AbstractSolar-powered electrocoagulation (EC) process is proven to be an alternative option for wet flue gas desulfurization (WFGD) wastewater treatment, due to simultaneous removal of multiple pollutants efficiently and reduce the operation costs significantly. Rapid and stable photoelectricity response is necessary for the removal efficiency of pollutants (eg. COD and turbudity), especially in low solar irradiation intensity. In this paper, dimensionally stable anode (DSA) and operation voltages in EC process driven by solar cells were investigated, for the purpose of the optimized removal of pollutants, including COD, turbidity, (free residual chlorine and chlorine dioxide). The results show that the removal efficiency of COD and turbidity can respectively reach 59.12% and 39.11% within 30 min of illumination time (the enhanced solar radiation = 915.8 W m−2), when Ti-based plates were adopted. The concentration of free residual chlorine and chlorine dioxide can reach 2.70 and 5.31 mg L−1, indicating that chlorine ions present in WFGD wastewater have been converted into active chlorine partly. Solar-powered EC equipped with Ti-based plates have a potential prospect in EC process for WFGD wastewater treatment.
1. IntroductionCoal-fired power plants (CFPPs) are still main power generation in China, and wet flue gas desulfurization (WFGD) facility is the most prevalent practice for flue gas purification. Wastewater discharge is necessary for WFGD system, in order to keep its substance balance. Despite small proportion in the total wastewater from CFPPs, WFGD wastewater requires further treatments, due to high hardness ions, soluble chlorides, suspended particulate matter, and trace amounts of heavy metals, etc [1]. Demands for developing novel technologies to treat WFGD wastewater cost-effectively and efficiently become urgent with increasingly stringent environment regulations [2].
Electrocoagulation (EC) is one of the most attractive techniques for wastewater treatment. The advantages include lower sludge production [3], simultaneous pollutants removal [4] and compact equipment layout [5]. When a sacrificial anode, e.g., iron or aluminum plates, is used while passing a direct current, hydrated cations (e.g., Al(OH)3 or Fe(OH)3 ) formed serves as coagulants to remove heavy metals, turbidity and etc, which means no chemical coagulants are needed. However, there are some challenges for EC to be put into practice of WFGD wastewater treatment, including electrode stability in WFGD slurry, large energy consumption and low performance for some elements (Cl−, Se) [6, 7]. In particular, EC application at CFPPs will also increase the air emissions per unit of electric generation, as Daniel claimed an tradeoff stategy of water-air emission have to be considered [8].
On the other hand, the installation capacity of solar and wind energy reaches 204GW and 210GW, and the curtailment rate of wind and solar energy are 4% and 2%, respectively [9]. The growth of renewable energy generated in China have already been accelerated greatly, and meanwhile, the electric power surplus has to be solved, owing to variability and intermittency of renewable energy. Some researchers found solar-powered EC system potential for wastewater treatment. Two types, direct and indictect connection between EC and solar panels, representing connections with and without battery as energy storage, are employed nowadays [10, 11]. The economization of battery in direct connection would substantially reduce the cost of investment and maintenance, and simplify charge control of battery. On the other hand, occasional meteorological/geographical conditions, duration time of EC influences the photovoltaic output current and, therefore, the efficiency of pollutants removal. Modenes et al. revealed that COD removal and huge reduction in sludge production (80%) with a total cost reduction by 4% in tannery effluent can be achieved only under more than 120 min solar irradiation, compared to conventional EC. However, the critical requirements of solar irradiation intensity is beyond the practice of EC directly integrated with solar panel [12]. Zhang et al. [13] found that the removal rate of phosphate from landscape water in directly solar-powered EC decreases rapidly in overcast day within 15 minutes. Photoelectricity conversion efficiency for an overcast day is 12.2% and energy for removing TP is 0.71 kWh/m3. The removal efficiency of pollutants (eg. COD and turbudity) in actual dye house effluents are strongly depended on the solar irradiation intensity and electrode pair combinations, which influences the photovoltaic output current and therefore energy consumption [14, 15]. Fe-Fe combination consumed the least amount of energy (0.7–4.3 kWh/m3 for phenol and 0.8–4 kWh/m3 for aldehyde). At the point of economic analysis, it indicates that no extra expenditure for the electrical energy consumed during the EC-PV water treatment and it means 15–17% off the total costs [16].
