozonation ActivitiesIn this study, phenol was selected as the aim pollutant because it is one of the most common industrial wastewater contaminates and would cause serious ecological and environmental problems [27]. The phenol degradated by catalytic ozonation was performed in a self-regulating quartz reaction with 1 L aqueous solution showed in Fig. S3. The concentration of ozone was adjusted via controlling the current and flow rate of the ozone generator and measured by ozone detector. When the ozone concentration is stable and then bubbled into the integrated adsorption-catalytic ozonation reactor to start the reaction. The pneumatic panel was adopted to ensure that gaseous ozone was completely mixed with aqueous solution. The pHzpc of the prepared catalysts (α-MnO2, Co-α-MnO2) in this study was 8.03 and 9.87, respectively. The pH value of the solution was set at 10.00 to achieve the zero potential of the catalyst surface, which enhanced the catalytic ability [28, 29]. During the reaction, ozone concentration and flow rate were set at 3.0 mg/L and 3.0 L/min, respectively. The tail gas was absorbed by potassium iodide solution. 2 g catalyst was mixed with 1 L phenol wastewater (initial concentration was 400 mg/L; pH = 10; 293 ± 1 K) in the reaction system. The residual phenol concentration of water samples were measured by ultraviolet spectrophotometry to evaluate removal performance. Phenol removals were also described with pseudo zero-order, first order and second order kinetics, and the kinetic models were expressed as Eq. (1), Eq. (2) and Eq. (3).
Where K1, K2, K3 are the pseudo zero-order, first-order and second-order rate constant, respectively (min-1); t represents the reaction time; C0 and Ct stand for the phenol concentration at 0 min and t min, respectively.
3. Results and Discussions3.1. Catalytic Degradation of PhenolEffect of adsorption, ozonation and catalytic ozonation on phenol removal are investigated and compared in this part, which are shown in Fig. 1. It is clear to see that the phenol removal efficiencies are all less than 5% by adsorption (showed in red and black line in Fig. 3). So, the contribution of catalyst adsorption on phenol removal efficiency could be neglectable. The phenol removal efficiencies during 40 min are in order of Co-α-MnO2 (97.47%) > α-MnO2 (81.01%) > single ozonation (58.55%).
The parameters of kinetic fitting for the removal phenol are shown in Table S1. The removal efficiency of catalytic ozonation on phenol well accords with the first-order reaction kinetics, while that of single ozonation accords with the zero-order reaction kinetics. The first order reaction kinetics shows that the removal efficiency of phenol by catalytic ozone is proportional to the first order of phenol concentration. The zero-order reaction kinetics shows that the removal efficiency of phenol by single ozone is propotional to the zero power of the phenol concentration. Based on first-order kinetic fitting, Co-α-MnO2 achieves the highest reaction rate constant for phenol removal (0.092 min−1), which is 2.09 and 4 times higher than that in α-MnO2 (0.044 min−1) and O3 (0.023 min−1), respectively. It can be further verified that the doping of Co on α-MnO2 efficiently improved the catalytic activities. The results might be attributed to the changes of catalyst construction, elementary composition and surface properties, et al., which will be detailly discussed in the following section.
3.2. Specific Surface and Pore VolumeThe calculated results of specific surface area and pore volume of α-MnO2 and Co-α-MnO2 are showed in Table 1. It can be seen that Co-α-MnO2 catalyst has a bigger of specific surface area (79.496 m2/g) and pore volume (0.0396 cm3/g) than that of α-MnO2. The larger the specific surface area and pore volume, the better catalytic activity can be obtained. The reason is that the large specific surface area and pore volume can promote the mass transfer rate between solid-liquid-gas [30]. So, catalytic activity of Co-α-MnO2 is greater than that of α-MnO2. N2 adsorption-desorption isotherm showed that two kinds of catalysts belong to typically III type (as showed in Fig. S4) [31], indicating that the interaction between catalysts and adsorbate is weak. The surface of the catalysts generally adsorbs small molecules such as H2O molecules, which decomposed to generate hydroxyl radicals to promote the catalytic ozonation [32]. The absorbed H2O always dissociate into OH− and H+ forming the surface hydroxyl groups with surface cations and oxygen anions. In the system, surface hydroxyl groups can decompose ozone through a series of reactions and produce active free radicals to promote catalytic ozonation.
