AbstractIn this study, the properties and adsorption behaviors of organoclays synthesized with two structurally different surfactants having the same carbon chain length (hexadecyltrimethylammonium bromide (HDTMAB) and benzyldimethylhexadecylammonium chloride (BDHAC)) were evaluated. The structural properties of the synthesized organoclays were examined using Fourier-transform infrared spectroscopy, zeta potential analysis, X-ray diffraction, and N2 adsorption-desorption test. The modified clays with increased surfactant loadings (from 0.5 CEC to 3 CEC) were prepared to find the optimal surfactant loading. As a result, 1.5 cation exchange capacity (CEC) was found to be the optimal surfactant loading. The organoclay prepared using HDTMAB (H-Bt) exhibited the maximum adsorption capacity of 114.3 mg/g for anionic Orange G dye, which was 1.74 times higher than that of the organoclay prepared using BDHAC (B-Bt). Furthermore, in the presence of a mixture of cationic and anionic dye molecules in water, H-Bt tended to remove more of the cationic target compound than B-Bt (i.e., methylene blue (MB)), suggesting that H-Bt can serve as a more efficient adsorbent for the uptake of environmental contaminants in practical scenarios. Moreover, this study examined the effects of surfactant molecules on the structural properties of organoclays and their adsorption behaviors in the uptake of dye molecules.
1. IntroductionDyes are widely used in the paper, textile, printing, dyeing, and paint industries [1]. Currently, due to a rapid industrial development worldwide, more than 700,000 tons of 100,000 types of commercial dyes are produced annually [2]. Such high levels of dye production can cause various problems, especially when dyes are discharged into lakes and rivers. Several dyes are resistant to decomposition because they are stable when exposed to light and heat [3]. This means that the discharged dyes remain in nature for extended periods and cause various problems owing to their poor biodegradability [4]. The dyes discharged into rivers block underwater light and affect surface water properties. In addition, several dyes are toxic, allergic, and contain carcinogens or heavy metals, which makes them ecologically hazardous [5]. Moreover, the presence of dyes in such water bodies is aesthetically unpleasant. For these reasons, it is essential to remove the dyes discharged into water bodies, and adsorption is one of the most effective remediation technologies due to its high removal efficiency, cost effectiveness, and ease of operation [6, 7].
Although various types of adsorbents, including commercial activated carbon, metal oxide-based, carbon-based, metal-organic framework (MOF), and polymer-based adsorbents, are available for dye removal [8], the use of naturally occurring materials has been widely investigated due to their abundance in nature, easy availability, and low cost. Especially, bentonite and montmorillonite have been treated as organoclays with various organic compounds in a significant amount of research [9]. Bentonite has several advantages for use as a support, including its lack of toxicity, chemical reactivity, and hydrophilicity allowing easy fixation of organic matter [10]. Bentonite is a silicate clay mineral, and montmorillonite is its main component. Because of its layered structure consisting of two tetrahedral silica sheets with a central Al octahedral sheet in parallel, bentonite expands easily when it absorbs moisture, and it has a high adsorption capacity. Furthermore, because of isomorphous cation substitution, bentonite has permanent negative charges that are naturally balanced by cations (Na+, Ca2+, etc.). These cations, which are present in the interlayer, can be easily replaced with organic cations (e.g., organic surfactants) through ion exchange mechanisms, and the modified clays are called organoclays. In this manner, bentonite can be converted into organoclays by intercalating a cationic surfactant and its improved adsorption capacity an anionic compound can be expected. The properties of the organoclays prepared in this manner can vary depending on the types of surfactants used during synthesis [11].
It has been demonstrated in many studies that organoclays exhibit high efficiency in the adsorption of anionic dyes [12, 13]. According to Juang et al. (2007) [14], organoclays synthesized with cationic surfactants, such as hexadecyltrimethylammonium bromide (HDTMAB) into bentonite, facilitate electrostatic adsorption with a positive surface charge in all pH ranges. This increases the adsorption capacity significantly compared to that of the corresponding natural material in anionic dye adsorption tests. In addition, it has been proven that the organoclays synthesized with HDTMAB have a great affinity for adsorbing various organic contaminants and heavy metals [11, 14–17]. Although many studies have reported the synthesis of organoclays intercalated with different cationic surfactants [18–21], investigations on the modification of clay minerals by using different types of surfactants with and without a benzyl group are limited. Therefore, the results of the present study will be helpful for designing and optimizing the synthesis processes of organoclays with suitable cationic surfactant molecules, which will be essential for improving the adsorption efficiency and elucidating the adsorption mechanism for the removal of environmental pollutants.
In this study, the anionic dye Orange G (OG) was chosen as the target contaminant for organoclays modified with two different surfactants. OG has been shown to be genotoxic to Swiss Albino rats [22] and might be dangerous for humans as well [23]. For this reason, OG has been used in various dye adsorption studies, and it is suitable for examining the electrostatic adsorption mechanism of cationic surfactant modified organoclays as a divalent anion. Bentonite was initially modified with two cationic surfactants, namely HDTMAB and benzyldimethylhexadecylammonium chloride (BDHAC). These two selected surfactants have the same carbon chain length, but BDHAC possesses a benzene ring at the end of the molecule, and HDTMAB does not. Before the adsorption study, the structural properties of bentonite, as well as those of the organoclays prepared with HDTMAB and BDHAC, were examined using several characterization methods. Eventually, the adsorption capacity of the characterized materials towards the model compound was evaluated in aqueous solutions.
2. Experiments2.1. MaterialsBentonite and the surfactants used to synthesize the organoclays, namely HDTMAB (C19H42BrN) and BDHAC (C25H46ClN), were purchased from Sigma-Aldrich Co. (USA) and used without further purification. The chemical structural formulas of the surfactants are shown in Fig. S1. The anionic dye Orange G (OG) was obtained from Thermo Fisher Scientific (USA), and the cationic dye Methylene Blue (MB) was obtained from Junsei Chemical Co. Ltd. (Japan). All reagents were used without further purification. Ultrapure water (deionized water; DI) was produced using a water purification system (Synergy®, Merck, USA).
2.2. Preparation of OrganoclayBentonite was converted into organoclay by following the simple exchange procedure reported in [24]. At first, bentonite (< 20 g/L) in deionized water (DIW) was stirred in a beaker for 10 min to disperse it. The surfactant was then mixed with bentonite in 0.5, 1, 1.5, 2 and 3 times the theoretical cation exchange capacity (CEC, 110 meq/100 g) of bentonite at 60 °C for 24 h. In this step, the beaker was wrapped with foil to prevent evaporation. The product was centrifuged at 4,000 rpm for 10 min, washed several times with DIW, and then dried at 60 °C for 24 h. The obtained mass of organoclay was powdered and sieved to obtain particle of sizes less than 500 μm. The prepared organoclays were named HDTMAB-modified bentonite (H-Bt) and BDHAC-modified bentonite (B-Bt), and they were well sealed prior to analysis.
2.3. CharacterizationTo characterize the prepared organoclays, different characterization methods, such as X-ray diffraction (XRD), surface area analysis (Brunauer, Emmett, and Teller (BET) method), zeta potential analysis, and Fourier transform infrared spectroscopy (FT-IR) were used. Powdered XRD patterns of the organoclays were collected using an X-ray diffractometer (XRD; Bruker DE/D8 Advance (Bruker AXS GmbH, Germany) equipped with a Cu Kα radiation source (λ = 1.54 Å) in the range of 5° to 80° (2θ) with a 5 mm air scattering slit and a 2.6 mm equatorial slit to measure the interlayer space of bentonite and the organoclays. Interlayer spacing (d001 spacing) was calculated using the Bragg equation (Eq. 1).
where d is the XRD (100) interlayer spacing (d001), θ is the X-ray incidence angle, λ is the Cu wavelength constant, 1.54 Å, and n is the reflection order, 1.
