AbstractIn this study, pretreated organic wastes such as waste paper cups, cardboard waste, and vegetable waste were screened for the growth and lipid production of oleaginous bacteria DS-7 (isolated from the dairy effluent scum). The pretreated vegetable waste was found to be the best feedstock for biomass and lipid production by the DS-7. Further, process parameters such as inoculation time, substrate concentration (w/v) (amount of pretreated vegetable waste), pH, and inoculum size were optimized using a multi-objective optimization technique to enhance the biomass and lipid productions. The optimization study successfully enhanced the biomass concentration (g/L) and lipid content (%) by 47.9% and 15.84%, respectively in comparison with the unoptimized state. The biomass and lipid productivities were 42% (1.449 g/L/d) and 51% (1.267 g/L/d) greater than unoptimized conditions. The characteristics of the biodiesel obtained from the valorization of vegetable waste were comparable to the standard. Thus, the vegetable waste can be utilized as a potential feedstock for microbial biodiesel production.
1. IntroductionThe increasing concentration of greenhouse gases in our atmosphere and the effect of global warming possess an immediate threat to the planet. CO2 constitutes the majority of the greenhouse gases 76.7% (v/v) present in our atmosphere and its concentration has been drastically increasing due to growing industrialization [1]. The extensive use of non-renewable energy sources (fossil fuel) is the major reason for the aggregation of these gases in our atmosphere. Moreover, continuous depletion of stored fossil fuels will soon cause the scarcity of fuel. In this regard, alternative fuels are getting the importance to replace fossil fuels.
As an alternative renewable fuel source, biofuels are gaining attention to deal with issues of fossil fuel usage including energy security, and environmental pollution [2]. Monoalkylesters of biodiesel is obtained through the transesterification process of lipids from animal and plant origin [3, 4]. Advantages like weather independence, no need for arable land, shorter replication time, able to use organic waste as a carbon source and easy scalability has made microbial oil more preferable than plant and animal oil. Less area requirement and waste to energy concepts are the major reasons for the increasing attention of microbial-derived oil [5–8]. Among the microbial sources, algae require exposure to sunlight for growth and a longer time for obtaining a considerable amount of biomass. These reasons challenged the scientific world to search for other alternative options to produce oil [5, 9].
Some bacteria from the actinomycetes group such as Streptomyces, Nocardia, Rhodococcus, Mycobacterium, Dietzia are capable to accumulate lipids and triacylglycerols (TAGs) under nitrogen-limiting conditions [5, 10–12]. The recent research reports on different microbial sources for lipid production have shown that Rhodococcus opacus PD630 grown on molasses [13], carob waste [14], detoxified sweet gum hydrolysate [15], cellobiose and pyrolysis oil [16] yielded lipids (% w/w, dry cell weight) of 87.0, 76.0, 28.6, 39.5 and 25.8%, respectively. Bacillus subtilis grown on cotton stalk hydrolysate produced biomass of 5.7 g/L with a lipid content of 39.8% [17]. Oleaginous bacteria Serratia sp. ISTD04 used municipal secondary sludge as growth media and produced biodiesel yield 11.21 ± 0.19% w/w [18]. Rhodococcus opacus used synthetic mineral media-based biomass gasification wastewater (BGWW) as a media for lipids accumulation and produced lipid yield 65.8% (w/w) of lipids [19]. Rhodococcus opacus utilized biodegrade anthracene as a carbon source to produce biodiesel. The initial concentration of anthracene used for biodiesel production was 50–500 mg L−1, and the maximum lipid accumulation was 70.6% (w/w) [20]. R. opacus grown on dairy wastewater produced lipid accumulation of 51% (w/w) [21]. Oleaginous microbes have also been studied for waste degradation and lipid production using various wastes including hydrolysate of food waste [22], food waste [23], domestic wastewater [17], activated sludge [24] and industrial wastewater [25]. Food waste contains more important nutrients including, carbohydrates, minerals, proteins, and lipids, which are suitable for the growth of oleaginous microbes. The vegetable and food wastes release is about 3.3 billion tons of CO2 every year and it contributes significantly to the carbon footprint of the environment [26, 27]. Thus, the cultivation of oleaginous bacteria on wastes can act as a pretreatment to reduce its organic content before being dumped or discharged into the environment and also helps in lipid production. However, search for new bacterial strain capable of valorizing low-cost and renewable carbon sources to obtain a better yield and lipid production is still necessary. In the present study, previously isolated high lipid-accumulating bacteria were utilized for the lipid accumulation using various wastes (vegetable waste, paper cup waste, cardboard, and dairy sludge waste). Finally, optimization of the process parameters using vegetable waste was carried out for further improvement in biomass and lipid production. The FAME (fatty acid methyl esters) of produced lipids was characterized for its usability as biodiesel. This is the first report on the valorization of waste paper cups, cardboard waste, and vegetable waste for the biodiesel production from the oleaginous bacteria (oleaginous bacteria DS-7 isolated from dairy effluent scum).
2. Materials and Methods2.1. Collection and Pretreatment of Organic WastesIn this study, three organic wastes namely waste paper cups, cardboard waste, and vegetable waste were evaluated as renewable substrates for the production of microbial lipids as biodiesel feedstocks. All organic wastes were collected from the NIT Rourkela campus, Rourkela, India. Waste paper cups and vegetable waste were collected from the tea shop and residential area, respectively. Air conditioner (AC) packing material was used as cardboard waste. The collected waste samples were washed thoroughly with distilled water to remove dirt and then dried before use. The cardboard and the paper cups were cut into small pieces (approximately 1 × 1 cm dimension) before the acid hydrolysis. The vegetable waste samples were dried thoroughly and ground to make powder.
