AbstractIn this study, the PDVF membranes were fabricated with a La-doped TiO2 (La-TiO2) photocatalytic coating in which La element was recovered from the waste fluorescent powder. The process of La element recovery was investigated under different conditions with different acid concentrations. La-TiO2 photocatalysts were facilely synthesized by the solvothermal method at 80°C followed by thermal sintering at 400°C. The La-TiO2 photocatalytic membrane fabrication was carried out by dip-coating the polyacrylic acid (PAA) plasma-grafted PVDF membrane into the suspension La-TiO2. The waste fluorescent powder was characterized by X-Ray Photoelectron Spectroscopy (XPS) and Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES) after dissolving in the aqua regia. The La-TiO2 photocatalysts were characterized by Fourier-Transform Infrared Spectroscopy (FTIR), X-ray diffraction (XRD), UV–Vis diffuse reflectance spectroscopy (DRS), and Scanning Electron Microscopy (SEM). The La-TiO2 coated PVDF membrane was used to study the filtration and visible light driven photocatalytic degradation of Reactive Blue 5 solution. The experimental results showed that La-TiO2 possesses high photocatalytic activity (efficiency of 100% after 6-h irradiation), and the best membrane (La-TiO2/PAA/PVDF) performance (efficiency of 87%) with the water contact angle of 11.4° was achieved. Thereby, it showed that the La-TiO2 photocatalytic membranes were successfully fabricated with antifouling, self-cleaning properties.
1. IntroductionIn water and wastewater treatments, membrane filtration is a relatively new method, which is an easy process and capable of separation in one or a few steps [1]. Various types of low-cost membranes usually used in this field include polymeric membranes [2] such as PTFE, PVDF, PES, and PAN, etc. However, due to these membranes’ intrinsic hydrophobicity, it is usually fouled by organic hydrophobic fouling in a long-term operation [3]. Membrane fouling is caused by the deposition and adsorption of foulants (particles, colloids, macromolecules such as proteins, polysaccharides, organic pollutants, microorganisms, inorganic salts) on the membrane surfaces or inside the pores [4–7]. This leads to a decreasing in the permeate flux, a change in selectivity and separability of the membrane, and affecting membrane life [3]. To solve this problem, researchers have been focusing heavily on developing a membrane with desirable properties such as antifouling and self-cleaning accompanied by the ability to oxidize pollutants [8]. Our group also studied this so-called photocatalytic membrane reactor from the beginning of development in this field [9]. However, at that moment, TiO2 photocatalyst was used as a coating on PAA grafted PVDF membrane, and UV light was utilized to activate the photocatalytic phenomenon for dye degradation [10]. Our other study focused on using ZnO nanoparticles as UV light-induced photocatalyst coated onto the PVDF membrane surface [11]. Both TiO2 and ZnO nanoparticles possess relatively high bandgap energy of above 3.2 eV, so their photocatalytic activity is only activated by UV light. Because the use of UV light costs more energy and is more challenging to build a UV light system than a visible light system, trends in recent research of the photocatalytic membrane are based on the visible-light-induced photocatalytic membrane [12]. There are two major approaches for the fabrication of photocatalytic membranes, including coating methods and blending methods [13, 14]. In the second method, usually due to the incompatible nature of organic polymer matrixes and inorganic nanoparticles, the accumulation will happen in the materials that have the same character. This leads to the failure of the microporous structure of the membrane and the low dispersion of photocatalyst nanoparticles on the membrane surfaces. Besides, this fabrication method may also lead to the covering of nanoparticles on the surface by thick layers of polymeric materials; so, it is hard for the light to contact the photocatalyst [15]. In the coating method, the surface photocatalyst does not strongly affect the membrane microporous structure, and it is fully exposed to light. However, this method’s problem is the adhesion stability of the nanoparticles to membrane surfaces to overcome the photocatalyst leaching under operating pressure. That was why stable chemical bonds, including covalent bonds and coordination complex bonds, were targeted to fabricate the photocatalytic membrane in this work. The covalent bond is the bond between one of the carbon atoms of PAA and a carbon atom of PVDF on the surface. The covalent bond formation between PAA and PVDF in this study is supported by the cold-plasma technique. In particular, the surface of the PVDF membrane is bombarded by Ar-plasma and some of the bonds are broken to form free radicals or centers that are positively or negatively charged. The activated membrane is then exposed to oxygen gas and oxygen-containing free radicals are formed such as −C-O·, −C-O-O· or less stable peroxides such as −C-O-OH, −C-O-O-C-. These free radicals or peroxides will act as the initiators for the free radical polymerization reactions to attach PAA chains to the PVDF membrane surface. The stable, coordinated complex bond is the bond between titanium ions, lanthanum ions on the covers of nanoparticles with the carboxyl functional groups of grafted PAA. Because multiple carboxyl functional groups are capable of forming strong complexes with metal ions, especially transition metals with d-orbitals in the outer shell. The oxygen atoms of the carboxyl group contain pairs of unbounded p-electrons and it will form a donor-acceptor complex with empty d-orbitals of the transition metal ion.