The Brunauer-Emmet-Teller (BET) surface areas were determined by conducting a N2 adsorption-desorption test by using a Belsorp-mini II (Bel Japan, Inc., Japan), and pore volumes and diameters were determined using the Barrett–Joyner–Halenda (BJH) method. The functional groups present in the organoclays were identified using Fourier transform infrared (FT-IR) spectroscopy (Spectrum Two, Perkin Elmer, UK) in the spectral range of 650–4000 cm−1 with a total 8 scans and a resolution of 4 cm−1. The surface charge of the clays was measured using a zeta potential analyzer (Zetasizer Lab, Malvern, UK), and all samples were finely pulverized in an agate mortar, after which they were pretreated with DIW to obtain a suspension with a concentration of 1 mg/mL.
2.4. Dye Adsorption ExperimentsBatch experiments were performed to optimize the CEC value for dye adsorption. First, 50 mL of 100 mg/L dye solution was mixed with 1 g/L of adsorbent in a 50 mL glass vial. Then, after stirring for 24 h, the mixed suspension was filtered through a 0.2 μm polyethersulfone (PES) microfilter, and its absorbance was analyzed with a UV-Visible spectrometer (Genesys 50, Thermo Scientific, UK) at the wavelengths of 478 nm for OG and 662 nm for MB. The dye removal efficiency was expressed as follows:
where is the removal efficiency (%), is the concentration before adsorption (mg/L) of the dye solution, and is the concentration after adsorption (mg/L).
Adsorption kinetic tests were performed to investigate and evaluate the effect of adsorbent contact time on the adsorption rate. Approximately 1 g/L of the two adsorbents was stirred with 250 mL of the OG solution with an initial concentration of 200 mg/L at 800 rpm for 6 h. Samples were collected at predetermined points in time (5, 15, 30, 45, 60, 90, 120, 180, 240, and 360 min). The adsorption results were fitted to pseudo first-order (Eq. 3) and pseudo second-order kinetic models (Eq. 4) [25–26].
where qt is the adsorbed amount at any time t (mg/g), qe is the equilibrium concentration (mg/g), k1 is the first-order rate constant (1/min), and k2 is the second-order rate constant (g/mg·min).
A batch test was conducted to determine the adsorption isotherm by using different OG initial concentrations (20–200 mg/L for H-Bt and 50–250 mg/L for B-Bt) with the same method and conditions. The adsorption isotherm results were analyzed using the Langmuir (Eq. 5) and Freundlich isotherm models (Eq. 6).
where qe is the quantity of adsorbate adsorbed per unit weight of solid adsorbent, qm is the maximum sorption capacity of the adsorbent (mg/g), Ce is the equilibrium concentration of the adsorbate in solution (mg/L), and KL is the Langmuir affinity constant. KF and 1/n are constants indicating the adsorption capacity and adsorption intensity, respectively.
In an evaluation of the ion selectivity of the adsorbents, the UV-Vis absorbance spectra were analyzed in the wavelength range of 400–800 nm to determine the changes in the absorbances of the dye solutions.
3. Results and Discussion3.1. Organoclay Properties Analysis3.1.1. Interlayer structure of the organoclaysThe XRD data of bentonite and organoclays prepared with different surfactant loadings were shown in Fig. 1. The patterns of the modified bentonites indicate the modification of clay by surfactants.
As shown in Fig. 1, d001 increased significantly after treatment with surfactants. Pristine bentonite had an interlayer space of 1.23 nm, which increased to 1.88 nm and 1.78 nm for H-Bt and B-Bt, respectively. As the surfactant loading increased, the basal spacing of the organoclays gradually increased, which can be attributed to changes in the structural configuration of the molecules within bentonite [27–28]. Based on the observed interlayer spacing of bentonite and organoclays, the basal spacing of 1.45 nm with a shoulder of 1.72 nm indicated the presence of more than one molecular arrangement (a monolayer and a lateral bilayer at the edge) in the bentonite interlayer at 0.5 CEC for H-Bt. The basal spacing of 1.84 nm at 1.0 CEC implied a lateral bilayer arrangement, and the orientation of the molecular layer remained the same when the surfactant loading was increased up to 1.5 CEC.
Compared to exchange of the HDTMAB cationic surfactant, the use of BDHAC cationic surfactant having a benzene ring resulted in a significantly increased basal spacing of 1.78 nm at 0.5 CEC. This represents the formation of a lateral bilayer in the interlayer of the clay. Furthermore, one more expansion was observed at 1.81 nm for 1.0 CEC-BDHAC, reflecting a lateral bilayer arrangement. However, no further expansion occurred, and the spacing decreased marginally to 1.78 nm at 1.5 CEC. This phenomenon can be explained based on the fact that at 1.5 CEC, organoclays with excess surfactant can be adsorbed on the surface of bentonite by the arrangement of surfactant, which was simulated using density functional theory (DFT) calculation in the previous study. [29, 30]. The molecular dynamic (MD) simulation demonstrated that monolayer surface arrangements were evidently observed at lower surfactant loadings and more complicated surface arrangements such as bilayer or paraffin-type initiated by increased surfactant molecules (> 100% of the CEC), affecting the thickness of the organic cation surface layers [30]. Thus, the clay interlayer was not significantly improved. According to this result, the structural properties of bentonite clay layers differ with different surfactant loadings (low surfactant loading at 0.5 CEC vs. high surfactant loading up to 1.5 CEC) and surfactant types (HDTMAB vs. BDHAC). In particular, organoclays prepared with higher surfactant loadings (i.e., 1.5 CEC) have a larger basal spacing, and they may have a greater number of sorption sites within the interlayer space, as well as on the clay surface; these aspects will be further examined by performing BET analysis.
3.1.2. Surface area and pore size analysisOther structural parameters such as specific surface area, pore volume, and average adsorption-desorption pore diameter values of the organoclays are summarized in Table 1.
As summarized in Table 1, the specific surface area, total pore volume, and average pore diameter of the organoclays are lower than those of bentonite. The surface areas of bentonite, H-Bt at 1.5 CEC, and B-Bt at 1.5 CEC were 42.96, 1.79, and 9.22 m2/g, respectively. The total pore volumes of bentonite, H-Bt, and B-Bt were 0.95, 0.004, and 0.015 cm3/g, respectively, and the average pore diameters were 8.84, 8.06, and 6.59 nm, respectively. Bentonite has the capability to expand the interlamellar space when ion exchange occurs with surfactant molecules, and it is widely known that the larger organic cations in the surfactants increase the spacing between the tetrahedral sheets [31], as confirmed by the XRD result obtained herein. Based on the increased d001 spacing of the modified clays with two cationic surfactants, the relatively large surfactant cations of HDTMAB and BDHAC were intercalated into the aluminosilicate sheets, resulting in expansion of the interlamellar space of bentonite [14]. This behavior led to increase the packing density of organoclays by increasing the surfactant loading in the clays and altering the arrangement of surfactant molecules from parallel to surfactant chains oriented at certain angles to the silicate surface [30]. This reduced the BET surface area and pore volume of the organoclays. For instance, the relatively large HDTMAB and BDHAC cations may have been compactly loaded and packed in the interlamellar spaces in the clay, and severe pore blocking may have occurred, which may have led to a decrease in the pore volume, average pore diameter, and BET surface area.
Interestingly, the pore packing of the organoclays prepared with HDTMAB was larger than those prepared with BDHAC, possibly because of a difference in the hydrophilicity levels of the two surfactants. The hydrophilic bentonite and the hydrophobic functional groups of BDHAC possessing a benzene ring caused the two surfactants to repel each other. This repulsion may have prevented deep insertion of BDHAC into the bentonite pores during synthesis. Particularly, the presence of the larger benzene ring (8.29Å) on BDHAC compared to the alkyl chain length (3.65 Å) led to the loading of a more significant amount of surfactant on the clay surface than in the interlayer spacing of the clay. For this reason, the average pore diameter of B-Bt decreased more significantly than that of H-Bt. In other words, a greater amount of HDTMAB can be loaded in the interlayer space than BDHAC, even at the same CEC level, and the pore volumes of the organoclays prepared with HDTMAB were smaller, although the pore diameter did not decrease significantly. This trend of average pore size is consistent with the tendency observed in the XRD patterns. Additionally, an increase in the cation coverage on the clay surface tended to decrease the hydrophobicity of organoclays [30], which may alter the rheological and swelling properties of the modified clays, particularly for those with the higher organic cation loading (> 1.0 CEC). Eventually, it leads to the weaker sorption capacity of organoclays to uptake organic pollutants. It appears that the loaded surfactant and the type of surfactant molecule influence the structure distribution of organoclays [29], as will be further verified with FT-IR.