Initially, 5 g of waste samples were soaked in different volumes of 5% H2SO4 (100, 200, and 300 mL). Changmai et al. [28] studied a two-step process as the oil has 45% of FFA content and used 5 wt% sulfuric acid which lessens the content of free fatty acid (FFA) of the oil [28]. Then the mixtures were autoclaved at 121°C for 2 h. After hydrolysis, the samples were filtered and centrifuged to obtain the hydrolysate product. The pH of the obtained hydrolysate products was adjusted in the range of 7–7.5 by using 5 M NaOH and 1 M HCI solution. The hydrolysates were then analyzed for reducing sugar content.
2.2. Preparation of Seed Culture of Oleaginous Bacteria DS-7The oleaginous bacterium DS-7, previously isolated from the dairy scum [8] was used in this study for the utilization of organic wastes for the lipid accumulation for biodiesel production. Seed cultures were prepared by adding a loop full of inoculum grown for 24 h in nutrient agar media plate to 50 mL of culture medium containing minimal salt medium (MSM) in a 100 mL Erlenmeyer flask. The MSM composed of: glucose; 10 g/L, MgCl2.6H2O; 0.2 g/L, Na2SO4; 0.2 g/L, KNO3; 0.2 g/L, ZnCl2; 0.01 g/L, FeCl3.6H2O; 0.01 g/L, MnCl2.4H2O; 0.01 g/L, CaCl2.2H2O; 0.01 g/L. The culture was incubated at 30°C and 70 rpm for 48 h.
2.3. Screening of Pretreated Organic Wastes for the Biomass Growth and Lipid ProductionThe bacterium DS-7 was grown on the organic waste hydrolysate for its biomass and lipid production. The experiments were performed in 250 mL Erlenmeyer flasks containing 100 mL of MSM containing organic waste hydrolysate as a carbon source in place of glucose. The final sugar concentrations (reducing sugar) in each flask were 10 g/L. The flasks were inoculated with ~10% of seed culture and then incubated in an orbital shaker at 30°C and 150 rpm for 96 h as reported earlier [8] to study the biomass production and total lipid accumulation by DS-7. A control set of experiments (without inoculation) with each waste hydrolysate were also incubated with test experiments. All the experiments were performed in duplicates. The biomass production and total lipid accumulation were analyzed after 96 h.
2.4. Multi-objective Optimization of Process Parameters Using Box Behnken ModelFour process parameters namely, incubation time, substrate concentration (vegetable waste hydrolysate), initial pH, and inoculum size were optimized by the Response Surface Methodology using Box Behnken design of experiments. The Box Behnken design represents the process parameters to be optimized in three coded values as −1, 0, and 1 [29]. The design fits the output data into the quadratic model to form the regression equation. The coded values along with the true values of process parameters optimized are presented in Table 2. A total of 27 sets of experiments were performed as per the Box Behnken designed of experiments using Minitab 17 in Table 1. All the experiments were performed in 250 mL Erlenmeyer’s flask containing 100 mL of media. The incubation temperature and shaking speed were fixed at 30°C and 150 rpm, respectively. The responses measured were biomass production and lipid content.
2.5. Model ValidationIn this study, we desired to maximize the amount of total biomass and lipid content produced by DS-7 using vegetable waste with the help of Minitab 17 software. The validity test was conducted using the optimum values of the selected process parameters obtained after optimization.
2.6. Analytical Methods2.6.1. Estimation of biomass, lipid content, and lipid productivityBiomass production was estimated using the cell dry weight method as described by [8]. The accumulated lipids were extracted according to the modified Bligh & Dyer (1959) methodology. The lipid content, biomass productivity and lipid productivity for different substrates were calculated as per the Eq. (1), Eq. (2), and Eq. (3), respectively [8]:
2.6.2. Reducing sugar utilizationTo estimate the reducing sugar, the samples were centrifuged at 10,000 rpm for 15 min to separate biomass. The supernatant was used for reducing sugar estimation by the dinitrosalicylic acid (DNS) method [21].
2.6.3. Characterization of FAME and lipid analysisThe lipid produced after optimization was converted into biodiesel through an acid-catalyzed transesterification process. Biodiesel was produced from lipid by the addition of 3 mL of methanol and 3 mL of H2SO4 (5% v/v) to the dried lipid. The mixture was incubated at 70°C for 3 h and was allowed to cool down to room temperature. Distilled water and hexane, each 3 mL was added and mixed well before centrifugation at 10,000 rpm for 5 min followed by recovery of the upper layer in a fresh tube and added with 2 mL of Milli Q water. The mixture was mixed well and centrifuged for 5 min at 10,000 rpm and the upper layer was stored for future analysis at 4°C. Thus, the biodiesel obtained was subjected to GC-MS FID (Gas chromatography – Mass Spectroscopy Flame Ionization Detector with HP-5 methyl siloxane capillary column with a specification of 30 m × 320 mm × 0.15 mm column HP, Palo Alto, USA). The properties of the obtained biodiesel like the degree of unsaturation (DU), kinematic viscosity, pour point (PP), cloud point (CP), iodine value (IV), saponification value (SV), and cetane number (CN) were calculated using the mathematical formula as shown in Table S1 [30].
