1. Introduction
With the rapid development of economy, the industrial products and by-products generated during human activities, especially in production of chemical, pharmaceutical, printing and dyeing and petrochemical industries, have been released into the natural water without treatment. Organic compounds in those industrial products and by-products are often toxic and difficult to biodegrade [1, 2]. Quinoline is such the compound and it is a typical nitrogen heterocyclic compound mainly resulting from coking, dye, oil refining and pharmaceutical wastewater [3, 4]. As a water-soluble and volatile compound [5], it often exists in soil, groundwater and air [4, 6] and, its concentration in coking wastewater is very high up to 86.8 mg·L−1 [7]. It has a relatively stable loop structure, thus it is difficult to biodegrade. Also, it is easy to cause an acidification and eutrophication of water [8]. Furthermore, its toxicity [9] includes causing cancer, teratogenesis and mutagenicity of animals and human [8, 10]. Overall, it has posed a threat to water ecological security and human health. Therefore, developing economical and efficient method for the remediation of quinoline wastewater pollution is needed.
The common method for the degradation of the quinoline includes physical, biological and advanced oxidation methods. The degradation of organic matter into small molecular compounds by traditional physical methods such as membrane technology, ion exchange and activated carbon adsorption [11, 12], is not able to achieve. The drawbacks for the biological method are that: the quinoline has an inhibiting effect on the microorganisms so that the structure of quinoline cannot be recognized by enzyme system of the microorganisms, thus we have to carry out a screen of special strains [13, 14]. Also, sludge acclimatization is time-consumption [15] and its degradation treatment is often conduced on a large-scale which is high cost [16]. Furthermore, some of organics are degraded into intermediates and they would be not easily accumulated [17].
Fortunately, advanced oxidation technologies, such as supercritical water oxidation [18, 19], ozone oxidation [6], ultraviolet radiation [20], photocatalytic oxidation [21, 22], electrocatalytic oxidation [10, 23] and wet oxidation [8, 24], have been applied to degradation of resistant organic wastewater by generating oxidative free radicals. But, photocatalytic oxidation has an inferior effect on treatment of wastewater containing poor light transmission; supercritical oxidation has a high requirement for reaction equipment; electrocatalysis and ultrasonic oxidation are only effective in laboratory at present; the wet oxidation and catalytic wet oxidation need to conduct under high temperature and high pressure [25]. For example, Nilam J [8] showed that using Ru/C catalyst to remove quinoline needed to complete under a temperature up to 493 K and oxygen partial pressure up to 0.69 MPa. Although catalytic wet peroxide oxidation has a strong oxidation capacity, high mineralization efficiency and fast reaction rate for quinoline, some existing problems such as complex catalyst preparation, high cost and easy precipitation of active components [26, 27] are not avoided. Fenton method is the cost-effective advanced oxidation technology which has been widely applied in water treatment. However, its solution is often yellowish resulting in from ion element and effective treatment often reaches under low pH around 2 to 4 [28], and finally numerous iron-containing sludge needs to further treat [29]. Therefore, it is a challenge to improve pH effect and obtain a cost-effective method for treatment of polluted water.
In this study, the degradation effect of quinoline by hydrogen peroxide catalyzed by copper sulfate was investigated, which was a new Fenton-like system. In the system, effect of the parameters affecting the catalysis effect of copper ion on hydrogen peroxide, such as dosage of copper sulfate as catalyst, H2O2 dosage, reaction temperature, initial concentration and pH, was investigated by measuring the removal of quinoline. Possible degradation paths and the catalytic mechanism were analyzed.
2. Materials and Methods
2.1. Materials and Instruments
All chemicals used in this study were of analytical reagent grade. The chemicals such as quinoline (C9H7N, Adamas Reagent Co.,Ltd), coumarin (C9H6O2, Adamas Reagent Co.,Ltd), 7-hydroxyl coumarin (C9H6O3, Acros), copper chloride (CuCl2·2H2O, Guangdong Chemical Reagent Engineering Research and Development Center), copper acetate (C4H6 CuO4·H2O, Tianjin Regent Chemicals Co.,Ltd), Ferrous sulfate, (FeSO4·7H2O), ferric sulfate (Fe2(SO4)3), manganese sulfate (MnSO4·H2O), were purchased from Chengdu Cologne Chemical Co., Ltd.. Other chemicals (such as nitric acid copper (Cu(NO3)2·3H2O), copper sulfate (CuSO4·5H2O), titanium dioxide (TiO2)) were purchased from Chengdu Kelon Chemical Reagent Factory, hydrogen peroxide (H2O2, 30%), and those such as (hydrochloric acid (HCl), sulfuric acid (H2SO4), sodium hydroxide (NaOH)) were purchased from Chongqing Chuandong Chemical (group) Co., Ltd..
2.2. Experimental
Experiment of degradation of quinoline wastewater by the Fenton-like catalytic oxidation was carried out in a corked conical flask within a thermostatic water bath. A fixed concentration of simulated quinoline solution (7.2–100 mg·L−1, pH 3.8–8.8) was prepared by adding quinoline and distilled water in a conical flask, following by a certain amount of catalyst copper sulfate (0.2–1.28 g·L−1,) and a certain amount of H2O2 (m/m, 30%, 78–470 mmol·L−1). Subsequently, the solution reacted for a certain time at a stirring speed of 120 rpm/min and a certain temperature (55–85°C). Then, the samples were centrifuged and the supernatant was collected for measurement of total organic carbon (TOC) and UV-Vis absorbance, respectively.
