AbstractN-doped and N-F co-doped TiO2/bentonite composites were synthesized via the gel-sol method. The morphology, structure and surface charge of the composite before and after adsorption were used to determine the effect N/F doping ratio on TC removal. The results showed that, compared with undoped samples, the TC adsorption on N doped composites was reduced by 24.44% on average. N-F co-doping significantly increased the TC adsorption when the Ti-N-F molar ratio was 1:1: 0.01, reaching a maximum TC adsorption of 64.00 mmol·kg−1. The coverage of the N doped TiO2 increases as the N doping ratio increases; the specific surface area increased by 2.03 % on average, but the number of surface negative charges decreased by 36.24 % on average. FT-IR results confirmed that N doping reduced the number of -OH groups on the N-doped composites. Additionally, fluorination of N-F co-doped TiO2 and bentonite surfaces inhibits hydrogen bonding and π-π interactions between the TC and the composites. As the N doping ratio increased, the coverage of N-F co-doped TiO2 on the composite surface increased, resulting in the TC adsorption decrease with the increases N doping ratio.
1. IntroductionDue to the limited global water supply, water pollution has become a major scientific concern [1–3]. Photocatalytic oxidation was developed as an environmentally friendly technology because it can completely mineralize most organic pollutants [4, 5]. In recent years, titanium dioxide (TiO2) has been widely used to deal with water pollution due to its low cost and high efficiency [4]. However, the large band gap and small particle size of TiO2 lead to a lower light utilization rate and difficulties in recovering the particles in practical applications.
Doping is the primary way to reduce the bandgap of TiO2. Prior work has shown that the surface structure of TiO2 is significantly changed by doping different elements such as nitrogen, iron, and chromium [6]. Shaban et al. [7] prepared Ni-doped TiO2 nanotubes and confirmed that the nanotube structure gradually disappeared after Ni doping and a nanosheet structure began to appear. They also found that the specific surface area of Ni-doped TiO2 decreased and the degree of nanosheet aggregation changed with the Ni doping ratio. Yatim et al. [8] prepared vanadium and nitrogen co-doped TiO2 via the sol-gel method. They found that, after V-N co-doping, the specific surface area of the TiO2 increased by 100.99% compared with P25 TiO2. Thus, single or co-doping can cause complex changes in the surface structure of TiO2.
Difficulty in separation and recovery is another serious problem related to TiO2 in practical application [9, 10]. Because clay minerals (e.g., montmorillonite, kaolin, zeolite, etc.) are cheap, stable, and easy to obtain [11], many researchers have also recently loaded TiO2 on clays with larger particle sizes and excellent adsorption capacities to prepare doped TiO2/clay composite catalysts [12]. For TiO2/clay composites, numerous studies have shown that doping changed the surface structure of both TiO2 and the clay matrix to varying degrees [13–15]. Yan et al. [16] coated copper/nitrogen (Cu/N) co-doped TiO2 onto the surface of diatomite granule; their results demonstrated that TiO2 was evenly distributed on the surface, which significantly increased the specific surface area of the composite. The research on fluorine/ tungsten (F/W) co-doped TiO2-SiO2 composites by Liu et al. [13] confirmed that the structural network of the composite aerogel became loose as they increased the F/Ti molar ratio and the average pore size of the composite increased as they increased the F doping due to the destruction of Si-O-Si bonds by hydrofluoric acid (HF).
Adsorption is an essential pre-step in catalysis. For excellent adsorption performance, pollutants must contact and react with the catalyst very quickly, resulting in high removal efficiency [13, 17]. The adsorption performance is mainly affected by the surface structure and surface charge of the catalyst. Doping affects the surface structure of both the doped TiO2 and the clay substrate for composite materials, resulting in a change in the adsorption performance of the composite.
Tetracycline (TC) is the most widely-used antibiotic worldwide. The extensive use and untreated emissions of TC have led to its widespread presence in the water-soil environment. There has been much research on the adsorption and degradation of TC by doped TiO2 [18–20]. Nitrogen is a promising doping element due to its lower ionization energy and atomic size similar to oxygen, which can reduce the bandgap of TiO2 [19] and improve visible spectrum absorption [21]. Previous studies have confirmed that nitrogen doping affects the oxygen-containing functional groups and surface charge on the surface of TiO2. Fluorine has also been proven to be an effective anion doping element in recent years. [17], Fluorine doping reduces the charge recombination rate of TiO2 and increases the electronegativity of the TiO2 surface [22], thereby promoting the migration of TiO2 holes to the surface. In addition, the corrosive effects of fluorine on the surface of TiO2 will also change the surface structure (i.e., specific surface area and porosity) [23]. Both nitrogen and fluorine doping affect the charge properties and surface structure of TiO2, which will directly affect the adsorption behavior of pollutants on doped TiO2 or doped TiO2 composite materials.
However, prior work has mainly focused on the influence of doping on the bandgap and the recombination rate of electron and hole [7, 13]. The effect of doping on the adsorption process has not been reported. Because doping can change the surface structure of both TiO2 and clay mineral substrates, this will inevitably affect the adsorption process. Although doping may improve the catalytic efficiency of the TiO2/clay composite catalyst, its adsorption capacity may also be suppressed. When TiO2 is co-doped with multiple elements, the doping ratios of different elements may also affect the adsorption performance of the composite. These issues are of great significance for further research on stable and efficient catalysts.
In this work, nitrogen (N) - fluorine (F) co-doped TiO2/bentonite composites were prepared via the sol-gel method. The adsorption kinetics and equilibrium adsorption of tetracycline (TC) were studied to determine the role of dopants on the adsorption of (TC) by the N-F co-doped TiO2/bentonite composites. The surface structure (scanning electron microscopy, specific surface area and pore size distribution) and surface electrical properties (cation exchange capacity) were evaluated, and Fourier-transform infrared spectroscopy (FT-IR) before and after adsorption were compared to explore the adsorption mechanism. The objective of this study was to investigate the effects of doping on adsorption and provide the theoretical basis for subsequent research on efficient and stable catalysts.
2. Materials and Methods2.1. MaterialsTetracycline was purchased from Shanghai Aladdin Biochemical Technology Co. Ltd. Tetrabutyl titanate (TBOT), ethanol, urea (CH4N2O) and ammonia fluoride (NH4F) were analytical reagent (A.R) reagents purchased from Sinopharm Chemical Reagent Co. Ltd., Shanghai, China.
The Na-bentonite used in this study was obtained from Xinyang Tong Chuang Co. Ltd. Henan, China, and was purified according to our previous work [24]. Its total carbon content (TCC), cation exchange capacity (CEC) and Brunauer-Emmett-Teller surface area (SBET) were 4.98 g kg−1, 100.3 cmol+·kg−1, and 50.61 m2·g−1, respectively.
2.2. Synthesis of Undoped, Single and Co-doped TiO2/ Bentonite Composites (BD-TiO2)Undoped, single N doped and N-F co-doped TiO2/bentonite composites (BD-TiO2) were synthesized via the sol-gel method [25] (Fig. 1). 34 mL TBOT was added to 28.5 mL anhydrous ethanol while stirring (solution A). A certain amount of urea, ammonia fluoride, 5.4 mL deionized water, and 2.5 mL acetic acid were added to 28.5 mL anhydrous ethanol under agitation (solution B). Then, solution B was added to solution A dropwise at 2 mL·min−1 under vigorous stirring. 1 g of purified Na-bentonite was added into the mixture of solution A and B, then the mixture was stirred until a gel was formed. Finally, the gels were dried and ground to a fine powder then calcined at 400°C for 3 h in air (HS-1200X-A Heng Su, Zheng Zhou, China). After synthesis, all samples were ground and sieved. Fine powders smaller than 200 mesh size were used in the experiments.