3.1.3. Confirmation of intercalated surfactant molecules within organoclaysFT-IR can be used to determine the molecular environment of intercalated surfactant molecules within organoclays [11, 19, 32] and several distinguishing regions: (i) OH stretching region (3700–3000 cm−1), (ii) CH stretching region (2900–2800 cm−1), (iii) HCH bending vibration (1520–1400 cm−1), and (iv) Si-O region [5].
Here, the functional groups of the adsorbents prepared using HDTMAB and BDHAC were examined with FT-IR, as presented in Fig. 2. A sharp, intense peak at 3627 cm−1 for bentonite was assigned to OH stretching vibrations of the structural hydroxyl group, and the hydroxyl band of the organoclays was relatively steady. The CH stretching region of the organoclays (i.e., H-Bt and B-Bt) prepared with 1.5 CEC loading is shown in Fig. 2. Compared to bentonite, the bands at 2917, 2919, and 2850 cm−1 were attributed to the methylene group in the surfactant molecules, and the organoclays modified with HDTMAB and BDHAC had antisymmetric Vas(CH2) and symmetric Vs(CH2) stretching modes [33]. The bands observed at 1468 and 1471 cm−1 for the organoclays were ascribed to the scissoring modes of the methylene group (−CH2) and HCH deformation of the surfactant molecules intercalated into bentonite, respectively [19]. Broad bands at 1113 and 998 cm−1 were distinctively observed for bentonite owing to the Si-O groups in the tetrahedral sheets, and the peak at 916 cm−1 represented the Al-Al-OH groups in bentonite [32]. These peaks were observed in cases of the organoclays as well, which confirmed the presence of bentonite clay even after loading the surfactant molecules. The observed FT-IR results clearly indicated the presence of surfactant molecules loaded on the bentonite, and the surfactant modification of bentonite was successfully achieved.
3.1.4. Surface charge analysis of adsorbentsThe zeta potential was measured for surface charge analysis of the clays before and after modification. As shown in Fig. 3, the measured zeta potential of unmodified bentonite was −32.7 mV due to its negatively charged nature [34]. By contrast, the measured zeta potentials of the organoclays modified with cationic surfactants, namely H-Bt and B-Bt, were +47.8 mV and +54.2 mV, respectively. This shift in surface charge indicates not only the presence of intercalated surfactant molecules within the interlayer spacing of bentonite but also the great potential of the electrostatic adsorption mechanism for anionic materials.
3.2. Dye Adsorption by Organoclays3.2.1. CEC ratio optimization for adsorption of anionic Orange G (OG) dyeBased on the characteristics of bentonite and the organoclays, we conducted adsorption experiments using the organoclays to remove anionic OG dye from aqueous solutions. The adsorption capacities of the organoclays prepared with different surfactant loadings of 0.5–2.0 CEC were tested for the removal of OG after 24 h, and the maximum percentages of OG adsorbed by the organoclays are presented in Fig. 4.
Fig. 4 shows the maximum OG removals achieved using H-Bt and B-Bt at 1.5 times the theoretical CEC (110 meq/100 g). Because similar or lower OG removal rates were achieved with ratios higher than 1.5, the optimal removal efficiency of OG was considered to be achieved at 1.5 CEC. At 1.5 CEC, H-BT removed 99% of OG while approximately 67% OG removal was achieved by B-Bt and this level remained unchanged, even when the surfactant loading was increased. These phenomena agree well with the XRD data presented in Fig. 2. Although the d-spacing of H-Bt increased up to 1.5 CEC, B-Bt exhibited the maximum value at 1.0 CEC. This can be ascribed to the hydrophilic affinity resulting from the structural differences between the two surfactants, as discussed earlier in the BET analysis section. Thus, HDTMAB can be loaded continuously in the interlayer space, whereas BDHAC, which has less affinity to hydrophilic bentonite, is partially modified between the interlayer as well as on the surface of the clay, which was not further improved by our current synthesis method. However, because both organoclays yielded the maximum removal rate at 1.5 CEC surfactant loading, 1.5 CEC can be considered the optimal surfactant loading ratio for the organoclays. Therefore, the organoclays prepared using both surfactants at 1.5 CEC were used for all subsequent adsorption experiments to test the uptake of OG dye from aqueous solutions.
3.2.2. OG adsorption isothermsThe adsorption isotherms of OG for the two organoclays were obtained in batch experiments conducted using different initial concentration ranges, and they are shown in Fig. 5. To determine the adsorption capacities and adsorption surface properties of the adsorbents for OG, the equilibrium experimental data were fitted to the Langmuir (Eq. 5) and Freundlich (Eq. 6) isotherm equations. The Langmuir equation applies to a homogeneous adsorption system, and it refers to a chemical monolayer adsorption site, while the Freundlich equation is suitable for a heterogeneous system with physical multilayer adsorption [35–36]. It is also well known that the Langmuir equation is suitable for electrostatic adsorption [37]. The values of KL, qm, KF, n, and the correlation coefficient (R2) for the Langmuir and Freundlich isotherms are given in Table S1.
As listed in Table S1, both H-Bt and B-Bt showed higher R2 values in the Langmuir model (0.976 and 0.972, respectively) than the Freundlich model (0.896 and 0.900). Hence, OG adsorption by H-Bt and B-Bt fitted better into the Langmuir model than the Freundlich model, indicating that the adsorbents are suitable for chemical monolayer adsorption. In relation to the positive surface charge of the organoclays examined in section 3.1.2, the primary mechanism of removing OG can be associated with electrostatic and chemical sorption. The maximum sorption capacity (qm) of H-Bt (114.3 mg/g) was higher than that of B-Bt (65.56 mg/g), which is consistent with the result presented in 3.2.1 (Fig. 4). Moreover, it provides substantial information on the mechanism that a greater amount of surfactants exists between the H-Bt interlayers, which is in good agreement with the results of XRD and BET analyses. Consequently, the qm of H-Bt was higher than that of B-Bt due to the larger amount of modified surfactant, and for this reason, it yielded a higher OG removal rate. According to Seniha Elmen et al. (2012) [38], the qm of HDTMA-Bentonite for OG adsorption was 104.48 mg/g, and the qm used in this study yielded similar results.
3.2.3. OG adsorption kineticsAdsorption kinetics were determined to compare the adsorption rates of H-Bt and B-Bt. Kinetics studies are performed to obtain important indicators for understanding the adsorption behavior of OG. Both pseudo first-order and pseudo second-order kinetic models were used to fit the experimental data by using an initial concentration of 200 mg/L. The results are shown in Fig. 6 and summarized in Table S2. According to the results obtained using the two different kinetic models, both organoclays followed a pseudo second-order model with high correlation coefficient (R2) values of 0.993 for H-Bt and 0.982 for B-Bt, whereas the pseudo first-order model yielded R2 values of 0.956 and 0.938, respectively. The high correlation coefficients obtained for the pseudo-second order model suggest that the adsorption of anionic OG dye onto H-Bt and B-Bt followed the pseudo second-order model more closely than the pseudo first-order model in this study. It is known that physical adsorption fits the pseudo first-order kinetic model, and chemical adsorption fits the pseudo second-order kinetic model [39, 40]. These kinetic fitting results indicated that OG adsorption on organoclays was mediated by chemical interaction, which correlates to the isotherm results.
The kinetic constants, as obtained using the pseudo second-order model, were 0.002509 g/mg·min and 0.00083 g/mg·min for H-Bt and B-Bt, respectively. The kinetic constant of H-Bt was approximately three times higher than that of B-Bt, indicating that H-Bt tended to adsorb more anionic dye than B-Bt.
