1. Introduction
As climate change due to global warming becomes more apparent, it is increasingly important to secure stable water resources. In this situation, the importance of desalination technology is increasing as a solution to the emerging water shortage problem [1]. Desalination technologies commonly used include evaporation, reverse osmosis (RO), electrodialysis (ED), electrodeionization (EDI), and ion-exchange resins (IX). In addition, capacitive deionization (CDI) technology has been increasingly studied since 2000 [2–5].
CDI is an electrochemical desalination technology that removes ions by electrostatic attraction between the electrode and ions through the application of an electric potential to a carbon electrode. Compared with other desalination technologies, CDI is known as an environmentally friendly desalination process that is energy efficient, has high water recovery, and does not discharge pollutants during the operation process. Due to these advantages, CDI is recognized as a promising desalination method in the future [2–7].
In CDI, ions are removed by an electrosorption reaction at the carbon electrodes. Therefore, the adsorption performance of the carbon electrode determines the desalination performance. For the past 20 years, various studies have been conducted to improve the adsorption capacity of carbon electrodes [7]. Various carbon electrodes with improved adsorption capacity have been developed by improving the physical (specific surface area, pore size) and electrochemical (electrical conductivity, surface functional groups) properties of carbon materials [8–12]. In addition, composite carbon electrodes with metal oxides (ZnO, TiO2, MnO2, etc.) or polymers (chitosan, polyaniline, ion-exchange polymer, etc.) have been introduced [13–16]. It has been reported that the salt adsorption capacity (SAC) of a carbon electrode can currently reach 30 mg/g or more due to the improved adsorption performance of the carbon electrode [5, 17].
A carbon electrode that can selectively remove specific ions from a mixed-ion solution can increase the desalination efficiency of a CDI system and reduce energy costs. Many studies have been conducted on the selective removal of ions using a CDI system [18–28]. Studies on the ion selectivity according to the structure of the carbon electrode and various ion-selective composite carbon electrodes (SCCEs) have been developed. Chen et al. [21] studied the correlation between the carbon structure and ion selectivity through CDI experiments for mixed-anion solutions. They reported that the main factors determining the ion selectivity of the carbon electrode were the ion valence and hydrated radius. Hawks et al. [24] were able to increase the selective removal rate of nitrate ions by using ultramicroporous (< 1 nm) carbon. On the other hand, Lee et al. [18] used a lithium manganese oxide electrode to obtain a lithium recovery rate that was 7 times higher than that of a general carbon electrode. Park et al. [23] prepared a reduced graphene oxide/hydroxylapatite composite (rGO/HA) electrode to increase the removal rate of fluoride ions. They confirmed that the removal rate of fluoride by the rGO/HA electrode showed an improvement of 4.9 times that of a general carbon electrode based on the CDI test results with a mixed solution containing fluoride, chloride and nitrate.
The ion selectivity of a carbon electrode is greatly influenced by the adsorption equilibrium between the carbon electrode and ions in the solution [19]. An SCCE with ion selectivity can be obtained by coating the surface of a carbon electrode with a material that has a high selectivity for specific ions. When a potential is applied to the SCCE, ions in the coated layer move to the carbon electrode and are adsorbed. Therefore, it can be expected that the ions to be removed are determined by the ion selectivity of the coated material. Using this concept, an SCCE was manufactured by coating a selective ion-exchange resin for the nitrate ion on a carbon electrode surface [19]. From the results of the desalination experiment with a mixed-anion solution, the selective removal rate of nitrate ions could be improved by more than two times that of an unmodified carbon electrode.
In this study, the ion selectivity of an SCCE according to the characteristics of anion exchange resins (AERs) was investigated. The ion selectivity may vary depending on the physicochemical properties of the AERs. The selectivities for chloride, nitrate, and sulfate ions were analyzed using commercial AERs with different ion exchange capacities (IECs) and water contents. Each AER powder was coated on a carbon electrode surface to prepare an SCCE. Desalination experiments were performed for the mixed-anion solution by using a CDI cell equipped with the prepared SCCE. Based on the desalination results, the correlation between the characteristics of the AERs and the removed ions was analyzed. In addition, the effect of the current density on the ion selectivity of the SCCE was studied.
2. Materials and Methods
2.1. Analysis of Physicochemical Properties and Adsorption Equilibrium of AERs
Three types of commercial AERs were selected to investigate ion selectivity according to the characteristics (ion exchange capacity, water content) of each AER. The AERs used in this experiment were Trilite AMP16 (Samyang Co., South Korea), Bonlite BHP55 (Boeun Chemicals Inc., South Korea), and Ionac SR7 (Sybron Chemicals Inc., USA). These resins have a structure in which quaternary amine groups are introduced as ion-exchange functional groups in a polystyrene matrix. For the analysis of physicochemical properties and the adsorption equilibrium of AERs, each resin was immersed in 2.0 M NaCl solution for 24 h so that the functional groups inside the resin were replaced by their Cl-form. Then, each resin was washed with distilled water to remove NaCl remaining on the resin surface. The washing was repeated until the electrical conductivity of the washing solution reached 25 μS/cm or less and then the resin was used in the experiment.
The IEC and water content of each AER were measured. To measure the IEC, a certain amount (about 0.2 – 0.3 g) of AER was added to 100 mL of a 1.0 M NaNO3 solution. The mixture was stirred for 24 h so that all functional groups of the AER were replaced with nitrate ions. After stirring, the concentration of chloride ions in the solution was analyzed to calculate the IEC value [29]. Moreover, the water content of the AER was calculated based on the difference in the weight before and after drying the resin. Briefly, after immersing a certain amount (about 1 g) of resin in distilled water, the resin was removed from the water, the water on the surface of the resin was removed, and the weight was measured. Then, after being placed in a drying oven at 80°C for 24 h, the water content was calculated by measuring the weight of the dried resin. Table 1 summarizes the physicochemical properties of the AERs used in this study.