Single N doped TiO2/bentonite composites were prepared without ammonia fluoride. no doped TiO2/bentonite composites were prepared without urea and ammonia fluoride. Pure TiO2 prepared without urea, ammonia fluoride, and Na-bentonite under similar conditions.
BD-TiO2 (NxFy) denotes the prepared N-F co-doped TiO2/bentonite composites, in which x and y represent the molar ratio of N and F to Ti. B indicates bentonite, and D indicates doped. For example, BD-TiO2 (N1F0.01) means that the molar ratio in the prepared sample is Ti: N: F = 1:1:0.01.
2.3. CharacterizationThe morphology of the composites was imaged by scanning electron microscopy (SEM, S-4800 Hitachi, Japan). The Brunauer-Emmett-Teller specific surface area (SBET), total pore volume and average pore size were determined by the specific surface aperture analyzer (Application V-Sorb 2800P, China). The CEC was determined by sodium-ammonium acetate method with flame photometer (FP-640 ShanHai YiFen, China). Fourier transform infrared spectrum (FT-IR) was performed by Bruker Tensor 27, Germany (wavenumber range: 4,000–400 cm−1), samples were diluted with KBr powder before the FT-IR spectra were recorded.
2.4. Batch Adsorption ExperimentAll adsorption experiments were carried out in the dark to avoid light degradation. Stock and working solutions were prepared freshly 1 h before use.
2.4.1. Effect of different N/F doping ratios on TC adsorptionTo evaluate the effect of the N/F doping ratio on TC adsorption, 0.1 g of pure TiO2, B-TiO2, single N doped sample or N-F co-doped sample was added into TC solution with different concentrations (pH = 7 and co-existing ionic strength was 0.01 mol·L−1 NaCl).
2.4.2. Effect of pH, temperature and ionic strength on adsorptionTo evaluate the effect of pH on the adsorption of TC, the initial pH values of the TC solutions at 0.67 mmol·L−1 were adjusted between 3 and 10 with 0.1 mol·L−1 HCl and NaOH. The experiment was conducted at 25°C and the co-existing ionic strength was 0.01 mol·L−1 NaCl. To evaluate the effects of temperature on TC adsorption, three different temperatures 10, 25 and 40°C were set, the TC solution was 0.67 mmol·L−1, pH = 7, and the co-existing ionic strength was 10 mol·mL−1 NaCl. To evaluate the effects of the co-existing ionic strength on the TC adsorption, the co-existing ionic strength (NaCl) of the TC solutions at 0.67 mmol·L−1 were set to 0, 0.01, 0.1 and 0.5 mol·L−1, pH = 7, and the experiment was conducted at 25°C.
2.4.3. Isothermal adsorptionIsothermal adsorption was carried out in batch experiments. The adsorption isotherm was investigated with initial TC concentrations of 0.03 to 0.67 mmol·L−1 under certain temperatures, pH values, and co-existing ionic strengths (NaCl).
For the isotherm adsorption study, 0.1 g samples and 20 mL of TC solution were added to a 50 mL polypropylene centrifuge tube. The centrifuge tubes were shaken horizontally (ZHICHENG ZWY-2012C, China) at 150 rpm for 1 h in the dark (determined by adsorption kinetic results, Text S1). After equilibrium was attained, the supernatants were filtered using a 0.45 μm filter. The TC concentration in the supernatant was analyzed using a UV-Vis Spectrophotometer at 358 nm (MAPADA UV-3200, China).
The adsorption of TC was determined by the difference between initial and equilibrium concentration:
where q is the TC adsorption of adsorbent (mmol kg−1), C0 and C are the initial and equilibrium concentrations of TC (mmol L−1), V is the volume of solution (mL), and m is the mass of adsorbent (g).
3. Results and Discussions3.1. MorphologySEM images of samples are shown in Fig. 2. The pure TiO2 had a spherical structure with an average particle size of 20–50 nm (Fig. 2(a)). Fig. 2(b) shows the undoped TiO2/bentonite composite (B-TiO2), whose particle size was similar to pure TiO2, indicating successful-bentonite loading. Fig. 2(c)–(e) shows the surface morphologies of BD-TiO2 samples with different N doping ratios (0.5, 1, and 2). At the N doping ratio increases, the particles size gradually decreases, while the coverage and accumulation of N doped TiO2 on the bentonite surface gradually increases. At an N doping ratio of 2, the bentonite surface was almost completely covered by N doped TiO2. Fig. 2(f) and Fig. 2(g) shows the morphological characteristics of BD-TiO2 (N1F0.01) and BD-TiO2 (N1F0.05), respectively. Compared with BD-TiO2 (N1), the coverage of N-F co-doped TiO2 on BD-TiO2 (N1F0.01) and BD-TiO2 (N1F0.05) surface was reduced, the exposed bentonite structure was loose, and large pores were visible. At an F doping ratio of 0.01, the particle size of N-F co-doped TiO2 on the BD-TiO2 (N1F0.01) surface was 25–50 nm, similar to the BD-TiO2 (N1) sample. At an F doping ratio of 0.05, the particle size of N-F co-doped TiO2 on the BD-TiO2 (N1F0.05) sample surface was smaller (20–30 nm).
The SEM results demonstrated that the degree of coverage of N doped TiO2 on the composite surface increased and the particle size of N doped TiO2 decreased as the N doping ratio increased. However, the degree of coverage of N-F co-doped TiO2 on the N-F co-doped TiO2/bentonite composites is reduced by co-doped F, and the bentonite structure becomes loose due to erosion by fluorine [13, 26].
3.2. BET Surface Area, Pore Size and CECThe N2 adsorption isotherm and pore width distribution curves of the TiO2, B-TiO2 and BD-TiO2 samples are shown in Fig. 2h and Fig. 2i. The pore width and pore volume are shown in Table 1. All samples showed an IV mesoporous linear N2 adsorption isotherm and H2 hysteretic loop, indicating that the pore size of all samples was evenly distributed. The pore structure was complicated; the mesopores were formed by the accumulation of spherical particles [27]. The porous structure formed by the doped TiO2 were consistent with the morphological characteristics observed by SEM. The average pore width of pure TiO2 and B-TiO2 were 4.59 nm and 8.23 nm, respectively. For N doped sample, the pore width increased from 8.10 to 8.93 nm, when the N doping ratio increased from 0.5 to 1 but decreased to 7.23 nm at an N doping ratio of 2. The pore volume gradually decreased as the N doping ratio increased (from 0.13 to 0.09 cm3·g−1). Compared with BD-TiO2 (N1), the pore widths of BD-TiO2 (N1F0.01) and BD-TiO2 (N1F0.05) were 12.55 and 14.51 nm, an increase of 40.54% and 62.49%, respectively. The pore volume of BD-TiO2 (N1F0.01) and BD-TiO2 (N1F0.05) increased by 33.33% on average. The pore width of the N doped samples first increased and then decreased, while the pore volume decreased, with an increase in the N doping ratio.