2.3. Analytical Methods
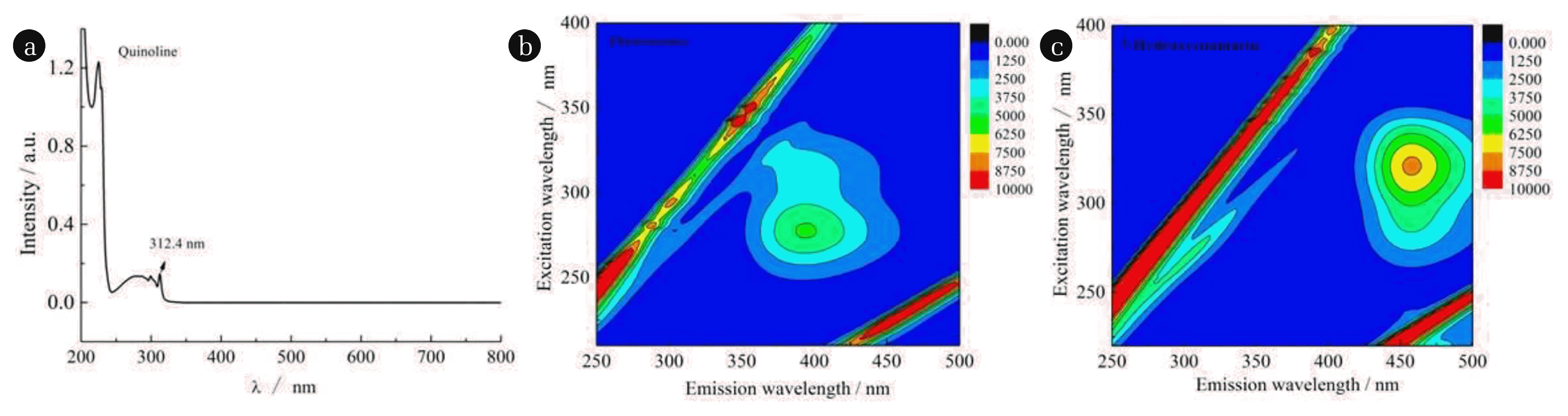
The UV-vis adsorption spectroscopy (UV-2550, Japan Shimadzu) of quinoline was measured, as shown in Fig. 1(a). A standard curve of the concentration of quinoline vs. absorbance at 312.4 nm of quinoline was created. The unknown quinoline concentration of samples through the absorbance and the curve was determined. TOC of samples was determined by total organic carbon (TOC) analyzer (TOC-VCPN, Japan Shimadzu). The concentration of H2O2 was calculated by titanium spectrophotometry method [30]. As reported in previous research [31], the concentration of hydroxyl radical (•OH) was determined by coumarin using a fluorescence spectrophotometer ((F-7100, Japan HITACHI)). Fluorescence scanning of coumarin and 7-hydroxycoumarin is shown in Fig. 1(b)–(c). The maximum excitation wavelength/emission wavelength of coumarin was at 277/392 nm (see Fig. 1(b)), the maximum excitation wavelength/emission wavelength of 7-hydroxycoumarin was at 321/452.6 nm (Fig. 1(c)). Degradation products were analyzed using gas chromatography-mass spectrometry (GC-MS, 7890b/5977b, Agilent USA). Thermostatic waterbath oscillator (SHA-C, Jintan Yichen Instrument Manufacturing Co., Ltd.) was used to control the reaction temperature. High speed centrifuge (TGL20M-II, Jintan Chengxi Chunlan Experimental Instrument Factory) was used in sample centrifugation.
3. Results and Discussion
3.1. Catalyst Screening
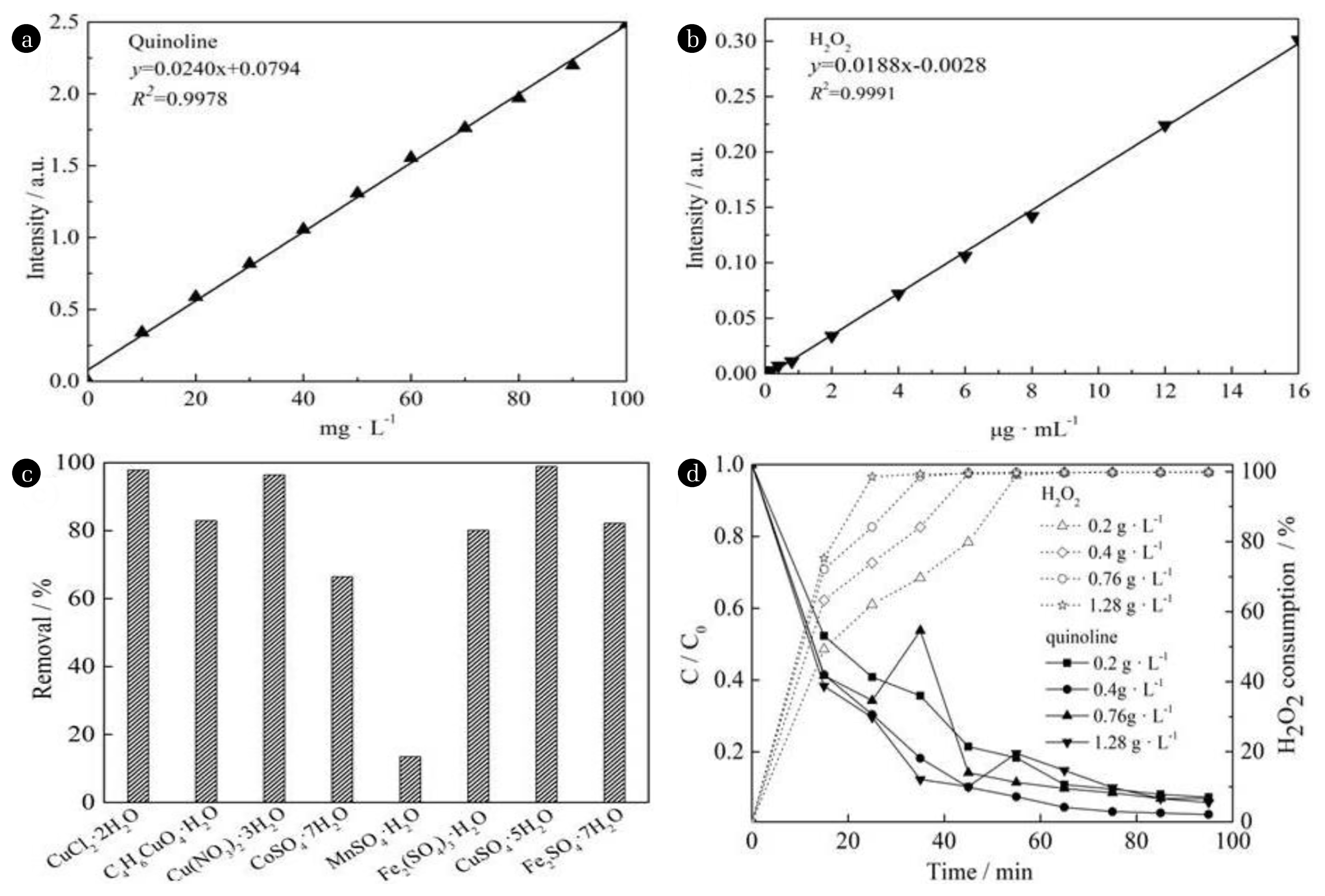
Fig. 2(a) and (b) is the standard curve of qunioline and H2O2, respectively. Fig. 2(c) is the effects of transition metal compounds on quinoline removal by Fenton-like system. As shown in Fig. 2(c), negligible difference was observed in the terms of the catalytic oxidation and degradation effects of copper sulfate, copper nitrate and copper chloride on quinoline. The removals were all above 95% upon 65 min reaction, and more than 99% H2O2 was consumed. However, the catalytic oxidation and degradation effect of copper acetate on quinoline was poor because of the only 82.9% removal. In addition, the catalytic oxidation and degradation performance of ferrous sulfate, ferric sulfate, cobalt sulfate and manganese sulfate were investigated, which indicated that manganese sulfate exhibited almost no catalytic oxidation and degradation ability to quinoline, the catalytic oxidation and degradation performance of cobalt sulfate was 30% lower than that of the above three copper compounds. Ferrous sulfate and ferric sulfate have certain ability of catalytic oxidation and degradation, but the water quality was muddy after the reaction, and the water quality after centrifugation was yellow, which was improved after 72 h precipitation. The results indicated that the three copper compounds (copper sulphate, nitric acid copper and copper chloride), with active component Cu2+, exhibited a higher catalytic oxidation degradation ability to quinoline, because chloride ion may have secondary pollution to water quality, and nitrate ion has a certain oxidation ability under acid condition, copper sulfate catalyst was selected to evaluate the ability of Fenton-like approach to the catalytic oxidation degradation.
3.2. Effect of Catalyst Dosage