The ion selectivity of each AER was analyzed through an adsorption equilibrium experiment in a mixed-anion solution. A mixed-anion solution with chloride, nitrate, and sulfate-ion concentrations of 3.0 meq/L was prepared by dissolving NaCl, NaNO3, and Na2SO4. A certain amount (approximately 0.2 g) of an AER was added to 300 mL of the prepared mixed solution. Then, the mixture was stirred for 24 h to reach adsorption equilibrium at room temperature. After reaching adsorption equilibrium, the concentration of each ion in the mixed solution was measured. The amount of each ion adsorbed to the AER was calculated through the change in the ion concentration of the mixed solution before and after reaching adsorption equilibrium. To confirm the reproducibility of the experimental results, the adsorption equilibrium experiment for each resin was repeated three times.
The ion concentration was analyzed using ion chromatography (Metrohm Compact IC) with a SI-90 4E column from Shodex Co.. The eluent was a solution of 1.8 mM Na2CO3 and 1.7 mM NaHCO3. The eluent was supplied to the column at a rate of 1.0 mL/min, and the concentrations of the chloride, nitrate, and sulfate ions were measured.
2.2. Fabrication of Ion-Selective Composite Carbon Electrodes
Activated carbon powder (CEP-21K, Power Carbon Technology Co., Korea) was mixed with polyvinylidene fluoride (PVdF, M.W. = 530,000, Aldrich) to prepare a carbon electrode for manufacturing the SCCE. PVdF was dissolved in di-methylacetamide (DMAc, Aldrich) and then mixed with activated carbon powder. Next, the mixture was stirred by a planetary centrifugal mixer (AR-100, THINKY Co., Japan) for 30 min to prepare a uniform electrode slurry. The electrode slurry was cast, using a doctor blade, to a thickness of 350 μm on a graphite sheet current collector (F02511, Dongbang Carbon Co.), and then the electrode was placed in a drying oven at 50°C for 6 h. The content of PVdF (polymer binder) in the prepared carbon electrode was 10 wt%.
SCCEs were prepared by coating an AER powder on the surface of a prepared carbon electrode. The AER was placed in a drying oven at 80°C for 24 h and then made into a fine powder using a grinder. The pulverized resin powder was sieved to a scale size of 50 μm and used for electrode coating. The AER powder and the PVdF solution were mixed to prepare a coating solution. The weight ratio of AER to PVdF (binder) was 5:1. After coating to a thickness of 100 μm on the carbon electrode surface, it was dried at 50°C for 6 h to complete the SCCE preparation.
2.3. Configuration of The CDI Unit Cell and Desalination Experiments
A CDI unit cell was prepared to confirm the ion selectivity of the prepared SCCE. The SCCE (anode), spacer, cation-exchange membrane, and carbon electrode (cathode) were arranged in order in a rubber gasket and fixed with plexiglass plates on both sides to make a CDI cell. The carbon electrode used in the CDI cell was 10 x 10 cm (100 cm2) in size, and the weight of the carbon layer was 0.92 g. More detail on the structure of the CDI cell can be found in our previous study [19, 30].
All CDI experiments were conducted in batch mode. The mixed solution (250 mL) was supplied to the cell at a constant flow rate (50 mL/min) by a peristaltic pump, and the effluent was circulated back to the feed tank. The concentrations of chloride, nitrate, and sulfate ions in the mixed solution were 5.0, 2.0, and 2.0 meq/L, respectively.
Power was supplied to the cell using a potentiostat (WPG100, WonA Tech Co.). The positive and negative terminals were connected to the SCCE and the carbon electrode in the CDI cell. Adsorption was performed until the cell potential reached 1.0 V while supplying a constant current. Then, the cell potential was immediately converted to 0.0 V, and desorption was carried out for 5 min. The adsorption and desorption processes were repeated three times in succession. During the adsorption and desorption process, changes in the cell potential were measured every 3 s. By connecting a conductivity probe to the interface, the conductivity of the mixed solution was automatically measured every 3 s. In addition, after each adsorption and desorption cycle, samples were collected from the feed tank, and the ion concentration was measured by ion chromatography to determine the amount of ions adsorbed and desorbed.