3.3. Crystal PhaseThe XRD results of α-MnO2 and Co-α-MnO2 catalysts are shown in Fig. 2. All patterns of the prepared α-MnO2 and Co-α-MnO2 catalyst samples can be indexed to body-centered tetragonal, which are same as that of the standard α-MnO2 (JCPDS NO.44-0141). An additional peak at 2θ of 65.8 in the Co-doped catalyst is presumed to be the formation of a similar CoMn2O4 structure [33]. In addition, there are no other additional characteristic peaks, indicating that Co mixed in the α-MnO2 mainly replaced Mn and entered the MnO2 skeleton structure or embedded in the pore structure of the catalyst tunnel, instead of existing on the surface of the catalyst in the form of cobalt oxide, and the replaced Mn may enter the hydrothermal synthesis solution in the process of reaction. The reason for the decrease of the grain size of Co-α-MnO2 from 320 Å to 287 Å may be that the doped metal ions enter the original phase, which limits the diffusion rate of Mn2+ and Mn7+ in the precursor prepared by the catalyst, and finally leads to the change of the lattice parameters and grain size of the catalyst. Also, the lattice parameters a, b, and c of Co-α-MnO2 become greater compared with that of α-MnO2 (as shown in Table 1), which show a distortion in the lattice cell. The increased relative intensity of α-MnO2 diffraction peak after Co doping also indicates the existence of lattice defects, which might promote the migration of adsorbed oxygen to decompose and transform organic matter and finally affected the catalytic performance of the catalyst [34, 35]. Therefore, Co-α-MnO2 has better catalytic ozonation of phenol than α-MnO2.
3.4. Surface Chemical CompositionsIn order to further investigate the elemental valence on the catalyst surface, Mn 2P, O1s and Co2p of both α-MnO2 and Co-α-MnO2 characterized by XPS were presented in Fig. 3(b), Fig. 3(c) and Fig. 3(d), respectively. Fig. 3(b) shows that the XPS spectrum of Mn 2p contains two main peaks at binding energies of 657.5 ± 7.5 and 642.5 ± 2.5eV, which could be attributed to Mn2P1/2 and Mn2p3/2. The binder energy of the peak at 641.6 eV, 642.5 eV and 643.4 eV are found in Mn2p3/2, which can be attributed to Mn3+ and Mn4+ [36, 37], respectively. As showed in Table S2, the relative content of Mn3+ of Co-α-MnO2 increases from 8.42% to 41.16% and the ratio of Mn3+/Mn4+ increases from 0.092 to 0.70 compared with α-MnO2. According to the results of the Table 2, the main valence state of Co in the catalyst is Co3+, which indicates that the doped Co2+ has a redox reaction in the catalyst preparation process and promotes the increase of the ratio of Mn3+/Mn4+ in Co-α-MnO2. The higher ratio of Mn3+/Mn4+ in Co-α-MnO2 is an important reason for the occurrence of more oxygen vacancy, which would promote the generation of reactive oxygen species and facilitate the migration and transformation of oxygen species [38, 39]. In addition, Mn3+ and other unsaturated metal ions behave as Lewis acid in the aqueous phase, which is easy to coordinate with water molecules and undergo chemical adsorption to form the surface hydroxyl group. Furthermore, the XPS spectrums of O1s for α-MnO2 and Co-α-MnO2 are showed in Fig. 3(c). The binding energy energies at the peak position 530.1 eV and 531.5 eV in the O1s spectrum, corresponding to the lattice oxygen and the adsorbed oxygen respectively [40]. Oxygen presenting form and relative content of oxygen calculated are showed in Table 2. Compared with α-MnO2, the ratio of adsorbed oxygen (Oads)/lattice oxygen (Olatt) of Co-α-MnO2 increases from 0.15 to 0.23, indicating that more Olatt would combine with H2O to produce surface hydroxyl, which is the active site for the catalyst to adsorb and decompose ozone to produce reactive oxygen, and then improve the efficiency of catalytic ozone oxidation. Surface hydroxyl group is well confirmed by the results of the following FT-IR analysis.