3. Results and Discussion3.1. Pretreatment of Waste SamplesThe collected wastes (paper cup waste, cardboard waste, and vegetable waste) were subjected to acid and heat treatment to breakdown the complex sugar molecules present in the lignocellulosic wastes into its constituent’s simple sugars. The reducing sugar of the hydrolyzed wastes was measured and is shown in Table S2. The results showed that 100 mL of 5% H2SO4 yields higher reducing sugar content than 200 or 300 mL H2SO4 (5%) in all cases except vegetable waste. Among all waste samples tested, vegetable waste had the highest reducing sugar yield (0.352 g/g), followed by cardboard waste (0.238 g/g) and paper cup waste (0.206 g/g). However, the yield of reducing sugar obtained from vegetable wastes in this study was relatively less compared to the yield of reducing sugar obtained from cauliflower waste (0.48–0.62 g/g) by pretreating using 4% (v/v) phosphoric acid followed by heat treatment at 121°C for 1 h [31]. Tadmourt et al. [32] has reported that paper waste when acid hydrolyzed at 96.31°C for 20.64 min with 1% H2SO4 (v/v), yielded 79.65% (w/v) of total sugars. The variations in reducing sugar yields could be due to the use of different waste compositions and pretreatment conditions [32]. Khattab et al. (2020) pretreated sugar beet wastes with different concentrations (0.5–3%) of H2SO4 followed by heat treatment using autoclaving and found that cellulose contents were completely hydrolyzed at all acid treatments. Similarly, the volume of acid (100 mL, 200 mL, and 300 mL) treatment did not affect the sugar yields significantly in this study. Therefore, the hydrolysis was done with 100 mL H2SO4 (5%) in all cases.
3.2. Biomass and Lipid Production by DS-7 Bacteria Using Different WastesThe biomass and lipid production by DS-7 strain was estimated in different waste hydrolysate media. It is observed from Fig. 1 that the maximum total biomass of 6.35 g/L was produced in cardboard waste hydrolysate followed by vegetable waste (3.96 g/L) and paper cup waste hydrolysates (3.29 g/L). Contrary to this, the maximum lipid accumulation was obtained in paper cup waste (78.11%) followed by vegetable waste (73.59%) and cardboard waste hydrolysate (40.94%) (Fig. 2). These results are in agreement with the previous report that biomass production and lipid accumulation are competitive [8]. The highest lipid productivity and yield were observed in vegetable waste hydrolysate (0.62 g/L/d and 0.015 g/g, respectively) followed by cardboard and paper cup waste hydrolysates (Table S3). The lipid contents obtained by the enzymatic hydrolysis of paper wastes reported by [33] were significantly lower (52.5%) compared to the lipid contents obtained in our study using acid treatment (78.1%). However, the lipid yields obtained using paper wastes hydrolysates obtained using enzymatic hydrolysis [33] and acid treatment were almost similar. Chatterjee and Mohan [34] have reported that vegetable waste hydrolysate by acid treatment yielded a very high concentration of reducing sugar (472.3 g/L) but the lipid yield of yeast Cryptococcus curvatus was only 28.3% [34]. In comparison to that vegetable waste hydrolysate in the present study yielded 73.5% lipid content, which was higher than other waste hydrolysates tested in the study as well. Therefore, further, attempts were made to improve both biomass production and lipid accumulation using optimization techniques in vegetable waste hydrolysate media.
3.3. Optimization of Selected Process Parameters to Maximize Total Biomass and Lipid ContentThe main objective of performing the optimization process was to maximize the total biomass and lipid content using the Box Behnken design of experiments [35]. The process parameters such as incubation time (72–120 h), amount of waste added (3.5–15% w/v), pH (6–8), and inoculum size (5–15%) were optimized for maximizing biomass production and lipid accumulation. A total of 27 sets of experiments were performed (in duplicates) as per the Box Behnken design and results (biomass production and lipid content) are presented in Table 1. It can be observed from Table 1 that total cell biomass was varied in the range of 1.25–10.61 g/L and the lipid content was varied in the range of 12.66–93.05% (w/w). Such large variations indicate that the selected process parameters had a significant effect on the biomass and lipid production by DS-7 microbe. When lipid content was as high as 93.05% (w/w) then the centrifugation method was used, so that bacterial get settled and attached to the wall. Multiple response surface regression analysis was performed to study the interaction between the process parameters (incubation time, amount of waste, pH, and inoculum size) and measured responses (total biomass production (g/L) and lipid content (% w/w)). For each response, coefficients of the regression equation were calculated and fitted into a second-order polynomial. The final regression equations for each response are given in Eq. (4) and Eq. (5).
The optimal values for which both total biomass and lipid content can be maximized at the same time were obtained by a multi-objective optimization curve. The optimal values thus obtained were: 120 h incubation time, 3.5% (w/v) of the amount of food waste hydrolysate, pH 8, and 6.31% (v/v) inoculum size. A confirmatory experiment was performed at these optimal values to validate the results.