Studies showed that doping TiO2 with rare earth metals could expand the range of light absorption of the photocatalyst and improve the separation of electron and hole pairs [16]. There have been many studies on doping TiO2 by Lanthanum as in [17–20]; however, the bandgap energy of La-doped TiO2 is still high above 3.2 eV. Amazingly, in another study of NO gas oxidation with La-TiO2, the photocatalytic activity could be activated by visible light with a bandgap energy of about 2.75 eV [21]. In this work, we also focused on doping the TiO2 photocatalyst with La3+ ions; however, La element was not from purified chemicals but the acid leaching recovery of lanthanum from fluorescent waste powder. The composition of metals in waste fluorescent powder includes the primary metals such as Y, Sr, Ca, Ba, Al, Mg, La, in which La metal accounts for about 1% of the total dissolved metals in aqua regia. This study shows the resource value of waste fluorescent powder in potential applications if this type of waste is appropriately and efficiently recovered and reused.
2. Materials and Methods2.1. MaterialsThe waste fluorescent powder was provided by Zhongtai Resources Technology Co., Ltd in Taiwan. Nitric acid (HNO3, 65%), hydrochloric acid (HCl, 37%), sulfuric acid (H2SO4, 98%), and commercial titanium dioxide (P25) were purchased from Sigma Aldrich. Lanthanum ion standard solution, yttrium ion standard solution, multi-metal ion standard solution, isopropyl alcohol, reactive blue 5 dye (RB5), acrylic acid (AA) derived from Merck. Titanium butoxide was supplied by Acros, and argon gas was supplied by Mingyang Special Gas Co., Ltd in Taiwan. Pure chemicals were used without any further purification.
2.2. Methods2.2.1. Acid leaching for lanthanum recoveryThe process of recovery of lanthanum from the waste fluorescent powder was investigated under different conditions such as inorganic acid types (H2SO4, HCl, and HNO3), the concentrations of inorganic acid (1M, 3M, and 5M), ratios of waste fluorescent powder to the volume of used inorganic acid (0.1–1.0 g/mL), the combinations of various inorganic acids through several steps (5M H2SO4 → 5M HNO3 → 5M HCl, 5M HNO3 → 1M HNO3 → 5M H2SO4, 1M HNO3 → 5M HNO3 → 5M HCl, and 7M HCl → 3M HCl → 1M HCl → 5M HNO3), and leaching reaction times with inorganic acids. An acid solution containing lanthanum recovered from optimal conditions was eliminated of other metal ions by an insoluble salt precipitation method. The solution containing lanthanum was then precipitated by evaporation of aqueous solvents to form a solid salt and dried in an oven for 24 h at 70°C.
2.2.2. Preparation of La-doped TiO29 mL of titanium n-butoxide was added to a beaker containing 65 mL of isopropanol and gently stirred to form a clear solution (denoted as solution A). Solution B was prepared by dissolving the salt of recovered lanthanum in 9 mL of HCl solution (concentrated HCl: H2O = 1:5) to form solutions with La3+ ions concentrations of 1wt%, 2wt%, 3wt%, and 5wt%. Solution A and solution B were then mixed and stirred continuously for 2 h at room temperature. The mixture was then added to the 50 mL stainless steel autoclave and placed in the oven at 80°C for 12 h. The solids obtained after the solvothermal reaction were vacuum filtered out, dried at 60°C for 24 h, and then heated at 400°C for 2 h. The photocatalyst products were denoted as La1-TiO2, La2-TiO2, La3-TiO2, and La5-TiO2, which correspond to the lanthanum concentration in solution B.
2.2.3. Fabrication of La-doped TiO2 coating on PAA plasma-grafted PVDF membraneThe PVDF membrane was activated by radio-frequency plasma with Ar gas and then exposed to oxygen. The specific parameters are as follows: two electrodes in the reaction chamber spaced 5 cm apart, PVDF membrane samples with an area of 5 × 5 cm2 and pore size of 0.45 μm, Ar gas flow rate at 20 sscm, pressure in reaction chamber before Ar gas applied at 0.3 Torr, plasma power of 100W, plasma activation time of 120 s. The PAA was then grafted onto the activated PVDF membrane surface similar to that in the previous study [10]. Different amounts of La-doped TiO2 (1 g, 2 g, 3 g, and 4 g), which possesses the best photocatalytic performance, were added to a beaker containing 100 mL of deionized water, and the mixture was ultrasonically treated for 1 h. The PAA plasma-grafted PVDF membrane was then dipped into the above mixtures for 60 min and dried in the air, followed by dipping into deionized water for 30 minutes and dried in air naturally. These modified membranes were denoted as 1gLa-TiO2/PAA/PVDF, 2gLa-TiO2/PAA/PVDF, 3gLa-TiO2/PAA/PVDF, and 4gLa-TiO2/PAA/PVDF, respectively, which correspond to the amount of La-doped TiO2 used to prepare the coating mixtures.
2.2.4. Photocatalytic performance test of La-doped TiO2 photocatalystsThe dye solution used in this experiment was a solution of RB5 with a concentration of 50 mg/L. 100 mL of RB5 dye solution was added to a 250 mL beaker, and the beaker was placed on a magnetic stirrer set at a stirring speed of 300 rpm. Next, 0.2 g of La-doped photocatalyst was added to the above beaker, and the mixture in the beaker continued to be stirred in the dark for 60 min. Then, the reaction system was irradiated by halogen lamp’s light for 6 h at room temperature. Every 1-h interval, the solution in the reaction mixture was withdrawn in a small volume by the pipette and centrifuged at 6,000 rpm, and then analyzed for the concentration of RB5 in the solution at that time by UV-Vis spectroscopy at the wavelength of 595 nm.