3.5. Surface Functional Groups PropertiesIn order to expose the influence of doping Co on surface functional groups of α-MnO2, the catalysts were characterized with FT-IR, and the results are present in Fig. 4. Although the position is slightly shifted, the intensities of stretching vibration of Mn-O and Mn-O-Mn bonds (467cm−1, 524 cm−1 and 719 cm−1) [41,42] are greatly improved, which is caused by the microstructure regulation due to the doping of Co on α-MnO2 and has been verified by the results of XRD. The characteristic peak of Mn-OH presents at the wavelength of 1042 cm−1, which is constructed by adsorbing water molecules on the catalyst surface and shedding hydrogen ions [43]. Compared with the α-MnO2, the peak intensity of Mn-OH in Co-α-MnO2 increases obviously, indicating that the adsorption capacity of α-MnO2 is improved by Co doping. The new peaks appeared at 879cm−1, 1387cm−1 corresponding to the stretching vibration of Co-O [44], further confirm the XRD results of Co replacing Mn into the structure of α-MnO2. The other three new peaks appeared at 1611cm−1, 2969cm−1and 3320cm−1 corresponded to the stretching vibration of the surface hydroxyl (O-H) [45], which are produced by flexural and telescopic vibration of adsorbed water on the catalyst surface. Therefore, in the Co-α-MnO2 catalytic ozonation system, more surface hydroxyl was generated, and they were showed as acidic or alkaline groups through proton exchange with aqueous solution, which promotes electron transfer and produces reactive oxygen species contributing to higher catalytic ozonation efficiency [46, 47].
3.6. Catalytic Stability and Reusability of CatalystUnder the same experimental conditions, five repeated experiments of catalytic ozone oxidation of phenol were carried out with the prepared Co-α-MnO2. The results of reusability of catalyst were shown in Fig. S5. With the increasing of reuse times loading from one to five, the phenol removal results after 40 min were 97.47 %, 93.66%, 91.83%, 86.72%, 83.80%, respectively. It can be found that the removal efficiency of phenol led to a gradually decrease with the increase of reuse times. The collision and friction between catalysts and the deposition of other substances on the surface or inside the pore of the catalyst lead to the disappearance of some surface-active sites, resulting in the loss of catalytic activity of some active sites of Co-α-MnO2. However, the catalytic activity of Co-α-MnO2 for ozonation of phenol after repeated use is still higher than that of α-MnO2. These results suggested that Co-α-MnO2 has excellent stability and reusability.
3.7. Mechanisms of Catalytic Ozonation3.7.1. Surface hydroxylation and ROSIn order to further recognize the role of surface hydroxyl of the catalysts, HPO42− was introduced and employed to occupy surface hydroxyl to deactivate the catalytic performance [48]. The results of masking experiments were showed in Fig. 5(a). In comparison to the experiments without masking agent, the phenol removal rates were much decreased because HPO42− was added to deactivate surface hydroxyl of catalyst. The removal rate of phenol obtained by single ozonation, O3/α-MnO2 and O3/Co-α-MnO2 were 52.74%, 57.62% and 64.58%, respectively, which are reduced by 5.81%, 23.39% and 32.89%, respectively. So, surface hydroxyl played a key role in these catalytic ozonation systems.