According to the morphological changes observed in the SEM images, the increased accumulation of doped TiO2 on the surface of the N doped samples led to an increase in the pore structure, thus increasing the pore width. As the N doping ratio increases, the greater coverage of N doped TiO2 causes the pores to be filled or blocked, resulting in reduced pore width and pore volume. For N-F co-doped samples, the erosion due to fluorine exposes the bentonite structure. The interlayer structure of the bentonite significantly increases the pore width and pore volume of the N-F co-doped samples and provides more adsorption sites for TC.
Table 1 also shows the SBET and CEC of TiO2, B-TiO2 and BD-TiO2 samples. Compared with TiO2, the CEC of B-TiO2 increased and the SBET decreased. This was ascribed to the larger particle size and pore size of bentonite; the SBET of B-TiO2 decreased by 26.49% compared to TiO2. However, there was a large number of negative charges on the bentonite surface, so the CEC of B-TiO2 increased by 45.06% compared with TiO2. The CEC of N doped composite samples decreased as N doping ratio increased. The mean reduction was 36.24 % compared with B-TiO2 due to the replacement of oxygen atoms in the TiO2 lattice by nitrogen, which reduces the number of oxygen-containing functional groups on the surface [9] and, thus, reduces in the negatively charged sites on the surface. For the N doped TiO2/bentonite composite samples, as the N doping ratio increased (0.5, 1 and 2), the SBET gradually increased (46.02, 46.33 and 48.06 m2 g−1), but only BD-TiO2 (N2) has a greater SBET value than B-TiO2 (46.49 m2 g−1). The SBET values of BD-TiO2 (N0.5) and BD-TiO2(N1) were lower than those of B-TiO2. The SBET was different from that measured in previous work on N doped TiO2 [6] or B/N co-doped TiO2 [28] due to the lower degree of coverage of N-doped TiO2 on the bentonite surface when the Ti/N molar ratio was 1:0.5 and 1:1.
Compared with BD-TiO2(N1), the CEC of BD-TiO2 (N1F0.01) and BD-TiO2 (N1F0.05) increased by 24.67% and 43.56 %, respectively. The SBET of BD-TiO2 (N1F0.01) and BD-TiO2 (N1F0.05) increased by 1.17 and 1.77 %, respectively. However, the eroding effect of fluorine on the surface of TiO2 and bentonite increased the pore size and volume of the composite and made the surface structure more complex (curled and loose as shown in the SEM images) and reduced the particle size of N-F co-doped TiO2. Thus, the SBET of the N-F co-doped composite significantly increased. The number of negative charges on the surface of the N-F co-doped TiO2 was increased by fluorination.
For N-doped TiO2/bentonite composites, N doping reduced the particle size of the TiO2, causing the specific surface area to increase; however, the pore structure of bentonite covered by N doped TiO2 resulted in a decrease in the average pore width. The increase in specific surface area of the N-F co-doped composite occurred due to the further reduction of the particle size and the F-driven erosion, which exposed the bentonite and significantly increased the number of macrospores on the surface of the composite, resulting in an increase in average pore width.
3.3. Adsorption StudiesThe adsorption isotherms and parameters of the composites are summarized in Fig. 3 and Table 2. The results of the adsorption experiments were fitted by the Langmuir and Freundlich models, and their corresponding coefficients of determination were R2 ≥ 0.8313 and 0.8595, respectively. The Freundlich model, which had the best fitting results, is expressed by equation (3):
Where qe is the equilibrium for TC adsorption and ce denotes the equilibrium concentration of TC in the aqueous phase. KF and 1/n represent the adsorption affinity and nonlinearity index of the Freundlich model, respectively.
Fig. 3(a) shows the influence of different N doping ratios on the TC adsorption. The TC adsorption of the N doped TiO2/bentonite composites was significantly higher than that of pure TiO2. The Freundlich model fit showed that the adsorption affinity (KF) of B-TiO2 increased by 132.85% compared to pure TiO2, confirming that bentonite significantly improved the TC adsorption capacity of the composites. The TC adsorption of N-doped TiO2/bentonite composite samples first increased and then decreased as the N doping ratio increased. Compared with B-TiO2, the TC adsorption of BD-TiO2 (N0.5), BD-TiO2 (N1) and BD-TiO2 (N2) decreased by 24.37, 19.41 and 29.53 %, respectively. The adsorption affinity (KF) gradually decreased from 62.92 to 60.93 mmol kg−1 L1/n mmol−1/n as the N doping ratio increased, which was similar to the CEC. Compared with BD-TiO2, the CEC of single N doped samples with different doping ratios (0.5,1, and 2) decreased by 19.64, 47.97, and 41.11%, respectively. Thus, the decrease in CEC of the N doped TiO2 composite samples was caused by N doping, which primarily caused the decrease in TC adsorption.
Fig. 3(b)–(d) shows the effect of N-F co-doping on TC adsorption, demonstrating that doping with both F and N (0.5 and 1 doping) improves the TC adsorption. Compared with BD-TiO2 (N0.5), the TC adsorption of BD-TiO2 (N0.5F0.01) and BD-TiO2 (N0.5F0.05) increased by 23.23% and 36.25%, respectively. Compared with BD-TiO2 (N1), the TC adsorption of BD-TiO2 (N1F0.01), and BD-TiO2 (N1F0.05) increased by 31.31% and 29.38%, respectively. However, when the N doping ratio was 2, the TC adsorption of BD-TiO2 (N2F0.01) increased by 15.34% and BD-TiO2 (N2F0.05) decreased by 0.80%, respectively. Comparing the KF (adsorption affinity) before and after F doping shows that, at an N doping ratio of 1, N-F co-doping increased TC adsorption, the KF increased by 55.28% on average. The maximum TC adsorption was 64.00 mmol·kg−1 for BD-TiO2 (N1F0.01) indicating that co-doping F increased TC adsorption. However, an excessive N doping ratio decreased the adsorption of TC. The 1/n values (Table 2) obtained from the Freundlich model fitted data were distributed from 0.1 to 0.5 indicating supportive sorption.
The isothermal adsorption results demonstrated that F doping increases the TC adsorption of N-F co-doped TiO2/bentonite samples for N doping ratio of 0.5 and 1. The increase in TC adsorption is due to (i) an increase in the CEC arising from F doping, causing an increase in the electrostatic attraction between the TC and N-F co-doped TiO2/bentonite samples, and (ii) the erosive effect of F on the surface of the N-F co-doped TiO2 and bentonite, which increased the SBET, pore size, and pore volume, providing more surface adsorption sites for TC [29].
However, at an N doping ratio of 2, the adsorption of TC on N-F co-doped composites began to decrease due to the high coverage of the N doped TiO2 on the composite samples. At the same time, when the negative charge on the N-F co-doped TiO2 surface was increased by fluorination, the hydrophilicity was also enhanced. The excessive coverage and increased hydrophilicity of the N-F co-doped TiO2 surface jointly weakened the distribution of TC and reduced the adsorption of TC.
3.4. Effect of pH, Temperature and Co-existing Ionic Strength on the TC Adsorption
Fig. 4(a)–(c) shows that the adsorption of TC on B-TiO2, BD-TiO2 (N1), and BD-TiO2 (N1F0.01) gradually increased with increasing temperature (10–40°C). The thermodynamic calculation results (Table S2) confirm that the adsorption of TC on the surface of the composite material is a spontaneous reaction with an increase in endothermic entropy, and the spontaneity of TC adsorption on the composite material increases with increasing temperature, which is consistent with the results of the adsorption experiment. TC can easily break the adsorption energy barrier at higher temperature [30], resulting in an increase in TC adsorption. Compared with B-TiO2, the TC adsorption spontaneity and the disorder of the solid-liquid interface on the N doped composite surface were reduced and the co-doping of F increases the adsorption spontaneous of TC.