2.2.5. Performance test of modified PVDF membranesThe modified PVDF membranes were characterized with a photocatalytic membrane reactor, as shown in Fig. 1. The RB5 dye solution used in the membrane performance test has a volume of 1 L with a concentration of 10 mg/L. The membrane was attached to a membrane module with two sides with an area of 10 × 10 cm2 on each side. The permeated liquid was seeped into the membrane by the pump pressure gradient, analyzed, and then circulated back into the feed solution. All membrane performance tests were performed at room temperature with stirring of a magnetic stirrer.
3. Results and Discussion3.1. Elemental Composition of Waste Fluorescent PowderThe waste fluorescent powder used in this study was derived from the recycling of waste lighting sources provided by Zhongtai Resources Technology Co., Ltd in Taiwan. To assist in utilizing the rare elements present in waste fluorescent powder as a doping element for TiO2 photocatalyst, this powder has been analyzed for its elemental composition by two different methods. The first method was X-ray photoelectron spectroscopy (XPS); the second method was Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES) after dissolving this powder in aqua regia. The XPS method allows analyzing the elemental composition of solid materials down to a depth of about 7.5 nm. The result of the XPS analysis was shown in Fig. 2, through which the composition of the chemical elements was determined as follows: phosphorus (P, 41.6%), oxygen (O, 30.2%), carbon (C, 15.5%), aluminum (Al, 6.8%), calcium (Ca, 2.1%), yttrium (Y, 2.0%), lanthanum (La, 0.5%), and others.
In the second metal composition determination, the method used aqua regia to detect heavy metals (NIEA S321.63B) according to the Taiwan Environmental Protection Agency regulation. The concentrations of metal ions in the solution after dissolving in aqua regia were determined by ICP-OES, as shown in Table S1. Accordingly, the percentage composition of metals in waste fluorescent powder was determined as follows: yttrium (Y, 48.6%), strontium (Sr, 19.4%), calcium (Ca, 18.6%), barium (Ba, 10.0 %), aluminum (Al, 1.9%), magnesium (Mg, 0.74%), lanthanum (La, 0.67%), manganese (Mn, 0.077%). There was a difference in the lanthanum content determined by the two methods above in terms of the nature of the method. The XPS method can identify all chemical elements on the surface layer of waste fluorescent powder, which was why we knew the content of non-metallic elements such as P, O, C, etc. While the ICP-OES method is only used to determine the concentration of certain metal ions contained in the solution. Therefore, the second method cannot determine the composition of all the elements contained in the waste fluorescent powder.
3.2. Lanthanum Recovery by Acid LeachingThe acid leaching process was investigated in individual acid solutions (HNO3, H2SO4, and HCl) at different concentrations (1M, 3M, and 5M) for 24 h with a sample weight of 15 g and volume of the acid solution of 45 mL. The data shown in Table S2 showed that when using H2SO4 solution, the dissolved La ion concentration was relatively low compared to that when using HNO3 and HCl solutions. This may be due to the fact that sulfate salt of lanthanum is less soluble in water, so although H2SO4 is a strong acid, it did not dissolve much La ions. This experimental result showed that La ions dissolved highest at the 5M concentration of both acid solutions (HNO3, HCl). The solubility of La ion was highest with 5M HNO3 acid solution (157.50 mg/L), which can be explained by the fact that HNO3 at high concentrations has oxidizing properties in addition to its strong acidity. The further investigation used 5M HNO3 solution at different volumes to dissolve samples with solid/liquid ratios such as 1:2, 1:3, 1:4, 1:5, 1:6, 1:7, 1:8, 1:9, and 1:10. Fig. 3(a) showed that when the acid content increased, the more dissolved La ions were; however, this relationship was not linear. The 1:5 ratio was chosen as the experimental condition for further investigation because it showed that La ion solubility was quite good, and the concentration of the solution after the dissolution was not too dilute. In the acid combination investigation with different concentrations, the combination of 1M HNO3 → 5M HNO3 → 5M HCl showed that the La ion solubility in 5 h was the highest concentration up to 362 mg/L. In this combination, the investigation of La ion dissolution at different time intervals (Fig. 3(b)) showed a significant increase in La ion concentration. However, this increase was only noticeable in the first 6 h, after which there was a very little increase (i.e., 599.5 mg/L at 6 h and 687 mg/L at 24 h). Therefore, to save reasonable process time, a 6-h dissolution interval by the combined procedure is sufficient for La element recovery for potential applications. This acid leaching combination was illustrated in Fig. S1. First, the waste fluorescent powder was added to 1M HNO3 solution to dissolve alkali metal and earth alkali metal ions. The remaining insoluble solids were then added to an oxidizing 5M HNO3 solution to remove organic contaminants such as oil and helped the next process have good contact between acid and La ions. Finally, the remaining insoluble solids dissolved La ions well in 5M HCl solution (687 mg/L after 24 h).