3. Results and Discussion
3.1. Ion Selectivities of The Anion-Exchange Resins
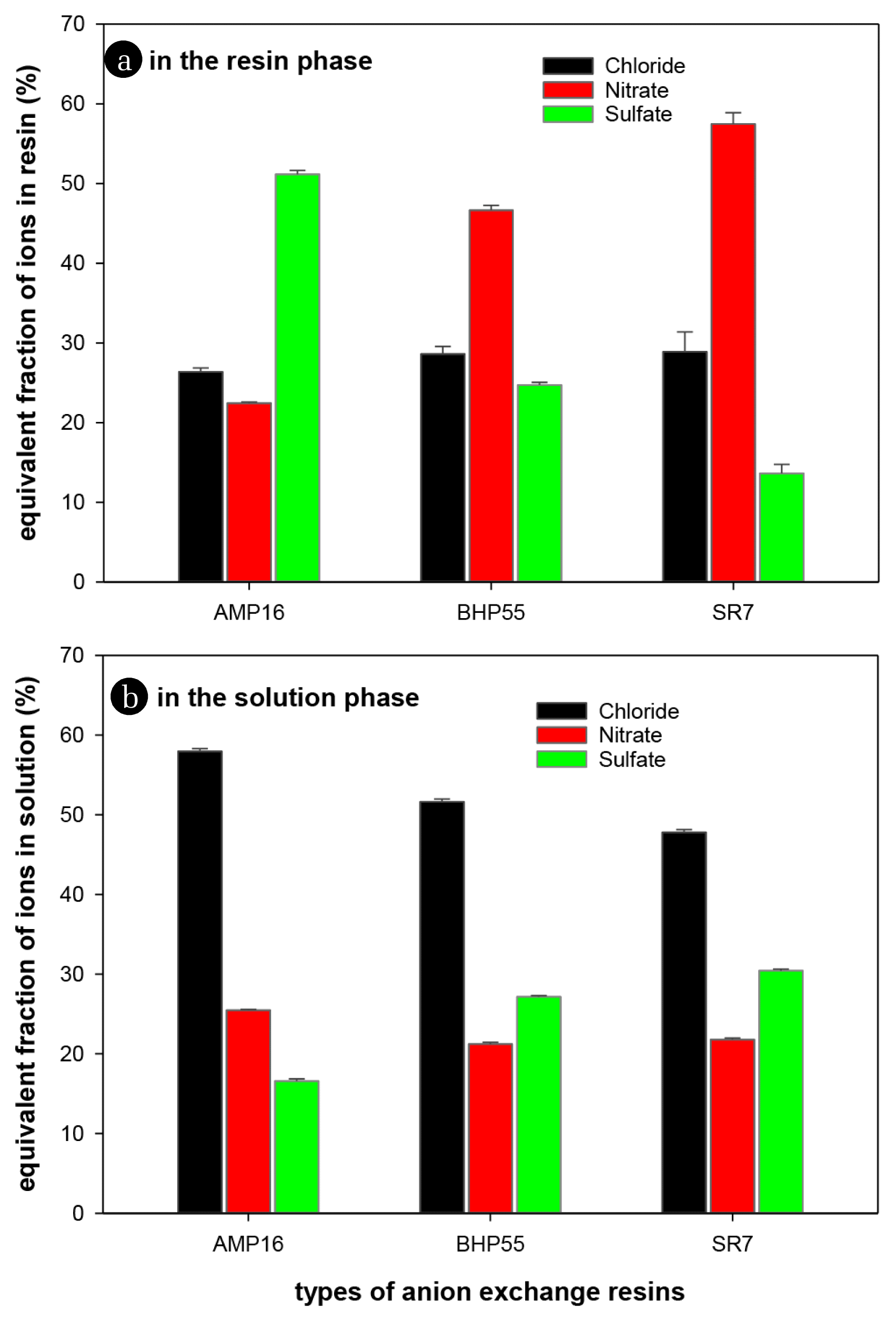
An AER was added to a mixed solution with a chloride, nitrate, and sulfate concentrations of 3.0 meq/L and stirred for 24 h so that the resin and the solution reached an adsorption equilibrium. The amount of ions adsorbed to the AER was calculated by analyzing the ion concentration of the mixed solution. Fig. 1 shows the equivalent fraction of each ion in the AER phase (a) and in the solution phase (b) at the adsorption equilibrium state.
Depending on the type of AER, there was a large difference in the equivalent fraction of ions adsorbed. In the case of the AMP16 resin, the proportion of sulfate ions was 51.2% of the total adsorbed ions. In the solution phase, the fraction of chloride ions was 57.9%, which was approximately 3.5 times higher than that of sulfate ions (16.6%), but the fraction of sulfate ions adsorbed on AMP16 resin was approximately twice as much as that of chloride ions (26.4%). These results show that the AMP16 resin had very high selectivity for sulfate ions.
On the other hand, the BHP55 and SR7 resins showed high selectivity to nitrate ions. In particular, the SR7 resin had very high selectivity for nitrate ions but very low selectivity for sulfate ions. For the SR7 resin, the equivalent fraction of nitrate ions in the solution phase was 21.8%, but the fraction in the resin phase was 57.5%. Despite the amount of chloride (47.8%) in the solution phase was more than twice as much as that of nitrate, the amount of nitrate adsorbed on the SR7 resin was approximately 2 times higher than that of chloride ions (28.9%). On the other hand, the fraction of divalent sulfate ions on the SR7 resin was only 13.6%, indicating very low selectivity for sulfate ions.
The ion selectivity of an AER is known to be influenced by the number of charges and the sizes of ions [31]. In particular, as the charge of an ion increases, the selectivity tends to increase. In fact, in the case of the AMP16 resin, the adsorption ratio of divalent sulfate ions was the highest. Among the nitrate and chloride ions, which are monovalent ions, chloride ions with their small ionic radius showed higher selectivity than nitrate ions. However, the ion selectivity of the BHP55 and SR7 resins deviated from the general adsorption tendency of resins. In particular, the selectivity of monovalent nitrate ions was higher than that of divalent sulfate ions.