The contribution of the respective catalyst adsorption, the volatilization of quinoline and the oxidation of separate H2O2 to the removal of quinoline wastewater was investigated before the study, and the results were 8.6, 8.9 and 8.5%, respectively after 2 h reaction. As shown in Fig. 2(d), more than 95% removal of three copper compounds to quinoline was achieved within 65 min, which indicated that the synergistic effect of catalyst and hydrogen peroxide was the key to the degradation of Fenton-like method to wastewater.
According to Fig. 2(d), the removal of quinoline showed much increment from 94.7% to 96.1% when the dosage of catalyst increased from 0.2 g·L−1 to 1.28 g·L−1 within the first 5 min of the reaction. However, the removals were all above 98.5% upon 65 min reaction, which demonstrated negligible little difference, indicating that the catalyst dosage exhibited less effect on the catalytic oxidation and degradation performance of quinoline along with the prolonged time. According to the highest removal of quinoline with 0.4 g·L−1 catalytic dosage in Fig. 2(d), 99.5% and 99.7% removals of quinoline were achieved after reaction of 65 and 95 min, respectively, and the removals of TOC were 87.2% and 88.7%, respectively. During the catalytic oxidation and degradation process of quinoline, the removal exhibited a remarkable reduction when 0.2 g L−1 catalyst dosage was applied upon 55 min reaction. At this time, the color of the solution became dark yellow which were not observed under the same condition when other catalyst dosages were used, indicating that the strong oxidizing •OH concentration wasn’t enough to accelerate oxidization and degradation of intermediate products under a lower catalyst dosage.
According to Fig. 2(d), in the early reaction time, the consumption of H2O2 was greatly affected by the catalyst dosage. After reacting for 15 min, the consumption rate of H2O2 increased from 49.4% to 75.3% along with the increment of the catalyst dosage. However, after reacting for 55 min, all of them reached more than 98.9%, which was slightly affected by the catalyst dosage. As shown in Fig. 2(d), within 15 min, more than 94.7% of the removal of quinoline could be achieved, which could be attributed to the low mass transfer resistance between Cu2+ and H2O2 under the Fenton-like reaction system, both could contact quickly and efficiently, leading to the accelerated transfer from H2O2 to strong oxidized •OH, enough strong oxidizing •OH could be produced in a short period of time which have an oxidizing reaction with quinoline quickly.
The removal of quinoline exhibited the best when the catalyst dosage was 0.4 g·L−1, which can be attributed to the equilibrium between the generation and disappear of •OH in the reaction system under the condition of the proper catalyst dosage. After the catalyst dosage continued to increase, excessive •OH might be produced. But they haven’t had enough time to participate in the organic oxidation reaction while part of them had a self-consumption reaction. On the other hand, the reaction of excessive •OH and H2O2 resulted in the generation of HOO· with a relatively poor oxidation capacity, and they may also reduce the catalytic capacity of the system.