3.3. Preparation and Characterization of La-doped TiO2La-doped TiO2 photocatalysts with different elemental lanthanum content were synthesized, and their characteristic properties and photocatalytic activity were examined. The photocatalytic activity of the La-doped catalysts under halogen lamp irradiation was investigated first (discussed below). Accordingly, the samples with the best photocatalytic activity would be characterized by different physical and chemical analysis methods. In X-ray diffraction analysis (Fig. 4(a)), both TiO2 and La1-TiO2 samples were prepared by the same method under the same conditions (calcinated at 400°C). The characteristic peaks of the anatase crystal of TiO2 were observed in both samples at 25.3°, 37.8°, and 48.1°, respectively, which corresponded to the lattice planes of (101), (004), and (200). The FWHM (full width at half maximum) of most of the characteristic peaks was relatively high; this could be explained by the fact that at 400°C, the anatase crystal property of TiO2 was not highly crystalline. According to many studies [22, 23], these characteristic peaks will be sharper (high crystallinity) if the calcination temperature is between 500°C and 600°C. Especially at a high temperature of about 600°C, there will be a crystalline transformation from anatase to rutile. The pure TiO2 and La-doped TiO2 (La1-TiO2) were also analyzed by FTIR spectroscopy, and the results of this analysis were shown in Fig. 4(b). These two samples’ characteristic peaks were nearly identical for the wavenumbers such as 3,410 cm−1, 1,631 cm−1, and 526 cm−1. The peaks at 3,410 cm−1 correspond to the stretching vibration of the O-H bond of the hydroxyl group. The peaks at 1,631 cm−1 correspond to the Ti-OH bond’s bending vibration due to the adsorption of the water molecule on the TiO2. Moreover, the peaks at 526 cm−1 correspond to the vibration mode of the Ti-O-Ti bond. In terms of peak intensity, the La1-TiO2 sample had higher intensity levels related to the hydroxyl group, which indicated that this sample adsorbed more water molecules than pure TiO2. This could lead to a beneficial reduction in the water contact angle of the La1-TiO2 modified PVDF membrane, which reduces membrane fouling. To determine the photocatalyst’s bandgap energy, the UV-Vis DRS (diffuse reflectance spectroscopy) spectra of the samples were analyzed. Fig. 4(c) showed how to resolve this bandgap energy value using a Tauc plot. The bandgap energies of the TiO2 and La1-TiO2 samples were about 3.12 eV and 2.78 eV, respectively. Therefore, it could be seen that the doping of La elements into the TiO2 crystal lattice reduced the bandgap energy. With this bandgap energy value of La1-TiO2, it could be activated photocatalytic activity under visible light irradiation. Fig. 5 showed the morphology and particle size of TiO2 P25 and La1-TiO2 nanoparticles. The particle size of TiO2 P25 announced by the supplier was about 21 ± 5 nm, while the size of La1-TiO2 nanoparticles in Fig. 5(b) was slightly larger than that of P25. This may be due to the high temperature (400°C) calcination used to prepare the La1-TiO2 sample.
The photocatalytic activity of La-doped TiO2 and TiO2 P25 photocatalysts (0.2 g) was investigated based on a solution of RB5 dye with a concentration of 50 mg/L under the irradiation of a halogen lamp (Fig. 6(a)). The light of the halogen lamps used has a range of wavelengths between 320 nm and 1,100 nm. A small band of light in the ultraviolet region could activate the photocatalytic activity of TiO2 P25, which was why this catalyst was still able to cause degradation of RB5 dye with an efficiency of 27% after 6 h irradiation. La-doped photocatalysts such as La1-TiO2, La2-TiO2, La3-TiO2, and La5-TiO2 caused decomposition of RB5 dyes with the efficiencies of 100%, 89%, 78%, and 54%, respectively. Thus, it can be concluded that the higher the La content in the above-doped catalysts, the lower the ability to catalyze the degradation of RB5 dye. This seemed to be that a high lanthanum content would cause the formation of the La2O3 phase rather than the doping of TiO2 crystals with La elements. The bandgap energy value of La2O3 is about 5.20 eV, so it is not favorable for photocatalytic activity under halogen lamp irradiation. Additionally, these attached La2O3 crystals could reduce the exposure of photocatalysts to light. A cycle test was performed to verify the photocatalytic activity’s stability for RB5 degradation using La1-TiO2 photocatalyst, as shown in Fig. 6(b). In these experiments, ten cycles were run over 70 consecutive hours with no significant decrease in the photocatalytic efficiency of La1-TiO2 photocatalyst; the decomposition efficiency of RB5 dye for the first cycle was 96%, and that of the 10th cycle was 84%.
3.4. Characterization of La1-TiO2/PAA/PVDF MembranesBased on the results of the investigation of the properties of La-doped TiO2 photocatalysts in the previous section, La1-TiO2 with the best photocatalytic activity was chosen to coat on the PAA grafted PVDF membrane. Modification of PVDF membrane with PAA and La-doped TiO2 coatings would cause significant changes in membrane properties such as hydrophilicity, permeability, antifouling properties, etc. These properties are closely related to the water contact angle (WCA) of the membrane surface and the photocatalytic property caused by photocatalyst coating. If the modified PVDF membrane can degrade the foulants attached to the membrane surface and in the feed solution, it is unlikely that membrane fouling formation is possible [24, 25]. Furthermore, a low WCA significantly limits the interactions of a nonpolar nature between the PVDF membrane surface and organic foulants [26]. This also has another beneficial effect of enhancing the water flux of membranes. The results of the WCA test of the membranes were shown in Fig. 7. Accordingly, the PVDF membrane with a hydrophobicity possesses the highest WCA of 121.1°; the modified membranes such as 1gLa-TiO2/PAA/PVDF, 2gLa-TiO2/PAA/PVDF, 3gLa-TiO2/PAA/PVDF showed WCAs of 76.4°, 11.4°, and 9.2°, respectively. It could be seen that the grafted layer of PAA did not significantly change the hydrophilic properties of the PVDF membrane; the most influencing factor was the content of La-doped TiO2 on the membrane surface.