The regression coefficients of each parameter along with their t value and p-value are shown in Table 2. The parameters having a lower p-value (p < 0.05) represent that the corresponding response is strongly dependent on that factor. From Table 2, it can be seen that amount of food waste hydrolysate added (% w/v) to the culture medium is having a significant effect on both biomass production (g/L) (square term, p = 0.021) and lipid content (% w/w) (both linear term, p = 0.006 and square term, p = 0.023). It is obvious as the amount of food waste hydrolysate added, which acts as a carbon source is essential for the growth of biomass and synthesis of lipid. In the optimization study, the amount of food waste hydrolysate was varied between 3.5 and 15% w/v, which correspond to approximately 10 and 40 g/L of reducing sugars, respectively. The optimum value of food waste hydrolysate obtained is 3.5% (w/v) i.e., 10 g/L, which was enough for microbial growth and lipid production in the studied conditions. Similarly, Rhodosporidium toruloides showed a non-linear increase in biomass and lipid production with an increase in substrate concentrations i.e., glucose and molasses (10–250 g/L) [36]. Among the other three parameters, incubation time significantly influenced the lipid content (p = 0.032) only; while pH significantly influenced the biomass production (p < 0.05 for both linear and interaction term). Inoculum size did not show any significant effect on either lipid or biomass production by DS-7. Similar observations have been reported by Juanssilfero et al. [37], where authors evaluated the effects of three different inoculums volume (0.5–0.6 g/L, 4.0–7.0 g/L, and 10.0–14.0 g/L) to ferment yeast Lipomyces starkey glucose as substrate [37]. The 10% (v/v) optimum inoculum concentration was used for lipid production using oleaginous yeast (Trichosporon fermentans and Trichosporon cutaneum) [38]. In this experimental work, they observed that fewer inoculums volume (< 5%) has prolonged the lag phase and took a long time to utilize the substrate whereas the higher inoculums volume is suitable for the fast utilization of substrate.
The results of the Box Behnken design thus produced were further analyzed using analysis of variance (ANOVA) (Table 3). From Table 3, it can be observed that the F value for both the models i.e., for total biomass production and lipid content were 4.07 and 3.94, respectively, and p-values were 0.010 and 0.011, respectively. This signifies that the predicted models are valid and can satisfactorily explain the experimental data. The goodness of fit was determined by R2 values, which were found to be 82.6% for biomass production and 82.12% for lipid content. This also represents that the model is fairly attributed to the tested parameters.
Three-dimensional surface diagrams were plotted to visualize and study the optimum values for each process parameter against the desired target response. The three-dimensional surface graphs were plotted by taking at a time two different process parameters in two different axes (X and Y) and the target response in the Z-axis keeping rest of the parameters at their center point values. Fig. 3 shows the three-dimensional surface plots for total biomass production. The interaction between the factors is not significant for total biomass production as it is observed from the surface plot as well as in Table 2. Fig. 3(f) shows that an increase in pH improves biomass production, whereas the change in inoculum size did not have any effect. It is also observed from Fig. 3(c) that an increase in incubation time increases biomass production, whereas an increase in inoculum size initially increases biomass production by 10%, and a further increase in inoculum size decreases the production. A low inoculum size could result in a long lag phase. The amount of waste added (% w/v) was not a significantly effective parameter for the biomass production since it was observed that the lower and higher level of this parameter was showing increased biomass response than the intermediate level (Fig. 3(a), (d), (e)). In Fig. 3(a), and 3(c) incubation time (h) showed higher biomass yield when grown for 120 h and it was observed that the increase in time increases biomass yield. In Fig. 3(b), there were no observable effects of incubation time but higher pH favors higher biomass yield same as observed in Fig. 3(d) and 3(f).
Fig. 4 shows the surface plots for lipid content. The overall interaction terms for lipid content were found to be significant from the p-value (0.039) in Table 3. Specifically, the interactions between incubation time with the amount of waste added, and pH with the amount of waste added was found to be significant from p-values in Table 2. It was observed from Fig. 4(e) that a lower to mid-value of the amount of waste is favorable for the higher lipid content, increasing the amount of waste further decreases the lipid content. The initial increase in inoculum size (from 5 to 10%) favored lipid production but further increase (to 15%) decreased production. Fig. 4(f) suggest that mid-value of pH and inoculum size produced maximum lipid yield whereas in Fig. 4(b) and 4(c) longer incubation time corresponds to higher lipid yield with pH having no significant impact in combination with incubation time in incase of Fig. 4(b) and higher inoculum size is suitable for higher lipid production in combination with incubation time in case of Fig. 4(c). Fig. 4(d) shows that a low level amount of waste and a high level of pH combination favors lipid production. Fig. 4(a) shows that 120 h incubation time and mid-level of waste (10% w/v) showed higher lipid production.
Multi-objective optimization was performed to obtain the optimal values for various process parameters such as incubation time (h), amount of waste used (% w/v), pH, and inoculum size (%) to maximize both the responses total biomass and total lipid content. Experiments were carried out at optimal values obtained from the model. The experiment results showed that the total biomass obtained was 7.602 g/L against the predicted value of 9.15 g/L whereas; total lipid content was found to be 87.45% (w/w) against the predicted value of 80.88% (w/w). The optimized biomass and lipid content were improved by 47.90% and 15.84%, respectively. Also, the optimized biomass and lipid productivities were greater than the un-optimized biomass and lipid productivity by 42% and 51%, respectively. Before optimization the biomass and lipid content were are 0.84 and 0.61 g/L/d and after optimization the biomass and lipid content were 1.45 and 1.27 g/L/d. For the acidogenic fermentation of food waste and vegetable waste pH range 6 to 7 are more specific and for the fermentation of sludge or wastewater, the pH ranges from 8 to 10 are more specific [39]. The optimum pH of 6.5 was reported for biodiesel production from Cryptococcus curvatus using vegetable waste hydrolysate [33]. Similarly, optimal pH 6 and temperature 28°C, was used for lipid production from yeast Yarrowia lipolytica by using synthetic and food waste-derived volatile fatty acids [40]. The yeast Y. lipolytica under optimum conditions yielded the total lipid contents of 31.62 ± 0.91%, 28.36 ± 0.74%, and 28.91 ± 0.66%, respectively of the dry mass at an initial concentration of 5, 2.5, and 2.5 g/L, respectively [40].