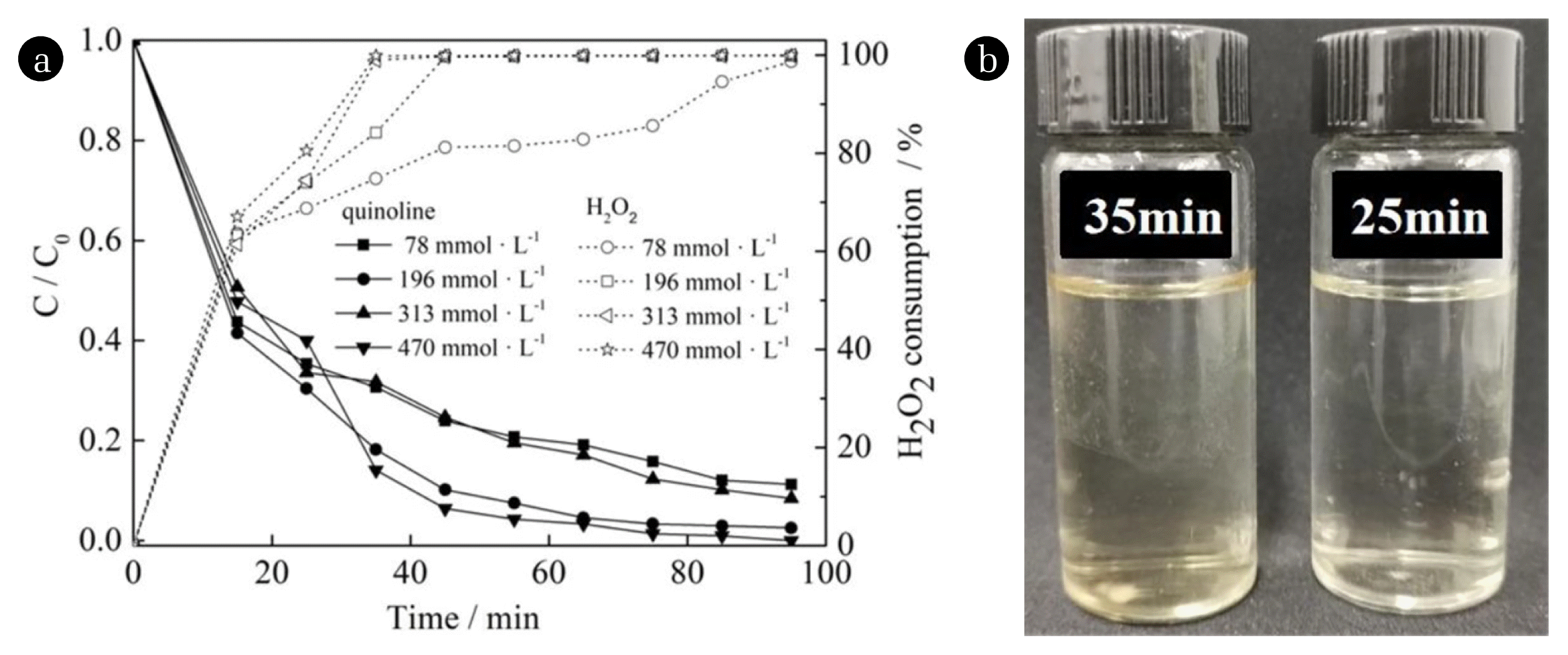
3.3. Effect of H2O2 Dosage
The theoretical demand equation of H2O2 was C9H7N + 23.5H2O29CO2 + 27H2O + NO2, where 196 mmol·L−1 was 10.7 times of the theoretical demand of H2O2. As shown in Fig. 3(a), H2O2 dosage didn’t show a great influence on the removal of quinoline, the difference in the removal was about 2% after 45 min of reaction. Relatively speaking, when the amount of H2O2 dosage was 196 mmol·L−1, the effect was slightly better. The removal of quinoline reached 95.8% after reacting for 15 min, and 99.5% after reacting for 65 min. This result indicated that a small amount of H2O2 showed a relatively strong catalytic oxidation and degradation ability under Fenton-like reaction system, which fully showed its superiority. When the dosage of H2O2 was 313 mmol·L−1 and 470 mmol·L−1, obvious pale yellow intermediate products were generated at 35 min and 25 min, respectively (as shown in Fig. 3(b)) and the color gradually disappeared after continuing reacting for a certain period of time.