Fig. S2 showed the experiment results comparing the photocatalytic activity of La1-TiO2 nanoparticles and 2gLa-TiO2/PAA/PVDF photocatalytic membrane. At the initial time (from 30 min to 90 min), the photocatalytic activity of the La1-TiO2 nanoparticles was better than that of the photocatalytic membrane. Still, after 180 min of irradiation, both samples’ photocatalytic degradation efficiency was almost the same. This proved that the photocatalytic membrane was no less photocatalytic than the photo catalyst dispersed in an aqueous solution.
Modified membranes with different loading levels of La1-TiO2 were used in the experiment to degrade RB5 dyes under halogen lamp irradiation (Fig. 8(a)). The first two characters in the modified membrane’s name stand for the mass of La1-TiO2 nanoparticles dispersed into the dipping solutions. The result was not as expected: the higher the amount of loading of La1-TiO2, the higher the modified membrane’s photocatalytic activity. The 2gLa-TiO2/PAA/PVDF membrane induced the degradation of RB5 dyes with the highest efficiency of 87%. However, the difference in these degradation efficiencies was not too high for the three membranes, such as 2gLa-TiO2/PAA/PVDF, 3gLa-TiO2/PAA/PVDF, and 4gLa-TiO2/PAA/PVDF (87%, 83%, and 78%, respectively). This could be explained that when the loading amount of La1-TiO2 was higher than the optimum, the aggregation of La1-TiO2 nanoparticles would form large particles on the membrane surface. These large particles were susceptible to be leached out due to pressure gradients during the filtration process. The higher loading of La1-TiO2 does not increase the light exposure probability of the La1-TiO2 nanoparticles on the membrane surface because of the thick layer of photocatalysts. The pure water flux experiments were conducted for 300 minutes, and their results are illustrated in Fig. 8(b). The modified PVDF membrane’s water flux was improved compared to the pristine PVDF membrane and was optimized with the 2gLa-TiO2/PAA/PVDF membrane. The 1gLa-TiO2/PAA/PVDF membrane showed lower water flux due to its relatively high WCA (76.4°). However, for the other two membranes (3gLa-TiO2/PAA/PVDF and 4gLa-TiO2/PAA/PVDF), the water flux value was lower; it could be explained that with a high loading of La1-TiO2, these nanoparticles could cause blocking of membrane pores.
4. ConclusionsThe La element was successfully recovered from waste fluorescent powder by the acid leaching method. La element recovery conditions were the combination of 1M HNO3 → 5M HNO3 → 5M HCl for 6 h with the solid/liquid ratio of 1:5. The success of the doping of TiO2 with the La element was confirmed by the results of analyzes such as XRD, FTIR, SEM, UV-Vis DRS. The results also showed that the La-doped TiO2 photocatalyst could also be photocatalytic activated by visible light with a bandgap energy of 2.78eV. The La1-TiO2 had the highest photocatalytic activity (100% after 6 h of visible light irradiation), and this activity was almost unchanged over ten cycles. The visible light driven photocatalytic La-TiO2/PAA/PVDF membranes were successfully fabricated. The 2gLa-TiO2/PAA/PVDF membrane possesses the highest photocatalytic activity (87%) and had the most increased pure water flux with a WCA of 11.4°. This indicated that the La-doped TiO2 coating had significantly improved the hydrophilicity and caused the antifouling properties of the membrane.
NotesAuthor Contributions H.T.N. (Ph.D. student) conducted the photocatalytic related experiment, wrote Experiment and Discussion sections; S.-Y.G. (Master) conducted acid leaching recovery of lanthanum, Sheng-Jie You (Ph.D.) wrote abstract, introduction, and conclusion sections, Ya-Fen Wang (Ph.D.) revised the manuscript draft. References1. Madsen HT. Chapter 6 - Membrane Filtration in Water Treatment - Removal of Micropollutants. Søgaard EG, editorChemistry of Advanced Environmental Purification Processes of Water. Elsevier; 2014. p. 199–248.
2. Khulbe KC, Feng C, Matsuura T. The art of surface modification of synthetic polymeric membranes. J Appl Polym Sci. 2010;115:855–895.
3. Jhaveri JH, Murthy ZVP. A comprehensive review on anti-fouling nanocomposite membranes for pressure driven membrane separation processes. Desalination. 2016;379:137–154.
4. Nasrollahi N, Ghalamchi L, Vatanpour V, Khataee A. Photocatalytic-membrane technology: a critical review for membrane fouling mitigation. J Ind Eng Chem. 2021;93:101–116.
5. Han SJ, Park JS. Understanding Membrane Fouling in Electrically Driven Energy Conversion Devices. Energies. 2021;14.
6. Wan Y, Xie P, Wang Z, et al. Application of UV/chlorine pretreatment for controlling ultrafiltration (UF) membrane fouling caused by different natural organic fractions. Chemosphere. 2021;263:127993.