3.2.4. Evaluation of dye ion-selective adsorptionAn adsorption test was conducted using a mixed sample of cationic and anionic dyes to investigate the ion selectivity of the adsorbents (Fig. 7). Methylene blue (MB), a cationic dye with a strong blue color, has a strong absorbance band at a wavelength of 662 nm, and the relatively pale orange anionic dye Orange G (OG) has a maximum absorbance peak at 478 nm. A comparison of the absorbance of OG and MB dyes before and after the adsorption test was conducted using the prepared organoclays, and changes in the absorbance of OG and MB were observed at 478 and 662 nm, respectively.
Both H-Bt and B-Bt organoclays removed considerable amounts of anionic dye molecules at 478 nm, suggesting that the organoclays prepared using two different types of surfactants (HDTMAB and BDHAC) can adsorb the anionic OG dye effectively (almost up to 100%). However, in terms of the uptake of cationic MB dye molecules, the removal efficiency of B-Bt was not significant, while H-Bt was able to adsorb more than 50% of the MB molecules in water. Based on these results, it can be concluded that the adsorption capacities of the organoclays intercalated with HDTMAB and BDHAC are close to 100% for anionic OG, and both organoclays are more efficient at adsorbing anionic contaminants from aqueous solutions than cationic pollutants. In other words, both H-Bt and B-Bt removed anionic pollutants more selectively than cations due to the high surface charges of the adsorbents (+47.8 mV for H-Bt, +54.21 mV for B-Bt), which have a tendency to adsorb anions electrostatically. Apparently, the pHs of dyes prepared separately and in the mixed form were measured at pH 6.7(OG)/7.2 (MB) and 7.0, respectively, and the effect of pH on the adsorption of cationic and anionic dye molecules by the pristine bentonite was expected to be minimal in the range of pH 3 – 12, in agreement with previous observations on other clays [41, 42].
Although both adsorbents commonly adsorbed almost all of the OG, as shown in Fig. 7, the adsorption behaviors of the two organoclays were different in the removal of cationic MB dye molecules. Additionally, the optimized organoclays can be an effective sorbent for the removal of anionic heavy metals (e.g., Cr(VI), Mo(VI), As(III), As(V)) due to the presence of positively charged exchange sites [43–45] via ion exchange, electrostatic interaction, and surface complexation. To a certain extent, the enhanced adsorption of Cu(II) and Zn(II) was reported as well as co-adsorption of organic pollutants and heavy metal ions were achieved by surfactant-modified clays [46, 47]. Therefore, from a practical perspective, it can be suggested that the organoclays synthesized with HDTMAB provide a higher potential for the uptake of a greater amount of environmental pollutants from real wastewater due to the obtained structural properties of the H-Bt organoclays, particularly the larger basal spacing of which increased during the synthesis. In particular, the utilization of modified clays can be appropriately considered for the adsorption of textile effluents, including dyes and heavy metals (e.g., Cu, Cd, Zn, Pb, and Ni originating mainly from color-pigments) during the fabric manufacturing processes [31, 48, 49].
4. ConclusionThis study comparatively evaluated the adsorption of anionic dye orange G (OG) from water using two organoclays. The organoclays were prepared by modifying bentonite with two surfactants (HDTMAB, BDHAC) having the same carbon chain length. As illustrated, the two surfactants have relatively similar structures, except that BDHAC possesses a benzyl group, which affects the resulting organoclays’ adsorption behaviors during OG removal.
Bentonite was converted into organoclays through intercalation with cationic surfactants, as confirmed by XRD, zeta potential, and FT-IR spectral analysis. The specific surface area, total pore volume, and average pore diameter of H-Bt decreased more significantly than those of B-Bt owing to differences in surfactant properties, which led to higher loading of HDTMAB molecules. The characterized organoclays were further tested for their adsorption capacity in removing anionic dye. The Langmuir isotherm model and the pseudo second-order kinetic model yielded the best fits, indicating that the adsorption behavior was chemical monolayer adsorption in the case of homogeneous systems; the electrostatic adsorption mechanism was demonstrated as well. In the mixed dye system, when a mixture of cationic and anionic dye molecules was present in water, H-Bt tended to remove more of the cationic target compound than B-Bt (i.e., methylene blue (MB)), while both organoclays removed similar amounts of OG. Finally, the results of this study emphasized the effects of surfactant molecules on the structural properties of modified clays and their adsorption behaviors in terms of the uptake of dye molecules from aqueous solutions.
AcknowledgementThis study was financially supported by Seoul National University of Science and Technology.
NotesAuthor Contributions N.C. (MSc student) conducted all the experiments and wrote the original draft. Y.S. (MSc student) assisted in some of the experiments. T-H.K. (Research Professor) conducted supplementary experiments and reviewed the data. Y.P. (Research Professor) conducted experimental supervision, manuscript review. Y.H. (Associate Professor) conducted conceptualization, manuscript review and financial support. References1. Nigam P, Mc Mullan G, Banat IM, Marchant R. Decolourisation of effluent from the textile industry by a microbial consortium. Biotechnol Letters. 1996;18(1)117–120. 10.1007/BF00137823
2. Jebapriya GR, Gnanadoss JJ. Bioremediation of textile dye using white rot fungi: a review. Int J Curr Res Rev. 2013;5(3)1.
3. Wang Y, Mu Y, Zhao Q-B, Yu H-Q. Isotherms, kinetics and thermodynamics of dye biosorption by anaerobic sludge. Sep Purif Technol. 2006;50(1)1–7. 10.1016/j.seppur.2005.10.012
4. Gita S, Hussan A, Choudhury T. Impact of textile dyes waste on aquatic environments and its treatment. Environ Ecol. 2017;35(3C)2349–2353.
5. Aljerf L. High-efficiency extraction of bromocresol purple dye and heavy metals as chromium from industrial effluent by adsorption onto a modified surface of zeolite: kinetics and equilibrium study. J Environ Manage. 2018;225:120–132. 10.1016/j.jenvman.2018.07.048
6. Ho Y, Chiang C. Sorption studies of acid dye by mixed sorbents. Adsorption. 2001;7(2)139–147. 10.1023/A:1011652224816
7. Pollard S, Fowler G, Sollars C, Perry R. Low-cost adsorbents for waste and wastewater treatment: a review. Sci Environ. 1992;116(1–2)31–52. 10.1016/0048-9697(92)90363-W
8. Dutta S, Gupta B, Srivastava SK, Gupta AK. Recent advances on the removal of dyes from wastewater using various adsorbents: A critical review. Mater Adv. 2021;10.1039/d1ma00354b
9. De Paiva LB, Morales AR, Díaz FRV. Organoclays: properties, preparation and applications. Appl Clay Sci. 2008;42(1–2)8–24. 10.1016/j.clay.2008.02.006
10. Öztürk N, Tabak A, Akgöl S, Denizli A. Newly synthesized bentonite–histidine (Bent–His) micro-composite affinity sorbents for IgG adsorption. Colloid Surf A: Physicochemical and Engineering Aspects. 2007;301(1–3)490–497. 10.1016/j.colsurfa.2007.01.026
11. Shah KJ, Pan S-Y, Shukla AD, Shah DO, Chiang P-C. Mechanism of organic pollutants sorption from aqueous solution by cationic tunable organoclays. J Colloid Interface Sci. 2018;529:90–99. 10.1016/j.jcis.2018.05.094
12. Huang Z, Li Y, Chen W, et al. Modified bentonite adsorption of organic pollutants of dye wastewater. Mater Chem Phys. 2017;202:266–276. 10.1016/j.matchemphys.2017.09.028
13. Bhatt AS, Sakaria PL, Vasudevan M, Pawar RR, Sudheesh N, Bajaj HC, Mody HM. Adsorption of an anionic dye from aqueous medium by organoclays: equilibrium modeling, kinetic and thermodynamic exploration. RSC Adv. 2012;2(23)8663–8671. 10.1039/C2RA20347B
14. Juang LC, Wang CC, Lee CK, Hsu TC. Dyes adsorption onto organoclay and MCM-41. J Environ Eng Manage. 2007;17(1)28–38.
15. Ceyhan Ö, BAYBAŞ D. Adsorption of some textile dyes by hexadecyltrimethylammonium bentonite. Turkish J Chem. 2001;25(2)193–200.