3.4. Effect of Reaction Temperature
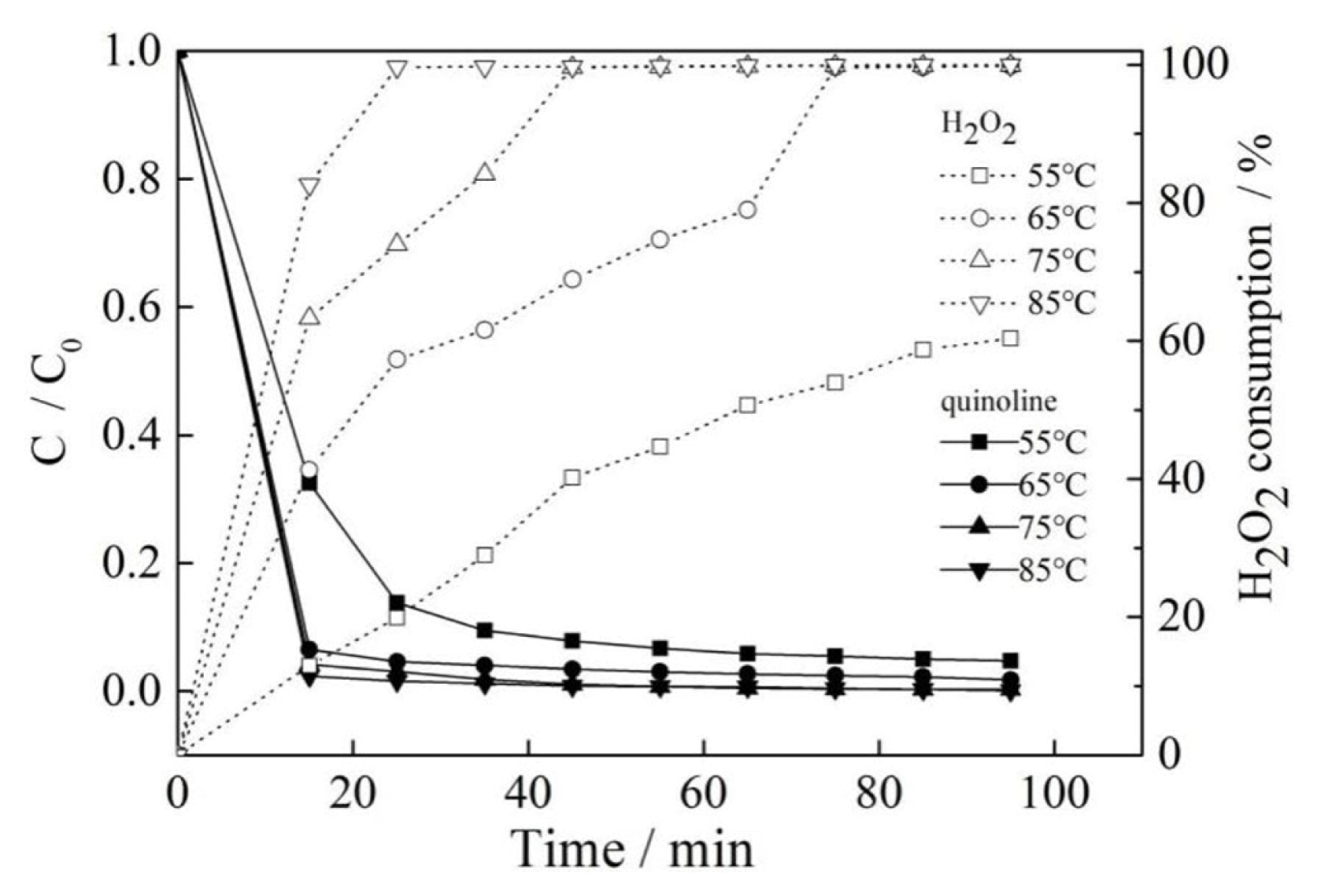
As shown in Fig. 4, after reacting for 15 min, when the reaction temperature increased from 55°C to 85°C, the removal of quinoline increased from 67.5% to 97.7%, and the consumption of H2O2 showed increment from 12.9% to 82.6%. That indicating that the reaction temperature had a great influence on the degradation effect of quinoline under the Fenton-like reaction system. When the reaction temperature was higher than 55°C, such as 65°C, 75°C and 85°C, no big difference of the removal of quinoline can be observed. The removal of quinoline increased by about 2% when the reaction temperature increased by every 10°C. However, the consumption of H2O2 still changes greatly accompanying with varied temperature. The consumption of H2O2 was 68.9% and 99.6%, respectively under 65°C and 75°C upon 45 min reaction with a difference of 30.7%, while there was almost no difference under the condition of respective 75°C and 85°C. So 75°C was a better reaction temperature. As shown in Fig. 4, both the removal of quinoline and the consumption of H2O2 increased along with the increment of reaction temperature. This means that it is difficult and not efficient for quinoline Fenton-like reaction under a lower reaction temperature, which could be attributed to the fact that it was not beneficial for the activation of H2O2 molecules to generate strong oxidizing •OH species and the activation of quinoline molecules at low reaction temperature. With the increase of the reaction temperature, the removal of quinoline increased significantly, but the curve of quinoline removal tended to be flat after exceeding 65°C, which indicated that there was not remarkable effect on the removal of quinoline along with the prolonged reaction time. The main reason would be that equilibrium of reaction for a certain time was achieved. Then, most of quinoline had been oxidized and the left was less contacting with •OH.