Previously, Nouri et al. [41] successfully optimized the culture condition of Sarocladium kiliense ADH17 using the RSM method to improve the lipid to dry weight ratio, biomass, and lipid production, which were improved by 32.5%, 45%, and 30%, respectively [41]. Optimization of medium components of Rhodosporidium toruloides A29 using the RSM method and scale-up to 30 L could achieve a 22-fold increase in lipid yield [42]. Under the optimum conditions obtained by the RSM method (glycerol 89 g/L, ammonium hydroxide 0.54 g/L, incubation time 66 h), Y. lipolytica could produce the biomass and lipid content of 25.0 ± 1.5 g/L and 52.7 ± 1.2% (w/w of dry biomass), which was increased by 64% and 20% compared to un-optimized conditions [43]. The lipid production of oleaginous yeast strain was improved from 0.21 to 0.441 g/g after the optimization of potato starch in an agro-industry waste medium [44]. Optimization of glycerol (industrial waste) derived medium using the Box-Behnken model of RSM for biomass and lipid production by oleaginous yeast Candida viswanathii Y-E4 improved total biomass and lipid content to 26.6 g/L, and 51.9%, respectively after 166 h, compared to 17.2 g/L of total biomass and 41% (w/w) lipid content, under un-optimized conditions.
3.4. GC Results and Biodiesel Parameter AnalysisThe fatty acid methyl ester of produced lipids as obtained from GC-MS was presented in Fig. S1. The major saturated fatty acids of biodiesel include 15.9% hexadecanoic acid (C16:0), and 15.21% heptadecanoic acid (C17:0). The major unsaturated compounds include 19.53%, oleic acid (C18:1), and 20.22% eicosenoic acid (C20:1). Apart from this, other carbon compositions (C13, C14, C15, C19, C21, and C25:1) were also found in the low range. The fatty acid composition showed that 47.72% of the total fatty acids were saturated and 42.29% were accounted for unsaturated fatty acids. Calorific value and Cetane number (CN) always depend on the fatty acid composition which is present in the feedstock in the form of triglycerides. In biodiesel production, unsaturation drops the energy content and saturation helps to increase the calorific value, and hydrocarbon chain C16:0 and C18:0 show high CN, which is important for biodiesel production [45].
The important parameters of the biodiesel are shown in Table 4 along with ASTM standard range. The estimated viscosity (~ 3.73 mm2/s) and CN (~ 76.17 min) fall within the range for the ASTM standard. A low value of the viscosity is indicative of good fuel properties (good flow properties with a high atomization quality, drop size, and high penetration ability of the fuel) that are due to the presence of fatty acids containing C20 to C25 carbon chains (~ 22.49%) [46]. The viscosity of biodiesel produced by bacterial strain DS-7 (grown on dairy wastewater) was found to be 19.49 mm2/s whereas the viscosity of biodiesel produced by yeast grown on crude glycerol, soybean, and sunflower was found to be 1.28, 4.20, and 3.90 mm2/s, respectively [47]. The high CN of 76.17 was estimated for biodiesel produced from DS-7 lipids grown on vegetable waste. High CN is preferable due to an easier cold start of the standard diesel engine with shorter ignition time and lesser white smoke, formation [47]. The CN of biodiesel produced from bacterial sources is found to be higher than the CN of yeast and plant biodiesel as seen in Table 4. This may be due to the difference in the percentage of saturated and unsaturated fatty acids present in the lipids of the organisms. The pour point (PP) and cloud point (CP) for biodiesel produced from DS-7 bacteria grown on vegetable waste were found to be 12.7 and 18°C, respectively. The values of PP and CP were found to be 6.44 and 0.17°C, respectively for biodiesel produced from DS-7 bacteria when grown on dairy wastewater [8]. Although the PP and CP of the produced biodiesel did not vary much from the standards, the higher values of PP and CP suggest that produced biodiesel may not be suitable to use in low-temperature regions. The iodine value (IV) (34.69 g I2/100 g oil) and saponification value (SV) (144.85 mg KOH/g) of the produced biodiesel were following the ASTM standard. The IV and SV values inversely influence the CN value of biodiesel. Although the maximum value for IV as per the ASTM standard is 120 g I2/100 g oil. However, the lower the IV value, the lower the chances of depositions and deterioration of the lubrication, which prevents the polymerization of biodiesel and clogging of the fuel injectors. Lower value IV is due to low unsaturation levels in the fatty acid composition [8,40]. It is also observed from previous studies that IV values of biodiesel from bacterial biodiesel are less than fungal and yeast biodiesel (Table 4). The SV value for the biodiesel from DS-7 grown on vegetable waste was found to be 144.85 mg KOH/g, which is slightly lower than ASTM standard values. The biodiesel from the same bacteria (DS-7) grown on dairy wastewater showed an SV value of 114.91 mg KOH/g [7]. The SV and IV values of biodiesel depend on the fatty acids composition of the lipids. The overall properties of biodiesel produced from DS-7 bacteria grown on vegetable waste suggest its suitability be used as an alternative the diesel fuel.