The surface hydroxyl group might be the main active cite for catalyst to absorb and further decomposed ozone to produce •OH. •OH and •O2− were active free radicals produced in the process of catalytic ozonation, which played an important role in the degradation of organic compounds. Tert-butanol and p-benzoquinone were introduced and employed to occupy •OH and •O2− in O3, O3/α-MnO2 and O3/Co-α-MnO2 systems, respectively. The results of masking experiments were showed in Fig. 5(b) and Fig. 5(c). After the addition of TBA or PBQ as masking agent, the removal rates of phenol decreased by 13.28%, 31.88%, 40.33% and 9.63%, 18.46% and 35.63%, respectively, which indicated that both •OH and •O2− were active oxygen influencing the degradation of phenol by catalytic ozonation. The addition of the catalyst promotes the decomposition of O3 to generate •OH and •O2−, while the doping of Co enhanced the catalytic activity of the catalyst.
3.7.2. Analysis of the catalytic mechanismThe masking experiment results demonstrated that the presence of surface hydroxyl group and reactive oxygen species promoted the degradation efficiency of phenol. Therefore, the mechanism of catalytic ozonation of phenol by α-MnO2 and Co-α-MnO2 was analyzed from the surface of polyphase catalysis (as showed in Fig. 6).
According to the adsorption-desorption isotherm of the catalyst, the interaction between the adsorption material and the adsorption gas is relatively weak, indicating that the interaction between the catalyst and the adsorbent is relatively weak (as showed in Fig. S4). The surface of the catalyst generally absorbs small molecules, so H2O molecules are preferentially adsorbed than O3 molecules. When introduced into an aqueous solution, H2O molecules will be strongly adsorbed on the surface of catalyst. The adsorbed H2O molecules will always dissociate into OH− and H+ [34] and form the surface hydroxyl group with the surface cation and oxygen anion respectively. The released HO3• will further decompose and release O2 and produce •OH. Then, the doping of Co could promote the generation of more surface hydroxyl groups, which promoted the catalytic ozonation process.
The surface hydroxyl group reacts with O3 to form a surface five-member ring, which will further decompose to release O2 and form surface HO2−. The HO2− formed will further react with O3 to form O3−• and HO2•, the generated HO2• will be converted to O2−• by releasing H+, O2−• will be oxidized by O3 to form O3−• and O2. The H+ generated during the above process and O3−• will combine with each other to form HO3• and further decompose to produce •OH and O2. In addition, O2−• in the presence of water molecules surface cations are formed, which in turn form surface hydrated cations (H2O+). With the release of •HO3, •OH and O2 are then produced, and H2O+ is converted to surface hydroxyl groups [49–51]. Therefore, the catalyst completed the surface hydroxyl regeneration and initiated the free radical chain reaction.
4. ConclusionPhenol removal was considerably enhanced to 97.47% by heterogeneous catalytic ozonation with Co-α-MnO2. Compared with α-MnO2, Co-doping produces Co-α-MnO2 with a larger specific surface area and pore volume, more crystal defects and oxygen vacancies, a higher relative content of Mn3+ and adsorbed oxygen (Oads), and more surface hydroxyl groups. The combined action of these factors finally improved the catalytic ozonation performance of Co-α-MnO2. Given that the better catalytic activity of Co-α-MnO2 catalyst could be obtained and then employed to improve the phenol removal performance. Masking experiments have shown that surface hydroxyl group and active free radicals (·OH and ·O2−) were involved in the catalytic ozonation of phenol. Furthermore, the primary reaction mechanism was proposed. The catalyst achieved the regeneration of surface hydroxyl group and the initiation of free radical chain reaction. The doping of Co could largely improve the density of surface hydroxyl groups and then facilitate the catalytic ozonation process.”
AcknowledgementsThanks for the Fundamental Research Funds for the Central Universities (2019XKQYMS78), the Open Sharing Fund for the Large-scale Instruments and Equipments of China University of Mining and Technology (CUMT).