The difference in ion selectivity depending on the type of AER is thought to be related to the IEC of an AER. In Table 1, the water content of AERs was in the range of 52.2–58.7%, thus showing similar values. However, the IEC of AMP16 was 4.23 meq/g, which was 1.6 times higher than that of SR7 resin. From the water content and IEC values, it can be seen that the concentration of ions in the resin phase decreases in the order of AMP16 > BHP55 > SR7. For divalent sulfate ions to be adsorbed on the resin, it is preferable to have a high concentration of ion-exchange functional groups around the sulfate ions to maintain electrical neutrality. As a result, it is judged that the selectivity of sulfate ions is proportional to the IEC value of the AER. On the other hand, it can be seen that the selectivity of nitrate ions is rather inversely proportional to the IEC. From the adsorption equilibrium results for AERs, it can be concluded that the ion selectivity of AERs is greatly affected by the IEC value. Moreover, the AMP16 and SR7 resins are expected to be effective in increasing the selective removal of sulfate and nitrate ions, respectively.
3.2. Desalination Characteristics of The CDI Cell Using a Selective Composite Carbon Electrode
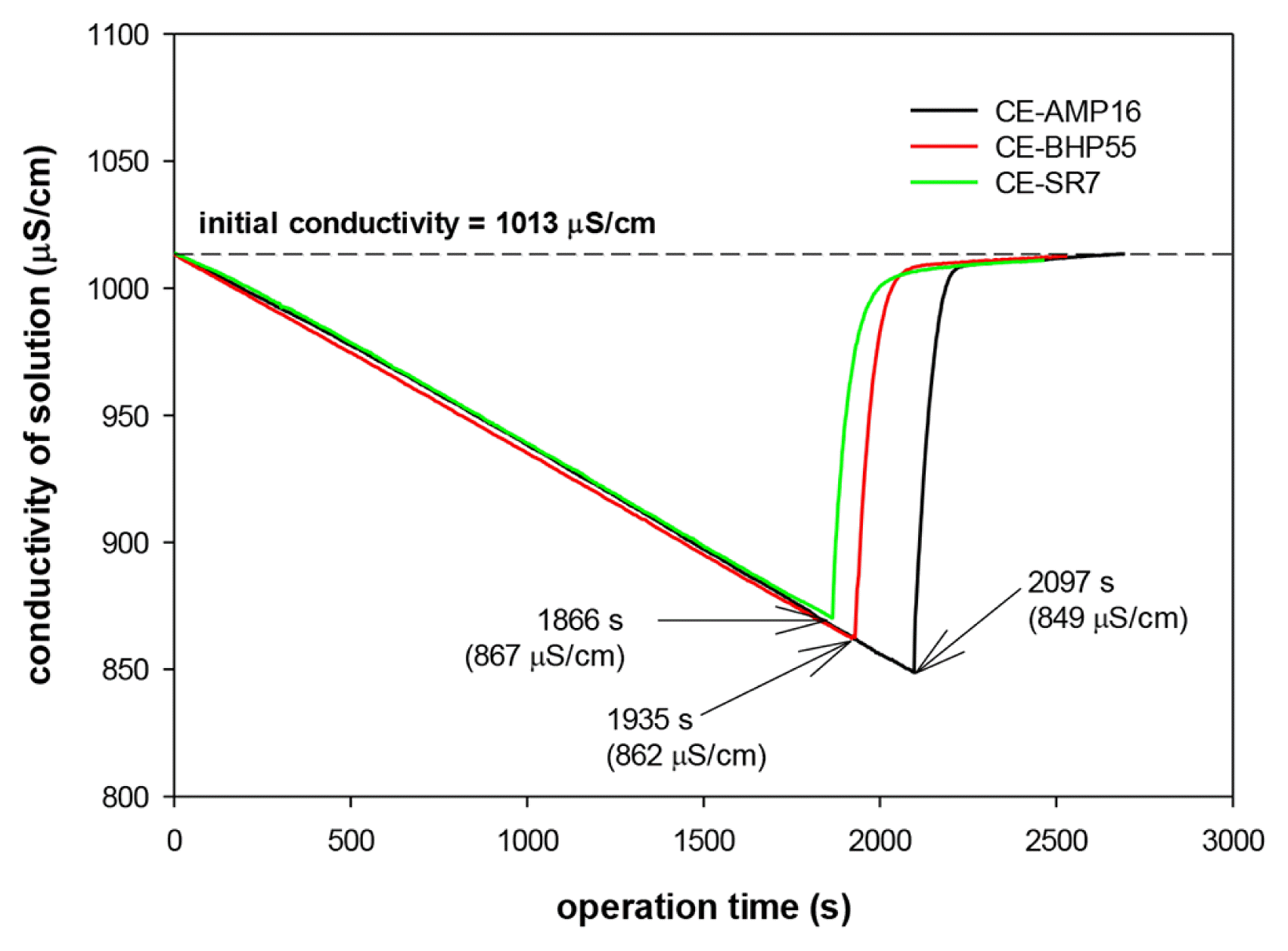
A CDI unit cell was fabricated with the prepared composite carbon electrode, and a desalination experiment was conducted while supplying a mixed solution with concentrations of 5.0, 2.0, and 2.0 meq/L chloride, nitrate, and sulfate ions, respectively. Fig. 2 shows the change in the conductivity of the mixed solution during the adsorption (current density = 2.0 mA/cm2) and desorption process (cell potential = 0.0 V).