To our knowledge, few attempts have been made to WFGD wastewater electrocoagulation treatments directly driven by solar energy so far, instead of external electricity supply. In this study, a modified electrocoagulation reactor equipped with dimensionally stable anode (DSA) Ti-substrate was investigated for the simultaneous removal of Cl−, COD and turbidity in corrosive slurry. The factors influencing performance, including types of electrode plates (aluminum plate, titanium plate and platinum-coating titanium plate) and operation voltages, were optimized. And then the optimized reactor directly connected with solar cells array was also established in practice.
2. Materials and Methods2.1. ChemicalsPotassium dichromate (K2Cr2O7) and manganese sulphate (MnSO4) were used as reagents in COD measurements. Glycine (C2H5NO2) and N,N-diethyl-p-phenylenediamine (DPD) were used to determine free residual chlorine and chlorine dioxide. Sodium hydroxide (NaOH, 10%), oxalic acid solution (H2C2O4, 15%), H2PtCl6 (in HCl 1:1, v/v), citric acid (C6H8O7), ethanol (EtOH), hydrochloric acid (HCl, 6%), deionized water (H2O) were purchased from Sinopharm (China).
2.2. Analytical MethodsChemical oxygen demand (COD) and turbidity in desulfurization wastewater were analyzed by COD tester (Model 5B-6C). Water quality analyzer (Model Q-CL501) was used to determine the concentration of free residual chlorine and chlorine dioxide. The pH value and conductivity were measured by a laboratory pH meter (Model PHSJ-3F) and a conductivity meter (Model DS-307). Solar radiation was determined by solar energy meter (Model TES-1333). The main parameters of original WFGD wastewater were given in Table 1.
2.3. Experimental Setup and ProcedureOptimization tests were performed in a homemade EC reactor, composed of a 2 L PMMA cubic tank, two Ti-substrate electrode plates (165 × 95 × 2 mm) and Fe induced electrode plate (20 × 95 × 2 mm). The Fe induced plate was placed between the two parallel Ti plates with 1cm of electrode space, and 3/4 part of all electrode plates were immerged vertically into wastewater with an electrode gap of 2–3 cm. 1.5 L WFGD wastewater collected from a 600 MW CFPP in China was added to the cubic tank.
A DC power supply (RXN-305D, China) under constant voltage or current mode was applied to optimize parameters of EC reactor, before connected with solar cells array. A set of solar cell array was composed of two PV panels (for each panel, Pmax = 150 W, Im = 8.34 A, Ump = 18.8 V, Uoc = 23.7 V, Isc = 9.17 A) with series and parallel connections. A PV panel is consisted of 4 × 9 monocrystalline silicon cells, each of which was 70 mm in length and 80 mm in width. A DC/DC Boost converter was used between the solar cell and load as shown in Fig. 1, in order to accomplish Maximum Power Point Tracking (MPPT). The load resistance was matched with the internal resistance of the PV mainly by adjusting the duty cycle D (0 ≤ D ≤ 1) of the Boost converter. Once the illumination intensity and atmosphere temperature change, the MPPT controller responds in real time and generates the corresponding pulse width modulation (PWM) wave to drive MOSFET in the boost drive circuit, so as to adjust the duty cycle D. Two types of illumination intensity conditions, high and low radiation intensity, were selected in this study for comparison. The whole equipment diagram was shown in Fig. 1.
2.4. Experimental ProcedureThree types of electrode plates were adopted in our experiments as anode, which include aluminum plate (Al), titanium plate (Ti, 99.6%) and platinum-coating titanium plate (Pt/Ti). Pt/Ti plate was prepared by immersion method. In order to prevent the corrosion caused by a large number of chloride ions in WFGD wastewater, Ti-substrate DSA electrode was coated with platinum, which can enhance the stability of the coating and can extend life of the electrode. The detail pretreatments were completed as follows. Ti plate was initially polished with sandpaper of different mesh numbers. After washing with deionized water, place the Ti plate in a 10% NaOH solution, and heat it in a water bath at 100°C for 1 h. And after being washed with deionized water, the Ti plate was placed in a 15% oxalic acid solution and was etched at 100°C for 2 h. Finally, it was washed with deionized water and ready for use. The Pt precursor solutions were obtained by dissolving H2PtCl6 (in HCl 1:1, v/v), in a citric acid and ethanol solution under constant stirring at 90–95°C. The etched Ti plate was immersed into the Pt precursor solutions for 5 min every time, and then was dried at 120°C. The dried plate was calcined at 500°C for 5 min in a preheated oven. These steps were repeated for 6 times until a thickness of 2 μm Pt layer was formed. All of electrode plates were polished to remove the passivation layer, and then were immersed into 6% HCl solution for about 10 min before experiments.