Fig. 4(d) and (e) shows the effects of pH and ionic strength on the TC adsorption of B-TiO2, BD-TiO2 (N1), and BD-TiO2 (N1F0.01). Fig. 4(d) shows that within the pH range of 4~8, the TC adsorption of B-TiO2, BD-TiO2 (N1) and BD-TiO2 (N1F0.01) gradually decreased with increasing pH. At pH > 8, the TC adsorption of B-TiO2, BD-TiO2 (N1), and BD-TiO2 (N1F0.01) significantly decreased. TC is an amphoteric molecule with a variety of functional groups. The electrical properties of TC change depending on the pH. At pH > 7, TC exists as an anion (TCH−/TC2−) [31], thus, the repulsion between the samples surface and TC was enhanced and the TC could not reach the sample surface, so the TC adsorption was significantly reduced.
Fig. 4(e) shows the effect of co-existing ionic strength on TC adsorption. As the co-existing ionic strength increased from 0.01 to 0.5 mol·L−1 (NaCl), the TC adsorption of B-TiO2, BD-TiO2 (N1), and BD-TiO2 (N1F0.01) gradually decreased. BD-TiO2 (N1) decreased more (19.54%) than B-TiO2 and BD-TiO2 (N1F0.01), which decreased by 11.32% and 12.37%, respectively. This can be ascribed to N doping reducing in the number of negative charges on the surface of BD-TiO2 (N1), which reduced the number of electrostatic attraction sites. The coexisting cations (Na+) will compete for these adsorption sites [32]. Thus, the effect of co-existing ionic strength on TC adsorption was more substantial. For B-TiO2 and BD-TiO2 (N1F0.01), the decrease in TC adsorption caused by coexisting cations was not clear due to the presence of more negatively-charged sites on the surface of B-TiO2 and BD-TiO2 (N1F0.01). Thus, N-F co-doped TiO2/bentonite composites have better adsorption capacities under high temperature (40°C), acidic (pH < 7) and low co-existing ionic strength (0.01 mol·L−1 NaCl) conditions.
3.5. FT-IR Analysis
Fig. 5(a) shows the FT-IR spectra of BD-TiO2 (N1) and BD-TiO2 (N1F0.01) before and after TC adsorption. The bands at 468 cm−1 represent the Ti-O stretching and Ti-O-Ti stretching modes. The peaks at around 1,000–1,100 cm−1 were assigned to Si-O stretching [33] and the peak at around 3,450 cm−1 was assigned to the stretching vibration of surface -OH groups [34]. For TC, the band at 3,405, 1,647 and 1,602 cm−1 can be assigned to the amide (NH2), dimethylamine, and C=O stretching, respectively [35].
Fig. 5(b) shows the Ft-IR spectrum of BD-TiO2 (N1). There were weak peaks corresponding to the stretching vibration of -OH group at 3,400 cm−1. However, -OH group peaks were observed for the BD-TiO2 (N1F0.01) sample at 3,420 cm−1, which indicated more surface -OH groups on the surface of the N-F co-doped samples [21]. As shown in Fig. 5(b), the TC has an -NH2 stretching vibration peak (sharp and large amplitude) at 3,405 cm−1 [36]. When TC adsorbed onto the surface of BD-TiO2 (N1) and BD-TiO2 (N1F0.01), the -OH peak approached 3405 cm−1. This indicates that the amide group of TC binds to the hydroxyl group on the surface of the BD-TiO2 samples by a hydrogen bond [37]. The peak shift range of -OH for BD-TiO2 (N1) after adsorption (3,400 to 3,404 cm−1) was more significant than that of BD-TiO2 (N1F0.01) (3,420 to 3,419 cm−1), which confirmed that F doping reduced the hydrogen bonding between TC and the N-F co-doped TiO2 composite. This may be due to fluorination, which enhances the hydrophilicity of the TiO2 surface [17].
The peaks of the test samples at 1,800–500 cm−1 are shown in Fig. 5(c). Compared with BD-TiO2 (N1), the Ti-O-Ti vibration peak of BD-TiO2 (N1F0.01) at 468 cm−1 shift to 467 cm−1, which is attributed to the interaction between some compounds and the TiO2 surface [38]; this confirmed the fluorination of the N-F co-doped TiO2 surface in this work. After TC adsorption, the Ti-O-Ti peaks of BD-TiO2 (N1) and BD-TiO2 (N1F0.01) shifted to 471 and 469 cm−1, respectively. This may be due to electrostatic attraction between TC and doped TiO2 on the composite surface. However, the shifting range of BD-TiO2 (N1F0.01) is lower than BD-TiO2 (N1) due to the lower surface coverage of N-F co-doped TiO2.
The Si-O peaks of BD-TiO2 (N1) and BD-TiO2 (N1F0.01) were at 1,042 and 1,048 cm−1, respectively, due to increased exposure of bentonite on the surface of BD-TiO2 (N1F0.01). After TC adsorption, the Si-O peak of BD-TiO2 (N1) and BD-TiO2 (N1F0.01) shifted to 1,046 and 1,042 cm−1, respectively. This indicates the breaking of the Si-O bond after adsorption [39], and TC is adsorbed on the surface of the bentonite by electrostatic attractions. The shift range of BD-TiO2 (N1F0.01) is more significant than BD-TiO2 (N1), indicating more Si-O bond action with TC on the BD-TiO2 (N1F0.01) surface. This confirms that there is a difference in the electrostatic attraction of TC on BD-TiO2 (N1) and BD-TiO2 (N1F0.01) surfaces. For the N doped composite, the electrostatic attraction is mainly between TC and N doped TiO2, while the electrostatic attraction between TC and bentonite is relatively weak. For the N-F co-doped TiO2 composite, the electrostatic attraction between TC and bentonite is stronger.
TC has a significant C=O group stretching vibration peak at 1,603 cm−1 [40], and the peak of BD-TiO2 (N1) and BD-TiO2 (N1F0.01) at around 1,630 cm−1 was shifted toward the low-frequency direction after TC adsorption. The peaks of BD-TiO2 (N1) and BD-TiO2 (N1F0.01) moved from 1,629 to 1,625 cm−1 and 1,628 to 1,626 cm−1, respectively. This confirmed that a portion of TC was adsorbed on BD-TiO2 (N1) and BD-TiO2 (N1F0.01) by π-π inductance coupling [6], and F co-doping also weakens π-π interactions [40]. FT-IR results suggest that the TC and N-F co-doped TiO2/bentonite primarily combine through electrostatic attraction, hydrogen bonding, and π-π interaction. Doping F reduces hydrogen bonding and π-π interaction between TC and N-F co-doped TiO2 but enhances electrostatic attraction.
3.6. Mechanism of the Effect of the N/F Co-doping Ratio on TC Adsorption
Fig. 3 shows that the adsorption isotherms of TC on undoped samples, N doped, and N-F co-doped composite samples were all non-linear. The Freundlich model was suitable for describing the adsorption of TC on all samples, indicating that the adsorption of TC occurred on a non-uniform surface [32]. TC is amphiphilic in nature with multiple ionizable functional groups dimethylamine, tricarbonyl methane, and phenolic diketone groups. These functional groups can be protonated or deprotonated to form the cationic species TCH3+ (pH < 3.3), the zwitterionic species TCH20/TCH2± (3.3 < pH < 7.7), or the anionic species TCH−, TC2− (pH > 7.7) (Qi et al. 2018). Therefore, at pH 7, the TC molecule and the adsorbent combine via hydrogen bonding, π-π bond interactions, and electrostatic attraction [31].