7. Zhang Z, Liu Y, Zhao B, Li J, Wang L, Ma C. Reduction of long-term irreversible membrane fouling: A comparison of integrated and separated processes of MIEX and UF. J Membr Sci. 2020;616:118567.
8. Zhao X, Zhang R, Liu Y, et al. Antifouling membrane surface construction: Chemistry plays a critical role. J Membr Sci. 2018;551:145–171.
9. Damodar RA, You SJ. Performance of an integrated membrane photocatalytic reactor for the removal of Reactive Black 5. Sep Purif Technol. 2010;71:44–49.
10. Cruz NKO, Semblante GU, Senoro DB, You SJ, Lu SC. Dye degradation and antifouling properties of polyvinylidene fluoride/titanium oxide membrane prepared by sol-gel method. J Taiwan Inst Chem Eng. 2014;45:192–201.
11. Laohaprapanon S, Vanderlipe AD, Doma BT, You SJ. Self-cleaning and antifouling properties of plasma-grafted poly(vinylidene fluoride) membrane coated with ZnO for water treatment. J Taiwan Inst Chem Eng. 2017;70:15–22.
12. Shi Y, Huang J, Zeng G, Cheng W, Hu J. Photocatalytic membrane in water purification: is it stepping closer to be driven by visible light? J Membr Sci. 2019;584:364–392.
13. Sakarkar S, Muthukumaran S, Jegatheesan V. Polyvinylidene Fluoride and Titanium Dioxide Ultrafiltration Photocatalytic Membrane: Fabrication, Morphology, and Its Application in Textile Wastewater Treatment. J Environ Sci. 2020;146:04020053.
14. Mohamad Said KA, Ismail AF, Karim ZA, Abdullah MS, Usman J, Raji YO. Innovation in membrane fabrication: Magnetic induced photocatalytic membrane. J Taiwan Inst Chem Eng. 2020;113:372–395.
15. Remanan S, Sharma M, Bose S, Das NC. Recent Advances in Preparation of Porous Polymeric Membranes by Unique Techniques and Mitigation of Fouling through Surface Modification. ChemistrySelect. 2018;3:609–633.
16. Yurtsever HA, Çiftçioğlu M. The effect of rare earth element doping on the microstructural evolution of sol-gel titania powders. J Alloys Compd. 2017;695:1336–1353.
17. Liqiang J, Xiaojun S, Baifu X, Baiqi W, Weimin C, Honggang F. The preparation and characterization of La doped TiO2 nanoparticles and their photocatalytic activity. J Solid State Chem. 2004;177:3375–3382.
18. Rostami M. Construction of La-doped TiO2@La-doped ZnO-B-doped reduced graphene oxide ternary nanocomposites for improved visible light photocatalytic activity. RSC Adv. 2017;7:43424–43431.
19. Zhu X, Pei L, Zhu R, Jiao Y, Tang R, Feng W. Preparation and characterization of Sn/La co-doped TiO2 nanomaterials and their phase transformation and photocatalytic activity. Sci Rep. 2018;8:1–14.
20. Wu HH, Deng LX, Wang SR, et al. The Preparation and Characterization of La Doped TiO2 Nanotubes and Their Photocatalytic Activity. J Dispersion Sci Technol. 2010;31:1311–1316.
21. Huang Y, Cao JJ, Kang F, You SJ, Chang CW, Wang YF. High selectivity of visible-light-driven la-doped TiO2 photocatalysts for NO removal. Aerosol Air Qual Res. 2017;17:2555–2565.
22. Lin J, Yu JC. An investigation on photocatalytic activities of mixed TiO2-rare earth oxides for the oxidation of acetone in air. Journal of Photochemistry and Photobiology A: Chemistry. 1998;116:63–67.
23. Abdullah H, Khan MR, Pudukudy M, Yaakob Z, Ismail NA. CeO2-TiO2 as a visible light active catalyst for the photoreduction of CO2 to methanol. Journal of Rare Earths. 2015;33:1155–1161.
24. Sidik DAB, Hairom NHH, Mohammad AW, et al. The potential control strategies of membrane fouling and performance in membrane photocatalytic reactor (MPR) for treating palm oil mill secondary effluent (POMSE). Chem Eng Res Des. 2020;162:12–27.