4. ConclusionsIn this research work three different wastes were pretreated and out of which vegetable wastes show higher reducing sugar yield 0.352 g/g. Maximum biomass and lipid produced in vegetable waste hydrolysate (0.62g/L/d and 0.015g/g) as compared to cardboard and paper waste. To improve the biomass and lipid production, Box Behnken design of experiments was used to optimize the process parameters (incubation time, inoculum size, pH, and amount of waste), which successfully improved the lipid content and biomass production by 15.84% and 47.90%, respectively. The lipid and biomass productivities were also improved by 51 and 41.87%, respectively. Further, the optimization value was analyzed using ANOVA which signifies that the model was fairly attributed to the tested parameters. The FAME analysis of produced biodiesel showed that, the lipids of DS-7 grown on vegetable waste majorly composed of palmitic acid (C16:0), heptanoic acid (C17:0), oleic acid (C18:1), and eicosenoic acid (C20:1). The estimated biodiesel properties suggested that the produced biodiesel is an ideal fuel that can replace conventional diesel fuel. Future research should be focused on the development of green methods to pretreat the vegetable waste and scale-up studies to produce biodiesel from this renewable waste.
AcknowledgmentThe authors would like to thank NIT Rourkela for institute funding and Institute’s Central Instrumental Facility (CIF) access.
NotesAuthor Contributions S.P. (B.Tech student) conducted experiments and collected data, analyzed samples. S.S. (M.Tech student) analyzed samples and data, writing draft, review, and editing. S.S. (Ph.D. student) analyzed samples and data, writing – draft, review, and editing. A.D. (Professor) supervision, writing – review, and editing. K.D. (Assistant professor) supervision, writing – review and editing, resources, project administration, conceptualization. References1. Dhillon RS, von Wuehlisch G. Mitigation of global warming through renewable biomass. Biomass Bioenerg. 2013;48:75–89.
2. Dutta K, Tsai CY, Chen WH, Lin JG. Effect of carriers on the performance of anaerobic sequencing batch biofilm reactor treating synthetic municipal wastewater. Int Biodeterior Biodegrad. 2014;95(PA)84–88.
3. Kumar V, Thakur IS. Biodiesel production from transesterification of Serratia sp. ISTD04 lipids using immobilised lipase on biocomposite materials of biomineralized products of carbon dioxide sequestrating bacterium. Bioresour Technol. 2020;307:123193.
4. Zahan KA, Kano M. Biodiesel production from palm oil, its by-products, and mill effluent: A review. Energies. 2018;11(8)1–25.
5. Qadeer S, Mahmood S, Anjum M, Ilyas N, Ali Z, Khalid A. Synchronization of lipid-based biofuel production with waste treatment using oleaginous bacteria: A biorefinery concept. Process Saf Environ Prot. 2018;115:99–107.
6. Jeevan Kumar SP, Banerjee R. Enhanced lipid extraction from oleaginous yeast biomass using ultrasound assisted extraction: A greener and scalable process. Ultrason Sonochem. 2019;52:25–32.
7. Yellapu SK, Klai N, Kaur R, Tyagi RD, Surampalli RY. Oleaginous yeast biomass flocculation using bioflocculant produced in wastewater sludge and transesterification using petroleum diesel as a co-solvent. Renew Energy. 2019;131:217–228.
8. Behera AR, Dutta K, Verma P, Daverey A, Sahoo DK. High lipid accumulating bacteria isolated from dairy effluent scum grown on dairy wastewater as potential biodiesel feedstock. J Environ Manage. 2019;252:109686.
9. Meng X, Yang J, Xu X, Zhang L, Nie Q, Xian M. Biodiesel production from oleaginous microorganisms. Renew Energy. 2009;34(1)1–5.
10. Hwangbo M, Chu KH. Recent advances in production and extraction of bacterial lipids for biofuel production. Sci Total Environ. 2020;734:139420.
11. Sathish Kumar R, Sureshkumar K, Velraj R. Optimization of biodiesel production from Manilkara zapota (L.) seed oil using Taguchi method. Fuel. 2015;140:90–96.
12. Alvarez HM, Steinbüchel A. Triacylglycerols in prokaryotic microorganisms. Appl Microbiol Biotechnol. 2002;60(4)367–376.
13. Tian S, Qian C, Yang X. Biodegradation of biomass gasification wastewater by two species of Pseudomonas using immobilized cell reactor. Appl Biochem Biotechnol. 2006;128(2)141–147.
14. Gouda MK, Omar SH, Aouad LM. Single cell oil production by Gordonia sp. DG using agro-industrial wastes. World J Microbiol Biotechnol. 2008;24(9)1703–1711.
15. Hetzler S, Steinbüchela A. Establishment of cellobiose utilization for lipid production in Rhodococcus opacus PD630. Appl Environ Microbiol. 2013;79(9)3122–3125.
16. Wei Z, Zeng G, Huang F, et al. Microbial lipid production by oleaginous Rhodococci cultured in lignocellulosic autohydrolysates. Appl Microbiol Biotechnol. 2015;99(17)7369–7377.
17. Zhang TY, Wu YH, Hu HY. Domestic wastewater treatment and biofuel production by using microalga Scenedesmus sp. ZTY1. Water Sci Technol. 2014;69(12)2492–2496.
18. Kumar M, Thakur IS. Municipal secondary sludge as carbon source for production and characterization of biodiesel from oleaginous bacteria. Bioresour Technol Reports. 2018;4:106–113.