The adsorption rate of ions in the CDI cell is proportional to the current supplied to the cell. As a result, the conductivity decreased linearly during the adsorption process. The conductivity of the solution was restored to the initial value (1,013 μS/cm) during the desorption process, which indicated that the adsorption and desorption proceeded smoothly in the CDI cell. Additionally, the adsorption time differs according to the types of composite carbon electrodes. The adsorption time was 2,094 s for the AMP16-coated composite carbon electrode (CE-AMP16) but was shortened to 1,866 s for the CE-SR7 electrode. As the adsorption time increases, the amount of charge supplied to the electrode increases, and more ions are adsorbed. In fact, the decrease in conductivity of the mixed solution during the adsorption process was 164, 151, and 146 μS/cm for the CE-AMP16, CE-BHP55, and CE-SR7 electrodes, respectively. The difference in the adsorption amount depending on the types of SCCE is attributed to the IEC of the coated AER. The adsorption capacity of a carbon electrode increases as the ion concentration of the solution increases [32]. In the case of an SCCE coated with a resin powder having a high IEC value, the concentration of ions at the surface of the carbon particles increases, which results in an increase in the adsorption capacity of the SCCE. In fact, the amount that can be adsorbed by the SCCE increases in proportion to the IEC of the AER.
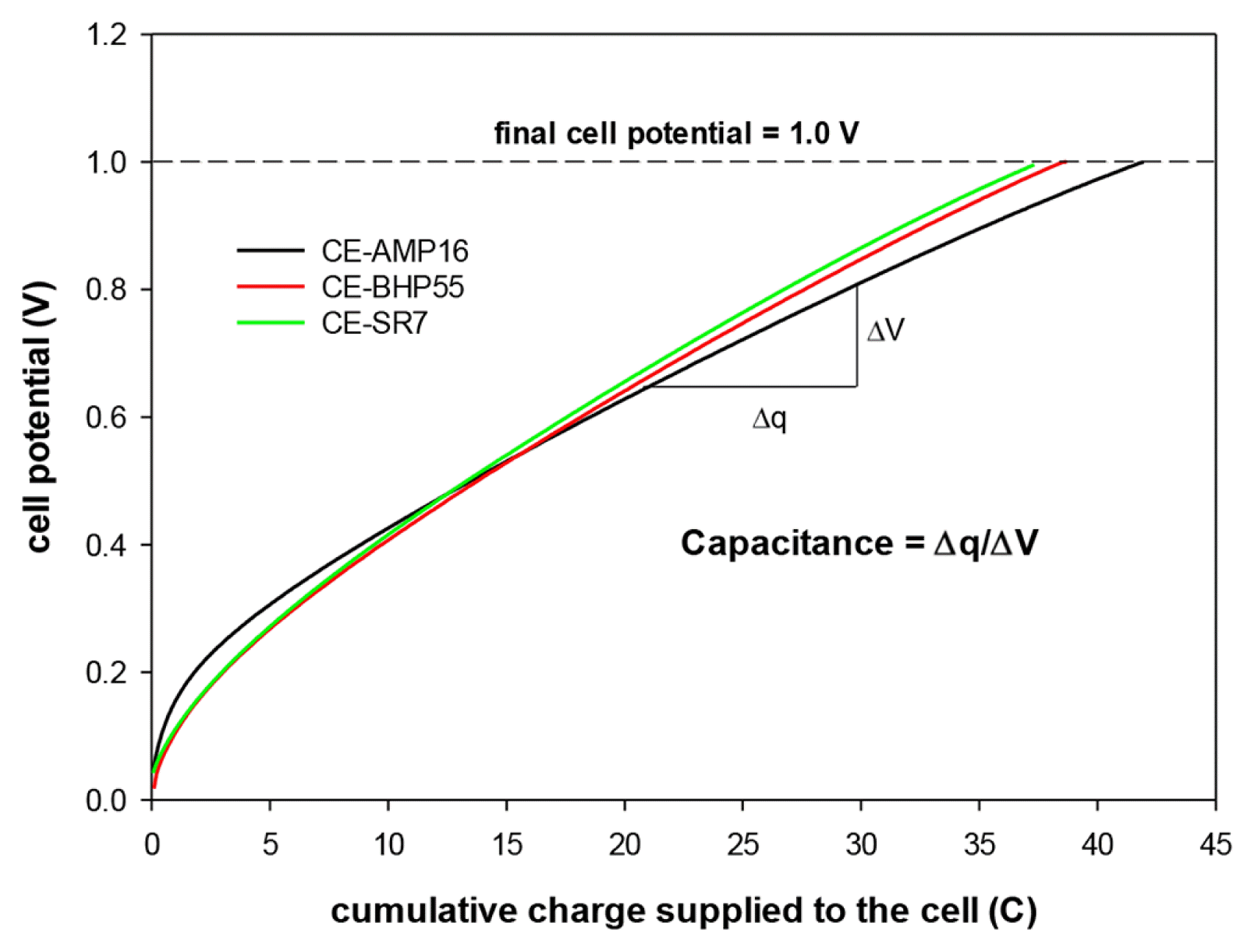
Fig. 3 shows the change in cell potential according to the amount of charge supplied to the cell during the adsorption process (at a constant current density of 2.0 mA/cm2). When current is supplied to the cell, electric charges accumulate in the carbon electrode, and the electrode potential increases; eventually, the cell potential increases proportionally. At the initial stage of the adsorption process, the increasing rate of the cell potential was somewhat high. This result is attributed to the change in concentration in the feed stream. When current is supplied to the cell, the concentration of the feed stream decreases as ions are adsorbed to the carbon electrode. As a result, the electrical resistance of the feed stream increases, thereby resulting in a high rate of increase of the cell potential.
In the region in which the cell potential increases linearly, the slope (the ratio of charge accumulated and cell potential) indicates the capacitance of the carbon electrode. The capacitance of the SCCEs decreases in the order of CE-AMP16 > CE-BHP55 > CE-SR7. This result is consistent with the conductivity result shown in Fig. 2.