NotesReferences1. Aziz KHM, Miessner H, Mueller S, et al. Comparative study on 2,4-dichlorophenoxyacetic acid and 2,4-dichlorophenol removal from aqueous solutions via ozonation, photocatalysis and non-thermal plasma using a planar falling film reactor. J. Hazard. 2018;343:107–115.
https://doi.org/10.1016/j.jhazmat.2017.09.025
2. Aziz KHM. Application of different advanced oxidation processes for the removal of chloroacetic acids using a planar falling film reactor. Chemosphere. 2019;228:377–383.
https://doi.org/10.1016/j.chemosphere.2019.04.160
3. Chen CM, Li Y, Ma WF, Guo SH, Wang QH, Li QX. Mn-Fe-Mg-Ce loaded Al2O3 catalyzed ozonation for mineralisation of refractory organic chemicals in petroleum refinery wastewater. Sep. Purif. Technol. 2017;183:1–10.
https://doi.org/10.1016/j.seppur.2017.03.054
4. Wang J, Chen H. Catalytic ozonation for water and wastewater treatment: recent advances and perspective. Sci. Total. Environ. 2020;704:135249.6
https://doi.org/10.1016/j.scitotenv.2019.135249
5. Hu L, Xia Z. Application of ozone micro-nano-bubbles to groundwater remediation. J. Hazard. Mater. 2018;342:446–453.
https://doi.org/10.1016/j.jhazmat.2017.08.030
6. Peng JL, Lai LD, Jiang X, Jiang WJ, Lai B. Catalytic ozonation of succinic acid in aqueous solution using the catalyst of Ni/Al2O3 prepared by electroless plating-calcination method. Sep. Purif. Technol. 2018;195:138–148.
https://doi.org/10.1016/j.seppur.2017.12.005
7. Zhang JL, Xiong ZK, Wei J. Catalytic ozonation of penicillin G using cerium-loaded natural zeolite (CZ): efficacy, mechanisms, pathways and toxicity assessment. Chem. Eng. J. 2020;383:123144.
https://doi.org/10.1016/j.cej.2019.123144
8. Zhang JM, Vasei M, Sang YH, Liu H, Claverie JP. TiO2@Carbon photocatalysts: the effect of carbon thickness on catalysis. Adv. Mater. Interfaces. 2016;8:1903–1912.
https://doi.org/10.1021/acsami.5b10025
9. Subramanian V, Wolf EE, Kamat PV. Catalysis with TiO2/gold nanocomposites. Effect of metal particle size on the Fermi level equilibration. J. Am. Chem. Soc. 2004;126( 15)4943–4950.
https://doi.org/10.1021/ja0315199
10. Emmanuel KTE, Ezugbe EO, Rathilal S, Asante-Sackey D. Removal of COD and SO4
2− from oil refinery wastewater using a photo-catalytic system-comparing TiO2 and zeolite efficiencies. Water. 2020;12:214.
https://doi.org/10.3390/w12010214
11. Yang Y, Cao H, Peng P, Bo H. Degradation and transformation of atrazine under catalyzed ozonation process with TiO2 as catalyst. J. Hazard. Mater. 2014;279:444–451.
https://doi.org/10.1016/j.jhazmat.2014.07.035
12. Du Jk, Xiao GF, Xi YX, et al. Periodate activation with manganese oxides for sulfanilamide degradation. Water. Res. 2020;169:115278.
https://doi.org/10.1016/j.watres.2019.115278
13. Liu D, Wang CR, Song YF, Wei YH, He L. Effective mineralization of quinoline and bio-treated coking wastewater by catalytic ozonation using CuFe2O4/Sepiolite catalyst: Efficiency and mechanism. Chem. 2019;227:647–656.