The mechanism of the effect of the N/F co-doping ratio on TC adsorption is shown in Fig. 6. For the N doped TiO2/bentonite composites, research by Yao et al. [41] and Chen et al. [9] confirmed that the N is mainly doped into the TiO2 lattice, replacing the oxygen atom to form Ti-N-O or N-Ti-O bonds. A decrease in the number of oxygen-containing functional groups (such as -OH) on the surface of N doped TiO2 reduces the number of hydrogen bonding sites on the surface. N doping inhibits the lattice growth of TiO2 [41]; therefore, as the N doping ratio increases, the particle size of the N doped TiO2 decreases, and the degree of coverage increases, resulting in an increase in SBET. But with the increasing coverage of N doped TiO2, the pores on the surface of the N-doped TiO2/bentonite composites were filled or blocked, resulting in decreases in pore size and pore volume. Thus, weakened of hydrogen bonding and reduced pore structure were the main reasons for the decrease in the TC adsorption after N doping. For N doped TiO2/bentonite samples, the effect of ionic strength on the TC adsorption became more significant due to the decrease in the number of surface negative charges.
For N-F co-doped TiO2/bentonite samples, fluorine was mainly doped by forming TiOF2 on the surface of TiO2. Due to the strong electronegativity of fluorine [13], the fluoride formed on the TiO2 surface significantly increased the negative charge on the surface of the N-F co-doped TiO2/bentonite composites and the number of electrostatic attraction sites increased. Moreover, due to erosion, the particle size of the N-F co-doped TiO2 on the composite surface was smaller and the interlayer structure of bentonite was more obvious, which enhanced the electrostatic attraction between TC and bentonite. This is consistent with the results reported by Liu et al. [13] on F/W co-doped TiO2-SiO2 composites. Erosion increased the pore width and pore volume of the N-F co-doped TiO2/bentonite composites, allowing the TC molecules (the size is about 12.6, 8.3 and 6.1 Å, calculation by Chem3D 15.1.0.144, PerkinElmer) to easily enter into the pores of the composite. In summary, the increased negative charges, enhanced electrostatic attraction and increased pore size were responsible for the increase in TC adsorption and initial adsorption rate for the N-F co-doped TiO2/bentonite composite. However, when the N doping ratio was 2, the adsorption of TC began to decrease after N-F co-doping due to the increase in the N-doping ratio; the coverage of N-F co-doped TiO2 on the bentonite surface gradually increased. The exposed bentonite structure covered by N-F co-doped TiO2 resulted in a weakening of the electrostatic attraction between TC and bentonite and a further weakening of hydrogen bonding.
4. ConclusionIn this work, the effects of different nitrogen and fluorine doping ratios on tetracycline adsorption by N doped TiO2/bentonite and N-F co-doped TiO2/bentonite composites were investigated and the mechanism was also evaluated. The TC adsorption by single N-doped TiO2/bentonite composites decreased due to the weakening of hydrogen bonds and the reduction of the pore size. The TC adsorption of N-F co-doped TiO2/bentonite composites was greater than that of single N-doped TiO2/bentonite composites. This was mainly due to the fluorination and erosion of fluorine on the co-doped TiO2 and bentonite surface, resulting in more electrostatic attraction sites, greater specific surface area, and increased pore size for N-F co-doped TiO2/bentonite composites. However, excessive N doping ratio suppressed the hydrogen bonding between TC and bentonite while reducing the pore size of N-F co-doped TiO2/bentonite composites, thereby inhibiting TC adsorption.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (No. 41271244).
NotesAuthor Contributions T.W. (Ph.D. student) conducted all the experiments and wrote the manuscript. Z.Z.M. (Professor) project administration, funding acquisition and revised the manuscript. X.X.W. (Master students) execution, data curation, and validation. A.A. (Associate Professor) revised the manuscript. X.W.C. (Master students) data curation, and validation. L. L. (Ph.D. student) data curation, and validation. References1. Ray JR, Shabtai IA, Teixido M, Mishael YG, Sedlak DL. Polymer-clay composite geomedia for sorptive removal of trace organic compounds and metals in urban stormwater. Water Res. 2019;157:454–462.
2. Li H, Shan C, Li W, Pan B. Peroxymonosulfate activation by iron(III)-tetraamidomacrocyclic ligand for degradation of organic pollutants via high-valent iron-oxo complex. Water Res. 2018;147:233–241.
3. Fan Y, Ji Y, Kong D, Lu J, Zhou Q. Kinetic and mechanistic investigations of the degradation of sulfamethazine in heat-activated persulfate oxidation process. J Hazard Mater. 2015;300:39–47.
4. Sharma PK, Cortes MALRM, Hamilton JWJ, Han Y, Byrne JA, Nolan M. Surface modification of TiO2 with copper clusters for band gap narrowing. Catal Today. 2019;321–322:9–17.
5. Dhakshinamoorthy A, Li Z, Garcia H. Catalysis and photocatalysis by metal organic frameworks. Chem Soc Rev. 2018;47(22)8134–8172.
6. Tang X, Wang Z, Wang Y. Visible active N-doped TiO2/reduced graphene oxide for the degradation of tetracycline hydrochloride. Chem Phys Lett. 2018;691:408–414.
7. Shaban M, Ahmed AM, Shehata N, Betiha MA, Rabie AM. Ni-doped and Ni/Cr co-doped TiO2 nanotubes for enhancement of photocatalytic degradation of methylene blue. J Colloid Interface Sci. 2019;555:31–41.
8. Yatim AAM, Ismail NA, Hamid MRY, et al. Vanadium and Nitrogen Co-Doped Titanium Dioxide (TiO2) with Enhanced Photocatalytic Performance: Potential in Wastewater Treatment. J Nanosci Nanotechnol. 2020;20(2)741–751.
9. Chen Y, Wu Q, Bu N, Wang J, Song Y. Magnetic recyclable lanthanum-nitrogen co-doped titania/strontium ferrite/diatomite heterojunction composite for enhanced visible-light-driven photocatalytic activity and recyclability. Chem Eng J. 2019;373:192–202.
10. Jo W-K, Natarajan TS. Influence of TiO2 morphology on the photocatalytic efficiency of direct Z-scheme g-C3N4/TiO2 photocatalysts for isoniazid degradation. Chem Eng J. 2015;281:549–565.
11. Bartolomei SS, Santana J, Valenzuela FR. Investigation of the effect of titanium dioxide and clay grafted with glycidyl methacrylate by gamma radiation on the properties of EVA flexible films. Radia Phys Chem. 2020;169:107973.
12. Ahmadi M, Ramezani MH, Jaafarzadeh N, et al. Enhanced photocatalytic degradation of tetracycline and real pharmaceutical wastewater using MWCNT/TiO2 nano-composite. J Environ Manage. 2017;186(Pt 1)55–63.
13. Liu J, Liu J, Shi F, et al. F/W co-doped TiO2-SiO2 composite aerogels with improved visible light-driven photocatalytic activity. J Solid State Chem. 2019;275:8–15.