Fig. 3(a) Amounts of recovered La ion per 1 g of the sample versus initial solid/liquid ratios (sample/HNO3 5M), (b) La ion concentrations in the combination of 1M HNO3 → 5M HNO3 → 5M HCl versus times. 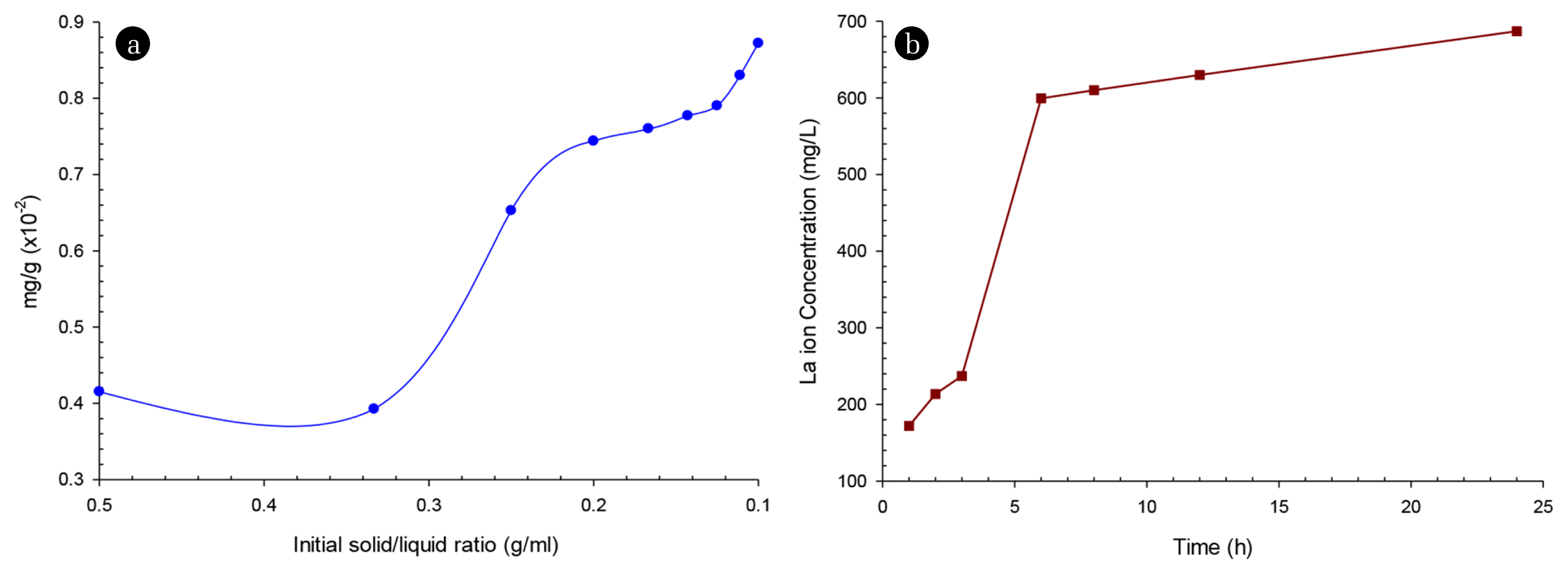
Fig. 4(a) XRD patterns, (b) FTIR spectroscopies, and (c) bandgap energy determination by Tauc plot of TiO2 and La1-TiO2 sample. 