16. Richards S, Bouazza A. Phenol adsorption in organo-modified basaltic clay and bentonite. Appl Clay Sci. 2007;37(1–2)133–142. 10.1016/j.clay.2006.11.006
17. Oyanedel-Craver VA, Smith JA. Effect of quaternary ammonium cation loading and pH on heavy metal sorption to Ca bentonite and two organobentonites. J Hazard Mater. 2006;137(2)1102–1114. 10.1016/j.jhazmat.2006.03.051
18. He H, Ma Y, Zhu J, Yuan P, Qing Y. Organoclays prepared from montmorillonites with different cation exchange capacity and surfactant configuration. Appl Clay Sci. 2010;48(1–2)67–72. 10.1016/j.clay.2009.11.024
19. Park Y, Ayoko GA, Horváth E, Kurdi R, Kristof J, Frost RL. Structural characterisation and environmental application of organoclays for the removal of phenolic compounds. J Colloid Interface Sci. 2013;393:319–334. 10.1016/j.jcis.2012.10.067
20. Zhou Q, He H, Frost RL, Xi Y. Adsorption of p-nitrophenol on mono-, di-, and trialkyl surfactant-intercalated organoclays: a comparative study. J Phys Chem C. 2007;111(20)7487–7493. 10.1021/jp0683364
21. Bujdáková H, Bujdáková V, Májeková-Koščová H, Gaálová B, Bizovská V, Boháč P, Bujdák J. Antimicrobial activity of organoclays based on quaternary alkylammonium and alkylphosphonium surfactants and montmorillonite. Appl Clay Sci. 2018;158:21–28.
https://doi.org/10.1016/j.clay.2018.03.010
22. Giri AK, Mukherjee A, Talukder G, Sharma A. In vivo cytogenetic studies on mice exposed to Orange G, a food colourant. Toxicology Letters. 1988;44(3)253–261. 10.1016/0378-4274(88)90164-6
23. Jović-Jovičić N, Milutinović-Nikolić A, Banković P, Mojović Z, Žunić M, Gržetić I, Jovanović D. Organo-inorganic bentonite for simultaneous adsorption of Acid Orange 10 and lead ions. Appl Clay Sci. 2010;47(3–4)452–456. 10.1016/j.clay.2009.11.005
24. Yang PJ. Development of Clay minerals based Adsorbent for Removal Volatile Organic Compounds thdissertation. Seoul: Seoul National Univ. of Sci. Technol; 2020.
25. Ho Y-S, McKay G. Sorption of dye from aqueous solution by peat. Chem Eng J. 1998;70(2)115–124. 10.1016/s0923-0467(98)00076-1
26. Lagergren S. Zur theorie der sogenannten adsorption geloster stoffe. Kungliga svenska vetenskapsakademiens Handlingar. 1898;24:1–39.
27. Yilmaz N, Yapar S. Adsorption properties of tetradecyl-and hexadecyl trimethylammonium bentonites. Appl Clay Sci. 2004;27(3–4)223–228. 10.1016/j.clay.2004.08.001
28. Lee SY, Cho WJ, Hahn PS, Lee M, Lee YB, Kim KJ. Microstructural changes of reference montmorillonites by cationic surfactants. Appl Clay Sci. 2005;30(3–4)174–180. 10.1016/j.clay.2005.03.009
29. Zhou Q, Zhu R, Parker SC, Zhu J, He H, Molinari M. Modelling the effects of surfactant loading level on the sorption of organic contaminants on organoclays. RSC Adv. 2015;5(58)47022–47030. 10.1039/C5RA05998D
30. Schampera B, Tunega D, Šolc R, Woche S, Mikutta R, Wirth R, Dultz S, Guggenberger G. External surface structure of organoclays analyzed by transmission electron microscopy and X-ray photoelectron spectroscopy in combination with molecular dynamics simulations. J Colloid Interface Sci. 2016;478:188–200. 10.1016/j.jcis.2016.06.008
31. Bouberka Z, Khenifi A, Mahamed HA, Haddou B, Belkaid N, Bettahar N. Adsorption of Supranol Yellow 4 GL from aqueous solution by surfactant-treated aluminum/chromium-intercalated bentonite. J Hazard Mater. 2009;162(1)378–385. 10.1016/j.jhazmat.2008.05.079
32. Alabarse FG, Conceição RV, Balzaretti NM, Schenato F, Xavier AM. In-situ FTIR analyses of bentonite under high-pressure. Appl Clay Sci. 2011;51(1–2)202–208. 10.1016/j.clay.2010.11.017
33. Park Y, Ayoko GA, Frost RL. Characterization of organoclays and adsorption of p-nitrophenol: Enviroinmetal application. J Colloid Interface Sci. 2011;360(2)440–456. 10.1016/j.jcis.2011.04.085
34. Mockovčiaková A, Orolínová Z, Škvarla J. Enhancement of the bentonite sorption properties. J Hazard Mater. 2010;180(1–3)274–281. 10.1016/j.jhazmat.2010.04.027
35. Kumar KV, Valenzuela-Calahorro C, Juarez J, Molina-Sabio M, Silvestre-Albero J, Rodriguez-Reinoso F. Hybrid isotherms for adsorption and capillary condensation of N2 at 77 K on porous and non-porous materials. Chem Eng J. 2010;162(1)424–429. 10.1016/j.cej.2010.04.058
36. Xin X, Si W, Yao Z, Feng R, Du B, Yan L, Wei Q. Adsorption of benzoic acid from aqueous solution by three kinds of modified bentonites. J Colloid Interface Sci. 2011;359(2)499–504. 10.1016/j.jcis.2011.04.044
37. Trogus F, Sophany T, Schechter R, Wade W. Static and dynamic adsorption of anionic and nonionic surfactants. Soc Petroleum Eng J. 1977;17(05)337–344. 10.2118/6004-PA
38. Elemen S, Kumbasar EPA, Yapar S. Modeling the adsorption of textile dye on organoclay using an artificial neural network. Dyes and Pigments. 2012;95(1)102–111. 10.1016/j.dyepig.2012.03.001
39. Morsi RE, Elsabee M. Polyaniline nanotubes: mercury and competative heavy metals uptake. Am J Polym Sci. 2015;5(5923.10)5923.