14. Shafiee S, Zarrebini M, Naghashzargar E, Semnani D. Antibacterial performance of nano polypropylene filter media containing nano-TiO2 and clay particles. J Nanopart Res. 2015;17(10)
15. Wang N, Feng J, Chen J, Wang J, Yan W. Adsorption mechanism of phosphate by polyaniline/TiO2 composite from wastewater. Chem Eng J. 2017;316:33–40.
16. Chen Y, Wu Q, Wang J, Song Y. Self-floating Cu/N co-doped TiO2/diatomite granule composite with enhanced visible-light-responsive photoactivity and reusability. J Chem Technol Biotechnol. 2019;94(4)1210–1219.
17. Samsudin EM, Abd Hamid SB, Juan JC, Basirun WJ, Centi G. Synergetic effects in novel hydrogenated F-doped TiO2 photocatalysts. Appl Surf Sci. 2016;370:380–393.
18. Bui TS, Bansal P, Lee B-K, Mahvelati-Shamsabadi T, Soltani T. Facile fabrication of novel Ba-doped g-C3N4 photocatalyst with remarkably enhanced photocatalytic activity towards tetracycline elimination under visible-light irradiation. Appl Surf Sci. 2020;506.
19. Ghoreishian SM, Ranjith KS, Lee H, et al. Hierarchical N-doped TiO2@Bi2WxMo1-xO6 core-shell nanofibers for boosting visible- light-driven photocatalytic and photoelectrochemical activities. J Hazard Mater. 2020;391.
20. Mai Hung TT, Nguyen TDC, Doan Van T, et al. Novel direct Z-scheme AgI/N-TiO2 photocatalyst for removal of polluted tetracycline under visible irradiation. Ceram Int. 2020;46(5)6012–6021.
21. Li G, Zou B, Feng S, et al. Synthesis of N-Doped TiO2 with good photocatalytic property. Physica B: Condensed Matter. 2020;588:412184.
22. Gao Q, Si F, Zhang S, Fang Y, Chen X, Yang S. Hydrogenated F-doped TiO2 for photocatalytic hydrogen evolution and pollutant degradation. Int J Hydrogen Energy. 2019;44(16)8011–8019.
23. Dozzi MV, D’Andrea C, Ohtani B, Valentini G, Selli E. Fluorine-Doped TiO2 Materials: Photocatalytic Activity vs Time-Resolved Photoluminescence. J Physic Chem C. 2013;117(48)25586–25595.
24. Li WB, Liu Z, Meng ZF, et al. Composite modification mechanism of cationic modifier to amphoteric modified kaolin and its effects on surface characteristics. Int J Environ Sci Technol. 2016;13(11)2639–2648.
25. Chen Y, Liu K. Preparation and characterization of nitrogen-doped TiO2/diatomite integrated photocatalytic pellet for the adsorption-degradation of tetracycline hydrochloride using visible light. Chem Eng J. 2016;302:682–696.
26. Li D, Ohashi N, Hishita S, Kolodiazhnyi T, Haneda H. Origin of visible-light-driven photocatalysis: A comparative study on N/F-doped and N–F-codoped TiO2 powders by means of experimental characterizations and theoretical calculations. J Solid State Chem. 2005;178(11)3293–3302.
27. Thiruppathi M, Selvakumar K, Arunpandian M, et al. An affordable photocatalyst for pharmaceuticals and superior electrocatalyst for methanol oxidation – A dual role by CuWO4 anchored bentonite clay. Colloids Surf, A. 2019;563:148–159.
28. Zeng X, Sun X, Yu Y, Wang H, Wang Y. Photocatalytic degradation of flumequine with B/N codoped TiO2 catalyst: Kinetics, main active species, intermediates and pathways. Chem Eng J. 2019;378:
29. Chang P-H, Li Z, Jean J-S, Jiang W-T, Wang C-J, Lin K-H. Adsorption of tetracycline on 2:1 layered non-swelling clay mineral illite. Appl Clay Sci. 2012;67–68:158–163.
30. Zhang T, Wu X, Shaheen SM, et al. Ammonium nitrogen recovery from digestate by hydrothermal pretreatment followed by activated hydrochar sorption. Chem Eng J. 2020;379:122254.
31. Zhao Y, Gu X, Gao S, Geng J, Wang X. Adsorption of tetracycline (TC) onto montmorillonite: Cations and humic acid effects. Geoderma. 2012;183–184:12–18.
32. Li Z, Schulz L, Ackley C, Fenske N. Adsorption of tetracycline on kaolinite with pH-dependent surface charges. J Colloid Interface Sci. 2010;351(1)254–260.
33. Chang P-H, Li Z, Jean J-S, Jiang W-T, Wang C-J, Lin KH. Adsorption of tetracycline on 2:1 layered non-swelling clay mineral illite. Appl Clay Sci. 2012;67–68:158–163.
34. Wang W, Lin J, Cheng J, et al. Dual super-amphiphilic modified cellulose acetate nanofiber membranes with highly efficient oil/water separation and excellent antifouling properties. J Hazard Mater. 2020;385:121582–121582.
35. Qi N, Wang P, Wang C, Ao Y. Effect of a typical antibiotic (tetracycline) on the aggregation of TiO2 nanoparticles in an aquatic environment. J Hazard Mater. 2018;341:187–197.
36. Yen CH, Lien HL, Chung JS, Yeh HD. Adsorption of precious metals in water by dendrimer modified magnetic nanoparticles. J Hazard Mater. 2017;322(Pt A)215–222.
37. Shi Y, Yang Z, Wang B, An H, Chen Z, Cui H. Adsorption and photocatalytic degradation of tetracycline hydrochloride using a palygorskite-supported Cu2O–TiO2 composite. Appl Clay Sci. 2016;119:311–320.
38. Lu D, Zhang G, Wan Z. Visible-light-driven g-C3N4/Ti3+-TiO2 photocatalyst co-exposed {001} and {101} facets and its enhanced photocatalytic activities for organic pollutant degradation and Cr(VI) reduction. Appl Surf Sci. 2015;358:223–230.
39. Wan D, Li W, Wang G, Chen K, Lu L, Hu Q. Adsorption and heterogeneous degradation of rhodamine B on the surface of magnetic bentonite material. Appl Surf Sci. 2015;349:988–996.
40. Wan Y, Bao Y, Zhou Q. Simultaneous adsorption and desorption of cadmium and tetracycline on cinnamon soil. Chemosphere. 2010;80(7)807–812.
41. Yao Y, Qin J, Chen H, et al. One-pot approach for synthesis of N-doped TiO2/ZnFe2O4 hybrid as an efficient photocatalyst for degradation of aqueous organic pollutants. J Hazard Mater. 2015;291:28–37.