https://doi.org/10.1016/j.watres.2019.115278
14. Oliveira JSD, Salla JDS, Kuhn RC, Jahn SL, Foletto EL. Catalytic ozonation of melanoidin in aqueous solution over CoFe2O4 catalyst. Mater. Res. 2019;22:e20180405.
https://doi.org/10.1590/1980-5373-mr-2018-0405
15. Niu Z, Yue T, Hu WJH, Sun W, Hu YH, Xu ZH. Covalent bonding of MnO2 onto graphene aerogel forwards: Efficiently catalytic degradation of organic wastewater. Appl. Sur. Sci. 2019;496:143585.
https://doi.org/10.1016/j.apsusc.2019.143585
16. Wang L, He H, Zhang CB, Sun L, Liu SJ, Wang SX. Antimicrobial activity of silver loaded MnO2 nanomaterials with different crystal phases against escherichia coli. J. Environ. Sci. 2016;41(3)112–120.
https://doi.org/10.1016/j.jes.2015.04.026
17. Wang C, Ma JZ, Liu FD, He H, Zhang RD. The effects of Mn2+ precursors on the structure and ozone decomposition activity of cryptomelane-type manganese oxide (OMS-2) catalysts. J. Phys. Chem. C. 2015;119(40)23119–23126.
https://doi.org/10.1021/acs.jpcc.5b08095
18. Zhu GX, Zhu JG, Jiang WJ, Zhang ZJ, Wang J. Surface oxygen vacancy induced α-MnO2 nanofiber for highly efficient ozone elimination. Appl. Cataly. B. Environ. 2017;209:729–737.
https://doi.org/10.1016/j.apcatb.2017.02.068
19. Wang YC, Li YZ, Lu ZG, Wang W. Improvement of O2 adsorption for α-MnO2 as an oxygen reduction catalyst by Zr4+ doping. Rsc Advances. 2016;8(6)2963–2970.
https://doi.org/10.1039/c7ra10079e
20. Uematsu T, Miyamoto Y, Ogasawara Y, Suzuki K, Yamaguchi K, Mizuno N. Molybdenum-doped α-MnO2 as an efficient reusable heterogeneous catalyst for aerobic sulfide oxygenation. Catal. Sci. Technol. 2016;6:222–233.
https://doi.org/10.1039/c5cy01552a
21. Anfar Z, Fakir AAE, Zbair M. New functionalization approach synthesis of sulfur doped, Nitrogen doped and Co-doped porous carbon: superior metal-free carbocatalyst for the catalytic oxidation of aqueous organics pollutants. Chem. Eng. J. 2021;405:126660.
https://doi.org/10.1016/j.cej.2020.126660
22. Lv AH, Hu C, Nie YL, Qu JH. Catalytic ozonation of toxic pollutants over magnetic cobalt-doped Fe3O4 suspensions. Appl. Catal. B. Environ. 2012;117:246–252.
https://doi.org/10.1016/j.apcatb.2012.01.020
23. Li SY, Li XK, Wu HT, Sun XL, Gu FL. Mechanism of synergistic effect on electron transfer over Co–Ce/MCM-48 during ozonation of pharmaceuticals in water. Acs. Appl. Mater. Inter. 2019;11:23957–23971.
https://doi.org/10.1021/acsami.9b02143.s001
24. Faleh S, Guiza M, Quederni A. An optimization study of cobalt supported on activated carbon for the catalytic ozonation of oxalic acid: effect of operating parameters and synergetic combination. Ozone-Sci. Eng. 2019;41(3)274–285.
https://doi.org/10.1080/01919512.2018.1533447
25. Kang JL, Hirata A, Kang LJ, Zhang XM, Hou Y. Enhanced supercapacitor performance of MnO by atomic doping. Angew. Chem. Int. Edit. 2013;125(6)1708–1711.
https://doi.org/10.1002/ange.201208993
26. Hu XL, Chen JX, Li SY, Chen YX, Qu WY. The promotional effect of copper in catalytic oxidation by Cu-doped α-MnO2 nanorods. Phy. Chem. 2020;124:701–708.