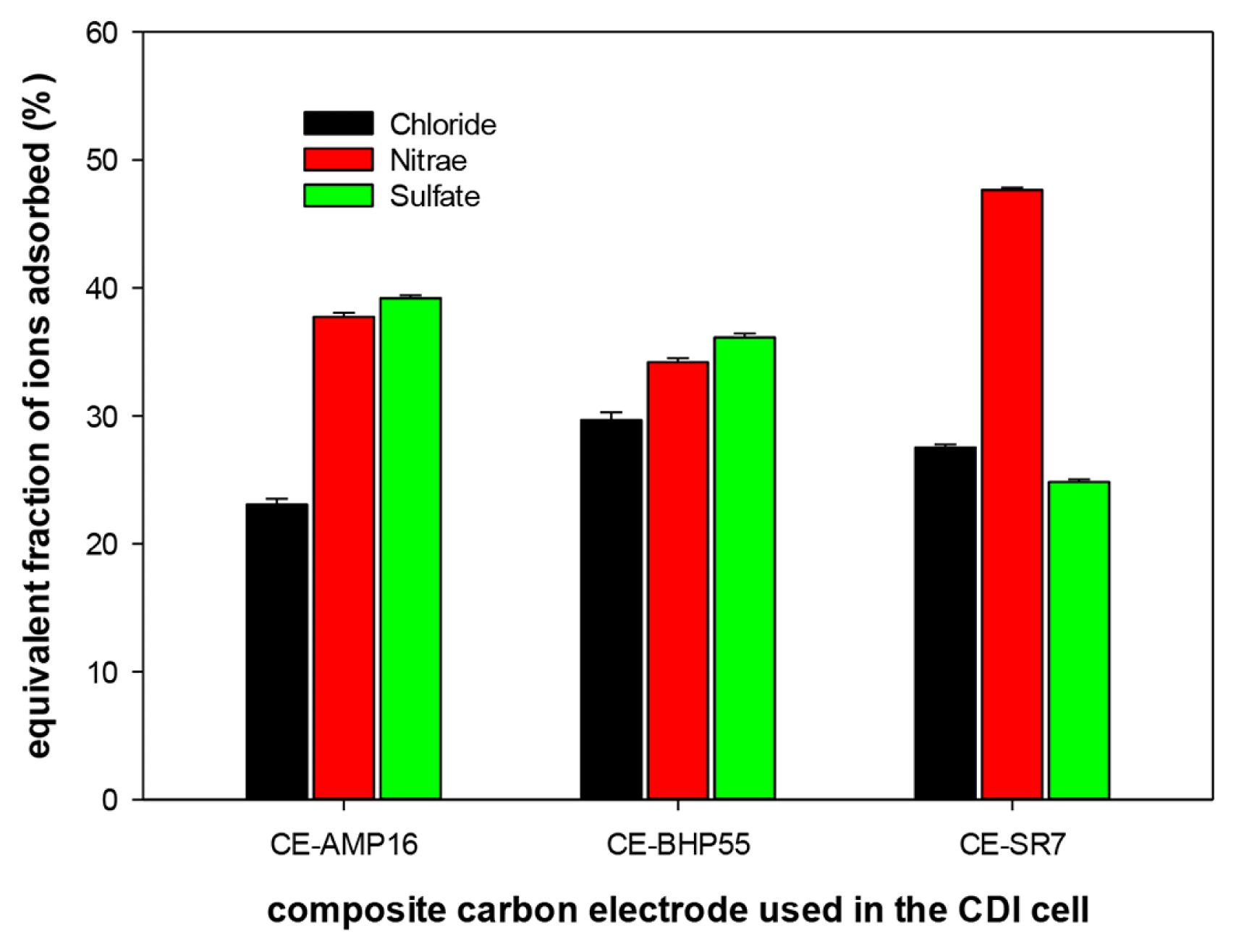
After the adsorption process was completed, the fraction of each ion adsorbed was analyzed. Fig. 4 shows the equivalent fraction of each anion adsorbed on the SCCE when adsorption was performed at a current density of 2.0 mA/cm2. The concentration ratio of chloride, nitrate, and sulfate ions in the initial mixed solution was 5:2:2. Thus, the chloride concentration was 2.5 times higher than that of the other two anions. However, the proportion of chloride ions adsorbed on the SCCE was less than 30% on all SCCEs. In particular, the fraction of chloride ions adsorbed was the lowest for the CE-AMP16 electrode, which has the highest IEC. However, CE-AMP16 shows a high removal performance (39.2%) for sulfate ions. Moreover, in the case of the CE-SR7 electrode, the adsorption ratio of nitrate ions was 47.7%, showing very high selectivity. Although the sulfate ion is divalent and the concentrations of sulfate and nitrate ions are the same in the mixed solution, the fraction of nitrate adsorbed was found to be almost two times higher than that of sulfate ions. It can be seen that the CDI experimental result is exactly the same as the adsorption equilibrium results of the AERs. Therefore, it is confirmed that the ion selectivity of the SCCE is governed by the ion selectivity of the AER coating.