3. Results and Discussion3.1. Effects of Voltage AppliedSince the output voltage of PV array varies from 0–20 V, three different voltages were selected in interval of 5 V. As can be seen from Fig. 2 (a), COD decreases rapidly within the first 10 min, when the voltage were 10, 15 and 20 V, respectively. After 10 minutes, COD and turbidity decrease very slowly. Removal of COD and turbidity increase with increasing voltages applied, and maximum removal efficiency reaches about 71.3% (133 mg L−1) and 50.0% (9.07 mg L−1) after 20 min, just satisfying the standard of WFGD effluent in China. Higher voltage favors COD and turbidity removal. It is worth noting that the removal efficiency is lower than our previous experiments directly electro-coagulation treatment of WFGD wastewater [17]. We attribute it to the configuration adopted and different principle involved. In this study, the contribution of electrode plates to flocculants is not derived from direct EC process, but an EC integrated with electrooxidation (EO), which would decrease COD removal to some degree and would extend the electrolysis time [18]. Similar removal efficiency can be achieved in longer time for treating laundry wastewater using aluminum plates by Fatemeh et al. [19] in electrocoagulation process (20V voltage; 45 min; two extra plates; distance between plates was 1.5 cm) [8, 19].
Instead of a direct sacrificed anode in EC system, induced Fe electrode plate was used in this study, to in-situ generate Fe2+ for coagulation. Aiming to explain the role of induced Fe electrode plate, pH variations with electrolysis time are also recorded synchronously as shown in Fig. 3(c). An increase in voltage causes to Fe dissolution on induced-electrode plate and to generate Fe2+, which combines with OH− to form ferric hydroxides with various hydration states[20]. The oxidation of Cl− in WFGD wastewater produces Cl2, which promotes the formation of Fe(OH)3 ((Eq. (1)–(2)) [21].
As a result, pH decreases rapidly with electrolysis time, due to consumption of OH− [22]. Iron hydroxide starts to polymerize and precipitate as coagulation, accompanying with the aggregation of organic pollutants, reducing pollutant load (e.g., organic matter, SO32− and S2O62−) in WFGD wastewater. Similar pH variations caused by water oxidation and chlorine formation have been revealed in previous literature [21, 23, 24]. pH value is also strongly related to the dissolution of Fe induced-electrode plate, which can be regarded as an indicator of the process of removing pollutants by electrocoagulation. It is noted that the trend of pH decline is more remarkable, a bit inconsistent with turbidity changes as shown in Fig. 2 (b) and (c). It is speculated that there are other factors contributing to the pH and will be discussed later.
3.2. Effects of Electrode PlatesThree types of anode plates, Al, Ti and Pt/Ti were applied for comparison, and the COD, turbidity as well as synchronous pH changes with anode types are illustrated in Fig. 3. Decline of COD and turbidity with electrolysis time are observed. However, trends of COD and turbidity exhibited on different types of electrode plate materials are discernible. For example, COD decreases in sequence of Al, Ti and Pt/Ti, while in sequence of Ti, Pt/Ti and Al for turbidity. The decreased pH is much greater for Al than other two electrode plates. When Al was used as anode plates, Al3+ is electrochemically generated through Al direct dissolution and the hydrolyzed Al(OH)3 acts as hydroxide flocculant to adsorb typical pollutants. Suspended substances can be removed in this way and residual turbidity value is the lowest. As one of dimension stable anodes, no metal cations are generated electrochemically in-situ when Ti-based anode was employed. Instead, main hydroxide flocculants are produced from induced-electrode plates as mentioned above. Hydrolysis is responsible for turbidity variations just as Fig. 3(b) shows. Oxidation occurs on the iron induced-electrode to form Fe2+ and Fe3+. The conversion of Fe2+ to Fe(OH)2 under neutral conditions is followed by rapid oxidation to Fe(OH)3, which results in a decrease in pH value. Under acidic conditions, COD will continue to decrease due to the strong oxidation of HClO.
3.3. Effect of Active Chlorine EvolutionsIt can be seen from Fig. 4 (a) that the concentration of active chlorine (eg., free residual chlorine and chlorine dioxide) reaches the maximum value after electrolysis for about 15 min. The concentration of active chlorine gradually decreases afterwards, and then tends to be stable, indicating that the conversion of chloride ions is almost completed. Other active chlorine species (eg., chlorite, chlorate, etc.) are not detectable. Under the condition of 15 V voltage, the concentration of free chlorine can reach 2.96 mg/L, and the concentration of chlorine dioxide can reach 6.25 mg/L. The similar tendency of active chlorine produced under higher voltage (20 V), and the highest concentration of free residual chlorine and chlorine dioxide reach 3.37 mg/L and 7.21 mg/L, respectively. It can be speculated that higher voltage is beneficial to the production of free chlorine and chlorine dioxide. The variations of active chlorine, that is first rising and then decline, are associated with active chlorine.