40. Ho Y-S. Review of second-order models for adsorption systems. J Hazard Mater. 2006;136(3)681–689. 10.1016/j.jhazmat.2005.12.043
41. Tonlé IK, Ngameni E, Tcheumi HL, Tchiéda V, Carteret C, Walcarius A. Sorption of methylene blue on an organoclay bearing thiol groups and application to electrochemical sensing of the dye. Talanta. 2008;74(4)489–497. 10.1016/j.talanta.2007.06.006
42. Gürses A, Doğar Ç, Yalçın M, Açıkyıldız M, Bayrak R, Karaca S. The adsorption kinetics of the cationic dye, methylene blue, onto clay. J Hazard Mater. 2006;131(1–3)217–228. 10.1016/j.jhazmat.2005.09.036
43. Rathnayake SI, Martens WN, Xi Y, Frost RL, Ayoko GA. Remediation of Cr (VI) by inorganic-organic clay. J Colloid Interface Sci. 2017;490:163–173. 10.1016/j.jcis.2016.11.070
44. Atia AA. Adsorption of chromate and molybdate by cetylpyridinium bentonite. Appl Clay Sci. 2008;41(1–2)73–84. 10.1016/j.clay.2007.09.011
45. Su J, Huang H-G, Jin X-Y, Lu X-Q, Chen Z-L. Synthesis, characterization and kinetic of a surfactant-modified bentonite used to remove As (III) and As (V) from aqueous solution. J Hazard Mater. 2011;185(1)63–70. 10.1016/j.jhazmat.2010.08.122