3.3. Change in Selectivity of The SCCE According to Current Density
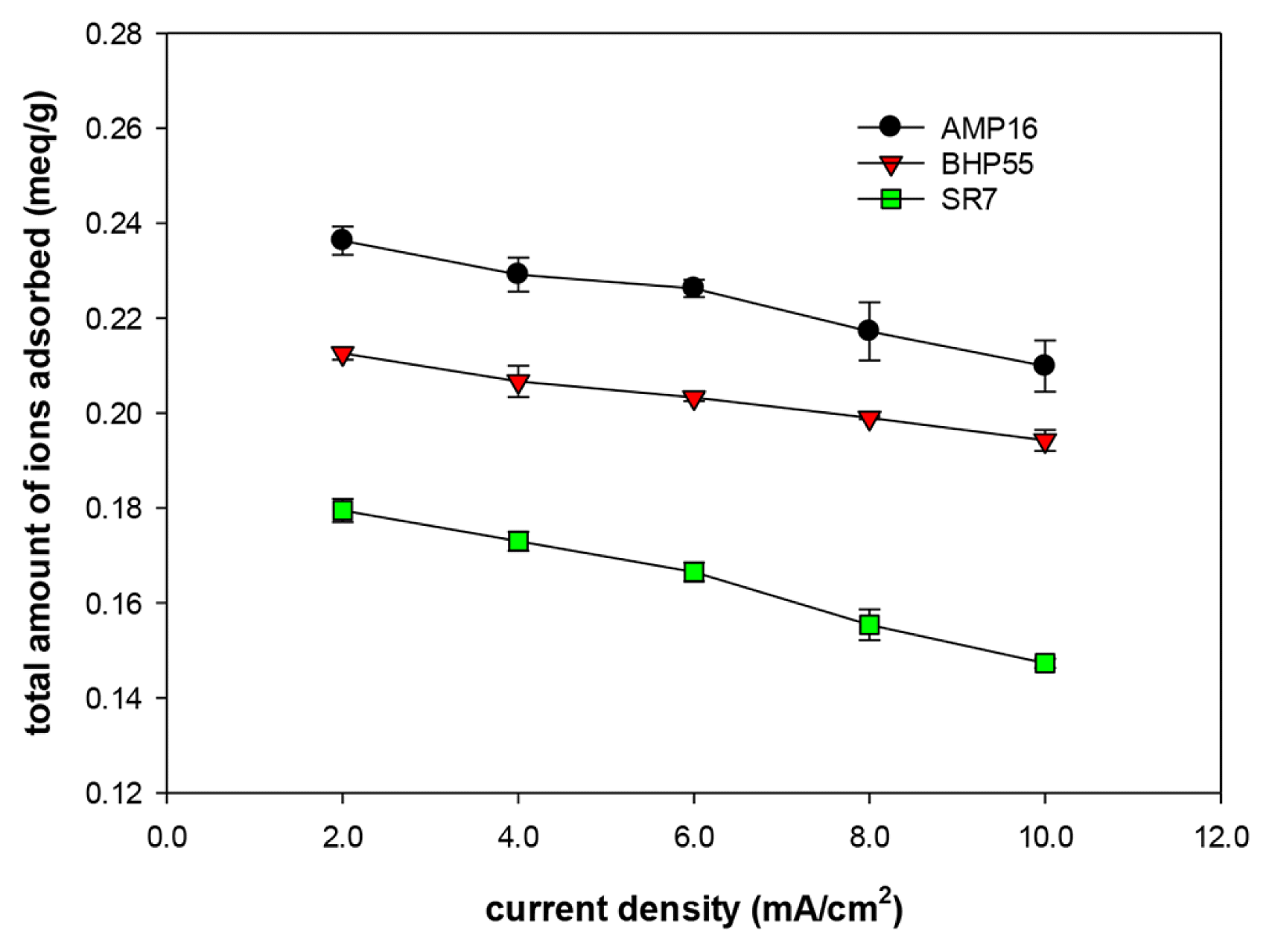
CDI desalination experiments were conducted while changing the current density and the concentration of each ion was measured at the end of the adsorption and desorption process. The total amount of adsorbed ions according to the SCCEs is shown in Fig. 5. The total amount of ions adsorbed at all current densities decreased in the order of CE-AMP16 > CE-BHP55 > CE-SR7. At a current density of 2.0 mA/cm2, the total adsorption amount for the CE-AMP16 electrode was 0.24 meq/g, which was 31.7% higher than that of the CE-SR7 electrode (0.18 meq/g). As explained earlier, this result is attributed to the high IEC of the AMP16 resin coated on the carbon surface. Moreover, it can be seen that the adsorption amount decreases as the current density increases for all SCCEs. The adsorption experiment was carried out until the cell potential reached 1.0 V. As the current density increases, the voltage drop due to the resistance elements (ion-exchange membrane, feed stream) inside the cell increases. As a result, the electrode potential that determines the adsorption amount decreases, resulting in a decrease in the adsorption amount as the current density increases.