19. Goswami L, Tejas Namboodiri MM, Vinoth Kumar R, Pakshirajan K, Pugazhenthi G. Biodiesel production potential of oleaginous Rhodococcus opacus grown on biomass gasification wastewater. Renew Energy. 2017;105:400–406.
20. Goswami L, Kumar RV, Arul Manikandan N, Pakshirajan K, Pugazhenthi G. Anthracene Biodegradation by Oleaginous Rhodococcus opacus for Biodiesel Production and Its Characterization. Polycycl Aromat Compd. 2019;39(3)207–219.
21. Kumar S, Gupta N, Pakshirajan K. Simultaneous lipid production and dairy wastewater treatment using Rhodococcus opacus in a batch bioreactor for potential biodiesel application. J Environ Chem Eng. 2015;3(3)1630–1636.
22. Pleissner D, Lau KY, Ki Lin CS. Utilization of food waste in continuous flow cultures of the heterotrophic microalga Chlorella pyrenoidosa for saturated and unsaturated fatty acids production. J Clean Prod. 2017;142:1417–1424.
23. Lau KY, Pleissner D, Lin CSK. Recycling of food waste as nutrients in Chlorella vulgaris cultivation. Bioresour Technol. 2014;170:144–151.
24. Wen Q, Chen Z, Li P, Duan R, Ren N. Lipid production for biofuels from hydrolyzate of waste activated sludge by heterotrophic Chlorella protothecoides
. Bioresour Technol. 2013;143:695–698.
25. Wu LF, Chen PC, Huang AP, Lee CM. The feasibility of biodiesel production by microalgae using industrial wastewater. Bioresour Technol. 2012;113:14–18.
26. Paritosh K, Kushwaha SK, Yadav M, Pareek N, Chawade A, Vivekanand V. Food Waste to Energy: An Overview of Sustainable Approaches for Food Waste Management and Nutrient Recycling. Biomed Res Int. 2017;2017:2370927.
27. Sibbi G. Bioenergy Production from Wastes by Microalgae as Sustainable Approach for Waste Management and to Reduce Resources Depletion. Int J Environ Sci Nat Resour. 2018;13(3)1–4.
28. Changmai B, Vanlalveni C, Ingle AP, Bhagat R, Rokhum L. Widely used catalysts in biodiesel production: A review. RSC Adv. 2020;10(68)41625–41679.
29. Ferreira SLC, Bruns RE. Box-Behnken design: An alternative for the optimization of analytical methods Box-Behnken design: An alternative for the optimization of analytical methods. Anal Chim Acta. 2007;597(2)179–186.
30. Kumar V, Muthuraj M, Palabhanvi B, Ghoshal AK, Das D. Evaluation and optimization of two stage sequential in situ transesterification process for fatty acid methyl ester quantification from microalgae. Renew Energy. 2014;68:560–569.
31. Majumdar S, Naha A, Bhattacharyya DK, Bhowal J. Effective delignification and decrystallization of cauliflower wastes by using dilute phosphoric acid for efficient enzymatic digestibility to produce fermentable sugars. Biomass Bioenerg. 2019;125:169–179.
32. Tadmourt W, Khiari K, Boulal A, Tarabet L. Waste paper valorization for bioethanol production: Pretreatment and acid hydrolysis optimization. Energy Sources, Part A Recover Util Environ Eff. 2020;1–20.
33. Zhou W, Gong Z, Zhang L, Liu Y, Yan J, Zhao M. Feasibility of lipid production from waste paper by the oleaginous yeast Cryptococcus curvatus
. BioResources. 2017;12(3)5249–5263.
34. Chatterjee S, Mohan SV. Microbial lipid production by Cryptococcus curvatus from vegetable waste hydrolysate. Bioresour Technol. 2018;254:284–289.
35. Kumar M, Khosla K, Thakur IS. Optimization of Process Parameters for the Production of Biodiesel from Carbon dioxide Sequestering Bacteria. J Energy Environ Sustain. 2017;3:43–50.
36. Singh G, Sinha S, Kumar KK, Gaur NA, Bandyopadhyay KK, Paul D. High density cultivation of oleaginous yeast isolates in ‘mandi’ waste for enhanced lipid production using sugarcane molasses as feed. Fuel. 2020;276:118073.
37. Juanssilfero AB, Kahar P, Amza RL, et al. Effect of inoculum size on single-cell oil production from glucose and xylose using oleaginous yeast Lipomyces starkeyi. J Biosci Bioeng. 2018;125(6)695–702.
38. Liu LP, Hu Y, Lou WY, Li N, Wu H, Zong MH. Use of Crude Glycerol as Sole Carbon Source for Microbial Lipid Production by Oleaginous Yeasts. Appl Biochem Biotechnol. 2017;182(2)495–510.
39. Zhang L, Loh KC, Kuroki A, Dai Y, Tong YW. Microbial biodiesel production from industrial organic wastes by oleaginous microorganisms: Current status and prospects. J Hazard Mater. 2021;402:123543.
40. Gao R, Li Z, Zhou X, Cheng S, Zheng L. Oleaginous yeast Yarrowia lipolytica culture with synthetic and food waste-derived volatile fatty acids for lipid production. Biotechnol Biofuels. 2017;10(1)1–15.
41. Nouri H, Moghimi H, Nikbakht Rad M, et al. Enhanced growth and lipid production in oleaginous fungus, Sarocladium kiliense ADH17: Study on fatty acid profiling and prediction of biodiesel properties. Renew Energy. 2019;135:10–20.