https://doi.org/10.1021/acs.jpcc.9b09891
27. Saputra E, Duan XG, Pinem JA, Bahri S, Wang SB. Shape-controlled Co3O4 catalysts for advanced oxidation of phenolic contaminants in aqueous solutions. Sep Purif Technol. 2017;186:
https://doi.org/10.1016/j.seppur.2017.02.057
28. Bai Z, Yang Q, Wang J. Catalytic ozonation of sulfamethazine using Ce0.1Fe0.9OOH as catalyst: mineralization and catalytic mechanisms. Chem Eng J. 2016;300:169–176.
https://doi.org/10.1016/j.cej.2016.04.129
29. Ikhlaq A, Waheed S, Joya K S, et al. Catalytic ozonation of paracetamol on zeolite A: non-radical mechanism. Catal Commun. 2018;112:15–20.
https://doi.org/10.1016/j.catcom.2018.01.010
30. Ghuge SP, Saroha AK. Catalytic ozonation of dye industry effluent using mesoporous bimetallic Ru-Cu/SBA-15 catalyst. Process. Saf. Environ. 2018;118:125–132.
https://doi.org/10.1016/j.psep.2018.06.033
31. Zhai HY, Hao Z. Synthesis of Cr, Ag Co-doped ZnS nanomaterials and its adsorption capability for reactive dyes. Spectrosc Spect Anal. 2017;37(08)2638–2644.
https://doi.org/10.3964/j.issn.1000-0593(2017)08-2638-07
32. Zhao L, Ma J, Sun ZZ. Mechanism of heterogeneous catalytic ozonation of nitrobenzene in aqueous solution with modified ceramic honeycomb. Appl. Catal. B. 2009(a);89:326–334.
https://doi.org/10.1016/j.apcatb.2008.12.009
33. Ji L, Lin J, Zeng HC. Metal-supported interactions in Co/Al2O3 catalysts: A comparative study on reactivity of support. J. Phys. Chem. B. 2000;104:1783–1790.
https://doi.org/10.1021/jp993400l
34. Yu YL, Li CT, Du XY, Zhu YC, Zhang YD, Li SH. Catalytic oxidation of toluene over MnO2 catalysts with different Mn (II) precursors and the study of reaction pathway. Fuel. 2020;262:116610.
https://doi.org/10.1016/j.fuel.2019.116610
35. Peña DA, Uphade BS, Smirniotis PG. TiO2-supported metal oxide catalysts for low-temperature selective catalytic reduction of NO with NH3: I. evaluation and characterization of first row transition metals. J. Catal. 2004;221(2)421–431.
https://doi.org/10.1016/j.jcat.2003.09.003
36. Zhou CS, Wang J, Liu X, Chen FY, Di YY. Magnetic and thermodynamic properties of α, β, γ and δ-MnO2
. New J Chem. 42(10)8400–8407.
https://doi.org/10.1039/c8nj00896e
37. Li YF, Liu ZP. Active site revealed for water oxidation on electrochemically induced δ-MnO2: role of spinel-to-layer phase transition. J. Am. Chem. Soc. 2018;140(5)1783–1792.
https://doi.org/10.1021/jacs.7b11393
38. Zhang J, Dong B, Ding D, He SL, Ge SJ. Removal efficiency and mechanism of bio-treated coking wastewater by catalytic ozonation using MnO2 catalyst modified with anionic precursors. Environ. Eng. Res. 2021;26(5)200394.
https://doi.org/10.4491/eer.2020.394
39. Liu Y, Wang JL. Reduction of nitrate by zero valent iron (ZVI)-based materials: A review. Sci. Total. Environ. 2019;671:388–403.