Active chlorine species produced differ significantly on various electrode plates. Free residual chlorine (eg., Cl2) produced on Pt/Ti is an order of magnitude higher than that on Ti, and 14 times compared to that on Al. This suggests Pt/Ti possesses higher electrochemical activity for chlorine reduction, which is confirmed previously [23, 24]. According to chlorine evolution (Eq. (3)–(5)), strong oxidizing ability attributed to ClO2 and ClO− facilitates oxidation of organic contaminants, and as a result, COD decreases significantly in presence of chloride ions on Ti-based electrode plate.
The production of ClO2 and ClO− also contributes to more H+ and therefore pH decreases greatly as shown in Fig. 2(c). Removal mechanism for COD is very unlike to that for turbidity removal, determining the performance difference shown in Fig. 3 (a) and (b). On the other hand, chloride ions oxidation, in spite of small proportion, is still welcome due to dechlorination especially for Ti-based anode plates.
3.4. Operations under Different Solar Radiation IntensitiesA small-scaled solar-powered EC system was employed and optimized parameters of EC system (eg., voltage, electrode plates) above were designated. The variations of COD, turbidity, active chlorine in 1.5 L WFGD wastewater as well as pH value were plotted in Fig. 5 (a)–(d), and at the same time the electrical output of PV panel was recorded. The influence of real operations of PV panel under different conditions on the pollutants in WFGD wastewater was analyzed. Two kinds of solar radiation intensities, I = 915.8 (sunny), 271.3 (cloudy) W m−2 were selected as working conditions. The changes of the voltage, current and power of PV panel are shown in Fig. 5 (e)–(g). When the solar radiation intensity is 271.3 W m−2, the PV panel’s voltage continues to decrease with electrolysis time, from 16.5 V to 12.2 V, and the current fluctuates slightly around 3.5 A. The maximum power of PV panel achieved is 62.7 W. When solar radiation intensity is 915.8 W m−2, voltage of PV panel decreases almost linearly with electrolysis time and the current increases slightly. The maximum power of PV panel under enhanced solar radiation intensity is double up to 123.4 W, compared to that of the worse solar radiation intensity. More stable operations can be observed under enhanced solar radiation intensity, particularly after 600 s.
COD values decrease rapidly before 540 s and afterwards remain stable with operations under both conditions. The maximum removal efficiency reaches 59.12% achieved under the enhanced solar radiation intensity, in concordance with the electrical output. The removal of COD is lower than that under fixed voltage applied above. The variations of turbidity, pH and active chlorine are desynchronized from that of COD. 39.11% of turbidity removal and active chorine generation can be achieved before 360 s (2.70 mg L−1 and 5.31 mg L−1 for free residual chlorine and chlorine dioxide, respectively), much faster response of these three parameters taking place, compared to COD change. This may be due to the complicated mechanism of COD degradation associated with participation of chlorine as discussed above [25].
With regard to energy consumption and energy conversion efficiency during EC process, they can be calculated as Eq. (6)–(7) presents [13].
When modified Ti electrode plates were employed for COD and turbidity degradation, the shortest electrolysis time required and the maximum removal efficiency achieved both are taken into account, and in this study energy consumption is minimized to 11.5 kWh m−3 (t = 540 s) under sunny condition. If the feed-in tariff for solar are USD$ 0.07 on the average, the energy expenditure is about USD$ 0.81 in case of solar. The energy consumption in this study is much higher than those achieved for other types of effluents, and however, electrolysis time required becomes shorter, so that rapid degradation can be achieved in good meteorological/geographical conditions [14, 16, 21]. Sunlight-to-electric energy conversion efficiency under two types of solar radiation conditions are calculated as 1.2% (sunny) and 4.4% (cloudy), respectively. Higher photoelectricity conversion efficiency obtained in cloudy day, lower than typical value of 15% for current monocrystal-line silicon photovoltaic modules [13, 26]. Even so, operations under sunny conditions are preferred for WFGD wastewater, due to good stability.