42. Saran S, Mathur A, Dalal J, Saxena RK. Process optimization for cultivation and oil accumulation in an oleaginous yeast Rhodosporidium toruloides A29. Fuel. 2017;188:324–331.
43. Sara M, Brar SK, Blais JF. Lipid production by Yarrowia lipolytica grown on biodiesel-derived crude glycerol: Optimization of growth parameters and their effects on the fermentation efficiency. RSC Adv. 2016;6(93)90547–90558.
44. Mirza S, Siddique S, Qamer HM, Doggar MG. Optimization of lipid production potential of oleaginous yeast by response surface methodology cultivated in agro-industrial waste. Int J Environ Sci Technol. 2019;16(7)3221–3230.
45. Giakoumis EG, Sarakatsanis CK. A comparative assessment of biodiesel cetane number predictive correlations based on fatty acid composition. Energies. 2019;12(3)1–30.
Fig. 3Surface plot of Biomass production. Interactive effect of (a) Incubation time and amount of waste added (b) Incubation time and pH (c) time and inoculum size (d) Amount of waste added and pH (e) Amount of waste added and inoculum size (f) pH and inoculum size on biomass production. 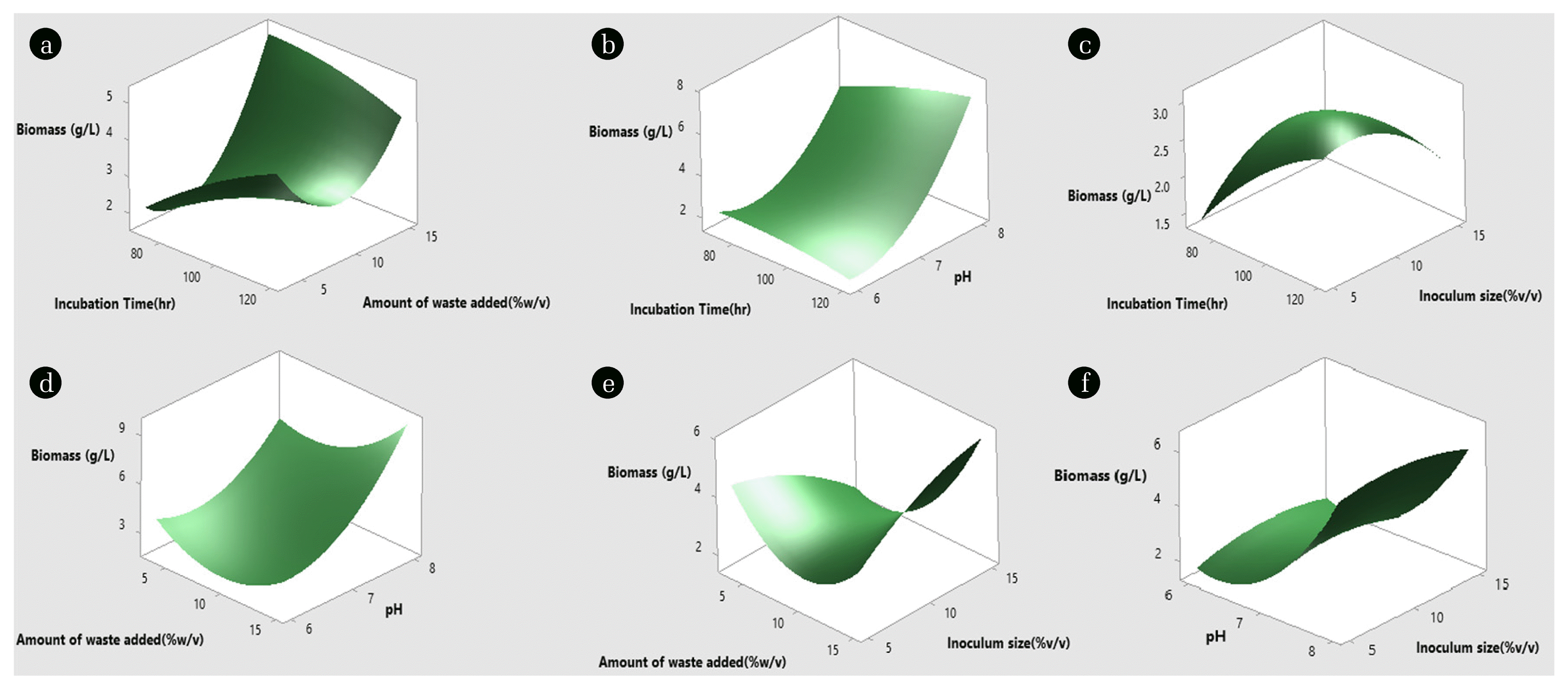
Fig. 4Surface plot of lipid content of DS-7. Interactive effect of (a) Incubation time and amount of waste added (b) Incubation time and pH (c) Incubation time and inoculum size (d) Amount of waste added and pH (e) Amount of waste added and inoculum size (f) pH and inoculum size on lipid content of DS-7. 
Table 1Total Biomass, Lipid Produced and Lipid Content Obtained after Optimization (Coded Values in Parenthesis) Table 2Coefficients of Regression Equations for Total Biomass and Lipid Content Table 3Analysis of Variance of Total Biomass and Lipid Content
Table 4Biodiesel Properties of FAME Obtained from DS-7 Bacteria Using Vegetable Waste
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||