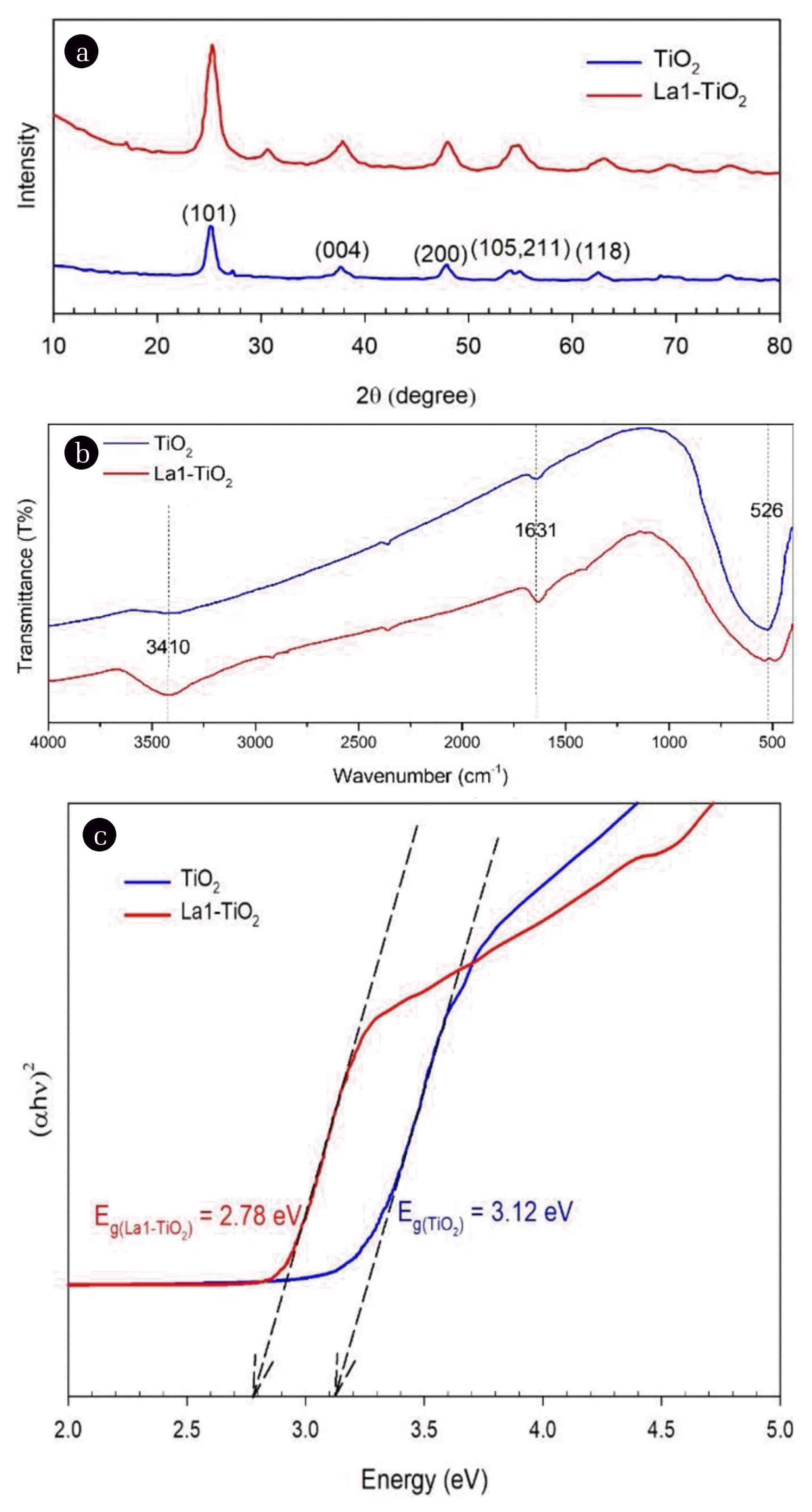
Fig. 6(a) Photocatalytic degradation efficiency and (b) Photocatalytic degradation cycle test of RB5 solution under irradiation of halogen lamp with La1-TiO2 sample. 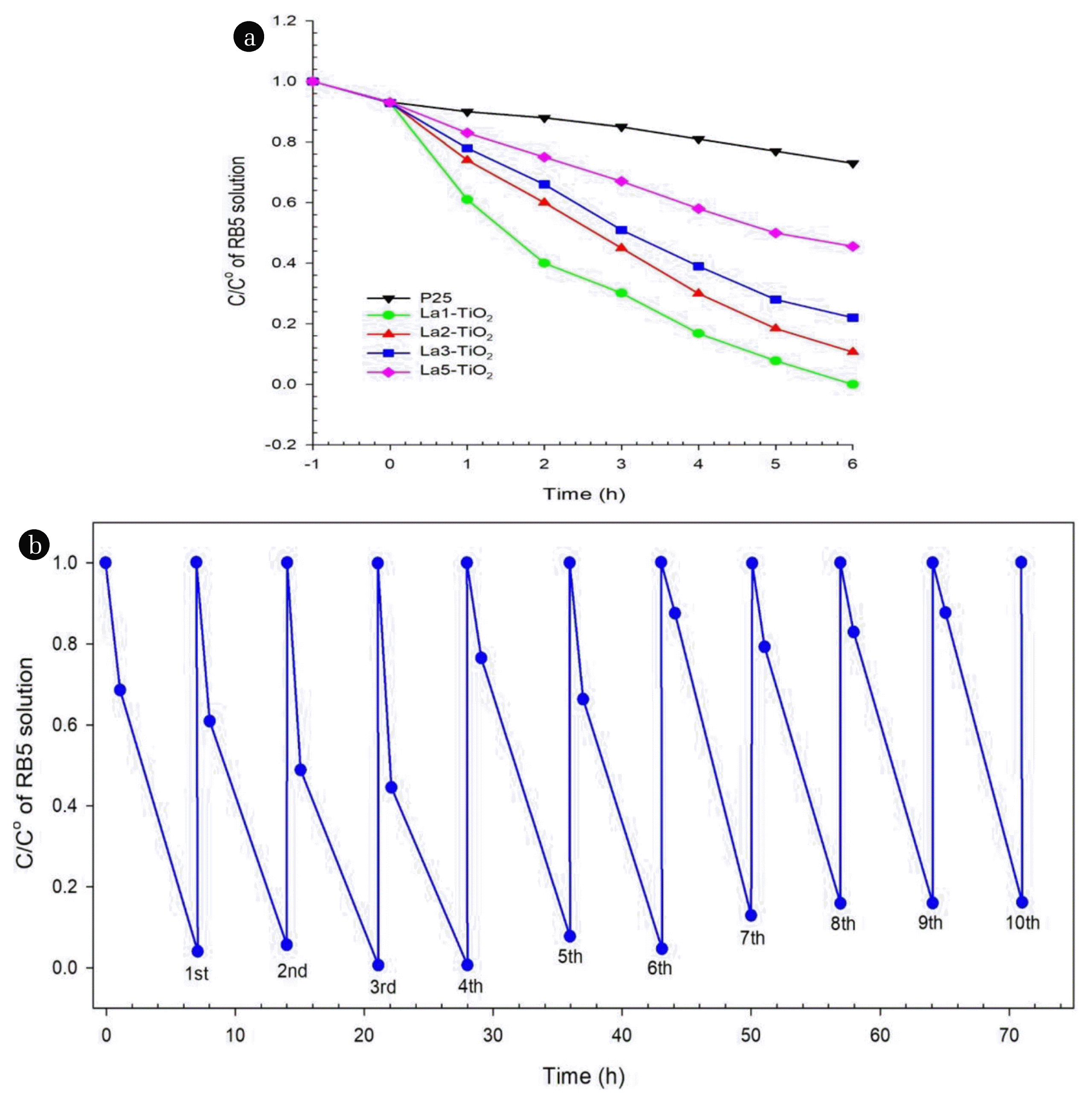
|
|
||||||||||||||||||||||||||||||||||||||||