3.5. Electrode Surface Changes
Fig. 6 shows the surface morphology of the electrode plates (Al, Ti and Pt/Ti) before and after EC process. Lower SEM magnification images (X100) can clearly presents the surface changes post-treatment. Some new solid phase stacks on the surface of electrode plates in Fig. 6 (d). Post-analysis of EDX shown in Fig. 7 confirms that it is derived by sulfate or sulfite present in WFGD wastewater. The coverage of sulfate or sulfite would passivate Al electrode plate soon. In case of Ti and Pt/Ti electrode plates as Fig. 6 (e) and (f), the delamination of surface can be found. Post-analysis of EDX indicates the dissolution of trace metal (eg., Fe and Zn) and substitution (eg., Mn) takes place, due to the increasingly acidic environment induced by EC conditions as discussed before.
4. ConclusionsThe EC configuration of DSA companied with Fe induced-electrode plates is applicative for treatment of wastewater from CFPPs, which contains various contaminants difficult to degrade, such as COD, turbidity and chloride, etc. Platinum-coating Ti substrate DSA is more active for COD degradation, assisted by active chlorine derived from chloride oxidation. Higher voltage is favorable for active chlorine production and contaminants degradation. When a real solar-powered EC system was built up on the basis of the optimized parameters, stable electrical output can be obtained under sunny condition (951.8W m−2), in favor of COD and turbidity removal in shorter time. A 59.12% of COD and 39.11% of turbidity are degraded at the optimal conditions.
AcknowledgmentsThe authors would acknowledge financial support from the National Natural Science Foundation (No. 51678291), the Six Top Talents Plan in Jiangsu Province (No. JNHB-029) and the Students’ Innovation Project Foundation in Jiangsu Province (Grant No. TB202012045).
NotesAuthor Contributions L.X. (MD. student) conducted experiments and wrote the manuscript. G.J.D. (MD. student) conducted PV experiments. L.H. (MD. student) built the PV array device. Z.H.Y. (Associated Professor) performed the water analysis. Y.Y. (Associated Professor) revised the manuscript. T.W.Y. (Professor) directed the whole plan and organized whole project. References1. Gingerich DB, Grol E, Mauter MS. Fundamental challenges and engineering opportunities in flue gas desulfurization wastewater treatment at coal fired power plants. Environ Sci Water Res Technol. 2018;4:909–925.
https://doi.org/10.1039/c8ew00264a
2. Ma SC, Chai J, Chen GD, Yu WJ, Zhu SJ. Research on desulfurization wastewater evaporation: Present and future perspectives. Renew Sust Energy Reviews. 2016;58:1143–1151.
https://doi.org/10.1016/j.rser.2015.12.252
3. Yavuz Y, Ögütveren ÜB. Treatment of industrial estate wastewater by the application of electrocoagulation process using iron electrodes. J Environ Manage. 2018;207:151–158.
https://doi.org/10.1016/j.jenvman.2017.11.034
4. Zhao S, Huang GH, Cheng GH, Wang YF, Fu HY. Hardness, COD and turbidity removals from produced water by electrocoagulation pretreatment prior to Reverse Osmosis membranes. Desalination. 2014;344:454–462.
https://doi.org/10.1016/j.desal.2014.04.014
5. Lakshmi PM, Sivashanmugam P. Treatment of oil tanning effluent by electrocoagulation: Influence of ultrasound and hybrid electrode on COD removal. Sep Purif Technol. 2013;116:378–384.
https://doi.org/10.1016/j.seppur.2013.05.026
6. Hansen HK, Peña SF, Gutiérrez C, Lazo A, Lazo P, Ottosen LM. Selenium removal from petroleum refinery wastewater using an electrocoagulation technique. J Hazard Mater. 2019;364:78–81.
https://doi.org/10.1016/j.jhazmat.2018.09.090
7. Kuokkanen V, Kuokkanen T, Rämö J, Lassi U, Roininen J. Removal of phosphate from wastewaters for further utilization using electrocoagulation with hybrid electrodes – Techno-economic studies. J Water Process Eng. 2015;8:e50–e57.
https://doi.org/10.1016/j.jwpe.2014.11.008
8. Gingerich DB, Sun X, Behrer AP, Azevedo IL, Mauter MS. Spatially resolved air-water emissions tradeoffs improve regulatory impact analyses for electricity generation. Proc Natl Acad Sci. 2017;114:1862–1867.
https://doi.org/10.1073/pnas.1524396114
9. NEO, National Energy Administration, China. Grid-connected operation of solar power in 2019 Internet. [c2021 cited 15 June 2021]. Available from: http://www.nea.gov.cn/2020-02/28/c_138827923.htm
10. Khemila B, Merzouk B, Chouder A, Zidelkhir R, Leclerc JP, Lapicque F. Removal of a textile dye using photovoltaic electrocoagulation. Sust Chem Pharm. 2018;7:27–35.