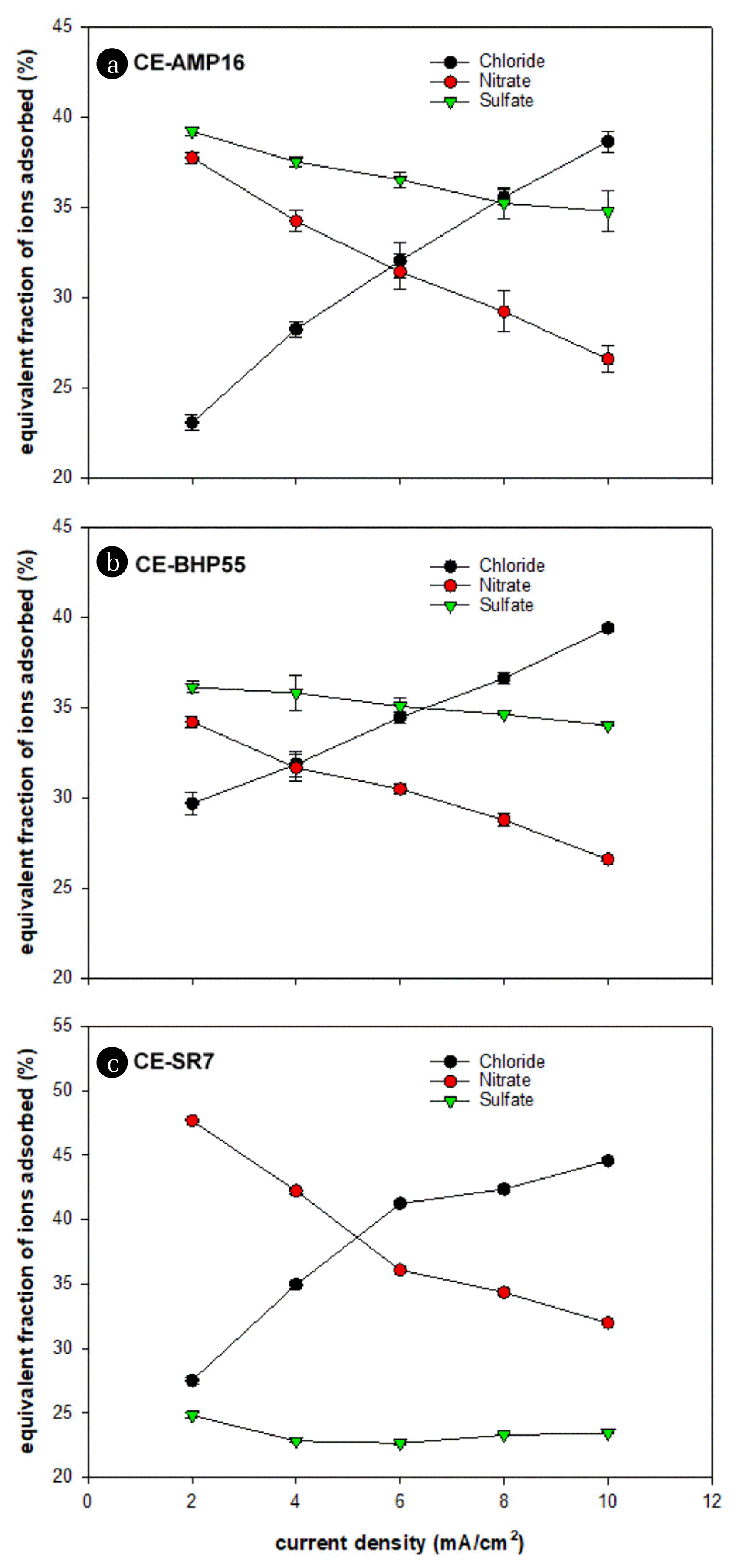
The change in ion selectivity of the SCCE according to the current density was examined. Fig. 6 shows the equivalent fraction of adsorbed ions according to the current density for the different SCCEs. The adsorbed fraction of each ion changes considerably according to the current density applied to the cell. For all SCCEs, as the current density increased, the fraction of adsorbed nitrate ions decreased, while the fraction of chloride ions tended to significantly increase. The adsorption fraction of sulfate ions also decreased as the current density increased but was not significantly different than that of nitrate ions. In particular, for the CE-SR7 electrode, the equivalent fraction of adsorbed sulfate ions was kept constant at approximately 23% at all current densities.
As the current density decreased, the fraction of adsorbed ions tended to coincide with the ion selectivity of the coated AER. However, as the current density increased, the fraction of adsorbed ions deviated from the adsorption equilibrium results of the AERs. This result is interpreted to be because the AER on the SCCE deviates from the adsorption equilibrium state as the current density increases. Before current is applied, the AER on the SCCE maintains an adsorption equilibrium with the ions in the feed solution. When supplying current to the cell, the ions in the resin move to the carbon, and new ions are adsorbed from the feed stream. To reach adsorption equilibrium between the AER and the ions in the mixed solution, a sufficient period of time is required. As the current density increases, the adsorption rate increases. As a result, it is concluded that the ions adsorbed to the AER at a high current density are affected by the concentration of ions in the feed stream rather than the adsorption equilibrium. In fact, it can be seen that the amount of adsorbed chloride ions increases as the current density increases. Although the ion selectivity decreases with the current density, the CE-AMP16 and CE-SR7 electrodes were effective in increasing the selective removal rate of sulfate and nitrate ions, respectively.
4. Conclusions
An SCCE was manufactured by coating AER powders with different physicochemical properties on a carbon electrode surface. In addition, desalination experiments were conducted in a CDI cell and analyzed the ion selectivity of the SCCE according to the characteristics of the AERs.
As a result of analyzing the ion selectivity of the AERs in a mixed-anion solution, the selectivity of divalent sulfate ions was high for the AMP16 resin with a high IEC value. On the other hand, the SR7 resin with a low IEC showed high selectivity for nitrate ions. As a result of the CDI experiment, the higher the IEC of the resin was, the higher the capacitance of the SCCE. In addition, the fraction of adsorbed ions was consistent with the ion selectivity of the AER. The SCCE coated with the AMP16 and SR7 resins showed high selectivity for sulfate and nitrate ions, respectively. However, as the current density supplied to the cell increased, the proportion of ions adsorbed tended to deviate from the adsorption equilibrium results of the AERs.
Through this study, it was confirmed that the selective removal rate of specific ions could be increased by using an SCCE coated with an AER powder. In addition, the ion selectivity of an AER was judged to be closely related to the ion concentration inside the resin. Therefore, SCCEs with high selectivity to specific ions by controlling the IEC and water content of the resin are expected to be manufactured.