Fig. 2The SEM images of TiO2 (a), B-TiO2 (b), BD-TiO2(N0.5) (c), BD-TiO2(N1) (d), BD-TiO2(N2) (e), BD-TiO2(N1F0.01) (f), BD-TiO2(N1F0.05) (g). The arrow shows the pore structure and bentonite structure of the sample surface. The N2 adsorption-desorption isotherms (h) and pore size distribution (i) of the BD-TiO2 samples. 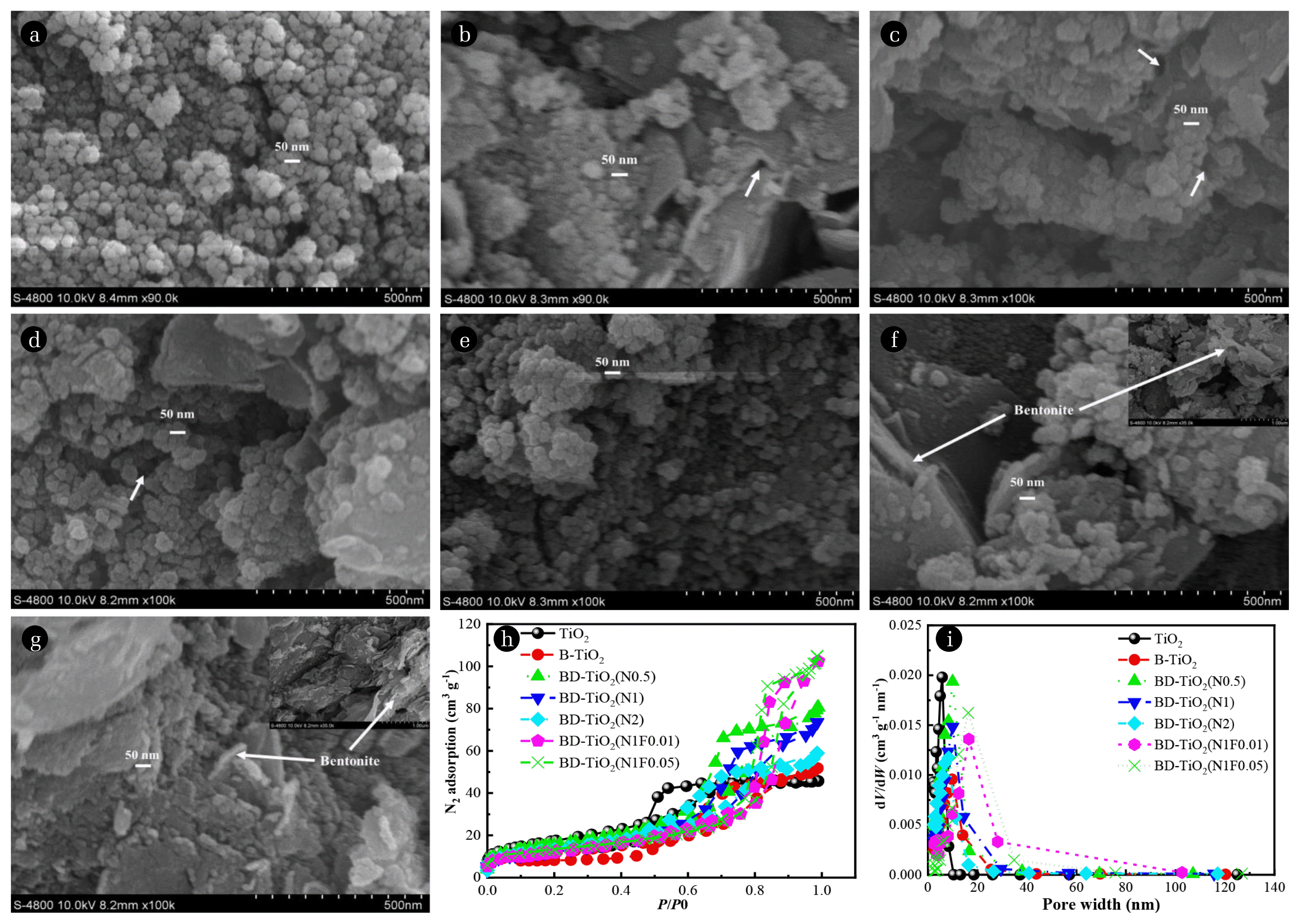
Fig. 3Adsorption isotherm of samples with different nitrogen doping ratios (a), with different fluorine doping ratios when nitrogen doping ratio is 0.5 (b), with different fluorine doping ratios when nitrogen doping ratio is 1 (c), with different fluorine doping ratios when nitrogen doping ratio is 2 (d). pH = 7, t = 25°C and co-existing ionic strength was 0.01 mol·L−1 NaCl. Line is Freundlich fit to the observed data. 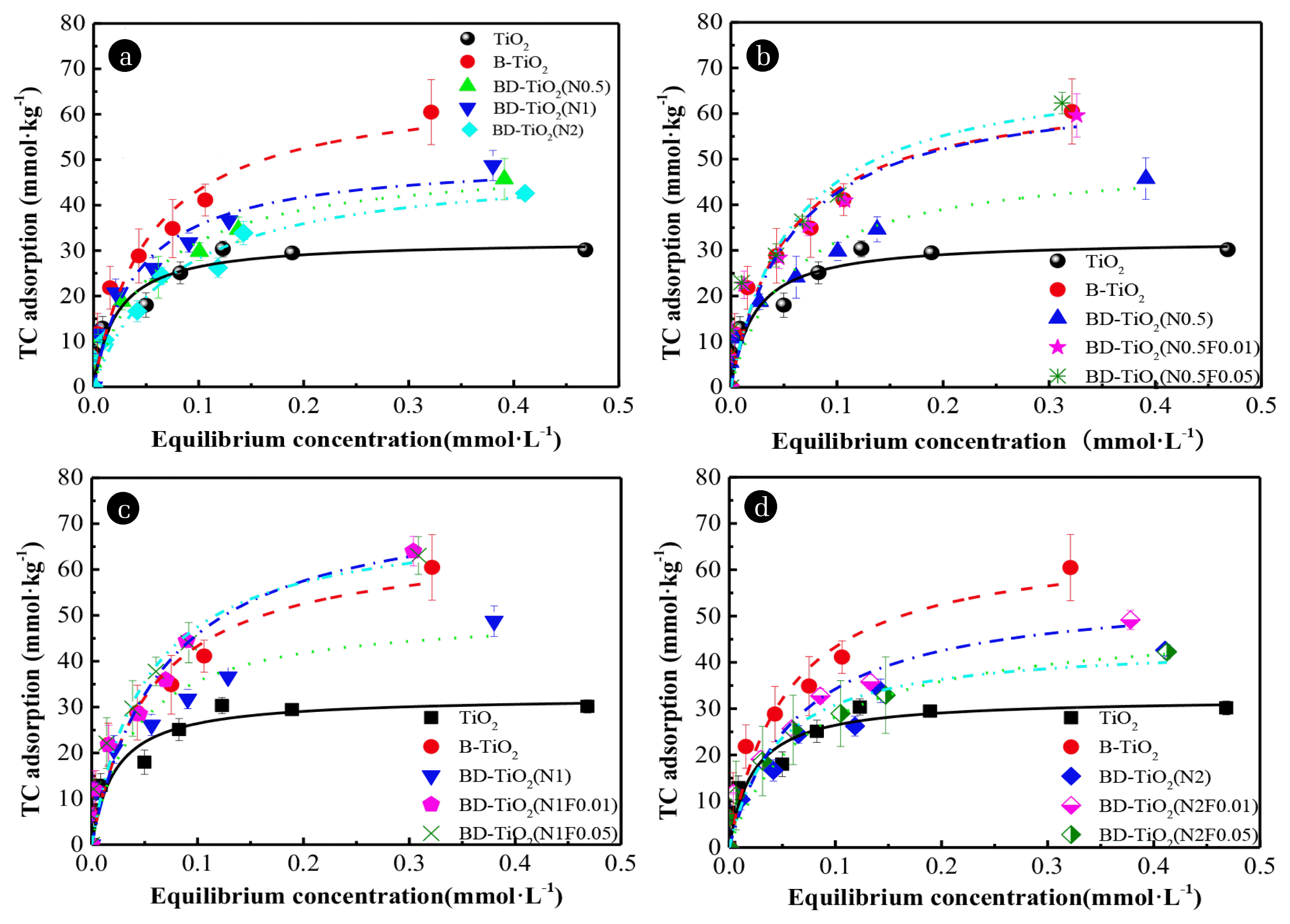