https://doi.org/10.1016/j.scp.2017.11.004
11. Valero D, Ortiz JM, Expósito E, Montiel V, Aldaz A. Electrocoagulation of a synthetic textile effluent powered by photovoltaic energy without batteries: Direct connection behaviour. Solar Energy Mater Solar Cells. 2008;92:291–297.
https://doi.org/10.1016/j.solmat.2007.09.006
12. Módenes AN, Espinoza-Quiñones FR, Borba FH, Manenti DR. Performance evaluation of an integrated photo-Fenton–Electrocoagulation process applied to pollutant removal from tannery effluent in batch system. Chem Eng J. 2012;197:1–9.
https://doi.org/10.1016/j.cej.2012.05.015
13. Zhang SX, Zhang J, Wang WQ, Li FR, Cheng XZ. Removal of phosphate from landscape water using an electrocoagulation process powered directly by photovoltaic solar modules. Sol Energy Mater Sol Cells. 2013;117:73–80.
https://doi.org/10.1016/j.solmat.2013.05.027
14. Marmanis D, Dermentzis K, Christoforidis A, Ouzounis K, Moumtzakis A. Electrochemical treatment of actual dye house effluents using electrocoagulation process directly powered by photovoltaic energy. Desalin Water Treat. 2015;56:2988–2993.
https://doi.org/10.1080/19443994.2014.966330
15. Olya ME, Pirkarami A. Electrocoagulation for the removal of phenol and aldehyde contaminants from resin effluent. Water Sci Technol. 2013;68:1940–1949.
https://doi.org/10.2166/wst.2013.439
16. Pirkarami A, Olya ME. Removal of dye from industrial wastewater with an emphasis on improving economic efficiency and degradation mechanism. J Saudi Chem Soc. 2017;21:S179–S186.
https://doi.org/10.1016/j.jscs.2013.12.008
17. Zhang HY, Shang ZT, Zhou C, Xue YB, Liu TF, Tan WY. Electrocoagulation Treatment of Wet Flue-Gas Desulfurization Wastewater Using Iron-Based Electrodes: Influence of Operating Parameters and Optimization. Int J Electrochem Sci. 2019;14:3114–3125.
https://doi.org/10.20964/2019.03.03
18. Asfaha YG, Tekile AK, Zewge F. Hybrid process of electrocoagulation and electrooxidation system for wastewater treatment: A review. Clean Eng Technol. 2021;4:100261.
https://doi.org/10.1016/j.clet.2021.100261
19. Janpoor F, Torabian A, Khatibikamal V. Treatment of laundry waste-water by electrocoagulation. J Chem Technol Biotechnol. 2011;86:1113–1120.
https://doi.org/10.1002/jctb.2625
20. Lü JC, Dou YM, Sun M, Jin C, Lan HC. The efficiency and mechanism for the field-induced electro-Fenton degradation of dimethyl arsenic. Acta Sci Circum. 2017;37:2152–2157.
https://doi.org/10.13671/j.hjkxxb.2016.0417
21. Hakizimana JN, Gourich B, Chafi MS, et al. Electrocoagulation process in water treatment: A review of electrocoagulation modeling approaches. Desalination. 2017;404:1–21.
https://doi.org/10.1016/j.desal.2016.10.011
22. Lakshmanan D, Clifford DA, Samanta G. Ferrous and Ferric Ion Generation During Iron Electrocoagulation. Environ Sci Technol. 2009;43:3853–3859.
https://doi.org/10.1021/es8036669
23. Karlsson RKB, Hansen HA, Bligaard T, Cornell A, Pettersson LGM. Ti atoms in Ru0.3Ti0.7O2 mixed oxides form active and selective sites for electrochemical chlorine evolution. Electrochim Acta. 2014;146:733–740.
https://doi.org/10.1016/j.electacta.2014.09.056
24. Scialdone O, Proietto F, Galia A. Electrochemical production and use of chlorinated oxidants for the treatment of wastewater contaminated by organic pollutants and disinfection. Current Opin Electrochem. 2021;27:100682.
https://doi.org/10.1016/j.coelec.2020.100682
25. Mascia M, Vacca A, Polcaro AM, Palmas S, Ruiz JR, Pozzo AD. Electrochemical treatment of phenolic waters in presence of chloride with boron-doped diamond (BDD) anodes: Experimental study and mathematical model. J Hazard Mater. 2010;174:314–322.