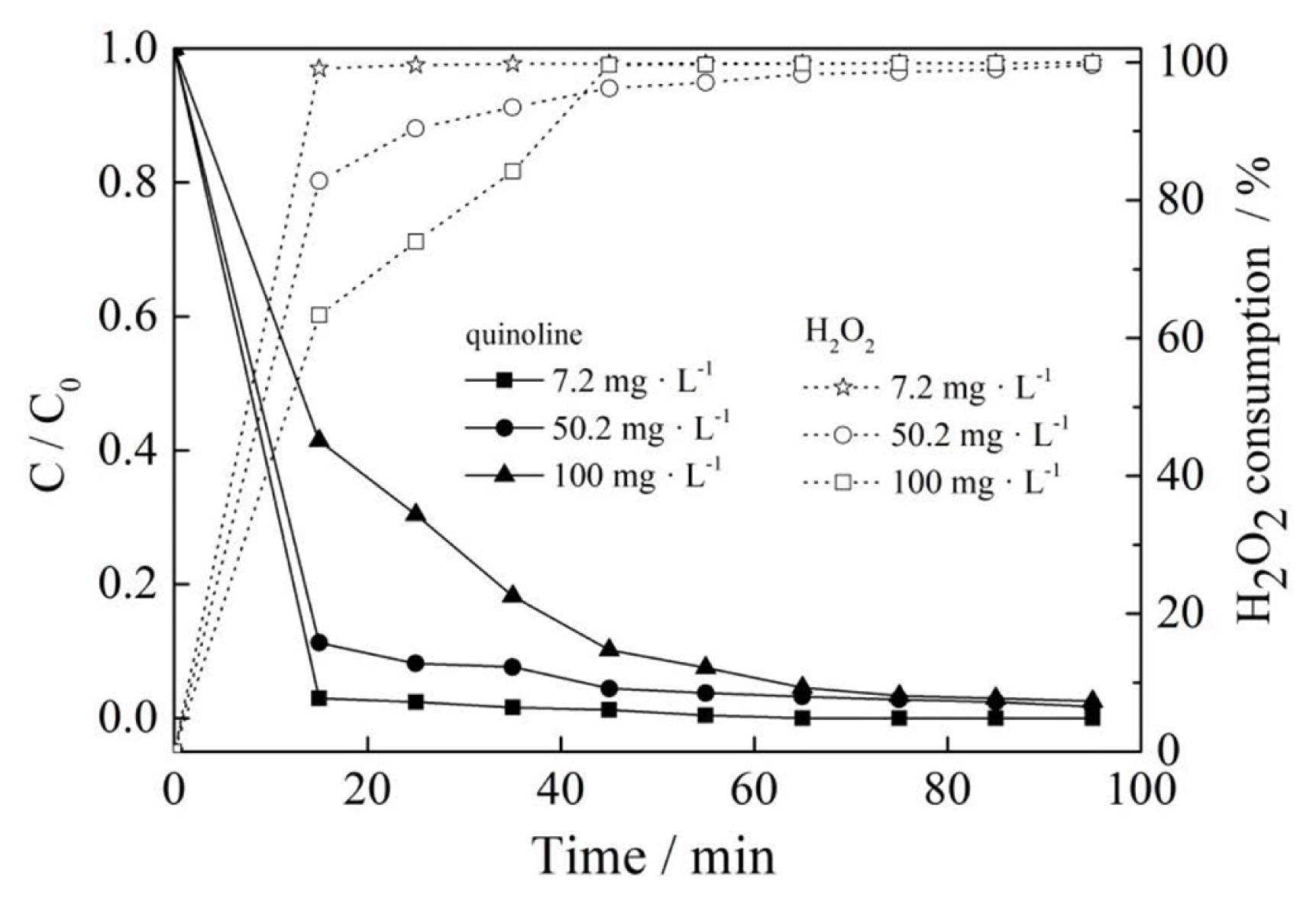
3.5. Effect of Initial Concentration of Quinoline
According to Fig. 5, the removal of quinoline decreased from 97.8% to 96.3% when the concentration of quinoline increased significantly from 7.2 mg·L−1 to 227.6 mg·L−1 upon 15 min reaction, and the removal of quinoline reduced from 100% to 99.5% upon 65 min reaction. The results demonstrated that no obvious difference for the removal of quinoline was achieved in the early stage or late stage. It could be noted that the change of quinoline concentration had a little influence on its removal in the range of quinoline concentration discussed above. However, as seen from Fig. 5, the H2O2 consumption decreased with the increase of the quinoline concentration. The concentration had a greater influence within 45 min. After the time, the consumption curve did not change significantly, which exceeded 97% after 55 min. The main reason for the decrease may be attributed to the increase of quinoline, which produced the intermediate products or bubbles forming a steric hindrance effect on preventing the full contact of Cu2+ from H2O2, thus leading to a slow decomposition of H2O2.
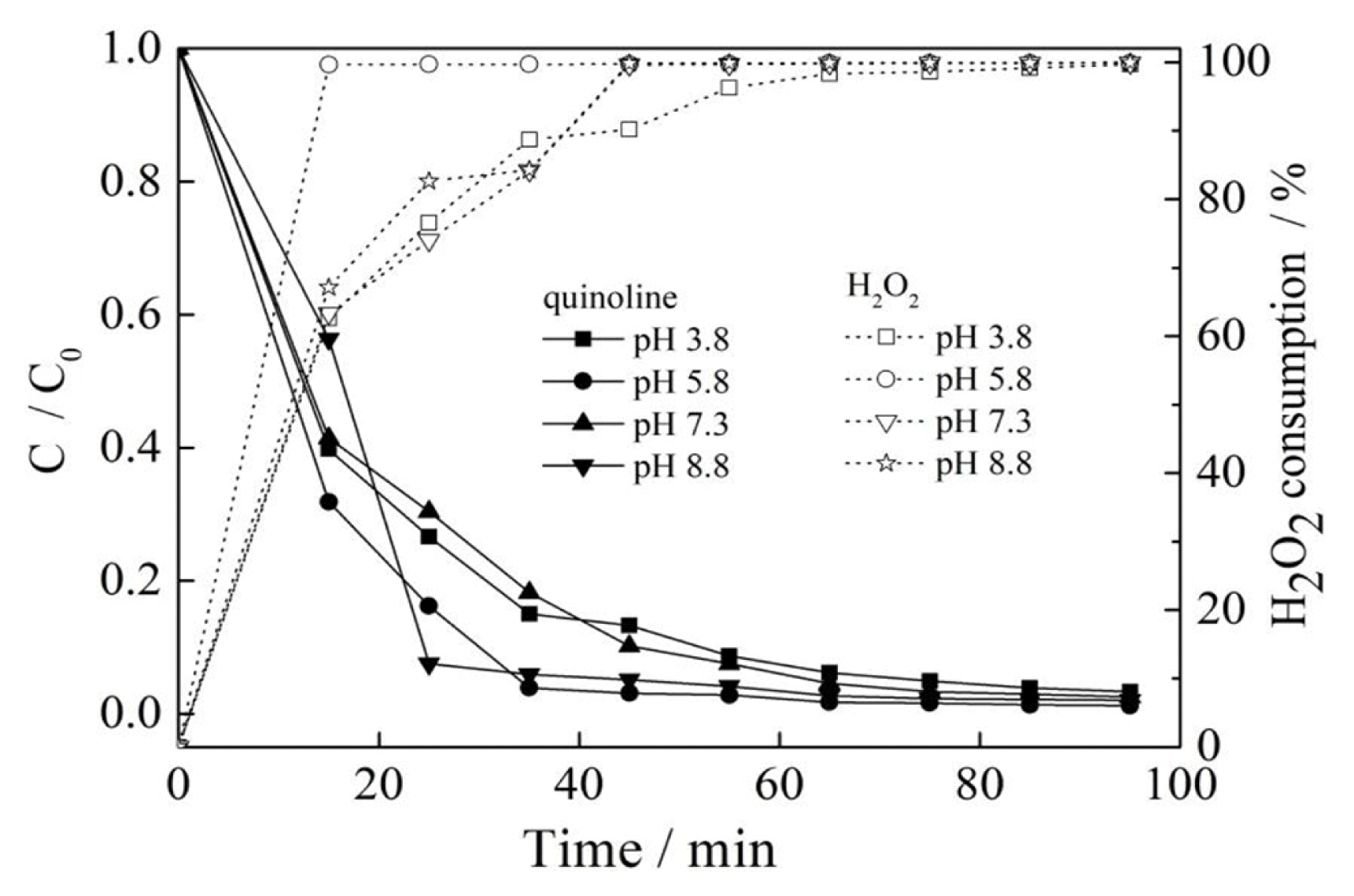
3.6. Effect of Initial pH
According to Fig. 6, when the pH was between 3.8 and 8.8, the removal of quinoline in Fenton-like reaction system was over 94.0%, it became higher than 99.0% upon 55 min reaction time. The results indicated that the pH exhibited almost no influence on the catalytic oxidation and degradation of quinoline within the researched pH range. As shown in Fig. 6, the consumption of H2O2 was greatly affected by the pH when the pH was in the range of 4.6 to 8.8. The consumption of H2O2 could be up to 99.6% when the pH was 5.8 upon 15 min reaction time. After that, the consumption curve tended to be flat, which might be ascribed to the complete consumption of H2O2. The consumptions of H2O2 were determined to be 62.6, 63.3 and 67.1% upon 15 min reaction time under the acidic condition with a pH of 3.8 and the alkaline condition with a pH of 7.3 and 8.8, respectively, which indicated that the