46. Tohdee K, Kaewsichan L. Enhancement of adsorption efficiency of heavy metal Cu (II) and Zn (II) onto cationic surfactant modified bentonite. J Environ Chem Eng. 2018;6(2)2821–2828. 10.1016/j.jece.2018.04.030
47. Jović-Jovičić N, Milutinović-Nikolić AD, Žunić M, Mojović Z, Banković P, Gržetić I, Jovanović DM. Synergic adsorption of Pb2+ and reactive dye—RB5 on two series of organomodified bentonites. J Contaminant Hyd. 2013;150:1–11. 10.1016/j.jconhyd.2013.03.004
Fig. 1XRD patterns of bentonite and organoclays ((a) H-Bt and (b) B-Bt) prepared using two different types of surfactants with different surfactant loadings. 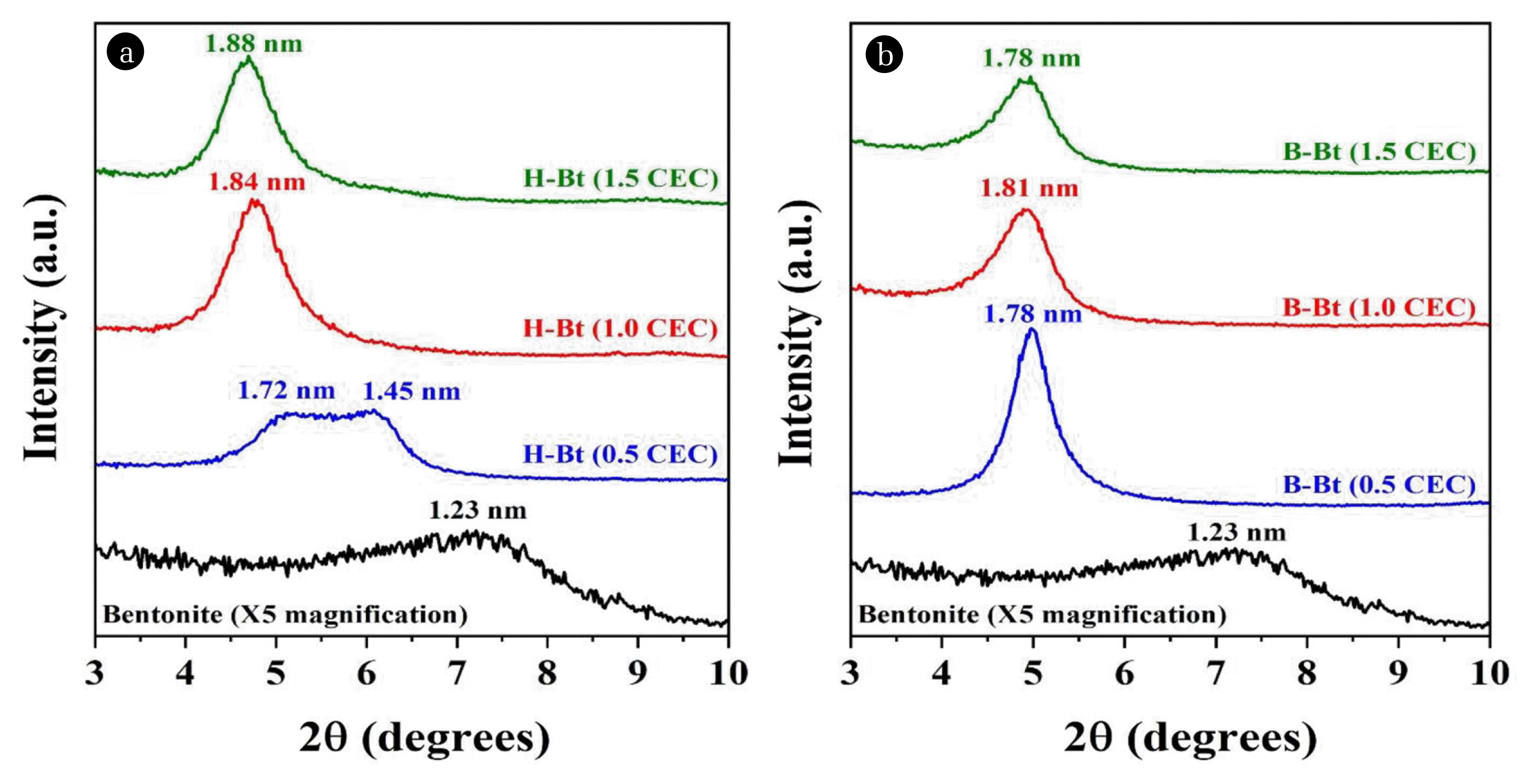
Fig. 2FT-IR spectra of bentonite and clays modified with HDTMAB (H-Bt) and BDHAC (B-Bt) at 1.5 CEC level. 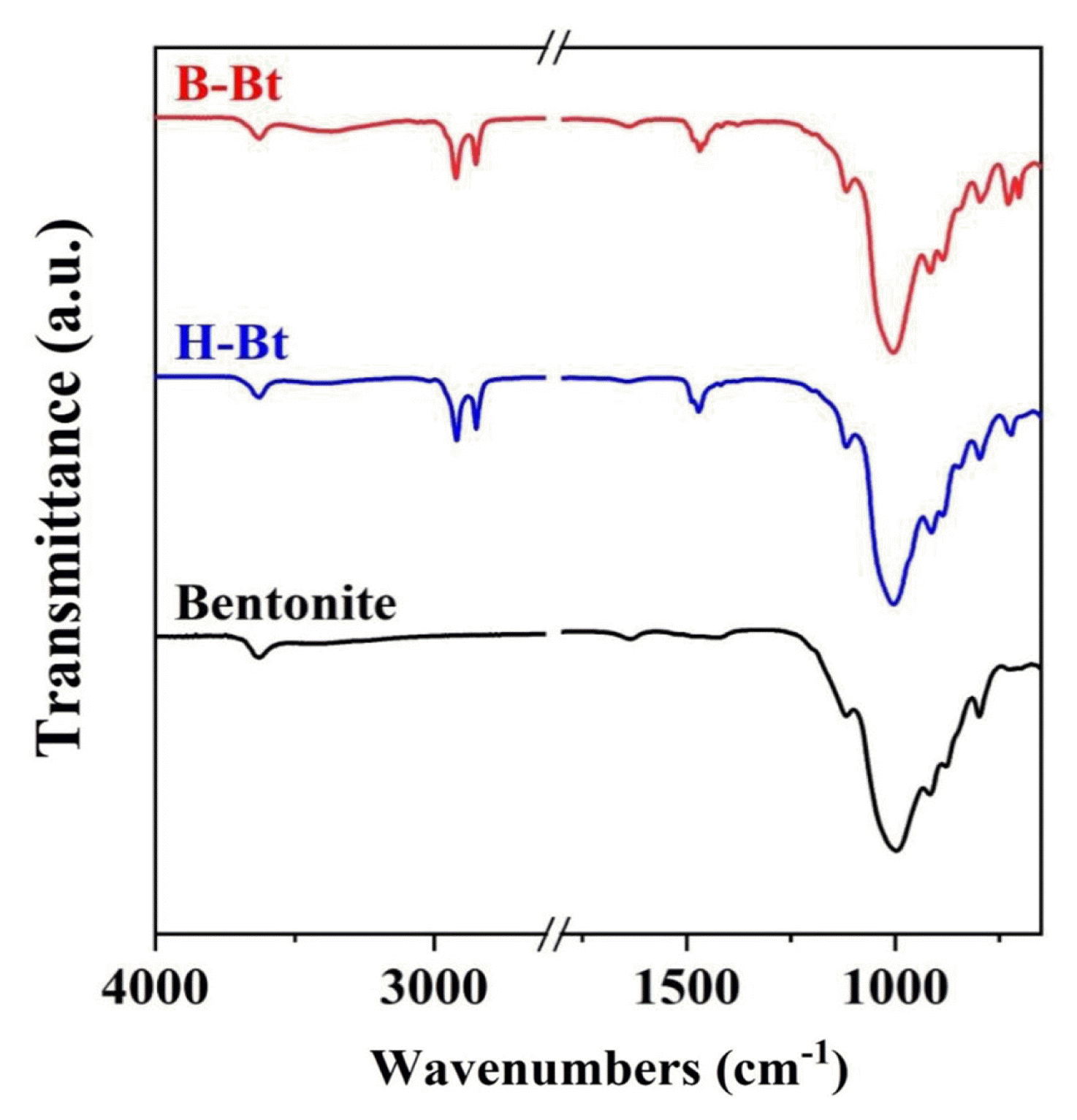
Fig. 4Orange G (OG) removal efficiency as a function of CEC ratio (Initial OG concentration: 100 mg/L, adsorbent concentration: 1 g/L, and contact time: 24 h). 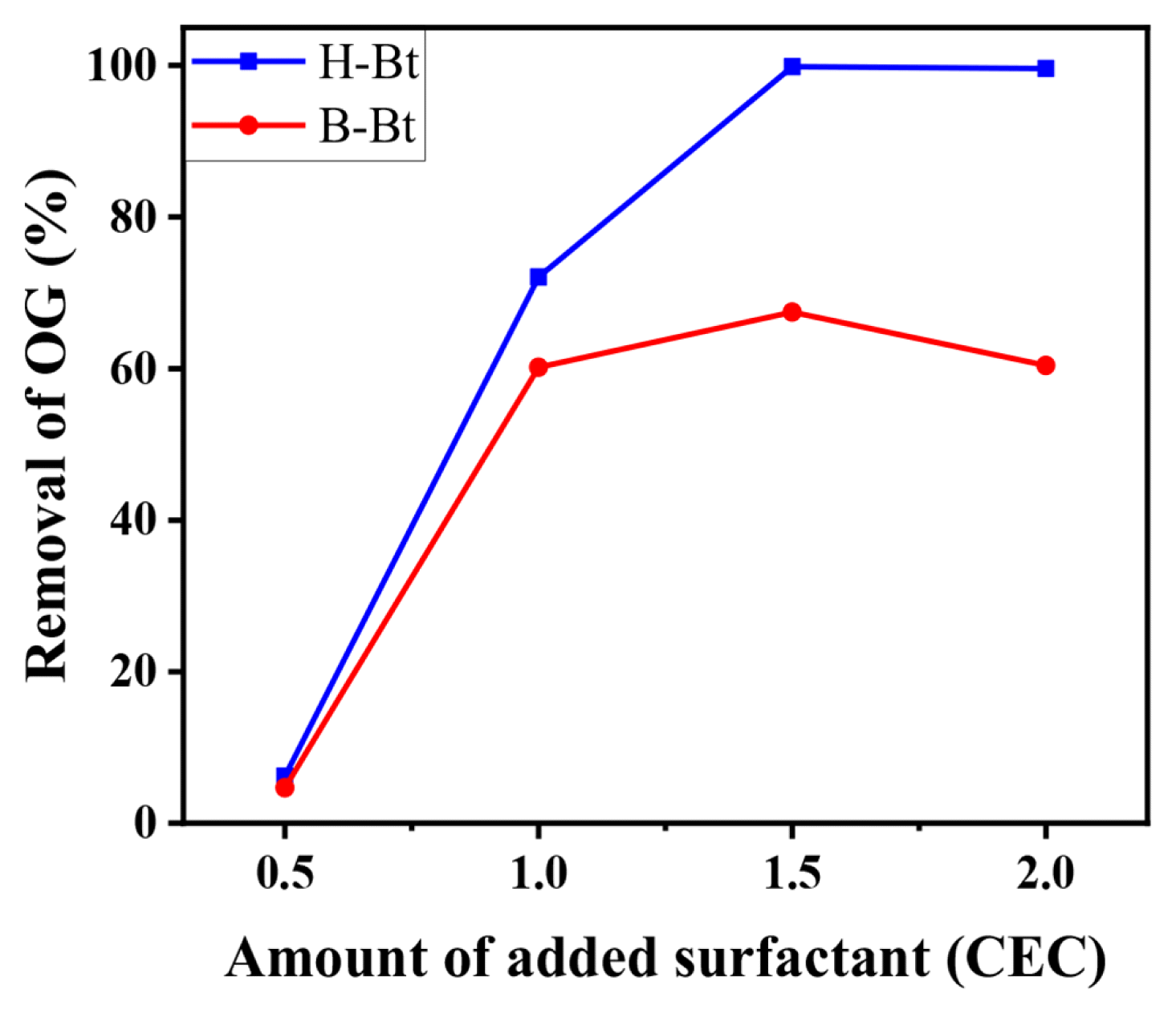