https://doi.org/10.1016/j.jhazmat.2009.09.053
26. Dominguez-Ramos A, Aldaco R, Irabien A. Photovoltaic solar electrochemical oxidation (PSEO) for treatment of lignosulfonate wastewater. J Chem Technol Biotechnol. 2010;85:821–830.
https://doi.org/10.1002/jctb.2370
Fig. 1Diagram of solar-powered electrocoagulation-electrooxidation WFGD wastewater system. (1. PV arrays; 2. Control box; 3. Converter; 4. Electrocoagulation-electrooxidation reactor; 5. DSA (platinum-coating titanium); 6. Fe induced-electrode; 7. Al cathode.)

Fig. 3Variations of COD (a), turbidity (b) and pH (c) of WFGD wastewater on electrode plates materials. 
Fig. 4Dependence of active chlorine (free residual chlorine and chlorine dioxide) generation on voltages (a) and electrode plates materials (b)–(d). 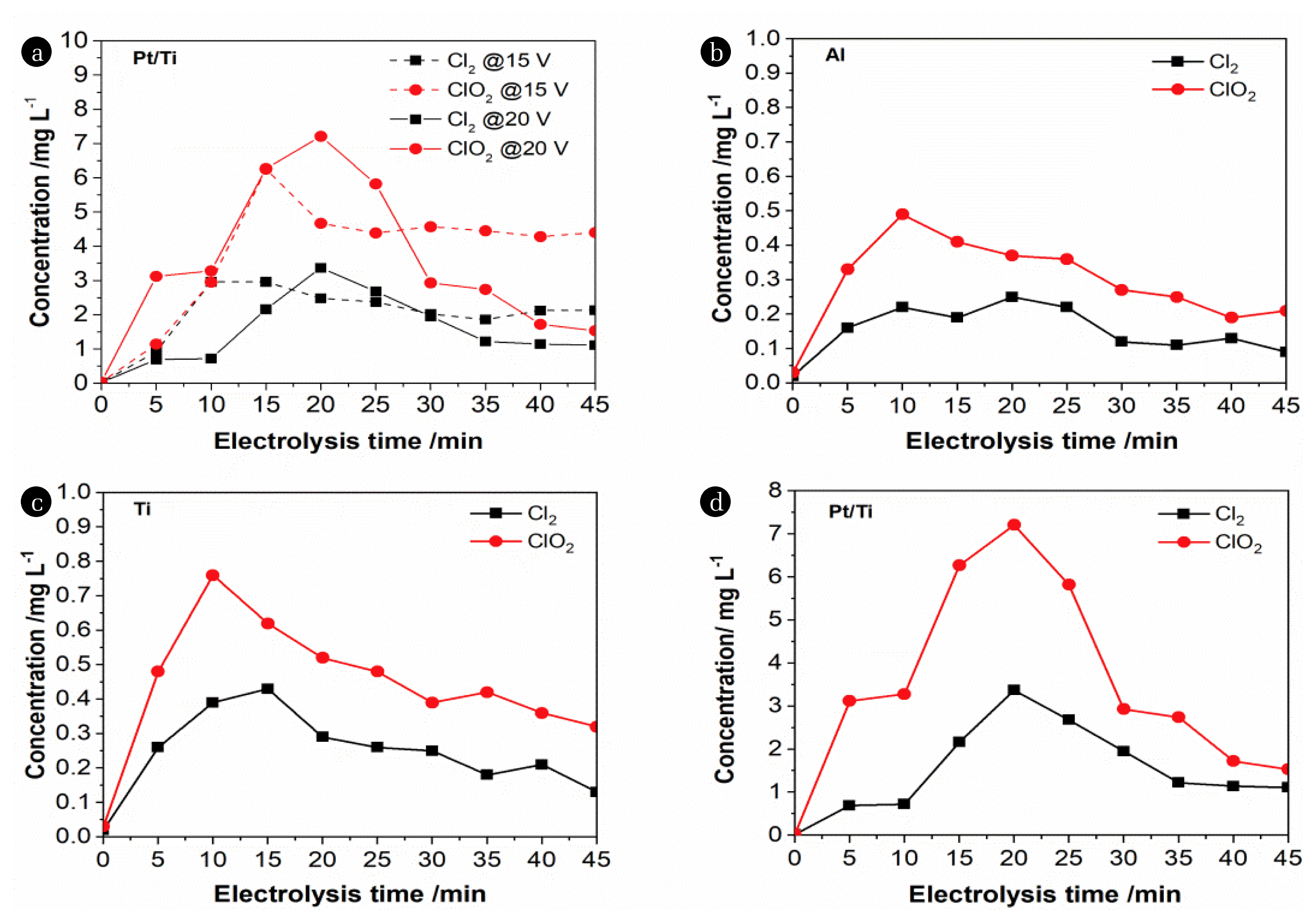
Fig. 6Surface morphology of electrode plates Al, Ti and Pt/Ti before (a)–(c) and after ((d)–(f)) EC treatments. 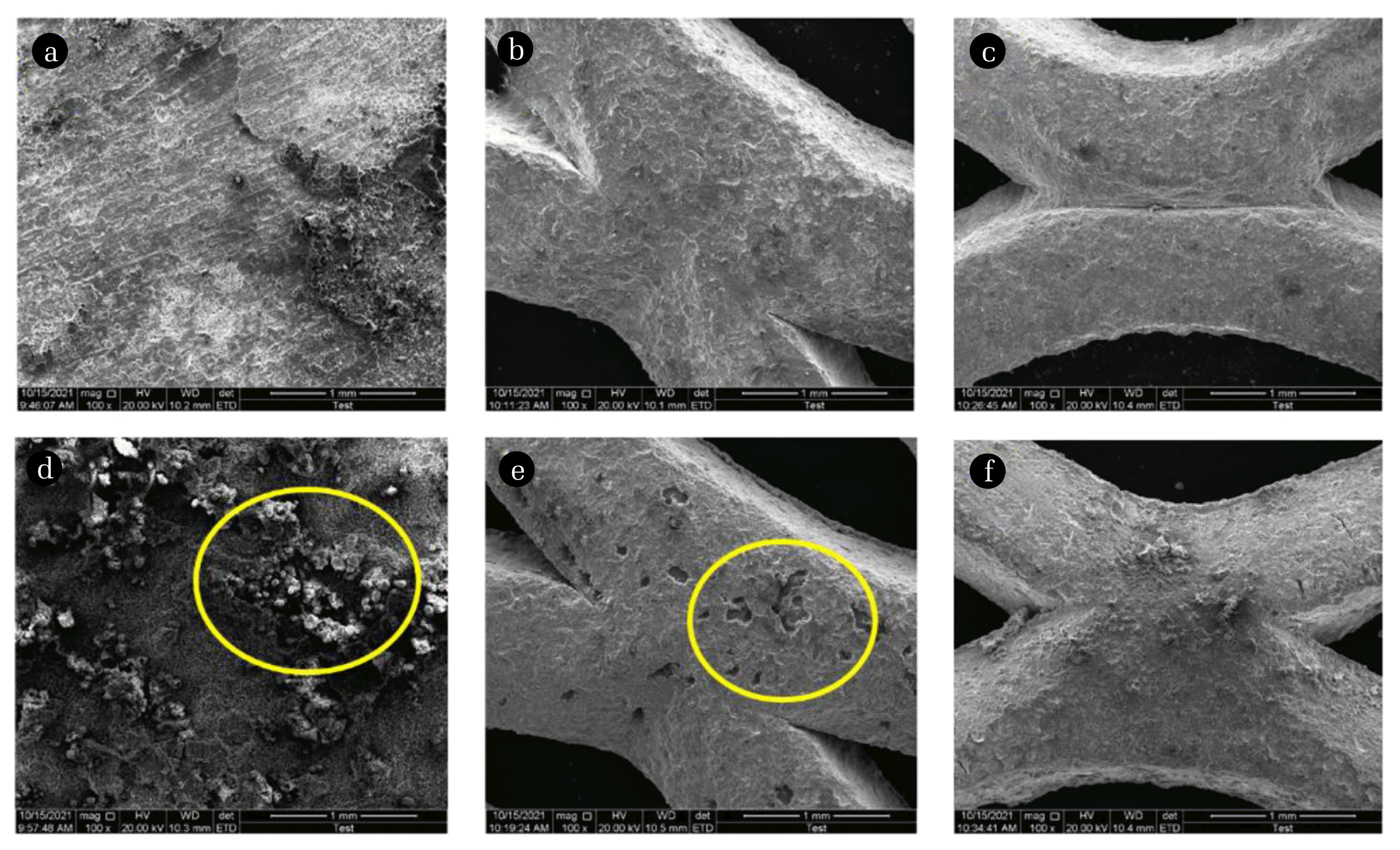
|
|
||||||||||||||||||||||||||||||||||||||