decomposition of H2O2 was affected almost in the same way under weak acidic and alkaline conditions in a relatively short period of reaction time. The consumption of H2O2 under the three pH conditions tended to be consistent after 65 min reaction time, reaching about 99%, this demonstrated that the environment of reaction might be almost same gradually after some intermediate products were generated and disappeared as the reaction continued. It can be found that the pH had a little influence on the final degradation result when the initial pH was between 3.8 and 8.8 after a long reaction time. According to the removal effect of quinoline by pH, as Fenton method generally reacts under the condition of pH 2–4, the Fenton-like method using copper sulfate as catalyst had wider pH adaptability and obvious advantages compared with Fenton method.
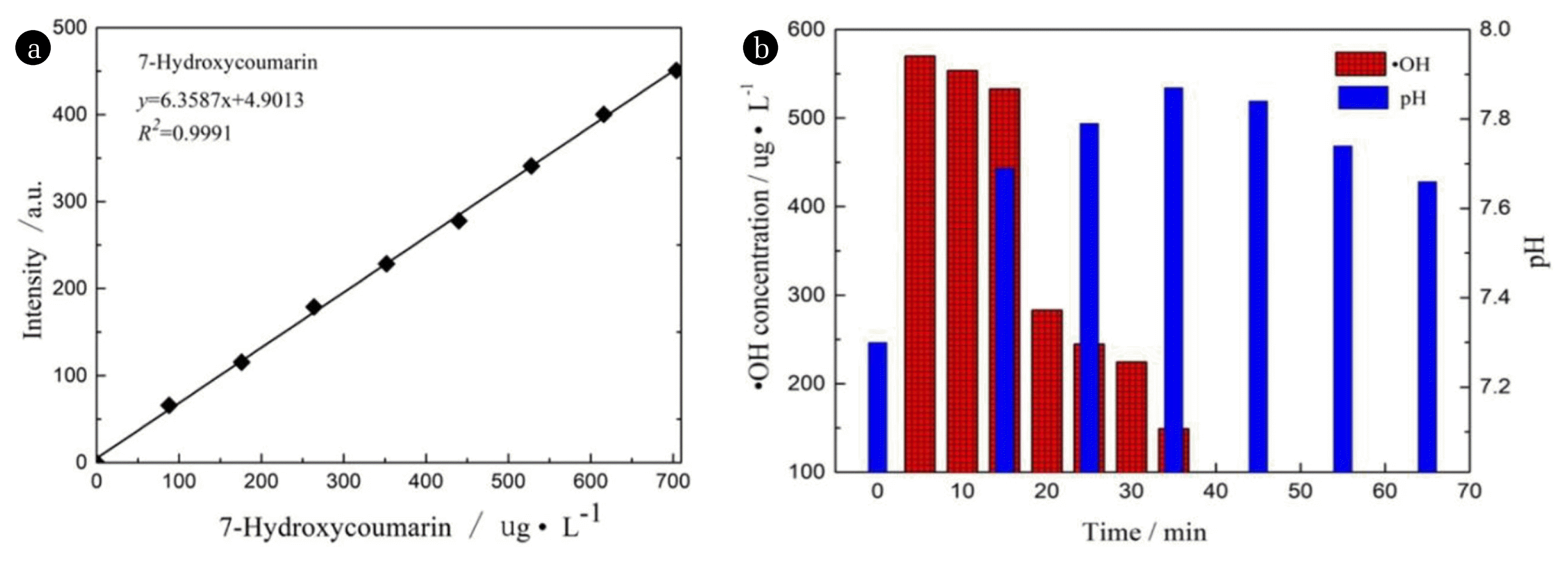
3.7. Hydroxyl Radical and pH Concentration Changes
The changes of pH and •OH concentration in the reaction process can provide basis for further investigation of degradation mechanism and pathway. In addition, with the progress of the reaction, whether the pH of the reaction system will exceed the pH adaptation range of the catalyst needs further analysis. Whether the •OH participate in the reaction is investigated by the change of the concentration of hydroxyl radicals. Fig. 7(a) is the standard curve of 7-hydroxycoumarin. Fig. 7(b) is the change of pH and •OH concentration during the reaction. As shown in Fig. 7(b), in the quinoline Fenton-like reaction system, the pH of the system first rose and then descended. Meanwhile the precipitation was observed during the reaction process that implies Cu(OH)2 precipitation was obtained under alkaline environment. Reducing Cu2+ active components and leading to decreased strong oxidizing •OH made them not enough to fully oxidize intermediates in a short time. Then the intermediates stayed for a longer time, and some acid intermediates were produced, leading to the decrease of the pH of the system. Fig. 7(b) demonstrated that the concentration of •OH gradually decreased along with prolonged reaction time in Fenton-like reaction system, implying that most •OH were participated in the reaction.