https://doi.org/10.1016/j.scitotenv.2019.03.317
40. Gao G, Shi JW, Liu C, Chen G, Fan ZY, Niu CM. Mn/CeO2 catalysts for SCR of NOx with NH3: comparative study on the effect of supports on low-temperature catalytic activity. Appl. Surf. Sci. 2017;411:338–346.
https://doi.org/10.1016/j.apsusc.2017.03.164
41. Abdel-Latif IA, Ismail AA, Bouzid H, Al-Hajry A. Synthesis of novel perovskite crystal structure phase of strontium doped rare earth mangantites using solgel method. J. Magn Magn. Mater. 2015;393:233–238.
https://doi.org/10.1016/j.jmmm.2015.05.078
42. Mishrap J. Electrochemical depositon of MWCNT-MnO2/PPy nano-composite application for microbial fuel cells. Inte. J. Hydrogen. Ene. 2016;41:22394.
https://doi.org/10.1016/j.ijhydene.2016.09.020
43. Han N, Wu XF, Chai LY, Liu HD, Chen YF. Counterintuitive sensing mechanism of ZnO nanoparticle based gas sensors. Sensors. Actuat. B. Chem. 2010;150(1)230–238.
https://doi.org/10.1016/j.snb.2010.07.009
44. Fu Y, Yun D, Tang JJ, Zhou SH, Wang BB. Oriented surface decoration of (Co-Mn) bimetal oxides on nanospherical porous silica and synergetic effect in biomass-derived 5-hydroxymethyl furfural oxidation. Mol. Catal. 2017;435:144–155.
https://doi.org/10.1016/j.mcat.2017.03.034
45. Kim YJ, Taniguchi Y, Murase K, Taguchi Y, Sugimura H. Vacuum ultraviolet-induced surface modification of cyclo-olefin polymer substrates for photochemical activation bonding. Appl. Surf. Sci. 2009;255(6)3648–3654.
https://doi.org/10.1016/j.apsusc.2008.10.009
46. Maehida M, Uto M, Kurogi D, Kijima T. Mn-CeO2 binary oxides for catalytic NOx sorption at low temperature Sorptive removal of NOx
. Chem. Mater. 2000;12:3158–3164.
https://doi.org/10.1021/cm000207r
47. Lu J, Wei X, Chang Y, Tian SG, Xiong Y. Role of Mg in mesoporous MgFe2O4 for efficient catalytic ozonation of acid orange II. J. Chem. Technol. Biotechnol. 2016;91(4)985–993.
https://doi.org/10.1002/jctb.4667
48. Nawaz F, Xie YB, Cao HB. Catalytic ozonation of 4-nitrophenol over an mesoporous-MnO2 with resistance to leaching. Catal. Today. 2015;258(2)595–601.
https://doi.org/10.1016/j.cattod.2015.03.044
49. Zhao L, Sun ZZ, Ma J. Novel Relationship between hydroxyl radical initiation and surface group of ceramic honeycomb supported metals for the catalytic ozonation of nitrobenzene in aqueous solution. Environm. Sci. Technol. 2009(b);43(5)4157–4163.
https://doi.org/10.1021/es900084w
50. Wang Y, Yang WZ, Yin XS, Liu Y. The role of Mn-doping for catalytic ozonation of phenol using Mn/γ-Al2O3 nanocatalyst: Performance and mechanism. J. Environ. Chem. Eng. 2016;4:3415–3425.
https://doi.org/10.1016/j.jece.2016.07.016
51. Zhu GX, Zhu JG, Jiang WJ, et al. Surface oxygen vacancy induced α-MnO2 nanofiber for highly efficient ozone elimination. Appl. Cataly. B: Environ. 2017;209(3)729–737.
https://doi.org/10.1016/j.apcatb.2017.02.068
Table 1Specific Surface Area, Pore Volume, Lattice Parameters and Grain Diameters of α-MnO2 and Co-α-MnO2. |
|
||||||||||||||||||||||||||||||||||||||||