Fig. 4Effect of temperature on TC adsorption, t=25°C and co-existing ionic strength was 0.01 mol·L−1 NaCl. (a) B-TiO2, (b) BD-TiO2 (N1), (c) BD-TiO2 (N1F0.01). Effect of pH (t=25°C and co-existing ionic strength was 0.01 mol·L−1 NaCl) (d) and co-existing ionic strength (pH=7 and t=25°C) (e) on the TC adsorption. TC concentration is 0.67 mmol L−1. 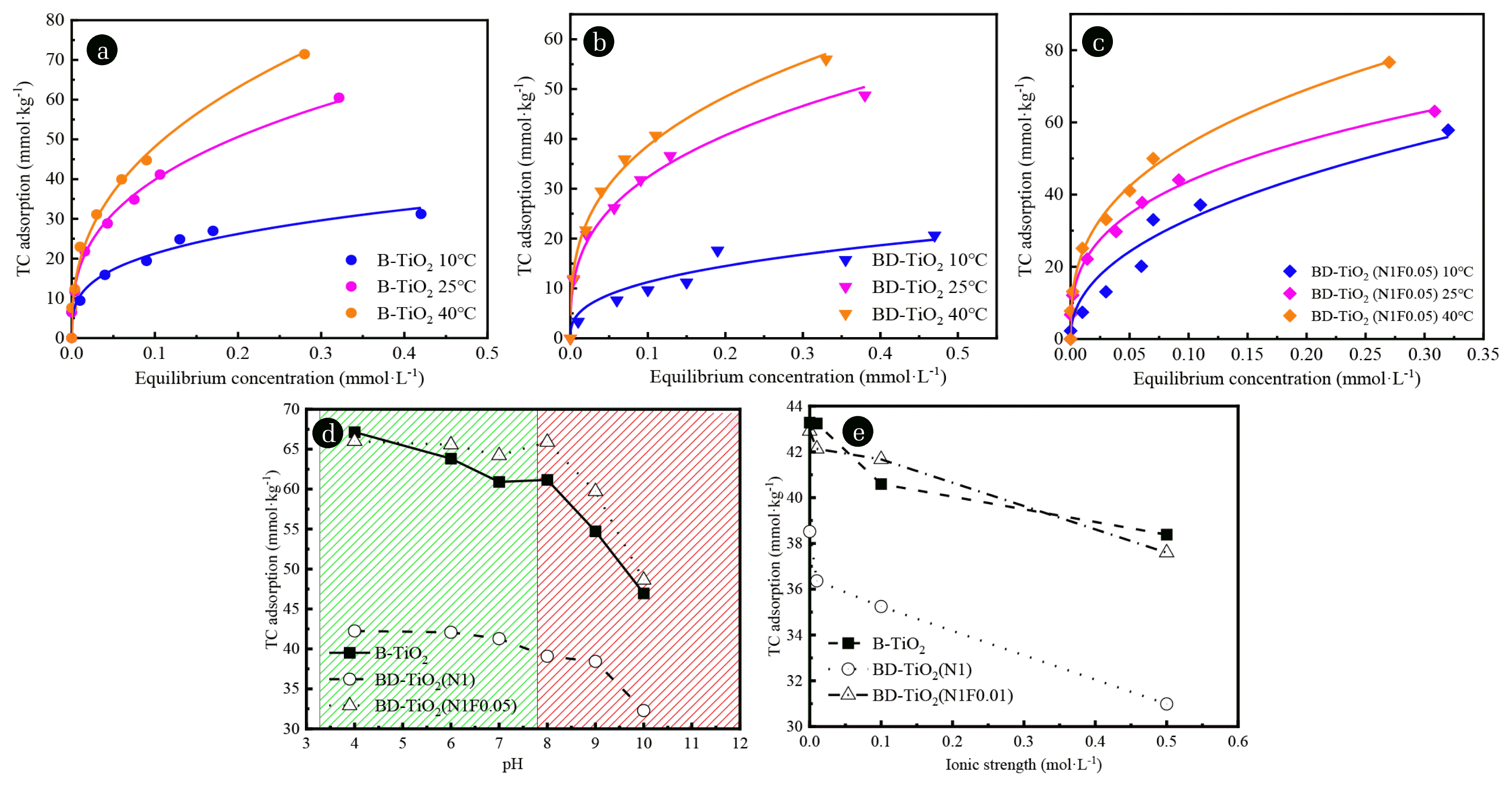
Fig. 5FT-IR spectrum for BD-TiO2(N1) and BD-TiO2(N1F0.01) (a) before and after adsorption, enlarged FT-IR spectrum (b) from 3,900 to 3,000 cm−1, (c) 1,800–400 cm−1. 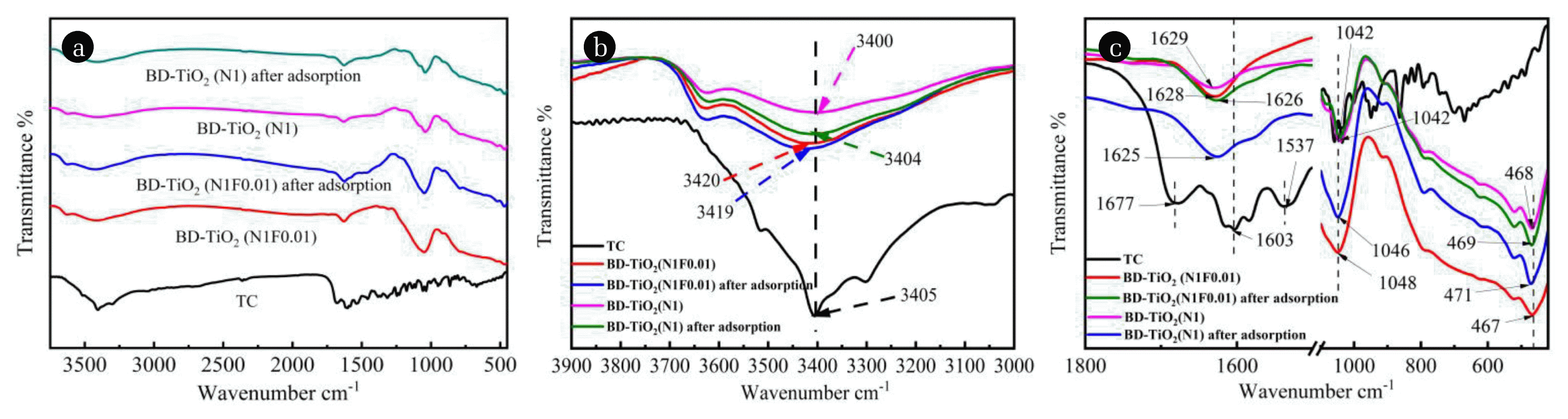
Table 1Specific Surface Area and CEC of TiO2, B-TiO2 and BD-TiO2 Samples Table 2The Parameters for the Langmuir and Freundlich Models
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||