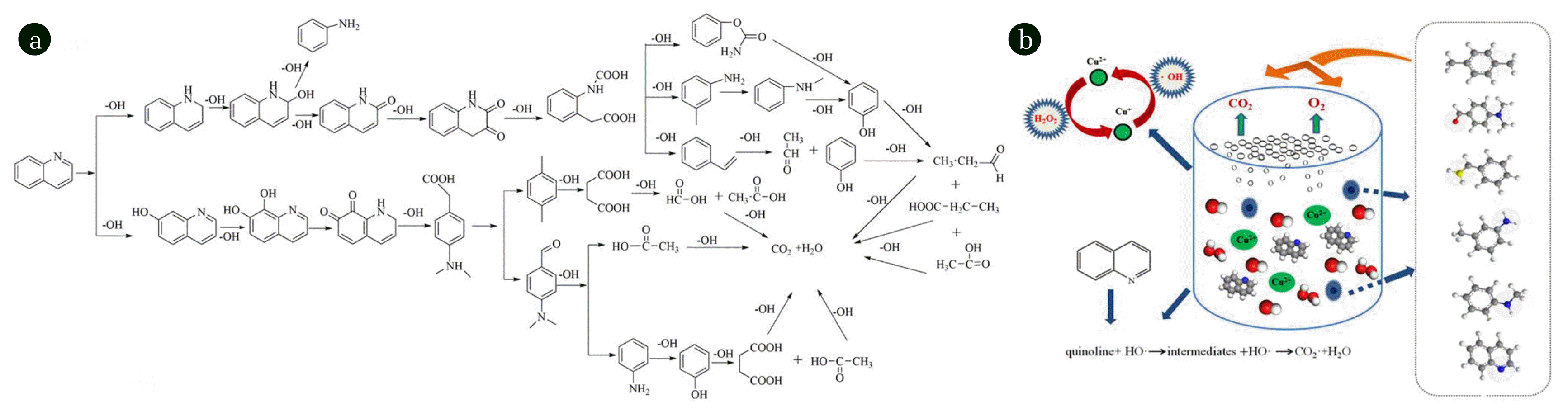
3.8. Degradation Pathways
The GC-MS analyses revealed the quinoline, p-xylene, aniline N-methyl, benzenamine 3-methyl-, carbamic acid phenyl ester-, styrene, benzaldehyde 4-(dimethylamino)-might form as intermediates. Their common characteristic is the disappearance of nitrogen-containing rings, which demonstrated the nitrogen-ring was firstly attacked by •OH during the reaction, opening the nitrogen- ring first and then further attacking the benzene ring. This is closely related to the high density of N-ring electron cloud in the quinoline structure, and the electrophilic addition reaction easily happened. The possible degradation pathways and scheme of quinoline are shown in Fig. 8(a) and (b). In conclusion, •OH plays a great role in the degradation of quinolone, the reaction between Cu2+/Cu+ takes place in the presence of H2O2 forming •OH and HO2·, the mechanism is illustrated as follows:
4. Conclusions
In earlier stage, increasing catalyst dosage was conducive to the increasing of reaction speed but in the final stage, it had no significant influence. H2O2 influence in degradation was not large, while the degradation increased with the increase of reaction temperature. Within the concentration range of this experiment, the change of quinoline concentration has little effect on degradation. The pH of the solution increased to the maximum and then decreased during the reaction. As a result, the removal efficiency of quinoline and TOC were 99.5% and 87.2%, respectively, when 0.4 g·L−1 catalyst, 196 mmol·L−1 H2O2 and 100 mg·L−1 quinoline was applied under 75°C reaction within 65 min. The Fenton-like method with copper sulfate as a catalyst took an obvious advantage over Fenton method for catalytic oxidative degradation of quinoline, which was able to react under raw solution pH 3.8–8.8. The degradation mechanisms showed that copper sulfate could generate •OH radicals through catalyzing H2O2 by Cu2+, which first attacked nitrogen ring, followed by benzene ring.