Fig. 5Langmuir and Freundlich isotherms of (A) H-Bt and (B) B-Bt obtained from the batch test (Initial OG concentration: 2 200 mg/L for H-Bt., 50 – 250 mg/L for B-Bt, adsorbents concentration: 1 g/L, contact time: 24 h). 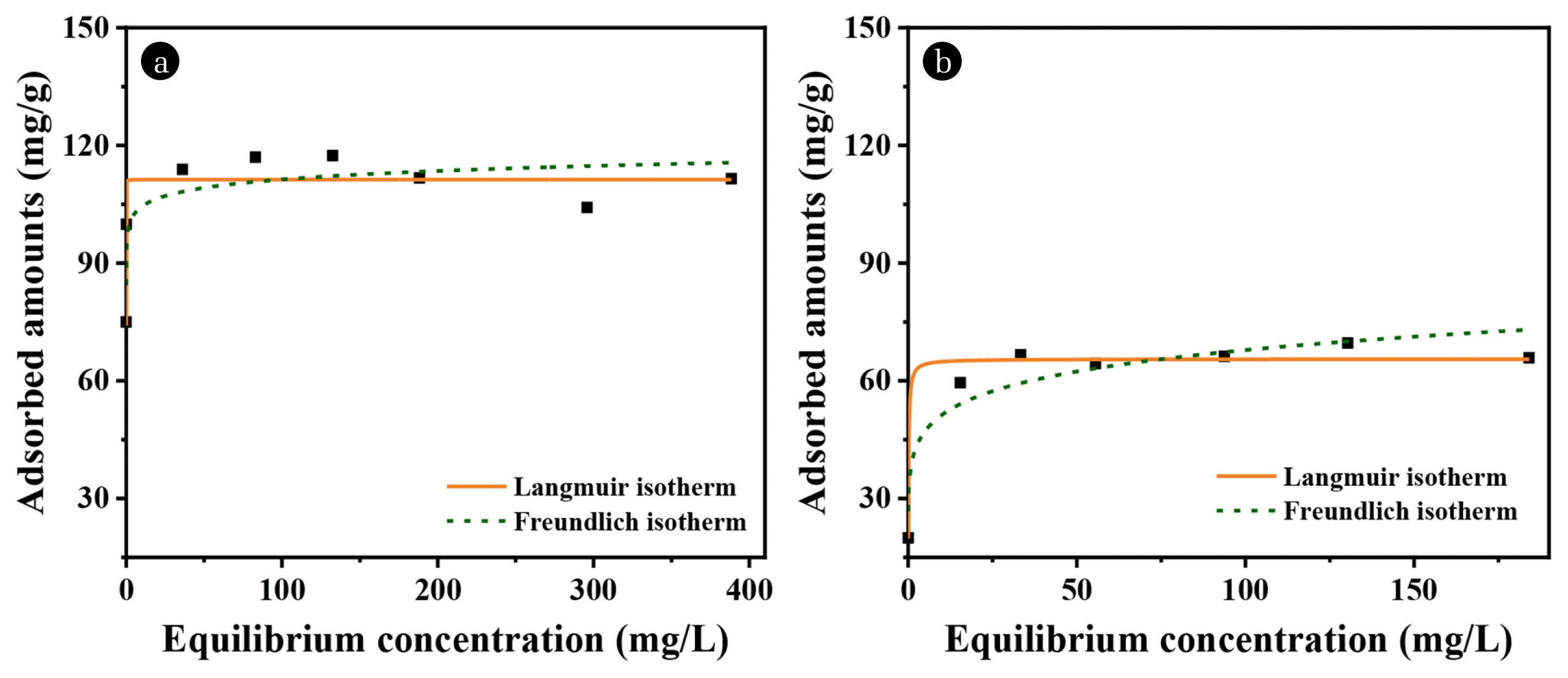
Fig. 6Adsorption kinetics of (A) H-Bt, and (B) B-Bt from the batch test (Initial OG concentration: 200 mg/L, adsorbent concentration: 1 g/L, and total contact time: 6 h. Analysis at 5, 15, 30, 45, 60, 90, 120, 180, 240, and 360 min). 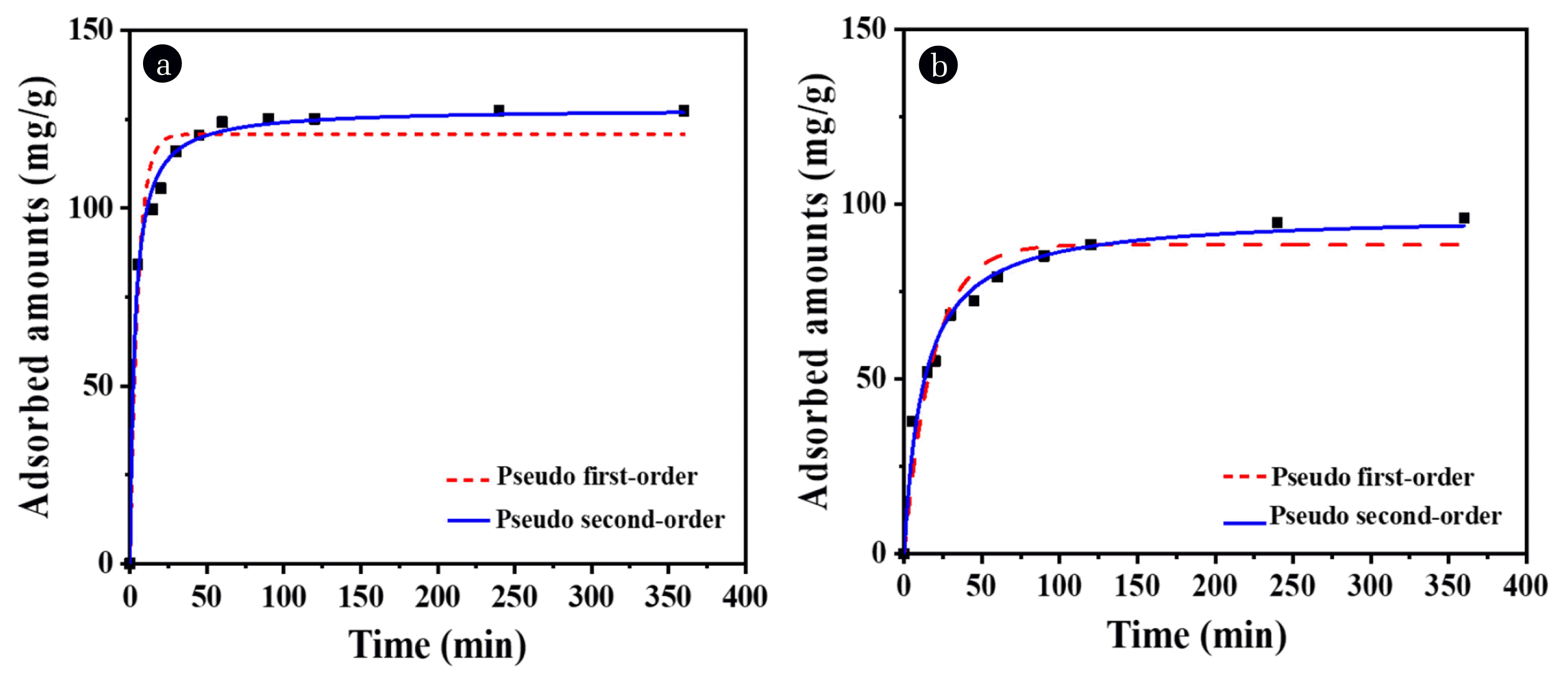
Fig. 7OG + MB absorbance in the 400 800 nm wavelength range after adsorption batch test (Initial OG + MB concentration: 100 mg/L, adsorbent concentration: 1 g/L, contact time: 24 h, UV-Vis scan). 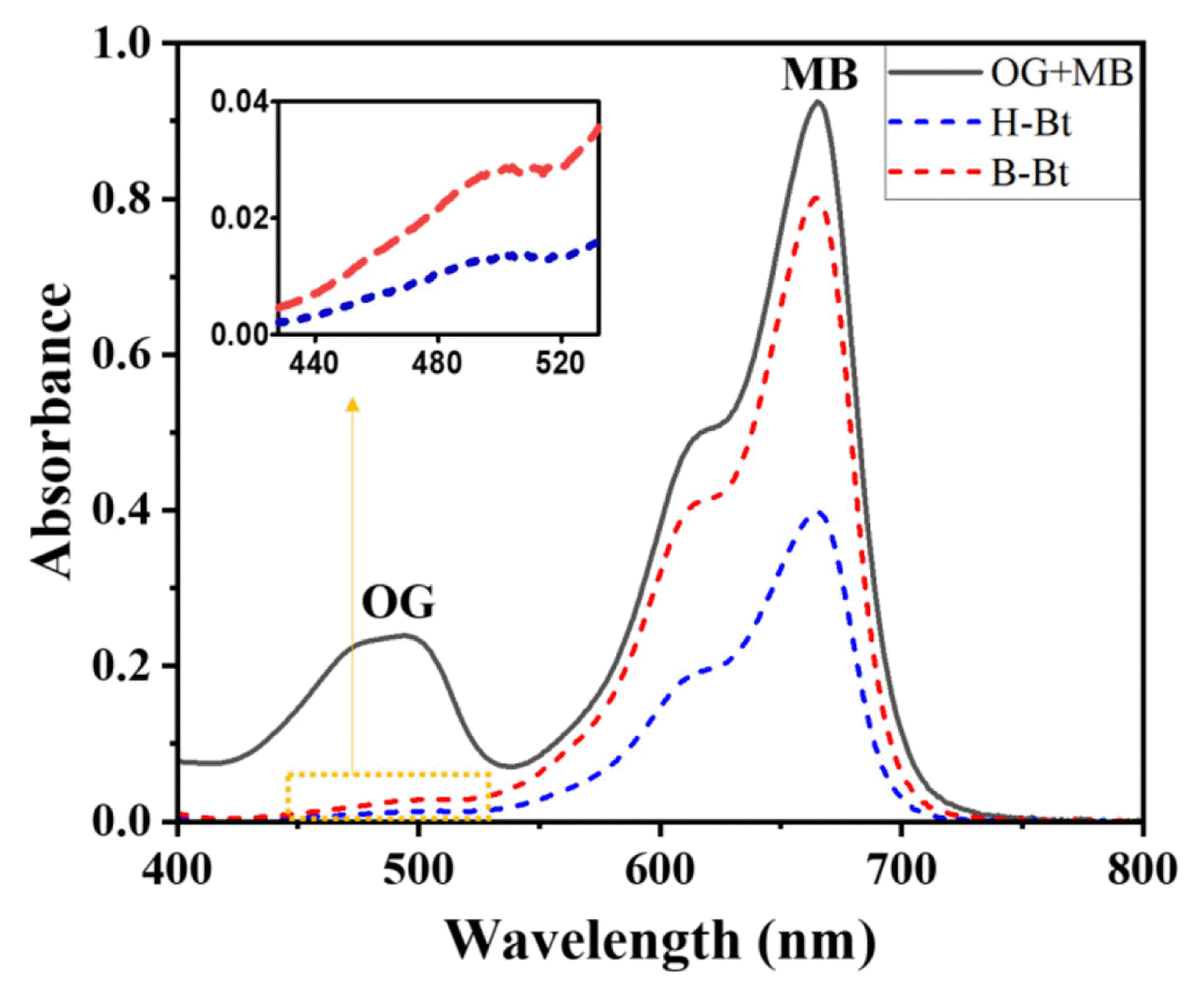
Table 1BET Surface Areas, Total Pore Volumes, and Pore Diameters of Bentonite Clay and the Organoclays Prepared with 1.5 CEC Surfactant Loading.
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||